Abstract
Smokers who can't reduce their cigarette intake using precessation nicotine patch treatment can benefit from alternative therapies, such as augmenting the patch with bupropion or switching to varenicline. An adaptive treatment plan can increase smoking cessation rates while minimizing risks and side effects.
Objective
The authors evaluated an adaptive smoking cessation treatment strategy in which nicotine patch treatment was initiated before a quit date, and then, depending on initial therapeutic response, either the nicotine patch was continued or alternative pharmacotherapies were provided.
Method
The study was a double-blind, parallel-arm adaptive treatment trial. A total of 606 cigarette smokers started open-label nicotine patch treatment 2 weeks before the quit date. Those whose ad lib smoking did not decrease by >50% after 1 week were randomly assigned to one of three double-blind treatments: nicotine patch alone (control condition); “rescue” treatment with bupropion augmentation of the patch; or rescue treatment with varenicline alone. Participants whose precessation smoking decreased >50% but who lapsed after the quit date were also randomly assigned to the two rescue treatments or to nicotine patch alone. Logistic regression analyses compared each rescue treatment against the control condition in terms of abstinence at the end of treatment (weeks 8–11) and at 6 months.
Results
Smokers who did not respond adequately to precessation nicotine patch benefited from bupropion augmentation; abstinence rates at end of treatment were 16% with nicotine patch alone and 28% with bupropion augmentation (odds ratio=2.04, 95% CI=1.03–4.01). Switching to varenicline produced less robust effects, but point abstinence at 6 months was 6.6% with the patch alone and 16.5% with a switch to varenicline (odds ratio=2.80, 95% CI=1.11–7.06). Postquit adaptive changes in treatment had no significant effects on any abstinence outcome.
Conclusions
It is possible to rescue a significant portion of smokers who would have failed to achieve abstinence if left on nicotine patch alone by identifying these smokers before their quit date and implementing adaptive changes in treatment.
Keywords: Smoking cessation, Nicotine patch, Varenicline, Bupropion, Biomarkers, Adaptive treatment, Carbon monoxide
Little information is available to guide health care providers when recommending which smoking cessation medication to use for a given smoker. While some algorithms have been suggested for smoking cessation treatment (1, 2), there is a paucity of experimental data validating these approaches.
Nicotine replacement therapy is widely used and, because of its safety and tolerability, has been available for many years as an over-the-counter treatment (3). Two prescription drugs, bupropion and varenicline, have also achieved widespread use (4–7). While efficacious, both varenicline and bupropion have “black box” warnings mandated by the Food and Drug Administration, as they have been associated with serious mood disturbances, including depressed mood and, rarely, suicidality (8–10). One recent meta-analysis linked varenicline with a slight excess risk for heart attack (11), and questions have been raised about its appropriateness as a first-line pharmacotherapy (8). Given the safety and tolerability profile of nicotine replacement therapy, our rationale in this study was to use nicotine replacement therapy as an initial line of treatment, and then identify early on which smokers are unlikely to benefit from nicotine alone.
To assess early therapeutic response to nicotine replacement therapy before the quit date, we monitored ad lib smoking during the first week of precessation open-label nicotine patch treatment by measuring expired-air CO levels. In previous studies, we observed that a reduction in ad lib smoking while on precessation nicotine patch treatment strongly predicted abstinence after the quit date (12–14). Our primary hypothesis was that smokers who did not respond to nicotine patch alone would achieve higher abstinence rates if they were switched to either of two alternative therapies (see phase 1 below). A secondary, exploratory hypothesis was that smokers who responded well initially to precessation nicotine patch treatment but who lapsed in the first week after their target quit date could be “rescued” by switching to alternative treatments at that point and setting a second quit date 1 week later (see phase 2 below). In contrast, for smokers who initially responded with sufficient smoking reduction with nicotine patch treatment, it was reasonable for them to remain on nicotine patch alone and not be exposed to the greater risks and side effects of the available prescription medications.
Method
Study Design
The study was a double-blind, parallel-arm adaptive treatment trial comprising two distinct phases (Figure 1).
Figure 1. CONSORT Diagrams Depicting Participant Recruitment, Eligibility Assessment, Allocation to Treatment Conditions, and Disposition in a Study of Adapting Smoking Cessation Treatmenta.
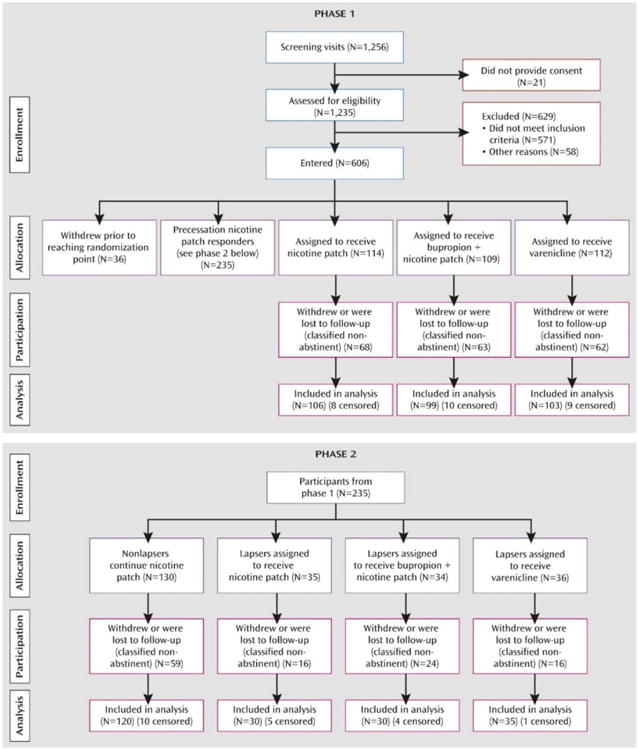
aIn phase 1, cigarette smokers received precessation nicotine patch treatment, and those whose ad lib smoking did not decrease by .50% after 1 week were randomly assigned to remain on nicotine patch treatment, switch to varenicline, or augment nicotine patch with bupropion. In phase 2, participants whose precessation smoking decreased .50% but who lapsed after the quit date were also randomly assigned to the two rescue treatments or to nicotine patch alone
Phase 1 assessed the effects of two potential precessation rescue treatments (varenicline or bupropion augmentation of nicotine patch) for smokers who did not respond adequately to open-label nicotine patch treatment before the target quit date. Smokers were classified as precessation “responders” or “nonresponders” to nicotine patch treatment, depending on whether their ad lib smoking (assessed by expired-air CO levels) decreased by more than 50% in the first week of precessation patch treatment. Nonresponders at that point (1 week before their target quit date) were randomly assigned to one of three double-blind treatment conditions: 1) a switch to varenicline (stopping nicotine patch treatment); 2) augmentation of nicotine patch with bupropion; and 3) continuation on nicotine patch alone. Varenicline was administered as a monotherapy because current labeling recommends against combining nicotine patch and varenicline because of an increased frequency of nausea and other side effects. Nicotine patch responders remained on open-label nicotine patches until Phase 2.
Phase 2 assessed the effects of postquit adaptive treatment changes for precessation nicotine patch responders who lapsed in the first week after the quit date. Lapsers were identified either by self-report of smoking (even a puff) within the first week after the quit date or by an expired-air CO level >10 ppm at 1 week after the quit date. These lapsers were scheduled for a second target quit date 1 week later and were randomly assigned to the same three double-blind treatment conditions as in phase 1. Nonlapsers remained on open-label nicotine patches for the duration of treatment.
Recruitment, Eligibility, and Compensation
Adult smokers expressing a desire to quit smoking were recruited through newspaper, radio, and television advertisements. To be eligible, participants had to be 18–65 years of age, report smoking an average of ≥10 cigarettes per day for 3 cumulative years, have an expired-air CO level ≥10 ppm, and have no exclusionary feature on history, physical examination, or laboratory evaluation (see the data supplement that accompanies the online edition of this article). After receiving a complete description of the study, participants provided written informed consent. Participants were compensated up to $320 for study participation.
Study Procedures
After screening and enrollment in the study, participants were seen weekly at our research center for 2 weeks before the quit date and attended four to six sessions after the quit date. Brief (<15 minutes) support was provided at each session, and clinical trial materials were dispensed. Smoking diaries and measures of expired-air CO levels, withdrawal symptoms, and other adverse effects were also collected. Six months after the target quit date, participants were contacted and those who reported point (7-day) abstinence were invited to return to the center for an assessment of expired-air CO level.
The study was conducted in a double-blind and double-dummy fashion; all participants who were assigned to a treatment condition received active nicotine skin patches (NicoDerm CQ, GlaxoSmithKline, Philadelphia) or placebo (Rejuvenation Laboratories, Salt Lake City), capsules containing varenicline (Pfizer, New York) or placebo, and bupropion sustained-release tablets (GlaxoSmithKline) or placebo (Glatt Pharmaceutical Services, Ramsey, N.J.). The recommended dosing titration schedule was used for both varenicline (0.5 mg once daily on days 1–3, 0.5 mg twice daily on days 4–7, and 1 mg twice daily through week 12) and bupropion (150 mg daily for 3 days, followed by 150 mg twice daily through week 12).
Initial nicotine patch dosing was based on initial expired-air CO reading; participants with CO levels >30 ppm at baseline received 42 mg/day (two 21 mg/day patches) daily, and the remaining participants received a single 21 mg/day patch daily. In the 21 mg/day condition, an active patch was applied each morning; in the 42 mg/day condition, an active 21 mg patch was applied each morning and a second patch at noon. This personalized dosing regimen was based on previous research suggesting that heavy smokers may not receive adequate nicotine replacement with a 21 mg patch (15, 16). Dose reductions were allowed in the event of side effects.
Statistical Analysis
For each of the two phases of the study, treatment groups were initially compared on demographic and smoking history variables, in order to identify potential confounding factors (none were identified).
To evaluate the main hypothesis of phase 1—that precessation adaptive treatment changes would rescue precessation nicotine patch nonresponders—abstinence outcomes were compared between each potential rescue treatment and the corresponding randomized nicotine patch group.
To evaluate the main hypothesis of phase 2—that postcessation adaptive treatment changes would rescue participants who lapsed in the first week after their quit date—abstinence outcomes were compared between each potential rescue treatment and the corresponding randomized nicotine patch group.
Logistic regression analyses were used to evaluate the primary outcome of continuous abstinence at end of treatment (abstinence during weeks 8–11 after the target quit date). Secondary outcomes included continuous 11 weeks of abstinence from the quit date, point (7-day) abstinence at 6 months, and continuous abstinence from the quit date at 6-months. Abstinence was identified by self-report of no smoking confirmed by end-expired CO levels ≤10 ppm. Participants who withdrew from the study or were lost to follow-up were classified as nonabstinent.
In addition to the above analyses, we sought confirmatory evidence in support of the two main assumptions underlying the adaptive treatment approach. In phase 1, to verify the assumption that precessation nicotine patch nonresponders would do poorly if left on nicotine patch alone, we compared the abstinence rate of precessation nicotine patch responders to that of nicotine patch nonresponders who were randomly assigned to remain on nicotine patch alone. Given that nicotine patch responders who lapsed after the quit date may have been assigned to treatments other than nicotine patch in phase 2, we eliminated the confounding effects of these postquit treatment changes by taking the outcome to be continuous abstinence from the initial quit date through end of treatment (11 weeks after the quit date). By using the continuous-abstinence endpoint, lapsers were considered nonabstinent before any potential confounding effects of postquit treatment changes could occur.
The second main assumption, in phase 2, was that participants who lapsed in the first week after the quit date who were assigned to nicotine patch alone would have a lower abstinence rate than participants on nicotine patch alone who had no lapses. To evaluate this assumption, abstinence outcome (4-week continuous abstinence at the end of treatment) was compared between lapsers and nonlapsers receiving nicotine patch alone in phase 2.
Forty-seven participants were excluded from data analyses (27 in phase 1 and 20 in phase 2) because of having initiated contraindicated medications (opiates, antidepressants, or other CNS-active medications) during the study or failing to meet other entry criteria. Another individual died before reaching the primary outcome point and was also censored from analysis. One participant was excluded from the analysis of percent change in CO level because of a value >35 standard deviations from the mean of the remaining sample.
Results
A total of 606 participants were enrolled in the study (see Figure 1). The demographic characteristics and smoking histories of participants were similar across treatment conditions for both phases (Table 1).
Table 1. Baseline Participant Characteristics for Phase 1 and Phase 2.
| PHASE 1 | Randomized pre-cessation conditions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nicotine Patch | Bupropion+Nicotine Patch | Varenicline | |||||||
|
|
|||||||||
| Gender (male) | n (%) | 51 | (48.1) | 53 | (53.5) | 62 | (60.2) | ||
| 21 mg nicotine patch | n (%) | 70 | (66.0) | 64 | (64.6) | 69 | (66.9) | ||
| Menthol cigarette brand | n (%) | 55 | (51.9) | 49 | (49.5) | 52 | (50.5) | ||
| Cigarettes/day | mean (SD) | 21.3 | (8.9) | 21.9 | (8.8) | 21.8 | (10.6) | ||
| Age | mean (SD) | 44.3 | (10.8) | 46.0 | (10.8) | 44.7 | (10.7) | ||
| Years smoked | mean (SD) | 26.0 | (11.2) | 26.8 | (10.6) | 25.9 | (11.0) | ||
| FTND score | mean (SD) | 5.8 | (2.0) | 5.8 | (2.0) | 5.8 | (1.7) | ||
| End-expired air CO | mean (SD) | 28.6 | (14.1) | 28.5 | (11.6) | 28.9 | (11.5) | ||
| Cotinine (ng/mL) | mean (SD) | 346 | (214) | 366 | (189) | 359 | (218) | ||
| Race/ethnicity | |||||||||
| European-American | n (%) | 67 | (63.2) | 61 | (61.6) | 66 | (64.1) | ||
| African-American | n (%) | 34 | (32.1) | 27 | (27.3) | 34 | (33.0) | ||
| Other | n (%) | 5 | (4.7) | 11 | (11.1) | 3 | (2.9) | ||
| PHASE 2 | Randomized post-cessation conditions | ||||||||
| Nicotine Patch | Bupropion+Nicotine Patch | Varenicline | Open-label Nicotine Patch | ||||||
| Gender (male) | n (%) | 11 | (36.7) | 15 | (50.0) | 18 | (51.4) | 60 | (50.0) |
| 21 mg nicotine patch | n (%) | 14 | (46.7) | 13 | (43.3) | 15 | (42.9) | 57 | (47.5) |
| Menthol cigarette brand | n (%) | 16 | (53.3) | 12 | (40.0) | 16 | (45.7) | 50 | (41.7) |
| Cigarettes/day | mean (SD) | 19.1 | (9.2) | 22.9 | (10.5) | 20.5 | (8.2) | 20.8 | (7.4) |
| Age | mean (SD) | 40.8 | (12.8) | 43.1 | (11.6) | 44.1 | (11.2) | 43.5 | (10.1) |
| Years smoked | mean (SD) | 22.7 | (12.0) | 24.7 | (11.4) | 24.6 | (10.5) | 24.6 | (10.3) |
| FTND score | mean (SD) | 5.7 | (1.9) | 6.3 | (2.0) | 5.9 | (2.3) | 5.6 | (1.6) |
| End-expired air CO | mean (SD) | 26.8 | (13.4) | 28.9 | (8.6) | 27.9 | (12.7) | 30.2 | (12.4) |
| Cotinine (ng/mL) | mean (SD) | 334 | (189) | 369 | (237) | 339 | (235) | 384 | (214) |
| Race/ethnicity | |||||||||
| European-American | n (%) | 18 | (60.0) | 20 | (66.7) | 21 | (60.0) | 89 | (74.2) |
| African-American | n (%) | 11 | (36.7) | 8 | (26.7) | 14 | (40.0) | 25 | (20.8) |
| Other | n (%) | 1 | (3.3) | 2 | (6.7) | 0 | (0.0) | 6 | (5.0) |
In phase 1, participants whose smoking did not decrease by >50% after 1 week of a 2-week precessation period of open-label nicotine patch treatment were randomly assigned to one of the three double-blind conditions; participants whose smoking did decrease by >50% remained on open-label nicotine patch until phase 2. In phase 2, participants who lapsed into smoking during the first week after the quit date were randomly assigned to one of the three double-blind conditions; those who did not lapse remained on open-label nicotine patch.
Phase 1: Evaluation of Precessation Rescue Effects
Among smokers who did not reduce their smoking sufficiently in the first week of nicotine patch, as assessed by CO level (nicotine patch nonresponders), abstinence rates were significantly higher after the adaptive treatment change to bupropion augmentation of nicotine patch, compared with nicotine patch alone (Table 2). Not only was the primary treatment outcome of continuous abstinence during weeks 8–11 after the quit date enhanced (p=0.04; odds ratio=2.06, 95% CI=1.05–4.07), but so too were the secondary outcomes of 11-week continuous abstinence from the quit date (p=0.01; odds ratio=3.36, 95% CI=1.34–8.39), 6-month point abstinence (p=0.02; odds ratio=2.93, 95% CI=1.16–7.41), and 6-month continuous abstinence from the quit date (p=0.01; odds ratio=5.19, 95% CI=1.43–18.81).
Table 2. Abstinence Rates for Participants in Phase 1 and Phase 2.
| Randomized conditions | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Phase 1 (pre-cessation NRT non-responders) | Phase 2 (post-cessation lapsers) | ||||||
|
| |||||||
| Abstinence outcome (%) | Nicotine Patch | Bupropion+ Nicotine Patch | Varenicline | NicotinePatch | Bupropion + Nicotine patch | Varenicline | Open-label Nicotine Patch |
| Weeks 8-11 (Primary outcome) | 16.0 | 28.3a | 23.3 | 26.7% | 26.7% | 37.1% | 59.2% |
| Quit date to week 11 | 6.6 | 19.2a | 11.7 | 23.3% | 20.0% | 28.6% | 51.7% |
| Quit date to 6 months | 5.8 | 13.1a | 5.8 | 10.0% | 10.0% | 14.3% | 20.0% |
| Point abstinence at 6 months | 6.6 | 17.2a | 16.5a | 13.3% | 10.0% | 20.0% | 21.7% |
P<0.05 vs. randomized nicotine patch condition within each phase
The precessation adaptive change in treatment to varenicline did not yield a significant effect on the primary outcome of continuous abstinence during weeks 8–11 or on the measures of continuous abstinence from the quit date, but there was a significant effect on point abstinence at 6 months (p=0.03; odds ratio=2.80, 95% CI=1.11–7.06). To examine whether the emergence of an effect of varenicline at 6 months may have been due to smokers' recovery from early lapses, a follow-up analysis compared 6-month point abstinence across conditions among smokers who lapsed at least once during the last 4 weeks of treatment (weeks 8–11). Among these participants, the percentage of smokers who ultimately achieved point abstinence at 6 months was 8.9% (7/79) in the varenicline condition, but only 1.1% (1/89) in the nicotine patch condition and 0% (0/71) in the bupropion plus nicotine patch condition (p=0.01 for varenicline compared with the other conditions; odds ratio=15.46, 95% CI=1.87–128.00). This 7%–8% difference between varenicline and the other treatment conditions accounts for most of the 10% higher 6-month point abstinence obtained with varenicline (Table 2).
Phase 2: Evaluation of Postcessation Rescue Effects
Postquit adaptive changes in treatment had no significant effects on any of the abstinence outcomes (Table 2).
Confirmation of Assumptions Underlying the Adaptive Treatment Design
The assumption underlying the design of phase 1 was that participants showing less of a decrease in ad lib smoking after 1 week of nicotine patch would have lower abstinence rates than those showing larger spontaneous reductions in smoking. This assumption was confirmed based on several analyses. First, among participants receiving nicotine patch, continuous smoking abstinence 11 weeks after the quit date was strongly predicted by the reduction of ad lib smoking 1 week before the quit date. Abstinence was significantly related to all measures of smoking reduction analyzed at this time point, including absolute CO level (p<0.0001; odds ratio=1.09, 95% CI=1.05–1.12), percent decrease in CO level (p<0.0001; odds ratio=1.04, 95% CI=1.03–1.06), and dichotomous classification of whether or not there was greater than 50% decrease in CO level (p<0.0001; odds ratio=6.76, 95% CI=2.98–15.32). When all three variables were entered into a multiple logistic regression analysis, percent reduction in CO level was the only predictor that remained significant (p=0.001). This result suggested that quantitative information about the extent of smoking reduction, above and beyond the dichotomization of >50% or not, has additional predictive value. Quartiles of percent CO level reduction, however, captured the relevant information, reflected by the findings that the correlation between the quartile score and abstinence was as high as for percent reduction (r=0.38 and r=0.36, respectively) and that when quartile was entered into a multiple logistic regression model with percent CO reduction, the percent reduction variable was rendered nonsignificant (p=0.16). Moreover, the quartile percent reduction in CO was a highly significant univariate predictor in its own right (p<0.0001; odds ratio=2.51, 95% CI=1.88–3.36). Figure 2 shows the monotonic increase in abstinence rate as a function of quartile percent reduction in CO level.
A similar analysis on prequit reduction in cigarettes per day yielded a similar conclusion. Abstinence was significantly correlated with all three indices reflecting cigarette reduction at week 1, including the absolute level of cigarettes per day, the percent reduction, and the dichotomous classification of whether or not there was a reduction >50%. As with CO level, when all three variables were entered into a multiple logistic regression analysis, only percent reduction remained significant. Moreover, as with CO level, a quartile score was defined and found to be largely redundant with the continuous index of percent reduction, as well as being a significant univariate predictor of smoking abstinence (p<0.0001; odds ratio=2.51, 95% CI=1.88–3.36). Figure 2 shows the relationship between quartile percent cigarette reduction and smoking abstinence.
The second assumption underlying the adaptive treatment design (phase 2), that participants who lapsed in the first week after their initial quit date would do worse at the end of treatment than those who did not lapse, was evaluated by comparing outcomes between the precessation nicotine patch responders who had a lapse and those who did not lapse and remained on nicotine patch alone. The lapsers showed a much lower rate of continuous 4-week abstinence at the end of treatment: 26.7% compared with 59.2% (p=0.002; odds ratio=0.25, 95% CI=0.10–0.61).
Adverse Events and Side Effects
Treatments were generally well tolerated; however, 25% of participants received dose reductions at some point during the study, with no difference between treatment conditions (range 20%–30%). A number of adverse events occurred that had no definitive causal relationship to treatment: one death believed to be due to stroke, two nonfatal heart attacks, one case of cancer, one participant with tachycardia with transient loss of consciousness, five participants reporting chest pain (including one left bundle branch block, one unifocal premature ventricular contractions, and one report of chest pain with vomiting and hallucinations), one participant with nausea/vomiting, three participants with hives or rash, one participant diagnosed with depression, and one participant reporting weakness, lightheadedness, and difficulty breathing.
Discussion
The results of this study show that smokers who fail to respond adequately to precessation nicotine patch treatment can benefit from being switched to alternative therapies. For these smokers, augmenting nicotine patch treatment with bupropion produced robust increases of approximately 10% in both primary and secondary abstinence outcomes, yielding an abstinence rate that was 2–3 times higher than if they remained on nicotine patch alone. Switching precessation nicotine patch nonresponders to varenicline produced less robust effects, but there was an enhancement in point abstinence at 6 months of about the same magnitude. Thus, it is possible to rescue a significant portion of smokers who would have failed to achieve abstinence if left on nicotine patch alone by identifying these smokers before their quit date and implementing adaptive changes in treatment.
The low success rate of precessation nicotine patch nonresponders who remained on nicotine patch alone replicates findings from previous research in our center. What is novel and important in phase 1 is that adaptive changes in treatment were implemented before the quit date, thereby averting failure instead of waiting for a relapse to occur before attempting to intervene. In so doing, we could minimize the deleterious effects of relapse on motivation and retention in treatment.
Phase 2, which explored the potential of post-quit date adaptive changes in treatment, yielded less promising results. Smokers who responded well to precessation nicotine patch but lapsed in the first week after the quit date did poorly relative to nonlapsers, extending the results of previous trials indicating that early lapses predict failure (17, 18). Unfortunately, however, there was no indication that either of the two potential rescue treatments increased abstinence rates relative to remaining on nicotine patch. In view of the small group sizes studied in this phase of the study, however, the results should be interpreted with caution. Nonetheless, this finding is similar to other negative results from studies attempting to provide alternative treatments after relapse to smoking (19).
In addition to showing that expired-air CO level is a clinically useful biomarker of response to precessation nicotine patch, the results suggest that other simple measures of ad lib smoking may prove useful as well to classify smokers as nicotine patch responders or nonresponders. For example, the decline in self-reported cigarettes per day was also predictive of outcome, and this measure might be more easily gathered in a variety of health care settings. For any measure of ad lib smoking, the question arises as to whether the absolute level or the relative change in the index is a better predictor of outcome. In this study, classification of responder status based on a relative change in expired-air CO level during the first week of precessation patch treatment was found to be a better predictor than absolute level of smoking at the end of week 1. Thus, although some clinicians have recommended using the level of smoking to guide adaptive treatment changes (1), the present data support percent reduction as a more predictive index. Additionally, we found that the relevant information contained in the percent CO reduction measure was captured by a quartile categorization.
Although the fraction of smokers who can be rescued by adaptive treatment with varenicline was similar to that for bupropion plus nicotine patch (based on 6-month point abstinence), it is possible that these smokers were different in terms of baseline characteristics. If so, and if the two distinct subsets could be identified at baseline, then a potentially greater increase in abstinence rates could be achieved by recommending the specific change in adaptive treatment most likely to succeed for a given smoker. Future development of adaptive smoking cessation treatment algorithms should incorporate additional predictive markers, including genotypic as well as phenotypic variables (14, 20). Ultimately, a combination of several predictor variables may be used to identify which smokers need adaptive treatment changes and which specific treatment will yield the highest probability of success with the smallest risk of adverse effects. This study had several strengths, including the unique feature of using an early prequit marker of response to nicotine patch to decide whether to modify treatment using prescription medications. In addition, the algorithm we used is supported by randomized controlled trial data, which has not been the case for previously proposed algorithms. The study also had limitations. One limitation, as noted earlier, was the relatively small group sizes in the postcessation randomization phase. Thus, we cannot confidently rule out a potential rescue effect for lapsers after the quit date. Second, the study was limited in using a tailored dosage of precessation nicotine patch therapy that is not in accordance with current product labeling in the United States (although precessation nicotine patch is approved in several other countries). Additionally, we did not ascertain the efficacy of other adaptive treatment strategies, such as increasing the length of precessation nicotine patch treatment or increasing nicotine dosage for initial nonresponders. Finally, we did not evaluate whether bupropion plus nicotine patch might prove more efficacious as an initial treatment for nicotine patch responders as well as nonresponders; however, our rationale was to avoid the additional risks and side effects of bupropion for nicotine patch responders, who had a reasonable chance of quitting using nicotine replacement alone.
In conclusion, our results support the use of an adaptive treatment regimen in which smokers initially receive precessation nicotine patch treatment, and if there is an insufficient smoking reduction within the first week, treatment is revised accordingly, either switching to varenicline or adding bupropion to nicotine patch. Further studies should confirm this finding, which has promise for practical implementation in health care settings to increase smoking cessation rates while minimizing treatment-related risks and side effects.
Supplementary Material
Acknowledgments
From the Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham, N.C.
Dr. Rose has served as a consultant for Targacept and Philip Morris USA and has a patent purchase agreement with Philip Morris International. Both authors have received research funding from Philip Morris USA.
Supported by a grant to Duke University from Philip Morris USA. Nicotine patches were donated by GlaxoSmithKline. The companies had no role in the planning or execution of the study, data analysis, or publication of results.
The authors thank Susan Claerhaut, Wendy Roberts, Kay Scime, Tanaia Loeback, Amanda Mitchell, Al Salley, and Eric Westman for their assistance in conducting this study.
References
- 1.Bittoun R. A combination nicotine replacement therapy (NRT) algorithm for hard-to-treat smokers. J Smok Cessat. 2006;1:3–6. [Google Scholar]
- 2.Hughes J. An algorithm for choosing among smoking cessation treatments. J Subst Abuse Treat. 2008;34:426–432. doi: 10.1016/j.jsat.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiore MC. Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guideline. Respir Care. 2000;45:1196–1199. [PubMed] [Google Scholar]
- 4.Boshier A, Wilton LV, Shakir SA. Evaluation of the safety of bupropion (Zyban) for smoking cessation from experience gained in general practice use in England in 2000. Eur J Clin Pharmacol. 2003;59:767–773. doi: 10.1007/s00228-003-0693-0. [DOI] [PubMed] [Google Scholar]
- 5.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 7.Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion for smoking cessation: a randomized trial. Arch Intern Med. 2004;164:1797–1803. doi: 10.1001/archinte.164.16.1797. [DOI] [PubMed] [Google Scholar]
- 8.Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S. Suicidal behavior and depression in smoking cessation treatments. PLoS ONE. 2011;6:e27016. doi: 10.1371/journal.pone.0027016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- 10.O'Malley SS. Varenicline and the evaluation of neuropsychiatric adverse events in smokers. Biol Psychiatry. 2011;69:1017–1018. doi: 10.1016/j.biopsych.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359–1366. doi: 10.1503/cmaj.110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- 13.Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine Tob Res. 2009;11:1067–1075. doi: 10.1093/ntr/ntp103. [DOI] [PubMed] [Google Scholar]
- 14.Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: interactions between nicotine dose, dependence, and quit-success genotype score. Mol Med. 2010;16:247–253. doi: 10.2119/molmed.2009.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DR. High-dose nicotine patch therapy: percentage of replacement and smoking cessation. JAMA. 1995;274:1353–1358. [PubMed] [Google Scholar]
- 16.Jorenby DE, Smith SS, Fiore MC, Hurt RD, Offord KP, Croghan IT, Hays JT, Lewis SF, Baker TB. Varying nicotine patch dose and type of smoking cessation counseling. JAMA. 1995;274:1347–1352. [PubMed] [Google Scholar]
- 17.Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation: who will quit with and without the nicotine patch. JAMA. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- 18.Westman EC, Behm FM, Simel DL, Rose JE. Smoking behavior on the first day of a quit attempt predicts long-term abstinence. Arch Intern Med. 1997;157:335–340. [PubMed] [Google Scholar]
- 19.Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, Kardinal CG, Knost JA, Tirona MT, Addo F, Morton RF, Michalak JC, Schaefer PL, Porter PA, Stella PJ. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. J Clin Oncol. 2003;21:914–920. doi: 10.1200/JCO.2003.08.160. [DOI] [PubMed] [Google Scholar]
- 20.Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, David SP, Niaura R, Lerman C. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65:683–693. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


