Abstract
In contrast to findings from cohorts comprised primarily of HIV-infected men, verbal memory deficits are the largest cognitive deficit found in HIV-infected women from the Women’s Interagency HIV Study (WIHS), and this deficit is not explained by depressive symptoms or substance abuse. HIV-infected women may be at greater risk for verbal memory deficits due to a higher prevalence of cognitive risk factors such as high psychosocial stress and lower socioeconomic status. Here, we investigate the association between perceived stress using the Perceived Stress Scale (PSS-10) and verbal memory performance using the Hopkins Verbal Learning Test (HVLT) in 1009 HIV-infected and 496 at-risk HIV-uninfected WIHS participants. Participants completed a comprehensive neuropsychological test battery which yielded seven cognitive domain scores, including a primary outcome of verbal memory. HIV infection was not associated with a higher prevalence of high perceived stress (i.e., PSS-10 score in the top tertile) but was associated with worse performance on verbal learning (p<0.01) and memory (p<0.001), as well as attention (p=0.02). Regardless of HIV status, high stress was associated with poorer performance in those cognitive domains (p’s< 0.05) as well as processing speed (p=0.01) and executive function (p<0.01). A significant HIV by stress interaction was found only for the verbal memory domain (p=0.02); among HIV-infected women only, high stress was associated with lower performance (p’s<0.001). That association was driven by the delayed verbal memory measure in particular. These findings suggest that high levels of perceived stress contribute to the deficits in verbal memory observed in WIHS women.
Keywords: HIV, Verbal memory, Stress, Women, Cognition
Introduction
Evidence of a marked sex differences in the stress response (Kajantie and Phillips 2006; Kudielka and Kirschbaum 2005) provides a strong rationale for studying the association between high levels of stress and cognitive performance in women separately from men. Compared to men, women show lower increases in cortisol, a steroid hormone released by the adrenal glands, following exposures to psychological stressors (Kirschbaum et al. 1992). Women, however, show a greater vulnerability to the cognitive effects of elevated cortisol (McCormick et al. 2007; Schoofs et al. 2013; Seeman et al. 1997; Shors et al. 2001; Wolf et al. 2001). Studies, for example, demonstrate a stronger association between elevated cortisol and deficits in verbal memory and executive function in women compared with men (McCormick et al. 2007; Seeman et al. 1997). A biological basis for this female vulnerability to the cognitive effects of stress is supported by evidence from rat models where acute stress increases learning and dendritic spine density in the hippocampus of male rats (Servatius and Shors 1994; Shors et al. 2001; Shors and Servatius 1997) but decreases learning and spine density in the hippocampus of female rats (Bangasser and Shors 2004; Shors et al. 1998, 2001).
Findings from cohorts comprised primarily of men indicate that deficits in learning and executive function are the most frequent cognitive impairments associated with HIV infection (Heaton et al. 2011; Woods et al. 2005). In contrast, a recent study of 1521 women from the Women’s Interagency HIV Study (WIHS) showed that the largest cognitive deficit associated with HIV infection was in the domain of verbal memory, particularly delayed verbal memory, as measured by the Hopkins Verbal Learning Test (HVLT) (Maki et al. 2015). Neuroimaging findings from the WIHS link alterations in hippocampal function during verbal encoding and recognition to deficits on the HVLT (Maki et al. 2009). Deficits in hippocampal function in HIV reflect neuroinflammation and neurodegeneration. Autopsy data reveal that microglial/macrophage activation is especially high in the hippocampus of HIV-infected individuals treated with highly active antiretroviral therapy (Anthony et al. 2005). Neurodegeneration in the hippocampus at the level of dendrites/neuronal cell bodies and presynaptic terminals is also a strong predictor of overall HIV-associated cognitive impairment (Moore et al. 2006). In predominantly white HIV-infected men who have sex with men, acute stressful life events are associated with worse executive function, attention, and processing speed, but not memory (Pukay-Martin et al. 2003). To date, however, no studies have examined the association between stressful life events and cognitive performance in HIV-infected women. This gap in the literature is surprising given that HIV-infected women report high rates of exposure to acute and chronic stressors including early childhood trauma, adult sexual assault, and physical violence as well as transactional sex, unemployment, poverty, and single parenting (Brief et al. 2004; Cohen et al. 2000). In the WIHS, 31 % of HIV-infected participants report childhood sexual abuse, 66 % report lifetime domestic violence, and 21 % report domestic violence within the previous 6 months (Cohen et al. 2000).
In the present investigation, we examine the association between perceived stress and cognitive performance in HIV-infected compared with HIV-uninfected women. Our primary aim was to investigate whether the central deficit in verbal memory observed in HIV-infected women in the WIHS is associated with high levels of perceived stress. We hypothesized that elevated stress would relate to worse performance on verbal memory and that the magnitude of the effect of verbal memory will be greater for HIV-infected versus HIV-uninfected women.
Methods
Study population
All participants were enrolled in the WIHS, a multi-site, longitudinal study of women with and at-risk for HIV. Participants were enrolled at six consortia (Chicago, Bronx, Brooklyn, Washington DC, San Francisco, Los Angeles) after signing institutional approved consent forms. Study methodology, standardized data collection, and training of interviewers have been previously described (Bacon et al. 2005; Barkan et al. 1998). Briefly, study visits were conducted every 6 months and include a detailed psychosocial, behavioral, and clinical survey, physical exam, and specimen collection.
This analysis included 1009 HIV-infected and 496 HIV-uninfected participants (mean age=46.2, 64 % African-American) who completed the first wave of neurocognitive testing implemented in the WIHS between April 2009 to April 2011 and met all inclusion criteria (see Maki et al. 2015). For cognitive testing, we targeted all active English-speaking participants (n=1908) who completed any of the four semiannual WIHS visits. Exclusion criteria were established in advance but applied after cognitive testing of the targeted group because variables acquired at the core semiannual visits (e.g., recent drug abuse) were needed to determine eligibility. Of 1908 eligible women, 1595 (84 %) women completed the cognitive test battery. We analyzed 1499 (1009 HIV-infected; 92 % of the cohort) after excluding 96 participants who met one or more of the following exclusion criteria: 1) missing data on the perceived stress scale (n=22); 2) presence of conditions that preclude completion of neurocognitive tests (e.g., hearing loss, impaired vision, being under the influence of illicit substances) (n=11); 3) history of stroke/CVA (n=13); and 4) self-reported use of antipsychotic medication in the past 6 months (n=50). Compared to women who did not complete the cognitive test battery, the women who completed the cognitive testing were more likely to be Black non-Hispanic, to be less educated, to have a lower annual household income, to be Hepatitis C virus (HCV) antibody positive, to smoke, to report recent crack, cocaine, and/or heroin and marijuana use, to be from the Bronx and Brooklyn study sites, and less likely to be HIV-infected or be from the LA and Chicago study sites (p’s<0.05).
Measures
Neuropsychological outcome measures
Seven cognitive domains were assessed. The primary cognitive domain of interest was verbal memory, which was assessed with the Hopkins Verbal Learning Test (HVLT), a 12-item list-learning task used to measure verbal learning and memory (Brandt and Benedict 2001). A verbal memory domain T-score was calculated by averaging derived T-scores (see statistical analysis section) for the following two HVLT indices: 1) number of words recalled after a 25-min delay (delayed recall) and 2) percent retention (delayed recall/maximum score on trial 2 or 3).
Secondary cognitive domains assessed included verbal learning, attention and concentration, executive functioning (behavioral inhibition, mental flexibility, working memory), psychomotor speed, verbal fluency, and fine motor skills. T-scores were also computed for each of the secondary cognitive domains. Each cognitive domain was computed by averaging the derived T-scores of all individual outcomes within each domain. Verbal learning was assessed with the following two HVLT indices: 1) Trial 1 (single trial learning) and 2) total words recalled across each of three learning trials (total learning). Attention and concentration were assessed with Trials 1 and 2 of the Stroop test (Comalli et al. 1962) (outcome=average time to complete Trials 1 and 2, Trials correlated at r=0.72), Trail Making Test part A (Reitan 1978) (outcome=time to complete), and the control/attention condition from the Letter-Number Sequence (LNS) test from the Wechsler Adult Intelligence Scale IV (WAIS IV) (outcome=total correct). Executive functioning was assessed with Trial 3, the color-word condition (interference) of the Stroop test (Comalli et al. 1962), which measures behavioral inhibition (outcomes=time to completion), Trail Making Test part B (Reitan 1978), which measures mental flexibility (outcome=time to complete), and the working memory condition of LNS (outcome=total correct). Psychomotor speed was assessed with the Symbol Digit Modalities Test (SDMT) (Smith 1968) (outcome=total number of boxes that were correctly filled within the time limit of 90 s). Verbal fluency was assessed with a letter fluency task (Benton 1968) (outcome=total words generated in response to the letters F, A, and S) and a category fluency task (outcome=total words generated in response to the semantic category of animals). Fine motor skills was assessed with the Grooved Pegboard Test (Reitan and Wolfson 1985) (outcome measure=average time to complete the dominant and nondominant hand). All timed outcomes were skewed to the right and therefore log transformed.
Primary explanatory variable
Perceived Stress Scale
The Perceived Stress Scale (PSS-10) (Cohen et al. 1983; Cohen and Williamson 1988) is a widely used self-report instrument measuring the degree to which situations the previous month in one’s life are appraised as stressful. Items assess the degree to which respondents have found their lives unpredictable (e.g., How often have you been upset because of something that happened unexpectedly?), uncontrollable (e.g., How often have you felt that you were unable to control the important things in your life?), and overloaded (e.g., How often have you felt difficulties were piling up so high that you could not overcome them?) in the last month. Each of the 10 items was rated on a 5-point Likert scale (0=never, 1=almost never, 2=sometimes, 3=fairly often, 4=very often). A total score was computed by summing item responses (reverse scored when needed), with higher scores indicating greater perceived stress (scores range from 0 to 40). A Cronbach Alpha of 0.88 indicated excellent internal consistency. Consistent with previous WIHS studies (Massad et al. 2011), we categorized perceived stress as higher when PSS-10 scores were in the top tertile (for the present sample≥18). Perceived stress was categorized as lower (low-to-moderate) when PSS-10 scores were <18 (bottom two tertiles).
Covariates
Sociodemographic variables and risk factors for cognitive impairment were selected based on a comprehensive analysis of the wave one findings (Maki et al. 2015) and included: annual household income; Center for Epidemiologic Studies Depression Scale (CES-D, cutoff score of 16); Hepatitis C virus antibody (HCV) status (indicative of HCV exposure); self-reported recent (within 6 months), former (>6 months), or never use of marijuana, crack, cocaine, and/or heroin and smoking; self-reported recent heavy alcohol use for women defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (>7 drinks/week or >4 drinks in one sitting); recent antidepressant use; and study geographic site. Additional HIV-related clinical variables of interest included combination antiretroviral therapy (cART) use (i.e., no cART therapy, cART therapy and self-reported <95 % adherent with medication as prescribed, cART therapy and self-reported ≥95 % adherent with medication as prescribed), duration of antiretroviral therapy use, current CD4 count <200 cells/mm3, current viral load (undetectable, <10,000 cp/ml, ≥10,000 cp/ml), and nadir CD4 count <200 cells/mm3 during WIHS study observation.
Statistical analysis
Differences between groups (HIV-uninfected not high stress; HIV-uninfected high stress; HIV-infected not high stress; HIV-infected high stress) in demographic characteristics were examined using two-way between subjects analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. In the absence of published cognitive test norms for low income minority women, we followed Heaton and colleagues (1991) and prior work within the WIHS (Maki et al. 2015; Manly et al. 2011; Rubin et al. 2014), using a regression approach to estimate premorbid levels of function for the total sample based on scores of the comparison group (HIV women). We did this by regressing age, years of education, race/ethnicity, and Reading Recognition subtest from the Wide Range Achievement Test-Revised (WRAT-R) (Wilkinson 1993), as a proxy for educational quality (Manly et al. 2002), on each cognitive outcome. The resulting unstandardized beta weights, constants, and standard errors were used to calculate predicted scores for each test that were then subtracted from each woman’s actual score and transformed to scores (using means of 50 and standard deviations of 10) that could be more easily compared across all cognitive outcomes.
In the overall sample, we used multivariable linear regression analyses to examine the separate and interactive associations of HIV status and perceived stress on the primary and secondary cognitive domains (p<0.05). Where significant effects were observed in cognitive domains, we also examined the separate and interactive associations of HIV and perceived stress on the individual outcomes (also T-scores) contributing to the domain scores. The model adjusted for confounds shown to be associated with cognitive functioning in the primary analysis of these cognitive data (Maki et al. 2015) including marijuana use; crack, cocaine, and/or heroin use; smoking; heavy alcohol use; antidepressant use; HCV antibody status; annual household income; and study site. We also adjusted for number of prior exposures to tests that were previously administered as part of an abbreviated WIHS cognitive battery ((Trail Making Test (range 1–5), and SDMT (range 1–5)), and previous cross-sectional and site-specific studies ((HVLT (range 1–2), Stroop test (range 1–4)). Interactions between HIV status and perceived stress were retained in the final model if p<0.10; stratum-specific estimates within HIV status are reported for interactions. For significant interactions, a final model was run for the HIV-infected women only including the additional covariates of recent CD4 count and HIV viral load, nadir CD4 count, and cART use and adherence. Planned exploratory analyses in HIV-infected women were also conducted to examine interactions between stress, HIV clinical characteristics (HIV viral load, recent and nadir CD4 count, cART use and adherence), and HCV status. Significance is defined as p<0.05 (two-sided). Cohen’s d effect sizes are also reported (small effect=0.2; medium effect=0.5; large effect=0.8) (Cohen 1992). Analyses were performed using SAS PROC GENMOD (version 9.2, SAS Institute Inc, Cary, NC).
Results
Table 1 shows demographic and clinical information for the HIV-infected (n=1003; 619 lower stress, 384 higher stress) and HIV-uninfected women (n=496; 317 lower stress, 179 higher stress). Participants ranged in age from 25 to 87 years (M=46.2, SD=9.4), with high minority representation (64 % African-American, 20 % Hispanic). Compared with HIV-uninfected women, HIV-infected women were significantly older, had a higher minority representation, were more likely to have positive HCV serology and to use antidepressant medication, and were less likely to engage in heavy alcohol use and marijuana (p’s<0.05; Table 1). The proportion of HIV-uninfected (36 %) and HIV-infected (38 %) women with higher perceived stress was similar (p=0.41). Regardless of HIV status, women with higher compared to lower perceived stress were older, less educated, had a lower annual household income, more likely to have positive HCV serology and to use antidepressant medication, more likely to smoke, use marijuana, crack cocaine, powder cocaine, and/or heroin and to have more depressive symptoms (p’s<0.05; Table 1). Among HIV-infected women, women with higher perceived stress had lower CD4 count and higher viral load than women with lower perceived stress (p’s<0.05; Table 1).
Table 1.
Background characteristics of HIV-infected and HIV-uninfected women as a function of perceived stress (lower vs. higher)
| HIV− |
HIV+ |
|||
|---|---|---|---|---|
| Characteristics | Lower (n=317) n (%) |
Higher (n=179) n (%) |
Lower (n=619) n (%) |
Higher (n=384) n (%) |
| Background characteristics | ||||
| Age, M (SD)a, b, c | 42.21 (10.36) | 45.94 (8.95) | 47.31 (9.19) | 47.70 (8.10) |
| WRAT-R, M (SD)b | 93.01 (16.94) | 89.73 (16.620 | 94.41 (17.46) | 89.03 (18.82) |
| Years of education, M (SD)b | 12.75 (2.54) | 11.99 (3.07) | 12.71 (2.86) | 11.95 (2.98) |
| Race/ethnicitya | ||||
| African-American, non-Hispanic | 207 (65) | 101 (56) | 416 (67) | 233 (61) |
| White, non-Hispanic | 26 (8) | 24 (13) | 80 (13) | 56 (15) |
| Hispanic | 72 (23) | 44 (25) | 96 (16) | 83 (22) |
| Other | 12 (4) | 10 (6) | 27 (4) | 12 (3) |
| Hepatitis C virus antibody (HCV)a, b | 56 (18) | 37 (21) | 178 (29) | 134 (35) |
| Recentd | ||||
| Average annual household income, M (SD)b |
3.91 (2.29) | 2.87 (1.96) | 4.06 (2.30) | 3.16 (2.05) |
| Alcohol usea | ||||
| Abstainer | 151 (48) | 92 (52) | 367 (59) | 239 (62) |
| Not heavy | 95 (30) | 36 (20) | 158 (26) | 85 (22) |
| Heavy | 71 (22) | 51 (28) | 94 (15) | 60 (16) |
| Antidepressant medication usea, b, c | 15 (5) | 33 (18) | 90 (15) | 89 (23) |
| Depressive symptoms (CES-D≥16)b | 32 (10) | 118 (66) | 67 (11) | 234 (61) |
| Perceived stress (PSS-10)b | 9.76 (5.23) | 23.24 (4.76) | 9.55 (5.03) | 22.58 (4.00) |
| Smokingb | ||||
| Never | 88 (28) | 26 (15) | 200 (32) | 71 (19) |
| Formere | 130 (41) | 102 (57) | 210 (34) | 201 (52) |
| Recent | 99 (31) | 51 (28) | 209 (34) | 112 (29) |
| Marijuana usea, b | ||||
| Never | 70 (22) | 28 (16) | 180 (29) | 82 (21) |
| Formere | 178 (56) | 112 (62) | 354 (57) | 237 (62) |
| Recent | 69 (22) | 39 (22) | 85 (14) | 65 (17) |
| Crack, cocaine, and/or heroin useb | ||||
| Never | 153 (48) | 50 (28) | 277 (45) | 121 (32) |
| Formere | 147 (47) | 107 (60) | 326 (53) | 225 (58) |
| Recent | 17 (5) | 22 (12) | 16 (2) | 38 (10) |
| HIV-related clinical characteristics | ||||
| Nadir CD4 count (cells/μl), M (SD) | – | – | 218 (158) | 208 (157) |
| CD4 count (cells/μl)b | – | – | ||
| >500 | 342 (55) | 169 (44) | ||
| ≥200 and <500 | 208 (34) | 155 (40) | ||
| <200 | 69 (11) | 60 (16) | ||
| Viral load (HIV RNA (cp/ml))b | – | – | ||
| Undetectable | 343 (55) | 182 (47) | ||
| <10,000 | 196 (32) | 141 (37) | ||
| ≥10,000 | 80 (13) | 61 (16) | ||
| Medication use | – | – | ||
| No cART | 141 (23) | 95 (25) | ||
| cART+ <95 % medication adherence | 66 (11) | 66 (17) | ||
| cART+ ≥95 % medication adherence | 412 (66) | 223 (58) | ||
| ART duration (years), M (SD) | – | – | 11.21 (4.59) | 11.01 (4.60) |
Heavy alcohol use=>7 drinks per week or >4 drinks at a sitting; Undetectable=<48 copies/ml; For income: 1=≤$6000; 2=$6001– $12000; 3=$12001–$18000; 4=$18001–$24000; 5=$24001– 30000; 6=$30001–$36000; 7=$36001–$75000; 8=>$75000
WRAT-R Wide Range Achievement Test Standard Score, CES-D Center for Epidemiologic Studies Depression Scale, PSS-10 Perceived Stress Scale, cART combination antiretroviral therapy, ART antiretroviral therapy
Main effect of HIV status is significant at p<0.05
Main effect of Perceived Stress is significant at p<0.05
Interaction between HIV status and perceived stress is significant at p<0.05
“Recent” refers to within 6 months of the most recent WIHS visit
“Former” refers to any previous use but not within the past 6 months
In multivariable analyses controlling for the full set of model variables, HIV-infected women performed significantly worse than HIV-uninfected women on the verbal learning (p=0.003, d=0.17) and memory (p<0.001, d=0.0.19) and attention and concentration (p=0.02, d=0.14) domains (Table 2). Consistent with our previous publication (Maki et al. 2015), HIV-infected women performed significantly worse than HIV-uninfected women on all individual HVLT indices (p’s<0.05), Stroop trials 1 and 2 (p=0.01), and LNS control (p=0.007).
Table 2.
Multivariable linear regression analyses for the primary and secondary outcome measures: separate and interactive associations of HIV status and stress on cognitive test performance
| Separate and interactive effects |
||||||
|---|---|---|---|---|---|---|
|
|
HIV infection (HIV+ vs. HIV−) |
Stress (higher vs. lower) |
HIV infection×stress | |||
| Cognitive domains | n | B (SE) | Cohen’s d | B (SE) | Cohen’s d | B (SE) |
| Primary outcome | ||||||
| Verbal memory | 1495 | −1.78 (0.52)*** | 0.19 | −1.54 (0.51)*** | 0.17 | −2.24 (1.05)* |
| HVLT: delayed free recall | 1495 | −1.94 (0.55)*** | 0.20 | −1.45 (0.55)** | 0.15 | −2.52 (1.11)* |
| HVLT: percent retention | 1495 | −1.62 (0.57)** | 0.16 | −1.64 (0.56)** | 0.16 | −1.95 (1.14)T |
| Secondary outcomes | ||||||
| Verbal learning | 1495 | −1.55 (0.51)** | 0.17 | −1.45 (0.51)** | 0.16 | −2.02 (1.04)T |
| HVLT: Trial 1 | 1495 | −1.66 (0.55)** | 0.17 | −1.81 (0.54)*** | 0.19 | −2.11 (1.11)T |
| HVLT: total Trials 1–3 | 1495 | −1.44 (0.55)** | 0.15 | −1.09 (0.54)* | 0.11 | −1.79 (1.10)T |
| Attention and concentration | 1320 | −1.01 (0.43)* | 0.14 | −1.21 (0.42)** | 0.17 | −0.34 (0.86) |
| Stroop Trials 1 and 2 | 1475 | −1.54 (0.62)* | 0.14 | −1.68 (0.61)** | 0.15 | −1.29 (1.25) |
| Trail Making Test part A | 1493 | −0.51 (0.57) | 0.05 | −1.05 (0.56)T | 0.10 | −1.17 (1.15) |
| LNS attention | 1334 | −1.64 (0.61)** | 0.16 | −1.10 (0.61)T | 0.11 | 0.30 (1.24) |
| Executive functions | 1252 | −0.44 (0.49) | 0.05 | −1.36 (0.49)** | 0.17 | 0.23 (0.99) |
| Stroop Trial 3 | 1430 | −0.50 (0.72) | 0.04 | −1.66 (0.71)* | 0.13 | −1.58 (1.45) |
| Trail Making Test part B | 1455 | −0.45 (0.58) | 0.04 | −1.42 (0.58)* | 0.14 | −0.56 (1.17) |
| LNS working memory | 1299 | −0.63 (0.65) | 0.06 | −0.81 (0.64) | 0.08 | 1.37 (1.12) |
| Psychomotor speed | 1487 | −0.80 (0.54) | 0.08 | −1.31 (0.54)* | 0.14 | −1.40 (1.09) |
| Fluency | 1486 | −0.21 (0.48) | 0.02 | −0.51 (0.42) | 0.06 | 0.59 (0.97) |
| Fine motor skills | 1442 | 0.21 (0.58) | 0.02 | −0.28 (0.58) | 0.03 | 0.04 (1.18) |
All models are adjusted for site, marijuana use, crack, cocaine, and/or heroin use, smoking, heavy alcohol use, antidepressants, HCV, income, study site, and number of previous cognitive test exposure
HVLT Hopkins Verbal Learning Test, LNS Letter-Number Sequence, Stress perceived stress scale, B parameter estimates, SE standard errors
p<0.001;
p<0.01;
p<0.05;
p>0.05;
p<0.10
Regardless of HIV status, perceived stress was significantly and inversely associated with cognitive performance (Table 2). Women with higher perceived stress performed worse than women with lower perceived stress on the verbal learning (p=0.004, d=0.16) and memory (p=0.003, d=0.17), attention and concentration (p=0.004, d=0.17), executive functioning (p=0.005, d=0.17), and psychomotor speed (p=0.01, d=0.14) domains. Specifically, women with higher perceived stress performed worse than women with lower perceived stress on all HVLT indices (p’s<0.05, d’s 0.11–0.19), Stroop trials 1 and 2 (p=0.01, d=0.15), Stroop trial 3 (p=0.02, d=0.13), Trails B (p=0.01, d=0.14), and SDMT (p=0.01, d=0.14).
Interactions between perceived stress and HIV status on cognition
As hypothesized, perceived stress interacted with HIV status to significantly influence the verbal memory domain, specifically on delayed recall (p’s < 0.05; Table 2/Fig. 1). Among HIV-infected women, those reporting the highest perceived stress performed significantly worse than women with perceived stress scores in the lowest two tertiles on the verbal memory domain (B=−2.24, SE=0.62, p<0.001; Cohen’s d=0.24). Follow-up analyses on individual HVLT indices revealed that this interaction was driven specifically by delayed recall (B=−2.24, SE=0.65, p<0.001; Cohen’s d=0.23). Both interactions remained significant (p’s < 0.05) even after adjusting for depressive symptoms (defined by a Center for Epidemiologic Studies Depression Scale score ≥16) as an additional covariate. The interaction between perceived stress and HIV status also remained significant on delayed recall even after controlling for the total words learned (p=0.04). In contrast, among the HIV-uninfected women, there were no significant differences on the verbal memory domain (B=−0.004, SE=0.88, p=0.99) or on delayed recall between women with higher and lower levels of perceived stress (B=0.28, SE=0.93, p=0.76). There were no other significant interactions between perceived stress and HIV status on the verbal learning, executive functioning, attention, fine motor skills, or processing speed domains.
Fig. 1.
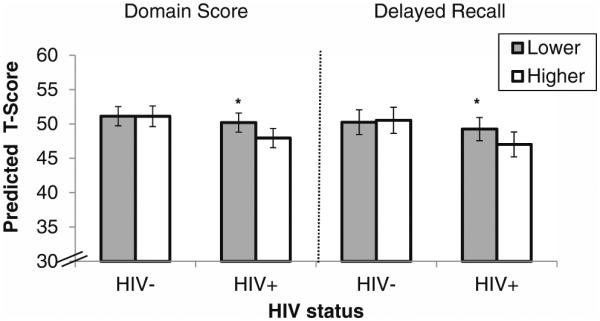
Perceived stress (lower vs. higher) is associated with the verbal memory domain and delayed recall only in the context of HIV. Note. *p<0.001. There was a significant interaction between perceived stress and HIV status on the verbal memory domain (p=0.03) and specifically on delayed recall (p=0.02). Among HIV-infected women, women with higher perceived stress performed worse than women with lower perceived stress on the verbal memory domain and on delayed recall (p’s< 0.001). Conversely, among HIV-uninfected women, women with higher and lower perceived stress performed similarly on the verbal memory domain and on delayed recall (p’s>0.76). The model is adjusted for marijuana use; crack, cocaine, and/or heroin use; smoking; hazardous alcohol use; antidepressants; HCV; income; study site; and number of exposures to the Hopkins Verbal Learning Test
In multivariable analyses of HIV-infected women only, those with higher perceived stress performed significantly worse than women with lower perceived stress on the verbal memory domain (B=−2.13, SE=0.62, p<0.001) and on delayed recall (B=−2.03, SE=0.65, p=0.002) after controlling for HIV disease characteristics, including current CD4 count, HIV viral load, cART medication use, and duration on ART. To ensure that the stress-cognition association was not driven by uncontrolled viremia, we conducted analyses among a subset of HIV-infected women with suppressed HIV RNA in plasma (n=525) and demonstrated that the association between perceived stress and performance on the verbal memory domain (p=0.01) and delayed recall in particular (p=0.01) remained significant.
Exploratory multivariable regression analyses tested whether perceived stress interacted with any HIV clinical characteristics and HCV antibody status on the verbal memory domain and on delayed recall specifically. There was a significant interaction between perceived stress and HIV viral load on the verbal memory domain (p=0.04; Fig. 2) and on delayed recall (p=0.04) after adjusting for other clinical characteristics. In follow-up analyses, stress had marked negative associations with the verbal memory domain (B=−4.54, SE=1.61, p=0.005) and delayed recall (B=−4.42, SE=1.70, p=0.009) in HIV-infected women with viral loads ≥10, 000 copies/ml. The magnitude of this difference was sizable (~4.5 T-score points or ~0.50 standard deviations). Perceived stress also interacted with HCV antibody status on the verbal memory domain (p=0.02) and on delayed recall (p=0.03). In follow-up analyses, stress was only negatively associated with the verbal memory domain (B=−4.20, SE=1.25, p=0.005) and delayed recall (B=−4.19, SE=1.31, p=0.001) in HIV-infected women who were HCV antibody positive.
Fig. 2.
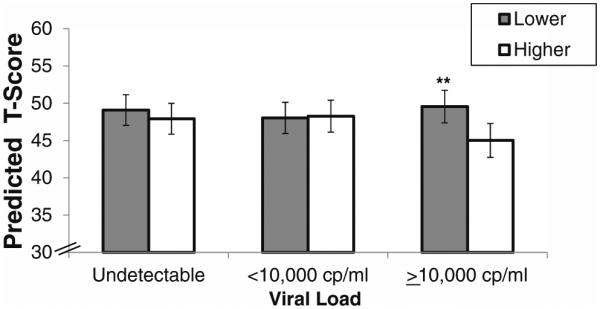
The interactive association between perceived stress (lower vs. higher) and viral load on the verbal memory domain in HIV-infected women. Note. **p<0.01. There was a significant interaction between perceived stress and viral load on the verbal memory domain (p=0.04). Among HIV-infected women with viral loads ≥10,000 cp/ml, those women with higher perceived stress performed significantly worse than women with lower perceived stress (B=−4.54, SE=1.61, p < 0.01). HIV-infected women with higher and lower perceived stress performed similarly if their viral loads were <10,000 cp/ml (B=0.23, SE=1.13, p=0.84) or undetectable (B=−1.17, SE=0.93, p=0.21). The model is adjusted for marijuana use; crack, cocaine, and/or heroin use; smoking; hazardous alcohol use; antidepressants; HCV; income; study site; number of exposures to the Hopkins Verbal Learning Test; recent CD4 count and viral load; nadir CD4 count; and cART use and adherence
Discussion
We found that regardless of HIV status women with higher levels of perceived stress performed worse than women with lower perceived stress on measures of verbal learning and memory, attention, processing speed, and executive function. Consistent with our previous study (Maki et al. 2015), HIV status was associated with poorer performance on measures of verbal learning and memory and attention. Most notably and as expected, there was a significant interaction between perceived stress and HIV status in that perceived stress was significantly associated with verbal memory among only the HIV-infected women. This association could not be attributed to higher levels of stress in HIV-infected women, as HIV-infected and HIV-uninfected women reported similar levels of perceived stress.
Consistent with prior literature (Seeman et al. 1997; VonDras et al. 2005; Wolf et al. 2001), women with higher levels of perceived stress in our cohort performed worse on measures of verbal learning and memory, attention, processing speed, and executive function compared to women with low perceived stress. These effects were small (d’s 0.14–0.19) with the largest effect being on verbal memory (d=0.19). Importantly, difficulties in verbal memory as well as executive functions and psychomotor speed are associated with increased risk of antiretroviral nonadherence (Hinkin et al. 2002, 2004). Insights into the neural circuitry of stress and memory associations are provided by studies in HIV-uninfected individuals. These studies have reported morphometric abnormalities in the medial prefrontal cortex (including the anterior cingulate) and hippocampus (Bremner 2007; Cohen et al. 2006; Lupien et al. 2009; McEwen 2000), brain areas important for attention, executive functions, and learning and memory (Carter et al. 1998; Kim and Diamond 2002). The prominent association between stress and memory might be due to stress-induced changes in the hippocampus, as exposure to psychological trauma is associated with smaller left, right, and total hippocampal volumes in HIV-uninfected adults (Woon et al. 2010). Perceived stress is also associated with grey matter volumes in the bilateral parahippocampal gyrus, fusiform cortex, and entorhinal cortex (Li et al. 2014). Midlife psychological stress is linked to late life brain temporal lobe atrophy and an increased risk of dementia (Johansson et al. 2010, 2012). Additionally, early life stress is linked to smaller anterior cingulate volumes in 1045 adults (Cohen et al. 2006). Laboratory-induced stressors such as social stress and restraint stress in animal studies also produce structural changes (e.g., dendritic remodeling) in the hippocampus (Buwalda et al. 2005; Luine et al. 1996). In humans, laboratory-induced stress (e.g., Trier Social Stress Test) produces impairments in memory, attention, and executive functioning (Kirschbaum et al. 1996; Olver et al. 2014; Plessow et al. 2011). Although previous studies demonstrate that psychosocial stressors impair working memory (Olver et al. 2014; Schoofs et al. 2008), we did not find an association between stress and working memory in the present cohort.
Consistent with our hypothesis, we found that perceived stress interacted with HIV status to influence verbal memory, in particular delayed verbal recall. This finding contrasts with a study in HIV-infected men where stress was associated with only attention, processing speed, and executive functioning (Pukay-Martin et al. 2012). The finding that stress is associated with deficits in delayed verbal memory lends new insights into the predominance of deficits in delayed verbal memory in WIHS women (Maki et al. 2015). Compared to men, women are more vulnerable to the negative effects of stress hormones on hippocampal-dependent tests (Seeman et al. 1997). This finding is notable in light of marked sex differences in the hypothalamic pituitary adrenal (HPA) axis response patterns (Kajantie and Phillips 2006; Kudielka and Kirschbaum 2005), the vulnerability of the hippocampus to stress (Alderson and Novack 2002; Diorio et al. 1993; Magarinos et al. 1987; McEwen 2007; McEwen et al. 1986; McEwen and Sapolsky 1995; Meaney and Aitken 1985; Sanchez et al. 2000), the profound effects of stress on immune function (DeVries et al. 2007), and the heightened vulnerability of individuals with post-traumatic stress disorder to the negative impact of glucocorticoids on verbal memory (Bremner et al. 2004; Grossman et al. 2006; Yehuda et al. 2007).
In the present study, perceived stress interacted with HIV status in relation to delayed verbal recall, but not learning, or retention on the HVLT. A pattern of impaired delayed recall, but not retention, suggests that HIV status and perceived stress interact to influence retrieval of information. Similar interactive effects of HIV status and perceived stress on acquisition (e.g., information not encoded as efficiently) and retention (e.g., information is not retained over a short time period) approached, but did not meet statistical significance (p’s< 0.10). Importantly, the interaction between perceived stress and HIV status remained for delayed verbal recall even after controlling for the total words learned. This finding suggests that the interaction on delayed verbal recall was not simply a result of poor acquisition. Taken together, the pattern of interactions provides tentative support that retrieval in particular is highly sensitive to the negative effects of stress among HIV-infected women.
The pattern of interactions on the HVLT outcomes also provides tentative support that stress and HIV among women might interact to particularly compromise hippocampal and prefrontal integrity and function and thus negatively influence performance on neurocognitive tests (Lupien et al. 2005, 2009; Lupien and Lepage 2001) which are associated with managing antiretroviral treatment (Hinkin et al. 2002, 2004) and are critically dependent on the integrity of prefrontal-hippocampal circuitry. In a previous WIHS functional magnetic resonance imaging (fMRI) study, hippocampal function correlated with both acquisition and delayed recall (Maki et al. 2009). Moreover, the hippocampus was overactive in HIV-infected women during delayed recall and the degree of activation correlated with impaired recall, which suggested a compensatory response (Maki et al. 2009). The hippocampus is particularly vulnerable to the effects of uncontrollable stress and excess cortisol (primary stress hormone) and is a prominent site of glucocorticoid receptors (Magarinos et al. 1987). Psychological stress and enhanced glucocorticoid levels disrupt long-term potentiation, suppress excitability, and cause neuronal death and atrophy in the hippocampus (Alderson and Novack 2002; McEwen and Sapolsky 1995).
Although viral load and HCV status did not individually predict verbal memory in HIV-infected women, these factors interacted with stress in analyses of verbal memory outcomes. Specifically, the associations of elevated perceived stress and verbal memory were quite marked among women whose viral loads were ≥10,000 cp/ml and among women who were HCV antibody positive. The viral load finding, though exploratory, is novel in the HIV adult literature, but a study of cognitive function found evidence for cognitive compromise, as measured by IQ, in HIV-infected children who were under high levels of stress (Hochhauser et al. 2008). In animal models, glucocorticoid exposures at levels similar to those following stressors but not at lower levels, exacerbated the deleterious effects of gp120, an HIV envelope protein, on hippocampal explants from the CA1 region of the hippocampus (Yusim et al. 2000), in part by increasing the release of toxins from microglia in the context of sustained stress (Brooke and Sapolsky 2002). The stronger associations between elevated stress and verbal memory observed in HCV antibody positive women compared to HCV antibody negative women is most likely due to HCV serology being a proxy of past intravenous drug use (Armstrong et al. 2006) rather than HCV status itself. In the present sample, 84 % of women who were HCV antibody positive also had a history of intravenous drug use. There is growing evidence that stress and drug use effect brain functioning including the prefrontal cortex and hippocampus (Sinha 2008; Sinha et al. 2005).
The present study has several limitations. First, the analyses were cross-sectional. We presumed that high levels of stress led to decreased cognitive functioning; however, it is possible that cognitive difficulties may have preceded high levels of stress for at least some women. Longitudinal assessments are underway to determine the robustness of these associations as well as mechanistic studies to understand the mechanisms by which stress can negatively impact cognition in HIV-infected women. Additionally, although stress is associated with depressive symptoms generally and in the present study, the interaction between HIV status and perceived stress on verbal memory in our study remained significant even after controlling for depressive symptoms.
Conclusions
To date, the HIV literature has examined the association between stress and cognition only in men. Here, we demonstrate that in contrast to those findings, women displayed a deficit in verbal memory that appears to relate to perceived stress. This finding suggests a female-related vulnerability to memory impairment related to stress. These findings underscore the importance of screening for and treating stress in HIV-infected women as it may increase vulnerability to memory dysfunction, resulting in decreased treatment adherence (Gorman et al. 2009) and poorer quality of life. Additionally, our findings suggest a possible movement toward written versus verbal instruction in the clinical setting to improve treatment adherence and long-term comprehension about health and treatment.
Acknowledgments
Dr. Rubin’s effort was supported by Grant Number 1K01MH098798-01 from the National Institute of Mental Health (NIMH) and by Grant Number K12HD055892 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institutes of Health Office of Research on Women’s Health (ORWH). This grant is also supported in part by the Chicago Developmental Center for AIDS Research (D-CFAR), an NIH-funded program (P30 AI 082151), which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NCCAM). Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen), U01-AI-034993; Metropolitan Washington WIHS (Mary Young), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Alexandra Levine and Marek Nowicki), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Leah H. Rubin, Department of Psychiatry, University of Illinois at Chicago, 912 S Wood St, Chicago, IL 60612, USA, lrubin2@uic.edulrubin@psych.uic.edu
Judith A. Cook, Department of Psychiatry, University of Illinois at Chicago, 912 S Wood St, Chicago, IL 60612, USA
Kathleen M. Weber, Bureau of Health Services of Cook County, The Core Center, Chicago, IL, USA
Mardge H. Cohen, Departments of Medicine Stroger Hospital and Rush University, Chicago, IL, USA
Eileen Martin, Department of Psychiatry, Rush University Medical Center, Chicago, IL, USA.
Victor Valcour, Department of Neurology, University of California, San Francisco, CA, USA.
Joel Milam, Institute for Health Promotion & Disease Prevention Research, University of Southern California, Los Angeles, CA, USA.
Kathryn Anastos, Departments of Medicine and Epidemiology & Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Mary A. Young, Department of Medicine, Georgetown University, Washington, DC, USA
Christine Alden, Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore, MD, USA.
Deborah R. Gustafson, Department of Neurology, SUNY-Downstate Medical Center, Brooklyn, NY, USA
Pauline M. Maki, Department of Psychiatry, University of Illinois at Chicago, 912 S Wood St, Chicago, IL 60612, USA Department of Psychology, University of Illinois at Chicago, Chicago, IL, USA.
References
- Alderson AL, Novack TA. Neurophysiological and clinical aspects of glucocorticoids and memory: a review. J Clin Exp Neuropsychol. 2002;24:335–355. doi: 10.1076/jcen.24.3.335.987. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. Acute stress impairs trace eye blink conditioning in females without altering the unconditioned response. Neurobiol Learn Mem. 2004;82:57–60. doi: 10.1016/j.nlm.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J, WIHS Collaborative Study Group The Women’s Interagency HIV Study. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6 [Google Scholar]
- Brandt J, Benedict RHB. Hopkins verbal learning test revised. PAR; Odessa: 2001. [Google Scholar]
- Bremner JD. Neuroimaging in posttraumatic stress disorder and other stress-related disorders. Neuroimaging Clin N Am. 2007;17:523–538. doi: 10.1016/j.nic.2007.07.003. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Afzal N, Nazeer A, Newcomer JW, Charney DS. Effects of dexamethasone on declarative memory function in posttraumatic stress disorder. Psychiatry Res. 2004;129:1–10. doi: 10.1016/j.psychres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Brief DJ, Bollinger AR, Vielhauer MJ, Berger-Greenstein JA, Morgan EE, Brady SM, Buondonno LM, Keane TM. Understanding the interface of HIV, trauma, post-traumatic stress disorder, and substance use and its implications for health outcomes. AIDS Care. 2004;16(Suppl 1):S97–S120. doi: 10.1080/09540120412301315259. [DOI] [PubMed] [Google Scholar]
- Brooke SM, Sapolsky RM. Glucocorticoid exacerbation of gp120 neurotoxicity: role of microglia. Exp Neurol. 2002;177:151–158. doi: 10.1006/exnr.2002.7956. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen M, Deamant C, Barkan S, Richardson J, Young M, Holman S, Anastos K, Cohen J, Melnick S. Domestic violence and childhood sexual abuse in HIV-infected women and women at risk for HIV. Am J Public Health. 2000;90:560–565. doi: 10.2105/ajph.90.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, McFarlane A, Bryant R, Gordon E, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health: Claremont Symposium on applied social psychology. Sage; Newbury Park, CA: 1988. [Google Scholar]
- Comalli PE, Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Craft TK, Glasper ER, Neigh GN, Alexander JK. 2006 Curt P. Richter award winner: social influences on stress responses and health. Psychoneuroendocrinology. 2007;32:587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol Rev. 2009;19:186–203. doi: 10.1007/s11065-009-9095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman R, Yehuda R, Golier J, McEwen B, Harvey P, Maria NS. Cognitive effects of intravenous hydrocortisone in subjects with PTSD and healthy control subjects. Ann N Y Acad Sci. 2006;1071:410–421. doi: 10.1196/annals.1364.032. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan battery: demographic corrections, research findings, and clinical applications. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, Thrasher D, Goetz MB, Stefaniak M. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochhauser CJ, Gaur S, Marone R, Lewis M. The impact of environmental risk factors on HIV-associated cognitive decline in children. AIDS Care. 2008;20:692–699. doi: 10.1080/09540120701693982. [DOI] [PubMed] [Google Scholar]
- Johansson L, Guo X, Waern M, Ostling S, Gustafson D, Bengtsson C, Skoog I. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain. 2010;133:2217–2224. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- Johansson L, Skoog I, Gustafson DR, Olesen PJ, Waern M, Bengtsson C, Bjorkelund C, Pantoni L, Simoni M, Lissner L, Guo X. Midlife psychological distress associated with late-life brain atrophy and white matter lesions: a 32-year population study of women. Psychosom Med. 2012;74:120–125. doi: 10.1097/PSY.0b013e318246eb10. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Li H, Li W, Wei D, Chen Q, Jackson T, Zhang Q, Qiu J. Examining brain structures associated with perceived stress in a large sample of young adults via voxel-based morphometry. Neuroimage. 2014;92C:1–7. doi: 10.1016/j.neuroimage.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magarinos AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiol Behav. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behav Brain Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Somoza G, De Nicola AF. Glucocorticoid negative feedback and glucocorticoid receptors after hippocampectomy in rats. Horm Metab Res. 1987;19:105–109. doi: 10.1055/s-2007-1011753. [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Valcour V, Martin E, Crystal HA, Young M, Weber KM, Manly J, Richardson J, Alden C, Anastos K. Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology. 2015;84(3):231–240. doi: 10.1212/WNL.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. J Int Neuropsychol Soc. 2002;8:341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Smith C, Crystal HA, Richardson J, Golub ET, Greenblatt R, Robison E, Martin EM, Young M. Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV− women: the Women’s Interagency HIV Study (WIHS) Neurocognitive Substudy. J Clin Exp Neuropsychol. 2011;33:853–863. doi: 10.1080/13803395.2010.547662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massad LS, Agniel D, Minkoff H, Watts DH, D’Souza G, Levine AM, Darragh TM, Young M, Cajigas A, Weber K. Effect of stress and depression on the frequency of squamous intraepithelial lesions. J Low Genit Tract Dis. 2011;15:42–47. doi: 10.1097/LGT.0b013e3181e66a82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Lewis E, Somley B, Kahan TA. Individual differences in cortisol levels and performance on a test of executive function in men and women. Physiol Behav. 2007;91:87–94. doi: 10.1016/j.physbeh.2007.01.020. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. [3H]Dexamethasone binding in rat frontal cortex. Brain Res. 1985;328:176–180. doi: 10.1016/0006-8993(85)91340-x. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Olver JS, Pinney M, Maruff P, Norman TR. Impairments of spatial working memory and attention following acute psychosocial stress. Stress Health. 2014 doi: 10.1002/smi.2533. [DOI] [PubMed] [Google Scholar]
- Plessow F, Fischer R, Kirschbaum C, Goschke T. Inflexibly focused under stress: acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to the stressor. J Cogn Neurosci. 2011;23:3218–3227. doi: 10.1162/jocn_a_00024. [DOI] [PubMed] [Google Scholar]
- Pukay-Martin ND, Cristiani SA, Saveanu R, Bornstein RA. The relationship between stressful life events and cognitive function in HIV-infected men. J Neuropsychiatry Clin Neurosci. 2003;15:436–441. doi: 10.1176/jnp.15.4.436. [DOI] [PubMed] [Google Scholar]
- Pukay-Martin ND, Pontoski KE, Maxwell MA, Calhoun PS, Dutton CE, Clancy CP, Hertzberg MA, Collie CF, Beckham JC. The influence of depressive symptoms on suicidal ideation among U.S. Vietnam-era and Afghanistan/Iraq-era veterans with posttraumatic stress disorder. J Trauma Stress. 2012;25:578–582. doi: 10.1002/jts.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Manual for administration of neuropsychological test batteries for adults and children. Neuropsychology Laboratories, Inc.; Tuscon, AZ: 1978. [Google Scholar]
- Reitan R, Wolfson D, editors. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Neuropsychology Press; Tuscon, AZ: 1985. [Google Scholar]
- Rubin LH, Sundermann EE, Cook JA, Martin EM, Golub ET, Weber KM, Cohen MH, Crystal H, Cederbaum JA, Anastos K, Young M, Greenblatt RM, Maki PM. Investigation of menopausal stage and symptoms on cognition in human immunodeficiency virus-infected women. Menopause. 2014 doi: 10.1097/GME.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs D, Pabst S, Brand M, Wolf OT. Working memory is differentially affected by stress in men and women. Behav Brain Res. 2013;241:144–153. doi: 10.1016/j.bbr.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Preuss D, Wolf OT. Psychosocial stress induces working memory impairmen ts in an n-back paradigm. Psychoneuroendocrinology. 2008;33:643–653. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab. 1997;82:2458–2465. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Shors TJ. Exposure to inescapable stress persistently facilitates associative and nonassociative learning in rats. Behav Neurosci. 1994;108:1101–1106. doi: 10.1037//0735-7044.108.6.1101. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Servatius RJ. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol Learn Mem. 1997;68:92–96. doi: 10.1006/nlme.1997.3763. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Smith A. The Symbol-Digit Modalities Test: a neuropsychologic test for economic screening of learning and other cerebral disorders. Learn Disord. 1968;3:83–91. [Google Scholar]
- VonDras DD, Powless MR, Olson AK, Wheeler D, Snudden AL. Differential effects of everyday stress on the episodic memory test performances of young, mid-life, and older adults. Aging Ment Health. 2005;9:60–70. doi: 10.1080/13607860412331323782. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. Wide range achievement test 3—administration manual. Jastak Associates, Inc; Wilimington, DE: 1993. [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Dawson MS, Morgan EE, Carey CL, Heaton RK, Grant I. Construct validity of Hopkins Verbal Learning Test-Revised component process measures in an HIV-1 sample. Arch Clin Neuropsychol. 2005;20:1061–1071. doi: 10.1016/j.acn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Harvey PD, Buchsbaum M, Tischler L, Schmeidler J. Enhanced effects of cortisol administration on episodic and working memory in aging veterans with PTSD. Neuropsychopharmacology. 2007;32:2581–2591. doi: 10.1038/sj.npp.1301380. [DOI] [PubMed] [Google Scholar]
- Yusim A, Franklin L, Brooke S, Ajilore O, Sapolsky R. Glucocorticoids exacerbate the deleterious effects of gp120 in hippocampal and cortical explants. J Neurochem. 2000;74:1000–1007. doi: 10.1046/j.1471-4159.2000.0741000.x. [DOI] [PubMed] [Google Scholar]


