Abstract
Introduction or background
The UK is at the forefront of mitochondrial science and is currently the only country in the world to legalize germ-line technologies involving mitochondrial donation. However, concerns have been raised about genetic modification and the ‘slippery slope’ to designer babies.
Sources of data
This review uses academic articles, newspaper reports and public documents.
Areas of agreement
Mitochondrial donation offers women with mitochondrial disease an opportunity to have healthy, genetically related children.
Areas of controversy
Key areas of disagreement include safety, the creation of three-parent babies, impact on identity, implications for society, definitions of genetic modification and reproductive choice.
Growing points
The UK government legalized the techniques in March 2015. Scientific and medical communities across the world followed the developments with interest.
Areas timely for developing research
It is expected that the first cohort of ‘three parent’ babies will be born in the UK in 2016. Their health and progress will be closely monitored.
Keywords: mitochondrial donation, reproduction, ethical issues, reproduction
Introduction
In February 2015, UK Parliament voted in favour, by a large majority, of changing the law to support the clinical application of novel in vitro fertilization (IVF) procedures involving mitochondrial donation. The result is that the UK remains at the vanguard of mitochondrial science—it is currently the only country in the world to legalize germ-line technologies.1 However, the techniques have attracted intense media interest, with ‘three-parent babies’ dominating the headlines and concerns raised about the possible ‘slippery slope’ to designer babies and human modification. This article explores the social and ethical issues surrounding the legalization of these techniques.
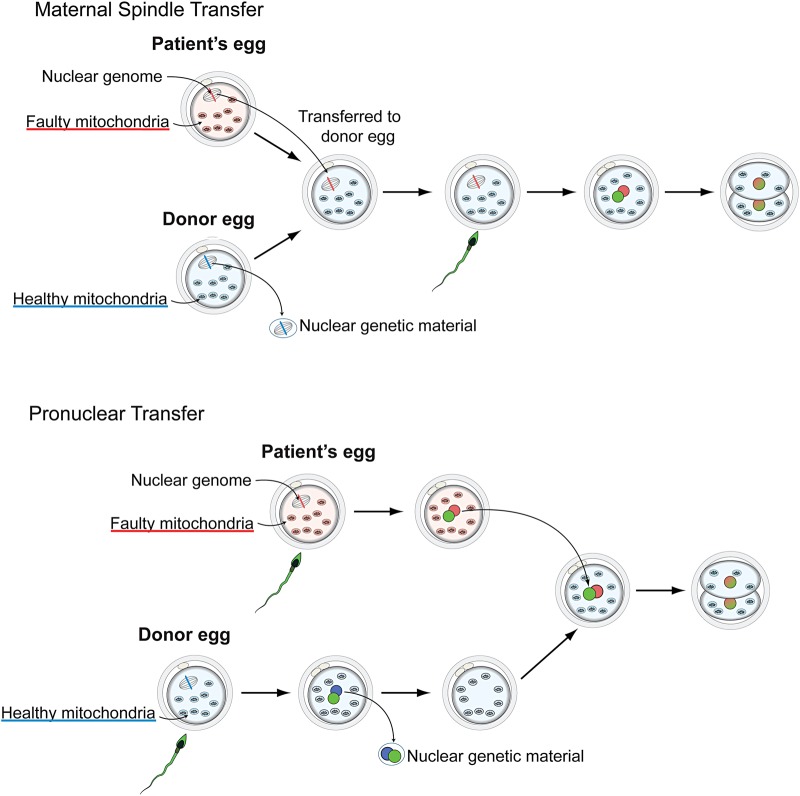
Mitochondria exist in the cytoplasm of a cell and are inherited through the female line. This means that the children of women with disease caused by mutations in mitochondrial DNA will inherit these mutations. There is no cure for mitochondrial disease, and treatment is limited,2 which means technologies that can prevent a child from inheriting the disease have been widely welcomed. Scientists at the Wellcome Trust Centre for Mitochondrial Research in Newcastle have developed two related techniques. Maternal spindle transfer involves removing the nucleus of an egg with faulty mitochondria from a woman with mitochondrial disease and transferring it into a donated e-nucleated egg containing healthy mitochondria. In contrast, pro-nuclear transfer occurs after fertilization (see Fig. 1). In both cases, the mitochondrial DNA from the donor could be inherited by future generations.
Fig. 1.
Maternal spindle transfer.
What is mitochondrial disease?
Mitochondria are small structures contained in the cytoplasm of a cell, producing energy in the form of adenosine triphosphate (ATP). Each cell contains hundreds to thousands of mitochondria, depending on the energy requirements of particular tissues. Mitochondrial DNA is made up of 37 genes, which are primarily responsible for maintaining the function of the mitochondria, making up <0.1% of our body's total DNA. Although the genetic contribution of mitochondria is small, the impact when they fail to function is considerable. Mitochondria dysfunction can be due to mutations in either nuclear or mitochondrial DNA sequences. This article focuses on diseases caused by mutations in mitochondrial DNA sequences. As mitochondria are derived through the oocyte (only one case of paternal inheritance of mitochondria DNA has been identified3) disease caused by mutations of mitochondrial DNA display a maternal inheritance pattern. Both sexes can inherit the disease but it is only women who are at risk of transmitting the disease to their children.
Although it is difficult to identify disease prevalence,4 it is estimated that one in 400 people carries a disease causing mitochondrial mutation.5 Mitochondrial disease is extremely variable according to which organs are affected and to what extent, and patients can be mildly, severely or fatally affected. Symptoms can include diabetes, epilepsy, digestive disorders, fatigue, cardiomyopathy, deafness, restricted sight and difficulties with mobility and balance. The term ‘mitochondrial disease’ was introduced in the late 1980s, but it encompasses a range of distinct disorders, including mitochondrial encephalomyopathy, lactic acidosis and stroke like episodes (MELAS), myoclonus epilepsy with RRF (MERRF), Leber's hereditary optic atrophy (LHON) and Leigh syndrome. The distinctions between these classifications and their implications for clinical management are beyond the scope of this paper and are described elsewhere.6–8
Why the controversy?
Mitochondrial donation is a germ-line technology and a change in law was required for it to be used in clinical practice. The techniques, and the scientists involved, have attracted both widespread support and consternation. The work at Newcastle is partly funded by Muscular Dystrophy UK and, based on the submissions to the public appeal for evidence,9 there is widespread support from patients with mitochondrial disease and their families. Indeed the need to listen to and support the wishes of parents and patients was noted in a letter from scientists, including five Nobel Prize winners.10 It also highlighted the importance of acting quickly, where parents ‘should not have to wait for the law to catch up’, now that the technology is available. Another letter written by 40 scientists from 14 different countries11 explained why they supported the techniques:
A positive vote would not only allow affected families to choose to use this new procedure under the care of the globally respected Newcastle team, with proper advice and safeguards; it would also be an international demonstration of how good regulation helps medical science to advance in step with wider society.
The legalization of mitochondrial disease has national and international significance. The process has been open to intense enquiry and mitochondrial donation is one of the most scrutinized techniques in recent history. Three scientific reviews by an expert panel,12 a dialogue exercise to assess public attitudes delivered by Sciencewise,13 a call for evidence on the ethical issues organized by the Nuffield Council on Bioethics,14 a public consultation and government guidance on draft regulations led by the UK Department of Health9,15 and several debates within the Houses of Parliament16,17 have been conducted. This article will explore six issues raised by mitochondrial donation and its debates. These are: concerns about safety; the creation of three-parent babies; the impact on a child's identity; the implications for society; definitions of genetic modification and reproductive choice.
Are the techniques safe?
The question of safety, of course, is a key issue for any new medical technologies, particularly those that involve reproduction. The techniques have been shown to be successful in mice18 and monkeys19 but animal models do not necessarily translate to human subjects.20 The Human Fertilisation and Embryology Authority (HFEA) regulates the use of gametes and embryos in assisted reproduction and research within the UK. At the request of the Department of Health, the HFEA conducted three scientific reviews. A panel of experts reported on, and assessed evidence about the safety and efficacy of mitochondrial replacement techniques. Questions were raised in particular about the role of mitochondrial DNA and the interactions between mitochondrial DNA and nuclear DNA. Two further concerns were raised. The first was whether the embryo was at risk if there was a mismatch between the mtDNA haplotype of the mitochondria donor and that of the intending mother.21,22 Although these concerns were later dismissed by the Chair of the HFEA's expert panel,23 the report acknowledged that there was a lack of research evidence and that licenced clinics could consider haplotype matching as a precaution. The second issue raised was the question of whether some of the faulty mitochondria would remain attached to the nucleus during the process of transfer. The panel concluded that although it is possible that mitochondria ‘carry over’ can occur, this would be such a small percentage that it would be unlikely to be problematic. It also noted that research had progressed during the last few years but further experiments were critical. Subsequently, it was made clear that these experiments did not need to be carried out before legislation.24 The report12 concluded that the techniques were potentially useful for a specific group of people, that is, for women who want to have a genetically related child and who are at risk of having a child with severe mitochondrial disease.
Significantly, the report found that the techniques were ‘not unsafe’. This highlights the difficulties in legislating for cutting edge, yet unproven, reproductive techniques. However, there is a history of related techniques that could be used as evidence. In the USA a procedure involving cytoplasm injection for fertility treatment in older women resulted in the birth of 17 children in 2000.25 Due to safety concerns the Food and Drug Administration (FDA) subsequently withdrew its licence, and it is reported that subsequent attempts in China did not result in any live births.26 It is only now, 15 years later, that a project is underway to monitor the health of the surviving children.27 The Department of Health has recommended that any child born through the new techniques of mitochondrial donation be involved in clinical studies to monitor their current and future health. Whether it is ethically acceptable to test children,28 whether follow-up will extend to future generations and whether incentives will be needed to encourage long-term participation are of course yet to be decided.
Three-parent babies
Relationships produced through donation, and the meanings we give them, are dependent on the legal, social and cultural context. We expect very different relationships between donor and recipient when the donation is blood, for example, to when it involves a living donation from a relative, or when it involves egg or sperm donation. Mitochondrial donation involves the transfer of genetic but not nuclear material, and this has led to uncertainty as to whether it should be regulated as egg donation or as tissue donation. Mitochondria play an important role in many bodily processes, and therefore the genetic contribution of the donor might be significant: there are complex interactions between nuclear DNA and mitochondrial DNA21 and organelles contained in the cytoplasm might introduce epigenetic alterations in nuclear DNA.29 Headlines of ‘three-parent babies’ dominated the debate, as they did more than 10 years ago when the techniques first started to be developed in the UK.30 Although there is a difference between a genetic parent and a social parent, focusing on biology alone suggests that all babies born through these techniques would be tri-parental.31 The Department of Health9 took a different view. Based on the extent of the genetic contribution and the function of the genes involved, it did not accept that the child born through mitochondrial donation would have three parents:
Genetically, the child will, indeed, have DNA from three individuals but all available scientific evidence indicates that the genes contributing to personal characteristics and traits come solely from the nuclear DNA, which will only come from the proposed child's mother and father. The donated mitochondrial DNA will not affect those characteristics. (DoH, 2014 p 15)
Following the change in law32 the debate has to some extent been settled. The relationship between child and donor is now defined as one where there is no legal obligation towards each other. Whether or not the child feels a genetic kinship with the mitochondria donor, knowing something about the donor might still be important, for example, in providing a fuller picture about the context of their conception.14 The legislation provides for this possibility, recommending the child has access to non-identifying information such as screening tests, family health and personal information provided by the donor.
Implications for identity
Alongside questions of parentage, the concept of identity has remained central within the debate. Once again, perspectives primarily differ according to the perceived significance of mitochondrial DNA. For example, if our character and physical appearance is considered to be solely determined by our nuclear genes then as the Department of Health suggested, altering mitochondrial genes might not have a significant impact on the child. But many reject this kind of genetic essentialism. Identity is difficult to define, but it is more than our character and physical traits.33 Reproductive medicine further complicates questions of identity. Being born without mitochondrial disease would, of course, have a significant impact on the child. As Bredenoord et al.34 highlight, ‘a person without a mtDNA disease will have a different life experience, a different biography and perhaps also a different character’. Through embryo selection or modification, many widely accepted reproductive technologies have the potential to alter an individuals' identity. Mitochondrial donation is therefore not necessarily a special case.14
Implications for society
Whereas the discussions about identity and donor–child relationships focus primarily on the personal impact of the techniques, concerns have also been raised about the wider implications for society. Writing in a letter to the Times, 55 Italian Members of Parliament35 urged British MPs to vote against legalisation, drawing attention to the pace of development and the consequences for ‘the whole of humanity’:
The creation of such embryos could have uncontrollable and unforeseeable consequences, affecting future generations, and modifying genetic heritage in an irreversible way, inevitably affecting the human species as a whole. It is a dangerous intervention involving genetic engineering, which affects the whole of humanity, and cannot possibly be contained within the confines of the UK.
Indeed, many of those who have spoken out against mitochondrial donation do so on the basis of personal beliefs. There appeared confusion within the church as to whether there were legitimate grounds to oppose the techniques. The Church of England36 had initially advised caution given the current level of knowledge, suggesting the need for further scientific review. In the House of Lords debate however, it was made clear that ‘despite some misleading headlines’, the Church was not opposed to mitochondrial donation. Although there can be blanket opposition to some aspects of assisted reproduction and embryo research based on the sanctity of human life, in the context of this discussion, one technique is considered particularly problematic. Maternal spindle transfer and pro-nuclear transfer both involve mitochondrial donation, but the latter involves creating and destroying an embryo in the process. As Fiona Bruce, Conservative MP for Congleton, highlighted in the House of Commons debate:
Let me be straightforward: I do oppose these proposals in principle. However, that should not prevent my concerns regarding their safety from being given a fair hearing. One of the two procedures that we are being asked to sanction today—pro-nuclear transfer—involves the deliberate creation and destruction of at least two human embryos, and in practice probably more, to create a third embryo, which it is hoped will be free of human mitochondrial disease. Are we happy to sacrifice two early human lives to make a third life?
Here the techniques are represented as two distinct processes, with one technique being considered unjustifiable. In contrast, the HFEA's reports into the safety of the techniques concluded that as there was not enough evidence of the efficacy of the techniques to suggest one was preferable to the other, both were recommended. This clear tension in perspectives and approaches highlights the complex tangle of ethical, legal and safety issues raised by mitochondrial donation.
Is this genetic modification?
Another area of controversy is the impact of the techniques on the human genome. The Department of Health9 identified that the techniques involved germ-line modification, ‘in that the result of mitochondrial donation – the avoidance of the transmission of a serious mitochondrial disease – will be passed down to future generations’. It concluded that the techniques did not involve genetic modification:
There is no universally agreed definition of ‘genetic modification’ in humans – people who have organ transplants, blood donations or even gene therapy are not generally regarded as being ‘genetically modified’. While there is no universally agreed definition, the Government has decided to adopt a working definition for the purpose of taking forward these regulations. The working definition that we have adopted is that genetic modification involves the germ- line modification of nuclear DNA (in the chromosomes) that can be passed on to future generations. This will be kept under review.
The UK government has strongly defended its decision to use the ‘working definition’ of genetic modification which excludes mitochondrial DNA. The techniques are viewed as replacing faulty mitochondrial genes, while leaving both the nuclear DNA and mitochondrial DNA intact.16 Leading scientists have questioned this position, accusing the government of dishonesty, misleading the public and acting by stealth.37
In anticipation of these concerns, the government and leading supporters have attempted to clearly demarcate the boundaries between mitochondrial donation and nuclear modification. Making clear that this is not genetic modification is politically prudent as it would have been unlikely that the public would accept attempts to approve the modification of the nuclear genome at this stage. Additionally, the legislation specifies who are eligible to use the techniques (women at risk of transmitting mitochondrial disease to their offspring). This would mean that those wishing to use the techniques to enhance fertility38 and lesbian couples who wish to use the techniques so that the child has a genetic contribution from both (one would be mitochondria donor) would not be permitted.
Genetic risk and reproductive choice
Medical innovations are often imagined as a utopia.30 This is the case with mitochondrial donation, which is represented as both a treatment and cure, potentially eliminating severe forms of mitochondrial disease8 and affecting the lives of thousands of women.39,40 The suggestion of technological determinism that if these techniques are available then this will ‘halt’ or ‘eradicate’ the disease is in contrast to the practical difficulties associated with mitochondrial disease. A complex relationship between genotype and phenotype, different mutation ratios in different organs, a wide range of symptoms and levels of severity present considerable difficulties for providing patients with genetic counselling about risk and severity.41–43 It is also in contrast with what we know about how individuals and families make sense of genetic risk in the context of living with disease.44,45
Importantly, severity of disease in offspring cannot be predicted on the basis of the severity of the disease of the mother. One reason put forward for this is the ‘bottleneck theory’.8,44 Put simply, replication of mitochondrial DNA between cells and redistribution during oocyte maturation can lead to extreme differences in levels of mutation. This means that an asymptomatic mother can have a very severely or fatally affected child. Indeed many of the parents whose stories have been the focus of the mitochondrial debate46–48 did not know that they were at risk of having a child with mitochondrial disease, until their child was diagnosed.
Where the risks are known, how individuals make sense of uncertainty and complexity of mitochondrial disease, how they assess the risk of having a child with mitochondrial disease and how they negotiate IVF technologies are important questions. This has been explored in multiple contexts according to different kinds of genetic disease and disease transmission.49–51 In contrast, there has been little reflection about the contexts within which reproductive decisions might be made by those with mitochondrial disease. There are alternatives to mitochondrial donation. However, oocyte donation does not offer the opportunity to have a genetically related child and relies on a supply of donor eggs,52 and prenatal diagnosis and pre-implantation genetic diagnosis would have limited success for those with homoplasmic mitochondrial disease.53 The Rare Mitochondrial Disease Service for Adults and Children, which has centres in Oxford, London and Newcastle, can provide genetic counselling for women at risk of carrying a mitochondria mutation and to assist with reproductive choices.
Widespread support for these techniques was evident in the large number of women who came forward to donate their eggs for this research following a public appeal by Newcastle University. However, here again there is controversy. Donating eggs is a complex process,54 with potential risks for the healthy donor, including ovarian hyperstimulation syndrome. Although the proposed compensation payment for egg donors of £500 is within current UK guidelines, the payment has raised concerns about exploitation.55 The progress of the techniques will rely on a supply of donated eggs, yet the egg donors and their health and safety have primarily been missing from the debate.56
Conclusion
Mitochondrial medicine represents a rapidly changing field with newly emerging tools for diagnosis and risk assessment. Maternal spindle donation and pro-nuclear donation were legalized in March 2015, as techniques that could allow women with mitochondrial disease the opportunity to have healthy, genetically related children. The process of legalization was not smooth nor a foregone conclusion. The wide reaching safety reviews, public dialogues, parliamentary debates and numerous calls for evidence have highlighted the robust nature of regulation in reproductive medicine in the UK. The extensive interest shown by the media, scientists, religious and special interest groups, patients and the general public demonstrate the strength of feeling in support of, and against, the introduction of these techniques and highlight the considerable challenges for policy-makers. Within the debate it is difficult to identify areas of agreement and many of the key elements have attracted equally strong supporters and detractors. The science is complicated, and there were disagreements about the function of mitochondria, its interactions with nuclear DNA and the health implications of mitochondrial donation. There were also disagreements about what definitions to use, what level of evidence is appropriate, what risks are appropriate to take and who should be protected.
Important conclusions have been drawn. The HFEA, in assessing safety and efficacy, concluded that the techniques were ‘not unsafe’. The use of language is interesting but for many was unsatisfactory. The ultimate test for proving whether any novel IVF techniques work is through human application, and this involves accepting that there will be risks. The Department of Health concluded that there was no significant genetic relationship between the child and donor. These are important pronouncements which will have a lasting impact on how society views the technology, and on patients and their families.
The techniques and surrounding debates have revealed considerable gaps between the capabilities of health technologies and clinical application. Mitochondrial donation has challenged our understanding of the symbolic importance of genetic material. Attitudes often differed about the implications of the genetic contribution. For those who believe the genetic contribution is important and suggests a genetic relationship between child and donor, mitochondrial donation appears similar to egg or sperm donation. For those who minimise the genetic contribution, mitochondrial donation is considered more like tissue/organ donation, which leads to very different conclusions about the relationship between the child and donor, and the potential to affect identity. Questions remain about how potential parents will make sense of reproductive risk in the context of complex and uncertain biomedical knowledge. Questions also remain about how individuals, families and wider society will respond to these technological solutions. As with many innovative medical technologies, the movement of mitochondrial donation from bench to bedside has and will continue to rely heavily on pioneering female patients who are willing to accept the risks.57 Being conceived through IVF or having three parents (for example, through step-families or adoption) is not unusual in our society. As Scully58 highlights, it is how society and families respond to mitochondrial donation as a ‘new kind of normal’ that will be important.
Acknowledgements
Dr Rebecca Dimond is funded by an ESRC future research leaders grant (ESRC ref 504751). The author thank Mary Herbert and Mahdi Lamb, Wellcome Trust Centre for Mitochondrial Research, who kindly gave permission to use the image.
References
- 1.Times. Britain is first to legalise three-parent IVF. 25 February 2015.
- 2.Hargreaves LP. Coenzyme Q10 as a therapy for mitochondrial disease. Int J Biochem Cell Biol 2014;49:105–11. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz M, Vissing J. Paternal inheritance of mitochondrial DNA. New Engl J Med 2002;347:576–80. [DOI] [PubMed] [Google Scholar]
- 4.Schaefer AM, McFarland R, Blakely EL et al. . Prevalence of mitochondrial DNA disease in adults. Ann Neurol 2008;63:35–9. [DOI] [PubMed] [Google Scholar]
- 5.Manwaring N, Jones MM, Wang JJ et al. . Population prevalence of the MELAS A3243G mutation. Mitochondrion 2007;7:230–3. [DOI] [PubMed] [Google Scholar]
- 6.DiMauro S. A history of mitochondrial diseases. J Inherit Metab Dis 2011;34:261–76. [DOI] [PubMed] [Google Scholar]
- 7.Kisler JE, Whittaker RG, McFarland R. Mitochondrial diseases in childhood: a clinical approach to investigation and management. Dev Med Child Neurol 2010;52:422–33. [DOI] [PubMed] [Google Scholar]
- 8.Chinnery PF, Hudson G. Mitochondrial genetics. Br Med Bull 2013;106:135–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Department of Health. Mitochondrial Donation: Government response to the consultation on draft regulations to permit the use of new treatment techniques to prevent the transmission of a serious mitochondrial disease from mother to child 22 July 2014. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/332881/Consultation_response.pdf (21 March 2015, date last accessed).
- 10.Sulston J, Deech R, Warnock M et al. . Three-person IVF. The Times letter. 28 January 2015 http://www.thetimes.co.uk/tto/opinion/letters/article4337474.ece (22 March 2015, date last accessed).
- 11.Carroll J, Christodoulou J, Egli D et al. . Parliament should approve regulations for mitochondrial donation. Guardian. 30 January 2015 http://www.theguardian.com/science/2015/jan/30/parliament-should-approve-regulations-for-mitochondrial-donation (22 March 2015, date last accessed).
- 12.HFEA. Third scientific review of the safety and efficacy of methods to avoid mitochondrial disease through assisted conception: update 2014. http://www.hfea.gov.uk/8807.html (21 March 2015, date last accessed).
- 13.HFEA. Mitochondria replacement consultation: Advice to Government. March 2013. http://www.hfea.gov.uk/docs/HFEA_Authority_meeting_March_2013_-_Mitchondria_report.pdf (21 March 2015, date last accessed).
- 14.Nuffield Council on Bioethics. Novel techniques for the prevention of mitochondrial DNA disorders: an ethical Review. London: Nuffield Council on Bioethics; 2012. http://www.nuffieldbioethics.org/mitochondrial-dna-disorders (21 March 2015, date last accessed). [Google Scholar]
- 15.Department of Health Mitochondrial Donation: A consultation on draft regulations to permit the use of new treatment techniques to prevent the transmission of a serious mitochondrial disease from mother to child. 27 February 2014 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/285251/mitochondrial_donation_consultation_document_24_02_14_Accessible_V0.4.pdf (21 March 2015, date last accessed).
- 16.Houses of Commons debate 3 February 2015. http://www.publications.parliament.uk/pa/cm201415/cmhansrd/cm150203/debtext/150203-0002.htm (23 March 2015, date last accessed).
- 17.House of Lords debate 24 February 2015. http://www.publications.parliament.uk/pa/ld201415/ldhansrd/text/150224-0002.htm (23 March 2015, date last accessed).
- 18.Sato A, Kono T, Nakada K et al. . Gene therapy for progeny of mito-mice carrying pathogenic mtDNA by nuclear transplantation. Proc Natl Acad Sci USA 2005;102:16765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tachibana M, Sparman M, Sritanaudomchai H et al. . Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 2009;461:367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis J, Atkinson P, Harrington J et al. . Representation and practical accomplishment in the laboratory: when is an animal model good-enough? Sociology 2013;47:776–92. [Google Scholar]
- 21.Reinhardt K, Dowling DK, Morrow EH. Mitochondrial replacement, evolution, and the clinic. Science 2013;341:1345–6. [DOI] [PubMed] [Google Scholar]
- 22.Burgstaller JP, Johnston IG, Jones NS et al. . mtDNA segregation in heteroplasmic tissues is common in vivo and modulated by haplotype differences and developmental stage. Cell Rep 2014;6:2031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenfield A. HFEA panel on mitochondrial replacement considered all submissions. Guardian 24 July 2014.
- 24.House of Commons Science and Technology Committee Oral Evidence: Mitochondrial Donation, HC 730 22 October 2014. http://www.parliament.uk/business/committees/committees-a-z/commons-select/science-and-technology-committee/inquiries/parliament-2010/mitochondrial-donation/ (25 March 2015, date last accessed).
- 25.Connor S. Three parent babies: ‘As long as she's healthy, I don't care’, says mother of IVF child. Independent 25 August 2013 Accessed 17/11/2014.
- 26.Check E. UK embryo licence draws global attention. Nature 2015;437:305–305. [DOI] [PubMed] [Google Scholar]
- 27.Connor S. Medical Dilemma of ‘three parent babies’: Fertility clinic investigates health of teenagers it helped to be conceived through controversial IVF technique. Independent 25 August 2014 Accessed 27/10/2014.
- 28.Bredenoord A, Braude P. Ethics of mitochondrial gene replacement: from bench to bedside. BMJ 2013;342:87–90. [DOI] [PubMed] [Google Scholar]
- 29.Chiaratti MR, Meirelles FV, Wells D et al. . Therapeutic treatments of mtDNA diseases at the earliest stages of human development. Mitochondrion 2011;11:820–8. [DOI] [PubMed] [Google Scholar]
- 30.Haran J, Kitzinger J, McNeil M et al. . Human Cloning in the Media. Oxon: Routledge, 2007. [Google Scholar]
- 31.Cohen J, Alikani M. The biological basis for defining bi-parental or tri-parental origin of offspring from cytoplasmic and spindle transfer. Reprod Biomed Online 2013;26:535–7. [DOI] [PubMed] [Google Scholar]
- 32.The Human Fertilisation and Embryology (Mitochondrial Donation) Regulations 2015 (SI 2015/572). http://www.legislation.gov.uk/uksi/2015/572/contents/made?page=5 (26 March 2015, date last accessed).
- 33.Baylis F. The ethics of creating children with three genetic parents. Reprod Biomed Online 2013;26:531–4. [DOI] [PubMed] [Google Scholar]
- 34.Bredenoord AL, Dondorp W, Pennings G et al. . Ethics of modifying the mitochondrial genome. J Med Ethics 2011;37:97–100. [DOI] [PubMed] [Google Scholar]
- 35.Roccella E, Buttiglione R, Picchi G et al. . Three-person DNA. The Times letter February 20th 2015 http://www.thetimes.co.uk/tto/opinion/letters/article4360729.ece (22 March 2015, date last accessed).
- 36.Knapton S. Three parent baby law is ‘irresponsible’ says Church of England ahead of vote 29 Jan 2015 http://www.telegraph.co.uk/news/science/11377992/Three-parent-baby-law-is-irresponsible-says-Church-of-England-ahead-of-vote.html (22 March 2015, date last accessed).
- 37.Connor S. 2014. Exclusive: Scientists accuse government of dishonesty over GM babies in its regulation of new IVF technique. The Independent 28 July.
- 38.Smyth C. Allow three-parent IVF to help older women too, says pioneer. The Times 9 Feb 2015 http://www.thetimes.co.uk/tto/health/news/article4348194.ece (25 March 2015, date last accessed).
- 39.Gallagher J. Thousands ‘need three-person babies’ BBC news website 29 January 2015. http://www.bbc.co.uk/news/health-31017999 (26 March 2015, date last accessed).
- 40.Gorman GS, Grady JP, Ng Y et al. . Mitochondrial donation: how many women could benefit?, N Engl J Med 2015;372:885–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bredenoord AL, Krumeich A, De Vries MC et al. . Reproductive decision-making in the context of mitochondrial DNA disorders: views and experiences of professionals. Clin Genet 2010;77:10–7. [DOI] [PubMed] [Google Scholar]
- 42.Brown DT, Herbert M, Lamb VK et al. . Transmission of mitochondrial DNA disorders: possibilities for the future. Lancet 2006;368:87–9. [DOI] [PubMed] [Google Scholar]
- 43.Poulton J, Chiaratti MR, Meirelles FV et al. . Transmission of mitochondrial DNA diseases and ways to prevent them. Plos Genet 2010;6:e1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsons E, Atkinson P. Lay constructions of genetic risk. Sociol Health Illn 1992;14:437–55. [Google Scholar]
- 45.Dimond R. Patient and family trajectories of mitochondrial disease: diversity, uncertainty and genetic risk. Life Sciences, Society and Policy 2013;9:1–11. [Google Scholar]
- 46.McVeigh K, Sample I. Parents call on MPs to back technique to end agony of rare childhood disease’ Guardian 22/10/2014.
- 47.BBC Radio 4 Today, Should ‘three parent IVF’ be allowed? Broadcast 11th March 2011. http://news.bbc.co.uk/today/hi/today/newsid_9421000/9421873.stm.
- 48.Driscoll M. Why we want the first three-parent baby. The Sunday Times. 1 February 2015 http://www.thesundaytimes.co.uk/sto/news/focus/article1514067.ece (22 March 2015, date last accessed).
- 49.Decruyenaere M, Evers-Kiebooms G, Boogaerts A et al. . The complexity of reproductive decision-making in asymptomatic carriers of the Huntington mutation. Eur J Hum Genet 2007;15:453–62. [DOI] [PubMed] [Google Scholar]
- 50.Myring J, Beckett W, Jassi R et al. . Shock, adjust, decide: reproductive decision making in cystic fibrosis (CF) carrier couples--a qualitative study. J Genet Couns 2011;20:404–17. [DOI] [PubMed] [Google Scholar]
- 51.Ormondroyd E, Donnelly L, Moynihan C et al. . Attitudes to reproductive genetic testing in women who had a positive BRCA test before having children: a qualitative analysis. Eur J Hum Genet 2012;20:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poulton J, Oakeshott P. Nuclear transfer to prevent maternal transmission of mitochondrial DNA disease. BMJ 2012;345:e6651. [DOI] [PubMed] [Google Scholar]
- 53.Nesbitt V, Alston CL, Blakely EL et al. . A national perspective on prenatal testing for mitochondrial disease. Eur J Hum Genet 2014;22:1255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haimes E. Juggling on a rollercoaster? Gains, loss and uncertainties in IVF patients’ accounts of volunteering for a UK ‘egg sharing for research'scheme. Soc Sci Med 2013;86:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knapton S. 2015 Three parent babies: women paid £500 to become ‘second mothers’. Telegraph. 24 Feburary.
- 56.Dickenson DL. The commercialization of human eggs in mitochondrial replacement research. New Bioeth 2013;19:18–29. [DOI] [PubMed] [Google Scholar]
- 57.Webster A. Innovative genetic technologies, governance and social accountability. In: Atkinson P, Glasner P, Lock M (eds). Handbook of Genetics & Society: Mapping the New Genomic Era. New York: Routledge, 2009. [Google Scholar]
- 58.Scully JL. p72 Nuffield Council on Bioethics (2012) Novel techniques for the prevention of mitochondrial DNA disorders: an ethical Review. London: Nuffield Council on Bioethics; http://www.nuffieldbioethics.org/mitochondrial-dna-disorders (21 March 2015, date last accessed). [Google Scholar]