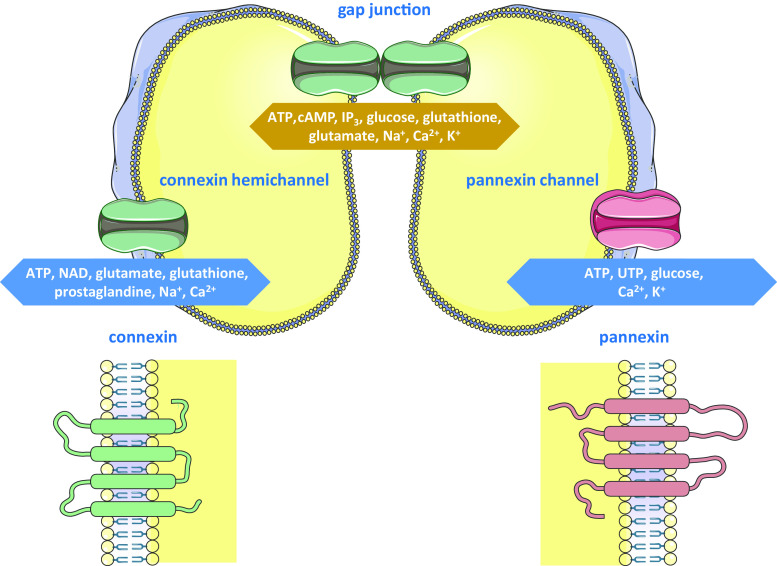
Homeostasis relies on the intimate interplay between extracellular, intracellular and intercellular signaling networks. Direct communication between adjacent cells is mediated by gap junctions [1–3]. They allow the intercellular diffusion of small (i.e. less than 1 kDa) and hydrophilic substances, including adenosine triphosphate (ATP), cyclic adenosine monophosphate, inositol triphosphate, glucose, glutathione, glutamate and several ions, like sodium, calcium and potassium (Fig. 1) [4]. This flux is called gap junctional intercellular communication (GJIC) and is considered as a basic mechanism in the maintenance of tissue functioning [1–3]. Over the last decades, GJIC has been shown indispensable for the establishment of metabolic or electrical intercellular coupling in all vital organs, such as the brain [5], the heart [6] and the liver [7], to name a few.
Fig. 1.
Structure and function of connexin-based and pannexin-based channels. Connexins form hemichannels that convey adenosine triphosphate (ATP), nicotinamide dinucleotide (NAD), glutamate, glutathione, prostaglandine, sodium and calcium ions between the cytosol and the extracellular environment. Similarly, pannexin channels provide a pathway for extracellular communication by controlling the flux of ATP, uridine triphosphate (UTP), glucose, calcium and potassium ions. Connexin hemichannels of adjacent cells can interact to form gap junctions, which control intercellular communication by mediating the exchange of ATP, cyclic adenosine monophosphate (cAMP), inositol triphosphate (IP3), glucose, glutathione, glutamate, sodium, potassium and calcium ions. Connexins and pannexins share a similar structure consisting of four transmembrane domains, two extracellular loops, one cytosolic loop, one cytosolic aminotail and one cytosolic carboxytail. In comparison with connexins, pannexins have longer extracellular loops and cytosolic carboxytail
Gap junctions arise from the head-to-head interaction of two hemichannels of neighboring cells, which in turn are composed of six connexin proteins. Today, more than 20 different connexins have been identified in humans and rodents, all of which are expressed in a cell-specific way [1, 8]. They are named based upon their molecular weight as predicted by cDNA sequencing. Thus, the most widespread connexin species has a molecular mass of 43 kDa and therefore is designated Cx43. Another connexin nomenclature system used in parallel is based upon genetic similarity, with alpha, beta, gamma, delta and epsilon groups, and order of discovery. According to this alternative system, Cx43 is called GJA1, whereby prefix GJ stands for gap junction [1, 9]. Despite the multitude of connexins presently identified, these proteins share a common molecular structure consisting of four transmembrane domains, two extracellular loops, one cytosolic loop, one cytosolic aminotail and one cytosolic carboxytail. Differences between connexin family members are mainly due to variations in the intracellular areas (Fig. 1) [1, 2].
GJIC is regulated by a plethora of mechanisms [10]. Long-term control hereby typically implies regulation of connexin gene expression. Both ubiquitous and tissue-specific transcription factors are in charge of the transcription of connexin genes [9]. Furthermore, epigenetic mechanisms, including histone acetylation, DNA methylation and microRNA-related control, are major determinants of connexin production at the most upstream regulatory level [9, 11]. Short-term control of GJIC, so-called gating, is driven by a number of factors, including transmembrane voltage, pH and calcium ions. Of all gating mechanisms, connexin phosphorylation, mainly occurring at the cytoplasmic carboxyterminal region, has gained most attention. All connexins are phosphoproteins, with the notable exception of Cx26. However, the regulation of GJIC by connexin phosphorylation is quite complex, as the outcome of this posttranslational modification, such as effects on gap junction activity or degradation, depends on several parameters, namely the type of connexin, the identity of the kinase and the cellular context [10, 12].
Although considered as merely structural precursors of gap junctions for a long time, an abundance of reports published in the last few years show that connexin hemichannels as such can provide a pathway for cellular communication, albeit between the cytosol of individual cells and their extracellular environment, and not between adjacent cells as is the case for GJIC. The messengers that are conveyed through connexin hemichannels are very similar to those involved in GJIC, including ATP, nicotinamide dinucleotide, glutamate, glutathione, prostaglandine, sodium and calcium ions (Fig. 1) [2, 8, 13]. Furthermore, connexin hemichannels are regulated by mechanisms that equally affect gap junctions. Nevertheless, an identical factor can have opposing effects on the two channels types, such as shown for certain inflammatory triggers [14, 15]. In line with this notion, connexin hemichannels, unlike their full channel counterparts, display a low open probability. In fact, connexin hemichannels seem to be preferably activated by pathological stimuli, including ischemia/reperfusion insults and oxidative stress, and thereby drive processes like cell death and inflammation [2, 8, 13]. Nonetheless, compelling evidence also shows physiological functions for connexin hemichannels, including in cell cycle progression [16], cochlear homeostasis [17], bone remodeling [18] and carbon dioxide sensing [19].
In 2000, a new set of connexin-like proteins was first described, namely the so-called pannexins [20]. Thus far, three pannexins have been identified in humans and rodents, called Panx1, Panx2 and Panx3. Their molecular architecture closely resembles that of connexins, yet pannexins have longer extracellular loops and carboxyterminal tails compared to connexins. As a matter of fact, the deviating composition of the extracellular loops is thought to underlie the observation that pannexins gather in a channel configuration reminiscent of connexin hemichannels, which do not dock with each other to form gap junctions [12, 21, 22]. Hence, ‘pannexin channels’ may be a more appropriate designation than ‘pannexin hemichannels’. Like connexin hemichannels, pannexin channels mediate the trafficking of a number of mediators between the intracellular and extracellular compartments, such as ATP, uridine triphosphate, glucose, calcium and potassium ions (Fig. 1) [23]. Clear-cut functions for Panx1 channels have now be defined in innate immunity by acting on inflammasomes [24, 25]. Other documented roles for pannexin channels relate to their capacity to control extracellular ATP release, an event critical for the development of a variety of cell types, such as keratinocytes [26], erythrocytes [27] and chondrocytes [28]. Pannexin channels are regulated by posttranslational modifications, among which glycosylation is the most prominent one [12, 29].
The connexin and pannexin research field has been surrounded by a lot of controversy in the last few years. In particular, the concept of functional connexin hemichannels has been debated heavily on several occasions [30, 31]. A major reason for this impediment is the lack of tools and technologies to distinguish between the different channel types, in casu between gap junctions and connexin hemichannels on the one hand, and between connexin-based and pannexin-based channels on the other hand [32, 33]. It should be noted that this hurdle is currently being tackled, as the first reports describing specific connexin hemichannel inhibitors that do not close the other channel types have recently been published [34, 35]. However, the picture is even more complicated by several studies showing nonchannel functions of connexins and pannexins. In this context, connexins, and to a lesser extent pannexins, are able to affect tissue homeostasis by mechanisms that do not relate to their channel-forming activities, such as by physically interacting with regulators of the cellular life cycle [36, 37].
This multi-author review edition of Cellular and Molecular Life Sciences entitled ‘Connexin and pannexin signaling in organ functionality’ focuses on the roles of connexins and pannexins in the maintenance of homeostasis. In particular, state-of-the-art overviews are provided on the multifaceted signaling capacities of connexins, pannexins and their channels in organ physiology for the most important organ systems, including the cardiovascular system, the respiratory system, the digestive system, the nervous system, the skeletal system, the excretory system, the reproductive system, the immune system, the endocrine system, the muscle system and the integumentary system. These cutting-edge overview papers are intended to encourage in-depth examination of the diverse signaling roles of connexins and pannexins in organ physiology as well as thorough exploration of their translational and clinical value in the upcoming years.
Acknowledgments
This work was financially supported by the grants of the University Hospital of the Vrije Universiteit Brussel-Belgium (“Willy Gepts Fonds” UZ-VUB), the Fund for Scientific Research-Flanders (FWO Grants G009514N and G010214N), the European Research Council (ERC Starting Grant 335476), the University of São Paulo-Brazil (USP) and the Foundation for Research Support of the State of São Paulo (FAPESP SPEC Grant 2013/50420-6).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Abbreviations
- ATP
Adenosine triphosphate
- cAMP
Cyclic adenosine monophosphate
- Cx
Connexin
- GJIC
Gap junctional intercellular communication
- IP3
Inositol triphosphate
- NAD
Nicotinamide dinucleotide
- Panx
Pannexin
- UTP
Uridine triphosphate
References
- 1.Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH. Gap junctions. Compr Physiol. 2012;2(3):1981–2035. doi: 10.1002/cphy.c110051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Decrock E, Vinken M, De Vuyst E, Krysko DV, D’Herde K, Vanhaecke T, Vandenabeele P, Rogiers V, Leybaert L. Connexin-related signaling in cell death: to live or let die? Cell Death Differ. 2009;16(4):524–536. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- 3.Vinken M, Decrock E, De Vuyst E, Ponsaerts R, D’hondt C, Bultynck G, Ceelen L, Vanhaecke T, Leybaert L, Rogiers V. Connexins: sensors and regulators of cell cycling. Biochim Biophys Acta. 2011;1815(1):13–25. doi: 10.1016/j.bbcan.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10(19):2045–2058. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- 5.Eugenin EA, Basilio D, Sáez JC, Orellana JA, Raine CS, Bukauskas F, Bennett MV, Berman JW. The role of gap junction channels during physiologic and pathologic conditions of the human central nervous system. J Neuroimmune Pharmacol. 2012;7(3):499–518. doi: 10.1007/s11481-012-9352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtenbach S, Kurtenbach S, Zoidl G. Gap junction modulation and its implications for heart function. Front Physiol. 2014;5:82. doi: 10.3389/fphys.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinken M, Henkens T, De Rop E, Fraczek J, Vanhaecke T, Rogiers V. Biology and pathobiology of gap junctional channels in hepatocytes. Hepatology. 2008;47(3):1077–1088. doi: 10.1002/hep.22049. [DOI] [PubMed] [Google Scholar]
- 8.Kar R, Batra N, Riquelme MA, Jiang JX. Biological role of connexin intercellular channels and hemichannels. Arch Biochem Biophys. 2012;524(1):2–15. doi: 10.1016/j.abb.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyamada M, Takebe K. Oyamada Y (2013) Regulation of connexin expression by transcription factors and epigenetic mechanisms. Biochim Biophys Acta. 1828;1:118–133. doi: 10.1016/j.bbamem.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Su V, Lau AF. Connexins: mechanisms regulating protein levels and intercellular communication. FEBS Lett. 2014;588(8):1212–1220. doi: 10.1016/j.febslet.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinken M, De Rop E, Decrock E, De Vuyst E, Leybaert L, Vanhaecke T, Rogiers V. Epigenetic regulation of gap junctional intercellular communication: more than a way to keep cells quiet? Biochim Biophys Acta. 2009;1795(1):53–61. doi: 10.1016/j.bbcan.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 12.D’hondt C, Iyyathurai J, Vinken M, Rogiers V, Leybaert L, Himpens B, Bultynck G. Regulation of connexin- and pannexin-based channels by posttranslational modifications. Biol Cell. 2013;105(9):373–398. doi: 10.1111/boc.201200096. [DOI] [PubMed] [Google Scholar]
- 13.Chandrasekhar A, Bera AK. Hemichannels: permeants and their effect on development, physiology and death. Cell Biochem Funct. 2012;30(2):89–100. doi: 10.1002/cbf.2794. [DOI] [PubMed] [Google Scholar]
- 14.De Vuyst E, Decrock E, De Bock M, Yamasaki H, Naus CC, Evans WH, Leybaert L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell. 2007;18(1):34–46. doi: 10.1091/mbc.E06-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27(50):13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franco L, Zocchi E, Usai C, Guida L, Bruzzone S, Costa A, De Flora A. Paracrine roles of NAD+ and cyclic ADP-ribose in increasing intracellular calcium and enhancing cell proliferation of 3T3 fibroblasts. J Biol Chem. 2001;276(24):21642–21648. doi: 10.1074/jbc.M010536200. [DOI] [PubMed] [Google Scholar]
- 17.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA. 2008;105(48):18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherian PP, Cheng B, Gu S, Sprague E, Bonewald LF, Jiang JX. Effects of mechanical strain on the function of gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem. 2003;278(44):43146–43156. doi: 10.1074/jbc.M302993200. [DOI] [PubMed] [Google Scholar]
- 19.Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010;588(20):3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10(13):R473–R474. doi: 10.1016/S0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 21.Penuela S, Gehi R. Laird DW (2013) The biochemistry and function of pannexin channels. Biochim Biophys Acta. 1828;1:15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 22.D’Hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions? BioEssays. 2009;31(9):953–974. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- 23.Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Vinken M, Rogiers V, Bukauskas FF, Bultynck G, Leybaert L. Paracrine signaling through plasma membrane hemichannels. Biochim Biophys Acta. 2013;1828(1):35–50. doi: 10.1016/j.bbamem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adamson SE, Leitinger N. The role of pannexin1 in the induction and resolution of inflammation. FEBS Lett. 2014;588(8):1416–1422. doi: 10.1016/j.febslet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makarenkova HP, Shestopalov VI. The role of pannexin hemichannels in inflammation and regeneration. Front Physiol. 2014;5:63. doi: 10.3389/fphys.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celetti SJ, Cowan KN, Penuela S, Shao Q, Churko J, Laird DW. Implications of pannexin 1 and pannexin 3 for keratinocyte differentiation. J Cell Sci. 2010;123(8):1363–1372. doi: 10.1242/jcs.056093. [DOI] [PubMed] [Google Scholar]
- 27.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006;103(20):7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwamoto T, Nakamura T, Doyle A, Ishikawa M, de Vega S, Fukumoto S, Yamada Y. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem. 2010;285(24):18948–18958. doi: 10.1074/jbc.M110.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penuela S, Simek J, Thompson RJ. Regulation of pannexin channels by posttranslational modifications. FEBS Lett. 2014;588(8):1411–1415. doi: 10.1016/j.febslet.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 30.Sáez JC, Leybaert L. Hunting for connexin hemichannels. FEBS Lett. 2014;588(8):1205–1211. doi: 10.1016/j.febslet.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54(7):758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 32.Bodendiek SB, Raman G. Connexin modulators and their potential targets under the magnifying glass. Curr Med Chem. 2010;17(34):4191–4230. doi: 10.2174/092986710793348563. [DOI] [PubMed] [Google Scholar]
- 33.Iyyathurai J, D’hondt C, Wang N, De Bock M, Himpens B, Retamal MA, Stehberg J, Leybaert L, Bultynck G. Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: gap junctions versus hemichannels. Neuropharmacology. 2013;75:491–505. doi: 10.1016/j.neuropharm.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 34.Wang N, De Vuyst E, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, Lai CP, De Bock M, Decrock E, Bol M, Vinken M, Rogiers V, Tavernier J, Evans WH, Naus CC, Bukauskas FF, Sipido KR, Heusch G, Schulz R, Bultynck G, Leybaert L. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2013;108(1):309. doi: 10.1007/s00395-012-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponsaerts R, De Vuyst E, Retamal M, D’hondt C, Vermeire D, Wang N, De Smedt H, Zimmermann P, Himpens B, Vereecke J, Leybaert L, Bultynck G. Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J. 2010;24(11):4378–4395. doi: 10.1096/fj.09-153007. [DOI] [PubMed] [Google Scholar]
- 36.Vinken M, Decrock E, Leybaert L, Bultynck G, Himpens B, Vanhaecke T, Rogiers V. Non-channel functions of connexins in cell growth and cell death. Biochim Biophys Acta. 2012;1818(8):2002–2008. doi: 10.1016/j.bbamem.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Zhou JZ, Jiang JX. Gap junction and hemichannel-independent actions of connexins on cell and tissue functions: an update. FEBS Lett. 2014;588(8):1186–1192. doi: 10.1016/j.febslet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]