Abstract
Cedecea neteri is a very rare human pathogen. We have isolated a strain of C. neteri SSMD04 from pickled mackerel sashimi identified using molecular and phenotypics approaches. Using the biosensor Chromobacterium violaceum CV026, we have demonstrated the presence of short chain N-acyl-homoserine lactone (AHL) type quorum sensing (QS) activity in C. neteri SSMD04. Triple quadrupole LC/MS analysis revealed that C. neteri SSMD04 produced short chain N-butyryl-homoserine lactone (C4-HSL). With the available genome information of C. neteri SSMD04, we went on to analyse and identified a pair of luxI/R homologues in this genome that share the highest similarity with croI/R homologues from Citrobacter rodentium. The AHL synthase, which we named cneI(636 bp), was found in the genome sequences of C. neteri SSMD04. At a distance of 8bp from cneI is a sequence encoding a hypothetical protein, potentially the cognate receptor, a luxR homologue which we named it as cneR. Analysis of this protein amino acid sequence reveals two signature domains, the autoinducer-binding domain and the C-terminal effector which is typical characteristic of luxR. In addition, we found that this genome harboured an orphan luxR that is most closely related to easR in Enterobacter asburiae. To our knowledge, this is the first report on the AHL production activity in C. neteri, and the discovery of its luxI/R homologues, the orphan receptor and its whole genome sequence.
Keywords: N-acyl-homoserine lactone, Quorum sensing, Food microbiology, Mass spectrometry, N-butyryl-homoserine lactone, Autoinducer
Introduction
Cedecea spp. are extremely rare Gram-negative bacteria that belong to the Enterobacteriaceae family (Berman, 2012). The representative of the genus is lipase-positive and resistant to colistin and cephalothin. The name Cedecea was coined by Grimont and Grimont, from the abbreviation of the Centers for Disease Control (CDC) (Grimont et al., 1981). Originally recognized as Enteric group 15, this genus is comprised of five species, out of which only three are valid, C. neteri, C. lapagei, C. davisae, while the other two were not validly published and are known as Cedecea species 3 and Cedecea species 5 (Brenner et al., 2005).
Cedecea species 4 was named as C. neteri in 1982 when its clinical significance was reported (Farmer 3rd et al., 1982). C. neteri was also found in a patient with systemic lupus erythematosus where it led to the patient’s death (Aguilera et al., 1995). Even though it was evident that C. neteri can act as human pathogen, its etiology is unknown and limited studies have been conducted on Cedecea spp. There were cases of isolation of Cedecea spp. from other sources except human (Jang & Nishijima, 1990; Osterblad et al., 1999).
Bacteria demonstrate a concerted gene regulation mechanism termed ‘Quorum Sensing’ (QS) that relies on the population density of the bacteria (Fuqua, Winans & Greenberg, 1996; Miller & Bassler, 2001; Schauder & Bassler, 2001). The mechanism of QS involves the production, release, detection, and response to small diffusible molecules known as autoinducers, such as N-acyl homoserine lactones (AHLs) commonly employed by Gram negative bacteria (Chhabra et al., 2005; Williams et al., 2007). AHL molecules are generally characterized by the length and saturation of the acyl side chains, which can vary from 4 to 18 carbons (Pearson, Van Delden & Iglewski, 1999), as well as the R-group substitution at the third carbon (Pearson, Van Delden & Iglewski, 1999; Waters & Bassler, 2005). QS has been shown to play a role in the regulation of a wide range of phenotypes, such as antibiotic biosynthesis, biofilm formation, pathogenesis, and bioluminescence (Fuqua, Winans & Greenberg, 1996; Salmond et al., 1995; De Kievit & Iglewski, 2000; Hastings & Nealson, 1977; Bainton et al., 1992; Eberl et al., 1996).
We have recently reported the isolation of C. neteri SSMD04 from Shime saba, a Japanese cuisine that involves marinating with salt and rice vinegar, enabling the usually perishable saba (mackerel) to be consumed in the form of sashimi (raw fish). The complete genome of C. neteri SSMD04 has been sequenced and published (Chan et al., 2014). This strain was isolated in a study to investigate the role of AHL-based QS in food spoilage and food safety (JY Tan, 2014, unpublished data). As a known human pathogen, C. neteri has never been reported to be isolated from food source. The adaptability of the bacterium to survive and colonize the two environments is an interesting aspect to be studied. It has been known that QS regulates virulence as well as food spoilage traits in some bacteria (Passador et al., 1993; Brint & Ohman, 1995; Skandamis & Nychas, 2012; Bruhn et al., 2004). This prompted us to test C. neteri SSMD04 for its QS activity. The genome sequence enabled investigation of QS related genes. Meanwhile, the presence of this bacterium in oily fish suggests possible lipolytic activity, which is present in representative of the genus Cedecea. Its lipase activity was also tested for this reason.
In this work, we show for the first time that C. neteri possesses an AHL QS system and identified a novel signaling synthase gene (cneI), its cognate receptor (cneR), and an orphan LuxR-type receptor gene.
Materials and Methods
Sample collection and processing
Shime saba sashimi sample was collected from a local supermarket in Malaysia and processed within half an hour following collection. Five grams of sample was stomached (mixing of sample in a sterile plastic bag by applying forces to the outside of the bag) using Stomacher® 400 circulator (Seward, West Sussex, UK) and homogenized in 50 ml of peptone water and then spread on MacConkey (MAC) agar. The culture plates were incubated overnight at 28 °C.
Bacterial strains, media and culture conditions
C. neteri SSMD04, Chromobacterium violaceum CV026, Erwinia carotovora GS101 and E. carotovora PNP22 were maintained in Luria Bertani (LB) medium at 28 °C. lux-based biosensor Escherichia coli (pSB401) was grown in LB supplemented with tetracycline (20 µg/mL) at 37 °C. All broth cultures were incubated with shaking (220 rpm).
Species identification of isolate SSMD04
16S rDNA phylogenetic analysis
Whole genome sequencing, assembly, annotation were performed as described previously (Chan et al., 2014). 16S rRNA gene sequence of C. neteri SSMD04 was searched using “Genome Browser” function in RAST (Aziz et al., 2012) after automated annotation. Other 16S rRNA gene sequences of Cedecea. spp. were retrieved from GenBank through text search. MEGA 6.0 (Tamura et al., 2013) was used to align the sequences with ClustalW and construct a Maximum likelihood tree using 1,000 bootstrap replications.
Biolog GEN III microbial identification system
Microbial identification using Biolog GEN III MicroPlate (Biolog, Hayward, California, USA) was carried out according to manufacturer’s protocol. In brief, overnight culture of C. neteri SSMD04 grown on Tryptic Soy Agar (TSA) was used to inoculate inoculating fluid (IF) A to a cell density of 90–98% transmittance. The inoculum was then pipetted into each well of the MicroPlate (100 µL per well) and incubated at 28 °C for 24 h. The MicroPlate was then read using the machine reader and software where the wells will be scored as ‘negative’ or ‘positive’ based on the colour change due to the reduction of tetrazolium redox dyes. This ‘Phenotypic Fingerprint’ was then used to identify the bacteria by matching it against the database in the system.
Detection of AHL production in C. neteri SSMD04
AHL-type QS activity of C. neteri SSMD04 was screened using biosensor C. violaceum CV026. This is performed by cross streaking C. neteri SSMD04 against C. violaceum CV026 (McClean et al., 1997). E. carotovora GS101 and E. carotovora PNP22 were used as positive and negative controls, respestively (Jones et al., 1993).
AHL extraction
C. neteri SSMD04 was cultured overnight at 28 °C in LB broth (100 mL) supplemented with 50 mM of 3-(N-morpholino)propanesulfonic acid (MOPS) (pH5.5). Culture supernatant was collected by centrifugation and organic compounds were subsequently extracted twice with equal volume of acidified ethyl acetate (AEA) (0.1% v/v glacial acetic acid). The extracts were air dried and reconstituted in 1 mL of AEA, transferred into sterile microcentrifuge tubes and air dried again. The extracts were later used for detection of AHL by lux-based biosensor E. coli (pSB401) as well as triple quadrupole LC/MS.
AHL identification by triple quadrupole LC/MS
AHL extracts were reconstituted in acetonitrile (ACN) prior to LC/MS analysis as described before (Lau et al., 2013), with slight modifications. In brief, mobile phase A was water with 0.1% v/v formic acid and mobile phase B used was ACN with 0.1% formic acid. The flow rate used was 0.5 mL/min. The gradient profile was set to: A:B 80:20 at 0 min, 50:50 at 7 min, 50:50 at 7.10 min, 80:20 at 12 min, 80:20 at 12.10 min, 20:80 at 14 min, 20:80 at 14.10 min. Precursor ion scan mode was carried out in positive ion mode with Q1 set to monitor m/z 90 to m/z 400 and Q3 set to monitor for m/z 102 which is characteristics of lactone ring moiety. ACN was also used as a blank.
Measurement of bioluminescence
E. coli (pSB401) (Winson et al., 1998) was used as biosensor for the detection of exogenous short chain AHLs present in the extracts. The biosensor strain was cultured in LB broth supplemented with tetracycline (20 µg/mL). The overnight culture was then diluted to an OD600 of 0.1 with fresh LB broth with tetracycline. The diluted E. coli culture was used to resuspend the extracts from Section ‘AHL extraction,’ prior to being dispensed into a 96-well optical bottom microtitre plate. Cell density bioluminescence measurements were carried out by Infinite M200 luminometer-spectrophotometer (Tecan, Männedorf, Switzerland) over a period of 24 h. Diluted E. coli culture without extracts was read for normalization and sterile broth was used as negative control. The results were displayed as relative light units (RLU)/OD495 nm against incubation time.
Lipase activity
The lipase activity of C. neteri SSMD04 was tested in a medium consisted of (in w/v) 0.05% yeast extract, 0.1% tryptone, 1% NaCl, and 1.5% agar supplemented with 0.5% (v/v) corn oil. The oil forms an opaque suspension in the agar. Bacteria culture was streaked on the agar and incubated at 28 °C for 48 h. The enzymatic activity is visualized by a halo zone formed around the colonies caused by the breakdown of lipids.
luxI/R homologues search and analysis
Whole genome of C. neteri SSMD04 was sequenced and annotated (Chan et al., 2014). The luxI/R homologues were searched on RAST using the “Genome Browser” function. The “Annotation Overview” function on RAST was used to make locus comparison of cneI/R pair and the orphan luxR with other genomes. Multiple sequence alignment of LuxR-type proteins was done with ClustalW. ESPript (Robert & Gouet, 2014) was used for the presentation of the alignment.
Results
As was mentioned in the introduction, C. neteri SSMD04 was isolated from shime saba in an attempt to recover AHL-producing bacteria from food. It was identified through 16S rRNA gene sequence and Biology Gen III microbial identification system. The novelty of this bacterium, coupled with the possible role it plays in food spoilage and known clinical aspects of other members of the same species, led to further analysis.
Species identification of C. neteri SSMD04
Seven 16S rRNA gene sequences were found in C. neteri SSMD04 genome, of which 6 were identical and the other has 2 SNPs. Both variants were included in phylogenetic analysis with other sequences of Cedecea spp. available in GenBank. As can be seen, 16S rRNA gene sequences of C. neteri SSMD04 and other C. neteri strains appeared in a monophyletic clade (Fig. 1).
Figure 1. Phylogenetic tree showing the position of C. neteri SSMD04 (green squares) relative to other Cedecea spp.
The maximum likelihood tree was inferred from 1,297 aligned positions of the 16S rDNA sequences using Hasegawa-Kishino-Yano substitution model. Boostrap values are represented at the nodes. The scale denotes the number of substitutions per nucleotide position. Serratia marcescens strain HokM was used as outgroup.
The Biology Gen III microbial identification system was also used to assess the identity of C. neteri SSMD04 biochemically. The system identified this strain to be C. neteri with a probability and similarity of 0.697. The positive reaction in sucrose well and D-sorbitol well agrees with the report of Farmer 3rd et al. (1982).
Detection of AHL-type QS activity in C. neteri SSMD04 using AHL biosensor
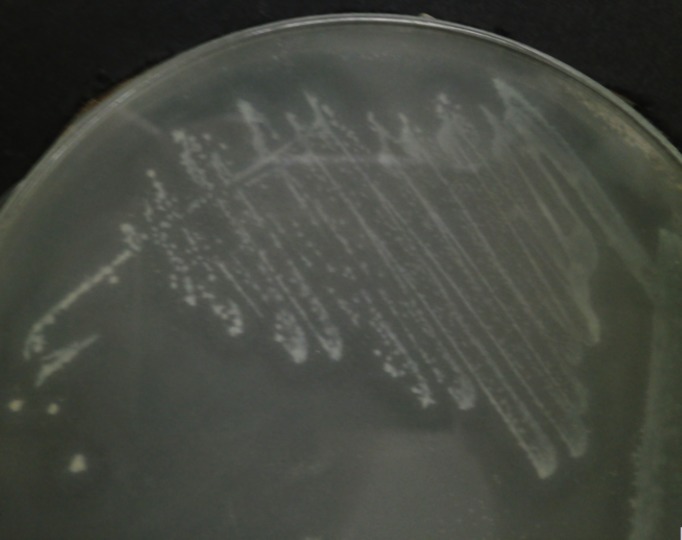
Since AHL-type QS has been known to regulate virulence and food spoilage traits in some bacteria, we hypothesized that C. neteri exhibits QS activity as well. In order to investigate the presence of AHL-type QS activity in C. neteri SSMD04, it was streaked on LBA against biosensor C. violaceum CV026, which would show purple pigmentation due to the production of violacein in the presence of short chain AHLs. The result shown in Fig. 2 indicated the presence of exogenous AHLs in C. neteri SSMD04 culture. E. carotovora GS101, a known AHL producing Erwinia strain, and E. carotovora PNP22, an AHL-synthase mutant were chosen as positive and negative controls, respectively.
Figure 2. Screening for AHL-type QS activity of C. neteri SSMD04 using the biosensor C. violaceum CV026.

The positions of C. neteri SSMD04, E. carotovora PNP22, E. carotovora GS101 are indicated by arrows, whereas C. violaceum CV026 was streaked perpendicularly against the tested strain at the periphery of the plate.
C. neteri SSMD04 also activated lux-based biosensor E. coli (pSB401) which produces bioluminescence in the presence of short chain AHLs (Fig. 3).
Figure 3. Detection of AHL by E. coli (pSB401).
Relative light unit (RLU)/OD495 against incubation time of cultures of E. coli (pSB401) in the presence of extracted AHLs (square plots) and negative control (circle plots).
AHL identification by triple quadrupole LC/MS
The use of both biosensors (C. violaceum CV026 and E. coli (pSB401)) does not give information on the specific type of AHL produced since each detects a variety of AHLs. Therefore, triple quadrupole LC/MS was employed in the identification of the AHL produced by C. neteri SSMD04. The extracted-ion chromatogram (EIC) generated from the triple quadrupole LC/MS system showed a peak with the same retention time as that of the synthetic N-butyryl-homoserine lactone (C4-HSL) standard, which was constantly present in all three replicates (Fig. 4). Analysis of the mass spectrum (MS) data revealed the presence of a peak with mass-to-charge ratio (m/z) of 172 (Fig. 5), which is consistent with the previously reported value (Ortori et al., 2011). This implication was strengthened by the presence of a product ion peak (m/z = 102), confirming the presence of a homoserine lactone ring, the invariant structure of AHLs. This result is in agreement with the results of the biosensor strains, C. violaceum CV026 and E. coli (pSB401), as both respond to C4-HSL.
Figure 4. EIC of C. neteri SSMD04 extract.

The data represented three replicates of the extract against synthetic C4-HSL, labelled as “std.” ACN was used as blank.
Figure 5. Mass spectrometry analysis of C. neteri SSMD04 spent supernatant extract.

Product ions of the peak seen which shows that the extract of C. neteri SSMD04 contains C4-HSL.
Lipase activity
C. neteri SSMD04 was streaked on medium containing 0.5% (v/v) corn oil and incubated overnight to test for its lipase activity. The halo zone was visible around the colonies after 24 h of incubation, but it was more visible after 48 h of incubation (Fig. 6).
Figure 6. C. neteri SSMD04 grown on medium containing 0.5% corn oil.
The halo zone around the colonies indicate lipase activity.
luxI/R homologues search and analysis
From the data generated by NCBI prokaryotic genome annotation pipeline, a 636 bp luxI homologue, hereafter named cneI, was found in the genome. This gene shares 70% base pair similarity with N-acyl homoserine lactone synthase croI of Citrobacter rodentium ICC168. Analysis of amino acid sequence of cneI using InterPro (Mitchell et al., 2015) identified the presence of an acyl-CoA-N-acyltransferase domain, the structural domain of N-acyl homoserine lactone synthetases (Gould, Schweizer & Churchill, 2004; Watson et al., 2002).
Eight bp away from cneI is a sequence encoding a hypothetical protein, potentially the cognate receptor, a luxR homologue (cneR). The coding region was found to be 705 bp long and share 70% similarity with croR of C. rodentium. Analysis of this protein reveals two signature domains, the autoinducer-binding domain and the C-terminal effector.
In order to investigate the relatedness of cneI/R and croI/R, the organization of cneI/R and their flanking region was examined and comparison to another C. neteri genome, M006, as well as C. rodentium ICC168 was made (Fig. 7). As can be seen, gene organization of cneI/R and their flanking region is highly similar in both C. neteri strains, but displays no similarity with croI/R and their flanking region. Interestingly, the genome of C. davisae DSM 4568 harbours no luxI/R homologous pair, suggesting that AHL-type quorum sensing is not present in all members of the genus Cedecea.
Figure 7. Schematic representation of cneI/R locus of C. neteri SSMD04 in comparison with C. neteri M006 and C. rodentium ICC168 (GenBank ID: CP0009458.1 and FN543502.1, respectively).
Species and strain are shown at the left. Each gene is numbered and labelled accordingly. Homologous genes are represented in the same colour. The arrows show the orientation of the gene. Overlapping genes are arranged below the line.
Apart from that, a sequence potentially encoding an orphan luxR type receptor (723 bp) was also found within the genome. This luxR homologue shares 69% sequence homology to luxR homologue of Enterobacter asburiae L1. Multiple sequence alignment of this orphan LuxR, CneR, and other canonical LuxR-type proteins (Fig. 8) reveals the presence of conserved sites, residues 57, 61, 70, 71, 85, 113, 178, 182, 188 (TraR sequence numbering used as a reference), which are present in at least 95% of LuxR-type proteins (Whitehead et al., 2001; Zhang et al., 2002).
Figure 8. Multiple sequence alignment of CneR, C. neteri SSMD04 orphan LuxR (Orphan) with five other canonical QS LuxR-type proteins.

TraR, CroR, LuxR, LasR, and RhlR (GenBank ID: 282950058, 59482356, 299361, 541657, 1117981, 740696391, 689266442, respectively) are LuxR-type proteins involved in quorum sensing from A. tumefaciens, C. rodentium, Vibrio fischeri, Pseudomonas aeruginosa (LasR and RhlR), respectively. Identical residues are denoted by a vertical filled bar, and conserved residues are denoted by unfilled box. TraR residue numbering is shown above the alignment as reference.
Orphan LuxR is known to be present in E. coli and Salmonella which allows response to exogenous AHLs (Ahmer, 2004). The function of the orphan LuxR in C. neteri SSMD04 is not known. However, genome comparison of C. neteri SSMD04 and M006, C. davisae DSM4568, C. rodentium ICC168, S. enterica subsp. enterica serovar Choleraesuis str. SC-B67, and E. coli K12 shows a considerable degree of conservation (Fig. 9). This is especially true for the 3 genomes of the genus Cedecea, despite the absence of canonical luxI/R pair in C. davisae DSM4568 genome.
Figure 9. Organization of C. neteri SSMD04 orphan luxR and its flanking genes in comparison with other selected species C. davisae DSM4568, C. neteri M006, C. rodentium ICC168, Salmonella enterica subsp. enterica serovar Ch.
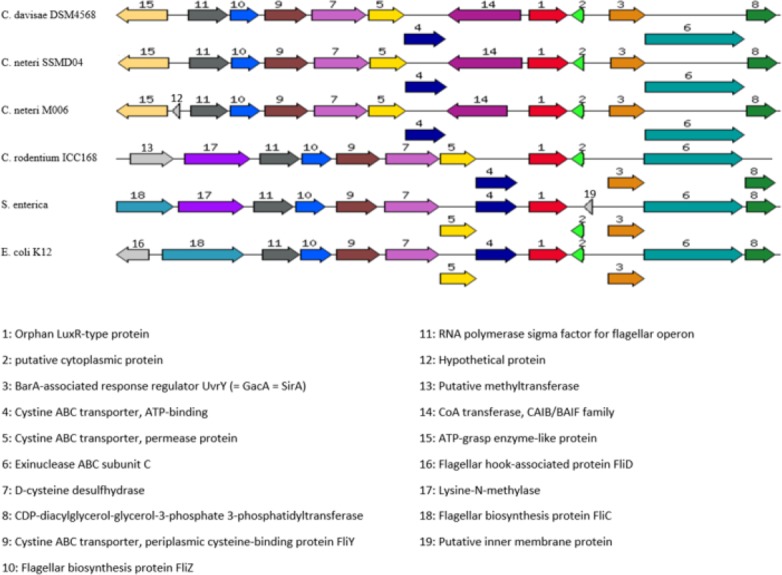
Organization of C. neteri SSMD04 orphan luxR and its flanking genes in comparison with other selected species C. davisae DSM4568, C. neteri M006, C. rodentium ICC168, Salmonella enterica subsp. enterica serovar Choleraesuis str. SC-B67 and E. coli K12 (GenBank ID: 513473511, 690276415, 282947233, 62178570, 556503834, respectively).
Discussion
Cedecea spp. are not well studied. Despite the evidence of their ability to infect human, their medical significance can be overlooked due the poor understanding of their physiology and etiology. Besides that, they are potentially challenging pathogens due to their resistance towards a wide range of antimicrobial agents, such as cephalothin, extended spectrum cephalosporins, colistin, and aminoglycosides (Mawardi et al., 2010; Abate, Qureshi & Mazumder, 2011; Dalamaga et al., 2008). To date, isolation of C. neteri from a non-clinical source has not been reported. Little is known about the mechanism of pathogenesis in this bacterium, and it has never been reported to be present in food. Nevertheless, the isolation of C. neteri SSMD04 from a food source expands the current knowledge on diversity of the genus Cedecea.
Although employed by a wide range of Gram-negative bacteria in gene regulation that allows the alteration of behaviour on a population level (Waters & Bassler, 2005), AHL-type QS activities have not been reported in C. neteri. Some bacteria utilizes QS to regulate virulence and thus gaining advantage of expressing virulence factors only when the population density is large enough to triumph the hosts’ immune system (Passador et al., 1993; Brint & Ohman, 1995; McClean et al., 1997; Thomson et al., 2000; Weeks et al., 2010). Regulation of virulence factors by QS has been extensively studied in Pseudomonas aeruginosa. The transcriptional regulator LasR in P. aeruginosa, a homologue of LuxR regulates expression of virulence genes such as lasB, lasA, aprA, and toxA in the presence of N-(-3-oxododecanoyl)-L-homoserine lactone (OC12-HSL), synthesized by LasI, a homologue of LuxI (Gambello & Iglewski, 1991; Gambello, Kaye & Iglewski, 1993; Pearson et al., 1994; Toder, Gambello & Iglewski, 1991). QS regulated virulence can also be seen in other bacteria such as Burkholderia cepacia, E. carotovora, and Agrobacterium tumefaciens (De Kievit & Iglewski, 2000). Given the understanding that C. neteri can act as human pathogen, it can be hypothesized that AHL-type QS activity in C. neteri is involved in the regulation of virulence factors. However, further studies on clinical as well as non-clinical strains would help in the solution of this hypothesis.
The whole genome sequence provides very valuable information in studying the genetic basis of QS in C. neteri SSMD04. The finding of cneI/R in this genome, lying adjacent to each other, demonstrated a common feature of luxI/R homologues (Brint & Ohman, 1995; Williamson et al., 2005). Analysis of the amino acid sequence of the cneI/R pair with InterPro agreed with their identity. The cneI/R pair was found to be most similar to croI/R in C. rodentium. C. rodentium has been found to produce C4-HSL as the major AHL and C6-HSL as the minor (Coulthurst et al., 2007). C. neteri SSMD04 also produces C4-HSL, but it does not produce detectable levels of C6. Nevertheless, chromosomal region comparison between two C. neteri genomes and C. rodentium shows no similarity in gene organization at the luxI/R homologues region. Interestingly, gene organization of the orphan luxR of C. neteri is highly similar to that of C. rodentium as well as E. coli and Salmonella. This probably allows C. neteri to interfere with AHLs produced by other bacterial species and thus improving population fitness.
The presence of lipase-positive C. neteri in marinated oily fish strongly suggests its role as a potential food spoilage agent, not only because of its ability to survive an extreme environment of high salinity and acidity, but also the fact that AHLs have long been associated with food spoilage via regulation of the proteolytic and lipolytic pathways (Skandamis & Nychas, 2012; Bruhn et al., 2004). Nevertheless, the roles of C. neteri in pathogenesis and food spoilage still require more information to be elucidated.
Conclusion
This study has confirmed the production of C4-HSL by C. neteri SSMD04 isolated from Shime saba sashimi. This is the first report of QS activity in C. neteri. However, the function of QS in C. neteri SSMD04 is still unknown. We hope that further studies coupled with the available genome information of C. neteri SSMD04 can help to elucidate the regulatory circuit of C. neteri SSMD04 by QS.
Supplemental Information
Funding Statement
The University of Malaya High Impact Research (HIR) Grants supported this work (UM-MOHE HIR Grant UM.C/625/1/HIR/MOHE/CHAN/14/1, no. H-50001-A000027; UM-MOHE HIR Grant UM.C/625/1/HIR/MOHE/CHAN/01, No. A000001-50001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Kian-Hin Tan performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables.
Jia-Yi Tan performed the experiments, wrote the paper, prepared figures and/or tables.
Wai-Fong Yin performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Kok-Gan Chan conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
We have submitted the whole genome sequence of C. neteri SSMD04 to NCBI:
NCBI Reference Sequence: NZ_CP009451.1
GenBank: CP009451.1
GI: 689262542.
References
- Abate, Qureshi & Mazumder (2011).Abate G, Qureshi S, Mazumder SA. Cedecea davisae bacteremia in a neutropenic patient with acute myeloid leukemia. Journal of Infection. 2011;63:83–85. doi: 10.1016/j.jinf.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Aguilera et al. (1995).Aguilera A, Pascual J, Loza E, Lopez J, Garcia G, Liaño F, Quereda C, Ortuño J. Bacteraemia with Cedecea neteri in a patient with systemic lupus erythematosus. Postgraduate Medical Journal. 1995;71:179. doi: 10.1136/pgmj.71.833.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmer (2004).Ahmer BMM. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Molecular Microbiology. 2004;52:933–945. doi: 10.1111/j.1365-2958.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- Aziz et al. (2012).Aziz RK, Devoid S, Disz T, Edwards RA, Henry CS, Olsen GJ, Olson R, Overbeek R, Parrello B, Pusch GD, Stevens RL, Vonstein V, Xia F. SEED servers: high-performance access to the SEED genomes, annotations, and metabolic models. PLoS ONE. 2012;7:e1216. doi: 10.1371/journal.pone.0048053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton et al. (1992).Bainton NJ, Stead P, Chhabra SR, Bycroft BW, Salmond GP, Stewart GS, Williams P. N-(3-oxohexanoyl)-L-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochemical Journal. 1992;288(Pt 3):997–1004. doi: 10.1042/bj2880997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman (2012).Berman JJ. Taxonomic guide to infectious diseases: understanding the biologic classes of pathogenic organisms. Amsterdam: Academic Press; 2012. [Google Scholar]
- Brenner et al. (2005).Brenner DJ, Krieg NR, Staley JT, Garrity GM. Bergey’s manual of systematic bacteriology vol. 2: the Protoeobacteria: Part B: the Gammaproteobacteria. New York: Springer; 2005. Cedecea; p. 683. [Google Scholar]
- Brint & Ohman (1995).Brint JM, Ohman DE. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. Journal of Bacteriology. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn et al. (2004).Bruhn JB, Christensen AB, Flodgaard LR, Nielsen KF, Larsen TO, Givskov M, Gram L. Presence of acylated homoserine lactones (AHLs) and AHL-producing bacteria in meat and potential role of AHL in spoilage of meat. Applied and Environmental Microbiology. 2004;70:4293–4302. doi: 10.1128/AEM.70.7.4293-4302.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan et al. (2014).Chan KG, Tan KH, Yin WF, Tan JY. Complete genome sequence of Cedecea neteri strain SSMD04, a bacterium isolated from pickled mackerel sashimi. Genome Announcements. 2014;2(6):e1216. doi: 10.1128/genomeA.01339-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra et al. (2005).Chhabra SR, Philipp B, Eberl L, Givskov M, Williams P, Camara M. Extracellular communication in bacteria. Chemistry of Pheromones and Other Semiochemicals II. 2005;240:279–315. doi: 10.1007/b98319. [DOI] [Google Scholar]
- Coulthurst et al. (2007).Coulthurst SJ, Clare S, Evans TJ, Foulds IJ, Roberts KJ, Welch M, Dougan G, Salmond GP. Quorum sensing has an unexpected role in virulence in the model pathogen Citrobacter rodentium. EMBO Reports. 2007;8:698–703. doi: 10.1038/sj.embor.7400984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalamaga et al. (2008).Dalamaga M, Karmaniolas K, Arsenis G, Pantelaki M, Daskalopoulou K, Papadavid E, Migdalis I. Cedecea lapagei bacteremia following cement-related chemical burn injury. Burns. 2008;34:1205–1207. doi: 10.1016/j.burns.2007.09.001. [DOI] [PubMed] [Google Scholar]
- De Kievit & Iglewski (2000).De Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infection and Immunity. 2000;68:4839–4849. doi: 10.1128/IAI.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl et al. (1996).Eberl L, Winson MK, Sternberg C, Stewart GS, Christiansen G, Chhabra SR, Bycroft B, Williams P, Molin S, Givskov M. Involvement of N-acyl-L-hormoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Molecular Microbiology. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- Farmer 3rd et al. (1982).Farmer JJ, 3rd, Sheth NK, Hudzinski JA, Rose HD, Asbury MF. Bacteremia due to Cedecea neteri sp. nov. Journal of Clinical Microbiology. 1982;16:775–778. doi: 10.1128/jcm.16.4.775-778.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua, Winans & Greenberg (1996).Fuqua C, Winans SC, Greenberg EP. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annual Review of Microbiology. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- Gambello & Iglewski (1991).Gambello MJ, Iglewski BH. Cloning and characterization of the Pseudomonas aeruginosalasR gene: a transcriptional activator of elastase expression. Journal of Bacteriology. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello, Kaye & Iglewski (1993).Gambello MJ, Kaye S, Iglewski BH. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infection and Immunity. 1993;61:1180–1184. doi: 10.1128/iai.61.4.1180-1184.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, Schweizer & Churchill (2004).Gould TA, Schweizer HP, Churchill ME. Structure of the Pseudomonas aeruginosa acyl-homoserinelactone synthase LasI. Molecular Microbiology. 2004;53:1135–1146. doi: 10.1111/j.1365-2958.2004.04211.x. [DOI] [PubMed] [Google Scholar]
- Grimont et al. (1981).Grimont PAD, Grimont F, Farmer JJ, 3rd, Asbury MA. Cedecea davisae gen. nov., sp. nov. and Cedecea lapagei sp. nov., New Enterobacteriaceae from clinical specimens. International Journal of Systematic Bacteriology. 1981;31:317–326. doi: 10.1099/00207713-31-3-317. [DOI] [Google Scholar]
- Hastings & Nealson (1977).Hastings JW, Nealson KH. Bacterial bioluminescence. Annual Review of Microbiology. 1977;31:549–595. doi: 10.1146/annurev.mi.31.100177.003001. [DOI] [PubMed] [Google Scholar]
- Jang & Nishijima (1990).Jang EB, Nishijima KA. Identification and attractancy of bacteria associated with Dacus dorsalis (Diptera: Tephritidae) Environmental Entomology. 1990;19:1726–1731. doi: 10.1093/ee/19.6.1726. [DOI] [Google Scholar]
- Jones et al. (1993).Jones S, Yu B, Bainton NJ, Birdsall M, Bycroft BW, Chhabra SR, Cox AJR, Golby P, Reeves PJ, Stephens S, Winson MK, Salmond GPC, Stewart GSAB, Williams P. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. The EMBO Journal. 1993;12:2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau et al. (2013).Lau YY, Sulaiman J, Chen JW, Yin WF, Chan KG. Quorum sensing activity of Enterobacter asburiae isolated from lettuce leaves. Sensors. 2013;13:14189–14199. doi: 10.3390/s131014189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawardi et al. (2010).Mawardi H, Pavlakis M, Mandelbrot D, Woo SB. Sirolimus oral ulcer with Cedecea davisae superinfection. Transplant Infectious Disease. 2010;12:446–450. doi: 10.1111/j.1399-3062.2010.00514.x. [DOI] [PubMed] [Google Scholar]
- McClean et al. (1997).McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143(Pt 12):3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- Miller & Bassler (2001).Miller MB, Bassler BL. Quorum sensing in bacteria. Annual Review of Microbiology. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- Mitchell et al. (2015).Mitchell A, Chang HY, Daugherty L, Fraser M, Hunter S, Lopez R, McAnulla C, McMenamin C, Nuka G, Pesseat S, Sangrador-Vegas A, Scheremetjew M, Rato C, Yong SY, Bateman A, Punta M, Attwood TK, Sigrist CJ, Redaschi N, Rivoire C, Xenarios I, Kahn D, Guyot D, Bork P, Letunic I, Gough J, Oates M, Haft D, Huang H, Natale DA, Wu CH, Orengo C, Sillitoe I, Mi H, Thomas PD, Finn RD. The InterPro protein families database: the classification resource after 15 years. Nucleic Acids Research. 2015;43:213–221. doi: 10.1093/nar/gku1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortori et al. (2011).Ortori CA, Dubern JF, Chhabra SR, Camara M, Hardie K, Williams P, Barrett DA. Simultaneous quantitative profiling of N-acyl-L-homoserine lactone and 2-alkyl-4(1H)-quinolone families of quorum-sensing signaling molecules using LC-MS/MS. Analytical and Bioanalytical Chemistry. 2011;399:839–850. doi: 10.1007/s00216-010-4341-0. [DOI] [PubMed] [Google Scholar]
- Osterblad et al. (1999).Osterblad M, Pensala O, Peterzens M, Heleniusc H, Huovinen P. Antimicrobial susceptibility of Enterobacteriaceae isolated from vegetables. Journal of Antimicrobial Chemotherapy. 1999;43:503–509. doi: 10.1093/jac/43.4.503. [DOI] [PubMed] [Google Scholar]
- Passador et al. (1993).Passador L, Cook JM, Gambello MJ, Rust L, Iglewski BH. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- Pearson et al. (1994).Pearson JP, Gray KM, Passador L, Tucker KD, Ebeerhard A, Iglewski BH, Greenberg EP. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, Van Delden & Iglewski (1999).Pearson JP, Van Delden C, Iglewski BH. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. Journal of Bacteriology. 1999;181:1203–1210. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert & Gouet (2014).Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Research. 2014;42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond et al. (1995).Salmond GP, Bycroft BW, Stewart GS, Williams P. The bacterial ‘enigma’: cracking the code of cell–cell communication. Molecular Microbiology. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- Schauder & Bassler (2001).Schauder S, Bassler BL. The languages of bacteria. Genes and Development. 2001;15:1468–1480. doi: 10.1101/gad.899601. [DOI] [PubMed] [Google Scholar]
- Skandamis & Nychas (2012).Skandamis PN, Nychas GJ. Quorum sensing in the context of food microbiology. Applied and Environmental Microbiology. 2012;78:5473–5482. doi: 10.1128/AEM.00468-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura et al. (2013).Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson et al. (2000).Thomson NR, Crow MA, McGowan SJ, Cox A, Salmond GP. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Molecular Microbiology. 2000;36:539–556. doi: 10.1046/j.1365-2958.2000.01872.x. [DOI] [PubMed] [Google Scholar]
- Toder, Gambello & Iglewski (1991).Toder DS, Gambello MJ, Iglewski BH. Pseudomonas aeruginosa LasA: a second elastase gene under transcriptional control of lasR. Molecular Microbiology. 1991;5:2003–2010. doi: 10.1111/j.1365-2958.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Waters & Bassler (2005).Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annual Review of Cell and Developmental Biology. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Watson et al. (2002).Watson WT, Minogue TD, Val DL, Von Bodman SB, Churchill ME. Structural basis and specificity of acyl-homoserine lactone signal production in bacterial quorum sensing. Molecular Cell. 2002;9:685–694. doi: 10.1016/S1097-2765(02)00480-X. [DOI] [PubMed] [Google Scholar]
- Weeks et al. (2010).Weeks JN, Galindo CL, Drake KL, Adams GL, Garner HR, Ficht TA. Brucella melitensis VjbR and C12-HSL regulons: contributions of the N-dodecanoyl homoserine lactone signaling molecule and LuxR homologue VjbR to gene expression. BMC Microbiology. 2010;10:167. doi: 10.1186/1471-2180-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead et al. (2001).Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum-sensing in Gram-negative bacteria. FEMS Microbiology Reviews. 2001;25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- Williams et al. (2007).Williams P, Winzer K, Chan WC, Camara M. Look who’s talking: communication and quorum sensing in the bacterial world. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:1119–1134. doi: 10.1098/rstb.2007.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson et al. (2005).Williamson LL, Borlee BR, Schloss PD, Guan C, Allen HK, Handelsman J. Intracellular screen to identify metagenomic clones that induce or inhibit a quorum-sensing biosensor. Applied and Environmental Microbiology. 2005;71:6335–6344. doi: 10.1128/AEM.71.10.6335-6344.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson et al. (1998).Winson MK, Swift S, Fish L, Throup JP, Jorgensen F, Chhabra SR, Bycroft BW, Williams P, Stewart GS. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiology Letters. 1998;163:185–192. doi: 10.1111/j.1574-6968.1998.tb13044.x. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2002).Zhang RG, Pappas KM, Brace JL, Miller PC, Oulmassov T, Molyneaux JM, Anderson JC, Bashkin JK, Winans SC, Joachimiak A. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature. 2002;417:971–974. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.