SUMMARY
Persistent mechanical hypersensitivity that occurs in the setting of injury or disease remains a major clinical problem largely because the underlying neural circuitry is still not known. Here we report the functional identification of key components of the elusive dorsal horn circuit for mechanical allodynia. We show that the transient expression of VGLUT3 by a discrete population of neurons in the deep dorsal horn is required for mechanical pain and that activation of the cells in the adult conveys mechanical hypersensitivity. The cells, which receive direct low threshold input, point to a novel location for circuit initiation. Subsequent analysis of c-Fos reveals the circuit extends dorsally to nociceptive lamina I projection neurons, and includes lamina II calretinin neurons, which we show also convey mechanical allodynia. Lastly, using inflammatory and neuropathic pain models, we show that multiple microcircuits in the dorsal horn encode this form of pain.
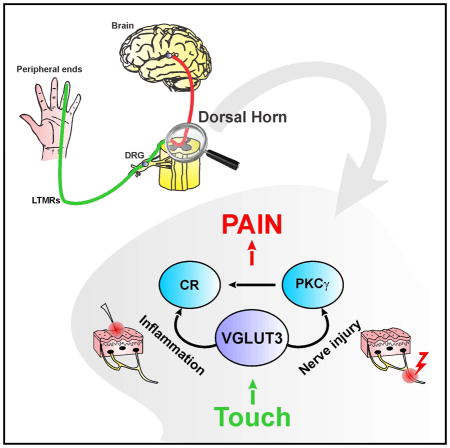
Graphical Abstract
INTRODUCTION
Mechanical pain conveyed acutely is beneficial, serving to warn the body of impending injury. However, persistent pain that results from injury or disease often becomes pathological, debilitating and is difficult to treat (Varrassi et al., 2010). Persistent pain states typically manifest as an increased sensitivity to thermal or mechanical stimuli. The latter allodynic state, in which innocuous touch or movement is perceived as painful, is one of the most clinically problematic forms of pain.
Persistent pain conditions are typically initiated by the dysregulation of primary sensory neurons leading to central sensitization within the spinal cord network and brain (Kuner, 2010; Prescott et al., 2014; von Hehn et al., 2012). The spinal cord dorsal horn is a major site of integration for somatosensory information and is composed of numerous excitatory and inhibitory interneuron populations and a relatively small number of output neurons (Todd, 2010). Information processing in this structure is crudely segregated, such that primary afferents conveying thermal and nociceptive information mainly innervate the superficial laminae, whereas those transmitting low threshold mechanical information generally target deeper laminae. Almost all output neurons are located in laminae I and III–V. The vast majority of lamina I projection neurons respond to noxious stimuli and express the receptor for neurokinin I (NK1R). These cells are thought to convey discriminative aspects of pain, such as the location and quality, as well as emotional aspects (Miraucourt et al., 2007; Todd, 2010). In the deep dorsal horn, most projection neurons are wide-dynamic range. These neurons respond to many different types of stimuli, possess very large receptive fields and code for stimulus intensity among other features (Craig, 2003).
One model proposed for the circuit level mechanisms underlying mechanical allodynia is based on the gate control theory, in which touch normally inhibits acute pain through direct activation of inhibitory interneurons (Melzack and Wall, 1965). Upon injury, however, mechanisms of disinhibition allow touch to instead directly activate pain circuits. Experimental evidence consistent with this theory has demonstrated the existence of a dorsally-directed polysynaptic pathway for mechanical pain that cannot be activated by innocuous mechanical input (touch) under normal conditions, due to a surrounding feed-forward inhibition, or “gate” (Duan et al., 2014; Lu et al., 2013; Torsney and MacDermott, 2006). Upon injury, various mechanisms trigger disinhibition, thus opening the gate and allowing the low threshold mechanoreceptors to engage the polysynaptic network to activate nociceptive-specific projection neurons in lamina I (Baba et al., 2003; Miraucourt et al., 2007; Torsney and MacDermott, 2006; Zeilhofer et al., 2012). This model provides a framework to begin to understand the anatomical substrates and mechanisms underlying mechanical allodynia, but we still do not know many of the fundamental aspects of the circuit, such as the identity of the neurons involved or how the circuit is organized.
Vesicular glutamate transporters (VGLUTs) package glutamate into synaptic vesicles for regulated release. We previously reported that VGLUT3, which has a relatively sparse distribution in the nervous system, is required specifically for acute mechanical pain and the persistent mechanical pain that develops in various models including inflammatory and neuropathic pain (Seal et al., 2009). All other somatosensory behaviors tested in mice lacking the transporter are normal, including thermal, itch, touch and persistent heat hypersensitivity.
Here, we took advantage of the specific requirement for VGLUT3 in mechanical pain to explore the cellular basis of the circuit using a number of complementary approaches. From the analyses, we have now identified a discrete population of spinal cord excitatory interneurons as the origin of the mechanical pain defects in VGLUT3 knockout (KO) mice. Interestingly, the cells reside in lamina III, a region important for touch, but largely ignored with respect to pain (but see Polgar et al., 2007a). We now also show that the cells participate in the persistent mechanical pain circuit and receive almost exclusively low threshold input, thus positioning them at a critical entry point to the circuit. We also identified additional excitatory populations that participate in the dorsal horn circuit for persistent mechanical hypersensitivity, including neurons in inner lamina II that express calretinin and were recently suggested to transmit only light acute mechanical pain (Duan et al., 2014). Lastly, we provide evidence that the neuronal composition of the circuit differs for different types of injury, indicating the existence of microcircuits for mechanical hypersensitivity. A better understanding of the microcircuits and their relationship to the injury will lead to more effective treatment strategies.
RESULTS
Characterization of Conditional VGLUT3 KO Mice
To identify the locus of the acute and persistent mechanical pain defects observed in global VGLUT3 KO mice, we used our floxed conditional KO line (VGLUT3fl/fl) (Figure S1A). Western blot analysis on brain lysates and immunohistochemistry performed on spinal cord slices from homozygous floxed mice indicate that VGLUT3 levels are not altered by loxP insertion (Figures S1B and S1C). We also produced a germline deletion, VGLUT3Δ/Δ, by crossing VGLUT3fl/fl to CMVCre mice. Biochemical and immunohistochemical analyses confirmed that this mouse line does not express the transporter (Figures S1B and S1C). Importantly, the VGLUT3Δ/Δ mice show attenuated acute mechanical pain in the Randall-Selitto assay as well as significantly reduced mechanical hypersensitivity in both the carrageenan model of inflammatory pain and the spared nerve injury (SNI) model of neuropathic pain (Figure S1D), similar to VGLUT3 global KO mice (Seal et al., 2008; Seal et al., 2009).
Cellular Origin of the Mechanical Pain Defects in VGLUT3 KO Mice
In adult mouse spinal cord, VGLUT3 is largely restricted to a discrete population of dorsal root ganglion (DRG) neurons, the C-low threshold mechanoreceptors (C-LTMRs) that innervate dorsal horn lamina II (Figure S1C) (Seal et al., 2009). To assess whether mechanical pain sensation requires VGLUT3 expression by these cells, we deleted the transporter in all DRG neurons using AdvillinCre mice (Figures 1A–1D). This line expresses Cre only in DRG and not spinal cord or brain (Figure 1A) (Hasegawa et al., 2007). Indeed, when the AdvillinCre mouse was crossed to the lsl-tdTomato reporter (Madisen et al., 2010) all DRG neurons expressed tomato (Figure 1B). As expected, VGLUT3 immunoreactivity was not detected in the dorsal horn of adult VGLUT3fl/fl;AdvillinCre mice (Figure 1C). Tail withdrawal thresholds of VGLUT3fl/fl;AdvillinCre mice did not differ from those of VGLUT3fl/fl control mice (Figure 1D), indicating that loss of VGLUT3 from DRG does not affect acute mechanical pain sensation. In both the carrageenan and SNI models, the ipsilateral paw withdrawal thresholds of the VGLUT3fl/fl;AdvillinCre mice were similar to VGLUT3fl/fl control mice and significantly reduced compared to the contralateral paw (Figure 1D), indicating that loss of VGLUT3 in DRG also does not affect mechanical hypersensitivity. Consistent with these results, loss of VGLUT3 from unmyelinated DRG neurons, including the C-LTMRs, using the SNScre line (Agarwal et al., 2004) also did not alter mechanical pain behavior (Figures S1E–S1H).
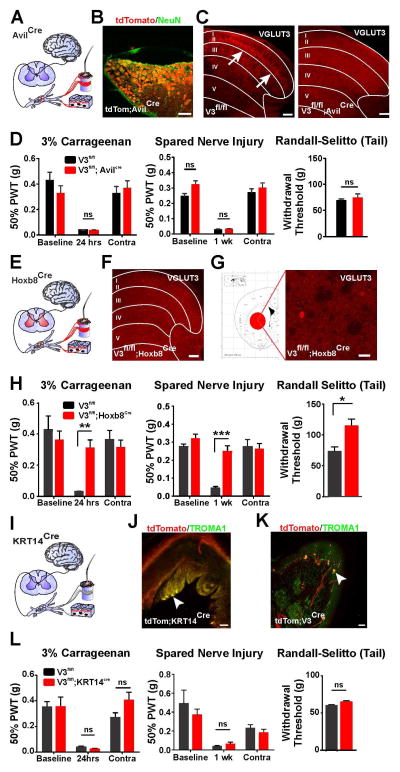
Figure 1. Mechanical Pain Defects Do Not Arise from DRG or Brain.
(A) Cartoon of AvilCre mice showing Cre (red) is restricted to primary afferents.
(B) All DRG neurons express tomato in lsl-tdTom;AvilCre mice.
(C) VGLUT3-IR is not detected in primary afferents (arrows) of VGLUT3fl/fl;AvilCre in the adult.
(D) Paw withdrawal thresholds (PWT) of VGLUT3fl/fl;AvilCre mice do not differ from control mice after carrageenan (n=12 both groups) or SNI (n=7 and n=9 respectively). Randall-Selitto thresholds also do not differ from controls (n=8 and n=6 respectively).
(E) Cartoon of Hoxb8Cre mice showing Cre (red) is expressed by spinal cord and DRG neurons, but not brain.
(F) VGLUT3fl/fl;Hoxb8Cre mice lack VGLUT3-IR in the dorsal horn at p10.
(G) VGLUT3-IR is still present in the striatum of VGLUT3fl/fl;Hoxb8Cre mice.
(H) PWTs of VGLUT3fl/fl;Hoxb8Cre mice are significantly greater than controls after carrageenan (n=11 and n=9 respectively) and SNI (n=9 and n=4 respectively). Randall-Selitto thresholds are also significantly elevated compared to controls (n=9 and n=7).
(I) Cartoon of KRT14Cre mice showing Cre (red) is restricted to keratinocytes and Merkel cells.
(J) As expected, tomato and the Merkel cell marker TROMA1-IR co-localize (arrowhead) in hindpaw glabrous skin of lsl-tdTom;KRT14Cre mice.
(K) Tomato and TROMA1-IR also co-localize (arrowheads) in hindpaw glabrous skin of lsl-tdTom;VGLUT3Cre mice.
(L) PWTs do not differ between VGLUT3fl/fl;KRT14Cre and control mice before or after carrageenan (n=10 and n=6 respectively) or SNI (n=3 both groups). Randall-Selitto thresholds also do not differ (n=7 and n=6 respectively).
All scale bars = 100 μm except in G (50 μm). Data are mean ± SEM. *p < 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Several brain regions implicated in pain processing express VGLUT3. Alternatively, dorsal horn neurons at early postnatal ages as well as Merkel cells, the end organ of slowly adapting mechanoreceptors, also express the transporter (Lou et al., 2013). To address whether VGLUT3 expressed by either of the latter two populations has a role in mechanical pain, we generated VGLUT3fl/fl;Hoxb8Cre mice (Figures 1E–1H). Hoxb8Cre mice express Cre in all neurons that lie caudal to cervical level 2–3, and not in the brain (Figure 1E) (Witschi et al., 2010). Immunoreactivity for VGLUT3 in the VGLUT3fl/fl;Hoxb8Cre mouse was absent from the lumbar dorsal horn, but still present in the striatum (Figures 1F and 1G). Withdrawal thresholds in both the acute and persistent mechanical pain assays were all markedly elevated in these mice compared to VGLUT3fl/fl controls (Figure 1H). To delete VGLUT3 specifically in Merkel cells, we crossed the VGLUT3fl/fl mice to the KRT14Cre line (Figure 1I), a line that expresses Cre specifically in keratinocytes and Merkel cells (Figures 1J and 1K) (Hafner et al., 2004). Mechanical pain behavior in VGLUT3fl/fl;KRT14Cre mice was similar to control VGLUT3fl/fl mice (Figure 1L). Taken together these data point to dorsal horn neurons as the likely locus of the pain defects.
Dorsal Horn Neurons Underlie the Defects in Mechanical Pain
To examine the distribution of neurons that transiently express VGLUT3 in the dorsal horn, we crossed our BAC transgenic VGLUT3Cre mice (Grimes et al., 2011) to the lsl-tdTomato mice. The resulting mice show both the adult and developmental expression of VGLUT3, as tomato is present in all cells that have ever expressed Cre. In the dorsal horn, tomato+ neurons are present in lamina III and to a lesser extent lamina II (Figure 2A). We also examined the dorsal horn of the VGLUT3EGFP BAC transgenic line, in which EGFP expression is under the direct control of VGLUT3 regulatory elements (Figure 2B) (Seal et al., 2009). In this line, we observe a peak number of EGFP+ cells at postnatal day (P) 10–12, but the reporter is present only in the central terminals of C-LTMRs in the adult (Figure 2B). We also analyzed VGLUT3 expression in the dorsal horn directly by taking advantage of the VGLUT3fl/fl;AdvillinCre mice. Because VGLUT3 is deleted from primary afferents in these mice, expression is thus restricted to spinal cord neurons. Here we observed VGLUT3 immunoreactivity that first appeared around P5, peaked around P10–12 and was significantly reduced by ~P20 (Figure 2C).
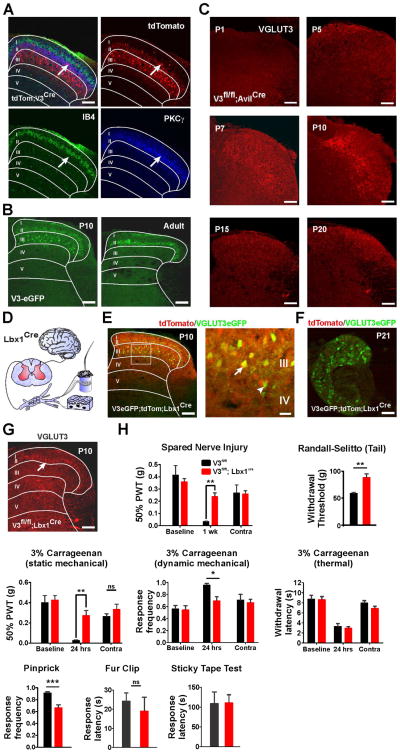
Figure 2. Somatosensory Behaviors of VGLUT3fl/fl;Lbx1Cre Mice.
(A) In lsl-tdTom;VGLUT3Cre mice, tomato+ neurons are located in laminae IIi–III and 16% co-localize with PKCγ-IR (arrow).
(B) In VGLUT3EGFP mice, EGFP is expressed by laminae IIi–III neurons at p10, but not adult.
(C) VGLUT3-IR in VGLUT3fl/fl;AvilCre mice peaks around P10 (middle row).
(D) In Lbx1Cre mice, only spinal cord neurons express Cre.
(E) EGFP and tomato co-localize at p10 in VGLUT3EGFP;lsl-tdTom;Lbx1Cre mice (arrow). A few EGFP+ neurons at the lamina IV border do not express tomato+ (arrowhead).
(F) Tomato is not present in DRG of adult VGLUT3EGFP;lsl-tdTom;Lbx1Cre mice.
(G) VGLUT3-IR in VGLUT3fl/fl;Lbx1Cre mice at P10 is present in primary afferents (arrow) and not spinal cord neurons.
(H) Baseline PWT of VGLUT3fl/fl;Lbx1Cre mice was similar to controls. After SNI (n=8 both groups), PWTs were significantly elevated compared to controls. Withdrawal responses in the Randall-Selitto assay (n=9 both groups) were also significantly higher than controls. After carrageenan, PWTs of VGLUT3fl/fl;Lbx1Cre mice (static mechanical) were elevated compared to controls (n=9 both groups). The mice also responded less frequently than controls to light dynamic mechanical stimulation with a cotton swab (n=11 and n=8 respectively). Latency to respond in the Hargreaves’ test did not differ from controls (n=11 and n=8 respectively). VGLUT3fl/fl;Lbx1Cre mice responded less frequently than controls to pinprick of the plantar hindpaw (n=11 and n=8 respectively). Sticky tape and fur clip measures of touch did not differ between VGLUT3fl/fl;Lbx1Cre mice and controls (both tests n=8 and n=7 respectively).
All scale bars = 100 μm except inset in E (20 μm). Data are mean ± SEM. *p<0.05, **p ≤ 0.01, ***p ≤ 0.001.
To determine whether mechanical pain sensation requires the transient expression of VGLUT3 by dorsal horn neurons, we used Lbx1Cre mice. In this line, Cre is restricted to only spinal cord dorsal horn neurons and is not in DRG or brain (Figure 2D) (Sieber et al., 2007). To confirm that the VGLUT3+ cells are in the Lbx1Cre lineage, we generated VGLUT3EGFP;lsl-tdTom;Lbx1Cre mice. Virtually all EGFP+ cells in the dorsal horn expressed tomato at P10 (Figure 2E). Importantly, no DRG neurons expressed tomato (Figure 2F). Consistent with the deletion of VGLUT3 in the dorsal horn and not in DRG, VGLUT3 immunoreactivity in VGLUT3fl/fl;Lbx1Cre mice at P10 appeared only as a discrete band in lamina IIi corresponding to C-LTMR afferents (Figure 2G). In the acute mechanical pain assay, tail withdrawal thresholds were significantly elevated compared to VGLUT3fl/fl controls (Figure 2H). Additionally, in the carrageenan and the SNI models of persistent pain, the von Frey thresholds of VGLUT3fl/fl;Lbx1Cre mice were significantly higher than control mice after injury. As expected, heat hypersensitivity, which is unaffected in global VGLUT3 KO mice, did not differ from controls. These data confirm that the dorsal horn neurons are the locus of the acute and persistent mechanical pain defects observed in global VGLUT3 KO mice.
To determine in more detail the mechanosensory behaviors that require VGLUT3, we tested the mice in several additional assays. VGLUT3fl/fl;Lbx1Cre showed reduced pinprick pain and impaired dynamic mechanical allodynia after carrageenan injection, but showed normal light touch-related behavior in the sticky tape and hair clip assays (Figure 2H).
VGLUT3 Marks a Population of Excitatory Interneurons in the Dorsal Horn
To determine whether VGLUT3 is expressed by inhibitory or excitatory interneurons, we performed double fluorescent in situ hybridizations on lsl-tdTom;VGLUT3Cre mice. Nearly all tomato+ cells co-expressed the excitatory (vglut2) and not the inhibitory marker (gad67) (Figure 3A). Tomato+ cells also did not express Pax2, another marker of inhibitory neurons (Figure 3B). To confirm that the mechanical pain defects are due to the loss of VGLUT3 from the excitatory neurons, we generated VGLUT3fl/fl;Tlx3Cre mice (Figures 3C–3E), in which Cre is only expressed by excitatory neurons (Xu et al., 2013). Immunoreactivity for VGLUT3 was absent in the dorsal horn of these mice at P10 (Figure 3D). In all three mechanical pain assays, withdrawal thresholds were significantly elevated compared to VGLUT3fl/fl controls (Figure 3E), consistent with the behavior of global VGLUT3 KO and VGLUT3fl/fl;Lbx1Cre mice.
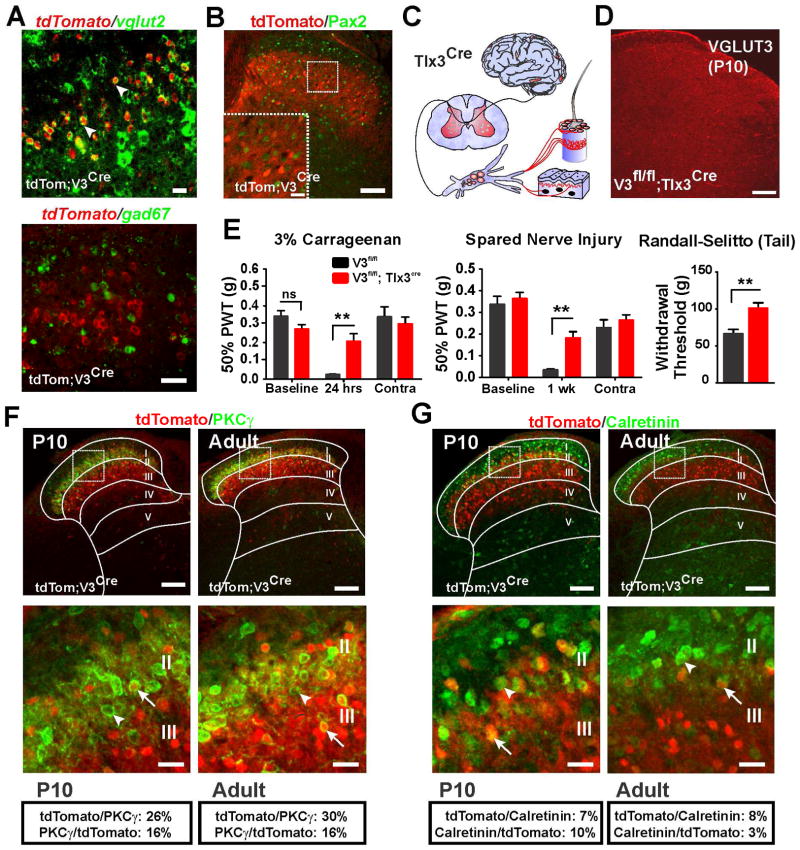
Figure 3. Excitatory Neurons in the Dorsal Horn Transiently Express VGLUT3.
(A) In situ hybridization of tomato co-localized with vglut2, but not gad67 in lsl-tdTom;VGLUT3Cre mice.
(B) The inhibitory neuron marker Pax2 does not colocalize with tomato in lsl-tdTom;VGLUT3Cre mice.
(C) Cartoon showing Tlx3Cre mice express Cre (red) only in excitatory neurons in the dorsal horn, DRG neurons and sparsely in brain.
(D) VGLUT3fl/fl;Tlx3Cre mice at P10 lack VGLUT3-IR in the dorsal horn.
(E) Baseline PWTs of VGLUT3fl/fl;Tlx3Cre mice were similar to controls. After carrageenan (n=12 both groups) or SNI (n=11 and n=8 respectively), PWTs of VGLUT3fl/fl;Tlx3Cre mice were significantly elevated compared to controls, as were Randall-Selitto tail withdrawal thresholds (n=9 both groups).
(F) Co-localization of tomato and PKCγ in lsl-tdTom;VGLUT3Cre mice at P10 and adult.
(G) Co-localization of tomato and calretinin in lsl-tdTom;VGLUT3Cre mice at p10 and adult.
All scale bars = 100 μm except A and insets in B, F and G (20 μm). Data are mean ± SEM. **p ≤ 0.01.
To further identify the transient VGLUT3 neurons, we examined co-expression of tomato with dorsal horn markers in lsl-tdTom;VGLUT3Cre mice (Figures 3F and 3G). At peak VGLUT3 expression (P10), few tomato+ cells express PKCγ (16%; 212 of 1311) or calretinin (10%; 108 of 1209). Similarly, tomato overlaps with ~25% (212 of 815) of the PKCγ population, and only 7% (108 of 1469) of calretinin neurons. The degree of overlap is similar in the adult.
Architecture and Afferent Innervation of the VGLUT3 KO Spinal Cord
Gross anatomical defects, such as the aberrant innervation by central terminals of DRG neurons or the loss of spinal cord neurons have been observed in mouse mutants with spinal cord gene deletions (Ross et al., 2010; Wang et al., 2013; Xu et al., 2013). Such changes could potentially contribute to disruption of the mechanical pain circuit in VGLUT3 KO mice. We therefore examined the distribution of molecular markers for spinal cord neurons (NeuN, vesicular GABA transporter, NK1R, VGLUT2 and PKCγ) and primary afferents (TRPV1, CGRP, isolectin-B4 and VGLUT1) in the dorsal horn of VGLUT3 KO and WT mice (Figure S2A) but observed no major differences. Crossed onto the global VGLUT3 KO strain, lsl-tdTom;VGLUT3Cre mice showed no change in the number of tomato+ dorsal horn neurons compared to mice WT for VGLUT3 (Figures S2B–S2C). Thus, deletion of VGLUT3 does not cause apoptosis or obvious changes in the architecture or afferent innervation of the dorsal horn, consistent with a more discrete defect in synaptic transmission.
Synaptic Transmission Defect is Consistent with Attenuated Mechanical Allodynia
The anatomical basis for light touch becoming painful after injury is thought to involve a disinhibition of the dorsal horn mechanical pain circuits that allows low threshold mechanoreceptors to activate pain transmitting projection neurons in lamina I (NK1R+) through a dorsally-directed polysynaptic network (Braz et al., 2014). Since the loss of VGLUT3 from dorsal horn excitatory neurons causes a behavioral impairment in mechanical hypersensitivity, we hypothesized that the low threshold A-fibers would not activate nociceptive lamina I neurons under conditions of disinhibition (the injured state) in the KO mice. To test this hypothesis, we used an in vitro spinal cord model of mechanical allodynia (Torsney and MacDermott, 2006). In this model, when inhibitory tone is normal, dorsal root stimulation at A-fiber intensities is unable to generate polysynaptic excitatory currents in nociceptive lamina I projection neurons. However, under pharmacological disinhibition, stimulation of the dorsal roots at A-fiber intensity reliably generates polysynaptic excitatory currents in the lamina I projection neurons, thus serving as the anatomical substrate for the perception of touch as painful after injury (Baba et al., 2003; Miraucourt et al., 2007; Torsney and MacDermott, 2006). This model is illustrated in Figure 4A.
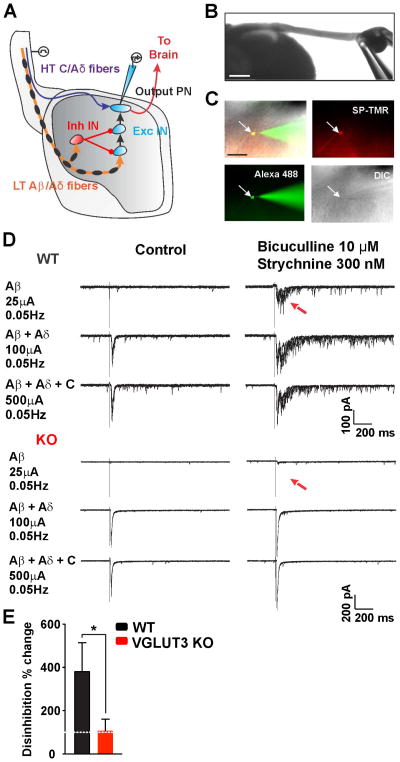
Figure 4. Loss of Polysynaptic Low Threshold Input to NK1R+ Lamina I Neurons.
(A) Schematic representation of the dorsal horn showing nociceptive inputs (High threshold (HT) C and Aδ fibers) to NK1R+ lamina I projection neurons (PN). Polysynaptic innocuous afferent (Low threshold (LT) Aβ and Aδ fibers) input to PNs through excitatory interneurons (Exc IN) is suppressed by local inhibitory interneurons (Inh IN). Disinhibition induced pharmacologically enables polysynaptic activation of NK1R+ lamina I neurons by LT inputs.
(B) In vitro spinal cord preparation at 4x magnification. Scale bar = 500 μm.
(C) Recorded lamina I NK1R+ neuron labeled by fluorescent SP-TMR and filled with Alexa-488. IR-DIC shows same neuron with patch pipette. Scale bar = 100 μm.
(D) Characterization of afferent inputs to NK1R+ lamina I neurons in control conditions (top left traces) and after pharmacological disinhibition (10 μm bicuculline, 300 nM strychnine) (top right traces). In WT slices, dorsal root stimulation at Aδ and C intensities evokes monosynaptic EPSCs in NK1R+ lamina I neurons. With pharmacological disinhibition, low intensity dorsal root stimulation generates Aβ and Aδ polysynaptic EPSCs in NK1R+ lamina I neurons. In VGLUT3 KO slices, Aδ and C stimulation generates monosynaptic events in NK1R+ lamina I neurons (bottom left traces). Under pharmacological disinhibition, low intensity dorsal root stimulation fails to generate EPSCs in NK1R+ lamina I neurons (bottom right traces).
(E) Percent increase in A-fiber-evoked EPSCs in NK1R+ lamina I neurons. Data are normalized to EPSCs measured before disinhibition. n = 7 (WT) and 8 (VGLUT3 KO) total cells from the same number of mice. Mann-Whitney test, *p<0.05.
Whole-cell patch clamp recordings were performed in transverse spinal cord slices with dorsal roots and DRGs still attached (Figure 4B). Roots were electrically stimulated at different intensities to recruit Aβ (25 μA) or Aβ and Aδ (100 μA) or Aβ, Aδ and C fibers (500 μA), respectively. To visualize neurons expressing NK1R, slices were pre-incubated with the fluorescent tetramethylrhodamine substance P conjugate, SP-TMR (Figure 4C) as previously described (Torsney and MacDermott, 2006). Under normal conditions, Aδ- and C-, but not Aβ-fiber stimulation produced monosynaptic glutamatergic EPSCs in NK1R+ lamina I neurons of both global VGLUT3 KO and control mice, similar to what has been observed in rats (Figure 4D, left traces) (Torsney and MacDermott, 2006). In the presence of the inhibitory receptor antagonists bicuculline (10 μM) and strychnine (300 nM), stimulation of dorsal roots at Aβ intensity reliably produced polysynaptic EPSCs in NK1R+ lamina I neurons in slices from WT mice (Figure 4D, upper right traces and Figure 4E). We also occasionally observed Aδ-induced polysynaptic EPSCs consistent with previous observations (Figure S2D) (Torsney and MacDermott, 2006). Strikingly, stimulation at A-fiber intensities failed to produce polysynaptic EPSCs in lamina I neurons of VGLUT3 KOs (Figure 4D, bottom right traces and Figure 4E). As a control, increasing the intensity of stimulation to recruit all A-fibers (100 μA) or all types of fibers (500 μA) produced monosynaptic EPSCs in the NK1R+ neurons similar to those observed in the absence of inhibitory blockers, showing that deletion of VGLUT3 selectively eliminated the polysynaptic EPSCs induced by Aβ-fibers. Importantly, conduction velocities as well as afferent thresholds measured for Aδ- and C-fibers did not differ between VGLUT3 KO and WT mice (Aδ threshold, 100 μA, p = 0.34; C threshold, 500 μA, p = 0.46; Figure S2E) and were similar to values previously reported (Torsney and MacDermott, 2006). The results are consistent with a severe impairment in neuronal transmission within the dorsal horn polysynaptic pathway that conveys mechanical hypersensitivity. In addition, the data provide support for altered excitatory transmission rather than a compensatory up-regulation of inhibition.
VGLUT3+ Dorsal Horn Neurons Convey Mechanical Hypersensitivity
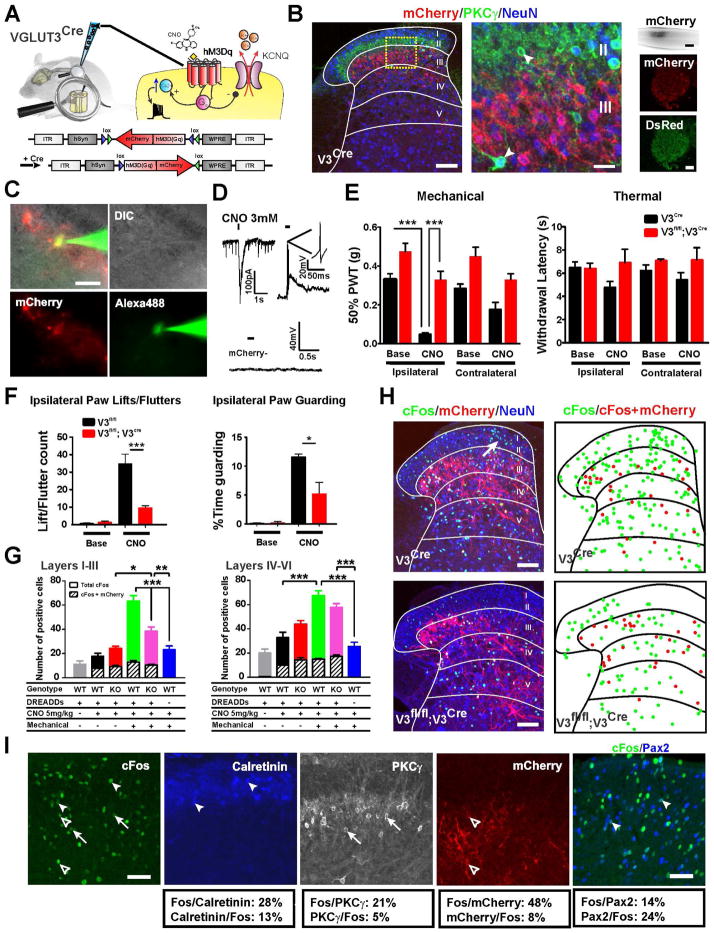
Our results show that normal mechanical pain sensation requires the transient postnatal expression of VGLUT3 by dorsal horn neurons, raising the possibility that the neurons themselves convey this type of pain. To test this hypothesis, we used a chemogenetic approach, targeting the excitatory designer receptor (DREADDs) hM3Dq to Cre expressing cells in lamina III of the VGLUT3Cre mice and then assessing whether activation of the cells with the receptor-specific ligand, clozapine-n-oxide (CNO), produces mechanical allodynia. First we verified that the Cre+ cells in the dorsal horn of our transgenic line match those responsible for the mechanical pain defects in VGLUT3 KO mice (Figures S3A and S3B). In the Randall-Selitto, carrageenan and SNI mechanical pain assays, VGLUT3fl/fl;VGLUT3Cre mice showed significantly elevated withdrawal thresholds compared to control mice (Figure S3B). Because all adult dorsal horn excitatory neurons express VGLUT2, we predicted that deletion of VGLUT2 from the transient VGLUT3 cells would also produce the mechanical pain defects (Figure S3C). Interestingly, while persistent mechanical hypersensitivity was reduced in the VGLUT2fl/fl;VGLUT3Cre mice (similar to knocking out VGLUT3), acute mechanical pain was normal. This unexpected finding is consistent with the idea that although both acute and persistent mechanical pain require the transient postnatal expression of VGLUT3 by these dorsal horn neurons, the cells themselves only transmit persistent pain in the adult.
To target the hM3Dq receptor specifically to the dorsal horn, we used a viral approach. We first determined that the AAV8 serotype coupled with the neuron-specific human synapsin promoter was highly specific for the infection of only dorsal horn neurons (and not DRG) (Figure S4). Three weeks after injection of the AAV8-hSyn-DIO-hM3Dq-mCherry virus into one side of the lumbar enlargement of P10 VGLUT3Cre mice (when Cre is at peak expression), the reporter was highly restricted to neurons in lamina III and importantly was not present in PKCγ neurons or DRG (Figures 5A and 5B). To confirm that hM3Dq functions properly in the dorsal horn, we performed patch clamp recordings in transverse spinal cord slices. As expected, application of 3 mM CNO produced an inward current in voltage clamp and generated action potentials in current clamp only in mCherry+ neurons (Figures 5C and 5D).
Figure 5. Activation of VGLUT3+ Dorsal Horn Neurons Produces Mechanical Allodynia and Reveals a Polysynaptic Mechanical Allodynia Circuit.
(A) Schematic of the VGLUT3Cre dorsal horn unilaterally injected at P10 with the Cre-dependent excitatory DREADDs virus (AAV8 hSyn-DIO- hM3Dq-mCherry).
(B) Lamina III, but not PKCγ (arrowheads) or DRG neurons express hM3Dq-mCherry. Scale bars = 100 μm except for inset (20 μm) and whole spinal cord (400 μm).
(C) Recording electrode filled with Alexa-488 targets a lamina III neuron expressing hM3Dq-mCherry. Second electrode used to puff on the specific hM3Dq ligand CNO can be seen in DIC picture. Scale bar = 50 μm.
(D) Direct application of 3 mM CNO to an hM3Dq-mCherry expressing neuron generates an inward current in voltage-clamp and series of action potentials in current-clamp. CNO applied to a cell lacking hM3Dq-mCherry does not elicit a change in membrane potential (bottom trace).
(E) PWTs for VGLUT3Cre and VGLUT3fl/fl;VGLUT3Cre mice expressing hM3Dq-mCherry were normal at baseline. After CNO (5 mg/kg) injection, only PWTs of VGLUT3Cre mice dropped dramatically. Hargreaves’ withdrawal latencies were unchanged for the two mouse lines (both tests n=7 and n=10 respectively).
(F) After CNO (5 mg/kg) injection, hM3Dq-expressing VGLUT3Cre mice show lifting, fluttering and guarding of the ipsilateral hindpaw. In contrast, hM3Dq-expressing VGLUT3fl/fl;VGLUT3Cre mice rarely exhibit the behaviors.
(G) Quantification of c-Fos+ cells in laminae I–III and laminae IV–VI of VGLUT3Cre (labeled WT) and VGLUT3fl/fl;VGLUT3Cre (labeled KO) mice expressing hM3Dq-mCherry. Hatched bars indicate number of c-Fos+ cells that also express mCherry for each condition.
(H) Induction of c-Fos cells in laminae I–III neurons of VGLUT3Cre, but not VGLUT3fl/fl;VGLUT3Cre mice (both expressing hM3Dq-mCherry) after CNO injection followed by mechanical stimulation. Scale bars = 100 μm.
(I) Co-staining of c-Fos with PKCγ (21%), calretinin (28%) and Pax2 (14%) neurons in laminae I–III of VGLUT3Cre mice expressing hM3Dq-mCherry after CNO injection followed by mechanical stimulation. Nearly half (48%) of the mCherry+ cells were also c-Fos+. Scale bars = 50 μm.
Data are mean ± SEM. *p<0.05, **p ≤ 0.01, ***p ≤ 0.001.
To test whether chemogenetic activation of the cells elicits mechanical pain behavior, we measured von Frey thresholds in the presence and absence of CNO (Figure 5E). In the absence of CNO, thresholds for both hindpaws were similar, suggesting that viral infection alone does not affect mechanical sensitivity. After CNO injection, thresholds were dramatically reduced at only the ipsilateral hindpaw, consistent with the induction of mechanical hypersensitivity. The injected mice also showed guarding and occasional fluttering of the ipsilateral hindpaw upon contact with an innocuous surface, indicative of mechanical allodynia (Figure 5F; Movie S1). In a test of heat hypersensitivity (Figure 5E), which is not altered in the global VGLUT3 KO, withdrawal latencies were the same for both hindpaws before and after CNO injection. Since deletion of VGLUT3 in these neurons impairs mechanical hypersensitivity (Figure S3B), we hypothesized that mice lacking the transporter would not show the drop in threshold. Indeed, injection of CNO in VGLUT3fl/fl;VGLUT3Cre mice expressing hM3Dq did not alter the mechanical threshold (Figure 5E). The mice also showed essentially no paw fluttering and significantly less guarding behavior compared to VGLUT3Cre mice (Figure 5F; Movie S1). The data thus provide strong evidence that transient VGLUT3 neurons in lamina III participate in the circuit for mechanical hypersensitivity.
c-Fos Reveals the Dorsally-directed Pathway For Mechanical Hypersensitivity
To identify additional dorsal horn neurons that participate in the mechanical allodynia circuit, we stained for c-Fos, a marker of neuronal activity, after activation of hM3Dq in VGLUT3Cre mice. After injection of CNO in anesthetized mice, c-Fos was surprisingly restricted almost exclusively to the mCherry+ cells (Figure 5G; Figure S5). This was also observed with the VGLUT3fl/fl;VGLUT3Cre mice. Since mechanical hypersensitivity in the VGLUT3Cre mice requires low threshold input, we followed CNO injection with walking the mice at a pace that was slow, but avoided guarding behavior. Under these conditions, a dramatic increase in c-Fos+ cells was observed in laminae I–II and to a lesser degree in lamina III in VGLUT3Cre, but not VGLUT3fl/fl;VGLUT3Cre mice (Figures 5G and 5H; Figure S5). Importantly, c-Fos expression was increased predominantly in the medial dorsal horn, an area corresponding to the distal part of the limb. Mice that did not receive CNO showed little to no c-Fos in the dorsal horn and mice that received mechanical stimulation, but not CNO, showed c-Fos only in deeper laminae, where innocuous mechanical circuits are active (Figure 5G; Figure S5).
To further identify the c-Fos+ neurons in laminae I–III, we co-stained for PKCγ, calretinin and Pax2. The c-Fos co-stained with ~48% of mCherry+, 21% of PKCγ+, 28% of calretinin+ and 14% of Pax2+ neurons (Figure 5I). Interestingly, c-Fos was also induced in an excitatory population in lamina III that lacked mCherry and PKCγ. Thus, we have now identified at least four distinct excitatory interneuron populations that reside within the circuit for mechanical hypersensitivity.
To identify neurons postsynaptic to the transient VGLUT3 population in lamina III, we injected a Cre-dependent virus encoding the anterograde trans-neuronal tracer, wheat-germ agglutinin (WGA) (Braz et al., 2002) in lsl-tdTom;VGLUT3Cre mice at P10 (Figure S6). WGA allowed us to refine the position of the neurons with respect to the VGLUT3Cre cells by observing the location of the tracer across time. At the earliest time point (3 dpi), WGA was detected almost exclusively in lamina III tomato+ neurons and not DRG. On days 4 and 5, we detected additional neurons in laminae III and IV as well as vertical cells in lamina IIo, which are all likely to be directly postsynaptic. By 6 and 21 dpi, WGA was still excluded from DRG, but was now detected in lamina II where it co-localized with PKCγ and calretinin as well as with inhibitory neurons throughout laminae II–III. We thus conclude that within lamina III, the transient VGLUT3 population is presynaptic to another unidentified population of excitatory neurons, which are presynaptic to lamina II calretinin and PKCγ neurons.
VGLUT3+ Lamina III Cells Receive A-fiber Input
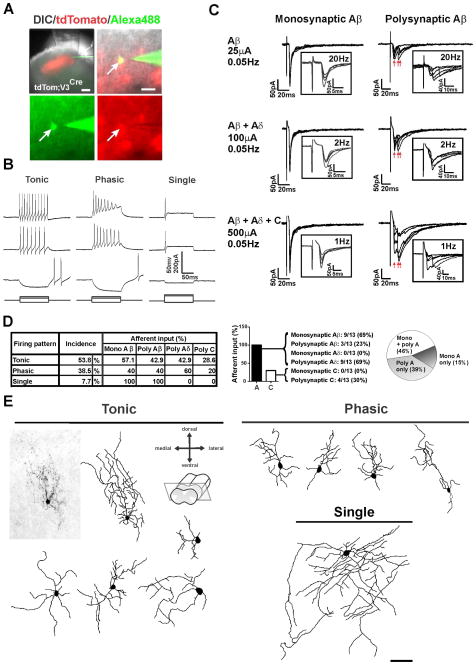
We next determined whether the transient VGLUT3 cells in lamina III directly receive low threshold input, a key component of the dorsal horn circuit for mechanical allodynia (Braz et al., 2014). Using lsl-tdTom;VGLUT3Cre mice, we recorded lamina III tomato+ neurons which exhibited equally phasic or tonic firing patterns in response to current injection (Figures 6A and 6B). Remarkably, dorsal root stimulation at A-fiber intensity induced EPSCs in all recorded tomato+ neurons (Figures 6C and 6D). About half of the cells (46%) received combined direct monosynaptic Aβ and polysynaptic Aδ inputs and a few received polysynaptic C-fiber input. The high proportion of cells receiving direct A-fiber input together with the behavioral results, suggest that the transient VGLUT3 population serves as an entry point to the A-fiber mediated, dorsally-directed circuit for mechanical allodynia.
Figure 6. Characterization of VGLUT3+ Neurons and Primary Afferent Input.
(A) Lsl-tdTom;VGLUT3Cre neurons were recorded using transverse spinal cord slices with the dorsal root and DRG still attached. Cells were filled with Alexa-488 and biotin for post-hoc reconstruction. Scale bars = 200 μm (upper left) and 50 μm.
(B) Example traces of tonic, phasic and single firing patterns recorded in tomato+ neurons.
(C) The tomato+ neurons receive monosynaptic or polysynaptic Aβ inputs.
(D) Incidence of firing patterns and afferent inputs of tomato+ neurons.
(E) Morphology of lamina III tomato+ neurons. Scale bar = 50 μm.
Subsequent morphological analyses revealed that the neurons are fairly homogeneous, showing a predominantly dorso-ventral dendritic arbor. Very few cells have a radial morphology and none resemble central or islet cells (Figure 6E). This finding is consistent with previous reports describing Lamina III excitatory interneurons with dorsally-directed processes (Polgar et al., 2007b; Schneider, 2008). Both phasic and tonic neurons showed similar morphologies and afferent input, but phasic neurons were more uniform with only dorsally-directed dendrites.
Lamina II Calretinin+ Neurons Convey Mechanical Hypersensitivity
Mechanical allodynia resulting from activation of the transient VGLUT3 neurons induced c-Fos expression in calretinin neurons, suggesting that these cells also transmit mechanical hypersensitivity. A small proportion of calretinin cells also transiently express VGLUT3 (Figure 3). We therefore first tested acute and persistent pain behaviors in VGLUT3fl/fl;CRCre mice, but observed no difference compared to VGLUT3fl/fl controls (Figures S7A and S7B).
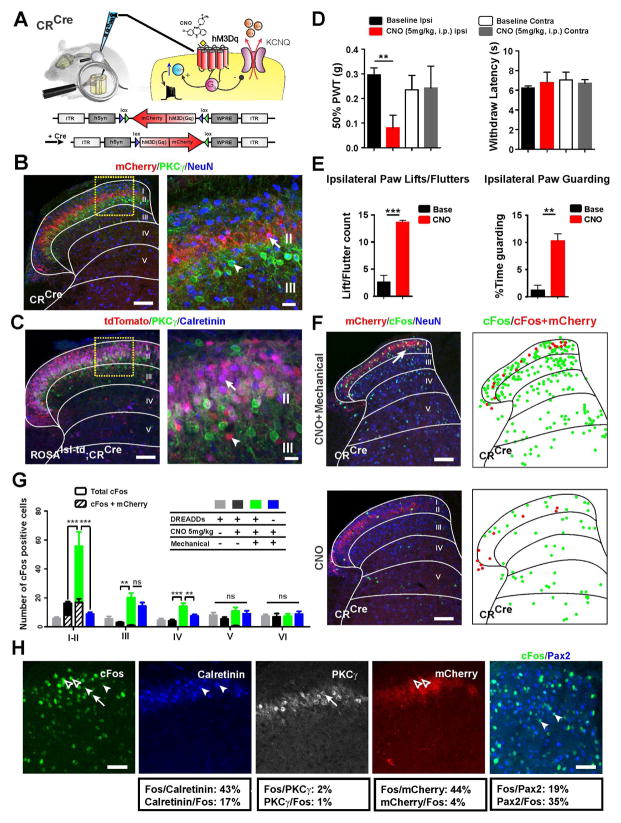
To directly manipulate calretinin neurons, we injected the hM3Dq virus into the dorsal horn of CRCre mice (Figure 7A). Expression of the mCherry was limited to a discrete layer of calretinin+ cells within the dorsal part of lamina IIi and was absent from PKCγ and DRG neurons (Figure 7B; Figures S7C and S7D). Importantly, mCherry expression corresponded to calretinin+ cells in the adult and not to neurons in lamina I, IIo and the ventral part of lamina II that transiently express calretinin during development and can be observed in lsl-tdTom;CRCre mice (Figure S7E). These adult calretinin neurons are largely distinct from an Lbx1+ population (Figure S7F) recently shown to affect acute pain (Duan et al., 2014). After CNO injection, the ipsilateral von Frey threshold dropped dramatically (Figure 7D). Behaviors such as paw guarding and fluttering were also observed in the ipsilateral paw (Figure 7E; Movie S2) consistent with mechanical hypersensitivity and similar to what we observed with VGLUT3Cre mice. Thermal sensitivity in these mice was unchanged (Figure 7D).
Figure 7. Activation of Calretinin+ Neurons Produces Mechanical Allodynia.
(A) Schematic of the CRCre dorsal horn unilaterally injected with the hM3Dq-mCherry virus.
(B) The mCherry localizes exclusively to the dorsal region of lamina IIi (arrow), and does not co-localize with PKCγ (arrowhead). Scale bars = 100 μm (left) and 20 μm (right).
(C) In lsl-tdTom;CRCre mice, tomato is expressed by neurons throughout lamina IIi and co-stains almost completely with calretinin, but very little with PKCγ. Scale bars = 100 μm (left) and 20 μm (right).
(D) PWTs of CRCre mice expressing hM3Dq-mCherry were normal at baseline, but dropped dramatically after injection of CNO (5 mg/kg). Hargreaves’ withdrawal latencies were unaffected (n=8 for both tests).
(E) After CNO (5 mg/kg) injection, CRCre mice expressing hM3Dq-mCherry demonstrate lifting, fluttering and guarding of the ipsilateral paw.
(F) Representative images and schematics of c-Fos in the dorsal horn of CRCre mice expressing hM3Dq-mCherry after CNO only (top) or CNO followed by mechanical stimulation (bottom). Scale bars = 100 μm.
(G) Quantification of c-Fos+ neurons in laminae I–II and laminae III–IV after CNO injection with or without mechanical stimulation, mechanical stimulation only or saline injection only.
(H) c-Fos+ neurons in laminae I–II induced by CNO plus mechanical stimulation co-stain with calretinin (17%) and Pax2 (35%), but much less with PKCγ (1%). Nearly half (44%) of the mCherry+ cells co-localized with c-Fos. Scale bars = 50 μm.
Data are mean ± SEM. **p ≤ 0.01, ***p ≤ 0.001.
We also examined the dorsal horn expression of c-Fos in these mice. In anesthetized animals injected with CNO, c-Fos was induced primarily in mCherry+ cells (Figures 7F and 7G; Figure S7G). However, coupling low threshold mechanical stimulation with CNO dramatically increased the number of c-Fos neurons in laminae I–II (Figures 7F and 7G; Figure S7H). This increase was absent in mice lacking hM3Dq or injected with saline (Figure 7G).
To further characterize the c-Fos+ cells, we co-stained for calretinin, PKCγ and Pax2 (Figure 7H). Approximately 30% of the calretinin neurons expressed mCherry and half of these showed c-Fos induction. Of the remaining mCherry-negative calretinin neurons, ~45% expressed c-Fos indicating a high degree of activation within the calretinin population. We also observed ~30% of c-Fos+ cells co-stained with Pax2. Most interestingly, very few of the c-Fos+ cells co-stained for PKCγ.
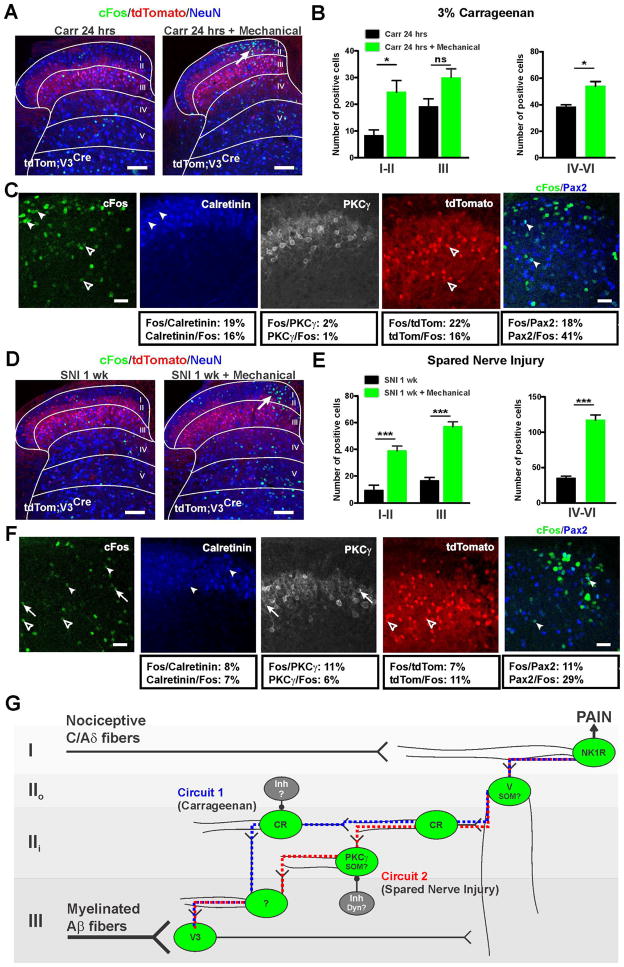
Our data thus far show that hM3Dq activation of either the transient VGLUT3 neurons in lamina III or the adult calretinin neurons in lamina II produces mechanical hypersensitivity, and that coupling a mechanical stimulus to the activation of these cells induces c-Fos in the dorsal horn circuit for mechanical allodynia. Interestingly, activation of the transient VGLUT3, but not the calretinin neurons, produces c-Fos in PKCγ neurons, suggesting the existence of more than one allodynic pathway. Since the discovery of PKCγ as a critical component of persistent mechanical pain (Malmberg et al., 1997), evidence for its contribution to allodynia produced by different types of injury has remained unclear (Gao and Ji, 2010; Zhao et al., 2011; Zou et al., 2011). We therefore compared the pattern of c-Fos induction in the carrageenan and SNI models (Figures 8A–8F).
Figure 8. Expression of c-Fos in inflammatory and neuropathic pain models reveals multiple microcircuits for mechanical allodynia.
(A) Representative pictures of c-Fos induced by carrageenan injection and with (right) or without (left) mechanical stimulation 24 hours later. Scale bars = 100 μm.
(B) Quantification of c-Fos+ dorsal horn cells after carrageenan injection and with (green bars) or without (black bars) mechanical stimulation 24 hours later.
(C) c-Fos+ cells colocalize with calretinin (16%), very few with PKCγ (1%) as well as tomato+ lamina III neurons (16%) and Pax2+ cells (41%). Scale bars = 50 μm.
(D) Representative pictures of c-Fos induced by SNI with (right) or without (left) mechanical stimulation delivered 1 week later. Scale bars = 100 μm.
(E) Quantification of c-Fos+ dorsal horn cells after SNI with (green bars) or without (black bars) mechanical stimulation delivered 1 week later.
(F) c-Fos co-localizes with cells expressing calretinin (7%), PKCγ (6%), tomato (11%) and Pax2 (29%). Scale bars = 50 μm.
(G) Schematic diagram of the circuit for mechanical allodynia. Transient VGLUT3 cells located in lamina III transmit the input from myelinated fibers to the more dorsal neural network underlying mechanical hypersensitivity. Neighboring cells in lamina III receive input from transient VGLUT3 neurons and relay the signal to lamina II cells including PKCγ+ and calretinin+ excitatory interneurons that act to refine the excitability of the circuit. Vertical cells in lamina IIo, which receive input from transient VGLUT3 cells and lamina II cells, integrate the signal and send an output to the nociceptive NK1R+ lamina I projection neurons. In persistent pain induced by nerve injury, the “gate” that controls PKCγ is opened resulting in activation of the VGLUT3-PKCγ-calretinin-vertical cell-NK1R pathway (red dotted line). During inflammation, the “gate” that controls calretinin neurons is opened resulting in the activation of the VGLUT3-calretinin-vertical cell-NK1R pathway (blue dotted line). V3: transient VGLUT3; SOM: somatostatin; CR: calretinin; Inh: inhibitory interneurons; Dyn: dynorphin; V: vertical; NK1R: neurokinin 1 receptor.
Data are mean ± SEM. *p<0.05, ***p ≤ 0.001.
Lsl-tdTom;VGLUT3Cre mice were injected with carrageenan with or without low threshold mechanical stimulation 24 hours later. As expected, the number of c-Fos+ cells in laminae I–III was increased dramatically in the mice that also received mechanical stimulation (Figures 8A and 8B). The distribution of c-Fos was strikingly similar to what was observed by activating the calretinin population together with mechanical stimulation, including a number of calretinin (~17%) and Pax2 (~41%) cells, but very few PKCγ neurons (~1%) (Figure 7H). Additionally, a significant number of c-Fos+ cells co-expressed tomato (16%), indicating activation of the transient VGLUT3 population.
For the SNI model, mice that received mechanical stimulation three days after surgery showed a substantial increase in c-Fos+ cells in laminae I–II (Figures 8D and 8E). In contrast to the inflammatory pain model, a significant number of PKCγ (11%) and fewer calretinin (8%) neurons expressed c-Fos (Figure 8F). Again, a substantial number of c-Fos+ cells co-expressed tomato (~11%). Together, our data show that distinct spinal circuits can lead to mechanical allodynia, with both requiring activation of the transient VGLUT3 neurons.
DISCUSSION
This study shows that the critical role of VGLUT3 in mechanical pain arises from its transient expression by dorsal horn excitatory interneurons. The cells directly receive Aβ primary sensory input and are required for polysynaptic transmission onto lamina I pain processing neurons after injury. Chemogenetic activation of transient VGLUT3 neurons in lamina III induces mechanical allodynia in a circuit that also includes PKCγ and calretinin cells in lamina II. Activation of calretinin neurons also induces mechanical hypersensitivity and engages a distinct, dorsally-directed pathway that does not include the PKCγ neurons. Further, we show that mechanical allodynia observed in models of inflammation and neuropathy is mediated by distinct neural circuits. Both require the participation of transient VGLUT3 neurons, but a distinct complement of second order neurons.
Role of VGLUT3 in the somatosensory system
Although VGLUT3 expression in the dorsal horn of adult mice is restricted to C-LTMRs, we now show that loss of the transporter from this population does not produce the mechanical pain defects observed in global VGLUT3 KO mice. Using a conditional VGLUT3 KO and several Cre-driver lines, including the spinal cord specific driver Lbx1Cre, we have determined that the defects are due to the loss of VGLUT3 from spinal cord neurons that express the transporter transiently during postnatal development. The finding, however, does not address whether the C-LTMRs themselves are important for pain. These DRG neurons also express VGLUT2 and tyrosine hydroxylase, which may provide alternative mechanisms for signaling (Li et al., 2011; Scherrer et al., 2010). Studies in humans and rodents suggest C-tactile afferents transmit pleasant touch sensation under normal conditions (Loken et al., 2009; Vrontou et al., 2013) and may have a role in suppressing pain potentially by activating islet cells (but also see Nagi et al) (Delfini et al., 2013; Liljencrantz et al., 2013; Nagi et al., 2011). Consistent with this idea, the C-LTMRs do not contact the pain-promoting PKCγ neurons that also reside in this layer (Peirs et al., 2014).
Our analysis of the dorsal horn architecture, including the pattern of primary afferent innervation and electrophysiological properties, showed no evidence of gross abnormalities in the VGLUT3 KO mice. However, using an in vitro model of mechanical allodynia, we find that low threshold A-fibers are unable to activate NK1R+ lamina I neurons when inhibitory transmission in the dorsal horn is completely blocked, indicating that the loss of mechanical hypersensitivity reflects a profound impairment in excitatory transmission between second order neurons. Interestingly, a small decrease in excitatory transmission in this polysynaptic pathway, such as blocking only NMDA receptors, can also prevent the activation of lamina I neurons (Torsney and MacDermott, 2006). Thus, a local change in synaptic transmission due to the absence of VGLUT3 could have a dramatic downstream impact. Importantly, the transient expression of VGLUT3 coincides with a critical period in the maturation of the dorsal horn, a time when the interneurons are establishing their mature firing patterns and connectivity (Fitzgerald, 2005). At the synaptic level, both NMDA and AMPA receptors are undergoing a series of changes, including the awakening of silent synapses and activity dependent alterations in subunit composition (Baba et al., 2000; Li and Zhuo, 1998). Precisely how VGLUT3 participates in these or other developmental changes to properly setup the mechanical pain circuit remains unclear and the subject of further investigation.
Spinal cord circuit for mechanical hypersensitivity
Original models of the dorsal horn circuit for mechanical allodynia have focused almost exclusively on a series of synaptically connected neurons that transmit touch information to superficial layers (Lu et al., 2013; Miraucourt et al., 2007; Torsney and MacDermott, 2006). This serial circuit starts with PKCγ neurons, which then activate central cells in lamina IIi, vertical cells in lamina IIo, and finally the lamina I projection neurons (Lu et al., 2013; Lu and Perl, 2005). More recently, additional circuits have been proposed (Braz et al., 2014; Yasaka et al., 2014) to support the idea that the induction and transmission of mechanical hypersensitivity within the dorsal horn involves integrating information through multiple mechanisms and neuronal networks.
Surprisingly, deep dorsal horn neurons, which receive the major input from low-threshold sensory neurons, have been largely ignored. We now show using a chemogenetic approach that the transient VGLUT3 neurons located in lamina III directly participate in the mechanical allodynia circuit. Interestingly, although loss of VGLUT3 from the laminae II–III dorsal horn neurons attenuates both acute and persistent pain, deletion of VGLUT2, the transporter that mediates glutamate signaling by these neurons in the adult, produces defects only in persistent mechanical pain, suggesting that the neurons themselves do not convey acute mechanical pain. This result is consistent with the lack of acute pain behavior (biting and licking) observed when we activate hM3Dq in the VGLUT3Cre mice. The paw guarding and fluttering behavior that we do observe in these mice is more consistent with the actions of a limb sensitive to touch.
We also now report that activation of calretinin neurons in lamina II of adult mice specifically induces mechanical allodynia. These neurons are distinct from the calretinin population recently shown to be important only for light mechanical pain (Duan et al., 2014). Thus, there are at least two distinct calretinin populations in lamina II, one that transiently expresses calretinin together with Lbx1 and is not involved in mechanical hypersensitivity, and a second population that permanently expresses calretinin, but never Lbx1 and is important for persistent mechanical pain. Additionally, the adult calretinin population as well as the transient VGLUT3 population are distinct from the somatostatin+ excitatory neurons in lamina II that express Lbx1, partially overlap with PKCγ neurons and likely vertical cells (Duan et al., 2014; Polgar et al., 1999) and were recently implicated in persistent mechanical pain.
The transient VGLUT3 cells in lamina III receive input from myelinated Aβ fibers, placing them at an entry point for the dorsally-directed mechanical allodynia circuit. The anterograde tracing and c-Fos results provide a map of the rest of the allodynia circuit starting from these lamina III transient VGLUT3 neurons. Interestingly, we find that the neurons are connected to a second population of excitatory interneurons in this same lamina. These latter, still unidentified neurons, likely serve as the connection between lamina III and the more dorsally located neurons in the pathway including PKCγ and calretinin neurons. The tracing study also shows a direct connection between the transient VGLUT3 neurons and cells in lamina IIo, which are likely to be vertical cells because unlike other neurons in lamina II, these cells have long ventrally-directed dendrites that reach into lamina III. Consistent with our observation, Kato et al identified a population of lamina III excitatory neurons that provides a major input to the lamina II vertical cells and thus a potentially shorter route to the nociceptive projection neurons (Kato et al., 2009).
It has been suggested that the dorsal horn circuit for mechanical allodynia is controlled by a feed-forward inhibition evoked by low threshold inputs that prevent touch from becoming painful (Braz et al., 2014; Zeilhofer et al., 2012). This concept has elicited much interest, as it is the central element of “the gate control theory of pain” published by Melzack and Wall in 1965. In this model, revisited in recent reviews (Braz et al., 2014; Zeilhofer et al., 2012), the “gate” represents a population of inhibitory interneurons that facilitate nociceptive circuits and also prevent the passage of touch-related information from deep neurons to more superficial pain-processing cells. PKCγ-expressing neurons reside at the border between touch and pain (lamina IIi) and have been suggested to be key elements of the circuit (Lu et al., 2013; Miraucourt et al., 2007). The cells have been modeled as initiating the activation of a dorsally-directed pathway transforming touch into pain when the “gate is opened”, i.e. when the inhibition controlling their activity is decreased by injury. Our data now refines how these neurons participate in mechanical allodynia. Firstly, we now show that neurons more ventrally-located than the PKCγ neurons are required to elicit mechanical pain behavior in the carrageenan and SNI models as well as to evoke EPSCs in lamina I in the in vitro assay of mechanical allodynia. Interestingly, these transient VGLUT3 neurons do not show evidence of being under significant feed-forward inhibition as they reliably exhibit evoked EPSCs and membrane depolarization upon dorsal root stimulation. Thus, although these neurons are likely to engage the pathway, the nexus of inhibition must instead be on the postsynaptic lamina III neurons or the excitatory populations in lamina II (Duan et al., 2014) including the PKCγ neurons, which are known to be under strong inhibitory control (Lu et al., 2013). Secondly, we observed c-Fos expression in PKCγ neurons in the SNI model of neuropathic pain, but not the carrageenan model of inflammatory pain suggesting that the involvement of these neurons in the two models of mechanical hypersensitivity may differ. The precise role of PKCγ neurons in different types of injury will require further investigation. From this work, we now propose that the mechanical allodynia circuit is composed of multiple overlapping microcircuits with distinct gates that are engaged by different types of injuries (See model in Figure 8). The more comprehensive view of the spinal cord circuitry provided by this study will help guide efforts to design and implement new treatment strategies.
EXPERIMENTAL PROCEDURES
Animals
Mice were treated in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Conditional VGLUT3 KO mice (VGLUT3fl/fl) were crossed to the Rosa26Cre line to generate VGLUT3Δ/Δ and to AdvillinCre, Hoxb8Cre, KRT14Cre, Tlx3Cre, Lbx1Cre, SNSCre or VGLUT3Cre to generate cell-specific deletions. Detailed description of VGLUT3fl/fl and other mouse lines can be found in Supplemental Experimental Procedures.
Surgical procedures
SNI was preformed as previously described. Spinal cord injections of AAV were performed at P9–10 or P15–16. Under isoflurane, midline incision was made without laminectomy and virus delivered (1 μl) slowly with a glass microelectrode (50 μm tip) between lumbar segments L4–L5. Silk sutures were used to close lassimus dorsi and skin and Ketofen was given before and one day after surgery. Behavior was tested 3 weeks later. For more details including virus titers see Supplemental Experimental Procedures.
Electrophysiological Recordings and Morphology
Transverse slices were made from lumbar spinal cord of P25–35 mice, keeping roots and DRG attached. Whole cell patch-clamp recordings were made with neurobiotin in the pipette when morphology was also performed. For details see Supplemental Experimental Procedures.
In Situ hybridization and Immunohistochemistry
ISH and IHC were performed as previously described (Seal et al., 2009). Induction of c-Fos was performed under urethane anesthetic or with mechanical stimulation by slowly walking mice on a treadmill (10 cm/s). Mice were perfused with 4% paraformaldehyde 90 minutes after the treatment (see Supplemental Experimental Procedures).
Statistics
Data are reported as mean ± SEM. Randall-Selitto, pinprick, sticky tape and fur clip were analyzed by two-tailed Student’s t-test. Von Frey, Hargreaves’ and spontaneous behavior were analyzed by two-way ANOVA with Bonferroni’s post-hoc test. Significance was considered p < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. R. Kuner for SNSCre mice, Dr. F. Wang for AdvillinCre mice, Dr. H. Zeilhofer for Hoxb8Cre mice, Dr. Q. Ma for Tlx3Cre mice, Dr. C. Birchmeier for the Lbx1Cre mice, Dr. T. Hnasko for VGLUT2fl/fl mice and Dr. R. Sharif-Naeini for the WGA virus. We thank S. Dettwyler, J. Caverhill and C. Eckard for technical support, B. Sharif and Dr. Sharif-Naeini for instruction on spinal cord injections, Dr. A. MacDermott for critical reading of the manuscript and the Pittsburgh Rodent Behavior Analysis Core. The work was funded by University of Pittsburgh Startup Funds, and grants from NINDs (NS084191), the UPMC Competitive Medical Research Fund, Virginia Kaufman Foundation, American Diabetes Foundation, Rita Allen Foundation and American Pain Society to RPS and China Scholarship Council Fellowship to XZ.
Footnotes
Conflicts of Interest: None to report
Supplemental information includes 7 Figures and 2 Movies with Legends, Experimental Procedures and References.
AUTHOR CONTRIBUTIONS
CP, SPGW, XZ and RPS designed experiments, CP, SPGW, XZ, CW, JG, NC, ACG, ZL and PM performed experiments, HH provided a reagent, CP, SGW, XZ and RPS analyzed data and CP, SGW and RPS wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal N, Offermanns S, Kuner R. Conditional gene deletion in primary nociceptive neurons of trigeminal ganglia and dorsal root ganglia. Genesis. 2004;38:122–129. doi: 10.1002/gene.20010. [DOI] [PubMed] [Google Scholar]
- Baba H, Doubell TP, Moore KA, Woolf CJ. Silent NMDA receptor-mediated synapses are developmentally regulated in the dorsal horn of the rat spinal cord. J Neurophysiol. 2000;83:955–962. doi: 10.1152/jn.2000.83.2.955. [DOI] [PubMed] [Google Scholar]
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting Pain and Itch Messages: A Contemporary View of the Spinal Cord Circuits that Generate Gate Control. Neuron. 2014;82:522–536. doi: 10.1016/j.neuron.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Rico B, Basbaum AI. Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice. Proc Natl Acad Sci. 2002;99:15148–15153. doi: 10.1073/pnas.222546999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Ann Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Delfini MC, Mantilleri A, Gaillard S, Hao J, Reynders A, Malapert P, Alonso S, Francois A, Barrere C, Seal R, et al. TAFA4, a chemokine-like protein, modulates injury-induced mechanical and chemical pain hypersensitivity in mice. Cell Rep. 2013;5:378–388. doi: 10.1016/j.celrep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell. 2014;159:1417–1432. doi: 10.1016/j.cell.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia. J Neurochem. 2010;115:505–514. doi: 10.1111/j.1471-4159.2010.06946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Seal RP, Oesch N, Edwards RH, Diamond JS. Genetic targeting and physiological features of VGLUT3+ amacrine cells. Vis Neurosci. 2011;28:381–392. doi: 10.1017/S0952523811000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Wenk J, Nenci A, Pasparakis M, Scharffetter-Kochanek K, Smyth N, Peters T, Kess D, Holtkotter O, Shephard P, et al. Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein. Genesis. 2004;38:176–181. doi: 10.1002/gene.20016. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Abbott S, Han BX, Qi Y, Wang F. Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. J Neurosci. 2007;27:14404–14414. doi: 10.1523/JNEUROSCI.4908-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G, Kawasaki Y, Koga K, Uta D, Kosugi M, Yasaka T, Yoshimura M, Ji RR, Strassman AM. Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn. J Neurosci. 2009;29:5088–5099. doi: 10.1523/JNEUROSCI.6175-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature. 1998;393:695–698. doi: 10.1038/31496. [DOI] [PubMed] [Google Scholar]
- Liljencrantz J, Bjornsdotter M, Morrison I, Bergstrand S, Ceko M, Seminowicz DA, Cole J, Bushnell MC, Olausson H. Altered C-tactile processing in human dynamic tactile allodynia. Pain. 2013;154:227–234. doi: 10.1016/j.pain.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Lou S, Duan B, Vong L, Lowell BB, Ma Q. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J Neurosci. 2013;33:870–882. doi: 10.1523/JNEUROSCI.3942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest. 2013;123:4050–4062. doi: 10.1172/JCI70026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II) J Neurosci. 2005;25:3900–3907. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS One. 2007;2:e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagi SS, Rubin TK, Chelvanayagam DK, Macefield VG, Mahns DA. Allodynia mediated by C-tactile afferents in human hairy skin. J Neurophysiol. 2011;589:4065–4075. doi: 10.1113/jphysiol.2011.211326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Patil S, Bouali-Benazzouz R, Artola A, Landry M, Dallel R. Protein kinase C gamma interneurons in the rat medullary dorsal horn: distribution and synaptic inputs to these neurons, and subcellular localization of the enzyme. J Comp Neurol. 2014;522:393–413. doi: 10.1002/cne.23407. [DOI] [PubMed] [Google Scholar]
- Polgar E, Campbell AD, MacIntyre LM, Watanabe M, Todd AJ. Phosphorylation of ERK in neurokinin 1 receptor-expressing neurons in laminae III and IV of the rat spinal dorsal horn following noxious stimulation. Mol Pain. 2007a;3:4. doi: 10.1186/1744-8069-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Fowler JH, McGill MM, Todd AJ. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- Polgar E, Thomson S, Maxwell DJ, Al-Khater K, Todd AJ. A population of large neurons in laminae III and IV of the rat spinal cord that have long dorsal dendrites and lack the neurokinin 1 receptor. Eur J Neurosci. 2007b;26:1587–1598. doi: 10.1111/j.1460-9568.2007.05793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, Ma Q, De Koninck Y. Normal and abnormal coding of somatosensory stimuli causing pain. Nat Neurosci. 2014;17:183–191. doi: 10.1038/nn.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, et al. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci. 2010;107:22296–22301. doi: 10.1073/pnas.1013413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SP. Local circuit connections between hamster laminae III and IV dorsal horn neurons. J Neurophysiol. 2008;99:1306–1318. doi: 10.1152/jn.00962.2007. [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber MA, Storm R, Martinez-de-la-Torre M, Muller T, Wende H, Reuter K, Vasyutina E, Birchmeier C. Lbx1 acts as a selector gene in the fate determination of somatosensory and viscerosensory relay neurons in the hindbrain. J Neurosci. 2007;27:4902–4909. doi: 10.1523/JNEUROSCI.0717-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrassi G, Muller-Schwefe G, Pergolizzi J, Oronska A, Morlion B, Mavrocordatos P, Margarit C, Mangas C, Jaksch W, Huygen F, et al. Pharmacological treatment of chronic pain - the need for CHANGE. Curr Med Res Opin. 2010;26:1231–1245. doi: 10.1185/03007991003689175. [DOI] [PubMed] [Google Scholar]
- von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrontou S, Wong AM, Rau KK, Koerber HR, Anderson DJ. Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature. 2013;493:669–673. doi: 10.1038/nature11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang J, Eberhart D, Urban R, Meda K, Solorzano C, Yamanaka H, Rice D, Basbaum AI. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron. 2013;78:312–324. doi: 10.1016/j.neuron.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witschi R, Johansson T, Morscher G, Scheurer L, Deschamps J, Zeilhofer HU. Hoxb8-Cre mice: A tool for brain-sparing conditional gene deletion. Genesis. 2010;48:596–602. doi: 10.1002/dvg.20656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Lopes C, Wende H, Guo Z, Cheng L, Birchmeier C, Ma Q. Ontogeny of excitatory spinal neurons processing distinct somatic sensory modalities. J Neurosci. 2013;33:14738–14748. doi: 10.1523/JNEUROSCI.5512-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasaka T, Tiong SY, Polgar E, Watanabe M, Kumamoto E, Riddell JS, Todd AJ. A putative relay circuit providing low-threshold mechanoreceptive input to lamina I projection neurons via vertical cells in lamina II of the rat dorsal horn. Mol Pain. 2014;10:3. doi: 10.1186/1744-8069-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Wildner H, Yevenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Leitges M, Gereau RWt. Isozyme-specific effects of protein kinase C in pain modulation. Anesthesiology. 2011;115:1261–1270. doi: 10.1097/ALN.0b013e3182390788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Song Z, Guo Q, Liu C, Zhang Z, Zhang Y. Intrathecal lentiviral-mediated RNA interference targeting PKCgamma attenuates chronic constriction injury-induced neuropathic pain in rats. Human Gene Ther. 2011;22:465–475. doi: 10.1089/hum.2010.207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.