Abstract
Mitochondria have emerged as critical platforms for antiviral innate immune signaling. This is due in large part to the mitochondrial localization of the innate immune signaling adaptor MAVS (mitochondrial antiviral signaling protein), which coordinates signals received from two independent cytosolic pathogen recognition receptors (PRRs) to induce antiviral genes. The existence of a shared adaptor for two central PRRs presents an ideal target by which the host cell can prevent cellular damage induced by uncontrolled inflammation through alteration of MAVS expression and/or signaling. In this review, we focus on the MAVS regulome and review the cellular factors that regulate MAVS by (1) protein–protein interactions, (2) alterations in mitochondrial dynamics, and/or (3) post-translational modifications.
Keywords: innate immunity, MAVS, RIG-I, MDA5
Introduction
The mitochondria, historically referred to as the “powerhouse of the cell” due to its prominent role in cell respiration, is a double-membrane-bound organelle that maintains its own genome and proteome and its own system for regulation of size, shape, and/or number through autophagy (in a process termed mitophagy) and mitochondrial fusion/fission [1–3]. In addition to regulating cellular metabolism, mitochondria are also involved in a wide variety of cellular processes that include cooperating with the endoplasmic reticulum (ER) to regulate both intracellular calcium signaling and lipid synthesis at the mitochondria-associated membrane (MAM) [4,5] and the control of cell death and antiviral signaling [6]. This review will discuss the important role of the mitochondria in the innate immune response to viral RNA (vRNA), with a special focus on the regulation of the first discovered protein linking the mitochondria to antiviral type I interferon (IFN) signals, the mitochondrial innate immune adaptor MAVS (mitochondrial antiviral signaling protein; previously known as IPS-1/VISA/Cardif) [7–10].
Recognition of pathogen-derived nucleic acids is among the most important processes of the host cell defense against invading viruses. Upon recognition of a viral invader, the transcription of a myriad of antiviral genes ensues, culminating in a cellular antiviral state that equips the cell to resist and/or suppress infection. It is important to note that sensors exist for pathogen-associated RNA and DNA, but this review will specifically focus on the recognition of vRNA. There are a number of excellent reviews that focus specifically on DNA sensors [11,12]. Endosomally localized toll-like receptors and the cytosolic sensors of the RIG-I-like receptor (RLR) pathway are the major sensors of vRNA [13,14]. The RLR signaling pathway is initiated by the recognition of distinct species of vRNA by one of two cytosolic sensors—retinoic acid-inducible gene-I (RIG-I) or melanoma differentiation-associated gene 5 (MDA5). RIG-I was the first identified sensor of the RLR pathway and consists of two N-terminal caspase activation and recruitment domains (CARDs), a central DEAD box helicase/ATPase domain, and a C-terminal regulatory domain necessary to prevent constitutive activation [15,16]. MDA5 shares structurally homology with RIG-I in that it contains two N-terminal CARD domains and a central DEAD box helicase/ATPase domain [17]. Other reviews in this issue provide specific details on the differences between RIG-I and MDA5 and on their mechanisms of vRNA recognition.
Although RIG-I and MDA5 differ in the types of vRNA they sense [15,18–24], they share a common mitochondria-localized adaptor, MAVS, which binds to both RLRs by CARD-mediated interactions [7–10]. In addition, MAVS has been shown to localize to peroxisomes and peroxisome-associated MAVS participates in a rapid IFN-independent response early following viral invasion. This is in contrast to the delayed yet stable IFN-dependent response propagated by mitochondria-localized MAVS [25]. The N-terminal CARD of MAVS mediates its interaction not only with RLRs but also with important downstream targets including TNF receptor associated factor (TRAF) 3 [7–10]. In addition, a proline-rich region is present just downstream of the CARD that also mediates interaction with various downstream targets [8,10,26]. The localization of MAVS to the mitochondrial membrane is mediated by a C-terminal transmembrane domain and is required for downstream antiviral signaling events [7–10]. Upon activation of the RLR pathway, MAVS-mediated antiviral signaling is propagated through assembly of a MAVS “signalosome” containing TRAF3, TRAF6, TRAF family member-associated nuclear factor κB (NF-κB) activator (TANK), and TNFR1-associated death domain protein (TRADD). The formation of a MAVS signaling complex results in the phosphorylation and nuclear translocation of interferon regulatory factor 3 (IRF3) by TANK binding kinase 1 (TBK1) and/or IKKε, as well as activation of NF-κB to induce type I interferons (IFNs) and pro-inflammatory cytokines [27–30]. It is important to acknowledge that autophagy and cell death are also known as innate antiviral responses to exogenous RNA, and they have been extensively reviewed elsewhere [1,2,6,31].
MAVS Regulation at the Mitochondrial Membrane
Because enhanced or insufficient levels of inflammation can elicit cell damage or inhibit the ability of cells to remove the invading threat, respectively, mechanisms must be in place to tightly regulate antiviral signaling. Regulation at the mitochondrial level is quite strategic given that signals propagated by independent cytosolic sensors converge on MAVS at the mitochondrial membrane. Therefore, regulators of MAVS exert a higher level of control than they might if they targeted upstream components of RLR signaling such as RIG-I or MDA5 individually. In the following sections, we detail the variety of mechanisms by which regulators specifically modulate MAVS expression and/or signaling, with a focus on those that regulate by (1) protein–protein interactions, (2) alterations in mitochondrial dynamics, and/or (3) post-translational modifications (Fig. 1 and Table 1).
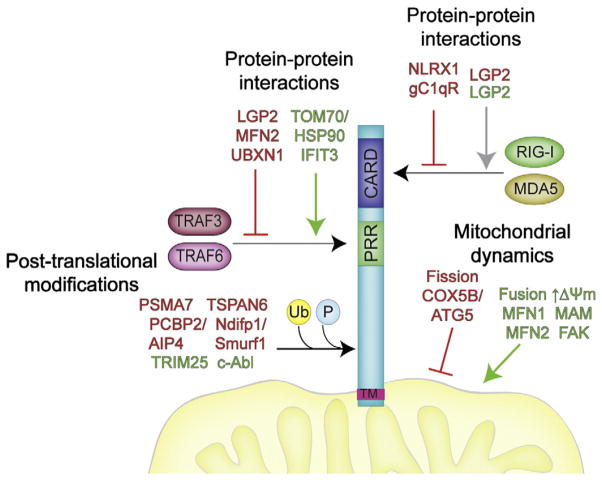
Fig. 1.
Mechanisms of MAVS regulation. There are multiple mechanisms by which MAVS is regulated to exert cellular control over innate immune signaling. MAVS can be regulated by host cell factors that inhibit MAVS signaling by direct protein–protein interactions, by altering mitochondrial properties or dynamics, or by post-translational modifications. PRR, proline-rich region; Ub, ubiquitination; P, phosphorylation. Positive regulators of MAVS signaling are shown in green text and negative regulators of MAVS signaling are shown in red text. Note that LGP2 is shown in both red and green given conflicting results on its role in the regulation of RLR signaling.
Table 1.
The MAVS regulome categorized by mechanism of regulation.
| Protein–protein interactions | Mitochondrial dynamics | Post-translational modifications (Ub or P) |
|---|---|---|
| LGP2 (+/−) [16,17,32–36] | Fusion (+)/fission (−) [44,58] | PSMA7 (−) (Ub) [73] |
| NLRX1 (−) [26,37–40] | MFN1 (+) [58,59] | PCBP2/AIP4 (−) (Ub) [76] |
| MFN1 (+) [58,59] | MFN2 (+) [44,58,61] | TRIM25 (+) (Ub) [81] |
| MFN2 (−) [43] | ↑Δψm (+) [44] | Ndifp1/Smurf1 (−) (Ub) [83] |
| Tom70/Hsp90 (+) [45] | MAM (+) [58,61] | TSPAN6 (−) (Ub) [86] |
| IFIT3 (+) [50] | FAK (+) [62] | PLK1 (−) (P)a [88] |
| gC1qR (−) [57] | COX5B/ATG5 (−) [67] | c-Abl (+) (P) [93] |
| UBXN1 (−) [54] |
Ub, ubiquitination; P, phosphorylation; (+), positive regulation; (−), negative regulation.
PLK1 does not directly phosphorylate MAVS but, rather, may require phosphorylation of MAVS for docking of PLK1 at an upstream site prior to PLK1 binding near the C terminus of MAVS where it exerts regulatory activity.
Regulation of MAVS by protein–protein interactions
As discussed earlier, RLR signaling begins with recognition of vRNA by the DExD/H box RNA helicases RIG-I and MDA5 and converges on MAVS via CARD-mediated interactions. In addition to RIG-I and MDA5, a third RNA helicase harboring a DExD/H box RNA helicase domain exists and is termed LGP2 (Laboratory of Genetics and Physiology 2). LGP2 exhibits 30–40% amino acid sequence identity to RIG-I and MDA5 and is capable of double-stranded RNA binding [17,32]. However, and quite importantly, LGP2 lacks a CARD with which to signal to downstream mediators of IFN induction, which has suggested a different function for LGP2 than for either RIG-I or MDA5. Consistent with a possible role in innate immune function, the expression of LGP2 is induced by type I IFNs, double-stranded RNA, and virus infection [17,32]. However, unlike RIG-I and MDA5, overexpression of LGP2 results in a downregulation of IFNβ promoter activity [17,32]. Indeed, LGP2 has been suggested to serve as a negative regulator of the RLR pathway via its interaction with MAVS at the mitochondrial membrane, thus preventing its vital association with the downstream signaling molecule TRAF3 [33]. A later study reported that MAVS homooligomerization of its N-terminal CARD domain, dependent on the C-terminal mitochondrial localization domain, resulted in more efficient signaling [34]. This suggests that, while LGP2 association with MAVS might prevent its association with TRAF3, it could also interfere with MAVS dimerization.
In addition to the negative regulation of MAVS, LPG2 has also been suggested to directly regulate RLRs themselves. LGP2−/− MEFs are more susceptible to synthetic RNA [poly(I:C)] stimulation of IFN production, and LGP2−/− mice are less sensitive to lethal vesicular stomatitis virus (VSV) infection, a rhabdovirus known to signal through RIG-I (presumably due to enhanced IFN production and subsequent infection control). However, these results were not observed with encephalomyocarditis virus, a picornavirus known to signal through MDA5 [35]. These seemingly disparate results suggest that RIG-I may actually serve as the target of LGP2-mediated downregulation of IFN production [16]. A later study showed that LGP2 was actually a positive regulator of RLR signaling, facilitating RNA sensing by RIG-I and MDA5, and was essential for the response of MDA5 to picornaviruses [36]. Thus, the role of LGP2 in innate immunity remains somewhat unclear and more work is needed to determine at which steps of the RLR pathway LGP2 exerts its effect.
Mitochondrially localized proteins represent logical candidates for the regulation of MAVS. The first mitochondrial protein that was identified as a negative regulator of MAVS was the nucleotide-binding domain and leucine-rich repeat containing family member, NLRX1 [26]. NLRXs are members of the NOD-like receptor family of cytosolic pattern recognition receptors (PRRs) initially believed to be involved in innate immunity independent of RLR signaling. This study successfully confirmed the putative localization of NLRX1 to the outer mitochondrial membrane and went on to show that it interacted with MAVS via its CARD, disrupting vital MAVS interactions with upstream signaling partners [26]. These data were corroborated in NLRX1−/− MEFs. IFNβ production was increased in NLRX1−/− MEFs infected with a variety of viruses known to engage RIG-I. However, there was no change in response to encephalomyocarditis virus, a virus known to engage MDA5 [37]. Interestingly, cells deficient in NLRX1 exhibited RIG-I/MAVS association even in the absence of infection whereas the MDA5/MAVS association was only present after viral infection [37]. The constitutive association between RIG-I and MAVS in the absence of NLRX1 could account for the increase in IFNβ in response to infection with RIG-I-engaging viruses but not with MDA5-engaging viruses. Conflicting results do exist, however, as subsequent studies in two independently derived NLRX1−/− MEFs found no potentiation of IFN induction or IRF3 phosphorylation in response to poly(I:C) stimulation or Sendai virus (SeV) infection compared to wild-type MEFs and no change in the serum level of IFNβ in NLRX1−/− mice compared to wild-type mice upon injection with poly(I:C) [38,39]. Another study reported NLRX1-mediated inhibition of RLR signaling to be an artifact of inhibition of luciferase activity, which is quite relevant since many of the previous studies used luciferase-based assays to measure RLR signaling [40]. Like LGP2 inhibition of MAVS activity, NLRX1 inhibition of MAVS activity has yielded conflicting results. It is certainly possible that NLRX1 has multiple regulatory roles, depending on whether positive or negative regulation is advantageous for the cell, but more studies are needed to reconcile disparate findings and elucidate the role of NLRX1 in MAVS signaling.
The mitofusins (MFN1 and MFN2) are residential outer mitochondrial membrane proteins that play roles in regulating mitochondrial dynamics by controlling fusion, and MFN2 has been reported to act as a mitochondria-ER-tethering protein [41,42]. While screening the MAVS mitochondrial supramolecular complex by mass spectrometry for MAVS interacting partners, MFN2 was identified as an interacting partner of MAVS [43]. Upon further investigation, overexpression of MFN2, but not MFN1, was found to inhibit RIG-I-, MDA5-, and MAVS-mediated type I IFN induction. Conversely, RNAi (RNA interference)-mediated silencing of MFN2 and studies in MFN2−/− MEFs showed that RLR signaling was enhanced in knockdown cells, a phenotype that was reversed upon addition of exogenous MFN2 into these cells. These results were corroborated in a later study [44]. Immunoprecipitation studies confirmed that MAVS and MFN2 interact and that this interaction was dependent upon the mitochondrial localization of MAVS and occurred between a central hydrophobic heptad repeat (HR1) region of MFN2 and a C-terminal region of MAVS [43].
The resident mitochondrial protein translocase of outer membrane 70 (Tom70) was identified as a novel MAVS interacting protein by immunoprecipitation and subsequent mass spectrometry [45]. Tom70 is a member of the Tom complex on the outer mitochondrial membrane that recognizes newly synthesized mitochondrial proteins in the cytosol and plays a role in translocating them to their final destination at the mitochondria [46]. Exogenous Tom70, but not the related mitochondrial protein Tom20, potentiated IFNβ induction in response to SeV and cytosolic poly(I:C) (both potent stimulators of RLR signaling [23]) stimulation whereas its depletion by RNAi resulted in an abrogated induction of IFNβ in response to these same stimuli. The interaction between MAVS and Tom70 was increased in response to SeV infection and cytosolic poly(I:C) and requires the mitochondrial localization of both proteins. Based upon previous work by the same group demonstrating that the cellular chaperone protein Hsp90 was constitutively complexed with IRF3/TBK1 [47] and the work of others showing that Hsp90 interacts with Tom70 [48], they next hypothesized that Tom70 could be involved in recruitment of IRF3/TBK1 to MAVS in order to facilitate signaling. Indeed, immunoprecipitation experiments confirmed that exogenous Hsp90 bound Tom70 and IRF3/TBK1 and that endogenous Tom70, but not Tom20, interacted with Hsp90 and IRF3/TBK1. Overall, the results of these studies proposed a model in which Tom70 interacts with MAVS and recruits both TBK1/IRF3 and the IKK complex to mitochondria by specifically recognizing and interacting with Hsp90, thus linking MAVS to downstream signaling.
Other studies have shown that antiviral interferon stimulated genes might also regulate MAVS in order to influence signaling. IFN-induced protein with tetratricopeptide repeats 3 (IFIT3) is an interferon-stimulated gene and therefore represents a classical candidate for positive feedback of RLR signaling through MAVS. IFIT proteins are known to be highly inducible by virus infection and IFNs [49]. IFIT3 localizes to the mitochondria where it interacts with both MAVS and TBK1. Overexpression of IFIT3 potentiated RLR signaling whereas its depletion attenuated it [50]. IFIT3 likely functions as a scaffold to facilitate the interaction of MAVS with TBK1 as the association between MAVS and TBK1 is weak in the absence of IFIT3 and strengthened by its presence. Thus, this study added IFIT3 to the list of positive regulators at the MAVS signalosome and has uncovered a novel role for IFIT3 in antiviral signaling.
Along with resident mitochondrial proteins, those that are relocalized to the mitochondria during viral infection represent other possible regulators of MAVS. The complement protein gC1qR (receptor for globular head domain of complement component C1q) was investigated for its role in antiviral signaling due to reports that it was involved in rubella and cytomegalovirus infection [51,52]. gC1qR is localized to the mitochondria, nucleus, cytoplasm, and cell surface [13,14,29,30,53–56]. Quite interestingly, it was shown that gC1qR was recruited to the mitochondria upon virus infection and poly(I:C) stimulation [57]. When overexpressed, gC1qR inhibited virus-activated, poly(I:C)-activated, and exogenous RLR-activated antiviral signaling. In addition, gC1qR and MAVS interacted weakly in uninfected cells; this interaction was greatly enhanced by viral infection. RNAi-mediated knockdown of gC1qR resulted in enhanced antiviral signaling in response to exogenous RIG-I or VSV infection and exogenous expression of qC1qR controlled VSV infection [57]. This study demonstrated a role for a complement receptor, which is known to act in various arms of innate immunity, in the regulation of RLR signaling through its interaction with MAVS.
Regulation of MAVS by mitochondrial dynamics
Utilizing protein–protein interactions as a means of regulating of MAVS-mediated innate immune signaling is clearly important. However, the physical properties of the mitochondria and the resulting changes in MAVS distribution and/or aggregation can also play an important role in its regulation. Initial evidence for the role of mitochondrial dynamics in MAVS signaling came from studies demonstrating that infection of cells with SeV or transfection of poly(I: C) resulted in elongation and/or fusion of mitochondria, leading the authors to conclude that activation of RLR signaling results in physical alterations in the mitochondria itself [58]. Indeed, this study also showed that phosphorylation of IRF3 was delayed in cells with fragmented mitochondria and that RLR signaling was attenuated by mitochondrial fragmentation but was enhanced upon mitochondrial fusion. Immunoprecipitation experiments showed that MAVS forms an interaction with mitofusin 1 (MFN1), a protein that regulates mitochondrial fusion events, suggesting a possible role for this interaction in the regulation of the mitochondrial dynamics that accompany antiviral signaling. Interestingly, a later study also reported on the interaction of MFN1 with MAVS and further showed that MFN1 acts as a positive regulator of MAVS-mediated antiviral signaling by redistributing MAVS to speckle-like aggregates observed upon activation of RLR signaling [59]. This could explain why MFN1 and mitochondrial fusion seem to be important for RLR signaling given that fusion of the mitochondria could facilitate MAVS aggregation. Others further investigated the role of MFNs in MAVS signaling using MEFs deficient in both MFN1 and MFN2 (MFNs-dm) [44]. These cells were unable to undergo mitochondrial fusion and were impaired in their ability to produce IFNβ and IL-6 in response to viral infection. In light of the results of these studies, it is likely that the role of MFNs in innate immune signaling is multifold. Not only do both MFN1 and MFN2 interact directly with MAVS to exert a regulatory role [43,58,59] but their activities in mitochondrial dynamics also appear to be important for MAVS functioning. This is in accordance with earlier reports that MAVS activation requires self-association into higher-order oligomers [34] and formation of large prion-like aggregates for potent propagation of antiviral signaling [60]. More recently, MAVS-containing prion-like aggregates were reported to be responsible for propagation of signaling downstream of MAVS by binding TRAF2, TRAF5, and TRAF6 in an ubiquitin-dependent manner [55]. Importantly, this study also provided evidence that TRAF2 and TRAF5 act redundantly with TRAF6 for activation of IRF3 [55]. Other studies have also pointed to a direct role for another mitochondrial process in RLR signaling as carbonyl cyanide m-chlorophenylhydrazone, a compound known to dissipate mitochondrial membrane potential (Δψm), resulted in suppressed innate immune signaling [44], thus suggesting that Δψm is another example of a mitochondrial process that is important for regulation of MAVS-mediated signaling. Taken together, these reports suggest that mitochondrial elongation and fusion may facilitate the aggregation of MAVS into active complexes primed for maximum signaling capacity.
In addition to regulating mitochondrial fusion, MFN2 is also important in the tethering of the mitochondria to the ER at the MAM. The MAM is emerging as an important subcellular domain in MAVS signaling. For example, virally infected cells exhibit increased numbers of ER contacts with elongated mitochondria compared to uninfected control cells, suggesting that ER–mitochondria contacts increase upon infection-induced mitochondrial fusion and elongation [58]. This is of particular significance given that the population of MAVS residing at the MAM is important for antiviral signaling [61].
Our own laboratory recently described a role for focal adhesion kinase (FAK) in the positive regulation of MAVS-mediated antiviral signaling [62]. FAK is a protein tyrosine kinase that localizes to points of contact between the extracellular matrix and the intracellular cytoskeleton known as focal adhesions. This study showed that FAK−/− MEFs were highly susceptible to RNA virus infection but exhibited no enhanced susceptibility to DNA virus infection. FAK−/− MEFs were attenuated in NF-κB and IFNβ signaling and expression of exogenous FAK enhanced activation of the NF-κB and IFN-β promoters in response to cytosolic poly(I:C). Surprisingly, coimmunoprecipitation studies and immunofluorescence microscopy showed that FAK relocalizes from focal adhesions to the mitochondrial membrane, where it interacts with MAVS, in a virus-infection-dependent manner. Although the precise function of FAK in MAVS-mediated signaling has yet to be determined, the distribution of MAVS and of the mitochondria itself are highly abnormal in cells deficient in FAK, suggesting that it may play a role in the regulation of mitochondrial dynamics necessary to facilitate antiviral signaling downstream of MAVS.
Regulation of reactive oxygen species (ROS) production has also been reported to play a role in the positive regulation of RLR signaling from the mitochondria [63–66]. While examining the mechanism for this phenomenon, Zhao et al. described cytochrome C oxidase 5B (COX5B) as a MAVS interacting partner responsible for repression of ROS and RLR signaling [67]. COX5B is a mitochondrial protein and is a member of the cytochrome c oxidase complex, the complex that catalyzes the last step in the electron transport chain [68]. Overexpression of COX5B decreased MAVS-mediated antiviral signaling without having any effect on toll-like receptor-mediated or TNF-α-induced signals, suggesting that the effect was specific to the RLR pathway [67]. Cells depleted of COX5B also exhibited enhanced antiviral signaling. Interestingly, in addition to its role in ATP production, COX5B has been shown to be involved in the negative regulation of ROS production [69]. To investigate the possible role of this pathway in COX5B-mediated regulation of MAVS, the authors utilized two compounds reported to alter ROS levels and found that an increase in ROS resulted in an increase in MAVS-mediated signaling and decreasing ROS levels resulted in a decrease of MAVS-mediated signaling. In addition, cells expressing exogenous MAVS produced higher levels of ROS, which was abrogated by exogenous COX5B [67]. Interestingly, COX5B expression was not induced by addition of purified IFNβ but was induced in the presence of overexpressed MAVS, suggesting that it is not an interferon-inducible gene but its expression is coordinated with MAVS expression for its specific negative regulation.
MAVS overexpression induces autophagy (or perhaps, more specifically, mitophagy) [67] and ROS production has also been associated with the induction of autophagy [70]. Because autophagy is involved in the removal of aggregated proteins [71,72], and the aggregation of MAVS during RLR activation is known to potentiate signaling [34,59,60], COX5B and regulators of autophagy such as ATG5 might regulate MAVS-mediated signaling by affecting MAVS aggregation upon its activation. Indeed, MAVS aggregation is affected by the expression of ATG5 and COX5B, with overexpression leading to decreased aggregation and depletion leading to increased aggregation [67]. These results suggest that COX5B works coordinately with ATG5 to negatively regulate MAVS-mediated antiviral signaling through an increased clearance of MAVS aggregates in addition to its role in repression of ROS production.
Regulation of MAVS by post-translational modifications
The post-translational control of proteins is a common means by which cells regulate diverse pathways and processes. It is thus not surprising that post-translational modifications of MAVS and/or its interacting partners are a key aspect of host cell regulation of antiviral signaling. A yeast two-hybrid screen for MAVS interacting partners identified the proteasomal component PSMA7 as a MAVS interacting partner [73]. PSMA7 is a subunit comprising the outer ring of the 20S catalytic core complex of the 26S proteasome and is involved in proteasomal activity regulation [74,75]. MAVS interaction with PSMA7 requires both the C-terminal transmembrane domain and the CARD region of MAVS [73]. Overexpression of PSMA7 reduced IFNβ induction and suppressed VSV infection whereas its silencing yielded the opposite results. Consistent with these findings, overexpression or depletion of PSMA7 decreased or increased endogenous MAVS protein levels, respectively [73]. Importantly, MAVS mRNA levels remained unchanged in response to these manipulations, suggesting that PSMA7 modulated MAVS levels post-transcriptionally. Indeed, PSMA7 overexpression induced the ubiquitination of MAVS, implicating the PSMA7-mediated proteasomal degradation of MAVS. However, given that PSMA7 protein has not been shown to be involved in the process of protein ubiquitination itself, it is clearly not the only player in this process. Thus, it remains to be seen if PSMA7 recruits enzymes of the ubiquitination pathway for MAVS ubiquitination prior to recruiting ubiquitinated MAVS to the proteasome for degradation.
The multi-protein requirement for ubiquitin-mediated degradation and negative regulation of MAVS is emerging as a common theme in the regulation of RLR signaling. After performing a yeast two-hybrid screen in search of interacting partners of MAVS, poly(rC) binding protein 2 (PCBP2) was identified as a negative regulator of MAVS [76]. PCBP2 is involved in RNA and DNA binding with many different purposes in the cell, including mRNA stability and translation regulation [77]. Overexpression of PCBP2 resulted in suppression of MAVS-mediated IFNβ induction but had no effect on TBK1-or IRF3-induced signaling. PCBP2 expression was highly inducible by interferon treatment and virus infection, and the interaction between endogenous PCBP2 and endogenous MAVS was inducible by SeV infection. Subcellular localization studies showed that endogenous PCBP2 localized primarily to the nucleus but relocalized to the cytoplasm where it colocalized with MAVS upon viral infection or MAVS overexpression. Despite lacking any ubiquitin ligase activity itself, PCBP2 overexpression induced a dramatic proteasome-dependent degradation of MAVS. Using mutational analysis of MAVS, the authors showed that ubiquitination of two specific lysine residues led to its degradation and that the levels of MAVS polyubiquitination were higher in the presence of overexpressed PCBP2. Given that PCBP2 is not an enzyme of the ubiquitination pathway, the authors hypothesized that PCBP2 could be acting as physical scaffold linking MAVS to an E3 ubiquitin ligase. Screening known E3 ubiquitin ligases for a candidate that both mediates degradation of MAVS and binds to PCBP2, the authors found the Nedd4-like E3 ubiquitin ligase AIP4. Overexpression of AIP4 partially abrogated IFNβ signaling and induced MAVS degradation in a manner dependent on its E3 ubiquitin ligase activity. Although AIP4 and MAVS were shown to interact, this interaction required PCBP2, suggesting that PCBP2 acts as a scaffold to facilitate AIP4-mediated degradation of MAVS. This was confirmed using in vitro ubiquitination assays which showed that PCBP2 expression greatly increased the AIP4-mediated ubiquitination and degradation of MAVS. Finally, type I IFN signaling was enhanced in Itch (the mouse homologue of AIP4)−/− MEFs further linking this E3 ligase to MAVS signaling. Collectively, this study nicely showed that PCBP2 acts as an adaptor for AIP4-mediated ubiquitination and subsequent proteasomal degradation of MAVS for negative regulation of RLR signaling, elucidating a quite novel and interesting mechanism of RLR regulation. A later report by the same group showed that poly(rC) binding protein 1, a protein highly similar to PCPB2 [78,79], is also involved in negative regulation of MAVS-mediated signaling using a similar mechanism [80]. However, unlike PCBP2, poly(rC) binding protein 1 is not induced by type I IFNs, leading the authors to conclude that it is a “housekeeper” of MAVS levels rather than a negative feedback inhibitor.
TRIM25-dependent ubiquitination of MAVS also plays a role in its regulation though a surprisingly different negative regulatory role than most other ubiquitin ligases [81]. Previous studies have linked this E3 ubiquitin ligase to the regulation of RIG-I signaling [82]. Overexpression of TRIM25 enhanced ubiquitination and degradation of MAVS and catalyzed the addition of ubiquitin to two residues within MAVS (K7 and K10) [81]. Functional studies showed that exogenous TRIM25 enhanced IFN-β promoter activity stimulated by cytosolic high-molecular-weight poly(I:C) (a known agonist of MDA5, used to avoid experimental complications from TRIM25 modification of RIG-I). Depletion of TRIM25 led to attenuation of IFN-β promoter activity stimulated by cytosolic high-molecular-weight poly(I:C) and decreased MAVS ubiquitination and degradation in response to cytosolic poly(I:C). Based on these observations, the authors proposed that, upon RLR activation, the MAVS signalosome was assembled at the mitochondria, and only upon TRIM25-mediated ubiquitination and subsequent proteasomal degradation of MAVS could it be released to translocate to the cytosol for phosphorylation of IRF3 and subsequent production of IFNβ. While this is a tempting and interesting hypothesis, it remains to be seen if the specific abrogation of MAVS degradation, perhaps by mutational analysis of ubiquitinated residues, maintains the same effect on IRF3-mediated IFNβ induction.
Ndfip1 has also been classified as a negative regulator of MAVS at the mitochondria through enhancement of ubiquitination and proteasomal degradation [83]. In light of mounting evidence linking E3 ubiquitin ligase activity to MAVS regulation, Ndifp1 is a logical candidate given its reported role in enhancement of protein ubiquitination through interaction with a family of E3 ubiquitin ligases known as Nedd4 ubiquitin ligases, particularly in signaling pathways [84,85]. Ndfip1 inhibited MAVS-mediated signaling in a proteasome-dependent manner. As these results pointed to ubiquitination-mediated proteasomal degradation as the mechanism of negative regulation of MAVS by Ndifp1, the authors next screened the four known members of the Nedd4 E3 ubiquitin ligase family for their ability to induce MAVS degradation in the presence of Ndifp1. The Nedd4 E3 ubiquitin ligase Smurf1 was shown to lead to degradation of MAVS, but not of RIG-I or TBK1, in the presence of Ndifp1. The interaction between Smurf1 and MAVS was increased in the presence of Ndifp1, as was the Smurf1-mediated ubiquitination of MAVS, indicating that Ndifp1 likely serves as an adaptor for recruitment of Smurf1 to MAVS. This study described a mechanism of MAVS negative regulation that is quite similar to that of PCBP2 and AIP4 as discussed above and provides yet another example of the complexity of ubiquitination in the regulation of MAVS signaling.
Adding a different twist to the recently emerging and growing role of ubiquitination in RLR signaling, tetraspanin protein 6 (TSPAN6) was recently described to play a role in MAVS-mediated RLR signaling [86]. TSPAN6 is a member of the membrane-embedded tetraspanin protein family that has been shown to have many different functions in the cell, including various roles in host immunity [87] Interestingly, TSPAN6 does not promote the ubiquitination of MAVS either directly or indirectly but is itself ubiquitinated in order to promote its association with MAVS and disrupt the mitochondria-localized signalosome. Overexpression of TSPAN6 resulted in a reduction of exogenous MAVS-induced signaling and was shown to interact with MAVS. TSPAN6 is ubiquitinated in response to RLR activation, which is involved in its association with MAVS. The authors propose that ubiquitination of TSPAN6 in the presence of viral infection promotes its recruitment to the mitochondria where it interacts with MAVS, abrogating the assembly of the signalosome and thus inhibiting antiviral signaling. The enzymes responsible for ubiquitination of TSPAN6 in the context of RLR activation remain to be discovered.
Like ubiquitination, phosphorylation represents a post-translational mechanism of protein regulation in many cellular processes. Yeast two-hybrid screening identified the Polo-like kinase 1 (PLK1) as an interacting partner for MAVS [88]. PLK1 is a serine/threonine Polo-like kinase [89–91]. Contrary to other known regulators of MAVS, induction of antiviral signaling did not enhance the association between MAVS and PLK1. PLK1 interacts with MAVS at two unique regions, downstream of the CARD region and just upstream of the C terminus (the interaction downstream of the CARD region is dependent upon phosphorylation of MAVS at position Thr234) [88]. The phosphorylation-independent C-terminal interaction was shown to be responsible for the attenuation of IFN signaling due to a disruption of MAVS–TRAF3 interaction. This finding has been corroborated by more recent work that has uncovered a second TRAF3 binding site in MAVS corresponding to this same region [92]. In fact, a more recent study has reported that an ubiquitin regulatory X domain-containing protein (UBXN1) also negatively regulates MAVS by binding it and blocking its interaction with TRAF3/TRAF6 at the C-terminal binding site, further supporting the importance of this region in MAVS-mediated signaling [54]. Work is ongoing to determine the specific kinases responsible for the primary phosphorylation of MAVS that facilitates PLK1 binding.
More recently, the tyrosine kinase c-Abl was identified as a MAVS-interacting partner that acts as a positive regulator of MAVS by direct interaction and phosphorylation [93]. c-Abl is a nuclear and cytoplasmic Src-like non-receptor protein tyrosine kinase that is known to serve many cellular functions [94]. The interaction between MAVS and c-Abl was shown to require both the transmembrane domain and CARD of MAVS, likely suggesting that mitochondrial localization of MAVS is required for this interaction. Depletion of c-Abl resulted in abrogation of MAVS signaling and pharmacological inhibition of c-Abl abrogated IFNβ production in response to VSV infection. The tyrosine phosphorylation of MAVS was enhanced by c-Abl expression, but not by a c-Abl mutant defective in kinase activity. In a later report, tyrosine-scanning mutational analysis revealed that inducible phosphorylation at Tyr9 of MAVS was involved in the recruitment of TRAF3/TRAF6 to propagate MAVS-mediated RLR signaling [95]. Whether c-Abl is involved in phosphorylation of Tyr9 of MAVS remains to be determined and would represent an interesting follow-up to these two studies.
Conclusion
Antiviral signaling is an extremely powerful cellular response that necessitates tight regulation in order to adequately neutralize invading threats while avoiding damage to the cell from excessive inflammation. A large portion of antiviral signaling regulation has evolved at the mitochondria due to its pivotal position in the antiviral signaling pathway. Strategically, this is a logical step for regulation because of the convergence of independent upstream sensors on the common mitochondrial signaling adaptor protein MAVS. As discussed above, the cell employs many diverse mechanisms to regulate MAVS, including protein–protein interactions for physical blockage of MAVS association with upstream or downstream signaling partners, alterations of mitochondrial physical dynamics as well as the physical distribution/aggregation of MAVS, and post-translational modifications such as phosphorylation and ubiquitination. Although remarkable progress has been made, there is still much to be learned regarding the myriad of mechanisms by which host cells regulate MAVS-mediated signaling. Ongoing work in the field will continue to identify MAVS regulators, hopefully providing a complete picture of the MAVS regulome.
Acknowledgments
We wish to thank Dr. Saumendra Sarkar and Katharine Harris (University of Pittsburgh) for helpful discussions and review of the manuscript. We apologize to any colleagues whose work we may have neglected to cite due to space limitations. Our work on antiviral pathways is supported by the National Institutes of Health (AI081759). In addition, C.B.C. is a recipient of the Burroughs Welcome Investigators in the Pathogenesis of Infectious Disease Award.
Abbreviations used
- ER
endoplasmic reticulum
- MAM
mitochondria-associated membrane
- vRNA
viral RNA
- RLR
RIG-I-like receptor
- RIG-I
retinoic acid-inducible gene-I
- MDA5
melanoma differentiation-associated gene 5
- CARD
caspase activation and recruitment domain
- IRF3
interferon regulatory factor 3
- TBK1
TANK binding kinase 1
- VSV
vesicular stomatitis virus
- SeV
Sendai virus
- FAK
focal adhesion kinase
- ROS
reactive oxygen species
- COX5B
cytochrome C oxidase 5B
- PCBP2
poly(rC) binding protein 2
- PLK1
Polo-like kinase 1
References
- 1.Cloonan SM, Choi AM. Mitochondria: sensors and mediators of innate immune receptor signaling. Curr Opin Microbiol. 2013;16:327–38. doi: 10.1016/j.mib.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cloonan SM, Choi AM. Mitochondria: commanders of innate immunity and disease? Curr Opin Immunol. 2012;24:32–40. doi: 10.1016/j.coi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–5. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–21. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13:607–25. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780–8. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- 7.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–72. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 8.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–8. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 9.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–82. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–40. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Rathinam VA, Fitzgerald KA. Innate immune sensing of DNA viruses. Virology. 2011;411:153–62. doi: 10.1016/j.virol.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38:870–80. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. Antiviral signaling through pattern recognition receptors. J Biochem. 2007;141:137–45. doi: 10.1093/jb/mvm032. [DOI] [PubMed] [Google Scholar]
- 14.Harris KG, Coyne CB. Enter at your own risk: how enteroviruses navigate the dangerous world of pattern recognition receptor signaling. Cytokine. 2013;63:230–6. doi: 10.1016/j.cyto.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–7. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 16.Saito T, Hirai R, Loo YM, Owen D, Johnson CL, Sinha SC, et al. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci USA. 2007;104:582–7. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–8. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 18.Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–7. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 19.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt A, Schwerd T, Hamm W, Hellmuth JC, Cui S, Wenzel M, et al. 5′-Triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc Natl Acad Sci USA. 2009;106:12067–72. doi: 10.1073/pnas.0900971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–7. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzri D, Gehrke L. Nucleotide sequences and modifications that determine RIG-I/RNA binding and signaling activities. J Virol. 2009;83:4174–84. doi: 10.1128/JVI.02449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–10. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–5. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 25.Dixit E, Boulant S, Zhang Y, Lee AS, Odendall C, Shum B, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141:668–81. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saha SK, Pietras EM, He JQ, Kang JR, Liu SY, Oganesyan G, et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 2006;25:3257–63. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–11. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–6. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 29.Michallet MC, Meylan E, Ermolaeva MA, Vazquez J, Rebsamen M, Curran J, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28:651–61. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Guo B, Cheng G. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J Biol Chem. 2007;282:11817–26. doi: 10.1074/jbc.M700017200. [DOI] [PubMed] [Google Scholar]
- 31.Richetta C, Faure M. Autophagy in antiviral innate immunity. Cell Microbiol. 2013;15:368–76. doi: 10.1111/cmi.12043. [DOI] [PubMed] [Google Scholar]
- 32.Rothenfusser S, Goutagny N, DiPerna G, Gong M, Monks BG, Schoenemeyer A, et al. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol. 2005;175:5260–8. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 33.Komuro A, Horvath CM. RNA- and virus-independent inhibition of antiviral signaling by RNA helicase LGP2. J Virol. 2006;80:12332–42. doi: 10.1128/JVI.01325-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang ED, Wang CY. MAVS self-association mediates antiviral innate immune signaling. J Virol. 2009;83:3420–8. doi: 10.1128/JVI.02623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkataraman T, Valdes M, Elsby R, Kakuta S, Caceres G, Saijo S, et al. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J Immunol. 2007;178:6444–55. doi: 10.4049/jimmunol.178.10.6444. [DOI] [PubMed] [Google Scholar]
- 36.Satoh T, Kato H, Kumagai Y, Yoneyama M, Sato S, Matsushita K, et al. LGP2 is a positive regulator of RIG-I-and MDA5-mediated antiviral responses. Proc Natl Acad Sci USA. 2010;107:1512–7. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–65. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rebsamen M, Vazquez J, Tardivel A, Guarda G, Curran J, Tschopp J. NLRX1/NOD5 deficiency does not affect MAVS signalling. Cell Death Differ. 2011;18:1387. doi: 10.1038/cdd.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soares F, Tattoli I, Wortzman ME, Arnoult D, Philpott DJ, Girardin SE. NLRX1 does not inhibit MAVS-dependent antiviral signalling. Innate Immun. 2012;19:438–48. doi: 10.1177/1753425912467383. [DOI] [PubMed] [Google Scholar]
- 40.Ling A, Soares F, Croitoru DO, Tattoli I, Carneiro LA, Boniotto M, et al. Post-transcriptional inhibition of luciferase reporter assays by the Nod-like receptor proteins NLRX1 and NLRC3. J Biol Chem. 2012;287:28705–16. doi: 10.1074/jbc.M111.333146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 42.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–10. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 43.Yasukawa K, Oshiumi H, Takeda M, Ishihara N, Yanagi Y, Seya T, et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signaling. 2009;2:ra47. doi: 10.1126/scisignal.2000287. [DOI] [PubMed] [Google Scholar]
- 44.Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signaling. 2011;4:ra7. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- 45.Liu XY, Wei B, Shi HX, Shan YF, Wang C. Tom70 mediates activation of interferon regulatory factor 3 on mitochondria. Cell Res. 2010;20:994–1011. doi: 10.1038/cr.2010.103. [DOI] [PubMed] [Google Scholar]
- 46.Baker MJ, Frazier AE, Gulbis JM, Ryan MT. Mitochondrial protein-import machinery: correlating structure with function. Trends Cell Biol. 2007;17:456–64. doi: 10.1016/j.tcb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Yang K, Shi H, Qi R, Sun S, Tang Y, Zhang B, et al. Hsp90 regulates activation of interferon regulatory factor 3 and TBK-1 stabilization in Sendai virus-infected cells. Mol Biol Cell. 2006;17:1461–71. doi: 10.1091/mbc.E05-09-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. doi: 10.1016/s0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- 49.Fensterl V, Sen GC. The ISG56/IFIT1 gene family. J Interferon Cytokine Res. 2011;31:71–8. doi: 10.1089/jir.2010.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu XY, Chen W, Wei B, Shan YF, Wang C. IFN-induced TPR protein IFIT3 potentiates antiviral signaling by bridging MAVS and TBK1. J Immunol. 2011;187:2559–68. doi: 10.4049/jimmunol.1100963. [DOI] [PubMed] [Google Scholar]
- 51.Beatch MD, Everitt JC, Law LJ, Hobman TC. Interactions between rubella virus capsid and host protein p32 are important for virus replication. J Virol. 2005;79:10807–20. doi: 10.1128/JVI.79.16.10807-10820.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marschall M, Marzi A, aus dem Siepen P, Jochmann R, Kalmer M, Auerochs S, et al. Cellular p32 recruits cytomegalovirus kinase pUL97 to redistribute the nuclear lamina. J Biol Chem. 2005;280:33357–67. doi: 10.1074/jbc.M502672200. [DOI] [PubMed] [Google Scholar]
- 53.Dedio J, Jahnen-Dechent W, Bachmann M, Muller-Esterl W. The multiligand-binding protein gC1qR, putative C1q receptor, is a mitochondrial protein. J Immunol. 1998;160:3534–42. [PubMed] [Google Scholar]
- 54.Wang P, Yang L, Cheng G, Yang G, Xu Z, You F, et al. UBXN1 interferes with Rig-I-like receptor-mediated antiviral immune response by targeting MAVS. Cell Rep. 2013;3:1057–70. doi: 10.1016/j.celrep.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S, Chen J, Cai X, Wu J, Chen X, Wu YT, et al. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. Elife. 2013;2:e00785. doi: 10.7554/eLife.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joseph K, Ghebrehiwet B, Peerschke EI, Reid KB, Kaplan AP. Identification of the zinc-dependent endothelial cell binding protein for high molecular weight kininogen and factor XII: identity with the receptor that binds to the globular “heads” of C1q (gC1q-R) Proc Natl Acad Sci USA. 1996;93:8552–7. doi: 10.1073/pnas.93.16.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu L, Xiao N, Liu F, Ren H, Gu J. Inhibition of RIG-I and MDA5-dependent antiviral response by gC1qR at mitochondria. Proc Natl Acad Sci USA. 2009;106:1530–5. doi: 10.1073/pnas.0811029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 2010;11:133–8. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Onoguchi K, Onomoto K, Takamatsu S, Jogi M, Takemura A, Morimoto S, et al. Virus-infection or 5′ppp-RNA activates antiviral signal through redistribution of IPS-1 mediated by MFN1. PLoS Pathog. 2010;6:e1001012. doi: 10.1371/journal.ppat.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–61. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horner SM, Liu HM, Park HS, Briley J, Gale M. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc Natl Acad Sci USA. 2011;108:14590–5. doi: 10.1073/pnas.1110133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bozym RA, Delorme-Axford E, Harris K, Morosky S, Ikizler M, Dermody TS, et al. Focal adhesion kinase is a component of antiviral RIG-I-like receptor signaling. Cell Host Microbe. 2012;11:153–66. doi: 10.1016/j.chom.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soucy-Faulkner A, Mukawera E, Fink K, Martel A, Jouan L, Nzengue Y, et al. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 2010;6:e1000930. doi: 10.1371/journal.ppat.1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci USA. 2009;106:2770–5. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonzalez-Dosal R, Horan KA, Rahbek SH, Ichijo H, Chen ZJ, Mieyal JJ, et al. HSV infection induces production of ROS, which potentiate signaling from pattern recognition receptors: role for S-glutathionylation of TRAF3 and 6. PLoS Pathog. 2011;7:e1002250. doi: 10.1371/journal.ppat.1002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tal MC, Iwasaki A. Autophagic control of RLR signaling. Autophagy. 2009;5:749–50. doi: 10.4161/auto.5.5.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao Y, Sun X, Nie X, Sun L, Tang TS, Chen D, et al. COX5B regulates MAVS-mediated antiviral signaling through interaction with ATG5 and repressing ROS production. PLoS Pathog. 2012;8:e1003086. doi: 10.1371/journal.ppat.1003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galati D, Srinivasan S, Raza H, Prabu SK, Hardy M, Chandran K, et al. Role of nuclear-encoded subunit Vb in the assembly and stability of cytochrome c oxidase complex: implications in mitochondrial dysfunction and ROS production. Biochem J. 2009;420:439–49. doi: 10.1042/BJ20090214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campian JL, Gao X, Qian M, Eaton JW. Cytochrome C oxidase activity and oxygen tolerance. J Biol Chem. 2007;282:12430–8. doi: 10.1074/jbc.M604547200. [DOI] [PubMed] [Google Scholar]
- 70.Huang J, Lam GY, Brumell JH. Autophagy signaling through reactive oxygen species. Antioxid Redox Signaling. 2011;14:2215–31. doi: 10.1089/ars.2010.3554. [DOI] [PubMed] [Google Scholar]
- 71.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:1017. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jia Y, Song T, Wei C, Ni C, Zheng Z, Xu Q, et al. Negative regulation of MAVS-mediated innate immune response by PSMA7. J Immunol. 2009;183:4241–8. doi: 10.4049/jimmunol.0901646. [DOI] [PubMed] [Google Scholar]
- 74.Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem. 1999;68:1015–68. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 75.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–47. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 76.You F, Sun H, Zhou X, Sun W, Liang S, Zhai Z, et al. PCBP2 mediates degradation of the adaptor MAVS via the HECT ubiquitin ligase AIP4. Nat Immunol. 2009;10:1300–8. doi: 10.1038/ni.1815. [DOI] [PubMed] [Google Scholar]
- 77.Makeyev AV, Liebhaber SA. The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA. 2002;8:265–78. doi: 10.1017/s1355838202024627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leffers H, Dejgaard K, Celis JE. Characterisation of two major cellular poly(rC)-binding human proteins, each containing three K-homologous (KH) domains. Eur J Biochem. 1995;230:447–53. [PubMed] [Google Scholar]
- 79.Kiledjian M, Wang X, Liebhaber SA. Identification of two KH domain proteins in the alpha-globin mRNP stability complex. EMBO J. 1995;14:4357–64. doi: 10.1002/j.1460-2075.1995.tb00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou X, You F, Chen H, Jiang Z. Poly(C)-binding protein 1 (PCBP1) mediates housekeeping degradation of mitochondrial antiviral signaling (MAVS) Cell Res. 2012;22:717–27. doi: 10.1038/cr.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castanier C, Zemirli N, Portier A, Garcin D, Bidere N, Vazquez A, et al. MAVS ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors. BMC Biol. 2012;10:44. doi: 10.1186/1741-7007-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–20. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Tong X, Ye X. Ndfip1 negatively regulates RIG-I-dependent immune signaling by enhancing E3 ligase Smurf1-mediated MAVS degradation. J Immunol. 2012;189:5304–13. doi: 10.4049/jimmunol.1201445. [DOI] [PubMed] [Google Scholar]
- 84.Mund T, Pelham HR. Control of the activity of WW-HECT domain E3 ubiquitin ligases by NDFIP proteins. EMBO Rep. 2009;10:501–7. doi: 10.1038/embor.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mund T, Pelham HR. Regulation of PTEN/Akt and MAP kinase signaling pathways by the ubiquitin ligase activators Ndfip1 and Ndfip2. Proc Natl Acad Sci USA. 2010;107:11429–34. doi: 10.1073/pnas.0911714107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Tong X, Omoregie ES, Liu W, Meng S, Ye X. Tetraspanin 6 (TSPAN6) negatively regulates retinoic acid-inducible gene I-like receptor-mediated immune signaling in a ubiquitination-dependent manner. J Biol Chem. 2012;287:34626–34. doi: 10.1074/jbc.M112.390401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–11. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 88.Vitour D, Dabo S, Ahmadi Pour M, Vilasco M, Vidalain PO, Jacob Y, et al. Polo-like kinase 1 (PLK1) regulates interferon (IFN) induction by MAVS. J Biol Chem. 2009;284:21797–809. doi: 10.1074/jbc.M109.018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng KY, Lowe ED, Sinclair J, Nigg EA, Johnson LN. The crystal structure of the human polo-like kinase-1 polo box domain and its phospho-peptide complex. EMBO J. 2003;22:5757–68. doi: 10.1093/emboj/cdg558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, et al. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell. 2003;115:83–95. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- 91.Garcia-Alvarez B, de Carcer G, Ibanez S, Bragado-Nilsson E, Montoya G. Molecular and structural basis of polo-like kinase 1 substrate recognition: implications in centrosomal localization. Proc Natl Acad Sci USA. 2007;104:3107–12. doi: 10.1073/pnas.0609131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paz S, Vilasco M, Werden SJ, Arguello M, Joseph-Pillai D, Zhao T, et al. A functional C-terminal TRAF3-binding site in MAVS participates in positive and negative regulation of the IFN antiviral response. Cell Res. 2011;21:895–910. doi: 10.1038/cr.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song T, Wei C, Zheng Z, Xu Y, Cheng X, Yuan Y, et al. c-Abl tyrosine kinase interacts with MAVS and regulates innate immune response. FEBS Lett. 2010;584:33–8. doi: 10.1016/j.febslet.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 94.Pendergast AM. The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res. 2002;85:51–100. doi: 10.1016/s0065-230x(02)85003-5. [DOI] [PubMed] [Google Scholar]
- 95.Wen C, Yan Z, Yang X, Guan K, Xu C, Song T, et al. Identification of tyrosine-9 of MAVS as critical target for inducible phosphorylation that determines activation. PLoS One. 2012;7:e41687. doi: 10.1371/journal.pone.0041687. [DOI] [PMC free article] [PubMed] [Google Scholar]