Abstract
Activation of primordial follicles into the growing pool, selection of the dominant follicle, and its eventual ovulation require complex endocrine and metabolic interactions as well as intraovarian paracrine signals to coordinate granulosa cell proliferation, theca cell differentiation, and oocyte maturation. Early preantral follicle development relies mostly upon mesenchymal-epithelial cell interactions, intraovarian paracrine signals, and oocyte-secreted factors, whereas development of the antral follicle depends on circulating gonadotropins as well as locally derived regulators. In women with polycystic ovary syndrome (PCOS), ovarian hyperandrogenism, hyperinsulinemia from insulin resistance, and altered intrafollicular paracrine signaling perturb the activation, survival, growth, and selection of follicles, causing accumulation of small antral follicles within the periphery of the ovary, giving it a polycystic morphology. Altered adipocyte-ovarian interactions further compound these adverse events on follicle development and also can harm the oocyte, particularly in the presence of increased adiposity. Finally, endocrine antecedents of PCOS occur in female infants born to mothers with PCOS, which suggests that interactions between genes and the maternal-fetal hormonal environment may program ovarian function after birth.
Keywords: Androgens, antimullerian hormone, growth differentiation factor-9, insulin, polycystic ovaries
Polycystic ovary syndrome (PCOS) is the most common cause of infertility in women (1), so it is essential to understand what endocrinologic, molecular, metabolic, and/or genetic factors contribute to this disease and how they alter ovarian follicle development (2–4). According to the Rotterdam criteria, PCOS is characterized by two of the following three features: [1] clinical or biochemical hyperandrogenism, [2] oligoanovulation, and [3] polycystic ovaries (PCO), excluding other endocrinopathies (5). Patients with PCOS also often present with elevated serum levels of luteinizing hormone (LH) and insulin. Although primordial follicles in the ovaries of PCOS patients leave the resting pool, most arrest at the small antral stage preceding dominant follicle selection, giving rise to many small follicles forming a ring around the ovary as a characteristic of PCO. Abnormal follicle growth in PCOS accompanies elevated levels of LH, insulin, and androgens; altered expression of transforming growth factor-β (TGF-β) family members; and comparatively low levels of follicle-stimulating hormone (FSH) (4, 6). Studies have indicated that the expression and impact of these factors may vary in PCOS patients by phenotype and may be further influenced by the degree of adiposity (7–10).
This review integrates what is currently known about normal follicular development with specific events that are altered in the follicles of PCOS patients. Mechanisms by which LH hypersecretion, hyperandrogenism, hyperinsulinemia, and TGF-β-related events alter ovarian follicle development are discussed, along with the potential roles of the insulin-like growth factor-I (IGF-I)/AKT/Forehead BoxO (FOXO) pathway and WNT signaling in these events. Due to space limitations, not all aspects of follicle development are covered in depth.
PREANTRAL FOLLICLE DEVELOPMENT
Overview
Primordial follicles are recruited into a cohort of growing follicles, from which one antral follicle is selected to become dominant while the others undergo atresia. Each primordial follicle contains an oocyte arrested at the diplotene stage of prophase one, surrounded by squamous granulose cells. With follicle growth, the oocyte begins to synthesize messenger ribonucleic acid (mRNA), while squamous granulosa cells enlarge into a complete single layer of cuboidal granulosa cells, forming the primary follicle (11, 12).
The Primordial to Primary Transition
Primordial follicles can remain in the quiescent “resting” stage for many years. The precise signals that initiate the transition of a primordial follicle to a growing primary follicle are incompletely understood but are independent of gonadotropins (13). Rather, factors derived from oocyte and granulosa cells activate primordial follicles or inhibit them (12, 14). In the oocyte, the PI3K pathway is critically involved in maintaining oocyte quiescence. Disruption of Pten, Foxo3, or other PI3K pathway genes in mice leads to oocyte activation, global transition of primordial follicles into the growing pool, and premature ovarian failure (15–18). Conversely, granulosa cell antimüllerian hormone (AMH) production, beginning in primary follicles, acts to reduce the number of primordial follicles leaving the resting pool (19–22). Although granulosa cell AMH produced in response to oocyte-derived factors (23, 24) inhibits primordial follicle growth, disruption of Amh gene expression does not cause a reciprocal activation of all primordial follicles, implying that other factors, including granulosa cell-derived kit ligand (KL) and its receptor c-kit on oocytes (11, 12, 25), also contribute to the initiation of primordial follicle development and oocyte growth (25–27). Whether this initial transition process is altered in the ovaries of PCOS patients remains unclear.
Formation of Primary Follicles
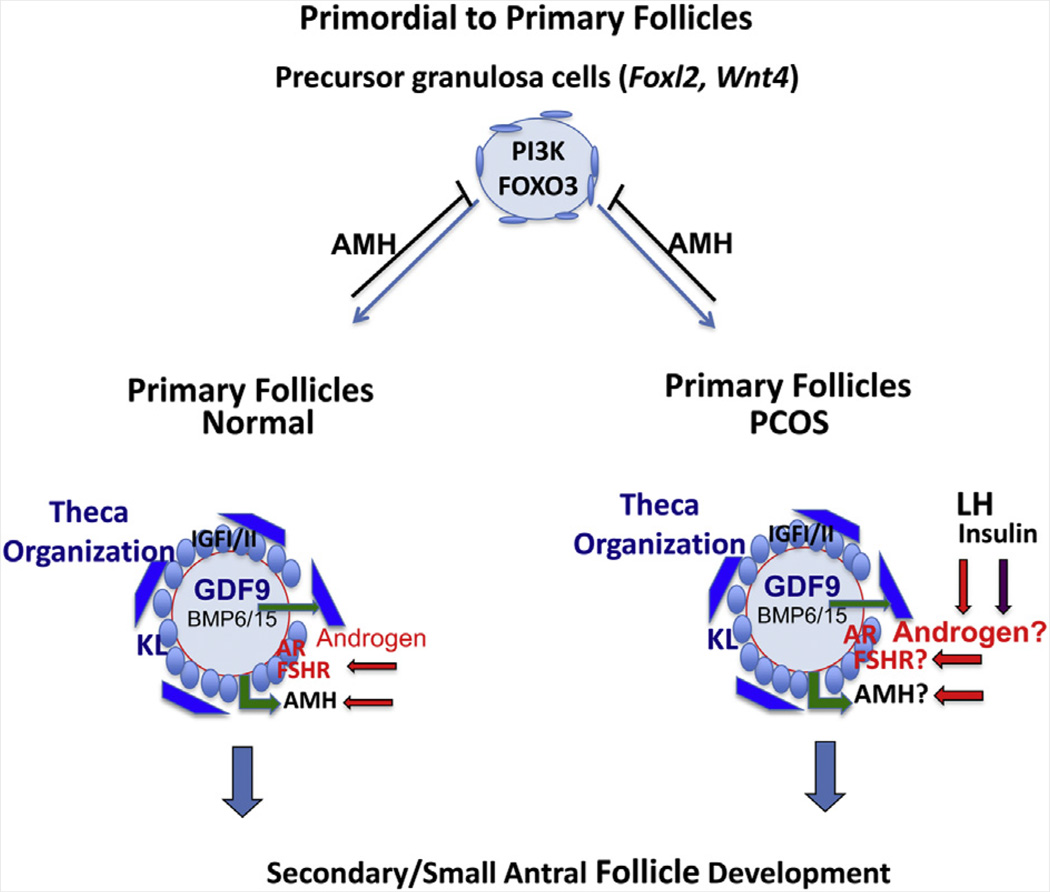
Once follicles leave the primordial stage to enter the growing pool, specific changes in the oocyte, granulosa cell, and theca cell functions occur, including [1] transition of granulosa cells from a flattened fibroblastic-like morphology to a cuboidal shape, [2] appearance of the zona pellucida, and eventually [3] formation of the theca cell layer external to granulosa cells and resting upon the basal lamina (Fig. 1).
FIGURE 1.
Transition of primordial to primary follicles. Embryonic specification of the female gonad and formation of primordial follicles depends on WNT4 and FOXL2. Quiescent primordial follicles leave the resting pool by mechanisms that involve changes in both oocyte (activation of the PI3K pathway) and somatic cell (levels of AMH) functions. Primary follicle formation is independent of gonadotropins but is associated with changes in granulosa cell function and morphology leading to expression of IGF-I/-II, FSHR, AMH, AR, and KL. Changes in oocyte functions lead to the production of the zona pellucida and expression of GDF9. KL and GDF9 coordinate to regulate formation of the theca cell layer. GDF9 also appears to enhance theca cell androgen production, which in turn can increase the expression of FSHR. In PCOS, high levels of insulin and LH may act on cognate receptors in theca cells, once organized, to further increase androgen production. Levels of FSHR (and AMH?) are elevated; however, some data indicate that AMH is lower (262). Large gray circle: oocyte; red line: zona pellucida; small blue circles: granulosa cells; dark blue boxes: theca cells. Oocyte factor and GDF9 signaling is denoted by green arrows; LH and androgen signaling by red arrows and letters; and insulin signaling by purple arrows. Blue arrows indicate that these primary follicles go on to develop into secondary follicles.
Formation of the theca cell layer in primary follicles critically depends on oocyte-derived growth differentiation factor 9 (GDF9) (28, 29) and the granulosa-derived factor KL (11, 12). In addition, GDF9 enhances androgen production in cultured small follicles by regulating theca cell androgen production either directly or indirectly (25, 30–32). Theca cell-derived androgens, in turn, serve essential regulatory roles by increasing the expression of FSH receptors (Fshr) in vivo and in vitro (33, 34). In primates, testosterone administration to adult female rhesus monkeys increases the number of growing preantral and small antral follicles (35, 36); up-regulates mRNA expression of FSH receptors, IGF-I receptors, and IGF-I in proliferating granulosa cells (35, 37, 38), and enhances IGF-I and IGF-I receptor mRNA expression in primordial follicle oocytes (37). These androgen actions likely occur via the androgen receptor (AR), which is present in human and mouse primary follicles (39, 40) and if disrupted in mice reduces Fshr and Kitl expression, impairs folliculogenesis, and induces premature ovarian failure (41–43). Moreover, targeted loss of AR signaling exclusively in murine granulosa cells of preantral and antral follicles also reduces fecundity, induces follicular atresia, and impairs oocyte fertilization as well as preimplantation embryogenesis (44, 45).
Receptors for LH (LHCGR) are present in theca (but not granulosa) cells of small secondary follicles (but not primordial follicles), allowing LH-stimulated androgen production in follicles at this early stage (46). The factors that induce LH receptors are unknown but could be GDF9 or other oocyte- or granulosa cell–derived factors (IGF-I or IGF-II?), retinoic acid signaling (47), or other yet to be identified factors.
Insulin also acts through its own receptors on theca cells, stroma, granulosa, and oocytes to promote the primordial to primary follicle transition (48, 49). This is important for many women with PCOS who have hyperinsulinemia from insulin resistance beyond that predicted by body mass index (BMI) alone, with 50% to 70% of such women demonstrating insulin resistance (50). Hyperinsulinemia in PCOS results from abnormal postreceptor signal transduction, which reduces insulin-mediated glucose uptake (9) without affecting steroidogenesis (51, 52). Thus, insulin excess stimulates theca cell CYP17a activity (53), amplifies LH- and IGF-I-stimulated androgen production (54, 55), elevates serum free testosterone levels through decreased hepatic sex hormone-binding globulin (SHBG) production, and enhances serum IGF-I bioactivity through suppressed IGF-binding protein (IGFBP) production, thereby perpetuating ovarian hyperandrogenism (52). High insulin levels could theoretically act through IGF-I receptors to exert some of these effects; insulin stimulation of human granulosa cell steroidogenesis, however, is mostly mediated through its own receptor because this action is inhibited by blocking with antibody to the insulin receptor, but not to the IGF-I-receptor (56). Insulin (at high levels) may also enhance GDF9-mediated increases in androgen production in primary and small follicles (30).
Given this background, hyperandrogenism, hyperinsulinemia, and/or LH hypersecretion in PCOS patients would be expected to stimulate early follicle development, increase Fshr expression prematurely or to an exaggerated level, and render granulosa cells prematurely more responsive to FSH, perhaps through enhanced FSH-stimulated cyclic adenosine 3′ :5′ monophosphate (cAMP) production (57, 58). Conversely, the ability of AMH to decrease the expression of Fshr and the response of granulosa cells to FSH (59) would be expected to blunt the effects of FSH, at least temporarily, in early growing follicles but not necessarily in PCOS follicles (as will be discussed).
In support of this, in vitro studies of PCOS theca cells show intrinsically increased androgen biosynthesis and augmented expression of several steroidogenic enzymes (60, 61), presumably from the enhancing effects of the retinoic acid pathway (47) and dysregulation of theca cell mitogen activated protein kinase signaling (62). However, androgen production has not been established for normal or PCOS primordial or primary follicles, and reports using in situ hybridization indicate that the ovaries of anovulatory women with PCOS have increased primary follicle growth with reduced oocyte GDF-9 mRNA (63, 64). Lower levels of GDF9 in PCOS follicles at this stage might be expected to reduce theca cell organization or androgen production, but this has not yet been investigated.
Insulin may compensate for reduced GDF9. Furthermore, histologic examination of human ovaries from women with PCOS has found an enhanced number of growing primary follicles and a reciprocally decreased proportion of primordial follicles, independent of ovulatory status or atresia (65, 66). This may be related to the lower level of AMH (but not necessarily AMHR2 levels) that is observed in primordial and transitional follicles in anovulatory PCOS ovaries (39, 67). Primary PCOS follicles also show increased granulosa cell proliferation and enhanced oocyte growth (63), with decreased atresia of preantral PCOS follicles grown in vitro, possibly from enhanced survival (68). However, the roles of GDF9, AMH, and AMHRII during the primordial to primary transition in normal and PCOS follicles remain to be clearly defined with a larger number of samples, especially for GDF9 and AMHR2 expression, and functional data. Studies in nonhuman primates and other species will be especially important in this regard.
The Primary to Secondary Follicle Transition
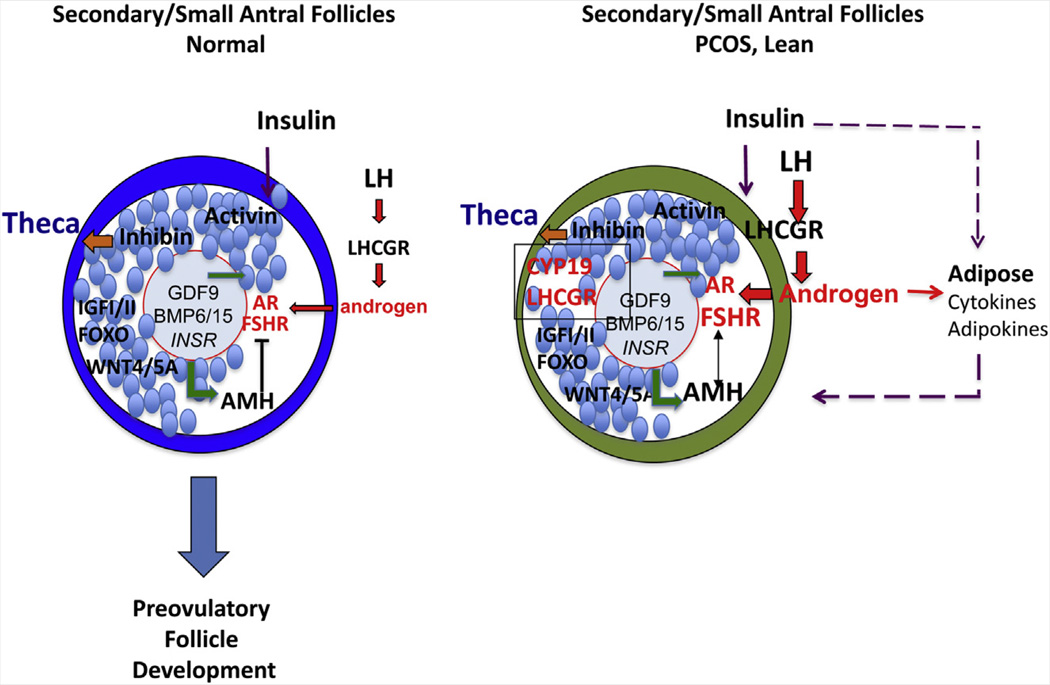
Secondary follicles develop over several months, acquire additional steroid receptors, and become physiologically coupled by gap junctions (Fig. 2 and Fig. 3) (12, 69). As these secondary follicles grow, they become more dependent on gonadotropins, likely because of the spatial changes within the follicle and receptor levels/activity. As granulosa proliferate and an antrum begins to form, the granulosa cells and the theca cells become spatially distanced from the oocyte and its secreted factors. Thus, at the secondary stage, LH may become more important than GDF9 in promoting theca androgen biosynthesis and affecting granulosa cell responsiveness to FSH relative to AMH.
FIGURE 2.
Transition from primary to secondary/small antral follicles. Granulosa cells proliferate and follicles grow in response to multiple factors including activin, IGF-I, WNT4/5a, and FSH. The transcription factor FOXO1 impacts both follicle growth and follicle atresia (see the text for details). Consequently, as the distance between the oocyte and the theca layer is increased, theca cell production of androgens becomes more dependent on LH and possibly inhibin-α. Levels of AMH increase and negatively regulate FSH action in granulosa cells to prevent premature maturation. In lean PCOS patients, elevated LH and insulin levels increase theca cell androgen production, which enhances FSHR expression and premature differentiation of granulosa cells via induction of aromatase (CYP19a1), LH receptor (LHCGR), and other genes including RUNX2 (102). WNT4/5a may contribute to the enhanced actions of FSH to induce CYP19a1. Enhanced FSH actions override those of elevated AMH. Large gray circle: oocyte; red line: zona pellucida; small blue circles: granulosa cells; large blue circle: normal theca cells; green circle: PCOS theca. Oocyte factor and GDF9 signaling is denoted by green arrows; inhibin by the orange arrow; LH and androgen signaling by red arrows and letters; and insulin signaling by purple arrows and letters. Large blue arrow indicates continued normal follicular growth.
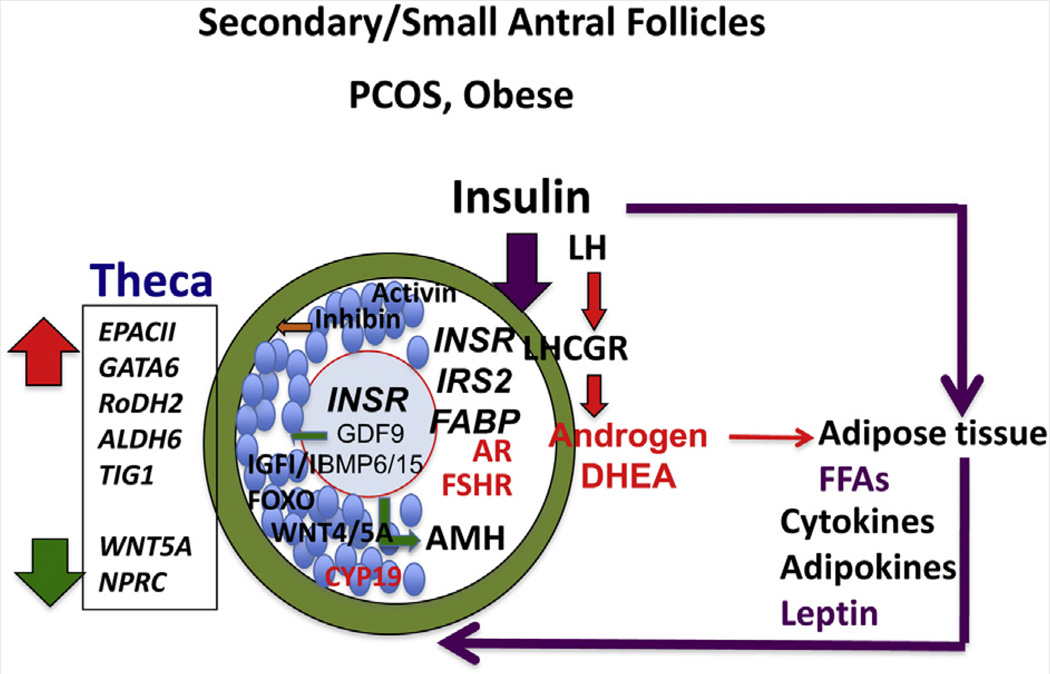
FIGURE 3.
Transition from primary to secondary/small antral follicles. In obese PCOS patients, hyperinsulinemia from adiposity-dependent insulin resistance and altered adipogenesis impair follicular development by mechanisms that appear to differ from those in lean PCOS patients. Insulin may have a greater influence than LH on theca, granulosa, and likely oocyte functions. Adipokines also likely alter each cell type within the follicle. Note that insulin receptor (INSR) expression may be elevated in oocytes of PCOS patients compared to non-PCOS patients (205). Cumulus cells of obese PCOS patients also express higher levels of INSR and specific fatty acid binding proteins (FABPs) (102). Theca cells produce elevated levels of androgens, especially DHEA, in response to LH and elevated expression of CYP17A1 and CYP11A1, which are targets of GATA6 and retinoic acid (RA) (47). The PCOS theca cells exhibit increased expression of enzymes controlling RA production and enhanced responses to RA, including steroidogenesis and the target gene TIG1. These responses may be related to cAMP signaling through exchange protein activated by cAMP II (EPAC II). Conversely, PCOS theca cells exhibit reduced expression of WNT signaling and cGMP signaling pathways, but the functional significance of this remains to be determined. Oocyte factor and GDF9 signaling is denoted by green arrows; inhibin by the orange arrow; LH and androgen signaling by red arrows and letters; and insulin signaling by purple arrows and letters.
Little is known about secondary follicle development in humans due to the scarcity of this follicle stage in archived ovarian tissue (65, 68). Nevertheless, IGF-II mediates FSH-induced growth of cultured human preantral follicles (70). In PCOS, therefore, when LH and/or androgens are elevated, an increased response of granulosa cells to FSH may lead to FSH-stimulated expression of aromatase (Cyp19) and other genes along with additional developmental changes (46, 71) that promote FSH action despite the repressor effects of AMH, particularly in the presence of insulin excess. This may allow FSH and AMH to functionally coexist.
The IGFI/II/FOXO Pathway
Insulin-like growth factor-I or IGF-II (in humans) is expressed in the granulosa cells of small growing follicles and can enhance FSH-mediated granulosa cell differentiation. This is related to activation of the PI3K and ERK1/2 pathways and to regulation of Forehead BoxO transcription factors. Both FOXO1 and to a lesser extent FOXO3 are highly expressed in granulosa cells of mouse, rat, nonhuman primates, and humans (72–75). These transcription factors have been evolutionarily conserved from Caenorhabditis elegans to man, are downstream of AKT in the PI3K pathway, and impact reproductive success (75–77). Therefore, FOXO1/3 are targets of both IGF-I/IGF-II and presumably the insulin pathways in granulosa cells.
Studies have shown that Foxo1/3 mutant mice are completely infertile and exhibit impaired follicular development as well as impaired apoptosis (77). Detailed molecular analyses of the mutant ovaries in these mice document that FOXO1/3 interact with activin to control genes that promote follicle growth and interact with bone morphogenetic protein-2 (BMP2) to direct apoptosis. What controls this switch remains to be determined but is obviously critical for determining the fate of follicles, of which 99% undergo apoptosis.
The roles of FOXO1/3, IGF-I, and IGF-II in preantral PCOS follicles have not been fully explored, although increased circulating IGF-I bioavailability in women with PCOS could have a permissive effect (78). Because IGF-I receptors are present on mouse oocytes, this signaling pathway may impact oocyte quality as well. In vitro studies using synthetic IGF-I analog with low IGFBP affinity show that an inappropriate increase in IGF bioavailability is detrimental to bovine oocytes of preantral follicles (79).
The WNT/Frizzled Pathway
The WNT/Frizzled pathway is essential for specification of the mouse and human embryonic ovary and is crucial for the transition of secondary follicles to the antral stage (80, 81). Its actions, due in part to activation of β-catenin (CTNNB1), include enhancement in gene expressions of Fshr and aromatase (Cyp19a1) (80, 82, 83). The actions of WNT4 are tightly linked to those of FOXL2, a transcription factor that also is essential for embryonic specification of the ovary and suppresses steroidogenic gene expression while promoting activin expression (73, 84). Microarray data show that WNT signaling is repressed in PCOS theca cells (85), with several genes of the WNT signaling pathway dysregulated in ovaries of both PCOS patients and androgen-treated female-to-male transsexuals, implicating an androgen influence on this pathway (86). However, the expression levels and functions of WNT4/5A in granulosa cells of normal and PCOS patients remain to be determined.
ANTRAL FOLLICLE DEVELOPMENT
Overview
Growth of follicles from the primordial to preovulatory stage in women takes approximately 6 months, with the final 2 weeks of antral follicular development depending on changing circulating gonadotropin levels (87). Antral follicle development is characterized by slower oocyte growth (maximum diameter 140 µm), extracellular fluid formation, granulosa cell differentiation into mural and cumulus phenotypes, and selection of a dominant follicle (11, 12). Early antral follicles in humans are responsive to FSH (12, 69). In follicles 6–8 mm in size, granulosa cells begin to express CYP19A1 (aromatase) (88), allowing theca-derived androgens to undergo aromatization to estrogens by FSH-stimulated granulosa cells. The LH-stimulated thecal cell androstenedione production via CYP17A1 is enhanced by granulosa cell-derived paracrine factors (89). These paracrine factors include inhibins, IGF-I, and IGF-II as well as retinoic acid, which stimulate thecal cell androgen production; conversely, follistatin binds to activin to inhibit its androgen-suppressing effect (90).
Progressive growth of the antral follicle coincides with gradual acquisition of oocyte developmental competence, defined as the ability of the oocyte to complete meiosis and undergo fertilization, embryogenesis, and term development (6). Oocytes from healthy large antral follicles, exposed to an appropriately timed progression of changes in the intrafollicular microenvironment, are more likely to fertilize and undergo successful embryogenesis than similarly matured oocytes from small antral follicles (6).
Hyperandrogenism
Elevated androgens appear to increase pituitary LH pulsatility and secretion, leading to enhanced theca cell stimulation through reduced hypothalamic sensitivity to steroid feedback, which can be established in utero or postnatally (91–95). Increased androgen biosynthesis appears to enhance the expression of FSH receptor, leading to an increased estradiol (E2) responsiveness of PCOS granulosa cells to FSH in vitro (96, 97). Despite this phenomenon, follicular arrest in PCOS patients occurs at the stage when granulosa cells normally begin to express aromatase and secrete E2 (6, 88). Androgen excess together with premature differentiation of granulosa cells overexpressing LH receptor preempts FSH action on E2 production in favor of progesterone synthesis (56, 98–101), perhaps in combination with a local inhibitor of FSH action (96). Elevated expression of LH receptor mRNA and genes associated with differentiation, such as STAR and RUNX2, have also been reported in the cumulus cells of lean PCOS patients but not of obese PCOS patients (102), indicating that altered cumulus cell functions likely impact oocyte quality and maturation in this context.
Androstenedione biosynthesis and the steroidogenic enzyme CYP17A1 are elevated in PCOS theca (60, 61, 96). As a result, serum androstenedione levels positively correlate with antral follicle number in normal women and those with PCOS (103, 104), while antiandrogen therapy to PCOS patients improves PCO morphology (105). Small PCOS follicles also have elevated 5α-reductase activity, which elevates 5a–reduced androgen levels to concentrations capable of inhibiting, rather than increasing, granulosa cell aromatase activity in vitro (58, 106, 107). Consistent with this finding, dihydrotestosterone (DHT) impairs gonadotropin-stimulated E2 secretion in female rhesus monkeys (108) and can inhibit gran-ulosa cell proliferation in rodents in certain contexts (109). Clearly the effects of DHT are dose and concentration dependent, and the beneficial effects of androgens can support follicle development (110) in part by regulating the FSH receptor (33, 34).
High androgens in small antral PCOS follicles may also decrease rates of in vitro maturation, fertilization, and embryo development compared with immature oocytes from normal women (111) by interfering with E2-mediated effects that promote oocyte cytoplasmic maturation (112–114).
Hyperinsulinemia
Insulin can enhance granulosa cell responsiveness to LH (56, 98), implying that premature follicle luteinization may occur in hyperinsulinemic PCOS patients (99). As evidence, cultured granulosa cells from small PCOS follicles exhibit premature responsiveness to LH due to early expression of LH receptors (3, 99, 100), leading to enhanced progesterone production (101). Such premature follicle luteinization facilitated by insulin excess may also affect human oocyte development as well because insulin receptors are present in oocytes (48). As an example, insulin together with FSH up-regulates LH receptor expression in cultured mouse cumulus-oocyte complexes and reduces blastocyst development (115). One caveat of these studies, however, is the extremely high levels of insulin that were used.
Hyperinsulinemic PCOS patients undergoing gonadotropin therapy develop a larger number of follicles between 12 and 16 mm in diameter (116). Of relevance, mRNA expressions of insulin receptor (INSR) and specific fatty acid binding proteins (FABP) are elevated in cumulus cells of obese PCOS compared to lean PCOS patients (102) and the INSR also may be elevated in oocytes of PCOS versus non-PCOS patients (205). These distinctly different patterns of insulin-related gene expression suggest that the cumulus cells and oocytes of obese PCOS patients have different responses to insulin than those of lean PCOS patients. Because insulin can bind directly to oocytes, a direct effect of insulin excess may alter oocyte quality (117).
Increased Vascular Endothelial Growth Factor (VEGF)
Vascular endothelial growth factor A (VEGF-A) and its related protein members (VEGF-B, VEGF-C, and VEGF-D) of the platelet-derived growth factor family are potent cytokines that promote angiogenesis and vascular permeability (118). Vascular endothelial growth factor gene expression occurs in normal and PCOS ovaries (119) and increases after human chorionic gonadotropin (hCG) administration in human luteinized granulosa cells (120, 121), elevating VEGF protein levels in blood and follicles as well as in peritoneal fluids of women at risk of or with ovarian hyperstimulation syndrome (OHSS) (121–123). Ovarian VEGF synthesis and vascular blood flow as well as serum VEGF levels are increased in PCOS patients (124–126). Consequently, enhanced VEGF-mediated vascular permeability and fluid extravasation can occur in PCOS patients undergoing controlled ovarian stimulation, increasing their risk of developing the complications of OHSS, including ascites, intravascular fluid depletion, hemoconcentration, hypercoagulation, hypotension, pleural effusion, hematologic and liver dysfunction, renal failure, and respiratory distress (127). This increased risk of OHSS also is linked with hyperinsulinemia because in vitro VEGF-A production by luteinized granulosa cells of PCOS patients compared with normal women is preferentially enhanced by synergistic interactions between LH/hCG and insulin (128). Whether other factors, including angiopoietins, further alter the ovarian vascular network in PCOS, as they do in the DHEA-treated rodent model of PCOS (129), remains to be determined.
Increased AMH Production
Antimüullerian hormone is normally produced by the granulosa cells of growing follicles (67, 130), so low AMH levels occur in primordial and primary follicles, increase to maximal levels in large preantral and small antral stages, and then decline in granulosa cells but not cumulus cells during final follicular maturation (67, 131–133). As a marker of growing follicles in normal women undergoing in vitro fertilization (IVF), serum AMH levels positively correlate with the number of antral follicles, the serum androgen concentration, and the oocytes retrieved, and negatively correlate with amount of recombinant human FSH administered (132, 134). Intrafollicular AMH levels also negatively correlate with FSH levels in the follicles of normal women undergoing IVF (135).
Women with PCOS have elevated circulating and intrafollicular AMH levels related to an increase in the number of follicles as well as from hypersecretion by granulosa cells themselves (59, 136–139). With AMH production in anovulatory PCOS significantly higher on the basis of the granulosa cell per se, raised serum AMH levels in PCOS patients represent both enhanced production by granulosa cells and an increased number of follicles. Consequently, serum AMH levels are elevated in normoandrogenic women with PCO undergoing ovarian stimulation for IVF, and are further increased in hyperandrogenic women with PCO, independent of antral follicle number (133). Some investigators have suggested that serum AMH levels (>35 pmol/L, or >5 ng/mL) may serve as a surrogate marker of PCOS, although insufficient data exist regarding the ability of circulating AMH determinations to discriminate PCOS phenotypes by patient age (140).
An inverse relationship between serum AMH and E2 levels in PCOS agrees with the ability of AMH to decrease FSH receptor mRNA expression and impair FSH-induced aromatase activity in vitro (59, 138, 139, 141). However, other data show that mRNA levels for Amh, Fshr, and Ar are all higher in small and large follicles obtained from hormone-stimulated PCOS patients compared with those from control patients (142). These results indicate that in PCOS antral follicles the inhibitory effects of AMH are reduced/altered. The question is why? The possible explanations are multiple and need to be resolved. For example, follicle development may differ among women, with lean PCOS patients exhibiting follicles at a more differentiated stage and obese patients having follicles at a less differentiated stage (see Fig. 2). Alternatively, androgens that enhance FSH-receptor expression (33) when combined with the mitogenic actions of growth factors (i.e., LH, epidermal growth factor, TGF-β, IGF-I, or insulin) on PCOS granulosa cells (6, 12, 48) may overcome AMH inhibition of FSH-dependent aromatase activity. In light of these considerations, serum AMH and LH levels are positively linked in severe PCOS (143, 144), with AMH secretion by cultured PCOS granulosa cells enhanced by LH (136), perhaps from premature differentiation due to early expression of LH receptors (3, 99, 100). Collectively, serum AMH concentrations in PCOS are [1] positively predicted by LH and testosterone levels as well as antral follicle number, [2] variably related to Fshr expression or action, [3] negatively predicted by BMI (144), and [4] variably reduced by weight loss (145, 146) or metformin (134, 147), suggesting complex interactions between LH, androgen, and insulin with ovarian function in obese women with PCOS.
Inhibins, Activins, and Follistatin
Granulosa cell-derived inhibins and activins belong to the TGF-β superfamily. Follistatin binds activin with high affinity to inhibit its action (148). In addition, FSH increases activins and inhibin, which in turn regulate granulosa cell, theca cell, and pituitary Fshb cell functions (29, 84).
Inhibin A occurs in follicles as small as 6.5 mm and increases in parallel with α-subunit and βA-subunit mRNA expression during antral follicle growth (149, 150). Inhibin A suppresses FSH synthesis by inhibiting Fshb gene transcription (151) and enhances LH-stimulated androgen biosynthesis (152). In contrast, intrafollicular inhibin B levels do not necessarily increase, nor does its βB-subunit mRNA expression vary, by follicle size (149, 150). Activin promotes follicle growth by stimulating granulosa cell proliferation while delaying luteinization, increasing granulosa cell FSH receptor expression and E2 synthesis (i.e., enhanced FSH-induced Cyp19, Ccnd2, and Inha and other genes) and decreasing androgen production (148, 153). Activin actions are balanced by bone morphogenetic proteins (BMPs) within the ovary, such as BMP6 and BMP15 (oocyte), BMP4 (theca), and BMP2 (apoptotic granulosa cells) (84, 154, 155). Collectively, activins promote follicular development by enhancing granulosa cell responsiveness to FSH and by suppressing androgen synthesis, while inhibins produced by the dominant follicle stimulate theca cell androgen production (89, 148–150).
Inhibin α-subunit and βA-subunit mRNA levels are reduced in granulosa cells of small PCOS follicles (156). Inhibin A and B concentrations also are decreased in some, but not all, small PCOS follicles, despite normal amounts of activin and follistatin unbound to activin (150, 157). Although exogenous FSH stimulates the growth of PCOS follicles, intrafollicular inhibin A levels in PCOS patients receiving gonadotropin-releasing hormone (GnRH) analog/gonadotropin therapy for IVF remain reduced (158). On the other hand, serum inhibin B levels arising from the multiple small follicles are elevated in PCOS patients (159) and are suppressed by exogenous hCG and endogenous insulin (160), linking defective inhibin biosynthesis with follicular arrest, likely by reducing FSH levels (151).
High follistatin and low activin A levels also have been reported in the circulation of some PCOS patients (161, 162). Follistatin levels in small human antral follicles, however, are 10-fold greater than those of activin A levels, which brings into question the role of activin A bioactivity in these follicles (163). Thus, the overall impact of the activin and inhibin pathways on PCOS remains unclear.
Adipocyte-Ovarian Interactions
Adiposity-dependent insulin resistance, although not necessarily intrinsic to PCOS, has become inextricably linked with the syndrome (164). Greater total and abdominal obesity as well as insulin resistance, menstrual irregularity, and hyperandrogenism occur in women with classic PCOS, while weight loss in obese women with PCOS lowers circulating LH, androgen, and insulin levels, thereby improving hirsutism and menstrual and ovulatory dysfunction as well as dyslipidemia (4, 165–169).
In addition to obesity, insulin resistance in PCOS is greater than that predicted by BMI, with 40% to 50% of women with PCOS being nonobese (170). Nevertheless, adipose function is important because adipose tissue secretes many factors that not only regulate adipocytes, macrophages, and pluripotential cells but also likely alter ovarian function in PCOS (171, 172). These factors may act indirectly or directly on granulosa cells (i.e., IL6, leptin, adiponectin) or cumulus cells and oocytes (leptin, adiponectin) (173). Adiponectin has many functions (174), including its ability to promote oocyte maturation and blastocyst formation (175), and is reduced in the circulation of women with PCOS (176), together with tumor necrosis factor-α-induced dysregulation of adiponectin secretion by PCOS adipocytes in vitro (177). In addition, leptin impairs FSH-stimulated steroidogenesis in human granulosa cells, thereby reducing ovarian responsiveness to FSH (178–181). Obesity also lowers LH pulse amplitude and alters LH pharmacokinetic structure (182, 183), contributing to reduced serum LH levels in obese women with PCOS (184, 185), who may be more susceptible to the modulatory effects of insulin excess than LH.
THE DIFFERENTIATED PREOVULATORY FOLLICLE
Overview
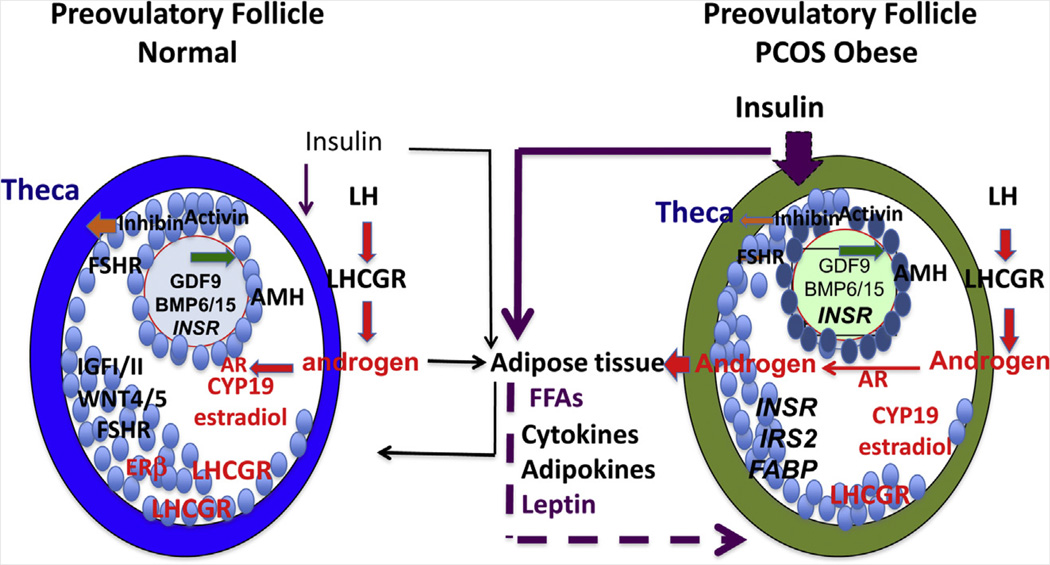
The hallmarks of normal preovulatory follicles are acquisition of LH receptors on mural granulosa cells, elevated expression of aromatase (Cyp19a1) with increased E2 production, and markedly enhanced responsiveness of granulosa cells to FSH and LH via increased cAMP production (Fig. 4) (186–189). Only preovulatory follicles ovulate in response to the LH surge, and only preovulatory follicles express high levels of LH receptor in granulosa cells, a response mediated by the coordinate actions of FSH and E2 or FSH and androgens (187).
FIGURE 4.
Preovulatory follicles. The hallmarks of preovulatory follicles are the induction of LH receptors (LHCGR) and elevated expression of aromatase (CYP19A1). In obese individuals with PCOS, preovulatory follicles that are selected and ovulate (either in response to weight reduction, exogenous hormone, and/or metformin) express high AMH and remain hyperandrogenic. Thus, despite lowered insulin action or improved adipogenic function to facilitate the growth of follicles that can be ovulated, theca androgen production is central to the PCOS condition. However, direct effects of insulin and/or androgens may harm oocytes at this late stage or earlier stages of follicular development. Oocyte factor and GDF9 signaling is denoted by green arrows; LH and androgen signaling by red arrows and letters; and insulin signaling by purple arrows.
With the midcycle LH surge, the preovulatory follicle shifts steroidogenesis from androgen and estrogen to progesterone production (87). Critical events induced in granulosa cells by the LH surge depend on induction of epidermal growth factor-like factors (amphiregulin, epiregulin, and β-cellulin) (190, 191), which activate the epidermal growth factor receptor, leading to activation of RAS and the MEK/MAPK1/3 (ERK1/2) pathway. This pathway is essential for initiation of meiosis, cumulus cell oocyte complex expansion, and follicle rupture (190–192). As a result, bidirectional cumulus-cell–oocyte signaling through cGMP (193–196) and gap junctions (197, 198) is reduced to permit the oocyte to undergo germinal vesicle breakdown and produce a haploid metaphase II oocyte (i.e., nuclear maturation) capable of cytoplasmic maturation, fertilization, and initial embryonic development (199–202).
Oocyte Developmental Competence
Terminally differentiated follicles of classic PCOS patients undergoing ovarian stimulation for IVF remain hyperandrogenic (203, 204) and contain normal appearing metaphase II oocytes with distinctly abnormal gene expression profiles (205) involving signal transduction, cell metabolism, DNA transcription, and RNA processing. These genes often contain promoter sequences with putative binding sites for androgen receptor, peroxisome proliferating receptor-γ, and/or peroxisome proliferating receptor γ-retinoid × receptor, linking hyperandrogenism with insulin resistance in the developmental fate of the oocyte. With androgen and insulin levels in the follicles of IVF patients determined by PCOS and BMI, respectively (203, 206), obese PCOS patients experience a high miscarriage rate after transfer of normal appearing embryos into a surrogate uterus (207).
Impaired oocyte developmental competence, however, is not a universal finding in PCOS, and it is related to many factors (207–213). For example, impaired oocyte competence has been linked with elevated follicle fluid levels of tumor necrosis factor-α (214) and interleukins (215), whereas other adipokines have a positive action on cumulus-oocyte functions (173, 175). This constellation of findings may involve ovarian cell lipotoxicity, as shown in mice fed a high-fat diet, whereby poor oocyte quality is associated with increased lipid content and reduced mitochondrial membrane potential, cumulus-oocyte complexes with increased gene expression of endoplasmic reticulum stress markers, increased granulosa and cumulus cell apoptosis, and abnormal embryogenesis (168, 216–220). The degree to which ovarian cell lipotoxicity contributes to impaired oocyte developmental competence in women with PCOS remains to be determined.
Ovarian Aging
Anovulatory women with PCOS can resume ovulation as serum AMH and androgen levels diminish with age (221–225). Despite an age-related decline in AMH, serum AMH levels remain twofold to threefold higher in women with PCOS compared with normal women in the fourth decade of life, predicting an estimated delay in menopause of about 2 years (134, 226, 227). Whether delayed ovarian aging in PCOS is biologically relevant is unclear, although PCOS patients undergoing ovarian stimulation for IVF between the ages of 22 and 41 years do not exhibit the reduced number of oocytes retrieved and live-birth rates typical of IVF patients of similar age with tubal factor infertility (228). Whether prolonged survival of PCOS follicles in vivo, despite increased primordial follicle recruitment, accounts for a larger follicle pool throughout reproductive life remains to be established (68).
ENDOCRINE ANTECEDENTS TO PCOS
Some evidence indicates that PCOS has been evolutionarily conserved and may have a genetic basis (4). In addition, infant girls born to mothers with PCOS exhibit overproduction of AMH, which persists into prepubertal life (229) and is variably improved, along with exaggerated E2 responsiveness to leuprolide administration when their mothers received metformin during pregnancy, particularly beginning at conception (230, 231). Increased ovarian volume and hyperinsulinemia also occur in the prepubertal daughters of mothers with PCOS, accompanied by hyperandrogenism later in puberty (232, 233).
These data also suggest that epigenetic changes in fetal life may impact the developmental origins of PCOS, assuming a critical time interval of fetal susceptibility beginning in midgestation when developmental programming occurs (91, 234). In humans, monkeys, and sheep, species characterized by ovarian follicular differentiation by birth, experimentally induced prenatal testosterone excess programs permanent PCOS-like phenotypes, including multifollicular ovaries (92, 234–237). This agrees with an increased prevalence of PCOS in women with classic congenital adrenal hyperplasia and congenital adrenal virilizing tumors (2, 238, 239). Such prenatal testosterone treatment in rhesus monkeys and sheep induces maternal glucose intolerance, causing transient hyperinsulinemia and decreased activin A availability in their respective female fetuses, which could affect ovarian steroidogenesis, follicular proliferation, and germ cell survival in utero (240–243). Because maternal androgen in normal pregnancy does not usually program PCOS in offspring due to placental aromatization (244), a plausible hypothesis for transgenerational programing of ovarian function is that metabolic disorders of pregnancy, including PCOS, induce androgen overproduction by the midgestational human fetal ovary, which reprograms ovarian function after birth in susceptible female offspring (4, 91).
FUTURE DIRECTIONS
Although PCOS is the leading cause of anovulatory infertility in women (1), the endocrine and molecular events that control this disease remain to be fully understood. With obesity as one of the fastest-growing medical problems worldwide,itiscrucial to understand how PCOS and obesity interact to disrupt the intrafollicular environment and oocyte development. Lean and obese PCOS patients likely represent different ends in a continuum of reproductive dysfunction that requires these two types of PCOS patients to be analyzed separately.
In lean PCOS patients, LH hypersecretion and ovarian hyperandrogenism appear to be the primary drivers of altered folliculogenesis, with insulin enhancing gonadotropin actions on ovarian steroidogenesis and granulosa cell differentiation (see Fig. 2). In obese PCOS patients, metabolic factors such as glucose-insulin homeostasis, adipogenic dysfunction, and possibly lipotoxicity may be more important than LH as primary drivers of altered folliculogenesis (see Fig. 3). Whereas loss of weight can restore ovulation and fertility in many obese PCOS patients, this is not true of the lean PCOS patients, in whom ovulation induction is necessary.
In lean and obese PCOS patients alike, we need to identify androgen target genes in all cell types of the human ovary. Is the FSH receptor gene the primary androgen target or are there more? Does testosterone, acting as an androgen or through aromatization, promote follicle survival, as it does for mouse and rhesus preantral follicles in vitro (40, 245, 246)? We also need to know the critical insulin target genes in these same ovarian cell types, using in vitro models whereby physiologic and pathophysiologic rather than pharmacologic amounts of insulin are used. Although high insulin levels might act through IGF-I receptors in follicular cells to exert some effects, too many in vitro studies have used 5–10 mg/mL of insulin, which are levels that far exceed the physiologic limit and therefore preclude meaningful interpretations.
In lean PCOS patients, we need to understand what controls the persistently elevated expression of intrafollicular AMH and whether it contributes to ovarian dysfunction, follicle survival, and impaired oocyte quality (247). Serum levels of LH and AMH are positively correlated in PCOS patients (144), agreeing with the ability of LH to stimulate AMH production by cultured PCOS granulosa cells (136). That such PCOS granulosa cells secrete AMH in response to LH implies the presence of LH receptors in these prematurely differentiated cells and further suggests that AMH overproduction may be under the tripartite control of LH, androgen, and insulin. However, AMH expression can also be regulated by BMPs (human), activin (mouse), FSH, and the FoxoBoxO transcription factors (77, 248), implicating SMADs and FOXOs as well as cAMP in regulating transcription of the AMH promoter (249). The AMH promoter contains orphan nuclear receptor (SF1 or LRH1) response elements and putative GATA and AP1 binding sites (249). Transcription factors, especially LRH1 and GATA4, are highly expressed in granulosa cells of growing follicles (75, 250, 251) and impact follicle development (252, 253), potentially interacting with FOXO1 or SMADs to regulate AMH expression. Increased phosphorylation of LRH1 is likely, given the high levels of LH and insulin that can activate the mitogen-activated protein kinase (MAPK) pathway (192). Estradiol has also been implicated in controlling the responsiveness of the AMH promoter to FSH (254). Do potential regulatory loops between activin, AMH, and FSH (or LH) exist in PCOS granulosa cells, and, if so, does it reflect higher levels of FSH receptor (induced by androgens), predict AMH or E2 responsiveness to FSH in vitro (96, 136), or interact with other inflammatory, adipogenic, or other metabolic factors?
Equally important are the regulatory mechanisms governing bidirectional cumulus cell-oocyte signaling. From an “oocentric” point of view, the ability of oocyte-derived factors such as BMP15 and GDF9 to regulate cumulus cell proliferation, differentiation, and steroidogenesis emphasizes the role of the oocyte in determining its own developmental fate and protecting itself against its own microenvironment (197, 198, 255). Oocyte-derived factors also appear to increase AMH in primary follicles, joined by granulosa- or theca-derived factors during secondary follicle growth, and by cumulus cells of growing and preovulatory follicles. Furthermore, studies have indicated that midrange follicular fluid levels of AMH are optimal for maximal oocyte quality and IVF success (256), suggesting that oocyte-mediated, paracrine control of follicle growth may account for the heterogeneity in follicular development among the cohort of PCOS follicles undergoing gonadotropin stimulation. Conversely, a granulosa cell-cumulus cell connection is required for generating cGMP that passes through gap junctions to suppress the resumption of meiosis in oocytes, at least in antral follicles (190, 193, 195).
Finally, because experimental constraints exist on studying human oocytes, animal models and in vitro follicle cultures must continue to pioneer the earliest aspects of ovarian and oocyte physiology (20, 40, 92, 197, 198, 234, 257–261). Such models need to explore how developmentally relevant endocrine/paracrine factors and genes interact to promote optimal epigenetic and genetic expression in the oocyte for successful fertilization and preimplantation embryogenesis. They also need to define critical times during fetal development when the maternal endocrine status might permanently alter the ovarian physiology of the fetus and modify ovarian function after birth. With such information, new clinical strategies targeting long-term correction of follicle development in PCOS could improve fertility, optimize follicular responsiveness to ovulation induction, and enhance pregnancy outcomes by IVF, while decreasing the risk of multiple gestation and its adverse consequences on maternal-fetal health.
Footnotes
D.A.D. has nothing to disclose. J.S.R. has nothing to disclose.
Presented at the 45th Annual Meeting of the Society for the Study of Reproduction and 18th Ovarian, Workshop, State College, PA, August 12-15, 2012; and the Androgen Excess and PCOS Society, Update Meeting, “Origins of PCOS,” Houston, TX, June 22, 2012.
REFERENCES
- 1.Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed) 1986;293:355–359. doi: 10.1136/bmj.293.6543.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumesic DA, Schramm RD, Abbott DH. Early origins of polycystic ovary syndrome. Reprod Fertil Dev. 2005;17:349–360. doi: 10.1071/rd04092. [DOI] [PubMed] [Google Scholar]
- 3.Franks S, Gilling-Smith C, Watson H, Willis D. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin North Am. 1999;28:361–378. doi: 10.1016/s0889-8529(05)70074-8. [DOI] [PubMed] [Google Scholar]
- 4.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 5.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 6.Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv. 2008;63:39–48. doi: 10.1097/OGX.0b013e31815e85fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmina E, Chu MC, Longo RA, Rini GB, Lobo RA. Phenotypic variation in hyperandrogenic women influences the findings of abnormal metabolic and cardiovascular risk parameters. J Clin Endocrinol Metab. 2005;90:2545–2549. doi: 10.1210/jc.2004-2279. [DOI] [PubMed] [Google Scholar]
- 8.Welt CK, Gudmundsson JA, Arason G, Adams J, Palsdottir H, Gudlaugsdottir G, et al. Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam criteria: the impact of weight on phenotype and metabolic features. J Clin Endocrinol Metab. 2006;91:4842–4848. doi: 10.1210/jc.2006-1327. [DOI] [PubMed] [Google Scholar]
- 9.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faddy MJ, Gosden RG. Modelling the dynamics of ovarian follicle utilization throughout life. In: Trounson AO, Gosden RG, editors. Biology and pathology of the oocyte role in fertility and reproductive medicine. Cambridge: Cambridge University Press; 2003. pp. 44–52. [Google Scholar]
- 12.Gougeon A. The early stages of folliclar growth. In: Trounson AO, Gosden RG, editors. Biology and pathology of the oocyte role in fertility and reproductive medicine. Cambridge: Cambridge University Press; 2003. pp. 29–43. [Google Scholar]
- 13.Oktay K, Briggs D, Gosden RG. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab. 1997;82:3748–3751. doi: 10.1210/jcem.82.11.4346. [DOI] [PubMed] [Google Scholar]
- 14.Horie K, Fujita J, Takakura K, Kanzaki H, Suginami H, Iwai M, et al. The expression of c-kit protein in human adult and fetal tissues. Hum Reprod. 1993;8:1955–1962. doi: 10.1093/oxfordjournals.humrep.a137967. [DOI] [PubMed] [Google Scholar]
- 15.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan SD, Castrillon DH. Insights into primary ovarian insufficiency through genetically engineered mouse models. Semin Reprod Med. 2011;29:283–298. doi: 10.1055/s-0031-1280914. [DOI] [PubMed] [Google Scholar]
- 17.Zheng W, Nagaraju G, Liu Z, Liu K. Functional roles of the phosphatidylino-sitol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol. 2012;356:24–30. doi: 10.1016/j.mce.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 19.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen APN. Control of primordial follicle recruitment by anti-mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 20.Visser JA, Joop IS, Laven SE, Themmen AP. Anti-müllerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinology. 2012;8:331–341. doi: 10.1038/nrendo.2011.224. [DOI] [PubMed] [Google Scholar]
- 21.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-müllerian hormone. Reproduction. 2002;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 22.Myers M, Middlebrook BS, Matzuk MM, Pangas SA. Loss of inhibin alpha uncouples oocyte-granulosa cell dynamics and disrupts postnatal folliculogenesis. Dev Biol. 2009;334:458–467. doi: 10.1016/j.ydbio.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266:201–208. doi: 10.1016/j.ydbio.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Johnson PA. Follicle selection in the avian ovary. Repro Dom Anim. 2012;47:283–287. doi: 10.1111/j.1439-0531.2012.02087.x. [DOI] [PubMed] [Google Scholar]
- 25.Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cells interactions during preantral follicular development. J Ovarian Research. 2009;2:1–7. doi: 10.1186/1757-2215-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- 27.Driancourt MA, Reynaud K, Cortvrindt R, Smitz J. Roles of KIT and KIT LIGAND in ovarian function. Rev Reprod. 2000;5:143–152. doi: 10.1530/ror.0.0050143. [DOI] [PubMed] [Google Scholar]
- 28.Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- 29.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orisaka M, Jiang JY, Orisaka S, Kotsuji F, Tsang BK. Growth differentiation factor 9 promotes rat preantral follicle growth by up-regulating follicular androgen biosynthesis. Endocrinology. 2009;150:2740–2748. doi: 10.1210/en.2008-1536. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi N, Orisaka M, Cao M, Kotsuji F, Leader A, Sakuragi N, Tsang BK. Growth differentiation factor-9 mediates follicle-stimulating hormone-thyroid hormone interaction in the regulation of rat preantral follicular development. Endocrinology. 2009;150:5566–5574. doi: 10.1210/en.2009-0262. [DOI] [PubMed] [Google Scholar]
- 32.Solovyeva EV, Hayashi M, Margi K, Barkats C, Klein C, Amsterdam A, et al. Growth differentiation factor-9 stimulates rat theca-interstitial cell androgen biosynthesis. Biol Reprod. 2000;63:1214–1218. doi: 10.1095/biolreprod63.4.1214. [DOI] [PubMed] [Google Scholar]
- 33.Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–2956. doi: 10.1210/jcem.84.8.5929. [DOI] [PubMed] [Google Scholar]
- 34.Luo W, Wiltbank MC. Distinct regulation by steroids of messenger RNAs for FSHR and CYP19A1 in bovine granulosa cells. Biol Reprod. 2006;75:217–225. doi: 10.1095/biolreprod.105.047407. [DOI] [PubMed] [Google Scholar]
- 35.Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83:2479–2485. doi: 10.1210/jcem.83.7.4917. [DOI] [PubMed] [Google Scholar]
- 36.Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999;61:353–357. doi: 10.1095/biolreprod61.2.353. [DOI] [PubMed] [Google Scholar]
- 38.Vendola K, Zhou J, Wang J, Bondy CA. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod. 1999;14:2328–2332. doi: 10.1093/humrep/14.9.2328. [DOI] [PubMed] [Google Scholar]
- 39.Rice S, Ojha K, Whitehead S, Mason H. Stage-specific expression of andro-gen receptor, follicle-stimulating hormone receptor, and anti-müllerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. J Clin Endocrinol Metab. 2007;92:1034–1040. doi: 10.1210/jc.2006-1697. [DOI] [PubMed] [Google Scholar]
- 40.Lenie S, Smitz J. Functional AR signaling is evident in an in vitro mouse follicle culture bioassay that encompasses most stages of folliculogenesis. Biol Reprod. 2009;80:685–695. doi: 10.1095/biolreprod.107.067280. [DOI] [PubMed] [Google Scholar]
- 41.Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, et al. Premature ovarian failure in androgen receptor-deficient mice. Proc Nat Acad Sci USA. 2006;103:224–229. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, et al. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Nat Acad Sci USA. 2004;101:11209–11214. doi: 10.1073/pnas.0404372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Molecular Endocrinology. 2010;24:1393–1403. doi: 10.1210/me.2010-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walters KA, Allan CM, Handelsman DJ. Androgen actions and the ovary. Biol Reprod. 2008;78:380–389. doi: 10.1095/biolreprod.107.064089. [DOI] [PubMed] [Google Scholar]
- 45.Walters KA, Middleton LJ, Joseph SR, Hazra R, Jimenez M, Simanainen U, et al. Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol Reprod. 2012;87:151–162. doi: 10.1095/biolreprod.112.102012. [DOI] [PubMed] [Google Scholar]
- 46.Richards JS, Jahnsen T, Hedin L, Lifka J, Ratoosh SL, Durica JM, Goldring NB. Ovarian follicular development: from physiology to molecular biology. Recent Prog Hormone Res. 1987;43:231–276. doi: 10.1016/b978-0-12-571143-2.50012-5. [DOI] [PubMed] [Google Scholar]
- 47.Wickenheisser JK, Nelson-DeGrave VL, Hendricks KL, Legro RS, Strauss JF, McAllister JM. Retinoids and retinol differentially regulate steroid biosynthesis in ovarian theca cells isolated from normal cycling women and women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4858–4865. doi: 10.1210/jc.2005-0330. [DOI] [PubMed] [Google Scholar]
- 48.Samoto T, Maruo T, Ladines-Llave CA, Matsuo H, Deguchi J, Barnea ER, Mochizuki M. Insulin receptor expression in follicular and stromal compartments of the human ovary over the course of follicular growth, regression and atresia. Endocr J. 1993;40:715–726. doi: 10.1507/endocrj.40.715. [DOI] [PubMed] [Google Scholar]
- 49.Kezele PR, Nilsson EE, Skinner MK. Insulin but not insulin-like growth factor-1 promotes the primordial to primary follicle transition. Mol Cell Endocrinol. 2002;192:37–43. doi: 10.1016/s0303-7207(02)00114-4. [DOI] [PubMed] [Google Scholar]
- 50.DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril. 2005;83:1454–1460. doi: 10.1016/j.fertnstert.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 51.Baillargeon JP, Nestler JE. Commentary: polycystic ovary syndrome: a syndrome of ovarian hypersensitivity to insulin? J Clin Endocrinol Metab. 2006;91:22–24. doi: 10.1210/jc.2005-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balen AH, Conway GS, Homburg R, Legro RS. Polycystic ovary syndrome: a guide to clinical management. London: Taylor and Francis; 2005. pp. 47–67. [Google Scholar]
- 53.Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85:139–146. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 54.Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjo T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril. 1993;59:323–331. doi: 10.1016/s0015-0282(16)55675-1. [DOI] [PubMed] [Google Scholar]
- 55.McGee EA, Sawetawan C, Bird I, Rainey WE, Carr BR. The effect of insulin and insulin-like growth factors on the expression of steroidogenic enzymes in a human ovarian thecal-like tumor cell model. Fertil Steril. 1996;65:87–93. doi: 10.1016/s0015-0282(16)58032-7. [DOI] [PubMed] [Google Scholar]
- 56.Willis D, Franks S. Insulin action in human granulosa cells from normal and polycystic ovaries is mediated by the insulin receptor and not the type-I insulin-like growth factor receptor. J Clin Endocrinol Metab. 1995;80:3788–3790. doi: 10.1210/jcem.80.12.8530637. [DOI] [PubMed] [Google Scholar]
- 57.Jonassen JA, Bose K, Richards JS. Enhancement and desensitization of hormone-responsive adenylate cyclase in granulosa cells of preantral and antral ovarian follicles: effects of estradiol and follicle-stimulating hormone. Endocrinology. 1982;111:74–79. doi: 10.1210/endo-111-1-74. [DOI] [PubMed] [Google Scholar]
- 58.Fitzpatrick SL, Richards JS. Regulation of cytochrome P450 aromatase messenger ribonucleic acid and activity by steroids and gonadotropins in rat granulosa cells. Endocrinology. 1991;129:1452–1462. doi: 10.1210/endo-129-3-1452. [DOI] [PubMed] [Google Scholar]
- 59.Pellatt L, Rice S, Dilaver N, Heshri A, Galea R, Brincat M, et al. Anti-müllerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril. 2011;96:1246–1251. doi: 10.1016/j.fertnstert.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 60.Nelson VL, Legro RS, Strauss JF, 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 61.Nelson VL, Qin KN, Rosenfield RL, Wood JR, Penning TM, Legro RS, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- 62.Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, et al. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19:379–390. doi: 10.1210/me.2004-0178. [DOI] [PubMed] [Google Scholar]
- 63.Stubbs SA, Stark J, Dilworth SM, Franks S, Hardy K. Abnormal preantral folliculogenesis in polycystic ovaries is associated with increased granulosa cell division. J Clin Endocrinol Metab. 2007;92:4418–4426. doi: 10.1210/jc.2007-0729. [DOI] [PubMed] [Google Scholar]
- 64.Filho FLT, Baracat EC, Lee TH, Suh CS, Matsui M, Chang RJ, et al. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1337–1344. doi: 10.1210/jcem.87.3.8316. [DOI] [PubMed] [Google Scholar]
- 65.Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 66.Cataldo NA, Dumesic DA, Goldsmith PC, Jaffe RB. Immunolocalization of Fas and Fas ligand in the ovaries of women with polycystic ovary syndrome: relationship to apoptosis. Hum Reprod. 2000;15:1889–1897. doi: 10.1093/humrep/15.9.1889. [DOI] [PubMed] [Google Scholar]
- 67.Stubbs SA, Hardy K, Da Silva-Buttkus P, Stark J, Webber LJ, Flanagan AM, et al. Anti-mu€llerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab. 2005;90:5536–5543. doi: 10.1210/jc.2005-0907. [DOI] [PubMed] [Google Scholar]
- 68.Webber LJ, Stubbs SA, Stark J, Margara RA, Trew GH, Lavery SA, et al. Prolonged survival in culture of preantral follicles from polycystic ovaries. J Clin Endocrinol Metab. 2007;92:1975–1978. doi: 10.1210/jc.2006-1422. [DOI] [PubMed] [Google Scholar]
- 69.Adashi EY. The ovarian follicular apparatus. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive endocrinology, surgery, and technology. Philadelphia: Lippincott-Raven; 1996. pp. 18–40. [Google Scholar]
- 70.Yuan W, Giudice LC. Insulin-like growth factor-II mediates the steroidogenic and growth promoting actions of follicle stimulating hormone on human ovarian pre-antral follicles cultured in vitro. J Clin Endocrinol Metab. 1999;84:1479–1482. doi: 10.1210/jcem.84.4.5727. [DOI] [PubMed] [Google Scholar]
- 71.Richards JS. Perspective: The ovarian follicle—a perspective in 2001. Endocrinology. 2001;142:2184–2193. doi: 10.1210/endo.142.6.8223. [DOI] [PubMed] [Google Scholar]
- 72.Arden KC. FoxOs in tumor suppression and stem cell maintenance. Cell. 2007;128:235–237. doi: 10.1016/j.cell.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 73.Pisarska MD, Barlow D, Huo FT. Minireview: Roles of the forkhead transcription factor FOXL2 in granulosa cell biology and pathology. Endocrinology. 2011;152:1199–1208. doi: 10.1210/en.2010-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pisarska MD, Kuo FT, Tang D, Zarrini P, Khan S, Ketefian A. Expression of forkhead transcription factors in human granulosa cells. Fertil Steril. 2009;91:1392–1394. doi: 10.1016/j.fertnstert.2008.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1 and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen and the gonadotropins. Mol Endocrinol. 2002;16:580–599. doi: 10.1210/mend.16.3.0806. [DOI] [PubMed] [Google Scholar]
- 76.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 77.Liu Z, Castrillon DH, Zhou W, Richards JS. FOXO1/3 depletion in granulosa cells alters follicle growth, death and regulation of pituitary FSH. Mol Endocrinol. 2013;27:238–252. doi: 10.1210/me.2012-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strauss JF, Williams CJ. The ovarian life cycle. In: Strauss JE, Barbieri RL, editors. Yen and Jaffe’s reproductive endocrinology: physiology, pathophysiology, and clinical management. 6th ed. Philadelphia: Saunders Elsevier; 2009. pp. 155–190. [Google Scholar]
- 79.Thomas FH, Campbell BK, Armstrong DG. Effects of IGF-I bioavailability on bovine preantral follicular development in vitro. Reproduction. 2007;133:1121–1128. doi: 10.1530/REP-06-0382. [DOI] [PubMed] [Google Scholar]
- 80.Boyer A, Lapointe E, Zheng X, Cowan RG, Quirk SM, DeMayo FJ, et al. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J. 2010;24:3010–3025. doi: 10.1096/fj.09-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyer A, Goff AK, Boerboom D. WNT signaling in ovarian follicle biology and tumorigenesis. Trends Endocrinol Metab. 2010;21:25–32. doi: 10.1016/j.tem.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 82.Fan HY, O’Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol. 2010;24:1794–1804. doi: 10.1210/me.2010-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parakh TN, Hernadez JA, Grammer JC, Weck J, Hunzicker-Dunn M, Zeleznik AJ, Nilson JH. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires beta-catenin. Proc Natl Acad Sci USA. 2006;103:12435–12440. doi: 10.1073/pnas.0603006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120:963–972. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, Urbanek M, et al. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem. 2003;278:26380–26390. doi: 10.1074/jbc.M300688200. [DOI] [PubMed] [Google Scholar]
- 86.Jansen E, Laven JS, Dommerholt HB, Polman J, van Rijt C, van den Hurk C, et al. Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol Endocrinol. 2004;18:3050–3063. doi: 10.1210/me.2004-0074. [DOI] [PubMed] [Google Scholar]
- 87.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 88.Jakimiuk AJ, Weitsman SR, Brzechffa PR, Magoffin DA. Aromatase mRNA expression in individual follicles from polycystic ovaries. Mol Hum Reprod. 1998;4:1–8. doi: 10.1093/molehr/4.1.1. [DOI] [PubMed] [Google Scholar]
- 89.Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the “two-cell, two-gonadotrophin” model revisited. Mol Cell Endocrinol. 1994;100:51–54. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 90.Zachow RJ, Magoffin DA. Ovarian androgen biosynthesis: paracrine/au-tocrine regulation. In: Azziz R, Nestler JE, Dewailly D, editors. Androgen excess disorders in women. Philadelphia: Lippincott-Raven; 1997. pp. 13–22. [Google Scholar]
- 91.Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Padmanabhan V, Manikkam M, Recabarren S, Foster D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol. 2006;246:165–174. doi: 10.1016/j.mce.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 93.McGee WK, Bishop CV, Bahar A, Pohl CR, Chang RJ, Marshall JC, et al. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod. 2012;27:531–540. doi: 10.1093/humrep/der393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blank SK, McCartney CR, Helm KD, Marshall JC. Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med. 2007;25:352–359. doi: 10.1055/s-2007-984741. [DOI] [PubMed] [Google Scholar]
- 95.Roland AV, Moenter SM. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology. 2011;152:618–628. doi: 10.1210/en.2010-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mason HD, Willis DS, Beard RW, Winston RM, Margara R, Franks S. Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotro-pins and sex steroids in follicular fluid. J Clin Endocrinol Metab. 1994;79:1355–1360. doi: 10.1210/jcem.79.5.7962330. [DOI] [PubMed] [Google Scholar]
- 97.Erickson GF, Magoffin DA, Garzo VG, Cheung AP, Chang RJ. Granulosa cells of polycystic ovaries: are they normal or abnormal? Hum Reprod. 1992;7:293–299. doi: 10.1093/oxfordjournals.humrep.a137638. [DOI] [PubMed] [Google Scholar]
- 98.Willis D, Mason H, Gilling-Smith C, Franks S. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab. 1996;81:302–309. doi: 10.1210/jcem.81.1.8550768. [DOI] [PubMed] [Google Scholar]
- 99.Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83:3984–3991. doi: 10.1210/jcem.83.11.5232. [DOI] [PubMed] [Google Scholar]
- 100.Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab. 2001;86:1318–1323. doi: 10.1210/jcem.86.3.7318. [DOI] [PubMed] [Google Scholar]
- 101.Franks S, Mason H, Willis D. Follicular dynamics in the polycystic ovary syndrome. Mol Cell Endocrinol. 2000;163:49–52. doi: 10.1016/s0303-7207(99)00239-7. [DOI] [PubMed] [Google Scholar]
- 102.Kenigsberg S, Bentov Y, Chalifa-Caspi V, Potashnik G, Ofir R, Birk OS. Gene expression microarray profiles of cumulus cells in lean and overweight-obese polycystic ovary syndrome patients. Mol Hum Reprod. 2009;15:89–103. doi: 10.1093/molehr/gan082. [DOI] [PubMed] [Google Scholar]
- 103.Dumesic DA, Damario MA, Session DR, Famuyide A, Lesnick TG, Thornhill AR, McNeilly AS. Ovarian morphology and serum hormone markers as predictors of ovarian follicle recruitment by gonadotropins for in vitro fertilization. J Clin Endocrinol Metab. 2001;86:2538–2543. doi: 10.1210/jcem.86.6.7605. [DOI] [PubMed] [Google Scholar]
- 104.Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod. 2003;18:598–603. doi: 10.1093/humrep/deg115. [DOI] [PubMed] [Google Scholar]
- 105.De Leo V, Lanzetta D, D’Antona D, la Marca A, Morgante G. Hormonal effects of flutamide in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:99–102. doi: 10.1210/jcem.83.1.4500. [DOI] [PubMed] [Google Scholar]
- 106.Jakimiuk AJ, Weitsman SR, Magoffin DA. 5α-reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84:2414–2418. doi: 10.1210/jcem.84.7.5863. [DOI] [PubMed] [Google Scholar]
- 107.Agarwal SK, Judd HL, Magoffin DA. A mechanism for the suppression of estrogen production in polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:3686–3691. doi: 10.1210/jcem.81.10.8855823. [DOI] [PubMed] [Google Scholar]
- 108.Zeleznik AJ, Little-Ihrig L, Ramasawamy S. Administration of dihydrotestos-terone to rhesus monkeys inhibits gonadotropin-stimulated ovarian steroidogenesis. J Clin Endocrinol Metab. 2004;89:860–866. doi: 10.1210/jc.2003-031292. [DOI] [PubMed] [Google Scholar]
- 109.Pradeep PK, Li X, Peegel H, Menon KM. Dihydrotestosterone inhibits granulosa cell proliferation by decreasing the cyclin D2 mRNA expression and cell cycle arrest at G1 phase. Endocrinology. 2002;143:2930–2935. doi: 10.1210/endo.143.8.8961. [DOI] [PubMed] [Google Scholar]
- 110.Gleicher N, Weghofer A, Barad DH. The role of androgens in follicle maturation and ovulation induction: friend or foe of infertility treatment. Reprod Biol Endocrinol. 2011;9:116–128. doi: 10.1186/1477-7827-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barnes FL, Kausche A, Tiglias J, Wood C, Wilton L, Trounson A. Production of embryos from in vitro-matured primary human oocytes. Fertil Steril. 1996;65:1151–1156. doi: 10.1016/s0015-0282(16)58330-7. [DOI] [PubMed] [Google Scholar]
- 112.Dumesic DA, Schramm RD, Abbott DH. Steroid and oocyte development. In: Filicori M, editor. Updates in infertility treatment 2004. Bologna, Italy: Medimond; 2004. pp. 457–475. [Google Scholar]
- 113.Tesarik J, Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80:1438–1443. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- 114.Tesarik J, Mendoza C. Direct non-genomic effects of follicular steroids on maturing human oocytes: oestrogen versus androgen antagonism. Hum Reprod Update. 1997;3:95–100. doi: 10.1093/humupd/3.2.95. [DOI] [PubMed] [Google Scholar]
- 115.Eppig JJ, O’Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod. 1998;59:1445–1453. doi: 10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- 116.Fulghesu AM, Villa P, Pavone V, Guido M, Apa R, Caruso A, et al. The impact of insulin secretion on the ovarian response to exogenous gonadotropins in polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:644–648. doi: 10.1210/jcem.82.2.3727. [DOI] [PubMed] [Google Scholar]
- 117.Acevedo N, Ding J, Smith GD. Insulin signaling in mouse oocytes. Biol Reprod. 2007;77:872–879. doi: 10.1095/biolreprod.107.060152. [DOI] [PubMed] [Google Scholar]
- 118.Cebe-Suarez S, Zehnder-Fiallman A, Mallmer-Hofer K. The role of VEGF receptors in angiogenesis: complex partnerships. Cell Mol Life Sci. 2006;63:601–615. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferrara N, Frantz G, LeCouter J, Dillard-Telm L, Pham T, Draksharapu A, et al. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am J Pathol. 2003;162:1881–1893. doi: 10.1016/S0002-9440(10)64322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neulen J, Yan Z, Raczek S, Weindel K, Keck C, Weich HA, et al. Human chorionic gonadotropin-dependent expression of vascular endothelial growth factor/vascular permeability factor in human granulosa cells: importance in ovarian hyperstimulation syndrome. J Clin Endocrinol Metab. 1995;80:1967–1971. doi: 10.1210/jcem.80.6.7775647. [DOI] [PubMed] [Google Scholar]
- 121.Wang TH, Horng SG, Chang CL, Wu HM, Tsai YJ, Wang HS, Soong YK. Human chorionic gonadotropin-induced ovarian hyperstimulation syndrome is associated with up-regulation of vascular endothelial growth factor. J Clin Endocrinol Metab. 2002;87:3300–3308. doi: 10.1210/jcem.87.7.8651. [DOI] [PubMed] [Google Scholar]
- 122.Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohi J, Simon C. The pathogenesis of ovarian hyperstimulation syndrome: in vivo studies investigating the role of interleukin-1β, interleukin-6, and vascular endothelial growth factor. Fertil Steril. 1999;71:482–489. doi: 10.1016/s0015-0282(98)00484-1. [DOI] [PubMed] [Google Scholar]
- 123.Abramov Y, Barak V, Nisman B, Schenker JG. Vascular endothelial growth factor plasma levels correlate to the clinical picture in severe ovarian hyperstimulation syndrome. Fertil Steril. 1997;67:261–265. doi: 10.1016/S0015-0282(97)81908-5. [DOI] [PubMed] [Google Scholar]
- 124.Kamat BR, Brown LF, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa, theca lutein cells. Role in corpus luteum development. Am J Pathol. 1995;146:157–165. [PMC free article] [PubMed] [Google Scholar]
- 125.Agrawal R, Sladkevicius P, Engmann L, Conway GS, Payne NN, Bekis J, et al. Serum vascular endothelial growth factor concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries. Hum Reprod. 1998;13:651–655. doi: 10.1093/humrep/13.3.651. [DOI] [PubMed] [Google Scholar]
- 126.Artini PG, Monti M, Matteucci C, Valentino V, Cristello F, Genazzani AR. Vascular endothelial growth factor and basic fibroblast growth factor in polycystic ovary syndrome during controlled ovarian hyperstimulation. Gynecol Endocrinol. 2006;22:465–470. doi: 10.1080/09513590600906607. [DOI] [PubMed] [Google Scholar]
- 127.Navot D, Bergh P, Lauger N. The ovarian hyperstimulation syndrome. In: Adashi E, Rock J, Rosenwaks Z, editors. Reproductive endocrinology, surgery, and technology. Philadelphia: Lippincott-Raven; 1996. pp. 2216–2232. [Google Scholar]
- 128.Stanek MB, Borman SM, Molskness TA, Larson JM, Stouffer R, Patton PE. Insulin and insulin-like growth factor stimulation of vascular endothelial growth factor production by luteinized granulosa cells: comparison between polycystic ovarian syndrome (PCOS) and non-PCOS women. J Clin Endocrinol Metab. 2007;92:2726–2733. doi: 10.1210/jc.2006-2846. [DOI] [PubMed] [Google Scholar]
- 129.Abramovich D, Irusta G, Bas D, Cataldi NI, Parborell F, Tesone M. Angiopoietins/TIE2 system and VEGF are involved in ovarian function in a DHEA rat model of polycystic ovary syndrome. Endocrinology. 2012;153:3446–3456. doi: 10.1210/en.2012-1105. [DOI] [PubMed] [Google Scholar]
- 130.Knight PG, Glister C. Local roles of TGF-beta superfamily members in the control of ovarian follicle development. Anim Reprod Sci. 2003;78:165–183. doi: 10.1016/s0378-4320(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 131.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 132.Fanchin R, Louafi N, Mendez Lozano DH, Frydman N, Frydman R, Taieb J. Per-follicle measurements indicate that anti-müllerian hormone secretion is modulated by the extent of follicular development and luteinization and may reflect qualitatively the ovarian follicular status. Fertil Steril. 2005;84:167–173. doi: 10.1016/j.fertnstert.2005.01.115. [DOI] [PubMed] [Google Scholar]
- 133.Eldar-Geva T, Margalioth EJ, Gal M, Ben-Chetrit A, Algur N, Zylber-Haran E, et al. Serum anti-müllerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod. 2005;20:1814–1819. doi: 10.1093/humrep/deh873. [DOI] [PubMed] [Google Scholar]
- 134.Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820–1826. doi: 10.1093/humrep/deh850. [DOI] [PubMed] [Google Scholar]
- 135.Dumesic DA, Lesnick TG, Stassart JP, Ball GD, Wong A, Abbott DH. Intrafollicular antimüllerian hormone levels predict follicle responsiveness to follicle-stimulating hormone (FSH) in normoandrogenic ovulatory women undergoing gonadotropin releasing-hormone analog/recombinant human FSH therapy for in vitro fertilization and embryo transfer. Fertil Steril. 2009;92:217–221. doi: 10.1016/j.fertnstert.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Mason H. Granulosa cell production of anti-müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–245. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- 137.Fallat ME, Siow Y, Marra M, Cook C, Carrillo A. Müllerian-inhibiting substance in follicular fluid and serum: a comparison of patients with tubal factor infertility, polycystic ovary syndrome, and endometriosis. Fertil Steril. 1997;67:962–965. doi: 10.1016/s0015-0282(97)81417-3. [DOI] [PubMed] [Google Scholar]
- 138.La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970–972. doi: 10.1016/j.fertnstert.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 139.Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum müllerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil Steril. 2002;77:141–146. doi: 10.1016/s0015-0282(01)02944-2. [DOI] [PubMed] [Google Scholar]
- 140.Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26:3123–3129. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 141.Dilaver NM, Goes D, Rice S, Mason HD. Anti-müllerian hormone may contribute to anovulation in PCOS via inhibition of human granulosa cell growth; Presented at the 94th Annual Meeting of the Endocrine Society; June 23–26, 2012; Houston, TX. abstract: MON-18. [Google Scholar]
- 142.Catteau-Jonard S, Jamin SP, Leclerc A, Gonzales J, Dewailly D, di Clemente N. Anti-müllerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4456–4461. doi: 10.1210/jc.2008-1231. [DOI] [PubMed] [Google Scholar]
- 143.Panidis D, Katsikis I, Karkanaki A, Piouka A, Armeni AK, Georgopoulos NA. Serum anti-müllerian hormone (AMH) levels are differentially modulated by both serum gonadotropins and not only by serum follicle stimulating hormone (FSH) levels. Med Hypotheses. 2011;77:649–653. doi: 10.1016/j.mehy.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 144.Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-müllerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296:E238–E243. doi: 10.1152/ajpendo.90684.2008. [DOI] [PubMed] [Google Scholar]
- 145.Thomson RL, Buckley JD, Moran LJ, Noakes M, Clifton PM, Norman RJ, Brinkworth GD. The effect of weight loss on anti-müllerian hormone levels in overweight and obese women with polycystic ovary syndrome and reproductive impairment. Hum Reprod. 2009;24:1976–1981. doi: 10.1093/humrep/dep101. [DOI] [PubMed] [Google Scholar]
- 146.Moran LJ, Noakes M, Clifton PM, Norman RJ. The use of anti-müllerian hormone in predicting menstrual response after weight loss in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:3796–3802. doi: 10.1210/jc.2007-1188. [DOI] [PubMed] [Google Scholar]
- 147.Romualdi D, De Cicco S, Tagliaferri V, Proto C, Lanzone A, Guido M. The metabolic status modulates the effect of metformin on the antimüllerian hormone-androgens-insulin interplay in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:E821–E824. doi: 10.1210/jc.2010-1725. [DOI] [PubMed] [Google Scholar]
- 148.Knight PG, Glister C. Potential local regulatory functions of inhibins, activins and follistatin in the ovary. Reproduction. 2001;121:503–512. doi: 10.1530/rep.0.1210503. [DOI] [PubMed] [Google Scholar]
- 149.Schneyer AL, Fujiwara T, Fox J, Welt CK, Adams J, Messerlian GM, Taylor AE. Dynamic changes in the intrafollicular inhibin/activin/follistatin axis during human follicular development: relationship to circulating hormone concentrations. J Clin Endocrinol Metab. 2000;85:3319–3330. doi: 10.1210/jcem.85.9.6767. [DOI] [PubMed] [Google Scholar]
- 150.Magoffin DA, Jakimiuk AJ. Inhibin A, inhibin B and activin A concentrations in follicular fluid from women with polycystic ovary syndrome. Hum Reprod. 1998;13:2693–2698. doi: 10.1093/humrep/13.10.2693. [DOI] [PubMed] [Google Scholar]
- 151.Bernard DJ, Fortin J, Wang Y, Lamba P. Mechanisms of FSH synthesis: what we know, what we don’t, and why you should care. Fertil Steril. 2010;93:2465–2485. doi: 10.1016/j.fertnstert.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 152.Smyth CD, Miro F, Whitelaw PF, Howles CM, Hillier SG. Ovarian theca-l/interstitial androgen synthesis is enhanced by a follicle-stimulating hormone-stimulated paracrine mechanism. Endocrinology. 1993;133:1532–1538. doi: 10.1210/endo.133.4.8404591. [DOI] [PubMed] [Google Scholar]
- 153.Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, et al. Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem. 2005;280:9135–9148. doi: 10.1074/jbc.M409486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pangas SA. Bone morphogenetic protein signaling transcription factor (SMAD) function in granulosa cells. Mol Cell Endocrinol. 2012;356:40–47. doi: 10.1016/j.mce.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pangas SA, Jorgez CJ, Tran M, Agno J, Li X, Brown CW, et al. Intraovarian activins are required for female fertility. Mol Endocrinol. 2007;21:2456–2471. doi: 10.1210/me.2007-0146. [DOI] [PubMed] [Google Scholar]
- 156.Fujiwara T, Sidis Y, Welt C, Lambert-Messerlian G, Fox J, Taylor A, Schneyer A. Dynamics of inhibin subunit and follistatin mRNA during development of normal and polycystic ovary syndrome follicles. J Clin Endocrinol Metab. 2001;86:4206–4215. doi: 10.1210/jcem.86.9.7798. [DOI] [PubMed] [Google Scholar]
- 157.Welt CK, Taylor AE, Fox J, Messerlian GM, Adams JM, Schneyer AL. Follicular arrest in polycystic ovary syndrome is associated with deficient inhibin A and B biosynthesis. J Clin Endocrinol Metab. 2005;90:5582–5587. doi: 10.1210/jc.2005-0695. [DOI] [PubMed] [Google Scholar]
- 158.Lambert-Messerlian G, Taylor A, Leykin L, Isaacson K, Toth T, Chang Y, Schneyer A. Characterization of intrafollicular steroid hormones, inhibin, and follistatin in women with and without polycystic ovarian syndrome following gonadotropin hyperstimulation. Biol Reprod. 1997;57:1211–1126. doi: 10.1095/biolreprod57.5.1211. [DOI] [PubMed] [Google Scholar]
- 159.Lockwood GM, Muttukrishna S, Groome NP, Matthews DR, Ledger WL. Mid-follicular phase pulses of inhibin B are absent in polycystic ovarian syndrome and are initiated by successful laparoscopic ovarian diathermy: a possible mechanism regulating emergence of the dominant follicle. J Clin Endocrinol Metab. 1998;83:1730–1735. doi: 10.1210/jcem.83.5.4756. [DOI] [PubMed] [Google Scholar]
- 160.Welt CK, Taylor AE, Martin KA, Hall JE. Serum inhibin B in polycystic ovary syndrome: regulation by insulin and luteinizing hormone. J Clin Endocrinol Metab. 2002;87:5559–5565. doi: 10.1210/jc.2002-020546. [DOI] [PubMed] [Google Scholar]
- 161.Norman RJ, Milner CR, Groome NP, Robertson DM. Circulating follistatin concentrations are higher and activin concentrations are lower in polycystic ovarian syndrome. Hum Reprod. 2001;16:668–672. doi: 10.1093/humrep/16.4.668. [DOI] [PubMed] [Google Scholar]
- 162.Eldar-Geva T, Spitz IM, Groome NP, Margalioth EJ, Homburg R. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum Reprod. 2001;16:2552–2556. doi: 10.1093/humrep/16.12.2552. [DOI] [PubMed] [Google Scholar]
- 163.Jeppesen JV, Nielsen ME, Kristensen SG, Yding Andersen C. Concentration of activin A and follistatin in follicular fluid from human small antral follicles associated to gene expression of the corresponding granulosa cells. Mol Cell Endocrinol. 2012;356:48–54. doi: 10.1016/j.mce.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 164.Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:162–168. doi: 10.1210/jc.2007-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guzick DS, Wing R, Smith D, Berga SL, Winters SJ. Endocrine consequences of weight loss in obese, hyperandrogenic, anovulatory women. Fertil Steril. 1994;61:598–604. [PubMed] [Google Scholar]
- 166.Huber-Buchholz MM, Carey DG, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab. 1999;84:1470–1474. doi: 10.1210/jcem.84.4.5596. [DOI] [PubMed] [Google Scholar]
- 167.Pasquali R, Antenucci D, Casimirri F, Venturoli S, Paradisi R, Fabbri R, et al. Clinical and hormonal characteristics of obese amenorrheic hyperandro-genic women before and after weight loss. J Clin Endocrinol Metab. 1989;68:173–179. doi: 10.1210/jcem-68-1-173. [DOI] [PubMed] [Google Scholar]
- 168.Purcell SH, Moley KH. The impact of obesity on egg quality. J Assist Reprod Genet. 2011;28:517–524. doi: 10.1007/s10815-011-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13:1502–1505. doi: 10.1093/humrep/13.6.1502. [DOI] [PubMed] [Google Scholar]
- 170.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 171.Monget P, Chabrolle C, Dupont J. Adipose tissue, nutrition and reproduction: what is the link? [in French] Bull Acad Natl Med. 2008;192:637–648. [PubMed] [Google Scholar]
- 172.Michalakis KG, Segars JH. The role of adiponectin in reproduction: from polycystic ovary syndrome to assisted reproduction. Fertil Steril. 2010;94:1949–1957. doi: 10.1016/j.fertnstert.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu Z, Richards JS. IL6: anautocrine regulator of the mouse cumulus cell-oocyte complex expansion process. Endocrinology. 2008;150:3360–3368. doi: 10.1210/en.2008-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Palin MC, Bourdignon VV, Murphy BD. Adiponectin and the control of female reproductive functions. Vitamin Horm. 2012;90:239–287. doi: 10.1016/B978-0-12-398313-8.00010-5. [DOI] [PubMed] [Google Scholar]
- 175.Richards JS, Liu Z, Kawai T, Tabata K, Watanabe H, Suresh D, et al. Adiponectin and its receptors modulate granulosa cell and cumulus cell functions, fertility, and early embryo development in the mouse and human. Fertil Steril. 2012;98:471–479. doi: 10.1016/j.fertnstert.2012.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Manneras-Holm L, Leonhardt H, Kullberg J, Jennische E, Oden A, Holm G, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:E304–E311. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 177.Chazenbalk G, Trivax BS, Yildiz BO, Bertolotto C, Mathur R, Heneidi S, Azziz R. Regulation of adiponectin secretion by adipocytes in the polycystic ovary syndrome: role of tumor necrosis factor-α. J Clin Endocrinol Metab. 2010;95:935–942. doi: 10.1210/jc.2009-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yildizhan R, Adali E, Kolusari A, Kurdoglu M, Yildizhan B, Sahin HG, Kamaci M. Ovarian stimulation in obese and non-obese polycystic ovary syndrome using a low-dose step-up regimen with two different starting doses of recombinant follicle-stimulating hormone. J Int Med Res. 2008;36:1197–1204. doi: 10.1177/147323000803600605. [DOI] [PubMed] [Google Scholar]
- 179.Butzow TL, Moilanen JM, Lehtovirta M, Tuomi T, Hovatta O, Siegberg R, et al. Serum and follicular fluid leptin during in vitro fertilization: relationship among leptin increase, body fat mass, and reduced ovarian response. J Clin Endocrinol Metab. 1999;84:3135–3139. doi: 10.1210/jcem.84.9.6004. [DOI] [PubMed] [Google Scholar]
- 180.Greisen S, Ledet T, Moller N, Jorgensen JO, Christiansen JS, Petersen K, Ovesen P. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79:931–935. [PubMed] [Google Scholar]
- 181.Gurbuz B, Yalti S, Ficicioglu C, Tasdemir S. The relation of serum and follicular fluid leptin and ovarian steroid levels in response to induction of ovulation in in vitro fertilization cycles. Eur J Obstet Gynecol Reprod Biol. 2005;118:214–218. doi: 10.1016/j.ejogrb.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 182.Pagan YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE. Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab. 2006;91:1309–1316. doi: 10.1210/jc.2005-2099. [DOI] [PubMed] [Google Scholar]
- 183.Srouji SS, Pagan YL, D’Amato F, Dabela A, Jimenez Y, Supko JG, Hall JE. Pharmacokinetic factors contribute to the inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome. J Clin Endocrinol Metab. 2007;92:1347–1352. doi: 10.1210/jc.2006-2716. [DOI] [PubMed] [Google Scholar]
- 184.Hall JE, Taylor AE, Hayes FJ, Crowley WF., Jr Insights into hypothalamic-pituitary dysfunction in polycystic ovary syndrome. J Endocrinol Invest. 1998;21:602–611. doi: 10.1007/BF03350785. [DOI] [PubMed] [Google Scholar]
- 185.Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, Hall JE. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2248–2256. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 186.Richards J. Hormonal control of follicular growth and maturation in mammals. New York: Plenum Press; 1978. [Google Scholar]
- 187.Richards J. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev. 1980;60:51–89. doi: 10.1152/physrev.1980.60.1.51. [DOI] [PubMed] [Google Scholar]
- 188.Richards JS. Hormonal control of ovarian follicular development: a 1978 perspective. Recent Prog Hormone Res. 1979;35:343–373. doi: 10.1016/b978-0-12-571135-7.50012-6. [DOI] [PubMed] [Google Scholar]
- 189.Zeleznik AJ. The physiology of follicle selection. Reprod Biol Endocrinol. 2004;2:31–38. doi: 10.1186/1477-7827-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Conti M, Hsieh M, Park JY, Su YQ. Role of the EGF network in ovarian follicles. Mol Endocrinol. 2005;20:715–723. doi: 10.1210/me.2005-0185. [DOI] [PubMed] [Google Scholar]
- 191.Shimada M, Gonzalez-Robayna I, Hernandez-Gonzalez I, Richards JS. Paracrine and autocrine regulation of EGF-like factors in cumulus oocyte complexes and granulosa cells: key role for prostaglandin synthase 2 (Ptgs2) and progesterone receptor (Pgr) Mol Endocrinol. 2006;20:348–364. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 192.Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang M, Su Q, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Robinson JW, Zhang M, Shuhaibar LC, Norris RP, Geerts A, Wunder F, et al. Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic GMP decrease that promotes resumption of meiosis in oocytes. Dev Biol. 2012;366:308–316. doi: 10.1016/j.ydbio.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vaccari S, Weeks JL, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81:595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Conti M, Hsieh M, Musa Zamah A, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356:65–73. doi: 10.1016/j.mce.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sugiura K, Eppig JJ. Society for Reproductive Biology Founders’ Lecture 2005. Control of metabolic cooperativity between oocytes and their companion granulosa cells by mouse oocytes. Reprod Fertil Dev. 2005;17:667–674. doi: 10.1071/rd05071. [DOI] [PubMed] [Google Scholar]
- 198.Webb R, Garnsworthy PC, Campbell BK, Hunter MG. Intra-ovarian regulation of follicular development and oocyte competence in farm animals. Theriogenology. 2007;68(Suppl 1):S22–S29. doi: 10.1016/j.theriogenology.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 199.Stebbins-Boaz B, Richter JD. Translational control during early development. Crit Rev Eukaryot Gene Expr. 1997;7:73–94. doi: 10.1615/critreveukargeneexpr.v7.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 200.Sirard MA, Desrosier S, Assidi M. In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology. 2007;68(Suppl 1):S71–S76. doi: 10.1016/j.theriogenology.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 201.Gandolfi TA, Gandolfi F. The maternal legacy to the embryo: cytoplasmic components and their effects on early development. Theriogenology. 2001;55:1255–1276. doi: 10.1016/s0093-691x(01)00481-2. [DOI] [PubMed] [Google Scholar]
- 202.Chen J, Melton C, Suh N, Oh JS, Horner K, Xie F, et al. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Devel. 2011;25:755–766. doi: 10.1101/gad.2028911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab. 2006;91:2327–2333. doi: 10.1210/jc.2005-2142. [DOI] [PubMed] [Google Scholar]
- 204.Teissier MP, Chable H, Paulhac S, Aubard Y. Comparison of follicle steroidogenesis from normal and polycystic ovaries in women undergoing IVF: relationship between steroid concentrations, follicle size, oocyte quality and fecundability. Hum Reprod. 2000;15:2471–2477. doi: 10.1093/humrep/15.12.2471. [DOI] [PubMed] [Google Scholar]
- 205.Wood JR, Dumesic DA, Abbott DH, Strauss JF., 3rd Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- 206.Phy JL, Conover CA, Abbott DH, Zschunke MA, Walker DL, Session DR, et al. Insulin and messenger ribonucleic acid expression of insulin receptor isoforms in ovarian follicles from nonhirsute ovulatory women and polycys-tic ovary syndrome patients. J Clin Endocrinol Metab. 2004;89:3561–3566. doi: 10.1210/jc.2003-031888. [DOI] [PubMed] [Google Scholar]
- 207.Cano F, Garcia-Velasco JA, Millet A, Remohi J, Simon C, Pellicer A. Oocyte quality in polycystic ovaries revisited: identification of a particular subgroup of women. J Assist Reprod Genet. 1997;14:254–261. doi: 10.1007/BF02765826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Koivunen R, Pouta A, Franks S, Martikainen H, Sovio U, Hartikainen AL, et al. Fecundability and spontaneous abortions in women with self-reported oligo-amenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Hum Reprod. 2008;23:2134–2139. doi: 10.1093/humrep/den136. [DOI] [PubMed] [Google Scholar]
- 209.Morin-Papunen L, Rantala AS, Unkila-Kallio L, Tiitinen A, Hippelainen M, Perheentupa A, et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): a multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab. 2012;97:1492–1500. doi: 10.1210/jc.2011-3061. [DOI] [PubMed] [Google Scholar]
- 210.Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:13–21. doi: 10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- 211.Sengoku K, Tamate K, Takuma N, Yoshida T, Goishi K, Ishikawa M. The chromosomal normality of unfertilized oocytes from patients with polycystic ovarian syndrome. Hum Reprod. 1997;12:474–477. doi: 10.1093/humrep/12.3.474. [DOI] [PubMed] [Google Scholar]
- 212.Ludwig M, Finas DF, al-Hasani S, Diedrich K, Ortmann O. Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients. Hum Reprod. 1999;14:354–358. doi: 10.1093/humrep/14.2.354. [DOI] [PubMed] [Google Scholar]
- 213.Tian L, Shen H, Lu Q, Norman RJ, Wang J. Insulin resistance increases the risk of spontaneous abortion after assisted reproduction technology treatment. J Clin Endocrinol Metab. 2007;92:1430–1433. doi: 10.1210/jc.2006-1123. [DOI] [PubMed] [Google Scholar]
- 214.Amato G, Conte M, Mazziotti G, Lalli E, Vitolo G, Tucker AT, et al. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol. 2003;101:1177–1182. doi: 10.1016/s0029-7844(03)00233-3. [DOI] [PubMed] [Google Scholar]
- 215.Gallinelli A, Ciaccio I, Giannella L, Salvatori M, Marsella T, Volpe A. Correlations between concentrations of interleukin-12 and interleukin-13 and lymphocyte subsets in the follicular fluid of women with and without polycystic ovary syndrome. Fertil Steril. 2003;79:1365–1372. doi: 10.1016/s0015-0282(03)00344-3. [DOI] [PubMed] [Google Scholar]
- 216.Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- 217.Minge CE, Bennett BD, Norman RJ, Robker RL. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reverses the adverse effects of diet-induced obesity on oocyte quality. Endocrinology. 2008;149:2646–2656. doi: 10.1210/en.2007-1570. [DOI] [PubMed] [Google Scholar]
- 218.Robker RL, Wu LL, Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol. 2011;88:142–148. doi: 10.1016/j.jri.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 219.Slawik M, Vidal-Puig AJ. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev. 2006;5:144–164. doi: 10.1016/j.arr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 220.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 221.Carmina E, Campagna AM, Lobo RA. A 20-year follow-up of young women with polycystic ovary syndrome. Obstet Gynecol. 2012;119:263–269. doi: 10.1097/AOG.0b013e31823f7135. [DOI] [PubMed] [Google Scholar]
- 222.Carmina E, Campagna AM, Mansuet P, Vitale G, Kort D, Lobo R. Does the level of serum antimüllerian hormone predict ovulatory function in women with polycystic ovary syndrome with aging? Fertil Steril. 2012;98:1043–1046. doi: 10.1016/j.fertnstert.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 223.Elting MW, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum Reprod. 2000;15:24–28. doi: 10.1093/humrep/15.1.24. [DOI] [PubMed] [Google Scholar]
- 224.Brown ZA, Louwers YV, Fong SL, Valkenburg O, Birnie E, de Jong FH, et al. The phenotype of polycystic ovary syndrome ameliorates with aging. Fertil Steril. 2011;96:1259–1265. doi: 10.1016/j.fertnstert.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 225.Liang SJ, Hsu CS, Tzeng CR, Chen CH, Hsu MI. Clinical and biochemical presentation of polycystic ovary syndrome in women between the ages of 20 and 40. Hum Reprod. 2011;26:3443–3449. doi: 10.1093/humrep/der302. [DOI] [PubMed] [Google Scholar]
- 226.Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC. Changes in anti-müllerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod. 2004;19:2036–2042. doi: 10.1093/humrep/deh373. [DOI] [PubMed] [Google Scholar]
- 227.Tehrani FR, Solaymani-Dodaran M, Hedayati M, Azizi F. Is polycystic ovary syndrome an exception for reproductive aging? Hum Reprod. 2010;25:1775–1781. doi: 10.1093/humrep/deq088. [DOI] [PubMed] [Google Scholar]
- 228.Mellembakken JR, Berga SL, Kilen M, Tanbo TG, Abyholm T, Fedorcsak P. Sustained fertility from 22 to 41 years of age in women with polycystic ovarian syndrome. Hum Reprod. 2011;26:2499–2504. doi: 10.1093/humrep/der214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sir-Petermann T, Codner E, Maliqueo M, Echiburu B, Hitschfeld C, Crisosto N, et al. Increased anti-müllerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:3105–3109. doi: 10.1210/jc.2005-2693. [DOI] [PubMed] [Google Scholar]
- 230.Crisosto N, Echiburu B, Maliqueo M, Perez V, Ladron de Guevara A, Preisler J, et al. Improvement of hyperandrogenism and hyperinsulinemia during pregnancy in women with polycystic ovary syndrome: possible effect in the ovarian follicular mass of their daughters. Fertil Steril. 2012;97:218–224. doi: 10.1016/j.fertnstert.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 231.Vanky E, Carlsen SM. Androgens and antimüllerian hormone in mothers with polycystic ovary syndrome and their newborns. Fertil Steril. 2012;97:509–515. doi: 10.1016/j.fertnstert.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 232.Sir-Petermann T, Codner E, Perez V, Echiburu B, Maliqueo M, Ladron de Guevara A, et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1923–1930. doi: 10.1210/jc.2008-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2008;93:1662–1669. doi: 10.1210/jc.2007-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 235.Abbott DH, Barnett DK, Levine JE, Padmanabhan V, Dumesic DA, Jacoris S, Tarantal AF. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod. 2008;79:154–163. doi: 10.1095/biolreprod.108.067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71:776–784. doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Veiga-Lopez A, Ye W, Padmanabhan V. Developmental programming: prenatal testosterone excess disrupts anti-müllerian hormone expression in preantral and antral follicles. Fertil Steril. 2012;97:748–756. doi: 10.1016/j.fertnstert.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendo-crine function in women. J Clin Endocrinol Metab. 1994;79:1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- 239.Yildiz BO, Azziz R. The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord. 2007;8:331–342. doi: 10.1007/s11154-007-9054-0. [DOI] [PubMed] [Google Scholar]
- 240.Abbott DH, Bruns CR, Barnett DK, Dunaif A, Goodfriend TL, Dumesic DA, Tarantal AF. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. Am J Physiol Endocrinol Metab. 2010;299:E741–E751. doi: 10.1152/ajpendo.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, et al. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84:87–96. doi: 10.1095/biolreprod.110.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Martins da Silva SJ, Bayne RA, Cambray N, Hartley PS, McNeilly AS, Anderson RA. Expression of activin subunits and receptors in the developing human ovary: activin A promotes germ cell survival and proliferation before primordial follicle formation. Dev Biol. 2004;266:334–345. doi: 10.1016/j.ydbio.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 243.Abi-Salloum B, Veiga-Lopez A, Abbott DH, Padmanabhan V. Developmental programming: gestational exposure to excess testosterone, by its androgenic action, disrupts maternal steroidal and metabolic environment in sheep. 94th Annual Meeting of the Endocrine Society; June 23–26, 2012; Houston, TX. abstract: MON-15. [Google Scholar]
- 244.Hickey M, Sloboda DM, Atkinson HC, Doherty DA, Franks S, Norman RJ, et al. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab. 2009;94:3714–3720. doi: 10.1210/jc.2009-0544. [DOI] [PubMed] [Google Scholar]
- 245.Rodrigues JK, Xu J, Yeoman RR, Zelinski MB, RL S. Testosterone promotes survival and growth of primate preantral follicles cultured individually in a three-dimensional matrix. 68th Annual Meeting of the American Society for Reproductive Medicine; October 20–24, 2012; San Diego, CA. abstract: P-30. [Google Scholar]
- 246.Murray AA, Gosden RG, Allison V, Spears N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil. 1998;113:27–33. doi: 10.1530/jrf.0.1130027. [DOI] [PubMed] [Google Scholar]
- 247.Xu J, Lawson M, Yeoman R, Stouffer R, M Z. Anti-müllerian hormone (AMH) alters preantral follicle survival and growth, plus inhibits steroid production in antral follicles during encapsulated 3-dimensional (3D) culture in primates. 68th Annual Meeting of the American Society for Reproductive Medicine; October 20–24, 2012; San Diego, CA. abstract: O-230. [Google Scholar]
- 248.Ogura-Nose S, Yoshino O, Osuga Y, Shi J, Hiroi H, Yano T, Taketani Y. Anti-müllerian hormone (AMH) is induced by bone morphogenetic protein (BMP) cytokines in human granulosa cells. Eur J Obstet Gyn Reprod Biol. 2012;164:44–47. doi: 10.1016/j.ejogrb.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 249.Taieb J, Grynberg M, Pierre A, Arouche N, Massart P, Belville C, et al. FSH and its second messenger cAMP stimulate the transcription of human anti-müllerian hormone in cultured granulosa cells. Mol Endocrinol. 2011;25:645–655. doi: 10.1210/me.2010-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Stocco C. Aromatase expression in the ovary: hormonal and molecular regulation. Steroids. 2008;73:473–487. doi: 10.1016/j.steroids.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Heikinheimo M, Ermolaeva M, Bielinska M, Rahman NA, Narita N, Huhtaniemi IT, et al. Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology. 1997;138:3505–3514. doi: 10.1210/endo.138.8.5350. [DOI] [PubMed] [Google Scholar]
- 252.Duggavathi R, Volle DH, Mataki C, Antal MC, Messeddeq N, auwerx J, et al. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22:1871–1876. doi: 10.1101/gad.472008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bennett J, Wu YG, Gossen J, Zhou P, Stocco C. Loss of GATA-6 and GATA-4 in granulosa cells blocks folliculogenesis, ovulation, and follicle stimulating hormone receptor expression leading to female infertility. Endocrinology. 2012;153:2474–2485. doi: 10.1210/en.2011-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Grynberg M, Pierre A, Rey R, Leclerc A, Arouche N, Hesters L, et al. Differential regulation of ovarian anti-müllerian hormone (AMH) by estradiol through α- and β-estrogen receptors. J Clin Endocrinol Metab. 2012;97:E1649–E1657. doi: 10.1210/jc.2011-3133. [DOI] [PubMed] [Google Scholar]
- 255.Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 256.Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022–2026. doi: 10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- 257.Luense LJ, Veiga-Lopez A, Padmanabhan V, Christenson LK. Developmental programming: gestational testosterone treatment alters fetal ovarian gene expression. Endocrinology. 2011;152:4974–4983. doi: 10.1210/en.2011-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mannerås L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M, Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 259.Van Houten ELAF, Kramer P, McLuskey A, Karels B, Themmen APN, Visser JA. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 2012;153:2861–2869. doi: 10.1210/en.2011-1754. [DOI] [PubMed] [Google Scholar]
- 260.Parrish EM, Siletz A, Xu M, Woodruff TK, Shea LD. Gene expression in mouse ovarian follicle development in vivo versus an ex vivo alginate culture system. Reproduction. 2011;142:309–318. doi: 10.1530/REP-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633–2639. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]