Abstract
Background:
The present study addressed the interesting findings of supplemental evaluation of hepatitis B “seroyield” donors.
Materials and Methods:
Each blood donor sample was tested for anti-human immunodeficiency virus type I (HIV-I)/HIV type II (HIV-II), HBsAg, and anti-hepatitis C virus (HCV) antibody by enhanced chemiluminescence method and subjected to individual donor-nucleic acid testing (NAT) for HIV-I, hepatitis B virus (HBV), and HCV. NAT test was performed using the eSAS system, Procleix Ultrio Assay, Novartis Diagnostics, CA, US. Confirmation of HBsAg was done using HBsAg Confirmatory Kit (Ortho Clinical Diagnostics, Johnson & Johnson, USA) and viral load assessment was done using Cobas TaqMan real time-polymerase chain reaction (RT-PCR) assay (Roche Molecular Systems, Branchburg, NJ, USA). To provide information on the stage of infection, specimens were tested for anti-HBc total (IgG + IgM), anti-HBc IgM and HBeAg. HBeAg-negative samples were tested for anti-HBe antibody.
Results:
A total of 60 hepatitis B seroyield donors which showed mean initial sample/cutoff of 1.6 with enhanced chemiluminescence assay were investigated further for confirmation of disease status. All 60 cases were confirmed positive with neutralization assay (VITROS HBsAg Confirmatory Kit) while no target was detected on viral load assessment with RT-PCR. Sixteen donors were HBeAg positive (4 IgM anti-HBc positive and 12 IgM anti-HBc negative) and 44 were IgM anti-HBc negative, anti-HBc total positive, and anti-HBe positive.
Conclusion:
About 7.7% of HBsAg positive and NAT nonreactive donors (nondetectable HBV DNA) could be potentially infectious (HBeAg positive), whereas rest of the donors were consistent with chronic HBV infection.
Keywords: Enhanced chemiluminescence assay, hepatitis B seroyield, supplemental evaluation
Introduction
Considerable improvement in blood safety is observed in recent years, and there is almost negligible transmission, especially of viral infections (hepatitis B virus [HBV], hepatitis C virus [HCV], and human immunodeficiency virus [HIV]) in the developed world.[1,2] However, in India, prevalence of these viral infections in donor and patient populations is high due to the lack of regular repeat voluntary blood donations and the lack of second tier nucleic acid testing (NAT) of the blood donors at pan India level.[3] As per our national guidelines, it is mandatory to screen the donated blood by serological assays for HBsAg, HCV antibody and HIV-I/II antibody.[4] Although the high sensitivity of the current serological assays has shortened the window period, they are still not able to identify the number of newly infected donors that makes these serological assays insufficient in preventing viral transmission by blood transfusion.
In recent years, newer highly sensitive screening method, for example, NAT to detect HIV-I, HCV RNA and HBV DNA, is in practice in some centers of India. NAT has added an additional layer of safety to the donated blood by further narrowing the window period. Benefit of NAT as a second tier of testing has been demonstrated through detection of units termed “NAT” yield, which is serology nonreactive, but NAT reactive. Single sample NAT (individual donor [ID]-NAT) can detect low levels of viral DNA and RNA, which is highly sensitive and specific for viral nucleic acids and also has the ability to detect viral mutants and occult infections.[5] This assay found to be more sensitive than even the most sensitive serological assays.
However, in some stages of infection, level of nucleic acids is extremely low that serologic screening rather than NAT screening may be more effective mainly in case of HBV infection. Studies indicate that 0.5-1.0% of anti-HBc-reactive; HBsAg-negative donations contain very low HBV DNA levels, which are unlikely to be detected by NAT.[6,7,8,9,10] This scenario where serology test shows positive result whereas NAT test demonstrates negative result is called as “seroyield.” In the present study, we address the issue of discrepant results of HBV between the enhanced chemiluminescence (serological assay) and NAT in terms of “seroyield.”
Materials and Methods
Study design
This prospective observational study was conducted at the department of transfusion medicine in a tertiary healthcare center in the National capital region of India between March 2010 and May 2012 (27 months). All blood donor samples were tested for HIV-I/II (anti-HIV-I/II), hepatitis B (HBsAg), and hepatitis C (anti-HCV) by enhanced chemiluminescence method on VitrosEciQ (Ortho Clinical Diagnostics, Johnson & Johnson, USA) using donor's serum sample. Simultaneously, ethylenediamine tetraacetic acid blood sample of the donor was subjected to ID-NAT for HIV-I, HBV and HCV. ID-NAT test was performed using the eSAS system, Procleix Ultrio Assay, Novartis Diagnostics, CA, US. All NAT yield cases were subjected further to the discriminatory assay for the detection of specific virus (Chiron Corporation, CA, USA), which is based on the principle of transcription-mediated amplification. A sample where the enhanced chemiluminescence method demonstrated positive result for the presence of HBV, whereas ID-NAT method demonstrated negative result for hepatitis B in a donor sample (seroyield) was further evaluated as per the study protocol.
Evaluation of “seroyield” samples
Evaluation of seroyield samples was done only in cases of HBV due to cost constraint and nonavailability of confirmatory kits for HCV.
All HBsAg positive cases were repeated on enhanced chemiluminescence method (VitrosEciQ Ortho Clinical Diagnostics, Johnson & Johnson, USA) and microparticle immunoassay (MEIA) (Abbott Architect). Confirmation of HBsAg was done using HBsAg ES confirmatory assay (VitrosEciQ Ortho Clinical Diagnostics, Johnson & Johnson, USA) and Cobas TaqMan (CTM) real time-polymerase chain reaction (RT-PCR) assay (Roche Molecular Systems, Branchburg, NJ, USA) for viral load assessment. To provide information on the stage of infection, specimens were tested for anti-HBc total (IgG + IgM), anti-HBc IgM and hepatitis B e antigen (HBeAg). HBeAg-negative samples were tested for anti-HBe antibody.
Serological assays
VitrosEciQ Immunodiagnostic system is a fully automated, random and stat access immunodiagnostic analyzer, which works on the principle of enhanced chemiluminescence for the in vitro screening of anti-HIV-I/II antibody, HBsAg, and anti-HCV antibody. The detection limit of the Vitros HBsAg assay was <0.16 ng/ml at the cutoff. The specificity of Vitros HBsAg assay was 99.98%.
Abbott Architect is an automated, chemiluminescent microparticle enzyme immunoassay (MEIA) for the qualitative determination of HBsAg. Calibration of this assay is based on the World Health Organization (WHO) International Reference Standard. Sensitivity of the Architect HBsAg assay is ≤0.05 IU/ml.
The Vitros Confirmatory Kit (HBsAg ES Confirmatory Assay, VitrosEciQ Ortho Clinical Diagnostics, Johnson & Johnson, USA) uses the principle of specific antibody neutralization to confirm the presence of HBsAg.
Nucleic acid assays
The Procleix Ultrio Assay is a qualitative in vitro nucleic acid amplification test for the detection of HIV-I RNA, HCV RNA, and HBV DNA in human plasma and specimens found to be reactive in the Procleix Ultrio Assay were run in individual HIV-I, HCV, and/or HBV discriminatory assays to determine if they were reactive for HIV-I, HCV, HBV or any combination of the three using the same assay procedure with one difference: HIV-I-specific, HCV-specific, or HBV-specific probe reagents were used in place of the Procleix Ultrio Multiplex Assay probe reagent.
The CTM (Roche Molecular Systems, Branchburg, NJ, USA) technology combines the extraction of total nucleic acids on the Cobas Ampliprep with RT-PCR on the CTM analyzer, using hydrolysis probe technology.
Hepatitis B virus
Cobas Ampliprep-Cobas TaqMan is an automated RT-PCR test based on a dual-labeled hybridization probe targeting the precore and core regions associated with an HBV DNA automated extraction based on the affinity of DNA for silica gel-covered magnetic beads. An internal quantitation standard (QS) is added to each sample during the processing step. After HBV DNA extraction with the Cobas ampliprep instrument, an RT-PCR test is performed by the CTM 48 analyzer with a multiplex TaqMan assay. Two targets, HBV DNA and the internal QS, are amplified.
For ensuring transfusion safety, blood units positive with either of the two tests or both the tests, were marked “positive” and discarded. The algorithm of the present study is shown in Figure 1.
Figure 1.
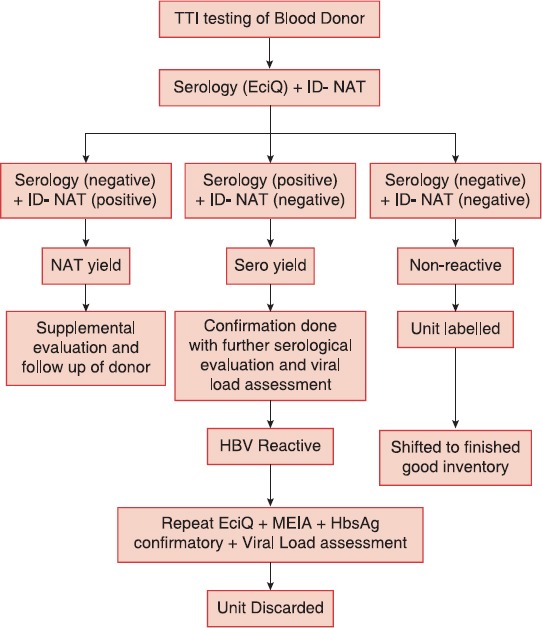
Algorithm
Results
Subject demographics
During the period of observation, a total of 48,441 blood donors were tested for transfusion transmitted viral infections. With regard to demographic information, mean age of the donors was 34.2 years and a large proportion of blood donors were male (94.2%, 45,631). Replacement donation by the family members and their relatives was the most common mode of blood donation (95.9%, 46,455). A total of 78.9% were first-time donors while 21.1% were repeat blood donors (data not shown in table).
Prevalence of transfusion-transmitted infection
Of the 48,441 study samples, 1000 samples were serologically reactive by enhanced chemiluminescence assay. Of the total samples studied, 112 (0.23%) were positive for the presence of HIV, 427 (0.88%) were positive for HBV and 461 (0.95%) were positive for the presence of HCV. A total of 414 (0.85%) samples were NAT reactive by the ultrio assay. Among the total of NAT ultrio reactive samples, 305 were reactive for HBV DNA (0.62%), 86 for HCV RNA (0.18%) and 23 were reactive for HIV-I RNA (0.04%). Enhanced chemiluminescence assay demonstrated simultaneous reactivity in a total of 97.3% of the total NAT ultrio samples (403/414) [Table 1, Figure 2].
Table 1.
Prevalence of transfusion transmitted viral infections
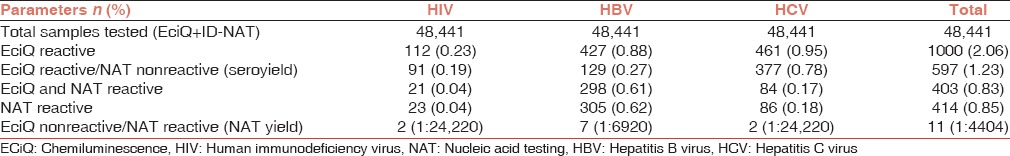
Figure 2.
(a-c) Prevalence of transfusion transmitted infections
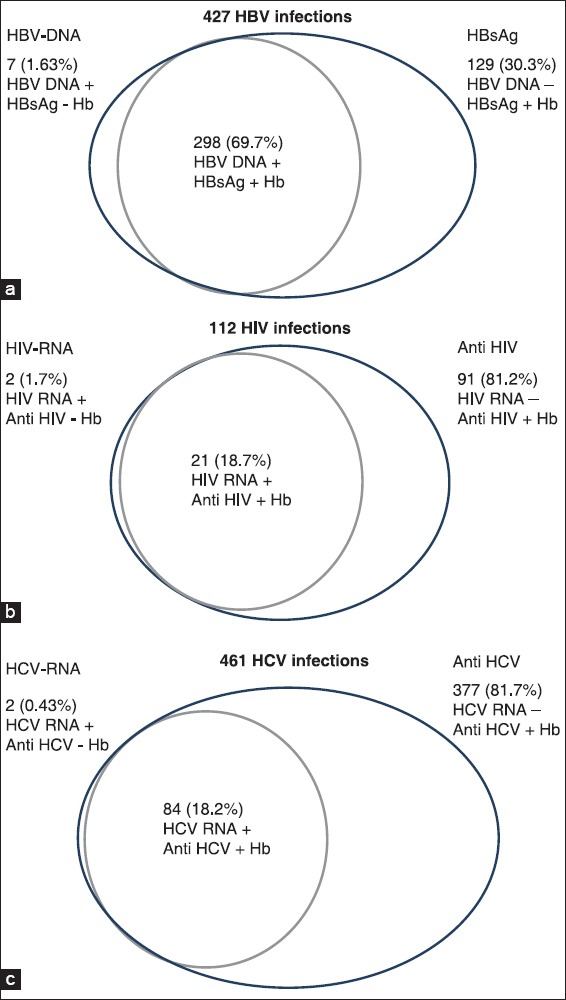
Of the 427 potential HBV-positive donors, 298 (69.7%) were concordantly HBV NAT and HBsAg positive. Seven (1.63%) HBV-infected donors were only HBV NAT positive and HBsAg was negative while 129 (30.3%) donors were HBsAg positive while HBV NAT negative (HBV seroyield). Of the 461 HCV infected donors 84 (18.2%) were both HCV RNA and anti-HCV reactive while 2 (0.43%) were only HCV RNA positive and anti-HCV negative. 377 (81.7%) HCV infected donors were only anti-HCV antibody positive. Of the 112 HIV-infected donors 21 (18.7%) were both HIV RNA and anti-HIV reactive while 2 (1.7%) donors were only HIV RNA positive and anti-HIV negative. A total of 91 (81.2%) HIV-infected donors were only anti-HIV antibody positive [Table 1, Figure 2].
Serologic profiles of HBsAg-positive donors
About 427 HBsAg positive donor samples were tested for anti-HBc total (IgG + IgM), anti-HBc IgM, HBeAg, and anti-HBe to assess the stage of HBV infection. Serologic profiles showed that 323 (76%) of HBsAg positive donors were anti-HBc IgM negative and anti-HBe positive, consistent with chronic HBV infection. Of which 96 (22.4%) donor samples were negative by NAT testing [Table 2].
Table 2.
Serologic profiles of HBsAg positive donors
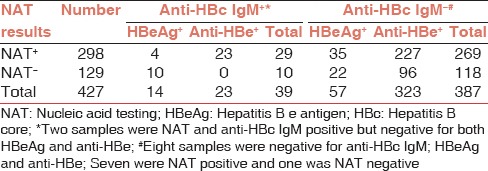
About 57 of the 427 samples (13.3%) were anti-HBc IgM negative, total anti-HBc positive and HBeAg positive, consistent with chronic hepatitis. Out of which 22 (5.1%) donor samples were NAT negative. Total of 39 HBsAg positive donors were also positive for anti-HBc IgM out of which 23 were anti-HBe positive, and 10 were HBeAg positive but NAT negative [Table 2].
Yields (nucleic acid testing yield and seroyield)
There were 11 (0.022%) NAT yield (sero-negative/NAT reactive) cases: 2 HIV, 7 HBV, and 2 HCV, which were positive by ID-NAT only. The individual NAT yield for the three viruses was as follows: HIV (0.004%), HCV (0.013%), and HBV (0.004%). Total of 597 cases were sero-reactive, but NAT nonreactive (seroyield): 91 HIV, 129 HBV and 377 HCV. The present study demonstrated a large numbers of “seroyield” donors, but due to cost constraint, and certain logistics further evaluation could be performed in 60 cases of HBV “seroyield” samples only [Table 1, Figure 2].
Hepatitis B virus seroyield cases
About 60 samples were further evaluated which showed mean initial sample/cutoff (S/CO) of 1.6 with enhanced chemiluminescence assay (VitrosEciQ), which was 1.4 on repetition with EciQ. All the 60 EciQ positive samples were also positive on chemiluminescent microparticle enzyme immunoassay while all 60 cases were confirmed positive with neutralization assay (Vitros HBsAg Confirmatory Kit), while no target were detected on viral load assessment with RT-PCR. Total of 16 cases were HBeAg positive (4 IgM anti-HBc positive and 12 IgM anti-HBc negative) and 44 were IgM anti-HBc negative, anti-HBc total positive, and anti-HBe positive [Table 3 and Figure 3].
Table 3.
Serologic profile of HBsAg positive donors

Figure 3.
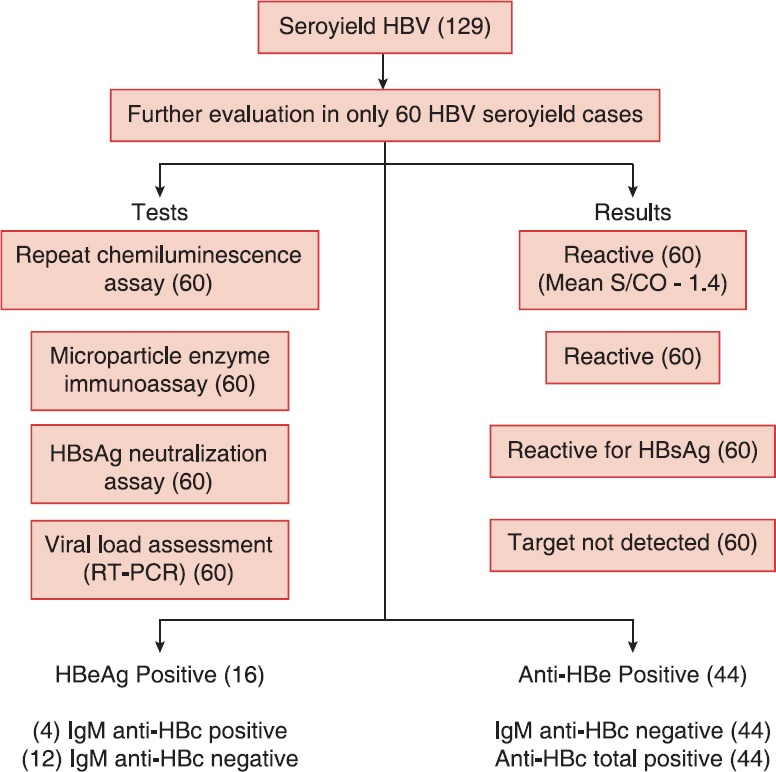
Evaluation of hepatitis B virus seroyield samples
Discussion
There is 1% chance of transfusion related events including transfusion transmitted infections (TTIs) with every unit of blood transfusion.[10] The risk of TTI has declined dramatically in developed countries over the past two decades as a result of a cumulative approach of remarkable improvement in repeat voluntary blood donation and simultaneous testing of blood donors with NAT technology. However, the picture in developing countries, like India, in terms of NAT testing is entirely different. In the present study, a total of 46,455 (95.9%) donations were made by replacement donors and only 1986 (4.1%) donations were contributed by voluntary blood donors. This finding was similar to results observed by Kakkar et al. (94.7%),[11] Singh et al. (84.43%),[12] Pahuja et al. (99.48%),[13] and Arora et al. (68.6%).[14]
In our study, the overall seroprevalence of HIV, HBsAg, and HCV were 0.23%, 0.88%, and 0.95%. These results were comparable to study done by Pahuja et al. from Delhi, who has noticed the seroprevalence of 0.56%, 2.23%, and 0.66%, respectively.[13] Similarly, Srikrishna et al.[15] have noted 0.44%, 1.86%, and 1.02% and Arora et al.[14] from Haryana has observed 0.3%, 1.7%, and 1% of seroprevalence of HIV, HBsAg, and HCV infection. Variations in seroprevalence in different areas might be due to the use of different screening methods and different generation of ELISA test kits, having different sensitivities and specificities. In our study, a total of 414 samples were NAT reactive out of which 403 samples were reactive by ECiQ also, giving the combined NAT yield of 11 (0.022%) (seronegative and NAT reactive) cases: 2 HIV, 7 HBV and 2 HCV which were positive by ID-NAT only. The NAT yield observed by other investigators were 0.034% (1 in 2972 donations) by Jain et al.[16] from Rajasthan, India and 0.038% (1 in 2622 donations) by Chatterjee et al.[17] The combined yield (seronegative/NAT reactive) for HIV-I, HCV and HBV was 0.065% observed by Makroo et al.[18] Agarwal et al.[19] have reported combined the NAT yield of 1 in 610 (0.16%) donations.
There was the lack of relationship between HBV DNA and HBsAg levels in donations from chronic carriers in the present study that was opposite to observation reported by Su et al.[20] where serum HBsAg marker has been used as a surrogate marker of HBV DNA levels mainly in HbeAg positive patients. Significant linear relationship between HBsAg assay S/CO values and HBV DNA copy/ml values during the ramp-up phase of acute infection (average ratio of HBsAg to DNA was 0.005-0.007 HBsAg per 100 DNA copies) were showed by Sato et al.[6] and Biswas et al.[21] Lack of correlation between HBV DNA and HBsAg levels observed in our study has also been reported by other studies. In a study by Kuhns et al.[22] ratio of HBsAg to DNA ranged from 0.188 to 2.8 × 104 HBsAg per 100 HBV DNA copies. Sato et al.[6] have also reported that blood donor samples from chronic carriers had HBsAg S/CO values that were disproportionately high compared to the low HBV DNA concentrations suggesting the amount of circulating HBsAg relative to HBV DNA varies widely depending on the stage of infection. Explanation for the lack of detectable HBV DNA in these donor samples given by Kuhns et al.[22] was either an extremely low level of HBV DNA or intermittent viremia while Allain et al.[23] explained this fact by considerable difference between release of viral structural proteins and the formation of full virions released in the circulation. They mentioned that nonencapsulated viral DNA tend to rapidly destroy, whereas in the absence of anti-HBs, viral surface antigen may remain in circulation for prolonged periods of time. This can explain rare cases of detectable HBsAg without detectable HBV DNA in chronic HBV infection. Other studies have also shown the presence of HBsAg-positive donors with very low or negative HBV DNA levels. Roth et al.[24] observed that only 298 out of 432 (69%) confirmed HBsAg-positive donations had detectable HBV DNA using a combination of mini-pool and single sample PCR assays. Al Shaer et al.[25] have reported 10 cases (5%) of HBV-infected donors with HBsAg-positive but no detectable HBV DNA while total of 36 cases of 12224 (0.29%) were HBsAg positive, but NAT nonreactive reported by Makroo et al.[18] Results observed in our study may also be false positive, but all HBsAg positive samples were confirmed HBsAg positive on repeat testing with ECiQ and were also reactive by the Abbott Architect HBsAg assay. There may be a problem of PCR inhibition also but the assay included an internal standard that was added to each sample and amplified.
Now, the problem arises whether these seroreactive, but NAT nonreactive (seroyield) units considered to be infectious or not and released to finished goods inventory or not. Considering the course of chronic HBV infection [Figure 4], in our study total of 129 (0.27%) HBV seroyield cases were identified out of which serologic profile [Table 2] of >90% cases were consistent with chronic HBV infection[26]. About 32 cases were HBeAg positive while NAT nonreactive consistent with immune clearance phase while 96 (74%) were anti-HBe positive HBeAg negative consistent with inactive carrier state. This inactive carrier state may persist indefinitely, in which the prognosis is generally favorable. This is also supported by a long-term follow-up study of 296 HBsAg-positive, healthy blood donors in Italy, whose survival was similar to that of 157 uninfected controls over a 30-year period, and in whom no episodes of hepatic decompensation occurred. However, 4-20% of inactive carriers have episodes of reversion to HBeAg positivity and 10-30% of inactive carriers have spontaneous reactivation of HBV replication and liver disease activity after years of quiescence.[27]
Figure 4.
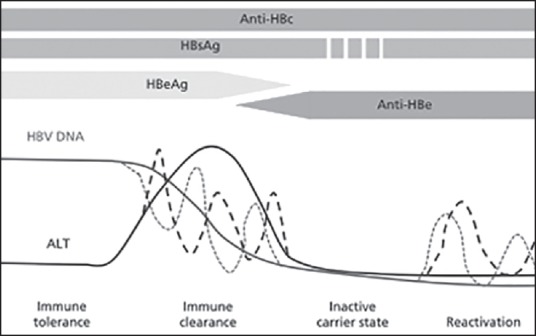
Course of chronic hepatitis B
Findings of the present study showed that HBsAg-positive donors can be NAT nonreactive (nondetectable HBV DNA). Approximately 0.27% of HBsAg-positive donors were negative by NAT testing, of which approximately 7.7% were considered to be highly infectious (HBeAg positive), while rest of the 90% of donors were consistent with chronic HBV infection, which may persist indefinitely or may show episodes of reversion or reactivation. Implementation of viral specific serology testing along with NAT testing reduces viral transmitted infection rate to a great extent, but at the same time large number of discrepant results are observed between the serology and NAT. Thus larger controlled trials are required for evaluation of these types of “seroyield” cases.
Footnotes
Source of Support: Nil
Conflicting Interest: None declared.
References
- 1.Jindal N, Arora U, Singh K. Prevalence of human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus in three groups of populations at high risk of HIV infection in Amritsar (Punjab), Northern India. Jpn J Infect Dis. 2008;61:79–81. [PubMed] [Google Scholar]
- 2.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Venkatakrishna Shyamala. Factors in enhancing blood safety by nucleic acid technology testing for human immunodeficiency virus, hepatitis C virus and hepatitis B virus. Asian J Transfus Sci. 2014 Jan-Jun;8:13–18. doi: 10.4103/0973-6247.126682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saran RK. New Delhi: Directorate General of Health Services, Government of India; 2003. Transfusion Medicine Technical Manual. [Google Scholar]
- 5.Hollinger FB, Sood G. Occult hepatitis B virus infection: A covert operation. J Viral Hepat. 2010;17:1–15. doi: 10.1111/j.1365-2893.2009.01245.x. [DOI] [PubMed] [Google Scholar]
- 6.Sato S, Ohhashi W, Ihara H, Sakaya S, Kato T, Ikeda H. Comparison of the sensitivity of NAT using pooled donor samples for HBV and that of a serologic HBsAg assay. Transfusion. 2001;41:1107–13. doi: 10.1046/j.1537-2995.2001.41091107.x. [DOI] [PubMed] [Google Scholar]
- 7.Kleinman SH, Kuhns MC, Todd DS, Glynn SA, McNamara A, DiMarco A, et al. Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: Implications for transfusion transmission and donor screening. Transfusion. 2003;43:696–704. doi: 10.1046/j.1537-2995.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 8.Kleinman SH, Busch MP. HBV: Amplified and back in the blood safety spotlight. Transfusion. 2001;41:1081–5. doi: 10.1046/j.1537-2995.2001.41091081.x. [DOI] [PubMed] [Google Scholar]
- 9.Busch MP. Should HBV DNA NAT replace HBsAg and/or anti-HBc screening of blood donors? Transfus Clin Biol. 2004;11:26–32. doi: 10.1016/j.tracli.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Garg S, Mathur DR, Garg DK. Comparison of seropositivity of HIV, HBV, HCV and syphilis in replacement and voluntary blood donors in western India. Indian J Pathol Microbiol. 2001;44:409–12. [PubMed] [Google Scholar]
- 11.Kakkar N, Kaur R, Dhanoa J. Voluntary donors-need for a second look. Indian J Pathol Microbiol. 2004;47:381–3. [PubMed] [Google Scholar]
- 12.Singh K, Bhat S, Shastry S. Trend in seroprevalence of Hepatitis B virus infection among blood donors of coastal Karnataka, India. J Infect Dev Ctries. 2009;3:376–9. doi: 10.3855/jidc.246. [DOI] [PubMed] [Google Scholar]
- 13.Pahuja S, Sharma M, Baitha B, Jain M. Prevalence and trends of markers of hepatitis C virus, hepatitis B virus and human immunodeficiency virus in Delhi blood donors: A hospital based study. Jpn J Infect Dis. 2007;60:389–91. [PubMed] [Google Scholar]
- 14.Arora D, Arora B, Khetarpal A. Seroprevalence of HIV, HBV, HCV and syphilis in blood donors in Southern Haryana. Indian J Pathol Microbiol. 2010;53:308–9. doi: 10.4103/0377-4929.64295. [DOI] [PubMed] [Google Scholar]
- 15.Srikrishna A, Sitalakshmi S, Damodar P. How safe are our safe donors? Indian J Pathol Microbiol. 1999;42:411–6. [PubMed] [Google Scholar]
- 16.Jain R, Aggarwal P, Gupta GN. Need for nucleic Acid testing in countries with high prevalence of transfusion-transmitted infections. ISRN Hematol. 2012;2012:718671. doi: 10.5402/2012/718671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee K, Coshic P, Borgohain M, Premchand, Thapliyal RM, Chakroborty S, et al. Individual donor nucleic acid testing for blood safety against HIV-1 and hepatitis B and C viruses in a tertiary care hospital. Natl Med J India. 2012;25:207–9. [PubMed] [Google Scholar]
- 18.Makroo RN, Choudhury N, Jagannathan L, Parihar-Malhotra M, Raina V, Chaudhary RK, et al. Multicenter evaluation of individual donor nucleic acid testing (NAT) for simultaneous detection of human immunodeficiency virus -1 & hepatitis B & C viruses in Indian blood donors. Indian J Med Res. 2008;127:140–7. [PubMed] [Google Scholar]
- 19.Agarwal N, Chatterjee K, Coshic P, Borgohain M. Nucleic acid testing for blood banks: An experience from a tertiary care centre in New Delhi, India. Transfus Apher Sci. 2013;49:482–4. doi: 10.1016/j.transci.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Su TH, Hsu CS, Chen CL, Liu CH, Huang YW, Tseng TC, et al. Serum hepatitis B surface antigen concentration correlates with HBV DNA level in patients with chronic hepatitis B. Antivir Ther. 2010;15:1133–9. doi: 10.3851/IMP1696. [DOI] [PubMed] [Google Scholar]
- 21.Biswas R, Tabor E, Hsia CC, Wright DJ, Laycock ME, Fiebig EW, et al. Comparative sensitivity of HBV NATs and HBsAg assays for detection of acute HBV infection. Transfusion. 2003;43:788–98. doi: 10.1046/j.1537-2995.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuhns MC, Kleinman SH, McNamara AL, Rawal B, Glynn S, Busch MP, et al. Lack of correlation between HBsAg and HBV DNA levels in blood donors who test positive for HBsAg and anti-HBc: Implications for future HBV screening policy. Transfusion. 2004;44:1332–9. doi: 10.1111/j.1537-2995.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 23.Allain JP, Hewitt PE, Tedder RS, Williamson LM. Evidence that anti-HBc but not HBV DNA testing may prevent some HBV transmission by transfusion. Br J Haematol. 1999;107:186–95. doi: 10.1046/j.1365-2141.1999.01665.x. [DOI] [PubMed] [Google Scholar]
- 24.Roth WK, Weber M, Petersen D, Drosten C, Buhr S, Sireis W, et al. NAT for HBV and anti-HBc testing increase blood safety. Transfusion. 2002;42:869–75. doi: 10.1046/j.1537-2995.2002.00128.x. [DOI] [PubMed] [Google Scholar]
- 25.Al Shaer L, AbdulRahman M, John TJ, AlHashimi A. Trends in prevalence, incidence, and residual risk of major transfusion-transmissible viral infections in United Arab Emirates blood donors: Impact of individual-donation nucleic acid testing, 2004 through 2009. Transfusion. 2012;52:2300–9. doi: 10.1111/j.1537-2995.2012.03740.x. [DOI] [PubMed] [Google Scholar]
- 26.Harvey J, Juan I. Rossi's Principles of Transfusion Medicine. UK: Blackwell Publishing Ltd; 2009. Transfusion-transmitted hepatitis; p. 723. [Google Scholar]
- 27.Manno M, Cammà C, Schepis F, Bassi F, Gelmini R, Giannini F, et al. Natural history of chronic HBV carriers in northern Italy: Morbidity and mortality after 30 years. Gastroenterology. 2004;127:756–63. doi: 10.1053/j.gastro.2004.06.021. [DOI] [PubMed] [Google Scholar]


