Abstract
Context
Stillbirth affects 1 in 160 pregnancies in the United States, equal to the number of infant deaths each year. Rates are higher than those of other developed countries and have stagnated over the past decade. There is significant racial disparity in the rate of stillbirth that is unexplained.
Objective
To ascertain the causes of stillbirth in a population that is diverse by race/ethnicity and geography.
Design, Setting, and Participants
A population-based study from March 2006 to September 2008 with surveillance for all stillbirths at 20 weeks or later in 59 tertiary care and community hospitals in 5 catchment areas defined by state and county boundaries to ensure access to at least 90% of all deliveries. Termination of a live fetus was excluded. Standardized evaluations were performed at delivery.
Main Outcome Measures
Medical history, fetal postmortem and placental pathology, karyotype, other laboratory tests, systematic assignment of causes of death.
Results
Of 663 women with stillbirth enrolled, 500 women consented to complete postmortem examinations of 512 neonates. A probable cause of death was found in 312 stillbirths (60.9%; 95% CI, 56.5%–65.2%) and possible or probable cause in 390 (76.2%; 95% CI, 72.2%–79.8%). The most common causes were obstetric conditions (150 [29.3%; 95% CI, 25.4%–33.5%]), placental abnormalities (121 [23.6%; 95% CI, 20.1%–27.6%]), fetal genetic/structural abnormalities (70 [13.7%; 95% CI, 10.9%–17.0%]), infection (66 [12.9%; 95% CI, 10.2%–16.2%]), umbilical cord abnormalities (53 [10.4%; 95% CI, 7.9%–13.4%]), hypertensive disorders (47 [9.2%; 95% CI, 6.9%–12.1%]), and other maternal medical conditions (40 [7.8%; 95% CI, 5.7%–10.6%]). A higher proportion of stillbirths in non-Hispanic black women compared with non-Hispanic white and Hispanic ones was associated with obstetric complications (43.5% [50] vs 23.7% [85]; difference, 19.8%; 95% CI, 9.7%–29.9%; P<.001) and infections (25.2% [29] vs 7.8% [28]; difference, 17.4%; 95% CI, 9.0%–25.8%; P<.001). Stillbirths occurring intrapartum and early in gestation were more common in non-Hispanic black women. Sources most likely to provide positive information regarding cause of death were placental histology (268 [52.3%; 95% CI, 47.9%–56.7%]), perinatal postmortem examination (161 [31.4%; 95% CI, 27.5%–35.7%]), and karyotype (32 of 357 with definitive results [9%; 95% CI, 6.3%–12.5%]).
Conclusions
A systematic evaluation led to a probable or possible cause in the majority of stillbirths. Obstetric conditions and placental abnormalities were the most common causes of stillbirth, although the distribution differed by race/ethnicity.
Stillbirth, Defined As Fetal death at 20 weeks’ gestation or later, is one of the most common adverse pregnancy outcomes in the United States and affects approximately 1 in 160 pregnancies.1 These approximately 26 000 stillbirths per year are equivalent to the number of infant deaths.2 The stillbirth rate in the United States is higher than that of many other developed countries.3–5 From 1990–2003, the stillbirth rate declined slowly but steadily, by an average of 1.4% per year. In contrast, the infant mortality rate declined twice as fast by an average of 2.8% per year.1 Since 2003 the stillbirth rate in the United States has remained stagnant at 6.2 stillbirths per 1000 births,1 59% higher than the Healthy People 2010 target goal of 4.1 fetal deaths per 1000 births.6
US stillbirth prevalence shows significant racial disparity. The stillbirth rate for non-Hispanic black women is 2.3-fold higher than that of non-Hispanic white women (11.13 compared with 4.79 fetal deaths per 1000 live births and fetal deaths).1 The rate for Hispanic women is 14% higher than for non-Hispanic white women (5.44 per 1000 live births and fetal deaths). Much of the racial disparity in stillbirth remains unexplained.7–11
The Stillbirth Collaborative Research Network (SCRN) was initiated by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to address this major public health issue. A workshop of experts convened by NICHD in 2001 concluded that vital records were inadequate to address the scope and causes of stillbirth.12 Therefore, one of the main objectives of SCRN was to ascertain the causes of stillbirth in a racially and geographically diverse population in the United States. To address this and other objectives, SCRN designed and conducted a multicenter population-based case-control study of stillbirths and live births enrolled at delivery. This article reports the causes of death among the stillbirths according to gestational age at delivery and race/ethnicity.
METHODS
Between March 2006 and September 2008, SCRN conducted a prospective population-based, case-control study of stillbirth, with enrollment of stillbirths and live births at the time of delivery. The study design and methods have been described in detail.13 This study only includes the cohort of stillbirths.
SCRN catchment areas were defined by state and county boundaries and included portions of 5 states: Rhode Island, Massachusetts, Georgia, Texas, and Utah. The study was conducted through 59 tertiary care and community hospitals that covered at least 90% of the stillbirth and live birth deliveries to residents in the catchment areas. Together, these hospitals had more than 80 000 deliveries per year.13 Women eligible to participate were residents of an SCRN catchment area who delivered at one of the study hospitals. A stillborn fetus was defined by Apgar scores of 0 at 1 and 5 minutes and no signs of life by direct observation. Deliveries resulting from the termination of a live fetus were excluded.
Gestational age was determined by the best clinical estimate using multiple sources including assisted reproductive technology with documentation of the day of ovulation or embryo transfer, first day of the last menstrual period, and obstetric sonograms.14 Although stillbirth was defined as death at 20 weeks’ gestation or later, fetal deaths between 18 weeks (plus 0 days) through 19 weeks (plus 6 days) gestation and without good dating criteria also were included to avoid missing additional fetal deaths that may have been greater than 20 weeks’ gestation.13
This study was approved by the institutional review boards of each clinical site, the 59 participating hospitals, and the data coordinating center. An advisory board reviewed the progress and safety of the study. All participants provided written informed consent. The institutional review board approved tracking of limited deidentified demographic data from women who declined participation.
Study components included a comprehensive standardized fetal postmortem examination and uniform placental pathology evaluation performed by a perinatal pathologist.15,16 A standardized maternal interview during the delivery hospitalization and detailed chart abstractions of prenatal office visits, antepartum hospitalizations, and the delivery hospitalization were performed. Maternal race/ethnicity was assessed to address racial disparity in stillbirth. Race/ethnicity was self-reported in response to options provided by the investigators. Collected biospecimens included maternal blood for serum and DNA, fetal blood from the umbilical cord (when available), placental tissue, and fetal tissue.
For stillbirths, a set of laboratory studies was recommended to clinicians practicing in all of the participating hospitals. These tests are part of the clinically recommended evaluation for stillbirth. 17 Perinatal postmortem examination, placental histopathology, fetal karyotype, testing for fetal-maternal hemorrhage, antibody screen, serologic test for syphilis, parvovirus serology, glycated hemoglobin, anticardiolipin antibodies, and toxicology screen were included. Studies were intended to screen for conditions known to be associated with stillbirth such as infections, chromosomal and fetal structural abnormalities, maternal-fetal hemorrhage, and maternal disease.
When possible, research samples were used to perform clinically indicated tests that were not obtained at the time of delivery. These included antibody screen, serologic test for syphilis, parvovirus serology, fructosamine (as a marker for hyperglycemia), and anticardiolipin antibodies.
SCRN investigators developed the initial causes of fetal death (INCODE) research tool to systematically assign causes of death using a priori definitions based on the best available evidence. 18 A condition was considered to be a probable cause of stillbirth if it had a high likelihood of directly causing the fetal death; if a condition was not a direct cause of the stillbirth, but possibly involved in a pathophysiologic sequence that led to the fetal death, it was considered a possible cause of death; and potentially important conditions that were present but did not meet criteria for probable or possible causes of death were recorded as present. Thus, INCODE acknowledges the uncertainty as to a specific cause of stillbirth from many potential causes. As an example, diabetes was considered a probable cause if the fetus had diabetic embryopathy with lethal anomalies or the mother had diabetic ketoacidosis; a possible cause if the mother had poor glycemic control documented and the fetus had abnormal growth; and condition present if the mother had good control or the fetus had no other abnormalities.18 In cases in which criteria were met for more than 1 cause of death, all were recorded without choosing a single cause as primary cause of death. The tool has content validity because it originated from a review of the published research to date and the agreement of the experts who comprised the network. INCODE is intended for use in cases of stillbirth with extensive evaluation including postmortem examination and placental histology. It also is intended as an evolving tool with plans to modify it as data from our study are analyzed.
Each case of stillbirth was reviewed centrally and in detail by 2 physicians (maternal-fetal medicine or neonatology specialties). Difficult cases were evaluated and adjudicated by a multidisciplinary panel with expertise in genetics and perinatal pathology.
Causes of death were grouped into broad categories for purposes of analysis: placental conditions; obstetric complications such as cervical insufficiency, placental abruption, preterm labor, and preterm premature rupture of membranes; fetal major structural malformations and/or genetic abnormalities; infections involving the fetus, placenta, or severe maternal systemic infection; maternal medical conditions including diabetes and antiphospholipid syndrome; hypertensive disorders (chronic hypertension and preeclampsia); umbilical cord abnormalities such as prolapse, strictures, and thrombosis; and other conditions such as hydrops and early amnion rupture sequence.
Statistical Analysis
Descriptive statistics were used to characterize the stillbirths on a range of demographic features and obstetrical and delivery services, with each stillbirth treated as an independent observation in a population consisting of the 5 catchment areas. Fisher exact and χ2 tests were used to assess associations. For P values less than .05 with multiple degrees of freedom, further consideration was given to 1 degree of freedom contrasts of interest. Cochran-Mantel-Haenszel methods (modified ridit scoring) were used to test for differences in trends and correlations. All P values were nominal and are provided for descriptive purposes. Point estimates and confidence intervals are given for contrasts of interest. Latent class analysis was used to identify cases that were similar to one another with respect to gestational age, timing of death (antepartum/intrapartum), and causes of death in amultivariable approach. Identified clusters may reflect an unmeasured (latent) grouping that might not otherwise be recognized. Models with 2 to 5 classes or clusters were estimated and the most appropriate model was selected based on model fit indices (Akaike, Bayesian, and sample-adjusted Bayesian information criterion measures) and the interpretability of the classes. The generalized estimating equations technique was used to compute robust variance estimates to account for dependencies due to multiple stillbirths within pregnancies. These estimates were used to confirm conclusions based on methods that treated the stillbirths as independent observations. Generalized estimating equations models were also used to evaluate associations with causes of stillbirth adjusted for differences by clinical site. SAS/STAT software version 9.2 of the SAS System for Windows was used for data analysis except for the latent class analysis, which was conducted using the MPLus software program.19
RESULTS
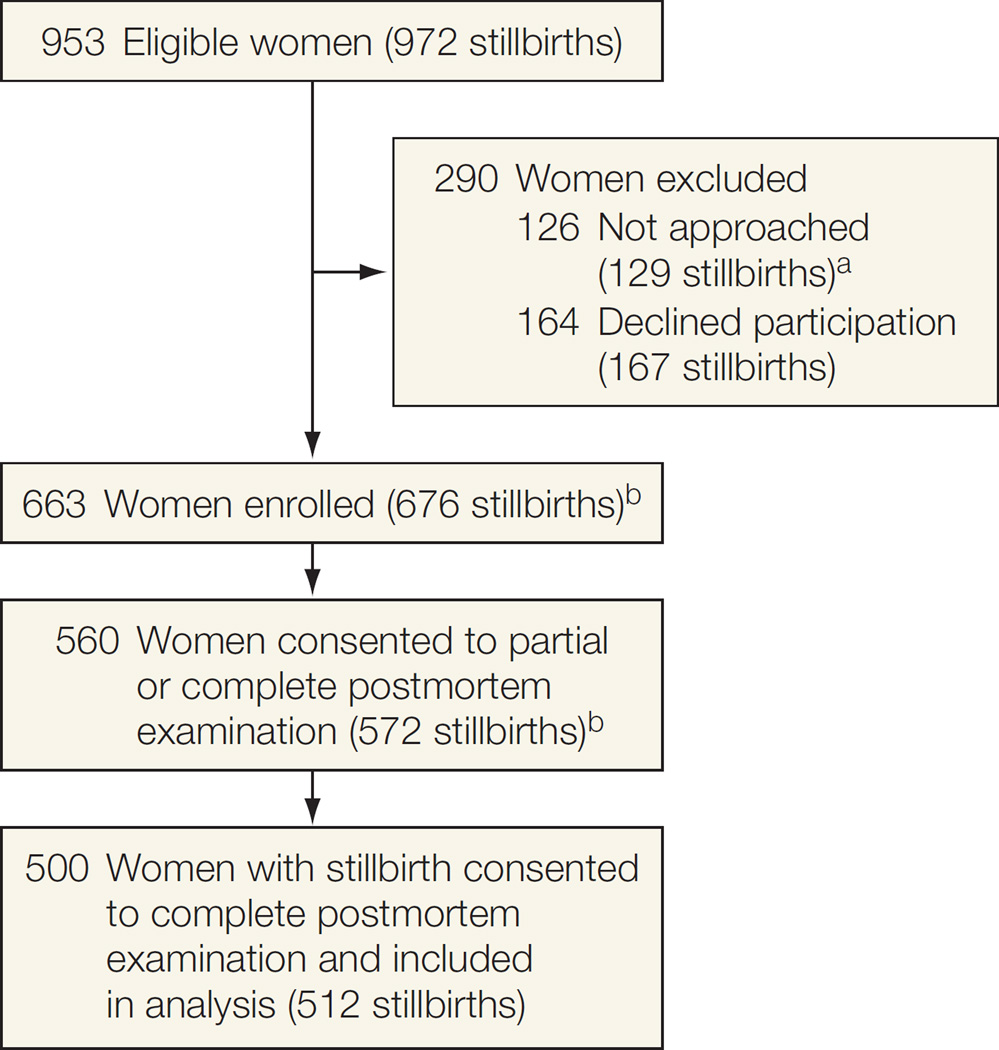
There were 953 eligible women with stillbirths (972 stillbirths) within the catchment areas over the surveillance period (Figure). Of these, 126 (13.2%) were not approached, either because they were not identified before discharge from the hospital or because the family or caregiver requested privacy. An additional 164 (17.2%) were approached but refused participation, leaving 663 (69.6%) women enrolled (676 stillbirths). Women who did not enroll in the study (n=290) did not differ from those enrolled according to maternal age, maternal race/ethnicity, insurance/method of payment, and gestational age at delivery (Table 1). Of the 663 women enrolled, 560 (84.0%) consented to a partial or complete postmortem examination. This report focuses on the 500 women (75.4%) who consented to their 512 stillborn neonates undergoing a complete postmortem examination. Of these 512 stillbirths, 425 (83.0%) occurred prior to the onset of labor and were considered antepartum stillbirths. Among stillbirth pregnancies, 465 were singleton, 34 were twin (22 with 1 stillbirth and 12 with 2 stillbirths), and 1 was triplet (with 1 stillbirth and 2 live births). Women with stillbirth who enrolled in the study and did or did not have a complete postmortem examination had similar age, race/ethnicity, marital status, insurance status, and income (Table 1). Those with a complete postmortem examination were slightly more likely to have received first- or second-trimester prenatal care (93.8% [469] vs 89.0% [145]; difference, 4.8%; 95% CI, 0.0%–10.1%; P=.04), and have a college education, (50.4% [238] vs 37.1% [52]; difference, 13.3%; 95% CI, 4.1%–22.5%; P=.01) than those declining complete postmortem examination.
Figure.
Study Enrollment
aNot approached either because they were not identified before discharge from the hospital or because the family or caregiver requested privacy.
bThe number of women (and stillbirths) enrolled are 70% of the eligible population. Of these, 84% of the women consented to postmortem examination of stillbirths (85% of the stillbirths).
c Sixty women consented to a partial postmortem examination and are not included in this analysis.
Table 1.
Comparison of Subgroups of Eligible Women With Stillbirtha
| Characteristic | SCRN Enrollment, No. (%) |
Complete Postmortem Examination, No. (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| No (n = 290) |
Yes (n = 663) |
P Value |
No (n = 163) |
Yes (n = 500) |
P Value |
|||
| Maternal age at delivery, y | ||||||||
| <20 | 40 (13.8) | 85 (12.8) | .64 | 26 (16.0) | 59 (11.8) | .30 | ||
| 20–34 | 196 (67.6) | 470 (70.9) | 106 (65.0) | 364 (72.8) | ||||
| 35–39 | 37 (12.8) | 80 (12.1) | 23 (14.1) | 57 (11.4) | ||||
| ≥40 | 17 (5.9) | 28 (4.2) | 8 (4.9) | 20 (4.0) | ||||
| Maternal race/ethnicity | ||||||||
| Non-Hispanic white | 95 (33.6) | 259 (39.2) | .39 | 54 (33.1) | 180 (36.1) | .45 | ||
| Non-Hispanic black | 79 (27.9) | 160 (24.2) | 30 (18.4) | 112 (22.4) | ||||
| Hispanic | 98 (34.6) | 218 (33.0) | 66 (40.5) | 171 (34.3) | ||||
| Other | 11 (3.9) | 23 (3.5) | 13 (8.0) | 36 (7.2) | ||||
| Marital status | ||||||||
| Not married or cohabitating | 31 (22.0) | 119 (25.1) | .29 | |||||
| Cohabitating | 44 (31.2) | 117 (24.7) | ||||||
| Married | 66 (46.8) | 238 (50.2) | ||||||
| Maternal education, y | ||||||||
| 0–11 (None, primary, some secondary) | 44 (31.4) | 101 (21.4) | .01 | |||||
| 12 (Completed secondary) | 44 (31.4) | 133 (28.2) | ||||||
| ≥13 (College) | 52 (37.1) | 238 (50.4) | ||||||
| Insurance/method of payment | ||||||||
| No insurance | 10 (4.0) | 52 (8.0) | .06 | 9 (5.6) | 31 (6.2) | .48 | ||
| Any public/private assistance | 137 (54.4) | 317 (48.5) | 92 (56.8) | 255 (51.3) | ||||
| VA/commercial health insurance/HMO | 105 (41.7) | 285 (43.6) | 61 (37.7) | 211 (42.5) | ||||
| Household income | ||||||||
| Public/private assistance only | 11 (7.9) | 40 (8.5) | .09 | |||||
| Public/private assistance and personal income | 63 (45.3) | 165 (35.2) | ||||||
| Personal income only | 65 (46.8) | 264 (56.3) | ||||||
| Prenatal care, first or second trimester | ||||||||
| Yes | 145 (89.0) | 469 (93.8) | .04 | |||||
| No | 18 (11.0) | 31 (6.2) | ||||||
| Gestational age, wk | ||||||||
| 18–19 | 9 (3.1) | 15 (2.3) | .15 | 5 (3.1) | 10 (2.0) | .26 | ||
| 20–23 | 105 (36.2) | 216 (32.6) | 62 (38.0) | 154 (30.8) | ||||
| 24–27 | 43 (14.8) | 108 (16.3) | 22 (13.5) | 86 (17.2) | ||||
| 28–31 | 25 (8.6) | 95 (14.3) | 26 (16.0) | 69 (13.8) | ||||
| 32–36 | 62 (21.4) | 119 (17.9) | 22 (13.5) | 97 (19.4) | ||||
| ≥37 | 46 (15.9) | 110 (16.6) | 26 (16.0) | 84 (16.8) | ||||
| Parity | ||||||||
| Nulliparous | 72 (44.7) | 228 (45.6) | .85 | |||||
| Multiparous | 89 (55.3) | 272 (54.4) | ||||||
| Plurality of index pregnancy | ||||||||
| Singleton | 155 (95.1) | 465 (93.0) | .35 | |||||
| Twins or triplets (only 1 triplet enrolled) | 8 (4.9) | 35 (7.0) | ||||||
| No. of stillbirths among multiples | ||||||||
| 1 | 7 | 23 | ||||||
| 2 | 1 | 12 | ||||||
Abbreviations: HMO, health maintenance organization; SCRN, Stillbirth Collaborative Research Network; VA, Veterans Administration.
Limited data were available at the time of screening on all 956 women with stillbirth who were eligible for the study. Empty table cells indicate data are not available.
Participants in this study comprised 180 (36.1%) non-Hispanic white, 171 (34.3%) Hispanic, 112 (22.4%) non-Hispanic black, and 36 (7.2%) women of other race/ethnicities. Their mean age was 27.4 years (range, 14–45 years), 238 (50.2%) were married, 469 (93.8%) had first or second trimester prenatal care, 211 (42.5%) had veterans’ benefits or private insurance, 255 (51.3%) received public assistance, and 31 (6.2%) were uninsured.
Almost one-third of stillbirths—160 (31.3%)—occurred between 20 and 24 weeks’ gestation and 259 (50.6%) occurred prior to 28 weeks’ gestation (Table 2). The gestational age of antepartum and intrapartum stillbirths differed significantly (P<.001) with 73 (83.9%) intrapartum stillbirths occurring at less than 24 weeks gestation vs antepartum stillbirths being relatively evenly distributed over all gestational ages.
Table 2.
Gestational Age at Stillbirth and Timing of Stillbirth in Relation to Labor and Race/Ethnicitya
| Race/Ethnicity, No. (%) |
Timing of Stillbirth, No. (%)b |
||||||
|---|---|---|---|---|---|---|---|
| Labor Characteristic |
Non-Hispanic White |
Non-Hispanic Black |
Hispanic | Other | Antepartum | Intrapartum | Total, No. (%) |
| Gestational age, wkb | |||||||
| 18–19 | 1 (0.5) | 8 (7.0) | 1 (0.6) | 0 | 3 (0.7) | 7 (8.0) | 10 (1.9) |
| 20–23 | 52 (28.4) | 48 (41.7) | 49 (27.8) | 11 (29.7) | 94 (22.1) | 66 (75.9) | 160 (31.3) |
| 24–27 | 38 (20.8) | 12 (10.4) | 31 (17.6) | 7 (18.9) | 84 (19.8) | 5 (5.7) | 89 (17.4) |
| 28–31 | 20 (10.9) | 19 (16.5) | 25 (14.2) | 8 (21.6) | 72 (16.9) | 0 | 72 (14.1) |
| 32–36 | 38 (20.8) | 14 (12.2) | 38 (21.6) | 7 (18.9) | 91 (21.4) | 6 (6.9) | 97 (19.0) |
| ≥37 | 34 (18.6) | 14 (12.2) | 32 (18.2) | 4 (10.8) | 81 (19.1) | 3 (3.4) | 84 (16.4) |
| Total No. | 183 | 115 | 176 | 37 | 425 | 87 | 512 |
| Antepartum | 166 (90.7) | 77 (67.0) | 150 (85.2) | 31 (83.8) | |||
| Intrapartum | 17 (9.3) | 38 (33.0) | 26 (14.8) | 6 (16.2) | |||
Race/ethnicity classification not available for 1 case.
Gestational age distribution varies by race/ethnicity (nominal P=.001) and intrapartum vs antepartum labor (nominal P<.001). Intrapartum/antepartum labor varies by race/ethnicity (nominal P<.001).
Non-Hispanic black women had a higher percentage of intrapartum stillbirths (33.0% [38] vs 9.3% [17] when compared with non-Hispanic white women; difference, 23.7%; 95% CI, 14.2%–33.3%; P<.001), and Hispanic women (14.8% [26]; difference, 18.2%; 95% CI, 8.2%–28.3%; P<.001). Stillbirths in non-Hispanic black women occurred earlier in gestation than those occurring in women of other race /ethnicities (P=.001 for differences in gestational age by race/ethnicity).
A probable cause of death was found in 312 of the stillbirths (60.9%; 95% CI, 56.5%–65.2%) and a possible or probable cause in 390 cases (76.2%; 95% CI, 72.2%–79.8%). More than 1 probable or possible cause of death was found in 161 stillbirths (31.4%; 95% CI, 27.5%–35.7%). The distribution of causes of death (probable and possible) within broad categories, stratified by relation to labor and gestational age, are shown in Table 3 and eTable 1 (available at http://www.jama.com). Obstetric complications were the most common category for cause of death (150 cases [29.3%; 95% CI, 25.4%–33.5%]), including abruption (38 cases [7.4%; 95% CI, 5.4%–10.1%]), complications of multiple gestation (31 cases [6.1%; 95% CI, 4.2%–8.6%]), and the constellation of preterm labor, preterm premature rupture of membranes, and cervical insufficiency, often in combination with chorioamnionitis (77 cases [15.0%; 95% CI, 12.1%–18.5%]). Placental abnormalities were implicated in 121 cases (23.6%; 95% CI, 20.1%–27.6%) including 24 with clinical evidence of uteroplacental insufficiency (4.7%; 95% CI, 3.1%–7.0%) and 39 with maternal vascular disorders (7.6%; 95% CI, 5.5%–10.4%). Other causes included fetal genetic/structural abnormalities in 70 cases (13.7%; 95% CI, 10.9%–17.0%), infection in 66 (12.9%; 95% CI, 10.2%–16.2%), umbilical cord abnormalities in 53 (10.4%; 95% CI, 7.9%–13.4%), hypertensive disorders in 47 (9.2%; 95% CI, 6.9%–12.1%), and maternal medical complications in 40 (7.8%; 95% CI, 5.7%–10.6%).
Table 3.
Probable and Possible Causes of Death by Timing of Stillbirth in Relation to Labor and Gestational Agea
| No. (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Timing of Stillbirthc |
Gestational Age, wkb |
||||||||
| Cause of Death | Total | Antepartum | Intrapartum | 18–19 | 20–23 | 24–27 | 28–31 | 32–36 | ≥37 |
| Obstetric complications | 150 (29.3) | 63 (14.8) | 87 (100.0) | 7 (70.0) | 82 (51.3) | 17 (19.1) | 15 (20.8) | 17 (17.5) | 12 (14.3) |
| Placental disease | 121 (23.6) | 111 (26.1) | 10 (11.5) | 4 (40.0) | 20 (12.5) | 32 (36.0) | 18 (25.0) | 26 (26.8) | 21 (25.0) |
| Fetal genetic/structural | 70 (13.7) | 66 (15.5) | 4 (4.6) | 0 | 22 (13.8) | 10 (11.2) | 10 (13.9) | 17 (17.5) | 11 (13.1) |
| Infection | 66 (12.9) | 43 (10.1) | 23 (26.4) | 2 (20.0) | 35 (21.9) | 7 (7.9) | 4 (5.6) | 8 (8.2) | 10 (11.9) |
| Umbilical cord abnormalities | 53 (10.4) | 46 (10.8) | 7 (8.0) | 1 (10.0) | 13 (8.1) | 10 (11.2) | 4 (5.6) | 13 (13.4) | 12 (14.3) |
| Hypertensive disorders | 47 (9.2) | 40 (9.4) | 7 (8.0) | 1 (10.0) | 6 (3.8) | 14 (15.7) | 12 (16.7) | 10 (10.3) | 4 (4.8) |
| Maternal medical complications | 40 (7.8) | 37 (8.7) | 3 (3.4) | 0 | 11 (6.9) | 9 (10.1) | 3 (4.2) | 8 (8.2) | 9 (10.7) |
| Other | 16 (3.1) | 16 (3.8) | 0 | 0 | 4 (2.5) | 3 (3.4) | 4 (5.6) | 3 (3.1) | 2 (2.4) |
| Any cause | 390 (76.2) | 303 (71.3) | 87 (100.0) | 9 (90) | 136 (85.0) | 62 (69.7) | 52 (72.2) | 71 (73.2) | 60 (71.4) |
| Total No. | 512 | 425 | 87 | 10 | 160 | 89 | 72 | 97 | 84 |
Some stillbirths had more than 1 probable or possible cause.
Different proportions by gestational age are noted for placental disease (nominal P=.001), infection (nominal P=.002), hypertensive disorder (nominal P=.004), and obstetric complications (nominal P<.001).
A higher proportion of causes of antepartum stillbirths were placental disease (nominal P=.003) and fetal abnormalities (nominal P=.007) and a lower proportion were infection (nominal P<.001) and obstetric complications (nominal P<.001) compared with intrapartum stillbirths.
The distributions of causes of death differed between antepartum and intrapartum stillbirths. All intrapartum stillbirths were classified as obstetric complications. A higher percentage of intrapartum stillbirths had infectious causes (26.4% [23] vs 10.1% [43] compared with antepartum stillbirths; difference, 16.3%; 95% CI, 6.6%–26.0%; P<.001). Antepartum stillbirths, when compared with intrapartum stillbirths, had a higher proportion of placental causes (26.1% [111] vs 11.5% [10]; difference, 14.6%; 95% CI, 6.7%–22.5%; P = .003) and fetal genetic/structural abnormalities (15.5% [66] vs 4.6% [4]; difference, 10.9%; 95% CI, 5.3%–16.5%; P=.007).
Placental disorders were associated with a higher proportion of stillbirths after 24 weeks’ gestation (28.4% [97] vs 14.1% [24]; difference, 14.3%; 95% CI, 7.2%–21.3%; P<.001). By contrast, stillbirths at less than 24 weeks’ gestation had a much higher proportion of obstetric complications (52.4% [89] vs 17.8% [61]; difference, 34.6%; 95% CI, 26.0%–43.1%; P<.001) and infections (21.8% [37] vs 8.5% [29]; difference, 13.3%; 95% CI, 6.4%–20.2%; P<.001).
Table 4 shows the probable and possible causes of death stratified by race/ethnicity. Non-Hispanic black women experienced a higher proportion of stillbirths associated with obstetric complications compared with non-Hispanic white women and Hispanic women combined (43.5% [50] vs 23.7% [85]; difference, 19.8%; 95% CI, 9.7%–29.9%; P<.001), and infections (25.2% [29] vs 7.8% [28]; difference, 17.4%; 95% CI, 9.0%–25.8%; P<.001). Conversely, cord abnormalities were associated with a higher proportion of stillbirths in non-Hispanic white and Hispanic women compared with non-Hispanic black and other women (12.8% [46] vs 4.6% [7]; difference, 8.2%; 95% CI, 3.4%–13.0%; P=.005). Categories of probable and possible causes of death did not differ by race/ethnicity in the subset of stillbirths that were antepartum or the subset that occurred after 24 weeks’ gestation.
Table 4.
Probable and Possible Causes of Death by Race/Ethnicitya
| No. (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cause of Death | Non-Hispanic White |
Non-Hispanic Black |
Hispanic | Other | Nominal P Value |
||||
| Obstetric complications | 41 (22.4) | 50 (43.5) | 44 (25.0) | 15 (40.5) | <.001 | ||||
| Placental disease | 40 (21.9) | 22 (19.1) | 47 (26.7) | 11 (29.7) | .35 | ||||
| Fetal genetic/structural | 25 (13.7) | 9 (7.8) | 31 (17.6) | 5 (13.5) | .13 | ||||
| Infection | 13 (7.1) | 29 (25.2) | 15 (8.5) | 8 (21.6) | <.001 | ||||
| Umbilical cord abnormalities | 23 (12.6) | 5 (4.3) | 23 (13.1) | 2 (5.4) | .05 | ||||
| Hypertensive disorders | 16 (8.7) | 14 (12.2) | 13 (7.4) | 4 (10.8) | .56 | ||||
| Maternal medical complications | 15 (8.2) | 8 (7.0) | 12 (6.8) | 5 (13.5) | .56 | ||||
| Other | 3 (1.6) | 5 (4.3) | 7 (4.0) | 1 (2.7) | .51 | ||||
| Any cause | 131 (71.6) | 94 (81.7) | 135 (76.7) | 29 (78.4) | .24 | ||||
| Total No. | 183 | 115 | 176 | 37 | |||||
Some stillbirths had more than 1 probable or possible cause. Race/ethnicity classification not available for 1 case.
After using generalized estimating equations to account for dependencies between twin stillbirths and adjusting for clinical site, differences in the causes of stillbirth remained significant (eTable 2). Cluster analysis was conducted to look for natural groupings of the cases according to gestational age, timing in relation to labor, and causes of death (Table 5). The 4-class solution was selected based on model fit indices and interpretability. A 2-class solution separated early from late gestational age at death; whereas a 3-class solution distinguished early, mid, and late gestation. The 4-class solution further split early gestations into 2 clusters. Class 1 contained 76 of the cases (14.8%; 95% CI, 11.9%–18.3%) including all intrapartum cases and those with gestational ages of less than 28 weeks. The most common causes of death were obstetric complications (100%) and infection (20 [26.3%]; 95% CI, 17.2%–37.9%) in class 1. Thirty-six of the stillbirths in class 1 were born to non-Hispanic black women (47.4%; 95% CI, 35.9%–59.1%). Class 2 contained 138 of the stillbirths (27.0%; 95% CI, 23.2%–31.1%), all of which occurred at less than 28 weeks’ gestation and all but 1 were antepartum. Ninety-seven occurred at less than 24 weeks’ gestation (70.3%; 95% CI, 61.8%–77.6%). The most common causes of death were placental disease (32 [23.2%; 95% CI, 16.6%–31.3%]), fetal genetic/structural abnormalities (24 [17.4%; 95% CI, 11.7%–25.0%]), and obstetric complications (24 [17.4%; 95% CI, 11.7%–25.0%]). Class 3 included 126 of the stillbirths (24.6%; 95% CI, 21.0%–28.6%) and ranged between 24 and 36 weeks’ gestation with 72 (57.1%; 95% CI, 48.0%–65.8%) occurring at 28 to 31 weeks. Placental disease (43 [34.1%; 95% CI, 26.1%–43.2%]) and hypertensive disorders (27 [21.4%; 95% CI, 14.8%–29.8%]) were more common in class 3. Class 4 stillbirths (n=172) occurred later in gestation (≥32 weeks) and were 33.6% (95% CI, 29.5%–37.9%) of stillbirths. There were more cord abnormalities in class 4 (25 [14.5%; 95% CI, 9.8%–20.9%]) than the other classes. Classes 2, 3, and 4 had similar race/ethnicity distributions.
Table 5.
Stillbirth Characteristics by Latent Classes Constructed From Gestational Age at Stillbirth, Timing of Stillbirth in Relation to Labor, and Probable and Possible Causes of Deatha
| Latent 4-Class Solution, No. (%)b |
||||
|---|---|---|---|---|
| Characteristic | Class 1 (n = 76) |
Class 2 (n = 138) |
Class 3 (n = 126) |
Class 4 (n = 172) |
| Clustering Characteristics | ||||
| Gestational age, wk | ||||
| 18–19 | 7 (9.2) | 3 (2.2) | 0 | 0 |
| 20–23 | 66 (86.8) | 94(68.1) | 0 | 0 |
| 24–27 | 3 (3.9) | 41 (29.7) | 45 (35.7) | 0 |
| 28–31 | 0 | 0 | 72 (57.1) | 0 |
| 32–36 | 0 | 0 | 9(7.1) | 88(51.2) |
| >37 | 0 | 0 | 0 | 84 (48.8) |
| Intrapartum | 76(100.0) | 1 (0.7) | 1 (0.8) | 9 (5.2) |
| Cause of death | ||||
| Obstetric complications | 76(100.0) | 24(17.4) | 23(18.3) | 27(15.7) |
| Placental disease | 5 (6.6) | 32 (23.2) | 43(34.1) | 41 (23.8) |
| Fetal genetic/structural | 2 (2.6) | 24(17.4) | 18(14.3) | 26(15.1) |
| Infection | 20 (26.3) | 21 (15.2) | 7 (5.6) | 18(10.5) |
| Umbilical cord abnormalities | 7 (9.2) | 12(8.7) | 9(7.1) | 25(14.5) |
| Hypertensive disorders | 3 (3.9) | 5 (3.6) | 27(21.4) | 12(7.0) |
| Medical complications | 2 (2.6) | 13(9.4) | 9(7.1) | 16(9.3) |
| Association Characteristics | ||||
| Race/ethnicity (P < .001)c | ||||
| Non-Hispanic white | 14(18.4) | 57(41.6) | 43(34.1) | 69(40.1) |
| Non-Hispanic black | 36 (47.4) | 28 (20.4) | 23(18.3) | 28(16.3) |
| Hispanic | 23 (30.3) | 42 (30.7) | 46 (36.5) | 65 (37.8) |
| Other | 3 (3.9) | 10(7.3) | 14(11.1) | 10(5.8) |
Some stillbirths had more than 1 probable or possible cause.
The 4-class solution was selected based on model fit indices (Akaike information criteria, Bayesian information criteria, and sample size-adjusted Akaike information criteria) and interpretability. The 2-class solution separated early from late gestational age at death; whereas the 3-class solution distinguished early, mid, and late. The 4-class solution further separated early by intrapartum/antepartum.
Race/ethnicity classification not available for 1 case.
The proportions of positive results for clinically indicated tests are shown in Table 6. Placental histology had the highest proportion of positive results (52.3% [268]; 95% CI, 47.9%–56.7%), defined as abnormalities contributing to a probable or possible cause of death. Perinatal postmortem examination had positive findings in 161 cases (31.4%; 95% CI, 27.5%–35.7%) and karyotype was abnormal in 32 of the 357 successful studies (9.0%; 95% CI, 6.3%–12.5%). Three hundred forty cases (66.4%; 95% CI, 62.1%–70.5%) had a positive result for at least 1 of these 3 tests. The remaining clinically indicated tests were positive in only 0.4% to 4.8% of stillbirths.
Table 6.
Results of Clinically Indicated Tests for Stillbirth Workup
| No. (%) |
|||
|---|---|---|---|
| Test | Tested Positivea | Positive Result Explanation | |
| Maternal (N = 500) | |||
| Antibody screen | 498 (99.6) | 18 (3.6) | Detection of antibodies: D, Kell, E, e, Cw, C, Ce, Kpa, Kpb, cE, k, Jk, s, Wra, Fya, M |
| Syphilis | 495 (99.0) | 2 (0.4) | Rapid plasma reagin reactive and fluorescent treponemal antibody positive |
| Parvovirus | 451 (90.2) | 9 (2.0) | IgM positive |
| Lupus anticoagulant | 190(38.0) | 6 (3.2) | Lupus anticoagulant present |
| Anticardiolipin antibodies | 458 (91.6) | 22 (4.8) | IgG ≥2000 mg/dL |
| Blood glucose screen | 455 (91.0) | 13 (2.9) | Hemoglobin A1C ≥6.5% of total hemoglobin and/or fructosamine ≥53 mg/L |
| Toxicology screen | 342 (68.4) | 12(3.5) | Detection of marijuana, cocaine, amphetamines and/or methamphetamine in the umbilical cord |
| Fetal-maternal hemorrhage | 218(43.6) | 10 (4.6) | Fetal blood detected (range, 3–165 mL in 5 of 10 cases with amount reported) |
| Fetal (N = 512) | |||
| Placental histology | 512(100.0) | 268 (52.3) | Possible or probable cause using INCODE instrument on placental histology |
| Autopsy | 512(100.0) | 161 (31.4) | Possible or probable cause using INCODE instrument on postmortem examination |
| Karyotype | 494 (96.5) | 32 (9.0) | Aneuploidy, unbalanced translocation, or other major abnormality |
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; INCODE, initial causes of fetal death. SI conversion factor: to convert fructosamine to µmol/L, multiply by 5.581.
Percent is calculated among those who were tested. For karyotype, percent is among 357 with a definitive result.
COMMENT
In this large US population-based cohort of stillbirths, systematic and thorough evaluation led to the ascertainment of a probable or possible cause of death in the vast majority of cases. Using INCODE, a rigorous classification tool developed from published evidence, 18 a probable cause of death was found in 61% of cases and a possible or probable cause was found in more than 76% of cases. These causes were differentially distributed across gestation and racial/ethnic groups, which has implications for monitoring and prevention.
The lack of information on causes of stillbirth has made it difficult to provide answers to families as well as design strategies for prevention. In the United States, evaluations for causes of stillbirth are often incomplete,20,21 eg, the rate of perinatal postmortem examination is estimated at less than 50% in all but a few dedicated centers.22 Reasons for failure to perform fetal autopsy include clinicians’ lack of knowledge, physician and patient discomfort with death and discussion of postmortem examination, concerns about cost, and limited availability of services.
Our data support performing perinatal postmortem examination, placental histology, and karyotype in all cases of stillbirth because the majority of stillbirths (66%) had at least 1 positive result out of these 3 components of the evaluation. Although other diagnostic tests have a lower yield, their utility should be considered in specific clinical scenarios according to cost and availability.
Placental disease was the leading cause of antepartum stillbirths (26%). This proportion was similar to that observed in a cohort of stillbirths in Sweden (23%).23 However, in a Dutch cohort of 750 antepartum stillbirths, 65% were attributed to placental abnormalities. 24 Placental disease has been recently recognized as an important contributor in antepartum stillbirths and those that would have been considered unexplained; however, the proportion of cases attributed to placental anomalies varies depending on the criteria used. Without clinical evidence of placental insufficiency (eg, fetal growth impairment, oligohydramnios, preeclampsia), it is difficult to determine whether specific placental abnormalities are associated with stillbirth since similar abnormalities are sometimes present in the placentas of normal pregnancies.
The proportion of SCRN cases attributed to infectious causes was similar to other recent studies in which 14% to 19% of stillbirths were because of infection. 23–26 The proportion of stillbirths from chromosomal abnormalities also was similar to other studies.23,27 A higher proportion of cases in our study were associated with obstetric abnormalities than previously reported in other studies. In part, this observation is likely due to the inclusion of intrapartum cases as well as the racial/ethnic diversity in our cohort. In addition, obstetric conditions have not been systematically evaluated in a large, population-based cohort in the United States.
Umbilical cord abnormalities accounted for 10% of our possible or probable causes of death, which is considerably higher than in previous studies,23,24 and were more common in stillbirths of greater than 32 weeks’ gestation. Nuchal cords are noted in almost one-fourth of uncomplicated pregnancies.28 Our criteria for considering a cord abnormality to be a cause of death were rigorous and included vasa previa, cord entrapment, and evidence of occlusion and fetal hypoxia, prolapse, or stricture with thrombi.18,29 Nuchal cord alone was not considered a cause of death. This important cause of stillbirth has been somewhat overlooked in prior studies because of the difficulty in differentiating between harmless nuchal cords and cord conditions associated with pathophysiology leading to stillbirth. As a potentially preventable cause of stillbirth, cord abnormalities deserve further investigation.
The consistent and persistent racial disparity in stillbirth (2.3-fold risk for non-Hispanic black compared with non-Hispanic white women in the United States in 2005)1 remains largely unexplained.7–11 This disparity is often attributed to poor access to prenatal care.7 However, racial disparity for stillbirth persists, even in women with prenatal care.9 This is the first US study with large, diverse, well-defined catchment areas describing the causes of stillbirth by race/ethnicity. Our findings strongly suggest that a majority of the excess rate of stillbirth in non-Hispanic black women is due to obstetric complications, infection, or both causes combined with stillbirth often occurring intrapartum and at less than 24 weeks’ gestation. The pathophysiology of these conditions is similar if not identical to the pathophysiology of spontaneous preterm birth, a condition with well-documented racial disparity. Non-Hispanic black women had a rate of spontaneous preterm birth of 18.3% compared with 11.5% for non-Hispanic white women in the United States in 2007.30 When conditions such as preterm labor, cervical insufficiency, preterm premature rupture of membranes, chorioamnionitis, and abruption lead to labor at a previable or periviable gestation, antepartum or intrapartum death is usually allowed to occur without obstetric intervention. If the same condition occurs at a viable gestation (eg, after 24 weeks’ gestation), cesarean delivery may lead to preterm birth rather than stillbirth. This observation allows us to target strategies intended to reduce the racial disparity in stillbirths. For example, measures that successfully reduce the rate of spontaneous preterm birth in non-Hispanic black women (such as treatment with progestational agents) could potentially reduce the rate of stillbirth as well.
The latent class analysis provides a new perspective to the heterogeneous nature of stillbirths. There are 4 distinct categories of stillbirths based on gestational age, race/ethnicity, and causes of death. This multivariable statistical technique has allowed us to recognize that there are 2 classes of early stillbirths (<28 weeks’ gestation) with different etiologies and racial composition. Class 1 includes intrapartum deaths, which are more common among non-Hispanic black women, and class 2, which includes antepartum deaths, which are more diverse in origin with only 17% attributed to obstetrical causes and almost one-fourth associated with placental disease. Fifty-seven percent of stillbirths with hypertensive disease designated as a cause were in class 3, which occurred primarily between weeks 24 and 31, while almost half of the deaths due to cord abnormalities occurred at later gestations (class 4). Placental disease was evenly distributed across gestation for antepartum stillbirths, possibly reflecting multiple mechanisms leading to stillbirth. This knowledge of the timing and duration of these conditions in relation to stillbirth is helpful in the development of new preventive strategies.
Our study had several limitations. A potential source of bias was that 30% of women experiencing stillbirth in our catchments were not enrolled. The study was conducted in 59 hospitals and many patients with stillbirth were hospitalized for only a short duration, often less than 24 hours. Despite intensive surveillance, on occasion study personnel were not notified of cases. Also, because stillbirth is an emotional event, some families were distraught and did not wish to participate in a research study at that time. Additionally, the caregiver could decide that the family should not be approached due to the circumstances. Importantly for our study, demographic characteristics were similar among women who did and did not enroll. The cases in this report were confined to the subset that underwent postmortem examination, another possible source of bias. Women who consented to autopsy were slightly more likely to have received early prenatal care and a larger percentage had a college education. Also, some of the stillbirths did not undergo some of the clinically indicated tests and documentation of conditions in prenatal and hospital records had differing levels of detail, which could have introduced bias in ascertaining cause. Some differences were noted in the identified causes of stillbirth by clinical site. Although these differences cannot be completely disentangled from patient characteristics in analysis, the differences in causes of stillbirth by race/ethnicity remained significant after adjustment for clinical site. Finally, the sample size was not large enough to ascertain rare causes of stillbirth.
There were numerous strengths of the study. Each patient had an extensive standardized evaluation for potential causes of stillbirth including postmortem examination, placental histology, karyotype, maternal interview, and abstraction of medical records. This allowed for a level of detail and accuracy that is not available from large databases, especially those using vital statistics. Indeed, information contained in fetal death certificates in the United States is often incomplete or inaccurate. 20 Our systematic approach to the evaluation of each case, which included a classification tool with rigorous criteria, an extensive review by 2 medical experts, and an adjudication process, was also a strength.
The study was population based and geographically, racially, and ethnically diverse, making the results more generalizable. The study was designed to have access to at least 90% of all deliveries in each catchment area, and almost all hospitals within each catchment area participated, including a large proportion of community hospitals. The proportion of non-Hispanic black women in our stillbirth cohort was similar to the proportion reported in US vital statistics (22.4% vs 25.4%) and the proportion Hispanic was greater (34.3% vs 20.8%). This enabled us to examine disparities by race/ethnicity in causes of death.1
The US stillbirth rate has remained unacceptably high, affecting 1 in 160 pregnancies each year. Reduction in the stillbirth rate will require thorough investigation into the cause of death. After a systematic and thorough evaluation, a cause of death was determined in the majority of cases of stillbirth in our study. Therefore, postmortem examination, placental histology, and karyotype are strongly recommended as part of the diagnostic evaluation. In addition, the development of interventions to prevent stillbirth should consider the observed differential distribution of causes of death as gestational age advances, as well as variation by race/ethnicity.
Acknowledgments
Funding/Support: This research was supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): U10-HD045953 Brown University; U10-HD045925 Emory University; U10-HD045952 University of Texas Medical Branch at Galveston; U10-HDO45955 University of Texas Health Sciences Center at San Antonio; U10-HD045944 University of Utah Health Sciences Center; and U01-HD045954 RTI International, RTP.
Role of the Sponsor: The Stillbirth Collaborative Research Network is solely responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Appendix
The Stillbirth Collaborative Research Network Writing Group: Radek Bukowski, MD, PhD, Department of Obstetrics and Gynecology, University of Texas Medical Branch at Galveston; Marshall Carpenter, MD, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Brown University School of Medicine, Providence, Rhode Island; Deborah Conway, MD, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Texas Health Science Center at San Antonio; Donald Coustan, MD, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Brown University School of Medicine; Donald J. Dudley, MD, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Texas Health Science Center at San Antonio; Robert L. Goldenberg, MD, Department of Obstetrics and Gynecology, Drexel University School of Medicine, Philadelphia, Pennsylvania; Carol J. Rowland Hogue, PhD, MPH, Department of Epidemiology, Rollins School of Public Health, and Women’s and Children’s Center, Emory University; Matthew A. Koch, MD, PhD, Statistics and Epidemiology Unit, Health Sciences Division, RTI International, Research Triangle Park, North Carolina; Corette B. Parker, DrPH, Statistics and Epidemiology Unit, Health Sciences Division, RTI International; Halit Pinar, MD, Division of Perinatal and Pediatric Pathology, Department of Pathology and Laboratory Medicine, Brown University School of Medicine; Uma M. Reddy, MD, MPH, Pregnancy and Perinatology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland; George R. Saade, MD, Department of Obstetrics and Gynecology, University of Texas Medical Branch at Galveston; Robert M. Silver, MD, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Utah School of Medicine, and Maternal Fetal Medicine at Intermountain Healthcare, Salt Lake City; Barbara J. Stoll, MD, Emory University School of Medicine and Department of Pediatrics, Children’s Healthcare Atlanta; Michael W. Varner, MD, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Utah School of Medicine, and Maternal Fetal Medicine at Intermountain Healthcare, Salt Lake City; and Marian Willinger, PhD, Pregnancy and Perinatology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Author Contributions: Dr Silver had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Silver, Bukowski, Carpenter, Conway, Dudley, Goldenberg, Hogue, Koch, Parker, Pinar, Reddy, Saade, Varner, Willinger.
Acquisition of data: Silver, Bukowski, Carpenter, Conway, Dudley, Goldenberg, Hogue, Koch, Parker, Pinar, Saade, Stoll, Varner.
Analysis and interpretation of data: Silver, Conway, Coustan, Dudley, Goldenberg, Hogue, Koch, Parker, Pinar, Reddy, Saade, Willinger.
Drafting of the manuscript: Silver, Coustan, Dudley, Hogue, Pinar, Reddy, Saade. Critical revision of the manuscript for important intellectual content: Bukowski, Carpenter, Conway, Coustan, Dudley, Goldenberg, Hogue, Koch, Parker, Pinar, Reddy, Saade, Stoll, Varner, Willinger.
Statistical analysis: Hogue, Koch, Parker, Reddy, Willinger.
Obtained funding: Silver, Bukowski, Carpenter, Dudley, Hogue, Koch, Parker, Pinar, Saade, Stoll, Varner.
Administrative, technical, or material support: Silver, Carpenter, Coustan, Dudley, Pinar, Reddy, Stoll, Varner, Willinger.
Study supervision: Carpenter, Coustan, Dudley, Goldenberg, Koch, Parker, Reddy, Saade, Varner, Willinger.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Stillbirth Collaborative Research Network: University of Texas Health Science Center at San Antonio: Donald J. Dudley, Deborah Conway, Karen Aufdemorte, Angela Rodriguez, Monica Pina; University of Utah School of Medicine: Robert M. Silver, Michael W. Varner, Kristi Nelson; Emory University School of Medicine and the Rollins School of Public Health: Carol J. Rowland Hogue, Barbara J. Stoll, Janice Daniels Tinsley, Bahig Shehata, Carlos Abramowsky; Brown University: Donald Coustan, Halit Pinar, Marshall Carpenter, Susan Kubaska; University of Texas Medical Branch at Galveston: George R. Saade, Radek Bukowski, Jennifer Lee Rollins, Hal Hawkins, Elena Sbrana; RTI International: Corette B. Parker, Matthew A. Koch, Vanessa R. Thorsten, Holly Franklin, Pinliang Chen; Pregnancy and Perinatalogy Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health: Marian Willinger, Uma M. Reddy; Drexel University School of Medicine: Robert L. Goldenberg.
Online-Only Material: eTable1, eTable 2, and the Author Video Interview are available at http://www.jama.com.
Additional Contributions: We acknowledge the members of the NICHD Scientific Advisory and Safety Monitoring Board, including Rev Phillip Cato, PhD; James W. Collins Jr, MD, MPH; Terry Dwyer, MD, MPH; William P. Fifer, PhD; John Ilekis, PhD; Marc Incerpi, MD; George Macones, MD, MSCE; Richard M. Pauli, MD, PhD; Raymond W. Redline, MD; Elizabeth Thom, PhD (chair); as well as all of the other physicians, study coordinators, research nurses, and patients who participated in the Stillbirth Collaborative Research Network. We also acknowledge Elizabeth Gates, MBA, University of Utah Health Sciences Center, for her editorial assistance. No compensation was received by any of these individuals in association with their contributions to this article.
References
- 1.MacDorman MF, Kirmeyer S. Fetal and perinatal mortality, United States, 2005. Natl Vital Stat Rep. 2009;57(8):1–19. [PubMed] [Google Scholar]
- 2.Macdorman MF, Mathews TJ. Recent trends in infant mortality in the United States. NCHS Data Brief. 2008;9(9):1–8. [PubMed] [Google Scholar]
- 3.Cousens S, Blencowe H, Stanton C, et al. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet. 2011;377(9774):1319–1330. doi: 10.1016/S0140-6736(10)62310-0. [DOI] [PubMed] [Google Scholar]
- 4.Health Data OECD. [Accessed August 14, 2011];2008 Statistics and indicators for 30 countries. http://www.ecosante.org/index2.php?base=OCDE&langh=ENG&langs=ENG.
- 5.Graafmans WC, Richardus JH, Macfarlane A, et al. EuroNatal Working Group. Comparability of published perinatal mortality rates in Western Europe: the quantitative impact of differences in gestational age and birthweight criteria. BJOG. 2001;108(12):1237–1245. doi: 10.1111/j.1471-0528.2001.00291.x. [DOI] [PubMed] [Google Scholar]
- 6. [Accessed August 14, 2011];Healthy People 2010. Second edition: understanding and improving health, objectives for improving health; volumes I and II. http://www.healthypeople.gov/Document/tableofcontents.htm#volume1.
- 7.Vintzileos AM, Ananth CV, Smulian JC, Scorza WE, Knuppel RA. Prenatal care and black-white fetal death disparity in the United States: heterogeneity by highrisk conditions. Obstet Gynecol. 2002;99(3):483–489. doi: 10.1016/s0029-7844(01)01758-6. [DOI] [PubMed] [Google Scholar]
- 8.Fiscella K. Racial disparity in infant and maternal mortality: confluence of infection, and microvascular dysfunction. Matern Child Health J. 2004;8(2):45–54. doi: 10.1023/b:maci.0000025726.53515.65. [DOI] [PubMed] [Google Scholar]
- 9.Healy AJ, Malone FD, Sullivan LM, et al. FASTER Trial Research Consortium. Early access to prenatal care: implications for racial disparity in perinatal mortality. Obstet Gynecol. 2006;107(3):625–631. doi: 10.1097/01.AOG.0000201978.83607.96. [DOI] [PubMed] [Google Scholar]
- 10.Willinger M, Ko CW, Reddy UM. Racial disparities in stillbirth risk across gestation in the United States. Am J Obstet Gynecol. 2009;201(5):469, e1–e8. doi: 10.1016/j.ajog.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowland Hogue CJ, Silver RM. Racial and ethnic disparities in United States: stillbirth rates: trends, risk factors, and research needs. Semin Perinatol. 2011;35(4):221–233. doi: 10.1053/j.semperi.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Hankins G, Willinger M, Spong CY, editors. Stillbirth after 20 weeks: introduction. Semin Perinatol. 2002;26(1):1–2. [Google Scholar]
- 13.Parker CB, Hogue CJR, Koch MA, et al. Stillbirth Collaborative Research Network. Stillbirth Collaborative Research Network: design, methods and recruitment experience. Paediatr Perinat Epidemiol. 2011;25(5):425–435. doi: 10.1111/j.1365-3016.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey JC, Klebanoff MA, Hauth JC, et al. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N Engl J Med. 2000;342(8):534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 15.Pinar H, Koch MA, Hawkins H, et al. The Stillbirth Collaborative Research Network (SCRN) placental and umbilical cord examination protocol [published online ahead of print June 29, 2011] Am J Perinatol. doi: 10.1055/s-0031-1281509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinar H, Koch MA, Hawkins H, et al. The Stillbirth Collaborative Research Network (SCRN) postmortem examination protocol [published online ahead of print August 3, 2011] Am J Perinatol [Google Scholar]
- 17.ACOG Practice Bulletin No. 102: management of stillbirth. Obstet Gynecol. 2009;113(3):748–761. doi: 10.1097/AOG.0b013e31819e9ee2. [DOI] [PubMed] [Google Scholar]
- 18.Dudley DJ, Goldenberg R, Conway D, et al. Stillbirth Research Collaborative Network. A new system for determining the causes of stillbirth. Obstet Gynecol. 2010;116(2 Pt 1):254–260. doi: 10.1097/AOG.0b013e3181e7d975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthen LK, Muthen BO. Mplus User’s Guide. 6th ed. Los Angeles, CA: Muthen &Muthen; 1998–2010. [Google Scholar]
- 20.Walsh CA, Vallerie AM, Baxi LV. Etiology of stillbirth at term: a 10-year cohort study. J Matern Fetal Neonatal Med. 2008;21(7):493–501. doi: 10.1080/14767050802086669. [DOI] [PubMed] [Google Scholar]
- 21.Heuser CC, Hunn J, Varner M, Hossain S, Vered S, Silver RM. Correlation between stillbirth vital statistics and medical records. Obstet Gynecol. 2010;116(6):1296–1301. doi: 10.1097/AOG.0b013e3181fb8838. [DOI] [PubMed] [Google Scholar]
- 22.Silver RM, Varner MW, Reddy U, et al. Work-up of stillbirth: a review of the evidence. Am J Obstet Gynecol. 2007;196(5):433–444. doi: 10.1016/j.ajog.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varli IH, Petersson K, Bottinga R, et al. The Stockholm classification of stillbirth. Acta Obstet Gynecol Scand. 2008;87(11):1202–1212. doi: 10.1080/00016340802460271. [DOI] [PubMed] [Google Scholar]
- 24.Korteweg FJ, Erwich JJHM, Holm JP, et al. Diverse placental pathologies as the main causes of fetal death. Obstet Gynecol. 2009;114(4):809–817. doi: 10.1097/AOG.0b013e3181b72ebe. [DOI] [PubMed] [Google Scholar]
- 25.Flenady V, Frøen JF, Pinar H, et al. An evaluation of classification systems for stillbirth. BMC Pregnancy Childbirth. 2009;9:24. doi: 10.1186/1471-2393-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Froen JF, Fretts RC, Flenady V. Definition and epidemiology of stillbirths. In: Fachinetti F, Dekker GA, Baronciani D, et al., editors. Stillbirth: Understanding and Management. Zug, Switzerland: Informa UK Ltd; 2010. pp. 1–15. [Google Scholar]
- 27.Korteweg FJ, Bouman K, Erwich JJHM, et al. Cytogenetic analysis after evaluation of 750 fetal deaths: proposal for diagnostic workup. Obstet Gynecol. 2008;111(4):865–874. doi: 10.1097/AOG.0b013e31816a4ee3. [DOI] [PubMed] [Google Scholar]
- 28.Carey JC, Rayburn WF. Nuchal cord encirclements and risk of stillbirth. Int J Gynaecol Obstet. 2000;69(2):173–174. doi: 10.1016/s0020-7292(99)00219-2. [DOI] [PubMed] [Google Scholar]
- 29.Parast MM, Crum CP, Boyd TK. Placental histologic criteria for umbilical blood flow restriction in unexplained stillbirth. Hum Pathol. 2008;39(6):948–953. doi: 10.1016/j.humpath.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 30.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2007. Natl Vital Stat Rep. 2010;58(24):1–85. [PubMed] [Google Scholar]