Abstract
This selective review aims to highlight some of the most recent empirical or theoretical advancements in the study of social relationships as buffers against stress and as protective factors against risk for disease, focusing on articles published between 2013 and 2015. The review summarizes novel findings showing that social relationships can protect individuals against negative health outcomes associated with chronic adversity and can be associated with reduced cumulative physiological damage (allostatic load). There is also evidence that some relationships can be a source of stress. Additionally, recent findings concerning the psychological and neurobiological mechanisms of action for social support, the developmental patterning of social stress-buffering and recent experimental studies attempting to change relationships to affect health are also reviewed.
Introduction
Socially integrated adults have 50% increased likelihood of survival in prospective studies compared to socially isolated individuals, and these effects on mortality are greater than those of health behaviors widely-recognized as risk factors for disease, such as obesity and alcohol consumption [1,2]. Three decades ago, an influential review [3] proposed that social relationships exert their beneficial effects on psychological and physical symptoms through two sets of mechanisms: direct effects (e.g., promoting individuals’ thriving and well-being [4]) and stress-buffering effects, whereby social support reduces or blocks exposure to stressful experiences or minimizes their impact on health (for a schematic depiction of possible pathways through which social relationships may affect physical health, see Figure 1). The present review focuses on the latter, describing recent developments in the study of social stress-buffering and its implications for physical health.
Figure 1.
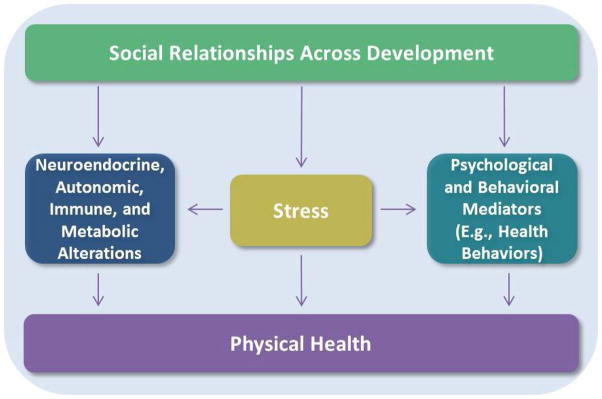
Conceptual model illustrating some of the pathways through which social relationships might impact physical health across development. Relationships can shape health by buffering or causing stress, but can also more directly affect physiological and psychological functioning, with implications for physical health. Bidirectional connections between several constructs depicted are likely, but are beyond the scope of this review.
Stress can be defined as a “real or interpreted threat to the physiological or psychological integrity of an individual which results in physiological and/or behavioral responses” [5]. These threats or challenges can be psychological or physical, but both types of stressors place demands on the organism that require mobilization of energy to promote adaptation. Stress-response systems like the hypothalamic-pituitary-adrenocortical (HPA) axis and sympathetic nervous system (SNS) are important mediators of these acute responses to threat in humans, and dynamically interact with immune, metabolic, and broader neural systems [6]. Stress-response systems are key actors in many disease processes [6,7], but their activity can be regulated and buffered by social relationships [8]. This selective review is intended to concisely highlight some of the most recent empirical or theoretical advancements in this area, focusing primarily on articles published between 2013 and 2015. Specifically, the review summarizes novel findings regarding social relationships as buffers against chronic stress and cumulative physiological damage, but also as potential sources of stress; it also reviews evidence on the psychological and neurobiological mechanisms of action for social support, the developmental patterning of social buffering, and recent experimental studies attempting to change relationships to affect health.
Social Relationships as Buffers for Health in Chronically Stressful Conditions
An exciting recent line of inquiry has begun to accumulate evidence that social relationships might offset or moderate the effects of living in chronically stressful conditions on physical health, with some studies probing potential explanatory mechanisms for these resilience effects. For instance, having supportive role models can buffer against the risk of systemic inflammation for adolescents experiencing low socioeconomic status [9]. Adults who report having received high levels of maternal nurturance during childhood appear protected from the increased risk of metabolic syndrome [10] and excessive pro-inflammatory signaling [11] associated with childhood poverty. In another study, adults who reported receiving more frequent hugs were buffered against the increased risk of upper respiratory infection associated with daily interpersonal tension and conflict [12]. Future studies should build upon these encouraging findings and extend these results using prospective, longitudinal designs and observational measures of relational quality obtained by coding interpersonal interactions occurring in the laboratory.
Social Relationships and Allostatic Load
As discussed above, acute stress responses involve neuroendocrine, autonomic, immune, and metabolic alterations that help the organism to mobilize and cope with threats. However, it is thought that chronic activation of these systems through long-term exposure to stressors can lead to “wear and tear” on the body (termed allostatic load)[6]. One test of the stress-buffering role of social relationships would be to examine the extent to which they can lower indices of allostatic load –i.e., measures of dysregulation across multiple biological systems. Recent research has risen to this challenge and shown that supportive close relationships (e.g., high-quality marriages, parental warmth) are indeed associated with lower scores on multi-system indices of biological risk, which include aggregate assessments of the HPA axis, SNS, cardiovascular, metabolic, and immune systems [13–15]. Future studies would benefit from examining this question with experimental designs (e.g., intervention studies), to test causality. Some of this recent research has also suggested that social strain might be a more powerful predictor of allostatic load than positive aspects of relationships [15]. Indeed, research on the pernicious correlates of poor-quality or absent social relationships for health has also burgeoned in recent years.
Relationships as Sources of Stress
A growing literature indicates that negative or deficient social interactions can become stressors in their own right and have deleterious consequences for physical health. For instance, social isolation and loneliness are associated with higher rates of morbidity and mortality, particularly in older adults [16,17]. Importantly, mechanistic animal studies that experimentally manipulate social isolation have provided causal evidence that social isolation alters the functioning of the HPA axis, consistent with extant investigations in humans [18], but more research is needed to fully specify the pathways from these alterations to disease endpoints and mortality.
Recent research has also made great strides in specifying some of the biological mediators through which stressful social experiences detrimentally affect physical health. For instance, one study showed that men and women experiencing high levels of hostility during a marital problem discussion and who had a history of mood disorders had significantly lower resting energy expenditure, higher insulin, and higher peak triglyceride responses after a high-fat meal [19]. Individuals who experience low subjective social status, low levels of perceived social support or high levels of loneliness also have stronger inflammatory responses to acute laboratory stressors (e.g., [20,21]), shorter leukocyte telomere length–an index of cellular aging [22,23], and differential expression of hundreds of gene transcripts in leukocytes and diseased tissues [24,25]. In the cardiovascular domain, a recent study showed that negative social interactions with friends, family, and partners prospectively predicted incident hypertension in older women [26].
Psychological Mediators of Social Stress-Buffering
Theoretical explanations for the stress-buffering role of social support often include psychological pathways–e.g., relationships can reduce perceived stress by affecting appraisal processes, promoting positive affect, or reducing depressive symptoms [27]. An intriguing recent review concluded that a substantial body of empirical research has been largely unable to provide supportive evidence for these psychological mediators between social relationships and health [27]. This may be due to the limited testing of mediating constructs, as recent projects have successfully identified such mediators between social support and some health outcomes–e.g., greater happiness, life satisfaction, mastery, and efficacy [28], hugging as a way to convey social support and caring [12], diminished distress associated with being ill [29], or reduced withdrawal symptoms during smoking cessation, which admittedly includes both psychological and somatic components [30]. Despite these more recent discoveries, the bulk of null findings when testing psychological mediators between social support and health outcomes [27] cannot be ignored, and might be alternatively explained by dissociations between self-reported states and physiology. Indeed, a recent review suggested that these dissociations are pervasive in stress research, with many studies using laboratory stress paradigms failing to find significant correlations between psychological and hormonal stress responses [31]. A solution to this problem would be to identify neural or hormonal measures that might directly mediate the effects of social support on physical health, an approach which has drawn increased attention in recent research.
The Neurobiology of Social Stress-Buffering
The brain plays a critical role in coordinating stress responses and encoding social information that may dampen stress responses [32]. A recent review [8] of the psychobiological mechanisms underlying the social buffering of HPA stress responses in human and nonhuman animals has implicated oxytocinergic systems and prefrontal neural networks as two potential neural substrates for the social regulation of the HPA axis [8]. Animal models support the role of the neuropeptide oxytocin in promoting social bonds and regulating stress responses [33]–e.g., oxytocin released in the paraventricular nucleus of the hypothalamus mediates the social buffering of the HPA axis in adult female prairie voles [34]. In humans, support from a parent via a phone conversation or physical presence can lower cortisol responses after a laboratory stressor and increase urinary oxytocin levels for children [35]. Evidence from social neuroscience [36] has also begun clarifying the neurocircuitry involved in processing social signals as safety cues that can quiet threat and stress-responding–e.g., the inhibitory role of the ventromedial prefrontal cortex for amygdala activity [36]. Despite the specificity of these results, social relationships and stress likely have pervasive effects across many neural regions–e.g., a recent study linked the diversity of one’s social network to white matter integrity [37]. More research is needed to fully characterize the neural activity responsible for initiating stress responses and transducing social input in ways that regulate stress responses across development.
Developmental Patterns
Interest in the legacy of early-life social experiences for later health has amplified in recent years. Reviews of this literature document the myriad ways in which childhood social adversity such as maltreatment or parental deprivation is associated with dysregulated stress and immune systems across the lifespan [38,39]. Positive social relationships may also exert long-lasting benefits–e.g., one study showed that perceived partner support buffered pregnant women against cortisol elevations related to distressing events, which may have implications for fetal development and presumably also for lifelong health [40]. Despite the potential public health relevance of understanding the social buffering of stress responses across development, until recently most of the experimental evidence had focused on adults or infants [41]. Some studies have begun charting the developmental course of these effects across childhood and adolescence. For example, one study revealed that parent support remains a potent buffer against HPA stress reactivity into late childhood, but may lose efficacy in adolescence [42]. Parallel neuroimaging findings showed that children under the age of 10 exhibit greater amygdala reactivity to images of their mother compared to a stranger, but this difference is no longer evident in adolescents [43]. Early-life social experiences likely also shape the effectiveness of social buffering. For instance, one report suggested that orphanage rearing may be associated with reduced differentiation in amygdala reactivity between mother and stranger stimuli [44] and an accelerated development of amygdala-prefrontal cortex circuitry, with potential implications for the development of anxiety [45]. Future research should continue to examine the neural circuitry underlying the social buffering of stress across development, and pair it with assessments of HPA or SNS reactivity.
Experimental Studies
The research described thus far could have multiple clinical and public health applications if the underlying links between social relationships and health are indeed causal. This is difficult to infer from correlational analyses, thus studies using an experimental design provide particularly powerful evidence that changing the quality of relationships could have salutary consequences for health. For instance, a recent randomized trial showed that a family-oriented intervention implemented with 11-year-old African American youth from the rural Southern United States reduced their inflammation levels 8 years later. The effects were partially mediated by improvements in parenting [46]. Additionally, a recent review discussed interventions that aimed to improve the social environment for children experiencing adversity (primarily through parent training) and that assessed HPA axis functioning as an outcome [47]. The review showed that the majority of these interventions were able to alter HPA axis functioning in children experiencing adversity compared to various comparison groups [47], and one recent study showed that such physiological changes can be long-lasting [48]. While these recent experimental findings are quite encouraging, more research is needed to specify the downstream consequences of these physiological effects for long-term physical health and risk of disease.
Conclusions and Future Directions
Research on the topic of social relationships, stress responses, and physical health has grown exponentially in the past decade. The most recent findings have greatly advanced our understanding of the biological and psychological mechanisms through which social relationships might protect against chronic stress and multi-system physiological dysregulation, the neurobiological underpinnings for their role in dampening physiological stress responses, how the social buffering phenomenon changes across development, and the extent to which it may be malleable through interventions. However, there is a paucity of research on the role of cultural contexts in moderating links between social relationships and health (for an exception, see [49]). Increasing attention should also be dedicated to uncovering how social media and online communication might alter the quality of social relationships and social networks (e.g., [50]), and specifically what the implications for physical health will be for generations that rely heavily on these means of communication. In sum, the recent developments reviewed here have answered important questions about the social buffering of stress and its health implications, while also inspiring a rich set of questions for future research.
Highlights.
Supportive relationships can protect against disease risk associated with adversity
Social relationships can also be a source of stress and physiological dysregulation
Psychobiological mechanisms of action for social support are increasingly revealed
There are developmental changes in the social buffering of stress responses
Novel interventions that improve relationships might also promote physical health
Acknowledgments
C. E. Hostinar’s effort on this manuscript was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number F32HD078048.
Footnotes
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review (2013–2015), have been highlighted as:
* of special interest
** of outstanding interest
- 1.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holt-Lunstad J, Smith TB. Social relationships and mortality. Soc Personal Psychol Compass. 2012;6:41–53. [Google Scholar]
- 3.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–57. [PubMed] [Google Scholar]
- 4.Feeney BC, Collins NL. A new look at social support: A theoretical perspective on thriving through relationships. Personal Soc Psychol Rev. 2014 doi: 10.1177/1088868314544222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwen BS. Stress, Definition and concepts of. In: Fink G, editor. Encyclopedia of Stress. Academic Press; 2000. pp. 508–509. [Google Scholar]
- 6.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller GE, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annu Rev Psychol. 2009;60:501–24. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- 8*.Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychol Bull. 2014;140:256–82. doi: 10.1037/a0032671. This review focused on integrating human studies and animal models of the social buffering of the HPA axis from infancy to adulthood, with the goal of identifying the psychobiological processes that might underlie these effects. The evidence reviewed implicated oxytocinergic systems and the prefrontal cortex, but also suggested major gaps in the literature that currently preclude a full specification of the neural circuitry through which social relationships regulate the HPA axis. Authors also proposed a developmental model of the social buffering of HPA activity, whereby early relationships are thought to shape the future effectiveness of social support in containing stress reactivity through multiple biological and psychological mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen E, Lee WK, Cavey L, Ho A. Role models and the psychological characteristics that buffer low-socioeconomic-status youth from cardiovascular risk. Child Dev. 2013;84:1241–52. doi: 10.1111/cdev.12037. [DOI] [PubMed] [Google Scholar]
- 10.Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011;22:1591–9. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2011;16:729–37. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen S, Janicki-Deverts D, Turner RB, Doyle WJ. Does hugging provide stress-buffering social support? A study of susceptibility to upper respiratory infection and illness. Psychol Sci. 2014 doi: 10.1177/0956797614559284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proc Natl Acad Sci U S A. 2013;110:17149–53. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks KP, Gruenewald T, Karlamangla A, Hu P, Koretz B, Seeman TE. Social relationships and allostatic load in the MIDUS Study. Heal Psychol. 2014;33:1373–1381. doi: 10.1037/a0034528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeman TE, Gruenewald TL, Cohen S, Williams DR, Matthews KA. Social relationships and their biological correlates: Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychoneuroendocrinology. 2014;43:126–38. doi: 10.1016/j.psyneuen.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110:5797–801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cacioppo JT, Cacioppo S. Social relationships and health: The toxic effects of perceived social isolation. Soc Personal Psychol Compass. 2014;8:58–72. doi: 10.1111/spc3.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733–67. doi: 10.1146/annurev-psych-010814-015240. This excellent and comprehensive review surveyed human and animal research on the neuroendocrine consequences of social isolation, in an attempt to explain its association with subsequent morbidity and mortality. The review concluded that the most consistent effects of social isolation on the activity of stress-response systems across studies and species appeared to involve the HPA axis, with more inconsistent results for catecholamine levels. An important contribution of this work was to show that the effects of social isolation are often dependent on the type and quality of the social bonds that are disrupted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Kiecolt-Glaser JK, Jaremka L, Andridge R, Peng J, Habash D, Fagundes CP, Glaser R, Malarkey WB, Belury MA. Marital discord, past depression, and metabolic responses to high-fat meals: Interpersonal pathways to obesity. Psychoneuroendocrinology. 2015;52:239–50. doi: 10.1016/j.psyneuen.2014.11.018. This noteworthy study recruited 43 healthy married couples and assessed a comprehensive set of metabolic and immune measures after consumption of a high-fat meal. Couples also engaged in a marital problem discussion two hours after the meal. Analyses showed that participants who displayed more hostile behaviors during this discussion and who also had a history of mood disorders had significantly lower post-meal resting energy expenditure, higher insulin, and higher peak triglyceride responses compared to participants lower in hostility. Among subjects with mood disorders, the energy expenditure result alone was estimated to translate to an additional 128 kcal for a 6.75-hour period, which could lead to a weight gain of roughly 7.6 pounds per year. These findings are significant because they specify some plausible pathways through which stressful social experiences might lead to obesity and associated chronic diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derry HM, Fagundes CP, Andridge R, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology. 2013;38:2676–85. doi: 10.1016/j.psyneuen.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaremka LM, Fagundes CP, Peng J, Bennett JM, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Loneliness promotes inflammation during acute stress. Psychol Sci. 2013;24:1089–97. doi: 10.1177/0956797612464059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll JE, Diez Roux AV, Fitzpatrick AL, Seeman T. Low social support is associated with shorter leukocyte telomere length in late life. Psychosom Med. 2013;75:171–177. doi: 10.1097/PSY.0b013e31828233bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zalli A, Carvalho LA, Lin J, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A. Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proc Natl Acad Sci. 2014;111:4519–4524. doi: 10.1073/pnas.1322145111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole SW. Social regulation of human gene expression: Mechanisms and implications for public health. Am J Public Health. 2013;103(Suppl):S84–92. doi: 10.2105/AJPH.2012.301183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole SW. Human social genomics. PLoS Genet. 2014;10:e1004601. doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sneed RS, Cohen S. Negative social interactions and incident hypertension among older adults. Heal Psychol. 2014;33:554–565. doi: 10.1037/hea0000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchino BN, Bowen K, Carlisle M, Birmingham W. Psychological pathways linking social support to health outcomes: A visit with the “ghosts” of research past, present, and future. Soc Sci Med. 2012;74:949–57. doi: 10.1016/j.socscimed.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crittenden CN, Pressman SD, Cohen S, Janicki-deverts D, Smith BW, Seeman TE. Social integration and pulmonary function in the elderly. Heal Psychol. 2014;33:535–543. doi: 10.1037/hea0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baek RN, Tanenbaum ML, Gonzalez JS. Diabetes burden and diabetes distress: The buffering effect of social support. Ann Behav Med. 2014;48:145–55. doi: 10.1007/s12160-013-9585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creswell KG, Cheng Y, Levine MD. A test of the stress-buffering model of social support in smoking cessation: Is the relationship between social support and time to relapse mediated by reduced withdrawal symptoms? Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell J, Ehlert U. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37:1111–34. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 32.McEwen BS. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci. 2012;109:17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AS, Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Horm Behav. 2012;61:320–30. doi: 10.1016/j.yhbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76:281–8. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seltzer LJ, Prososki AR, Ziegler TE, Pollak SD. Instant messages vs. speech: Hormones and why we still need to hear each other. Evol Hum Behav. 2012;33:42–45. doi: 10.1016/j.evolhumbehav.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muscatell KA, Eisenberger NI. A social neuroscience perspective on stress and health. Soc Personal Psychol Compass. 2012;6:890–904. doi: 10.1111/j.1751-9004.2012.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molesworth T, Sheu LK, Cohen S, Gianaros PJ, Verstynen TD. Social network diversity and white matter microstructural integrity in humans. Soc Cogn Affect Neurosci. 2015 doi: 10.1093/scan/nsv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. This brief but highly informative review summarized human studies linking childhood adversity (e.g., maltreatment, poverty, institutionalization) to immune dysregulation later in life. The immune indices covered in this review included systemic inflammation and inflammatory responses to microbial challenge, telomere length, latent herpesvirus activation, and measures of immune responses to tumors. This review also addressed the question of potentially mediating mechanisms, including psychological and physiological stress sensitivity, access to stress-buffering resources, and epigenetic modifications. The paper concluded with some very useful suggestions for future work in this area, including the need to empirically test, in humans, whether early-life adversity is related to later immune dysregulation via alterations in HPA and autonomic nervous system activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehlert U. Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology. 2013;38:1850–7. doi: 10.1016/j.psyneuen.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Giesbrecht GF, Poole JC, Letourneau N, Campbell T, Kaplan BJ. The buffering effect of social support on hypothalamic-pituitary-adrenal axis function during pregnancy. Psychosom Med. 2013;75:856–62. doi: 10.1097/PSY.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 41.Hostinar CE, Gunnar MR. Future directions in the study of social relationships as regulators of the HPA axis across development. J Clin Child Adolesc Psychol. 2013;42:564–75. doi: 10.1080/15374416.2013.804387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev Sci. 2014 doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, et al. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci. 2014;25:2067–78. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsavsky AK, Telzer EH, Shapiro M, Humphreys KL, Flannery J, Goff B, Tottenham N. Indiscriminate amygdala response to mothers and strangers after early maternal deprivation. Biol Psychiatry. 2013;74:853–60. doi: 10.1016/j.biopsych.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110:15638–43. doi: 10.1073/pnas.1307893110. This innovative report revealed that children who had experienced early-life maternal deprivation (i.e., orphanage rearing) before adoption into stable homes exhibited atypical patterns of medial prefrontal cortex-amygdala functional connectivity during an fMRI task involving emotional faces. Specifically, the observed negative coupling between these neural regions, which is more typical in later development, was suggestive of accelerated frontoamygdala development due to early-life social adversity. Cortisol levels after the MRI scan mediated the associations between early-life social experience and functional connectivity. These findings are consistent with rodent models of early-life maternal deprivation, leading authors to suggest that this accelerated development may be an ontogenetic adaptation to early adversity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Miller GE, Brody GH, Yu T, Chen E. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proc Natl Acad Sci U S A. 2014;111:11287–92. doi: 10.1073/pnas.1406578111. This groundbreaking study reported results from a randomized control trial designed to improve family relationships and psychosocial competence in low-SES African American youth from the rural Southern United States. Results showed that a 7-week family-oriented intervention conducted when youth were roughly 11-years-old predicted lower inflammation levels 8 years later. The effects were partially mediated by improvements in parenting–i.e., receiving more nurturing and involved parenting, and less harsh or inconsistent parenting, and were strongest for youth living in the most impoverished circumstances. These results are important because random assignment within this intervention provides support for the causal role of family relationships in altering future inflammation levels, a risk factor for the development of many chronic diseases of aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Slopen N, McLaughlin KA, Shonkoff JP. Interventions to improve cortisol regulation in children: A systematic review. Pediatrics. 2014;133:312–26. doi: 10.1542/peds.2013-1632. This very informative review identified 19 articles reporting results from experimental and quasi-experimental intervention studies that assessed changes in cortisol regulation for children experiencing adversity. The majority of these studies reported at least some significant changes in post-intervention cortisol levels, with 8 studies suggesting that the experimental groups were more likely to resemble the low-risk comparison groups after treatment. The review concluded with several recommendations for future research, including the need to test specific components that might mediate the intervention effects, and to expand the range of stress biomarkers investigated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernard K, Hostinar CE, Dozier M. Intervention effects on diurnal cortisol rhythms of Child Protective Services-referred infants in early childhood: Preschool follow-up results of a randomized clinical trial. JAMA Pediatr. 2015;169:112–9. doi: 10.1001/jamapediatrics.2014.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park J, Kitayama S, Karasawa M, Curhan K, Markus HR, Kawakami N, Miyamoto Y, Love GD, Coe CL, Ryff CD. Clarifying the links between social support and health: Culture, stress, and neuroticism matter. J Health Psychol. 2013;18:226–35. doi: 10.1177/1359105312439731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valkenburg PM, Peter J. Online communication among adolescents: an integrated model of its attraction, opportunities, and risks. J Adolesc Heal. 2011;48:121–7. doi: 10.1016/j.jadohealth.2010.08.020. [DOI] [PubMed] [Google Scholar]


