Summary
Interstrain differences in antigenic surface proteins may reflect immunological pressure or differences in receptor specificity of the antigen. Treponema denticola exhibits considerable interstrain variability in its major surface protein (Msp), but no studies have addressed this issue in dentilisin (CTLP), a surface protease complex that has a significant role in T. denticola – host interactions in periodontal disease. Furthermore, the genome annotation of the prcB-prcA-prtP operon encoding dentilisin contains apparent errors and lacks a deduced PrtP amino acid sequence. To address these issues we analyzed the protease operon from diverse T. denticola strains, as well as clones of the ATCC 35405 Type strain from which the genome sequence and original Genbank prtP sequence were derived. 6xHis-tagging of the PrtP C-terminus in ATCC 35405 demonstrated absence of the “authentic frameshift” in PrtP reported in the genome databases. We propose that T. denticola genome annotations be updated to reflect this new information. PrcB and the PrtP N-terminal region that includes the catalytic domain were highly conserved in common laboratory strains and clinical isolates of T. denticola. Dentilisin proteolytic activity varied considerably between strains. Antibodies against PrcB, PrcA and PrtP from the Type strain recognized these proteins in most T. denticola strains. PrtP varied up to 20% over the C-terminal 270 residues between strains. The PrtP C-terminal 8-residues (DWFYVEYP) was present in all strains, with two strains contained an additional Y-residue preceding the stop codon. Such conserved PrtP domains may be required for interactions with PrcA and PrcB, or for substrate interactions.
Introduction
The Treponema denticola outer membrane lipoprotein-protease complex (dentilisin) is comprised of the polypeptide products of the monocistronic prcB-prcA-prtP operon (Godovikova et al., 2010, Bian et al., 2005, Ishihara et al., 1996) that is both unique to and conserved in several oral Treponema species (Correia et al., 2003). Dentilisin contributes to periodontal disease by degrading components of serum and extracellular matrix (McDowell et al., 2009, Mäkinen et al., 1995, Uitto et al., 1995), and by disrupting intercellular junctions (Ellen et al., 2000, Chi et al., 2003) and dysregulation of tissue homeostasis control (Miao et al., 2011). Interstrain differences in antigenic surface proteins may reflect responses to immunological pressure or differences in host cell or tissue receptor specificity of the antigen. While T. denticola exhibits considerable interstrain variability in the Msp major surface protein (Fenno et al., 1997), no studies have addressed interstrain differences in the T. denticola dentilisin complex (CTLP), which is antigenically prominent (Capone et al., 2005) and plays a role in interactions with host tissue (reviewed in (Fenno, 2012)). We aimed to characterize sequence variability in the locus encoding the protease complex and relate this to the levels of protease activity in diverse strains.
One of the significant challenges in the era of microbial genomics is confirmation of annotated genome information by demonstration of function and activity of products of annotated genes. Automated genome analysis and annotation provides unprecedented amounts of information at the genomic level but prediction of gene function, especially in the case of “hypothetical proteins,” remains problematic (Sivashankari & Shanmughavel, 2006). Experimental confirmation of predicted gene structure and function is necessarily even more of an issue due to the rapidly expanding amount of unconfirmed genome annotation in the databases. Genomes are annotated based on accepted best practices and the best information available at the time. It is the responsibility of the research community to identify annotation errors in order to prevent their propagation as future annotations are constructed.
The operon encoding the dentilisin complex (CTLP) is encoded by TDE0760-TDE0762 in the annotated T. denticola genome (Seshadri et al., 2004). TDE0760, which we recently demonstrated to encode the acylated, 22-kDa outer membrane protein PrcB (Godovikova et al., 2010), is annotated as a 17.6-kDa conserved hypothetical non-acylated periplasmic protein with limited homology to a very small Pfam group (PF01833) characterized as containing a domain with an immunoglobulin-like fold found in some cell surface receptor and intracellular transcription factors. TDE0761, which we have shown to encode the acylated outer membrane protein PrcA (Godovikova et al., 2011a, Lee et al., 2002), is annotated differently by the Center for Microbial Resources at the J. Craig Venter Institute (CMR; cmr.jcvi.org) and the Oral Pathogen Sequence Database at Los Alamos National Laboratory (Oralgen; oralgen.lanl.gov). PrcA is described as a potential member of Pfam PF04773 (which includes the FecR sensor protein involved in dicitrate transport across the inner membrane (Oralgen annotation)) and Pfam PF00041 (due to presence of a potential fibronectin Type III domain (CMR annotation)). TDE0762 is annotated in both databases as a serine protease (PrtP, dentilisin) containing an “authentic frameshift.” While the graphic display of TDE0762 on the CMR site identifies a Type II signal peptide, no predicted PrtP amino acid sequence is shown and PrtP can be retrieved neither from protein databases by BLAST search with the PrtP amino acid sequence (Altschul et al., 1990) nor by searching the T. denticola genome using an algorithm designed specifically to identify lipoproteins in spirochete genomes (Setubal et al., 2006). We are hardly the first to note that significant annotation errors plague the genome databases (Perrodou et al., 2006, Brenner, 1999). We believe it is particularly appropriate to address the issue of PrtP annotation because the dentilisin protease complex is a significant virulence determinant of T. denticola pathogenesis in periodontal disease.
Herein we provide experimental data demonstrating the identity and amino acid sequence of PrtP, including showing the absence of the putative “authentic frameshift” that has resulted in exclusion of this significant microbial virulence determinant from genome-based databases. We then summarize our experimental results showing function and behavior of PrcB, PrcA and PrtP in contrast to the limited and incorrect information available in genomic databases. Furthermore, we characterize conservation, variability and expression of the prcB-prcA-prtP locus in T. denticola, demonstrating that this locus unique to a particular group of mammalian host-associated spirochetes encodes a highly conserved protease activity.
Methods
Bacterial strains and growth conditions
T. denticola strains (Table 1), were grown in NOS broth medium or NOS/GN semisolid medium under anaerobic conditions as previously described (Haapasalo et al., 1991, Chan et al., 1997), with erythromycin (Em, 40 μg ml-1) added as appropriate. Cultures were examined by darkfield microscopy for purity and typical strain morphology.
Table 1. T. denticola strains and plasmids used in this study.
| Strain | Features | Source (reference) |
|---|---|---|
| 35405 | Type strain | ATCCa (Chan et al., 1993) |
| K1 | prtP mutant | H. Kuramitsu (Ishihara et al., 1998) |
| 35404 | ATCCa (Chan et al., 1993) | |
| 33520 | ATCCa (Jacob et al., 1979) | |
| 33521 | ATCCa (Jacob et al., 1979) | |
| ASLM | clinical isolate | W. Loesche (Ohta et al., 1986) |
| SP82 | clinical isolate | W. Loesche (Salvador et al., 1987) |
| OTK | clinical isolate | R. Johnson (Fenno et al., 1997) |
| CF417 | prcB-6xHis | (Godovikova et al., 2010) |
| CF646 | prtP-6xHis | This study |
ATCC, American Type Culture Collection, Rockville, Md.
E. coli JM109 (Yanisch-Perron et al., 1985) and E. coli Rosetta™(DE3)/pLysS (Novagen, Inc., Madison, WI, USA) were used as hosts for cloning and expression of recombinant proteins, respectively. E. coli was grown on LB agar or broth medium with ampicillin (50 μg ml-1), kanamycin (30 μg ml-1) and chloramphenicol (34 μg ml-1) as appropriate. Plasmid vector pSTBlue-1 (Novagen) was used for direct cloning of polymerase chain reaction (PCR) products, and 6xHis-tagged constructs were made in pET28b (Novagen, Inc., Madison, WI, USA).
Construction of plasmids for expression and mutagenesis studies
DNA encoding the C-terminal region of prtP was amplified from T. denticola genomic DNA using primers CX616 and CX822 (Table 2), and the resulting PCR product carrying 5′ NcoI and 3′ XhoI engineered restriction sites was cloned in pET28b (Novagen) such that in the resulting plasmid (pCF617), a partial prtP open reading frame including a C-terminal 6xHistidine tag (6xHis) was expressed from the vector-encoded T7 promoter. To construct a DNA molecule capable of transferring this tagged prtP to T. denticola we employed a variation on overlap extension (OE) PCR methodology described by Shevchuk et al. (Shevchuk et al., 2004). An OEPCR product was generated by combining three PCR products: A: prtP6xHis, B: ermB, encompassing its Shine-Dalgarno sequence and coding region and C: downstream of prtP through the 5′ end of TDE0765. Primers used to generate these fragments (listed in Table 2) contain engineered overlapping 10-12 bp complementary to the adjacent PCR product. In the first step, a 100 μl PCR reaction containing templates A, B and C in a molar ratio of 5:1:5, and was carried out for 10 cycles in the absence of oligonucleotide primers. One μl of this product was used as template for a 35-cycle PCR using primers CX859 and CX819 complementary to the 5′ end of fragment A and the 3′ end of fragment C, respectively. The resulting PCR product was purified and cloned in pSTBlue-1 (Novagen) yielding pCF640, which carries ermB inserted between the 3′ end of prtP- 6xHis tag and DNA downstream of prtP including TDE0763, TDE0764 and the 5′ end of TDE0765.
Table 2. Oligonucleotide primers used in this study.
| Primer | Sequencea | Notesb |
|---|---|---|
| CX816 | CGGGACTTCCATGGCAACCCCATTTG | mid-prtP w/NcoI [F] |
| CX822 | GCTCGAGCGGATATTCTACATAGAACCAATC | prtP 3′ end w/XhoI [R] |
| CX834 | GATAACTGCAGATCAAATGAAGGCTCTGC | mid-prtP w/ PstI P1 [F] |
| CX847 | cttcttaattacTCAGTGGTGGTGGTGGT | prtP6xHis-ermB P2 [R] |
| CX848 | CACCACTGAgtaattaagaaggagtgattac | prtP6xHis-ermB [F] |
| CX849 | ATATTAACGcctaataatttatctacattcc | ermB-TERMprtP [R] |
| CX850 | gataaattattaggCGTTAATATGGGTAATTAGG | ermB-TERMprtP [F] |
| CX819 | CTTCAATGGGAAGAAGGAAG | past prtP-->tuf [R] |
| CX859 | GATCAAATGAAGGCTCTGC | mid-prtP [F] |
| CX636 | GCGCCGCCCTAATTAC | past prtP [R] |
| CX637 | GTTTGGTACGGTCGAAAC | prtP 1188 [F] |
Engineered restriction enzyme sites underlined; Upper case: prtP homology; Lower case: ermB homology.
Target; orientation with respect to prtP: forward [F]; reverse [R]).
Allelic replacement mutagenesis
Defined isogenic mutants were constructed as described previously (Li et al., 1996, Fenno et al., 1998b), by electroporation of T. denticola with linear DNA fragments consisting of the selectable erm cassette cloned between DNA fragments flanking the target gene. Plasmid pCF640 was digested with EcoRI prior to electroporation of T. denticola to separate the vector and insert fragments. Mutants were selected for resistance to Em (EmR) in NOS/GN agar (Chan et al., 1997). Mutations were verified by PCR analysis and by DNA sequencing of the target region in genomic DNA of the mutants.
Preparation of T. denticola extracts
T. denticola cultures were harvested by centrifugation at 10,000 × g (10 min, 4°C), washed 1 × in PBS and suspended in PBS at an optical density of 0.2 at 600 nm. Whole cell lysates were prepared by sonication prior to suspension in sample buffer. For some experiments, Triton X-114 extraction and phase partitioning of outer membrane proteins were performed as described for T. pallidum (Cunningham et al., 1988) with slight modifications (Fenno et al., 1998a, Miao et al., 2011). The final detergent phase extract was then precipitated in acetone and resuspended in electrophoresis sample buffer.
DNA sequence analysis
Templates for DNA sequencing, including plasmid DNA and PCR products generated from T. denticola genomic DNA, were sequenced at the University of Michigan DNA Sequencing Core Facility. Sequences of both DNA strands of regions of interest were obtained and analyzed using DNAStar-Lasergene software. DNA sequences of the prcB ORF in T. denticola strains ATCC 33520 and OTK were previously assigned Genbank accession numbers FJ555200 and FJ555201, respectively (Godovikova et al., 2010). DNA sequences encoding prcB-prcA-prtP of strains 33520, 33521, 35404, ASLM, SP82 and OTK have been assigned Genbank accession numbers JX984656 - JX98466, respectively. The prcB-prcA-prtP locus in 35405 was determined to match that in the published genome sequence (Genbank AE017226.1 and data not shown). Annotation of the open reading frames was done using the FGENESB algorithm trained to the T. denticola genome sequence (Softberry, Inc., Mt. Kisco, NY). A predicted σ-70 class promoter upstream of prcB was identified using BPROM software (Softberry, Inc.). Identification of potential signal peptidase cleavage sites was done using PSORT (Emanuelsson et al., 2007, Nakai & Kanehisa, 1991), LipoP (Juncker et al., 2003), and SpLip (Setubal et al., 2006).
Protein electrophoresis and immunoblotting
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblotting were done as described previously (Fenno et al., 1996). T. denticola cultures were harvested in the presence of 2 mM phenylmethylsulfonyl fluoride (PMSF) by centrifugation at 10,000 × g (10 min, 4°C), washed 1× in PBS and suspended in PBS at an optical density of 0.2 at 600 nm, then subjected to Triton X-114 extraction (Cunningham et al., 1988) without partitioning to aqueous and detergent phases. Equal volumes of samples were prepared in standard SDS-PAGE sample buffer containing ß-mercaptoethanol and 2 mM PMSF. Prior to electrophoresis, samples were either heated at 100° C for 5 min or held on ice. Proteins blotted to nitrocellulose membranes were detected with rabbit polyclonal antibodies raised against recombinant T. denticola proteins (Fenno et al., 1996, Godovikova et al., 2011b, Godovikova et al., 2010, Bian et al., 2005), followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Thermo Scientific, Rockford, IL). 6xHis-tagged proteins were detected HisProbe HRP reagent (Thermo Scientific). Protein bands of interest were visualized using SuperSignal West Pico chemiluminescent substrate (Thermo Scientific).
Protease activity assay
PrtP-dependent hydrolysis of the chromogenic substrate succinyl-L-alanyl-L-alanyl-L-prolyl-L-phenylalanine-p-nitroanilide (SAAPFNA) in 5-day T. denticola cultures was assayed by change in absorbance at 405 nm, as described previously (Uitto et al., 1988, Bian et al., 2005). SAAPFNA activity is expressed as percent of SAAPFNA activity of strain 35405 defined as 100%. Error bars represent the standard error over four independent experiments including triplicate samples.
Results and Discussion
T. denticola TDE0762 (prtP) does not contain an “authentic frameshift”
The DNA sequence of T. denticola prtP was first reported 1996 and assigned Genbank accession D83264 (Ishihara et al., 1996). The T. denticola ATCC 35405 genome was published in 2004 (Seshadri et al., 2004). In addition to the complete genome sequence (Genbank accession NC_002967), two major websites maintain the annotated genome sequence: the Center for Microbial Resources at the J. Craig Venter Institute (cmr.jcvi.org) and the Oral Pathogen Sequence Database at Los Alamos National Laboratory (Oralgen; oralgen.lanl.gov). While the DNA sequence of the genomic region including TDE0762 (prtP) has remained unchanged in the databases, the reported TDE0762 DNA sequence at these sites has varied over the last six years, and TDE0762 (prtP) is annotated as a “pseudogene” (Genbank) or as containing an “authentic frameshift” (CMR, Oralgen). In standard genome annotation methodology, open reading frames are detected using Glimmer (Salzberg et al., 1998). Detection of a mismatch in any particular open reading frame between the genomic sequence and a previously deposited Genbank sequence results in annotation of the gene as containing an “authentic frameshift” (Nelson et al., 2003). One consequence of this convention is that the amino acid sequence encoded by such a gene is not reported in the genome annotation and is generally absent from protein databases.
While neither Genbank, CMR nor Oralgen currently post the deduced amino acid sequence (CDS) encoded by TDE0762, several have been posted at various times over the past few years. Except for the full-length 766-residue PrtP briefly posted on the TIGR (now CMR) site in 2005, the others appear to have been truncated to approximate the length of prtP found in the first submitted prtP sequence (Genbank D83264), which reports prtP as encoding a 722-residue protein. Our DNA sequencing results in ATCC 35405 confirmed the reported genomic DNA sequence. Both our sequence and the genome databases show 3 differences compared with Genbank D83264: two 3-base changes substitutions (1414-1416:TAT vs ATA; 1494-1497: GAA vs CGA) and a single additional “G” in the D83264 sequence (position 2109). At the protein level, this results in I472 (D83264) vs V472, E499 (D83264) vs R499 and a frameshift in D83264 resulting in mismatches beyond residue 703 of the protein sequences deduced from the databases (Figure 1). It must be noted that the T. denticola genome sequence does not contain an in-frame stop codon at the point identified as the end of the coding sequence by both CMR and Oralgen, but that both databases arbitrarily truncate the prtP coding region after codon 721 (Oralgen) or 722 (CMR). However, both the genome sequence and our sequencing results suggest that, rather than the 722-residue PrtP reported in D83264 and implied in the genome databases, PrtP is a 766-residue protein whose sequence beyond residue 703 differs from that reported in D83264.
Figure 1.

Comparison of the T. denticola ATCC 35405 deduced PrtP amino acid sequences from residue 681. The top line is PrtP as reported in Genbank D83264. The middle line is the amino acid sequence deduced from TDE0762 as reported in the CMR genome database. Deduced translation of the Oralgen TDE0762 results in an amino acid sequence identical to that of CMR, except that the C-terminal histidine residue is absent. The bottom line is from the T. denticola genome sequence including DNA 3′ to identified TDE0762 until an in-frame stop codon. This sequence is confirmed in the present study. The difference between the sequences shown results from inclusion of an additional guanosine at nucleic acid residue 2109 in D83264, as discussed in the text.
To ensure that the mismatch between the original Genbank submission and the genomic sequence was not due to a mutation acquired during subculture of ATCC 35405 in separate laboratories, we next determined the DNA sequence of the 3′ region of prtP in T. denticola K1, an isogenic mutant of T. denticola ATCC 35405 that carries an antibiotic resistant marker inserted in the 5′ region of prtP (Ishihara et al., 1998). The K1 strain is derived from the ATCC 35405 clone that was the source of the D83264 prtP sequence. The DNA sequences of prtP from base 1290 through the end of the predicted prtP ORF shown in our 35405 clone and in the genomic sequence were identical in T. denticola K1 (data not shown). This strongly suggests that the prtP sequence deposited as Genbank D83264 contains sequencing errors, resulting in prediction of premature C-terminal truncation of the PrtP ORF.
Finally, to provide experimental evidence of the lack of an “authentic frameshift” in prtP, we constructed isogenic T. denticola mutant strain CF646 carrying a C-terminal 6xHis tag immediately before the prtP stop codon at base 2199 (following deduced amino acid codon 766 in the TDE0762 open reading frame). If native PrtP is truncated at residue 722, as shown in the original Genbank record and as suggested by current genome databases, then PrtP in the CF646 mutant would not include the C-terminal 6xHis tag. As shown in Figure 2, left panel, the presence of 6xHis tagged full length PrtP in CF646 clearly demonstrates that TDE0762 encodes a 766-residue PrtP protein and that the reported “authentic frameshift” in the genome databases is most likely the result of a sequencing error in the original Genbank entry. We believe that these data support revised annotation of the T. denticola dentilisin locus in curated genome databases. We have submitted requests to Oragen, CMR and the Human Oral Microbiome Database (homd.org) for updates reflecting this new information.
Figure 2.
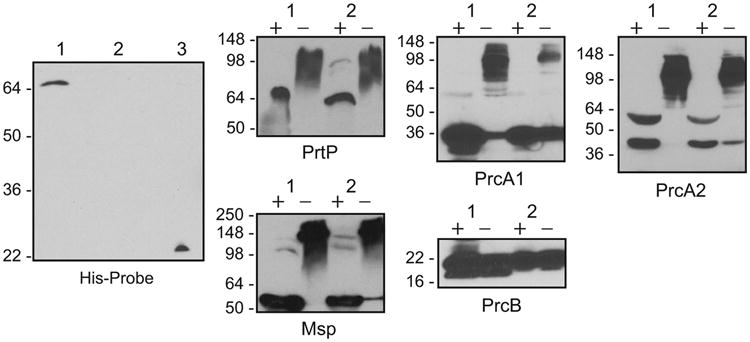
Outer membrane protein expression in T. denticola 35405 and CF646. The panels consist of blots probed HisProbe reagent (left panel) or with antibodies to the T. denticola proteins or polypeptides indicated. Molecular standards in kDa are shown to the left of each blot. Lanes: 1, T. denticola CF646; 2, T. denticola 35405; 3, T. denticola CF417. In blots probed with antibodies, samples were either heated (+) or not heated (-) prior to electrophoresis.
Expression of T. denticola outer membrane complex proteins in CF646
Molecular “tags” can interfere with expression and function of some proteins. To determine whether inclusion of the 6xHis tag at the C-terminus of PrtP affected expression of the outer membrane complexes in T. denticola, we assayed expression of individual protease complex components and the major surface protein Msp in parent and mutant strains. Samples were prepared for electrophoresis both with and without heating to detect both denatured proteins and protein complexes. As shown in Figure 2B, all proteins tested are expressed similarly in both 35405 and CF646, and there are no apparent differences in migration of outer membrane protein complexes between parent and mutant strains. Taken together, this suggests that the PrtP C-terminal 6xHis tag does not interfere with expression and assembly of the protease complex.
Conservation of the prcB-prcA-prtP locus in T. denticola
To characterize interstrain conservation of the protease locus, we sequenced the prcB-prcA-prtP region encoding the three lipoproteins comprising the dentilisin complex in well-established laboratory strains and in more recent isolates. As shown in Fig. 3, the deduced amino acid sequences of all three proteins show high levels of conservation, particularly in PrcB (89% identical at the amino acid level among the seven strains). PrcA is 77% identical among the seven strains, and much of the variability is in three regions (151-180, 328-362 and 512-545). In PrtP, the predicted catalytic triad (Asp203, His258, Ser447) is conserved in all strains. Consistent with conservation of proteolytic function, most PrtP interstrain variations are in the C-terminal 250 residues. Several strains showed insertions or deletions of between one and eight residues compared with the Type strain ATCC 35405. Apparent “hot spots” for this type of variation are in the regions of residue 235, 295, 562, 692, 724 and 754. Strains ASLM and SP82 also contained a C-terminal Y in addition to the conserved –DWFYVEYP C-terminus present in all strains. PrtP in 35405 is comprised of 766 amino acid residues. PrtP amino acid content ranged from 759 residues in 33521 to 776 residues in ASLM.
Figure 3.
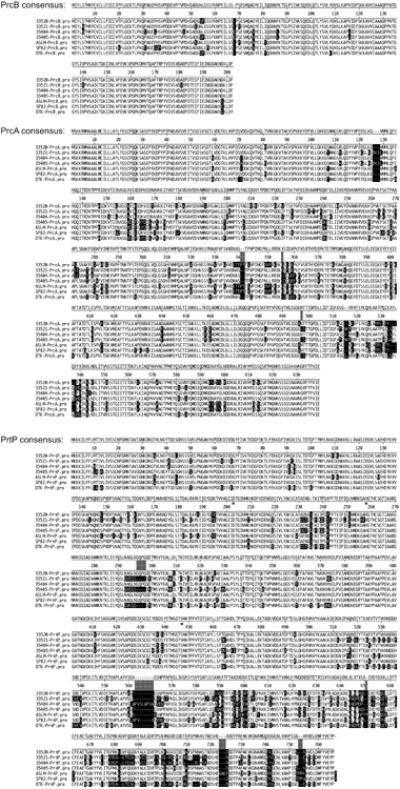
Conservation of PrcB, PrcA and PrtP in T. denticola strains. Deduced amino acid sequences were aligned using Clustal-W. The consensus sequence of each protein, numbered as for 35405, is shown above the aligned sequences. Mismatches from the consensus are shaded black, and gaps in the consensus are shaded grey. Predicted PrtP active site residues (Asp203, His258 and Ser447) are indicated by asterisks below the consensus sequence.
PrcB, PrcA and PrtP in all strains contain Type II signal peptidase cleavage sites. Our previous studies showed that each protein segregates to the detergent phase of Triton X-114 extracts (Fenno et al., 1998a, Godovikova et al., 2011b, Godovikova et al., 2010, Lee et al., 2002). In the current genome annotation, only PrcA is annotated as a lipoprotein. PrcB is reported as a cytoplasmic protein whose N-terminus is at residue 38 of the PrcB protein as reported here and previously (Godovikova et al., 2010). As noted earlier, the absence of annotation of PrtP protein is most likely due to previous sequencing errors in prior studies (Ishihara et al., 1996). From a functional standpoint the variability in the PrtP C-terminal region is suggestive of at least two possibilities. Variability in regions of a protein not essential for enzymatic function may result from selective immunological pressure, resulting in antigenic variation between strains. Alternatively, sequence variations may reflect functional differences in binding to host substrates or other protein interactions. The dentilisin complex (PrcA, PrcB and PrtP) is part of a very high molecular weight outer membrane complex that also includes the oligomeric Msp protein (Godovikova et al., 2011b). The gene encoding the major surface protein (Msp) in each strain examined here falls within one of the three previously identified msp types (Fenno et al., 1997) as do those of more than 30 clinical isolates (Fenno et al., 2001 and data not shown). While the data are somewhat limited, phylogenetic trees for each protein do not reflect any consistency in strain-dependent relationships between PrtP, PrcA and PrcB sequences (data not shown), nor is there any discernable relationship between the patterns of interstrain PrtP homology (Fig. 3) and interstrain Msp homology (Fenno et al., 1997). Thus, while it is clear that Msp and dentilisin components interact at the molecular level (Godovikova et al., 2011b), these interactions are likely between highly conserved domains of the relevant proteins. The protease complex is reported to specifically bind and degrade fibrinogen (Bamford et al., 2007), and its ability to degrade a range of other proteins and bioactive peptides (Mäkinen et al., 1995, Uitto et al., 1988) is suggestive of specific binding of these or other substrates. We speculate that the C-terminal region of PrtP is involved in substrate binding and that interstrain differences in binding or proteolytic activity of the dentilisin complex is due to strain-dependent sequence differences in this region. To date, no studies have systematically examined the function of the PrtP C-terminus.
As part of the Human Oral Microbiome Project (Dewhirst et al., 2010, Human Microbiome Project Consortium, 2012), the genomes of most of the strains examined here are being sequenced by the Broad Institute of Harvard and MIT (http://www.broadinstitute.org/). We have compared our DNA sequences with those of the provisional genome contigs and find them generally in close agreement. Genbank genome accession numbers of T. denticola strains in this study to date are as follows: NC_002967 (ATCC 35405), AGDU01000000 (ATCC 35404), AGDS01000000 (ATCC 33520), AGDT01000000 (ATCC 33521) and AGDR01000000 (ASLM), AGDY01000000 (OTK)
Protease complex protein expression
In addition to signal peptide cleavage and acylation, two of the three proteins of the protease complex undergo further posttranslational processing. In T. denticola 35405, PrtP is cleaved at residue 159 (Ishihara et al., 1996) to yield an acylated 16-kDa N-terminal polypeptide (PrtP-N) and 65-kDa mature PrtP (Godovikova et al., 2011b). PrcA undergoes PrtP-dependent cleavage (Lee et al., 2002) to acylated PrcA1 (approximately 30-kDa) and non-acylated PrcA2 (approximately 40-kDa). To determine the polypeptide profile of the dentilisin complex in diverse T. denticola strains, we probed immunoblots with antibodies specific for individual components of the T. denticola 35405 dentilisin complex.
As shown in Fig. 4A, there is considerable variation both in the level of detection obtained and in the relative molecular weights of some of the dentilisin complex polypeptides. PrcB, which is highly conserved in all strains, was detected equally in all strains. This strongly suggests that the dentilisin complex proteins are expressed at similar levels in all strains and is consistent with the presence of an identical sigma-70 type predicted promoter sequence upstream of prcB in all seven strains (data not shown). PrtP was detected in all strains though reactivity was relatively low in all but strain 35405, which was the source of the immunizing antigen. This is consistent with the interstrain variation evident in the PrtP C-terminal 250 residues. The N-terminal (PrcA1) and C-terminal (PrcA2) polypeptides resulting from PrtP-dependent processing of PrcA (Lee et al., 2002) were detected by at least one of the two specific antibodies tested in all strains. Interestingly, while PrcA2 was not recognized in strain 33520, anti-PrcA1 recognized the expected 30-kDa band. In all strains except 33520, anti-PrcA2 recognized either or both PrcA2 (approximately 40-kDa) and the full length PrcA molecule. Similarly, anti-PrcA1 recognized a PrcA1 of approximately 30-kDa in all strains except 33521, in which full length PrcA was detected. Anti-PrtP-N, raised against the acylated 16-kDa N-terminal polypeptide released upon PrtP activation (Ishihara et al., 1996, Godovikova et al., 2011b), reacted with all strains (data not shown).
Figure 4.
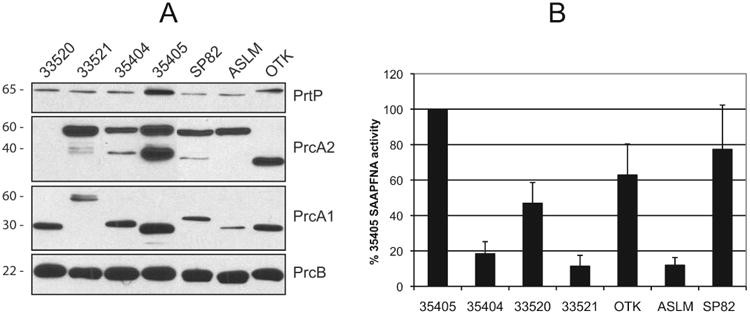
Protease expression and activity in T. denticola strains. Panel A: Immunoblots of T. denticola strains probed with antibodies specific for components of the dentilisin complex. Blots were probed with polyclonal antibodies specific for the proteins indicated to the right of each blot. All antibodies were raised against T. denticola 35405 proteins expressed in E. coli. Strain names are listed above each lane. Approximate sizes in kDa of the reactive bands are indicated to the left of each blot. Panel B: PrtP protease activity in 5-day T. denticola cultures. Protease activity of individual strains, assayed by change in absorbance at 405 nm, is expressed as a percentage relative to the SAAPFNA activity of strain 35405. Error bars represent the standard error of four independent experiments including triplicate samples.
Protease complex activity
To determine relative levels of dentilisin activity in T. denticola strains, we assayed cleavage of a chromogenic substrate (SAAPFNA) by T. denticola cultures in late logarithmic growth phase. We previously reported that transcription of prcA and prtP and protease activity increased as the growth phase progressed, likely in response to depletion of available nutrients (Bian et al., 2005). As shown in Fig. 4B, all strains exhibited chymotrypsinlike activity, though activity varied considerably between strains with the Type strain (35405) and strains OTK and SP82 exhibiting the highest activity. Interestingly the three other ATCC strains and strain ASLM showed markedly lower activity. Proteolytic activity does not appear to be related specifically to overall homology with 35405 PrtP (Fig. 3). It is not apparent whether protease activity has any relationship with passage number or total length of time in culture. Compared with the four ATCC strains, strains OTK, ASLM and SP82 are relatively low-passage isolates. While the putative promoter sequence upstream of prcB is identical in all seven strains, it is not known what specific environmental sensing and signaling mechanisms influence expression of the protease operon. Studies are in progress to characterize transcription of the prcB-prcA-prtP operon in these strains under a range of environmental conditions.
Acknowledgments
This work was supported by Public Health Service grant DE018221 (National Institute of Dental and Craniofacial Research), and by the Office of the Vice President for Research (University of Michigan). The authors thank Brian T. Foley of Los Alamos National Laboratories for helpful discussions.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bamford CV, Fenno JC, Jenkinson HF, Dymock D. The chymotrypsin-like protease (CTLP) complex of Treponema denticola ATCC 35405 mediates fibrinogen adherence and degradation. Infect Immun. 2007;75:4364–4372. doi: 10.1128/IAI.00258-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian XL, Wang HT, Ning Y, Lee SY, Fenno JC. Mutagenesis of a novel gene in the prcA-prtP protease locus affects expression of Treponema denticola membrane complexes. Infect Immun. 2005;73:1252–1255. doi: 10.1128/IAI.73.2.1252-1255.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner SE. Errors in genome annotation. Trends Genet. 1999;15:132–133. doi: 10.1016/s0168-9525(99)01706-0. [DOI] [PubMed] [Google Scholar]
- Capone R, Wang HT, Ning Y, Sweier DG, Lopatin DE, Fenno JC. Recognition of Treponema denticola outer membrane proteins by human antibodies. J Dent Res. 2005;84(Spec Iss A):2841. [Google Scholar]
- Chan EC, Siboo R, Keng T, Psarra N, Hurley R, Cheng SL, Iugovaz I. Treponema denticola (ex Brumpt 1925) sp. nov., nom. rev., and identification of new spirochete isolates from periodontal pockets. Int J Syst Bacteriol. 1993;43:196–203. doi: 10.1099/00207713-43-2-196. [DOI] [PubMed] [Google Scholar]
- Chan ECS, DeCiccio A, McLaughlin R, Klitorinos A, Siboo R. An inexpensive solid medium for obtaining colony-forming units of oral spirochetes. Oral Microbiol Immunol. 1997;12:372–376. doi: 10.1111/j.1399-302x.1997.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Chi B, Qi M, Kuramitsu HK. Role of dentilisin in Treponema denticola epithelial cell layer penetration. Res Microbiol. 2003;154:637–643. doi: 10.1016/j.resmic.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Correia FF, Plummer AR, Ellen RP, Wyss C, Boches SK, Galvin JL, Paster BJ, Dewhirst FE. Two paralogous families of a two-gene subtilisin operon are widely distributed in oral treponemes. J Bacteriol. 2003;185:6860–6869. doi: 10.1128/JB.185.23.6860-6869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TM, Walker EM, Miller JN, Lovett MA. Selective release of the Treponema pallidum outer membrane and associated polypeptides with Triton X-114. J Bacteriol. 1988;170:5789–5796. doi: 10.1128/jb.170.12.5789-5796.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen RP, Ko KS, Lo CM, Grove DA, Ishihara K. Insertional inactivation of the prtP gene of Treponema denticola confirms dentilisin's disruption of epithelial junctions. J Mol Microbiol Biotechnol. 2000;2:581–586. [PubMed] [Google Scholar]
- Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- Fenno JC. Treponema denticola interactions with host proteins. Journal of Oral Microbiology. 2012;4:9929. doi: 10.3402/jom.v4i0.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno JC, Hannam PM, Leung WK, Tamura M, Uitto VJ, McBride BC. Cytopathic effects of the major surface protein (Msp) and the chymotrypsinlike protease (CTLP) of Treponema denticola. Infect Immun. 1998a;66:1869–1877. doi: 10.1128/iai.66.5.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno JC, Lee SY, Bayer CH, Ning Y. Treponema msp's from subgingival plaque and cultivated strains. J Dent Res. 2001;80:S53. [Google Scholar]
- Fenno JC, Müller KH, McBride BC. Sequence analysis, expression and binding activity of recombinant major outer sheath protein (Msp) of Treponema denticola. J Bacteriol. 1996;178:2489–2497. doi: 10.1128/jb.178.9.2489-2497.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno JC, Wong GWK, Hannam PM, McBride BC. Mutagenesis of outer membrane virulence determinants of the oral spirochete Treponema denticola. FEMS Microbiol Lett. 1998b;163:209–215. doi: 10.1111/j.1574-6968.1998.tb13047.x. [DOI] [PubMed] [Google Scholar]
- Fenno JC, Wong GWK, Hannam PM, Müller KH, Leung WK, McBride BC. Conservation of msp, the gene encoding the major outer membrane protein of oral Treponema spp. J Bacteriol. 1997;179:1082–1089. doi: 10.1128/jb.179.4.1082-1089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godovikova V, Goetting-Minesky MP, Fenno JC. Composition and localization of the Treponema denticola lipoprotein protease complex. J Dent Res. 2011a;90 2963-IADR. [Google Scholar]
- Godovikova V, Goetting-Minesky MP, Fenno JC. Composition and localization of Treponema denticola outer membrane complexes. Infect Immun. 2011b;79:4868–4875. doi: 10.1128/IAI.05701-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godovikova V, Wang HT, Goetting-Minesky MP, Ning Y, Capone RF, Slater CK, Fenno JC. Treponema denticola PrcB is required for expression and activity of the PrcA-PrtP (dentilisin) complex. J Bacteriol. 2010;192:3337–3344. doi: 10.1128/JB.00274-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo M, Singh U, McBride BC, Uitto VJ. Sulfhydryl-dependent attachment of Treponema denticola to laminin and other proteins. Infect Immun. 1991;59:4230–4237. doi: 10.1128/iai.59.11.4230-4237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium. A framework for human microbiome research. Nature (London) 2012;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Kuramitsu HK, Miura T, Okuda K. Dentilisin activity affects the organization of the outer sheath of Treponema denticola. J Bacteriol. 1998;180:3837–3844. doi: 10.1128/jb.180.15.3837-3844.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Miura T, Kuramitsu HK, Okuda K. Characterization of the Treponema denticola prtP gene encoding a prolyl-phenylalanine-specific protease (dentilisin) Infect Immun. 1996;64:5178–5186. doi: 10.1128/iai.64.12.5178-5186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Allen AL, Nauman RK. Detection of oral anaerobic spirochetes in dental plaque by the indirect fluorescent-antibody technique. J Clin Microbiol. 1979;10:934–936. doi: 10.1128/jcm.10.6.934-936.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncker AS, Willenbrock H, Von Heijne G, Brunak S, Nielsen H, Krogh A. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 2003;12:1652–1662. doi: 10.1110/ps.0303703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Bian XL, Wong GW, Hannam PM, McBride BC, Fenno JC. Cleavage of Treponema denticola PrcA polypeptide to yield protease complex-associated proteins PrcA1 and PrcA2 is dependent on PrtP. J Bacteriol. 2002;184:3864–3870. doi: 10.1128/JB.184.14.3864-3870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ruby J, Charon N, Kuramitsu H. Gene inactivation in the oral spirochete Treponema denticola: construction of an flgE mutant. J Bacteriol. 1996;178:3664–3667. doi: 10.1128/jb.178.12.3664-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen PL, Mäkinen KK, Syed SA. Role of the chymotrypsin-like membrane-associated proteinase from Treponema denticola ATCC 35405 in inactivation of bioactive peptides. Infect Immun. 1995;63:3567–3575. doi: 10.1128/iai.63.9.3567-3575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Huang B, Fenno JC, Marconi RT. Analysis of a unique interaction between the complement regulatory protein factor H and the periodontal pathogen Treponema denticola. Infect Immun. 2009;77:1417–1425. doi: 10.1128/IAI.01544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao D, Fenno JC, Timm JC, Joo NE, Kapila YL. Treponema denticola chymotrypsin-like protease (dentilisin) induces MMP-2-dependent fibronectin fragmentation in periodontal ligament cells. Infect Immun. 2011;79:806–811. doi: 10.1128/IAI.01001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K, Kanehisa M. Expert system for predicting protein localization sites in gram- negative bacteria. Proteins. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- Nelson KE, Fleischmann RD, DeBoy RT, Paulsen IT, Fouts DE, Eisen JA, Daugherty SC, Dodson RJ, Durkin AS, Gwinn M, Haft DH, Kolonay JF, Nelson WC, Mason T, Tallon L, Gray J, Granger D, Tettelin H, Dong H, Galvin JL, Duncan MJ, Dewhirst FE, Fraser CM. Complete genome sequence of the oral pathogenic pacterium Porphyromonas gingivalis strain W83. J Bacteriol. 2003;185:5591–5601. doi: 10.1128/JB.185.18.5591-5601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Mäkinen KK, Loesche WJ. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986;53:213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrodou E, Deshayes C, Muller J, Schaeffer C, Van Dorsselaer A, Ripp R, Poch O, Reyrat JM, Lecompte O. ICDS database: interrupted CoDing sequences in prokaryotic genomes. Nucleic Acids Res. 2006;34:D338–343. doi: 10.1093/nar/gkj060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador SL, Syed SA, Loesche WJ. Comparison of three dispersion procedures for quantitative recovery of cultivable species of subgingival spirochetes. J Clin Microbiol. 1987;25:2230–2232. doi: 10.1128/jcm.25.11.2230-2232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg SL, Delcher AL, Kasif S, White O. Microbial gene identification using interpolated Markov models. Nucleic Acids Res. 1998;26:544–548. doi: 10.1093/nar/26.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri R, Myers GS, Tettelin H, Eisen JA, Heidelberg JF, Dodson RJ, Davidsen TM, DeBoy RT, Fouts DE, Haft DH, Selengut J, Ren Q, Brinkac LM, Madupu R, Kolonay J, Durkin SA, Daugherty SC, Shetty J, Shvartsbeyn A, Gebregeorgis E, Geer K, Tsegaye G, Malek J, Ayodeji B, Shatsman S, McLeod MP, Smajs D, Howell JK, Pal S, Amin A, Vashisth P, McNeill TZ, Xiang Q, Sodergren E, Baca E, Weinstock GM, Norris SJ, Fraser CM, Paulsen IT. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc Natl Acad Sci U S A. 2004;101:5646–5651. doi: 10.1073/pnas.0307639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setubal JC, Reis M, Matsunaga J, Haake DA. Lipoprotein computational prediction in spirochaetal genomes. Microbiology. 2006;152:113–121. doi: 10.1099/mic.0.28317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchuk NA, Bryksin AV, Nusinovich YA, Cabello FC, Sutherland M, Ladisch S. Construction of long DNA molecules using long PCR-based fusion of several fragments simultaneously. Nucleic Acids Res. 2004;32:e19. doi: 10.1093/nar/gnh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivashankari S, Shanmughavel P. Functional annotation of hypothetical proteins - A review. Bioinformation. 2006;1:335–338. doi: 10.6026/97320630001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto VJ, Grenier D, Chan EC, McBride BC. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988;56:2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto VJ, Pan YM, Leung WK, Larjava H, Ellen RP, Finlay BB, McBride BC. Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infect Immun. 1995;63:3401–3410. doi: 10.1128/iai.63.9.3401-3410.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


