Abstract
Cell survival depends on the maintenance of mitochondrial integrity controlled by a well-balanced interplay between anti- and pro-apoptotic B cell lymphoma 2 (Bcl2) family members. Given their frequent deregulation in human pathologies, including autoimmunity and cancer, significant research efforts have increased our molecular understanding of how Bcl2 proteins control cell death. This has fostered the development of small non-peptidic compounds, so-called BH3-mimetics, that show excellent prospects of passing clinical trials and entering daily use for targeted therapy. Possible limitations in clinical application may, to a certain degree, be predicted from loss-of-function phenotypes gathered from studies using gene-modified mice that we attempt to summarize and discuss in this context.
Keywords: apoptosis, Bcl-2 family, cancer, mouse models, targeted therapy
Introduction
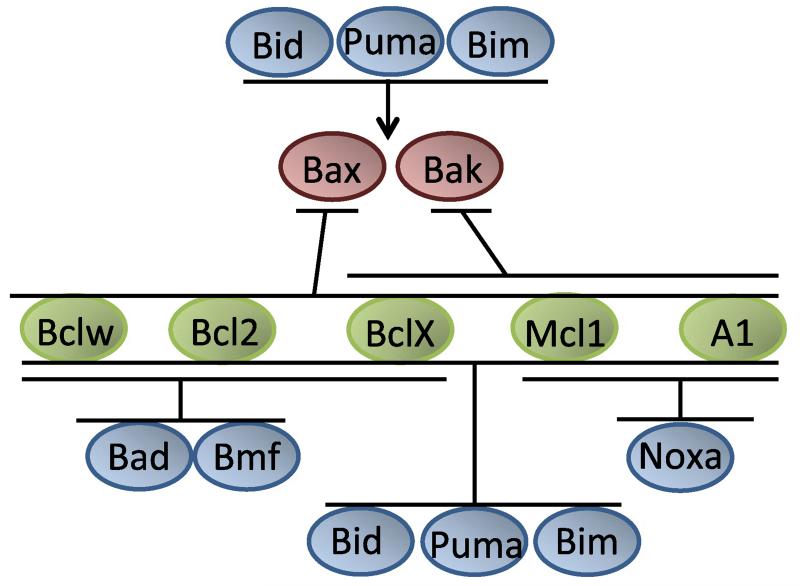
Within the B cell lymphoma 2 (Bcl2) family, BH3-only proteins, such as Bim, Puma or Bid, act as sentinels, activated in response to a broad range of developmental or environmental cues to trigger mitochondrial apoptosis. They do so by neutralizing anti-apoptotic Bcl2 proteins and by directly activating the highly redundant but rate-limiting cell death effectors Bax and Bak (Fig. 1). Once activated, these form homodimers that then assemble into higher order oligomers enabling activation of proteases of the caspase family (Casp-9, -3, -6, -7) and cell death [1,2].
Fig. 1.
Liaisons in the Bcl2 family. The Bcl2 family can be categorized into three classes of proteins that control mitochondrial cell death by complex protein–protein interactions, indicated here. These interactions are based on different affinities between individual family members and are influenced by various post-translational protein modifications. Anti-apoptotic Bcl2, BclX, Bclw, Mcl1 and A1/Bfl1 show only partially overlapping binding preference for pro-apoptotic Bax and Bak. Selective binding is also intricate to individual BH3-only proteins that act as sensors of cell stress and bind selectively to one or more anti-apoptotic Bcl2 proteins while some can also interact directly with Bax or Bak to promote mitochondrial outer membrane permeabilization. For detailed information on the molecular mode of action, see [1,2].
Upon ectopic expression in tissue culture, however, pro-survival members of the Bcl2 family (Bcl2, BclX, Mcl1, A1/Bfl1, Bclw and BclB) are all able to mediate cell death resistance to a broad range of genotoxic stimuli. Yet, overexpression phenotypes often fail to faithfully reflect the physiological role(s) of a protein. Nonetheless, first in vivo studies in transgenic mice galvanized critical roles in cell death control but also pointed towards possible differences in physiological function [3]. However, only loss-of-function studies provided clear evidence that each individual Bcl2 family member can exert highly selective roles in apoptosis signalling, often even in a strictly cell-type-dependent manner (Table 1).
Table 1.
Summary of Bcl2 family protein knockout lines generated and phenotypes observed. A more detailed description of the major phenotypic changes and possible implications for targeted therapy can be found in the text.
| Bcl2 −/− |
|
[7-9,29,31-33] |
| Bcl2fl/fl LysM-Cre |
|
[35] |
| BclX −/− |
|
[5,6,53] |
| Bcl-xfl/fl Lck-Cre |
|
[46] |
| BclXfl/fl CD4-Cre |
|
[46] |
| BclXfl/fl Rag1-Cre |
|
[47] |
| BclXfl/fl Aicda-Cre |
|
[48] |
| BclXfl/fl MMTV-Cre |
|
[50,55] |
| BclXfl/fl Pf4-Cre |
|
[52,120] |
| BclXfl/fl K5-Cre |
|
[56,57] |
| BclXfl/fl Sftpc–Cre |
|
[60] |
| BclXfl/fl Alb-Cre |
|
[58,59] |
| BclXfl/fl Six3-Cre |
|
[61,63] |
| BclXfl/fl TH-Cre |
|
[62] |
| BclXfl/fl CathepsinK-Cre |
|
[62] |
| Mcl1 −/− |
|
[4] |
| Mcl1fl/null Mx-Cre |
|
[73,76,77] |
| Mcl1fl/fl CreER |
|
[46,79] |
| Mcl1fl/fl Rag1-Cre |
|
[47] |
| Mcl1fl/null CD19-Cre |
|
[76] |
| Mcl1fl/fl Aicda-Cre |
|
[48] |
| Mcl1fl/null Lck-Cre |
|
[76] |
| Mcl1fl/fl CD4-Cre |
|
[46] |
| Mcl1fl/fl Ncr-1-Cre |
|
[49] |
| Mcl1fl/fl LysM-Cre |
|
[74,75] |
| Mcl1fl/fl Cpa3-Cre |
|
[80] |
| Mcl1fl/fl Pf4-Cre |
|
[52,120] |
| Mcl1fl/fl Alb-Cre |
|
[59,81] |
| Mcl1fl/fl Ckmm-Cre |
|
[85] |
| Myh-CreER |
|
[85] |
| Mcl1fl/fl Nestin-Cre |
|
[86] |
| Mcl1fl/fl Foxg1-Cre |
|
[86] |
| Mcl1fl/fl CamKIIα Cre |
|
[88] |
| A1a −/− |
|
[10] [99,100] |
| A1 RNAi |
|
[101,102] |
Unfortunately, peri-implantation stage embryonic lethality of mice lacking myeloid cell leukemia 1 (Mcl1) [4], the early embryonic death of BclX-deficient embryos [5,6] and the severely reduced lifespan of mice lacking Bcl2 [7-9] have slowed down our progress in understanding the role of the individual Bcl2 pro-survival proteins in development, tissue homeostasis and disease. Similarly, gene quadruplication of the Bcl2a1 locus encoding for A1/Bfl1 in mice has prevented classical gene targeting studies leaving the physiological role of this protein largely undefined [10,11]. Deletion of Bclw, on the one hand, has revealed essential roles in spermatogenesis [12,13], while loss of Boo/Diva, the mouse homologue of BCLB/BCL2L10 in humans, on the other hand, showed no obvious defects [14], leading to a drop in research efforts aiming to address the role of the latter two proteins in regulating mitochondrial apoptosis.
In this review, we aim to give an overview of our current knowledge of pro-survival Bcl2 family proteins in normal physiology, as evidenced by gain- or loss-of-function studies in mice, and discuss possible implications for Bcl2-targeting therapy [15].
B cell lymphoma 2 (Bcl2)
Bcl2 was the first discovered regulator of apoptosis when it was found as translocated and subsequently overexpressed in patients suffering from follicular B cell lymphoma, 30 years ago [16,17]. High-level expression of BCL2 was confirmed in numerous human tumours sparking extensive gain- and loss-of-function studies in different model systems. Early studies in mice have focused largely on the effects of Bcl2 overexpression in the immune system (Eμ-BCL2; H2K-BCL2; hBCL2-Ig; Vav-BCL2 transgenic mice) highlighting important roles in lymphocyte development and the induction and maintenance of tolerance. These mice developed autoimmune phenotypes as well as a predisposition for spontaneous and oncogene-driven lymphoma development and drug resistance [18-23]. Similar predispositions have been reported subsequently in mice expressing BCL2 in epithelial cells such as the mammary gland that showed increased rates of breast cancer upon concomitant MYC or SV40 LT overexpression [24,25]. Paradoxically overexpression of BCL2 in the liver delayed diethylnitrosamine (DEN) driven hepatocellular carcinoma [26]. This phenomenon may actually be due to the lack of compensatory proliferation upon DEN treatment by BCL2 [27]. Drug resistance phenotypes caused by BCL2 overexpression were also recapitulated in human disease [28].
While the vast body of evidence describing the anti-apoptotic effects of Bcl2 in various cell types and tissues predicted a prominent role in embryonic development and tissue homeostasis, loss-of-function analyses revealed a surprisingly restricted role in normal physiology. Bcl2 knockout mice showed largely normal embryogenesis but newborns displayed postnatal growth retardation, melanocyte loss and early death. Reduced lifespan was due to impaired renal cell differentiation and increased apoptosis leading to fatal polycystic kidney disease [7,8], but the severity of this phenotype [29] and phenotypes reported for the gastrointestinal tract [9] or in the postnatal nervous system [29] showed significant variability between studies, most probably due to differences in genetic background of the individual knockout strains [30].
In accordance with the noted massive splenic involution, no Bcl2−/− mice were able to maintain their lymphoid system. At the age of 4 weeks Bcl2 deficiency already caused massive loss of mature lymphocytes, accompanied by a relative increase in myeloid cells. Notably, the few surviving lymphocytes from Bcl2−/− mice did not exhibit any disadvantage when activated with mitogens [7-9].
Transplantation of bone marrow (BM) or fetal liver derived hematopoietic stem cell (HSC) enriched populations showed that Bcl2 loss favoured the generation of myeloid over lymphoid cells in a cell autonomous manner [31,32]. Transplanted Bcl2−/− HSCs failed to produce T cells and were unable to maintain B cell numbers. However, the presence of B cells in the periphery indicated that Bcl2 is an important pro-survival factor during early B cell development and homeostatic survival of mature B cells but no longer essential in activated B cells or Ig-producing plasma cells. Of note, adoptive transfer experiments suggest minor roles in activated and regulatory T cells [33].
Together these and subsequent studies pinpointed essential roles for Bcl2 in (most) mature lymphocyte subsets, melanocytes and kidney epithelium. Importantly, all these phenotypes were restored by loss of one or two alleles of Bim, the most critical physiological Bcl2 antagonist [34]. However, due to the drastically limited lifespan of Bcl2−/− mice, its physiological role in many important cell types is still poorly understood. Conditional Bcl2fl/fl mice have been made available [35] and will allow detailed follow-up studies investigating the role of Bcl2 in a cell-type and tissue-dependent context.
B cell lymphoma like 1 (Bcl2L1/BclX)
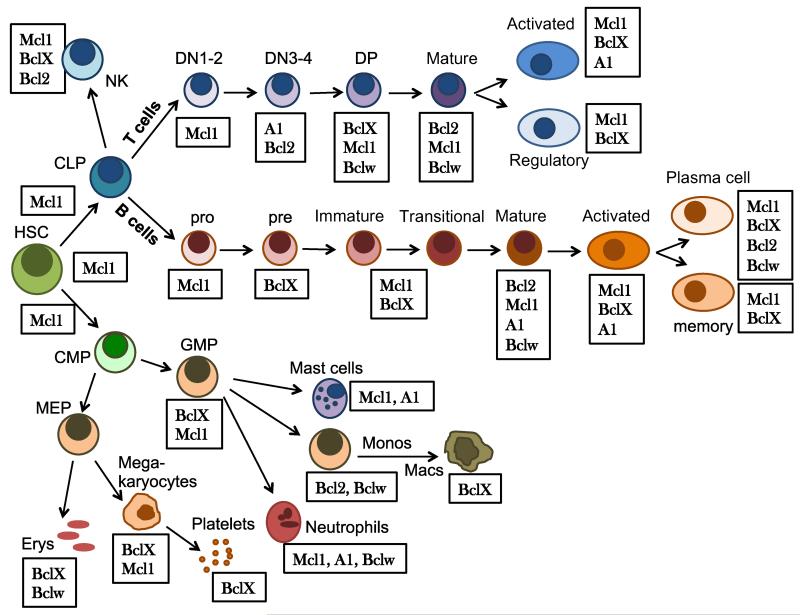
Bcl2’s next of kin, BclX, was noted early on to display a largely reciprocal expression pattern with Bcl2 in developing lymphocytes (Fig. 2). In fact, Bcl2 appears to be exchanged for BclX on a regular basis when massive expansion of lymphocyte precursors or mature lymphocytes is required, e.g. at the pre-B to naïve B cell transition in bone marrow, the CD4/CD8 double-negative to CD4/CD8 double-positive transition in the thymus or upon antigen encounter in spleen or reactive lymph nodes [36-38]. The reasons for this remain poorly understood. It may be possible, though, that next to reported negative effects of Bcl2 on proliferation (e.g. via blocking Ca2+ release from the endoplasmic reticulum, increased p27 levels or repressed autophagy), changes in the expression pattern of pro-apoptotic genes may select for a switch to BclX in activated lymphocytes (Fig. 1).
Fig. 2.
Dynamic expression changes of Bcl2 family proteins in haematopoiesis. Expression of individual Bcl2 family proteins is dynamically regulated in the developing hematopoietic system in response to a broad range of developmental cues, including cytokines, interactions of blood cells with stroma or antigen receptor expression and reactivity. Despite the fact that usually more than one anti-apoptotic Bcl2 family member is expressed at a given developmental stage, loss of function usually affects only a subset of blood cells. For example, why loss of Mcl1 is usually fatal, despite the fact that Bcl2 or BclX is still present, is poorly understood. Similarly, the need to change the panel of pro-survival proteins expressed during, for example, lymphocyte development is in many instances still unclear. In this figure we rank the expressed proteins according to the severity of the reported knockout phenotypes reported. Note also that the knockout of many of the Bcl2 proteins listed as expressed in a certain cell type has no effect on the survival of these cells, at least under homeostatic conditions (see also Table 1).
In analogy to studies on Bcl2, a series of transgenic mouse lines was generated addressing the role of BclX in lymphocyte development, immunity and malignant disease (SV40-Eμ-BclX; Eμ-tkP-BclX; Lck-BclX). These models largely recapitulated findings in BCL2 transgenic mice, although some of the phenotypes observed were more variable [37-42]. Combined overexpression of BclX and BCL2 further enhanced cell death resistance of lymphocytes, e.g. that of B cells exposed to high concentrations of anti-IgD, normally driving their depletion in vivo, or upon cytokine deprivation in vitro, suggesting functional redundancy [37]. Functional differences between BclX and BCL2 were reported upon overexpression in the liver that sparked some controversy as to whether Bcl2 family proteins are able to block death receptor mediated apoptosis [43-45]. Similarly, while BCL2 prevented DEN-driven liver cancer, BclX overexpression did not [44]. Ultimately, these discrepancies, next to a difference in transgene expression levels, may in part be explained by differences in binding to the BH3-only protein Bid (activated by caspase-8) or Bak by BclX (but less stringently by Bcl2), as well as possible anti-proliferative effects of Bcl2.
Ubiquitous BclX deficiency, however, is embryonic lethal and mice die at embryonic day 13 post fertilization. Analysis of foetuses revealed strong accumulation of apoptotic cells in the fetal liver and defective primitive and definite erythropoiesis as well as massive cell death in the central nervous system (CNS). Chimeric mice generated by transplantation of BclX−/− embryonic stem cells into C57BL/6 or RAG2−/− blastocysts showed reduced numbers of pre-B and immature B cells in the bone marrow and a shifted T cell ratio in the thymus [5,6]. This was accompanied by a significant loss of the T and B cells in the spleen while lymph nodes of BclX−/− chimeric mice remained unaffected. Moreover, bone marrow B cells and immature thymocytes manifested highly increased spontaneous apoptosis rates ex vivo while mature lymphocytes showed largely normal cell death responses. These experiments proved that BclX-deficient lymphocytes can mature, but in strongly reduced numbers. Once mature, they no longer depend on BclX but on Bcl2 and Mcl1. Similarly, despite the fact that BclX is prominently expressed in double-positive thymocytes, conditional deletion of BclX by CD4-Cre mediated recombination led only to a minor reduction in cell numbers. However, this can be explained by functional redundancy with Mcl1 at that stage as only combined deletion of both genes causes the near complete loss of CD4 + CD8 + double positive thymocytes [46]. Thus, lack of BclX shortens the lifespan and increases the apoptosis rate of immature lymphocytes. This notion is also supported by the finding that Cre expression, under control of the Rag1 promoter in BclXfl/fl mice, does not affect pro-B cell numbers, but these cells cannot progress to the pre-B cell stage, consistent with the onset of BclX expression downstream of a functional pre-BCR [47]. Conditional ablation of BclX in germinal centre (GC) B cells using Aicda-Cre showed minimal impact on GC or memory B cell formation [48] nor did Ncr1-Cre mediated deletion affect natural killer (NK) cell numbers [49].
A crucial physiological role for BclX was revealed in BclXfl/fl MMTV-Cre mice [50] and was confirmed later in a targeted N-ethyl-N-nitrosourea (ENU) screen that highlighted its role as a timer for platelet lifespan [51]. In the latter, ENU mutagenesis introduced destabilizing point mutations into BclX (C15T or N182I) that led to accelerated degradation of BclX thereby triggering Bak-dependent apoptosis. BclX also determines megakaryocyte function, as assessed by platelet factor 4 (Pf4)-Cre deletion, and, similar to immature thymocytes, controls their survival in conjunction with Mcl1 [52]. It may be speculated that BclX may exert a similar timer function in erythrocytes, as its absence only affects advanced erythroid differentiation stages, causing the massive anaemia observed upon its deletion during embryogenesis [53] and in BclXfl/fl MMTV-Cre mice [50]. However, results from clinical trials with navitoclax (see below), which is able to target BclX, did not show anaemia as a major side effect. Thus, whether all platelet, enucleated reticulocyte or erythrocyte death is mediated by simple degradation of BclX or whether BH3-only proteins can modulate time to death upon hypoxia, injury or during inflammation still remains to be investigated.
Less is known about the physiological functions of BclX outside the hematopoietic compartment. Haplo-insufficiency reduces fertility in male mice associated with Bax-dependent testicular degeneration [54]. Conditional deletion of BclX was performed in the skin using K5-Cre and in the mammary gland epithelium by MMTV-Cre. This study revealed normal mammary gland development but exacerbated involution phenotypes upon forced weaning [55]. K5-Cre mediated keratinocyte-specific BclX deletion did not compromise skin barrier function although more apoptotic cells were noted in the epidermis and BclX−/− keratinocytes were found to be more susceptible to UVB radiation damage in vitro [56] but less susceptible to UVB or 7,12-dimethylbenz[α]anthracene (DMBA)-driven skin cancer in vivo [57]. Loss of BclX in hepatocytes triggered increased cell death and fibrosis [58]. Of note, this effect was rescued by co-deletion of Bax/Bak (hepatocytes actually express little/no Bak) and, interestingly, also by co-deletion of the BH3-only protein Bid, found present in its active form at low levels in the liver [59]. Finally, deletion of BclX in the respiratory epithelium caused the death of about 50% of newborns but a significant number developed to adulthood. In summary, this study proposed that BclX is dispensable for normal lung maturation but functions to protect respiratory epithelial cells against oxygen-induced toxicity [60].
Timed depletion of BclX in the nervous system leads to interesting phenotypes, e.g. the loss of retinal ganglion cells (RGC) in the developing embryo when using Six3-Cre mediated mosaic deletion, leading to reduced thickness of the retina. However, BclX dependence of maturing RGCs was lost in adult mice where deletion was mediated by tamoxifen using a Cre-ERT2 allele [61]. Similarly, deletion of BclX in dopaminergic neurons using rat tyrosine hydroxylase promoter (TH-Cre mice) showed that it is required for the survival of catecholaminergic cells in the developing substantia nigra [62]. Hence, in post-mitotic neurons with established synaptic connections in the adult animal the role of BclX in survival may no longer be as prominent as during embryogenesis and Mcl1 may take over a more critical role (see below). Of note, concomitant loss of Bim acting upstream of MOMP can ameliorate several defects caused by loss of BclX, including the fetal liver apoptosis and testicular atrophy noted in BclX+/− mice, but not, however, neuronal loss during development [63].
Myeloid cell leukemia 1 (Mcl1)
Based on published findings one is tempted to speculate that, for reasons still poorly understood, Mcl1 is the most crucial pro-survival protein in the Bcl2 family as its deletion in the zygote stops embryogenesis already at the blastocyst stage [4]. Furthermore, most cells upon conditional deletion in the adult mouse succumb to pre-mature cell death. Notably, rescue from death usually requires co-deletion of Bax and Bak, while co-deletion of one or more BH3-only proteins, such as Bim and/or Puma, usually fails to do so. Whether this is due to proposed effects on mitochondrial structure and respiration remains to be clarified [64].
In contrast to Bcl2 or BclX, Mcl1 expression in lymphocytes is broad with little variation under steady state or during development with a propensity to higher levels in stem/progenitor cells (Fig. 2). While pro-B cells express little Mcl1, pre-B, immature, follicular and marginal zone B cells express higher but similar levels [65]. Similarly, most thymocyte and mature T cell subsets show comparable Mcl1 expression [46] and the protein is also prominently expressed outside the hematopoietic compartment. A crucial feature of Mcl1 is its short half-life allowing swift integration of different signalling inputs by stabilizing post-translational modification or its rapid proteasomal degradation. Reduction in Mcl1 expression is frequently a prerequisite for cell death induction in response to various forms of stress, including cytokine deprivation, UV radiation [66] or extended mitotic arrest [67], assigning a molecular ‘timer’ function to Mcl1 [68].
Similar to observations made in BCL2 or BclX transgenic mice, MCL1 overexpression led to splenomegaly with extended extra-medullary haematopoiesis correlating with enhanced leukocyte survival ex vivo [69,70]. Vav-Mcl1 transgenic mice, similarly to hMCL1 transgenic mice, were also predisposed to different types of late onset B cell lymphomas that in the first model were mainly derived from pre-B or stem/progenitor cells [69] while the latter model presented frequently with follicular lymphoma and diffuse large B cell lymphomas later in life [71]. H2K-MCL1 transgenic mice showed accelerated Eμ-Myc-driven lymphomagenesis comparable to those overexpressing BCL2 but, due to Mcl-1 shorter half-life, MCL1-overexpressing tumours were more prone to cell death upon drug treatment impinging on protein stability, such as etoposide or vincristine, than those from BCL2 transgenic mice [69,72].
Mcl1 loss-of-function was studied extensively in different models (Table 1). Poly-IC-triggered timed deletion in Mcl1fl/− Mx-Cre mice resulted in rapid mortality 12 to 21 days after Mcl1 ablation. These mice were highly anaemic and exhibited symptoms of bone marrow failure, due to impaired stem cell survival upon Mcl1 depletion. qPCR analysis of Mcl1 mRNA levels in cells from wild-type mice confirmed high expression levels in HSCs induced by stem cell factor (SCF) treatment, moderate levels in common lymphoid progenitors or common myeloid progenitors and low expression in megakaryocyte-erythrocyte progenitors and granulocyte-monocyte progenitors. Notably, HSC and committed progenitor populations were all diminished upon Mcl1 deletion, while erythropoiesis was not affected [73]. Low-level Mcl1 expression may explain in part the lack of phenotype in monocytes/macrophages upon LysM-Cre mediated deletion [74,75] or in megakaryocytes upon Pf4-Cre mediated deletion [52].
Similarly, Lck- or CD19-Cre mediated deletion of the Mcl1 locus arrested T and B cell development at the DN2/3 to DN4 transition in the thymus or the pre-pro-B cell stage in the bone marrow, respectively, and caused a subsequent reduction of all mature lymphocyte subsets [46,76]. Interestingly, the surviving cells had overcome the Mcl1 deletion. This observation is consistent with the Stat5a-driven onset of Mcl1 expression in early B cell development, after successful rearrangement of the Ig heavy chain [47]. Moreover, a reduction in B cell numbers was already noted in Mcl1 hemizygous mice, indicating a gene dosage effect, a phenomenon not noted in T cells [76]. However, these models did not allow assessment of the requirement for Mcl1 in mature T or B cell survival. Adoptive transfer of Mcl1f/− Mx-Cre lymphocytes in Rag2−/− mice and subsequent treatment with poly-IC resulted in severe lymphopenia within 2 weeks, indicating beyond doubt that Mcl1 is also critical for the survival of mature lymphocytes [73,76]. Similarly, lymphocytic choriomeningitis virus (LCMV) driven interferon production triggering Mx-Cre deletion of Mcl1 led to a lack of virus-specific CD4+ and CD8+ T cells that could only partially be rescued by concomitant overexpression of BclX, usually strongly induced upon T cell activation [77]. In contrast, BCL2 transgene expression did not rescue Mcl1-deficient T cells from developmental cell death while co-deletion of Bak, its key effector target, partially restored T cell numbers [78].
As Mcl1 expression, along with BclX, was noted to be higher in GC B cells than non-GC B cells, the consequences of loss of BclX and Mcl1 in GC formation, class switching and B cell memory formation were compared. Mcl1 deletion was thereby targeted to B cells initiating somatic hypermutation or class-switch recombination using Aicda-driven Cre recombinase [48]. Alternatively, B cells from CreERT2Mcl1fl/fl mice were adoptively transferred into isogenic recipients that were then immunized with NP-KLH, and tamoxifen treatment was used to assess the impact of Mcl1 deletion on B cell survival. Strikingly, no antigen-specific isotype-switched B cells or GC-derived memory B cells were detected and serum IgG1 (but not IgM) levels were low in Aicda-Cre/Mcl1f/f mice after immunization. CreERT2 mediated deletion of adoptively transferred B cells confirmed Mcl1 dose dependence in pre-formed GC and memory B cells [48]. In a follow-up the same group showed that timed Mcl1 deletion limits formation of plasma blasts in vitro as well as of pre-formed plasma cells in the bone marrow and in the spleen [79]. BclX, on the other hand, only appeared critical for the survival of long-lived plasma cells in the bone marrow but not GC or memory B cell formation [48,79]. The role of Bcl2 in all these processes and possible redundancies with Mcl1 remain to be investigated.
Deletion of Mcl1 in innate immune cells including granulocytes by LysM-Cre or in mast cells by Cpa3-Cre deletion confirmed essential survival roles while monocytes and macrophages became only Mcl1-dependent upon microbial challenge [74,80]. A recent report also documents crucial roles in NK cell survival rendering Mcl1fl/fl Ncr1-Cre mice highly susceptible to tumour metastasis but resistant in models of multi-bacterial sepsis [49].
The facts that Bcl2 family proteins can co-regulate the cell death of hepatocytes under inflammatory conditions and growth factor treatment induces Mcl1 levels in primary hepatocytes, prompted studies investigating its role in liver homeostasis. Mcl1fl/fl Alb-Cre mice presented with reduced liver size due to spontaneous hepatocyte apoptosis subsequently triggering increased rates of compensatory proliferation that fostered liver cancer in aged mice [81]. In addition, mice lacking Mcl1 in the liver were more susceptible to Concanavalin A (ConA) - driven hepatitis [82], demonstrating a relevant contribution of the intrinsic cell death pathway to this type of liver damage [83]. In line with a possible redundancy with BclX that triggers similar phenotypes when deleted, double deficiency in both genes using Alb-Cre causes liver failure and perinatal death [84].
Highly detrimental also are the consequences of Mcl1 deletion using muscle creatine kinase promoter driven Cre (Ckmm-Cre) in the heart and skeletal muscle, leading to early postnatal death with signs of severe cardiomyopathy and fibrosis. Timed ablation of Mcl1 using Myh6-Mer-Cre-Mer (Myh-CreER) mice that express a tamoxifen-inducible version of Cre under control of the cardiac-specific a-myosin heavy chain promoter triggered dilated cardiomyopathy, a phenomenon that can be counteracted by Bcl2 transgene expression [85]. Additionally, Mcl1fl/fl Myh-CreER mice suffered from loss of heart muscle contractility leading to heart failure within 3 weeks of tamoxifen administration. Co-deletion of Bax and Bak could rescue these effects but the noted distortion of mitochondrial ultrastructure and respiratory capacity was not fully restored [85].
Finally, neuroscientists also explored the role of Mcl1 in the survival of neuronal precursor cells (NPCs) that have the capacity to regenerate damaged regions in the brain. The size of the NPC pool is in part controlled by apoptosis and reducing NPC apoptosis may enhance regenerative capacity after injury. Nestin-Cre mediated deletion of Mcl1 impaired neurogenesis in the embryo leading to early lethality [86] and subsequent analyses also documented a critical role for Mcl1 in adult NPC survival [87]. Ablation of Mcl1 in cortical neurons of the cortex using a CamKIIα-Cre BAC transgene, on the other hand, triggered an autophagic stress response and caused early postnatal death of these animals (< 8 weeks) [88]. Whether Mcl1 is indeed a negative regulator of autophagy or whether absence of Mcl1 leads to increased cell death susceptibility for neurons that try to overcome this stress (e.g. impaired respiratory capacity) by activating autophagy remains to be dissected experimentally.
Bcl2a1 (A1/Bfl1)
While humans and rat encode A1 in a single gene locus, mice harbour three functional genes encoding for A1 protein (A1a, A1b and A1d) and one pseudogene (A1c) [10]. All isoforms are highly conserved at the DNA and protein level, suggesting a large degree of functional redundancy, and show > 70% homology to human A1/BFL-1 [89]. Another limitation is that commercial antibodies recognizing endogenous A1/Bfl1 are not of great quality and hence most expression data are based on mRNA analysis. Unquestionably, expression levels increase upon successful rearrangement of the T cell receptor β (TCR-β) chain and pre-TCR expression in developing thymocytes [90] and upon TCR mediated activation in T cells [91,92]. During B cell maturation A1 mRNA levels gradually increase and activated B cells show highest levels [93] but A1 is downregulated again in plasma cells by the transcription factor Blimp-1. Myeloid cells express A1 either constitutively, e.g. granulocytes, or in response to inflammatory cytokines (e.g. TNF, G-CSF) or TLR ligation (LPS) in macrophages, or upon FcεRI mediated activation in an NF-AT-dependent manner in mast cells [89]. Outside of the haematopoietic system A1 is usually poorly expressed but is found in some solid tumours, best documented in melanoma [94,95]. Similar to Mcl1, A1 has a very short half-life and is subjected to rapid proteasomal turnover suggesting critical roles in adaptation and selection processes upon antigen challenge, inflammation or drug treatment [96]. The nature of E3-ligases involved in A1 turnover, however, is currently unknown.
In Eμ-A1a transgenic mice, which do overexpress the mouse A1a isoform, defective pro- to pre-B cell transition was reported, leading to the accumulation of pro-B cells (B220+CD43+IgM−) and reduced numbers of mature B cells in the periphery. However, this phenotype was quite variable between different founder lines [97]. Bone marrow B lineage cells showed reduced spontaneous apoptosis ex vivo and thymocytes were less sensitive to spontaneous death, γ-irradiation or dexamethasone, again in support of redundancy with Mcl1, Bcl2 and BclX. Similarly, Lck-driven expression of A1a in T cells caused cell death resistance of thymocytes, resting and activated T cells in response to different triggers of mitochondrial cell death [98]. Neither study reported on pronounced lymphadenopathy that might enable spontaneous tumour formation indicating that the expression levels achieved were insufficient to facilitate transformation.
Targeted deletion of A1a in mice was shown to affect neutrophil survival in vitro but failed to reveal other defects in lymphocyte development or homeostasis that might be explained by the rather poor expression of A1a in T and B cells and functional redundancy with A1b and A1d [11]. Mast cells derived from these mice lacked the ability to respond with increased survival upon ex vivo stimulation of FcεRI [99] and peritoneal macrophages showed a similar deficit upon exposure to microbial challenge [100].
A recent study using in vivo RNAi to knockdown all A1 isoforms present in mice revealed signs of delayed thymic development and impaired B cell homeostasis [101]. Two different model systems were generated and both were predicted to lead to a constitutive knockdown of A1 in all hematopoietic cells but the phenotypes observed were only partially overlapping. While in both models a reduction of the colony formation potential of granulocyte progenitors in the bone marrow was noted the effects on mature granulocyte survival varied significantly. More convincingly, A1 RNAi diminished the numbers of mature follicular B cells in the spleen and impaired their activation by mitogens [101]. Moreover, B cells displayed enhanced apoptosis rates upon BCR ligation suggesting that, in contrast to Bcl2 or BclX, Mcl1 and A1 ensure the survival of mature B cells upon mitogen-induced stimulation. Similarly, mast cell homeostasis and survival upon activation were impaired in mice with a constitutive A1 knockdown leading to protection from systemic and cutaneous anaphylaxis [102]. This suggests that mast cell homeostasis is also co-regulated by Mcl1 and A1 [80]. However, due to limitations of the employed RNAi systems the generation of sophisticated conditional alleles of A1 in mice or a Cas/CRISPR knockout rat model are needed to increase our understanding of A1 in cell death control.
Implications for BCL2 targeting therapy
A critical question often asked in biomedical research is, of course, can we learn anything from the analysis of all these mouse mutants that would help us to predict the efficacy or possible side effects of BCL2-targeted therapy? As BH3-only proteins usually antagonize one or more anti-apoptotic BCL2 pro-survival proteins, can we anticipate that mimicking their function in patients causes some of the knockout or hypomorphic phenotypes observed in mice?
A number of so-called BH3-mimetics are currently in pre-clinical and clinical development, mainly for the treatment of malignant disorders [103]. The best and most specific agents of this group share the mechanism of structurally displacing BH3-domains from the hydrophobic groove of anti-apoptotic Bcl2 family members, breaking up or preventing protein–protein interactions and thereby promoting Bax/Bak-dependent apoptosis [104-106]. Ideally, BH3-mimetics display specificity for one anti-apoptotic protein over the others. While in theory this might then affect all cells expressing the relevant anti-apoptotic protein, in practice it turns out that these treatments derive some measure of specificity for tumour cells providing a therapeutic window [103]. Reminiscent to this, not all healthy cells that express a certain anti-apoptotic Bcl2 family protein suffer consequences from its deletion. In fact sensitive tumour cells may have a balance between pro- and anti-apoptotic proteins that is much closer to the threshold for cell death than is the case in normal tissues, rendering them sensitive to drug treatment. This ‘primed to death’ phenotype may be mimicked to a degree in those cells that do suffer from gene ablation in mice, because they are in a vulnerable state near an apoptotic threshold, e.g. under the influence of developmental cues. Both seem to be the case and at least for some tumours early clinical experiences hint at feasibility and initial measures of success of the overall approach [103].
However, the development of these agents is in its early stages and has already encountered some major obstacles that have led to discontinuation of developmental programmes for some substances. A major obstacle in clinical development was, paradoxically, an extremely fast efficacy, which in a clinical context produces significant problems with so-called tumour lysis syndromes, but this problem was successfully met by application schedules using slow initial dose escalation [103,106]. In addition, the first generation BH3-mimetic navitoclax (which was derived from the lead compound ABT-737) was acting as a so-called ‘Bad-like mimetic’, thus achieving a significant inhibition of BCLX, BCL2 and Bcl2L2 [105,107]. This turned out to lead to problematic thrombocytopenia by directly killing platelets [108] and this side effect led to the discontinuation of the navitoclax programme in hematological malignancies in favour of the development of the Bcl2-specific ABT-199 [103]. However, ABT-263 is still investigated in smaller trials in solid tumours, usually in combination therapy [109,110]. A number of BH3-mimetics with other specificities are currently in development and pre-clinical analyses as well as clinical trials will discover their critical side effects [103]. The question is whether the knowledge acquired in loss-of-function models, as reviewed in this paper, may help to foresee problems in clinical development of these agents and whether this may aid the choice of directions these programmes should take.
The phenotypes of animals may present relevant pointers as to what side effects may develop when attacking a BCL2 family protein with a BH3-mimetic in patients. However, the description of phenotypes of mice usually carries some biases, warranting a note of caution. For example, embryonic or early postnatal phenotypes may not allow observation of important phenotypic changes that would be otherwise clear consequences of the alteration in an adult animal (or human in the end). Furthermore, the gene function during embryogenesis may not be very informative regarding the consequences of targeting in the adult organism (beyond the obvious extreme caution one has to take not to employ any of these drugs during pregnancy). Also, the dominance of some phenotypes may mask and distract from clinically more relevant alterations not noted or overlooked when analysing these animal models.
For example, early data on BclX targeting were hampered by the embryonic lethality from a neurological phenotype. It was thus slightly surprising to find that ABT-263 had a profound effect on platelets. This thrombocytopenia was clearly puzzling at the time but might have been predicted by careful evaluation of the early data presented from BclXfl/fl MMTV-Cre mice reporting a decline in the number of circulating platelets [50]. The relevance of this finding was clarified only later in an ENU screen revealing destabilizing point mutations in BclX as a cause for the thrombocytopenia observed in these mouse mutants [51]. However, patients were already on clinical trial at that time [108], some of whom also presented with severe neutropenia that remains unexplained. By contrast the severe developmental consequences of targeting BclX and Bcl2 on the developing neuronal system and on developing kidneys did not clearly predict relevant CNS or renal toxicities in clinical trials with ABT-263 or ABT-199, although the CNS penetrance of the drugs may play an important role. Such predictions would have required the generation of tissue specific gene targeted mice. In practice, one prediction from the tissue specific targeting experiments outlined above would be testicular degeneration or osteopaenia upon prolonged BCLX inhibition [54,111]. However, the former should not be a concern to the usually advanced-age cancer patient. Skin hypersensitivity and sunburns may be another side effect, impinging on quality of life during therapy. On the other hand, neuronal complications may arise, similar to those in mice that lack BclX in retinal ganglion cells, upon axon injury [61]. While we do not know of reports to this end from clinical trials, these are examples for possible side-effects, predicted based on data from mouse model analysis.
The story, however, is even more complex. Observations from doubly targeted mice, such as the Bcl2−/−Bim−/− deficient mice, show that ultimately it is the balance between specific pro-apoptotic BH3-only proteins and anti-apoptotic Bcl2 family members, rather than the lack of one of the latter group, that determines the outcome. One major lesson to be learned here is that not all tissues have the same thresholds. Indeed, loss of one allele of Bim rescued all phenotypes in the kidney and haematopoietic system almost completely, whereas the melanocyte loss in the hair follicles was only prevented when Bim was removed completely [34]. This suggests important gene dosage effects that may not become apparent (or predictable) by in vivo targeting of the BCL2 rheostat on one side alone. The gene dosage effects, however, may also play to our advantage. Based on observations in Mcl1-deficient models it seems likely that targeting Mcl1 specifically may carry an enormous toxic potential in a large number of tissues (see Table 1). However, given a specific vulnerability of certain tumour models, loss of a small part of Mcl1 protection in ‘incomplete drug targeting’ may not be enough to create a relevant toxic effect in healthy tissue while it may be very effective in tumours, as has been proposed for murine models of Burkitt’s lymphoma [112], B-acute lymhoblastic laeukemia (B-ALL) [113] or acute myeloid leukaemia (AML) [114].
It is also very important to determine the specificity of BH3-mimetics for binding to anti-apoptotic Bcl2 family members, since this carries important information on possible mechanisms of drug resistance. Clearly, overexpression of Mcl1 or A1/Bfl1 will be able to serve as a mechanism of resistance regarding a substance like ABT-199 that is unable to bind to these pro-survival molecules [115,116], but this is also true with regard to toxicities, so that normal tissues with constitutively high Mcl1 expression may not experience drug-related toxicities. The genetic models surveyed in this review thus contain information on where a key resistance player shows important functional dominance. One may conclude that such tissues may have relevant means to escape toxicities when treated with a certain type of BH3-mimetic. Of note, basic scientists can also learn from side effects in (pre)clinical studies about the BCL2 dependence of certain cell types. This is nicely exemplified by the prevention of tamoxifen-driven endometrial hyperplasia by ABT-737-driven apoptosis, suggesting BclX or Bcl-w dependence, as ABT-199 was not reported to show such an effect [117].
A final important aspect is the fact that the Bcl2 family is an important determinant of the outcome of a number of developmental checkpoints, among them immune checkpoints that are still very active in adults. Toxicities and side effects are thus not simply determined by loss of certain cells from a tissue. Immune defects and autoimmunity may be important consequences of altering the BCL2 balance and need to be taken into account [118]. First evidence, again from animal models, is provided by the observation that ABT-737 treatment impacts severely on the persistence of memory B cells, the establishment of bone marrow plasma cells, as well as the induction of a cytotoxic T cell response, and hence adoptive immunity [119]. Which of these effects are mediated by inhibition of BclX, Bcl2 or both remains to be sorted out. Of note, however, lymphocytopenia was also reported in a significant number of patients on the navitoclax phase I study [108].
Taken together the knowledge gained from Bcl2 mouse mutants is clearly of significant value and provides a first guide of what to expect, but will fall short in details in the light of the complexity of the interactions in the Bcl2 family network, precluding reliable and precise predictions of the effects of pharmacological targeting of the family in patients. Thus, the level of sophistication that animal models need to develop, in order to help physicians leave the path of trial and error, still needs to increase significantly. Yet, these models are simply indispensable to create an understanding about the role of these proteins in normal physiology that ultimately is the key to exploring their role in human pathology and their drug-target potential.
Acknowledgements
The work in our laboratories on Bcl2 family proteins is supported by the Austrian Science Fund (FWF; FOR2036_I1298); MS is supported by the Doctoral College Molecular Cell Biology and Oncology (FWF; W1101); ST is the recipient of a DOC Fellowship of the Austrian Academy of Science (ÖAW). We apologize to all scientists in the field whose work we were unable to cite because of space constraints.
Abbreviations
- AML
acute myelois leukaemia
- B-ALL
B acute lymhoblastic leukaemia
- BCR
B cell receptor
- BM
bone marrow
- ConA
concanavalin A
- DMBA
7,12-dimethylbenz[α]anthracene
- DN
double negative
- DP
double positive
- LCMV
lymphocytic choriomeningitis virus
- NP-KLH
4-Hydroxy-3-nitrophenylacetyl-Keyhole Limpet Hemocyanin
- RGC
retinal ganglion cell
- SCF
stem cell factor
- Treg
regulatory T cell
References
- 1.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2013;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 2.Tait SW, Green DR. Mitochondrial regulation of cell death. Cold Spring Harb Persp Biol. 2013;5:1–12. doi: 10.1101/cshperspect.a008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strasser A, O’Connor L, Huang DCS, O’Reilly LA, Stanley ML, Bath ML, Adams JM, Cory S, Harris AW. Lessons from bcl-2 transgenic mice for immunology, cancer biology and cell death research. Behring Inst Mitt. 1996;97:101–117. [PubMed] [Google Scholar]
- 4.Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- 5.Motoyama N, Wang FP, Roth KA, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x deficient mice. Science (New York, NY) 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 6.Ma A, Pena JC, Chang B, Margosian E, Davidson L, Alt FW, Thompson CB. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci USA. 1995;92:4763–4767. doi: 10.1073/pnas.92.11.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama K-I, Nakayama K, Izumi N, Kulda K, Shinkai Y, Louie MC, Fields LE, Lucas PJ, Stewart V, Alt FW, et al. Disappearance of the lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science (New York, NY) 1993;261:1584–1588. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- 9.Kamada S, Shimono A, Shinto Y, Tsujimura T, Takahashi T, Noda T, Kitamura Y, Kondoh H, Tsujimoto Y. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus andspleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 1995;55:354–359. [PubMed] [Google Scholar]
- 10.Hatakeyama S, Hamasaki A, Negishi I, Loh DY, Sendo F, Nakayama K. Multiple gene duplication and expression of mouse bcl-2-related genes, A1. Int Immunol. 1998;10:631–637. doi: 10.1093/intimm/10.5.631. [DOI] [PubMed] [Google Scholar]
- 11.Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K-I, Hatakeyama S. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J Exp Med. 1998;188:1985–1992. doi: 10.1084/jem.188.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, de Kretser D, Metcalf D, Köntgen F, Adams JM, et al. Apoptosis regulator Bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci USA. 1998;95:12424–12431. doi: 10.1073/pnas.95.21.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, MacGregor GR. Testicular degeneration in Bclw-deficient mice. Nat Genet. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- 14.Russell HR, Lee Y, Miller HL, Zhao J, McKinnon PJ. Murine ovarian development is not affected by inactivation of the bcl-2 family member diva. Mol Cell Biol. 2002;22:6866–6870. doi: 10.1128/MCB.22.19.6866-6870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green DR, Walczak H. Apoptosis therapy: driving cancers down the road to ruin. Nat Med. 2013;19:131–133. doi: 10.1038/nm.3076. [DOI] [PubMed] [Google Scholar]
- 16.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science (New York, NY) 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 17.Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, Korsmeyer SJ. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985;41:899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- 18.McDonnell TJ, Deane N, Platt FM, Nuñez G, Jaeger U, McKearn JP, Korsmeyer SJ. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 19.McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18) Nature. 1991;349:254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- 20.Strasser A, Harris AW, Cory S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 21.Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, Harris AW. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci USA. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 23.Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA. 1999;96:14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jager R, Herzer U, Schenkel J, Weiher H. Overexpression of Bcl-2 inhibits alveolar cell apoptosis during involution and accelerates c-myc-induced tumorigenesis of the mammary gland in transgenic mice. Oncogene. 1997;15:1787–1795. doi: 10.1038/sj.onc.1201353. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Lewis B, Capuco AV, Laucirica R, Furth PA. WAP-TAg transgenic mice and the study of dysregulated cell survival, proliferation, and mutation during breast carcinogenesis. Oncogene. 2000;19:1010–1019. doi: 10.1038/sj.onc.1203271. [DOI] [PubMed] [Google Scholar]
- 26.Pierce RH, Vail ME, Ralph L, Campbell JS, Fausto N. Bcl-2 expression inhibits liver carcinogenesis and delays the development of proliferating foci. Am J Pathol. 2002;160:1555–1560. doi: 10.1016/S0002-9440(10)61101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgartner F, Villunger A. Apoptosis: a barrier against cancer no more? Hepatology. 2011;54:1121–1124. doi: 10.1002/hep.24637. [DOI] [PubMed] [Google Scholar]
- 28.Frenzel A, Grespi F, Chmelewskij W, Villunger A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis. 2009;14:584–596. doi: 10.1007/s10495-008-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaelidis TM, Sendtner M, Cooper JD, Airaksinen MS, Holtmann B, Meyer M, Thoenen H. Inactivation of bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early postnatal development. Neuron. 1996;17:75–89. doi: 10.1016/s0896-6273(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 30.Manzl C, Baumgartner F, Peintner L, Schuler F, Villunger A. Possible pitfalls investigating cell death responses in genetically engineered mouse models and derived cell lines. Methods. 2013;61:130–137. doi: 10.1016/j.ymeth.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuzaki Y, Nakayama K-I, Nakayama K, Tomita T, Isoda M, Loh DY, Nakauchi H. Role of bcl-2 in the development of lymphoid cells from the hematopoietic stem cell. Blood. 1997;89:853–862. [PubMed] [Google Scholar]
- 32.Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003;101:2393–2400. doi: 10.1182/blood-2002-07-2132. [DOI] [PubMed] [Google Scholar]
- 33.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, Humblet-Baron S, Schonefeldt S, Herold MJ, Hildeman D, et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouillet P, Cory S, Zhang L-C, Strasser A, Adams JM. Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev Cell. 2001;1:645–653. doi: 10.1016/s1534-5807(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 35.Thorp E, Li Y, Bao L, Yao PM, Kuriakose G, Rong J, Fisher EA, Tabas I. Brief report: increased apoptosis in advanced atherosclerotic lesions of Apoe−/− mice lacking macrophage Bcl-2. Arterioscler Thromb Vasc Biol. 2009;29:169–172. doi: 10.1161/ATVBAHA.108.176495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merino R, Ding L, Veis DJ, Korsmeyer SJ, Nunez G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994;13:683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grillot DAM, Merino R, Pena JC, Fanslow WC, Finkelman FD, Thompson CB, Núñez G. bcl-x exhibits regulated expression during B cell development and activation and modulates lymphocyte survival in transgenic mice. J Exp Med. 1996;183:381–391. doi: 10.1084/jem.183.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grillot DAM, Merino R, Nuñez G. Bcl-xL displays restricted distribution during T cell development and inhibits multiple forms of apoptosis but not clonal deletion in transgenic mice. J Exp Med. 1995;182:1973–1983. doi: 10.1084/jem.182.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-x(L) and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang W, Mueller DL, Pennell CA, Rivard JJ, Li YS, Hardy RR, Schlissel MS, Behrens TW. Frequent aberrant immunoglobulin gene rearrangements in pro-B cells revealed by a bcl-xL transgene. Immunity. 1996;4:291–299. doi: 10.1016/s1074-7613(00)80437-9. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi T, Honda H, Hirai H, Tsujimoto Y. Overexpressed Bcl-xL prevents bacterial superantigen-induced apoptosis of thymocytes in vitro. Cell Death Differ. 1997;4:159–165. doi: 10.1038/sj.cdd.4400214. [DOI] [PubMed] [Google Scholar]
- 42.Fang W, Weintraub BC, Dunlap B, Garside P, Pape KA, Jenkins MK, Goodnow CC, Mueller DL, Behrens TW. Self-reactive B lymphocytes overexpressing Bcl-xL escape negative selection and are tolerized by clonal anergy and receptor editing. Immunity. 1998;9:35–45. doi: 10.1016/s1074-7613(00)80586-5. [DOI] [PubMed] [Google Scholar]
- 43.Huang DC, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, Tschopp J, Strasser A. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-xL. Proc Natl Acad Sci USA. 1999;96:14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de La Coste A, Mignon A, Fabre M, Gilbert E, Porteu A, Van Dyke T, Kahn A, Perret C. Paradoxical inhibition of c-myc-induced carcinogenesis by Bcl-2 in transgenic mice. Cancer Res. 1999;59:5017–5022. [PubMed] [Google Scholar]
- 45.Rodriguez I, Matsuura K, Khatib K, Reed JC, Nagata S, Vassalli P. A bcl-2 transgene expressed in hepatocytes protects mice from fulminant liver destruction but not from rapid death induced by anti-Fas antibody injection. J Exp Med. 1996;183:1031–1036. doi: 10.1084/jem.183.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malin S, McManus S, Cobaleda C, Novatchkova M, Delogu A, Bouillet P, Strasser A, Busslinger M. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat Immunol. 2010;11:171–179. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vikstrom I, Carotta S, Luthje K, Peperzak V, Jost PJ, Glaser S, Busslinger M, Bouillet P, Strasser A, Nutt SL, et al. Mcl-1 is essential for germinal center formation and B cell memory. Science (New York, NY) 2011;330:1095–1099. doi: 10.1126/science.1191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sathe P, Delconte RB, Souza-Fonseca-Guimaraes F, Seillet C, Chopin M, Vandenberg CJ, Rankin LC, Mielke LA, Vikstrom I, Kolesnik TB, et al. Innate immunodeficiency following genetic ablation of Mcl1 in natural killer cells. Nat Commun. 2014;5:4539. doi: 10.1038/ncomms5539. [DOI] [PubMed] [Google Scholar]
- 50.Wagner KU, Claudio E, Rucker EB, 3rd, Riedlinger G, Broussard C, Schwartzberg PL, Siebenlist U, Hennighausen L. Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development (Cambridge, England) 2000;127:4949–4958. doi: 10.1242/dev.127.22.4949. [DOI] [PubMed] [Google Scholar]
- 51.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 52.Debrincat MA, Josefsson EC, James C, Henley KJ, Ellis S, Lebois M, Betterman KL, Lane RM, Rogers KL, White MJ, et al. Mcl-1 and Bcl-x(L) coordinately regulate megakaryocyte survival. Blood. 2012;119:5850–5858. doi: 10.1182/blood-2011-12-398834. [DOI] [PubMed] [Google Scholar]
- 53.Motoyama N, Kimura T, Takahashi T, Watanabe T, Nakano T. bcl-x prevents apoptotic cell death of both primitive and definitive erythrocytes at the end of maturation. J Exp Med. 1999;189:1691–1698. doi: 10.1084/jem.189.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rucker EB, 3rd, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, Hennighausen L. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol. 2000;14:1038–1052. doi: 10.1210/mend.14.7.0465. [DOI] [PubMed] [Google Scholar]
- 55.Walton KD, Wagner KU, Rucker EB, 3rd, Shillingford JM, Miyoshi K, Hennighausen L. Conditional deletion of the bcl-x gene from mouse mammary epithelium results in accelerated apoptosis during involution but does not compromise cell function during lactation. Mech Dev. 2001;109:281–293. doi: 10.1016/s0925-4773(01)00549-4. [DOI] [PubMed] [Google Scholar]
- 56.Umeda J, Sano S, Kogawa K, Motoyama N, Yoshikawa K, Itami S, Kondoh G, Watanabe T, Takeda J. In vivo cooperation between Bcl-xL and the phosphoinositide 3-kinase-Akt signaling pathway for the protection of epidermal keratinocytes from apoptosis. FASEB J. 2003;17:610–620. doi: 10.1096/fj.02-0597com. [DOI] [PubMed] [Google Scholar]
- 57.Kim DJ, Kataoka K, Sano S, Connolly K, Kiguchi K, DiGiovanni J. Targeted disruption of Bcl-xL in mouse keratinocytes inhibits both UVB- and chemically induced skin carcinogenesis. Mol Carcinog. 2009;48:873–885. doi: 10.1002/mc.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takehara T, Tatsumi T, Suzuki T, Rucker EB, 3rd, Hennighausen L, Jinushi M, Miyagi T, Kanazawa Y, Hayashi N. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 59.Hikita H, Takehara T, Kodama T, Shimizu S, Hosui A, Miyagi T, Tatsumi T, Ishida H, Ohkawa K, Li W, et al. BH3-only protein bid participates in the Bcl-2 network in healthy liver cells. Hepatology. 2009;50:1972–1980. doi: 10.1002/hep.23207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staversky RJ, Vitiello PF, Yee M, Callahan LM, Dean DA, O’Reilly MA. Epithelial ablation of Bcl-XL increases sensitivity to oxygen without disrupting lung development. Am J Respir Cell Mol Biol. 2010;43:376–385. doi: 10.1165/rcmb.2009-0165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harder JM, Ding Q, Fernandes KA, Cherry JD, Gan L, Libby RT. BCL2L1 (BCL-X) promotes survival of adult and developing retinal ganglion cells. Mol Cell Neurosci. 2012;51:53–59. doi: 10.1016/j.mcn.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM. Bcl-x is required for proper development of the mouse substantia nigra. J Neurosci. 2005;25:6721–6728. doi: 10.1523/JNEUROSCI.0760-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akhtar RS, Klocke BJ, Strasser A, Roth KA. Loss of BH3-only protein Bim inhibits apoptosis of hemopoietic cells in the fetal liver and male germ cells but not neuronal cells in bcl-x-deficient mice. J Histochem Cytochem. 2008;56:921–927. doi: 10.1369/jhc.2008.951749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, Temirov J, Cleland MM, Pelletier S, Schuetz JD, et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14:575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baumgartner F, Woess C, Pedit V, Tzankov A, Labi V, Villunger A. Minor cell-death defects but reduced tumor latency in mice lacking the BH3-only proteins Bad and Bmf. Oncogene. 2013;32:621–630. doi: 10.1038/onc.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 68.Fava LL, Villunger A. Stop competing, start talking! EMBO J. 2014;33:1849–1851. doi: 10.15252/embj.201489466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campbell KJ, Bath ML, Turner ML, Vandenberg CJ, Bouillet P, Metcalf D, Scott CL, Cory S. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells and enhances drug-resistance. Blood. 2010;116:3197–3207. doi: 10.1182/blood-2010-04-281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou P, Qian L, Bieszczad CK, Noelle R, Binder M, Levy NB, Craig RW. Mcl-1 in transgenic mice promotes survival in a spectrum of hematopoietic cell types and immortalization in the myeloid lineage. Blood. 1998;92:3226–3239. [PubMed] [Google Scholar]
- 71.Zhou P, Levy NB, Xie H, Qian L, Lee CY, Gascoyne RD, Craig RW. MCL1 transgenic mice exhibit a high incidence of B-cell lymphoma manifested as a spectrum of histologic subtypes. Blood. 2001;97:3902–3909. doi: 10.1182/blood.v97.12.3902. [DOI] [PubMed] [Google Scholar]
- 72.Brunelle JK, Ryan J, Yecies D, Opferman JT, Letai A. MCL-1-dependent leukemia cells are more sensitive to chemotherapy than BCL-2-dependent counterparts. J Cell Biol. 2009;187:429–442. doi: 10.1083/jcb.200904049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science (New York, NY) 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 74.Steimer DA, Boyd K, Takeuchi O, Fisher JK, Zambetti GP, Opferman JT. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood. 2009;113:2805–2815. doi: 10.1182/blood-2008-05-159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 77.Tripathi P, Koss B, Opferman JT, Hildeman DA. Mcl-1 antagonizes Bax/Bak to promote effector CD4(+) and CD8(+) T-cell responses. Cell Death Differ. 2013;20:998–1007. doi: 10.1038/cdd.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dunkle A, Dzhagalov I, He YW. Mcl-1 promotes survival of thymocytes by inhibition of Bak in a pathway separate from Bcl-2. Cell Death Differ. 2010;17:994–1002. doi: 10.1038/cdd.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peperzak V, Vikstrom I, Walker J, Glaser SP, LePage M, Coquery CM, Erickson LD, Fairfax K, Mackay F, Strasser A, et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol. 2013;14:290–297. doi: 10.1038/ni.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lilla JN, Chen CC, Mukai K, Benbarak MJ, Franco CB, Kalesnikoff J, Yu M, Tsai M, Piliponsky AM, Galli SJ. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weber A, Boger R, Vick B, Urbanik T, Haybaeck J, Zoller S, Teufel A, Krammer PH, Opferman JT, Galle PR, et al. Hepatocyte-specific deletion of the antiapoptotic protein myeloid cell leukemia-1 triggers proliferation and hepatocarcinogenesis in mice. Hepatology. 2010;51:1226–1236. doi: 10.1002/hep.23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vick B, Weber A, Urbanik T, Maass T, Teufel A, Krammer PH, Opferman JT, Schuchmann M, Galle PR, Schulze-Bergkamen H. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49:627–636. doi: 10.1002/hep.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaufmann T, Jost PJ, Pellegrini M, Puthalakath H, Gugasyan R, Gerondakis S, Cretney E, Smyth MJ, Silke J, Hakem R, et al. Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity. 2009;30:56–66. doi: 10.1016/j.immuni.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hikita H, Takehara T, Shimizu S, Kodama T, Li W, Miyagi T, Hosui A, Ishida H, Ohkawa K, Kanto T, et al. Mcl-1 and Bcl-xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. Hepatology. 2009;50:1217–1226. doi: 10.1002/hep.23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Bathina M, Lynch J, Koss B, Calabrese C, Frase S, Schuetz JD, Rehg JE, Opferman JT. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes Dev. 2013;27:1351–1364. doi: 10.1101/gad.215855.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung EC, Kelly MA, MacKenzie AE, Park DS, Opferman JT, et al. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci. 2008;28:6068–6078. doi: 10.1523/JNEUROSCI.4940-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malone CD, Hasan SM, Roome RB, Xiong J, Furlong M, Opferman JT, Vanderluit JL. Mcl-1 regulates the survival of adult neural precursor cells. Mol Cell Neurosci. 2012;49:439–447. doi: 10.1016/j.mcn.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 88.Germain M, Nguyen AP, Le Grand JN, Arbour N, Vanderluit JL, Park DS, Opferman JT, Slack RS. MCL-1 is a stress sensor that regulates autophagy in a developmentally regulated manner. EMBO J. 2011;30:395–407. doi: 10.1038/emboj.2010.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ottina E, Tischner D, Herold MJ, Villunger A. A1/Bfl-1 in leukocyte development and cell death. Exp Cell Res. 2012;318:1291–1303. doi: 10.1016/j.yexcr.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mandal M, Borowski C, Palomero T, Ferrando AA, Oberdoerffer P, Meng F, Ruiz-Vela A, Ciofani M, Zuniga-Pflucker JC, Screpanti I, et al. The BCL2A1 gene as a pre-T cell receptor-induced regulator of thymocyte survival. J Exp Med. 2005;201:603–614. doi: 10.1084/jem.20041924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Verschelde C, Walzer T, Galia P, Biemont MC, Quemeneur L, Revillard JP, Marvel J, Bonnefoy-Berard N. A1/Bfl-1 expression is restricted to TCR engagement in T lymphocytes. Cell Death Differ. 2003;10:1059–1067. doi: 10.1038/sj.cdd.4401265. [DOI] [PubMed] [Google Scholar]
- 92.Tomayko MM, Punt JA, Bolcavage JM, Levy SL, Allman DM, Cancro MP. Expression of the Bcl-2 family member A1 is developmentally regulated in T cells. Int Immunol. 1999;11:1753–1761. doi: 10.1093/intimm/11.11.1753. [DOI] [PubMed] [Google Scholar]
- 93.Trescol-Biemont MC, Verschelde C, Cottalorda A, Bonnefoy-Berard N. Regulation of A1/Bfl-1 expression in peripheral splenic B cells. Biochimie. 2004;86:287–294. doi: 10.1016/j.biochi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 94.Haq R, Yokoyama S, Hawryluk EB, Jonsson GB, Frederick DT, McHenry K, Porter D, Tran TN, Love KT, Langer R, et al. BCL2A1 is a lineage-specific antiapoptotic melanoma oncogene that confers resistance to BRAF inhibition. Proc Natl Acad Sci USA. 2013;110:4321–4326. doi: 10.1073/pnas.1205575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vogler M. BCL2A1: the underdog in the BCL2 family. Cell Death Differ. 2011;19:67–74. doi: 10.1038/cdd.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herold MJ, Zeitz J, Pelzer C, Kraus C, Peters A, Wohlleben G, Berberich I. The stability and anti-apoptotic function of A1 are controlled by its C terminus. J Biol Chem. 2006;281:13663–13671. doi: 10.1074/jbc.M600266200. [DOI] [PubMed] [Google Scholar]
- 97.Chuang PI, Morefield S, Liu CY, Chen S, Harlan JM, Willerford DM. Perturbation of B-cell development in mice overexpressing the Bcl-2 homolog A1. Blood. 2002;99:3350–3359. doi: 10.1182/blood.v99.9.3350. [DOI] [PubMed] [Google Scholar]
- 98.Gonzalez J, Orlofsky A, Prystowsky MB. A1 is a growth-permissive antiapoptotic factor mediating postactivation survival in T cells. Blood. 2003;101:2679–2685. doi: 10.1182/blood-2002-04-1229. [DOI] [PubMed] [Google Scholar]
- 99.Xiang Z, Ahmed AA, Moller C, Nakayama K, Hatakeyama S, Nilsson G. Essential role of the prosurvival bcl-2 homologue A1 in mast cell survival after allergic activation. J Exp Med. 2001;194:1561–1569. doi: 10.1084/jem.194.11.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kausalya S, Somogyi R, Orlofsky A, Prystowsky MB. Requirement of A1-a for bacillus Calmette-Guerin-mediated protection of macrophages against nitric oxide-induced apoptosis. J Immunol. 2001;166:4721–4727. doi: 10.4049/jimmunol.166.7.4721. [DOI] [PubMed] [Google Scholar]
- 101.Ottina E, Grespi F, Tischner D, Soratroi C, Geley S, Ploner A, Reichardt HM, Villunger A, Herold MJ. Targeting antiapoptotic A1/Bfl-1 by in vivo RNAi reveals multiple roles in leukocyte development in mice. Blood. 2012;119:6032–6042. doi: 10.1182/blood-2011-12-399089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ottina E, Lyberg K, Sochalska M, Villunger A, Nilsson G. Knockdown of the anti-apoptotic Bcl-2-family member A1/Bfl-1 protects mice from anaphylaxis. J Immunology. 2015 doi: 10.4049/jimmunol.1400637. pii: 1400637. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anderson MA, Huang D, Roberts A. Targeting BCL2 for the treatment of lymphoid malignancies. Semin Hematol. 2014;51:219–227. doi: 10.1053/j.seminhematol.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 104.Lessene G, Czabotar PE, Sleebs BE, Zobel K, Lowes KN, Adams JM, Baell JB, Colman PM, Deshayes K, Fairbrother WJ, et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat Chem Biol. 2013;9:390–397. doi: 10.1038/nchembio.1246. [DOI] [PubMed] [Google Scholar]
- 105.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 106.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 107.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 108.Wilson WH, O’Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, Tulpule A, Dunleavy K, Xiong H, Chiu YL, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vlahovic G, Karantza V, Wang D, Cosgrove D, Rudersdorf N, Yang J, Xiong H, Busman T, Mabry M. A phase I safety and pharmacokinetic study of ABT-263 in combination with carboplatin/paclitaxel in the treatment of patients with solid tumors. Invest New Drugs. 2014;32:976–984. doi: 10.1007/s10637-014-0116-3. [DOI] [PubMed] [Google Scholar]
- 110.Cleary JM, Lima CM, Hurwitz HI, Montero AJ, Franklin C, Yang J, Graham A, Busman T, Mabry M, Holen K, et al. A phase I clinical trial of navitoclax, a targeted high-affinity Bcl-2 family inhibitor, in combination with gemcitabine in patients with solid tumors. Invest New Drugs. 2014;32:937–945. doi: 10.1007/s10637-014-0110-9. [DOI] [PubMed] [Google Scholar]
- 111.Iwasawa M, Miyazaki T, Nagase Y, Akiyama T, Kadono Y, Nakamura M, Oshima Y, Yasui T, Matsumoto T, Nakamura T, et al. The antiapoptotic protein Bcl-xL negatively regulates the bone-resorbing activity of osteoclasts in mice. J Clin Investig. 2009;119:3149–3159. doi: 10.1172/JCI39819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kelly GL, Grabow S, Glaser SP, Fitzsimmons L, Aubrey BJ, Okamoto T, Valente LJ, Robati M, Tai L, Fairlie WD, et al. Targeting of MCL-1 kills MYC-driven mouse and human lymphomas even when they bear mutations in p53. Genes Dev. 2014;28:58–70. doi: 10.1101/gad.232009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koss B, Morrison J, Perciavalle RM, Singh H, Rehg JE, Williams RT, Opferman JT. Requirement for antiapoptotic MCL-1 in the survival of BCR-ABL B-lineage acute lymphoblastic leukemia. Blood. 2013;122:1587–1598. doi: 10.1182/blood-2012-06-440230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xiang Z, Luo H, Payton JE, Cain J, Ley TJ, Opferman JT, Tomasson MH. Mcl1 haploinsufficiency protects mice from Myc-induced acute myeloid leukemia. J Clin Investig. 2010;120:2109–2118. doi: 10.1172/JCI39964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2011;115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, Cohen GM. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 117.Vaillant F, Merino D, Lee L, Breslin K, Pal B, Ritchie ME, Smyth GK, Christie M, Phillipson LJ, Burns CJ, et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell. 2013;24:120–129. doi: 10.1016/j.ccr.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 118.Tischner D, Woess C, Ottina E, Villunger A. Bcl-2-regulated cell death signalling in the prevention of autoimmunity. Cell Death Dis. 2010;1:e48. doi: 10.1038/cddis.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carrington EM, Vikstrom IB, Light A, Sutherland RM, Londrigan SL, Mason KD, Huang DC, Lew AM, Tarlinton DM. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc Natl Acad Sci USA. 2010;107:10967–10971. doi: 10.1073/pnas.1005256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Josefsson EC, James C, Henley KJ, Debrincat MA, Rogers KL, Dowling MR, White MJ, Kruse EA, Lane RM, Ellis S, et al. Megakaryocytes possess a functional intrinsic apoptosis pathway that must be restrained to survive and produce platelets. J Exp Med. 2011;208:2017–2031. doi: 10.1084/jem.20110750. [DOI] [PMC free article] [PubMed] [Google Scholar]