Abstract
Early amplitude-integrated electroencephalography (aEEG) has been widely used in term infants with brain injury to predict neurodevelopmental outcomes; however, the prognostic value of early aEEG in preterm infants is unclear. We evaluated how well early aEEG could predict brain damage and long-term neurodevelopmental outcomes in very preterm infants compared with brain imaging assessments. We found that severe aEEG abnormalities (p = 0.000) and aEEG total score < 5 (p = 0.006) within 72 h after birth were positively correlated with white-matter damage, but aEEG abnormalities were not associated with intracranial hemorrhage (p = 0.186). Severe abnormalities in aEEG recordings, head ultrasound, and cranial magnetic resonance imaging (MRI) were all positively correlated with poor outcome at 18 months corrected age. The predictive power of poor outcomes of the aEEG and MRI combination was the same as the aEEG, MRI, and head ultrasound combination with a sensitivity of 52.4%, specificity of 96.2%, positive predictive value of 78.6%, and negative predictive value of 88.4%. These results indicate that severely abnormal aEEG recordings within 72 h after birth can predict white-matter damage and long-term poor outcomes in very preterm infants. Thus aEEG can be used as an early marker to monitor very preterm infants.
Preterm birth rates have increased globally since 1990, and about 15 million preterm neonates are currently born every year1,2,3. Modern advances in prenatal and neonatal intensive care have led to an increase in the survival rate of these premature infants4. However, neonates born preterm have increased risk of both short-term complications and long-term neurodevelopmental disorders such as cerebral palsy and intellectual disabilities5,6,7, and prematurity is still the leading cause of neonatal death and the second cause of childhood death under the age of 5 years8. Quality of life in premature infants who suffer from perinatal brain injury has become a major concern with clear social relevance. Although there are many causes for preterm labor9, all brain injuries in preterm infants consist mainly of periventricular leukomalacia (PVL) and intracranial hemorrhage (ICH)10.
Early diagnosis and early treatment of brain damage in preterm infants has the potential to improve their long-term quality of life11. However, the clinical manifestation of brain damage in preterm infants is not specific and not typical. The majority of ICH occurs in the first 72 hours after birth, especially in the first 24 hours12, and PVL occurs later13, thus early identification of preterm brain damage through clinical manifestations is difficult. The current clinical method for early diagnosis of brain damage in preterm infants is still mainly through neuroimaging – including head ultrasound (HUS) and magnetic resonance imaging (MRI) – but using neuroimaging for the diagnosis of severe brain damage has some limitations. Evaluation of brain damage, especially white-matter damage (WMD), by head ultrasound usually requires monitoring for more than 2 weeks14, and head MRI is difficult to perform in newborn preterm infants who need breathing support. It is necessary, therefore, to explore other methods of early prediction of brain damage in preterm infants.
Amplitude-integrated electroencephalography (aEEG) has the advantages of being simple to perform, of allowing continuous bedside monitoring, and of providing easily interpreted results, all of which make this an important method in the neonatal intensive care unit for monitoring brain function. It has also been shown that aEEG classifications correlate strongly with the clinical degree of hypoxic-ischemic encephalopathy and neurological outcomes in full-term infants15. Research on the use of aEEG in preterm infants has shown impressive predictive value for short-term and later outcomes, although such studies have only included limited numbers of patients16,17,18,19. The role of aEEG for predicting long-term adverse neurological outcome in preterm infants needs to be further confirmed by comparing it with the results from neuroimaging.
The aim of this study was to evaluate whether aEEG recordings within 72 h after birth predict brain damage and neurodevelopmental outcome in preterm infants with gestational age (GA) less than 32 weeks, and to compare these outcomes to head ultrasound and MRI assessments.
Results
Baseline characteristics
A total of 346 infants were admitted to the NICU during the study period. Four infants were excluded based on the exclusion criteria and 342 were eligible, of which 18 were lost to follow-up and excluded from the analysis. A total of 324 preterm infants with an average GA of 30.0 ± 1.1 weeks (27.1–31.6 weeks) and average birth weight of 1377 ± 288 g (700–2200 g) were included (Table 1). The male to female ratio was 1.8:1. A total of 152 very preterm infants underwent aEEG within 72 h, 337 preterm infants underwent head ultrasonography, 210 preterm infants underwent MRI at 40 weeks of corrected age, 152 preterm infants underwent both aEEG and head ultrasonography, 101 preterm infants underwent both aEEG and MRI, 210 preterm infants underwent both head ultrasonography and MRI, and 101 preterm infants underwent all three examinations. There were 31 preterm infants who died at an average age of 15.2 ± 12.5 days, of which 15 died from brain damage and 16 died from other causes including respiratory failure (n = 7), sepsis (n = 6), perforation of the digestive tract (n = 1), or pulmonary hemorrhage (n = 2), and these were excluded from the analysis of neurodevelopmental outcomes. A total of 308 infants were considered for long-term analysis, but 18 were lost to follow-up. Excluding those lost to follow-up, there were 134 follow-ups for infants who underwent aEEG within 72 h (88.15%), 293 follow-ups for infants who underwent head ultrasonography (86.94%), and 207 follow-ups for infants who underwent brain MRI at 40 weeks of corrected age (98.57%) (Fig. 1).
Table 1. Epidemiological data of the study group.
| All infants (n = 324) | Favorable outcome (n = 262) | Poor outcome (n = 62) | |
|---|---|---|---|
| Gestational age (weeks) | 30.0 ± 1.1 | 30.2 ± 1.0 | 29.5 ± 1.2* |
| Birth weight (g) | 1377 ± 288 | 1562 ± 198 | 1093 ± 131* |
| Male sex, n (%) | 208 (64.2) | 167 (63.7) | 41 (66.1) |
| 1-minute Apgar < 3′, n (%) | 16 (4.9) | 10 (3.8) | 6 (9.7)* |
| 1-minute Apgar 3–7′, n (%) | 152 (46.9) | 104 (39.7) | 48 (77.4)* |
| 1-minute Apgar > 7′, n (%) | 156 (48.2) | 148 (56.5) | 8 (12.9)* |
| Mechanical ventilation, n (%) | 126 (38.9) | 75 (28.6) | 51 (82.3)* |
| Sepsis, n (%) | 58 (17.9) | 32 (12.2) | 26 (41.9)* |
| BPD, n (%) | 38 (11.7) | 26 (9.9) | 12 (19.4)* |
| NEC, n (%) | 11 (3.4) | 8 (3) | 3 (4.8) |
| Severe anemia, n (%) | 118 (36.4) | 88 (33.6) | 30 (48.4)* |
| Cholestatic syndrome, n (%) | 20 (6.2) | 15 (5.7) | 5 (8.1) |
| Persistent hypoxemia, n (%) | 96 (29.6) | 68 (26.0) | 28 (45.2)* |
| Persistent hypercapnia, n (%) | 30 (9.3) | 17 (6.5) | 13 (21.0)* |
| Persistent hypoglycemia, n (%) | 7 (2.2) | 6 (2.3) | 1 (1.6) |
| Parent’s monthly income | |||
| <3000 yuan, n (%) | 42 (13.0) | 30 (11.5) | 12 (19.4) |
| 3000–8000 yuan, n (%) | 201 (62.0) | 161 (61.5) | 40 (64.5) |
| >8000 yuan, n (%) | 81 (25.0) | 71 (27.0) | 10 (16.1) |
| Parent’s education level | |||
| Middle school, n (%) | 45 (13.9) | 34 (13.0) | 11 (17.7) * |
| High school, n (%) | 172 (53.1) | 132 (50.4) | 40 (64.5) * |
| University, n (%) | 107 (33.0) | 96 (36.6) | 11 (17.7) * |
BPD: bronchopulmonary dysplasia. NEC: necrotizing enterocolitis. Poor outcome: death or survival with cerebral palsy, hypophrenia, or audio-visual disorder. Favorable outcome: survival without cerebral palsy, hypophrenia, or audio-visual disorder. *P < 0.05 significant difference between infants with favorable outcome versus poor outcome at 18 months corrected age.
Figure 1. Study flow.
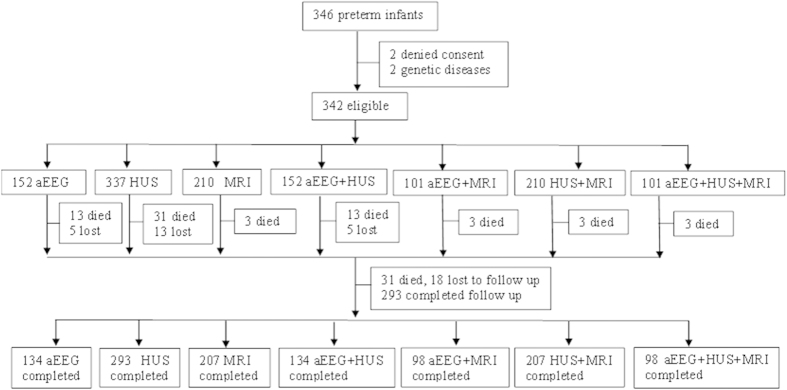
A schematic flowchart describing the recruitment and neurodevelopmental follow-up evaluation from birth to 18 months of corrected age for the preterm infants. Lost to follow-up means that contact with the family was lost during the follow-up period. aEEG: amplitude-integrated electroencephalography; HUS: head ultrasonography; MRI: magnetic resonance imaging.
aEEG recordings and brain damage in preterm infants
There was a significant association between the classification of aEEG recordings within 72 h after birth and the degree of WMD in preterm infants. Although aEEG recordings were severely abnormal in 77.8% (7/9) of the infants with grade III or IV ICH, the degree of abnormality of the aEEG recordings in the first 72 h of life were not associated with ICH classification (Table 2). Logistic regression analysis showed that severe aEEG abnormality was positively correlated with WMD (adjusted regression coefficients: 1.66; S.E.: 0.40; p = 0.000; OR: 5.28; 95% CI [2.41–11.57]). This means that severe aEEG abnormalities could predict WMD. Further analysis with the aEEG scoring system showed that cycling score, narrow bandwidth score, and total score on aEEG within 72 h in infants with severe WMD were significantly lower than the scores of those without or with mild WMD (Table 3). Logistic regression analyses showed that an aEEG total score < 5 positively correlated with WMD (adjusted regression coefficients: 1.39; S.E.: 0.50; p = 0.006; OR: 4.01; 95% CI [1.50–10.68]). However, ICH was not associated with aEEG scores. This indicates that an aEEG total score < 5 could predict WMD.
Table 2. aEEG classification compared to brain damage severity in preterm infants.
| WMD |
ICH |
|||||
|---|---|---|---|---|---|---|
| No WMD | Mild | Severe | No ICH | ICH I-II | ICH III-IV | |
| Normal aEEG (n = 10) | 6 | 1 | 3 | 9 | 1 | 0 |
| Mildly abnormal aEEG (n = 70) | 13 | 38 | 19 | 36 | 32 | 2 |
| Severely abnormal aEEG (n = 67) | 6 | 19 | 42 | 31 | 29 | 7 |
| P-value | 0.000 | 0.186 | ||||
WMD: white-matter damage. ICH: intracranial hemorrhage. P-value: assessed the association between the classification of aEEG with the degree of WMD and ICH using chi-square test, and P < 0.05 was considered significant.
Table 3. Relationship between 72 h aEEG scores and brain damage in preterm infants.
| No WMD n = 25 | Mild WMD n = 58 | Severe WMD n = 64 | P-value | No ICH n = 76 | ICH I-IIn = 62 | ICH III-IV n = 9 | P-value | |
|---|---|---|---|---|---|---|---|---|
| Co | 1.95 ± 0.41 | 1.80 ± 0.58 | 1.64 ± 0.59 | 0.105 | 1.75 ± 0.58 | 1.78 ± 0.56 | 1.50 ± 0.55 | 0.535 |
| Cy | 1.81 ± 1.44 | 1.77 ± 1.50 | 0.77 ± 1.25* | 0.006 | 1.22 ± 1.40 | 1.24 ± 1.46 | 1.17 ± 1.84 | 0.920 |
| LB | 1.21 ± 0.71 | 1.14 ± 0.60 | 0.96 ± 0.62 | 0.233 | 1.09 ± 0.75 | 1.04 ± 0.48 | 1.00 ± 0.63 | 0.910 |
| B | 1.68 ± 0.82 | 1.59 ± 0.89 | 1.23 ± 0.58* | 0.022 | 1.48 ± 0.79 | 1.36 ± 0.75 | 1.33 ± 0.52 | 0.709 |
| T | 6.05 ± 2.48 | 6.15 ± 2.89 | 4.60 ± 2.34* | 0.012 | 5.45 ± 2.85 | 5.32 ± 2.37 | 4.83 ± 2.92 | 0.859 |
Co: continuity score. Cy: sleep-wake cycling score. LB: lower border amplitude score. B: narrow bandwidth score. T: total score. WMD: white matter damage. ICH: intracranial hemorrhage. P-value: compared aEEG scores with WMD and ICH using one-way ANOVA. *P < 0.05, compared with no and mild WMD using LSD-t test.
aEEG recordings and poor outcome
Of the 152 preterm infants who underwent aEEG within 72 h after birth, 5 were lost to follow-up, 13 died (5 of them from brain damage), 134 surviving infants were followed up to 18 months of age, and 139 infants (including 5 that died from brain damage) were included in the statistical analysis of poor outcome. Abnormal aEEG recordings within 72 h were significantly associated with poor outcomes, including cerebral palsy and hypophrenia in preterm infants at 18 months corrected age (Table 4).
Table 4. aEEG, HUS, and MRI examination and poor outcome at 18 months in preterm infants.
| Poor outcome | CP | Hypophrenia | Audio-visual disorder | Death | |
|---|---|---|---|---|---|
| aEEG | |||||
| Normal (n = 9) | 0 | 0 | 0 | 0 | 0 |
| Mildly abnormal (n = 67) | 6 | 2 | 4 | 1 | 2 |
| Severely abnormal (n = 63) | 18 | 10 | 14 | 1 | 3 |
| P-value | 0.005 | 0.018 | 0.009 | 0.928 | 0.721 |
| HUS | |||||
| Normal (n = 46) | 1 | 1 | 1 | 0 | 0 |
| Mildly abnormal (n = 144) | 15 | 1 | 8 | 5 | 3 |
| Severely abnormal (n = 118) | 30 | 14 | 16 | 1 | 12 |
| P-value | 0.013 | 0.000 | 0.008 | 0.203 | 0.003 |
| MRI | |||||
| Normal (n = 148) | 11 | 2 | 10 | 1 | 0 |
| Mildly abnormal (n = 32) | 5 | 3 | 4 | 1 | 0 |
| Severely abnormal (n = 30) | 13 | 11 | 10 | 3 | 3 |
| P-value | 0.000 | 0.000 | 0.000 | 0.005 | 0.000 |
CP: cerebral palsy, aEEG: amplitude-integrated electroencephalography, HUS: head ultrasound, MRI: cranial magnetic resonance imaging. Hypophrenia: MDI < 70 according to Bayley Scales. Death: patients died from brain damage. Poor outcome: patients died from brain damage or survived with one or more of CP, hypophrenia, or audio-visual disorder. P-value: assessed the association between classifications of aEEG/HUS/MRI with poor outcomes using chi-square test, and P < 0.05 was considered significant.
Head ultrasound and poor outcome
Of 337 infants who underwent head ultrasound within 1 month after birth, 13 were lost to follow-up, 31 died (15 from brain damage), 293 surviving infants were followed up to 18 months of corrected age, and 308 infants were included in the statistical analysis of poor outcome. Abnormalities in the head ultrasound within 1 month were statistically associated with poor outcomes, including cerebral palsy, hypophrenia, and death from brain injury in preterm infants at 18 months corrected age (Table 4).
Head MRI and poor outcome
Of 210 infants who underwent head MRI at 40 weeks corrected age, 3 infants died from brain damage, 207 surviving infants were followed up to 18 months of age, and 210 infants were included in the statistical analysis of poor outcome. Abnormality of head MRI at 40 weeks corrected age was statistically associated with poor outcomes, including cerebral palsy, hypophrenia, audio-visual disorder, and death in preterm infants at 18 months corrected age (Table 4).
Combined examinations and poor outcomes
The combined examinations and long-term outcomes were analyzed for different combinations of assessments (Table 5). Of 101 preterm infants who underwent all three examinations, 3 infants died (2 from brain damage), 98 surviving infants were followed up to 18 months of age, and 100 infants were included in the statistical analysis of poor outcomes. Results from preterm infants who underwent both aEEG and MRI were the same as those who underwent all three examinations. Of 152 preterm infants who underwent both aEEG and head ultrasound, 5 were lost to follow-up, 13 infants died (5 from brain damage), 134 surviving infants were followed up to 18 months of age, and 139 infants were included in the statistical analysis of poor outcomes. Of 210 preterm infants who underwent both head ultrasound and MRI, 3 infants died (2 from brain damage), 207 surviving infants were followed up to 18 months of age, and 209 infants were included in the statistical analysis of poor outcomes. Abnormalities in the combinations of two or three examinations were statistically associated with poor outcomes, including cerebral palsy and hypophrenia in preterm infants at 18 months corrected age (Table 5).
Table 5. Prediction of poor outcome by combined aEEG, HUS, and MRI in preterm infants.
| Combined examinations (n) | Poor outcome % (n/total) | CP % (n/total) | Hypophrenia % (n/total) | Audio-visual Disorder n/total | Death n/total |
|---|---|---|---|---|---|
| HUS + MRI (209) | 45.2% (14/31)* | 68.8% (11/16)* | 41.7% (10/24)* | 2/5 | 2/2 |
| aEEG + HUS (139) | 66.7% (16/24)* | 83.3% (10/12)* | 66.7% (12/18)* | 1/2 | 3/5 |
| aEEG + MRI (100) | 52.4% (11/21)* | 83.3% (10/12)* | 50% (9/18)* | 1/2 | 1/2 |
| aEEG + HUS + MRI (100) | 52.4% (11/21)* | 83.3% (10/12)* | 50% (9/18)* | 1/2 | 1/2 |
| P-value | 0.159 | 0.098 | 0.303 | 0.999 | 0.619 |
HUS + MRI, aEEG + HUS, aEEG + MRI, aEEG + HUS + MRI: infants undergoing different combinations of examinations. CP: cerebral palsy, aEEG: amplitude-integrated electroencephalography, HUS: head ultrasound, MRI: cranial magnetic resonance imaging. Hypophrenia: MDI < 70 according to Bayley Scales. Death: patients died from brain damage. Poor outcome: patients died from brain damage or survived with one or more of CP, hypophrenia, or audio-visual disorder. n/total: patients with severe abnormalities of different combinations/all patients. P-value: compared the assessments of poor outcomes with severe abnormalities of different combinations using chi-square tests. *P < 0.05 compared abnormalities of different combinations with poor outcomes using chi-square test.
Predictors of poor outcomes
Univariate analysis of poor outcome was performed considering 22 items, including GA, birth weight, severe asphyxia, mechanical ventilation >7 days, respiratory distress syndrome grade 3 or 4, severe anemia, persistent hypoxemia, persistent hypercapnia, persistent hypoglycemia, sepsis, bronchopulmonary dysplasia, necrotizing enterocolitis, cholestasis, family socioeconomic status, parent education level, severe abnormality in aEEG, HUS, MRI, aEEG + HUS, aEEG + MRI, HUS + MRI, and aEEG + HUS + MRI. All combinations of examinations were analyzed separately, and p-values < 0.05 were required for inclusion in the logistic regression analysis. Logistic regression analyses showed that severe aEEG abnormality (adjusted regression coefficients: 1.61; S.E.: 0.63; p = 0.011, OR: 5.00; 95% CI [1.45–17.22]), severe HUS abnormality (adjusted regression coefficients: 1.03; S.E.: 0.41; p = 0.011, OR: 2.80; 95% CI [1.26–6.20]), and severe MRI abnormality (adjusted regression coefficients: 2.82; S.E.: 0.69; p = 0.000, OR: 16.75; 95% CI [4.35–64.50]) were significantly high risk factors of poor outcome. Severe abnormality of aEEG + MRI was a significantly high risk factor of poor outcome (adjusted regression coefficients: 3.92; S.E.: 0.96; p = 0.000, OR: 50.54; 95% CI [7.72–331.09]), which was the same as aEEG + HUS + MRI. Severe abnormalities of aEEG + HUS (adjusted regression coefficients: 2.09; S.E.: 0.63; P = 0.001, OR: 8.05; 95% CI [2.34–27.71]) and HUS + MRI (adjusted regression coefficients: 3.36; S.E.: 0.75; p = 0.000, OR: 28.75; 95% CI [6.68–123.76]) were also positively correlated with poor outcome. We took severe abnormalities of different combinations as predictors, and there was no significant predictive difference among different combinations of the assessments (Table 5). The sensitivity, specificity, positive predictive value, and negative predictive value for predicting poor outcomes and cerebral palsy for all combinations of assessments are shown in Table 6.
Table 6. Prediction of CP or poor outcome at 18 months corrected age in preterm infants.
| Predictor | CP |
Poor outcomes |
||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| aEEG (n = 139) | 83.3 | 58.2 | 15.9 | 97.4 | 75.0 | 60.9 | 28.6 | 92.1 |
| HUS (n = 308) | 87.5 | 64.5 | 11.9 | 98.9 | 65.2 | 66.4 | 25.4 | 89.7 |
| MRI (n = 210) | 68.8 | 90.2 | 36.7 | 97.2 | 50.0 | 92.1 | 53.3 | 91.1 |
| aEEG + HUS (n = 139) | 83.3 | 78.7 | 27.0 | 98.0 | 66.7 | 79.1 | 40.0 | 91.9 |
| aEEG + MRI (n = 100) | 83.3 | 96.6 | 76.9 | 97.7 | 52.4 | 96.2 | 78.6 | 88.4 |
| HUS + MRI (n = 209) | 68.8 | 93.8 | 47.8 | 97.3 | 45.2 | 93.8 | 56.0 | 90.8 |
| aEEG + HUS + MRI (n = 100) | 83.3 | 96.6 | 76.9 | 97.7 | 52.4 | 96.2 | 78.6 | 88.4 |
The predictors are defined as severe abnormalities in aEEG recordings within 72 h, HUS, or MRI individually or in combination. CP: cerebral palsy, aEEG: amplitude-integrated electroencephalography HUS: head ultrasound, MRI: cranial magnetic resonance imaging, PPV: positive predictive value, NPV: negative predictive value. Poor outcome: patients died from brain damage or survived with one or more of CP, hypophrenia, or audio-visual disorder.
Discussion
This study used a relatively large sample to show that severe abnormalities of early aEEG can predict white-matter injury and long-term outcomes in preterm infants. The specificity of aEEG to predict poor outcomes was similar to head ultrasound but lower than cranial MRI, and the sensitivity of aEEG was highest compared to MRI and HUS.
The survival of premature infants has improved dramatically due to advances in neonatal intensive care. However, preterm infants are at increased risk of dangerous complications occurring during the neonatal period that often cause brain injury such as ICH and PVL, and these are the most important risk factors for long-term neurologic sequelae such as cerebral palsy, cognitive deficits, and learning impairments20. Neuroimaging has played an important role in the diagnostic evaluation and monitoring of preterm infants10, but even though HUS and cranial MRI have predictive value for long-term outcomes there remains a subset of infants with impairments in childhood who demonstrate no significant brain injury or alterations upon neuroimaging21. Neural dysfunction as reflected by aberrations in aEEG has been widely used as an early marker of brain injury in full-term infants suffering from asphyxia, but the use of aEEG in preterm infants is not very common. Recent studies have found that abnormal aEEG in preterm infants is associated with brain injury and later outcome16,22,23, and the use of aEEG at earlier time points after birth might open a potential time window for neuroprotective interventions.
The optimal time after birth for taking aEEG recordings to predict adverse outcomes is still unclear17,18,24. Recent studies have assessed aEEG recordings within 72 h after birth and found reasonable predictive value for these aEEG recordings in predicting poor outcomes18,22,25. Preterm infants are most vulnerable to high-risk perinatal factors within the first 72 h after birth, and most ICH in preterm infants occurs during this period of time12. In addition, abnormalities in aEEG recordings taken during this time can predict hospitalization time in preterm infants26. In this study, we used aEEG recordings taken within the first 72 h of life to evaluate the predictive value of abnormal aEEG compared to standard imaging assessments. Univariate analysis showed that the degree of abnormality in the aEEG recordings in the first 72 h of life was positively associated with the degree of WMD. Multivariate analysis showed that severe aEEG abnormality was a significant higher risk of WMD and can be taken as an early predictor of WMD in preterm infants. We also found that cycling score, narrow bandwidth score, and total score on aEEG within 72 h in infants with severe WMD were significantly lower than in infants with no or mild WMD. This indicates that preterm infants with severe WMD have a delay in brain maturation27. Kato T28 reported 1 case of preterm infant developed PVL with GA 29 weeks, whose aEEG recordings in the first 1 hour was at the inhibitory state. In this study, we found that an aEEG total score <5 was a significant risk for WMD. These early maturity changes in aEEG recordings can help predict the occurrence of WMD in preterm infants.
The abnormal aEEG recordings had no association with the classification of ICH. This means that a preterm infant with severely abnormal aEEG recordings soon after birth will not necessarily develop severe ICH, which is different from other reports showing that early aEEG recordings are associated with ICH17,29. One possible reason for this is that aEEG records the activity of the brain cortex and reflects activity from the underlying cortex such as WMD. Although grade IV ICH might be reflexed by aEEG, there was only one patient with grade IV ICH in our study, and the influence of grade I–III ICH on the electrophysiological activity of the brain cortex might be less. Another reason for this might be that there were only nine very preterm infants with severe ICH (grade III–IV) in our study, which might affect the statistical results. Further study with a larger sample is necessary to confirm these results.
The predictive value of early aEEG for long-term outcomes is still controversial. Recent studies22,30 reported that abnormal aEEG recordings within 24 hours after birth in infants with GA 22–30 weeks or 27–32 weeks were associated with long-term adverse neurodevelopmental outcomes. However, another study reported that aEEG recordings in preterm infants with GA 28–36 weeks could not predict outcome at 18 to 22 months of age31. In the current study, we found that severe abnormalities in aEEG recordings within 72 h after birth were positively correlated with poor outcomes at 18 months of corrected age in preterm infants less than 32 weeks GA. The contradictory predictive values could be related to different GA of the selected preterm infants because EEG activity is related to brain maturation.
Neuroimaging of preterm infants has become part of routine clinical care in the NICU, and HUS is still considered the method of first choice. However, sequential HUSs are required to evaluate preterm brain injury and to predict outcome. The sensitivity and specificity of HUS in preterm infants ranges from 45% to 90%16. In this study, the sensitivity and specificity of HUS for cerebral palsy were 87.5% and 64.5%, respectively, which were a little bit higher than the assessment by aEEG. MRI is increasingly used for preterm infants to detect brain injury and to evaluate long-term outcomes, but it can only be performed when the infant has stabilized or is at term equivalent age10. MRI has been reported to have high predictive value with a sensitivity of 84% and specificity of 89% for preterm infants when performed at term equivalent age32. In our cohort, the specificity was similar to the previous report, but with a lower sensitivity for predicting poor outcomes or cerebral palsy. The predictive value of combining aEEG and cerebral MRI was the same as the combination of all three assessments with the highest sensitivity (83.3%) and specificity (96.6%) as well as positive predictive value (76.9%) and negative predictive value (97.7%) for cerebral palsy in preterm infants.
In summary, severe abnormalities in aEEG recordings within 72 h after birth could predict WMD in preterm infants with GA less than 32 weeks. Severe abnormalities in aEEG recordings, HUS, and cranial MRI were all positively correlated with poor outcome at 18 months corrected age. The predictive value of combined aEEG and MRI for poor outcome in preterm infants less than 32 weeks is similar to all three examinations combined together. We conclude that aEEG has the potential for use as an early marker to monitor preterm infants in the NICU.
Patients and Methods
Subjects
Neonates with GA < 32 weeks admitted to the NICU within 72 h after birth at the Third Affiliated Hospital of Zhengzhou University, China, from January to December 2012 were screened for eligibility for this prospective study cohort. Neonates with congenital brain malformations, chromosomal diseases, genetic diseases, or metabolic diseases were excluded. This study was approved by the Human Research Ethics Committee and Clinical Trials Committee of the hospital in accordance with the Helsinki Declaration. Written informed parental consent was obtained for all neonates.
aEEG
aEEG traces were recorded within 72 h (43 infants within 24 h, 67 infants between 24 h and 48 h, and 37 infants between 48 h and 72 h) after birth using a NicoletOneTM device (Nicolet Biomedical Inc., Madison, WI, US). Each recording lasted for 4–24 hours (91 infants were recorded for 4–10 h, 35 infants were recorded for 10–16 h, and 21 infants were recorded for 16–24 h). The average recording time was 10.9 ± 5.5 h, and all aEEG recordings were obtained by two investigators. The aEEG recordings were assessed using a combination of criteria for amplitude, background activity, and sleep-wake cycling (SWC): (1) normal: continuous normal amplitude (upper margin >10 μV and lower margin >5 μV), SWC matched to corresponding age, and no electrographic seizures; (2) mildly abnormal: discontinuous activity and mildly abnormal amplitude (upper margin >10 μV and lower margin <5 μV) with immature and delayed SWC or normal amplitude with electrographic seizures; (3) severely abnormal: discontinuous activity and severely abnormal amplitude (upper margin <10 μV and lower margin <5 μV) without SWC, including burst-suppression (discontinuous activity with lower margin at 0–1 μV constantly and a burst amplitude >25 μV), flat trace (electrical silence), continuous low voltage (continuous very low amplitude activity at about 5 μV or below 5 μV), or mildly abnormal amplitude with electrographic seizures33,34. aEEG scores were assigned according to a scoring system described by Burdjalov et al. to assess brain maturity35. The scores include four individual scores for continuity, cycling, amplitude, and bandwidth of the lower border, which can be summed into a total score ranging from 0 to 13 with higher scores being correlated to increasing postconceptional age.
Head ultrasonography
HUS was performed within 3 days after birth and then weekly until 4 weeks after birth with a GETM Voluson (General Electric Company, Fairfield, CT, US) with a 7.5-MHz transducer. ICH was classified as grade I to IV according to Papile et al.36. The WMD was classified into the following three types according to de Vries37: (1) no WMD (no enhancement of the echo of cerebral white matter); (2) mild WMD (transient and slight enhancement of the echo within 3 days after birth but decreasing or disappearing after 7–10 days); and (3) severe WMD (significant enhancement of the echo and PVL or a decrease in cerebral white-matter volume in the primary lesion after 3–4 weeks). Severe abnormal HUS was defined in this study as grade III or IV ICH or severe WMD.
Head MRI
Head MRI was performed at 40 weeks corrected age using a 1.5 T MRI (General Electric Company). Abnormalities on MRI at term equivalent were assessed blindly by the scores of white matter and gray matter38. Severely abnormal MRI was defined in this study as PVL or severe dilatation of the lateral ventricles.
Follow-up
All of the preterm infants were tracked every month for 6 months and then every 3 months until 18 months corrected age. All of the surviving preterm infants were evaluated through gross neurologic assessment and mental developmental index (MDI) testing. Assessment of neuromotor disability was based on the presence of cerebral palsy and functional disability. Mental or psychological development was evaluated with the Bayley Scales of Infant Development, Second Edition39. Good outcome was defined as survival without neurodevelopmental impairment, which includes one or more of the following: cerebral palsy, hypophrenia (MDI < 70, Bayley scales), or audio-visual disorder. Poor outcome was defined as survival with one or more of cerebral palsy, hypophrenia, or audio-visual disorder or death due to brain damage.
Statistical analysis
Statistical analysis was performed using SPSS version 19.0. Quantitative data are presented as means ± standard deviation. Differences between groups were evaluated using one-way ANOVA and LSD t-test. A p-value < 0.05 was considered significant. Qualitative data were compared using the chi-square tests, and a p-value < 0.05 suggested an association between the two groups. Logistic regression analyses were used to assess risk factors of WMD and poor outcome. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated using diagnostic tests.
Additional Information
How to cite this article: Song, J. et al. Early amplitude-integrated electroencephalography predicts brain injury and neurological outcome in very preterm infants. Sci. Rep. 5, 13810; doi: 10.1038/srep13810 (2015).
Acknowledgments
This study was supported by the Science and Technology Bureau of Zhengzhou (131PCXTD621), the Department of Health (201201002), and the Department of Science and Technology of Henan Province (134200510023), China, the Swedish Research Council (K2012-99X-21988-01-3), and Swedish governmental grants to scientists working in health care (ALFGBG-429271). We thank Prof. Kaijuan Wang from the Department of Epidemiology of Zhengzhou University for help with statistical analysis.
Footnotes
Author Contributions C.Z. and X.W. designed the research. J.S., F.X., L.G., J.G., L.X. and Y.Z. performed the clinical data analysis and took the aEEG recordings. J.S., L.W. and W.Z. performed the aEEG analysis. J.S. and C.Z. performed data analysis. J.S., C.Z. and X.W. wrote the paper. All authors read and discussed the manuscript.
References
- Han W. et al. Trends in live births in the past 20 years in Zhengzhou, China. Acta Obstet Gynecol Scand 90, 332–337 (2011). [DOI] [PubMed] [Google Scholar]
- Kinney M. V., Lawn J. E., Howson C. P. & Belizan J. 15 Million preterm births annually: what has changed this year? Reprod Health 9, 28 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172 (2012). [DOI] [PubMed] [Google Scholar]
- Platt M. J. Outcomes in preterm infants. Public Health 128, 399–403 (2014). [DOI] [PubMed] [Google Scholar]
- Allin M. P. Preterm babies grown up: understanding a hidden public health problem. Psychol Med 40, 5–7 (2010). [DOI] [PubMed] [Google Scholar]
- Pavlova M. A. & Krageloh-Mann I. Limitations on the developing preterm brain: impact of periventricular white matter lesions on brain connectivity and cognition. Brain 136, 998–1011 (2013). [DOI] [PubMed] [Google Scholar]
- Sun H. et al. Characteristics of respiratory distress syndrome in infants of different gestational ages. Lung 191, 425–433 (2013). [DOI] [PubMed] [Google Scholar]
- Liu L. et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379, 2151–2161 (2012). [DOI] [PubMed] [Google Scholar]
- Romero R., Dey S. K. & Fisher S. J. Preterm labor: one syndrome, many causes. Science 345, 760–765 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benders M. J., Kersbergen K. J. & de Vries L. S. Neuroimaging of white matter injury, intraventricular and cerebellar hemorrhage. Clin Perinatol 41, 69–82 (2014). [DOI] [PubMed] [Google Scholar]
- Leuchter R. H. et al. Association between early administration of high-dose erythropoietin in preterm infants and brain MRI abnormality at term-equivalent age. Jama 312, 817–824 (2014). [DOI] [PubMed] [Google Scholar]
- Linder N. et al. Risk factors for intraventricular hemorrhage in very low birth weight premature infants: a retrospective case-control study. Pediatrics 111, e590–595 (2003). [DOI] [PubMed] [Google Scholar]
- Agarwal P., Sriram B., Lim S. B., Tin A. S. & Rajadurai V. S. Borderline viability–neonatal outcomes of infants in Singapore over a period of 18 years (1990–2007). Ann Acad Med Singapore 42, 328–337 (2013). [PubMed] [Google Scholar]
- Ingram M. C., Huguenard A. L., Miller B. A. & Chern J. J. Poor correlation between head circumference and cranial ultrasound findings in premature infants with intraventricular hemorrhage. J Neurosurg Pediatr 14, 184–189 (2014). [DOI] [PubMed] [Google Scholar]
- Lawrence R., Mathur A., Nguyen The Tich S., Zempel J. & Inder T. A pilot study of continuous limited-channel aEEG in term infants with encephalopathy. J Pediatr 154, 835–841.e831 (2009). [DOI] [PubMed] [Google Scholar]
- Klebermass K. et al. Amplitude-integrated EEG pattern predicts further outcome in preterm infants. Pediatr Res 70, 102–108 (2011). [DOI] [PubMed] [Google Scholar]
- Soubasi V. et al. Early abnormal amplitude-integrated electroencephalography (aEEG) is associated with adverse short-term outcome in premature infants. Eur J Paediatr Neurol 16, 625–630 (2012). [DOI] [PubMed] [Google Scholar]
- Bowen J. R., Paradisis M. & Shah D. Decreased aEEG continuity and baseline variability in the first 48 hours of life associated with poor short-term outcome in neonates born before 29 weeks gestation. Pediatr Res 67, 538–544 (2010). [DOI] [PubMed] [Google Scholar]
- Cui H., Ding Y., Yu Y. & Yang L. Changes of amplitude integration electroencephalogram (aEEG) in different maturity preterm infant. Childs Nerv Syst 29, 1169–1176 (2013). [DOI] [PubMed] [Google Scholar]
- Hintz S. R. et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics 135, e32–42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy A. et al. Neuroimaging biomarkers of preterm brain injury: toward developing the preterm connectome. Pediatr Radiol 42 Suppl 1, S33–61 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom S. et al. Early single-channel aEEG/EEG predicts outcome in very preterm infants. Acta paediatrica 101, 719–726 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Zhu C., Xu F., Guo J. & Zhang Y. Predictive value of early amplitude-integrated electroencephalography for later diagnosed cerebral white matter damage in preterm infants. Neuropediatrics 45, 314–320 (2014). [DOI] [PubMed] [Google Scholar]
- West C. R., Harding J. E., Williams C. E., Gunning M. I. & Battin M. R. Quantitative electroencephalographic patterns in normal preterm infants over the first week after birth. Early Hum Dev 82, 43–51 (2006). [DOI] [PubMed] [Google Scholar]
- Griesmaier E. et al. Differences in the maturation of amplitude-integrated EEG signals in male and female preterm infants. Neonatology 105, 175–181 (2014). [DOI] [PubMed] [Google Scholar]
- Sommers R., Tucker R., Harini C. & Laptook A. R. Neurological maturation of late preterm infants at 34 wk assessed by amplitude integrated electroencephalogram. Pediatr Res 74, 705–711 (2013). [DOI] [PubMed] [Google Scholar]
- Natalucci G. et al. Delayed cyclic activity development on early amplitude-integrated EEG in the preterm infant with brain lesions. Neonatology 103, 134–140 (2013). [DOI] [PubMed] [Google Scholar]
- Kato T. et al. Amplitude-integrated electroencephalogram 1 h after birth in a preterm infant with cystic periventricular leukomalacia. Brain Dev 35, 75–78 (2013). [DOI] [PubMed] [Google Scholar]
- Sohn J. A. et al. Developmental change of amplitude-integrated electroencephalographic activity in preterm infants with intraventricular hemorrhage. Early Hum Dev 89, 961–966 (2013). [DOI] [PubMed] [Google Scholar]
- Kidokoro H. et al. Absent cyclicity on aEEG within the first 24 h is associated with brain damage in preterm infants. Neuropediatrics 41, 241–245 (2010). [DOI] [PubMed] [Google Scholar]
- Welch C., Helderman J., Williamson E. & O’Shea T. M. Brain wave maturation and neurodevelopmental outcome in extremely low gestational age neonates. J Perinatol 33, 867–871 (2013). [DOI] [PubMed] [Google Scholar]
- Woodward L. J., Anderson P. J., Austin N. C., Howard K. & Inder T. E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355, 685–694 (2006). [DOI] [PubMed] [Google Scholar]
- al Naqeeb N., Edwards A. D., Cowan F. M. & Azzopardi D. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics 103, 1263–1271 (1999). [DOI] [PubMed] [Google Scholar]
- Hellstrom-Westas L. & Rosen I. Continuous brain-function monitoring: state of the art in clinical practice. Semin Fetal Neonatal Med 11, 503–511 (2006). [DOI] [PubMed] [Google Scholar]
- Burdjalov V. F., Baumgart S. & Spitzer A. R. Cerebral function monitoring: a new scoring system for the evaluation of brain maturation in neonates. Pediatrics 112, 855–861 (2003). [DOI] [PubMed] [Google Scholar]
- Papile L. A., Burstein J., Burstein R. & Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92, 529–534 (1978). [DOI] [PubMed] [Google Scholar]
- de Vries L. S. Neurological assessment of the preterm infant. Acta paediatrica 85, 765–771 (1996). [DOI] [PubMed] [Google Scholar]
- Inder T. E., Wells S. J., Mogridge N. B., Spencer C. & Volpe J. J. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr 143, 171–179 (2003). [DOI] [PubMed] [Google Scholar]
- Palisano R. et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39, 214–223 (1997). [DOI] [PubMed] [Google Scholar]


