Abstract
Background
Older human immunodeficiency virus (HIV)-1 transgenic rats are a model for HIV-1 associated neurocognitive disorders (HAND). They show behavioral changes, neuroinflammation, neuronal loss, and increased brain arachidonic acid (AA) enzymes. Aspirin (acetylsalicylate, ASA) inhibits AA oxidation by cyclooxygenase (COX)-1 and COX-2.
Hypothesis
Chronic low-dose ASA will downregulate brain AA metabolism in HIV-1 transgenic rats.
Methods
Nine month-old HIV-1 transgenic and wildtype rats were given 42 days of 10 mg/kg/day ASA or nothing in drinking water; eicosanoids were measured using ELISAs on microwaved brain extracts.
Results
Brain 15-epi-lipoxin A4 and 8-isoprostane concentrations were significantly higher in HIV-1 transgenic than wildtype rats; these differences were prevented by ASA. ASA reduced prostaglandin E2 and leukotriene B4 concentrations in HIV-1 Tg but not wildtype rats. Thromboxane B2, 15-HETE, lipoxin A4 and resolvin D1 concentrations were unaffected by genotype or treatment.
Conclusion
Chronic low-dose ASA reduces AA-metabolite markers of neuroinflammation and oxidative stress in a rat model for HAND.
Keywords: HIV-1, transgenic, PGE2, 15-epi-lipoxin A4, 8-isoprostane, brain, chronic aspirin, rat, low dose, neuroinflammation, HAND
Introduction
Human immunodeficiency virus (HIV)-1-infected patients are at risk of developing HIV-1 associated neurocognitive disorders (HAND) [1, 2]. These disorders progress over time, ranking from mild neurocognitive impairment to HIV-1-associated dementia, which involves severe cognitive dysfunction in multiple domains [3]. The introduction of antiretroviral therapy (ART) has reduced the prevalence of dementia. However, as the lifespan of HIV-1 infected patients has been prolonged by ART, the prevalence of HAND with aging remains high [4].
HAND probably arises from direct and maintained viral invasion of the central nervous system (CNS), which is an important reservoir for HIV-1 virus regardless of plasma viral suppression or cumulative time on ART. In the North-East AIDS Dementia consortium, over 50% of viremically controlled HAND patients had detectable virus in the cerebrospinal fluid (CSF) [5]. Aberrant macrophage and T-lymphocyte activation in the CSF continued despite viremic control by ART [6], and brain atrophy correlated with the CSF level of quinolinic acid, evidence of activated CNS macrophages and microglia associated with neuroinflammation and excitotoxicity [7].
HIV-1 infection of the brain stimulates both the innate and adaptive immune systems.. Brain damage and neuronal loss are associated with activation of microglia, astrocytes and invasive macrophages, which release toxic quantities of agents such as nitric oxide, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and interleukin (Il)-1β [8]. Additionally, preclinical evidence indicates that there is secondary activation of phospholipases A2 (PLA2) that release of the n−6 polyunsaturated fatty acid arachidonic acid (AA) from membrane phospholipids [9]. AA and many of its eicosanoid products are proinflammatory. They include prostaglandin (PG)E2 formed preferentially by cyclooxygenase (COX)-2, thromboxane (TX)B2 formed preferentially by COX-1, 15 (S)-HETE formed by 15-lipoxygenase (LOX), lipoxin (LX)A4 formed from 15-HETE by 5-LOX [10], leukotriene (LT)B4 also derived from AA by 5-LOX [11], 15-epi-LXA4 synthesized from 15 (R)-HETE by acetylated COX-2 [12, 13], and isoprostanes produced by non-enzymatic pathways [14, 15]. COX-2 expression is increased following injection of HIV-1 Tat or gp-120 proteins into rodent brain, and in other HIV-1 rodent models [16–20].
Evidence for neuroinflammation in HIV-1 patients, and the recognized relation between neuroinflammation and upregulated AA metabolism in animal HIV-1 models and human neurodegenerative disease [21–23], suggest that antiinflammatory drugs that target the brain AA cascade might be of clinical relevance. One such drug is aspirin (ASA, acetylsalicylic acid), whose antiinflammatory actions in peripheral inflammation are well described [24, 25]. ASA inhibits COX-1, which converts AA to TXB2, while acetylating and inhibiting COX-2, which converts AA to PGE2 and also becomes capable of converting docosahexaenoic acid (DHA) to antiinflammatory 17R-hydroxy-containing di- and tri-hydroxy-docosanoids termed resolvins [26]. Evidence in animals and humans indicates that even low dose aspirin can exert behavioral and other biological effects in the intact CNS [27–32].
To provide a basis for testing potential efficacy of ASA in HIV-1 patients with HAND, we examined in the present study the effect of low-dose ASA on brain AA and DHA metabolism in a transgenic (Tg) rodent model of HIV-1, and in control rats. The human HIV-1 provirus, carrying seven out of the nine HIV-1 genes after functional deletion of the infectious genes Gag and Pol is constitutively expressed by the HIV-1 Tg rat. The HIV-1 Tg rat develops neuropathology as it ages, thus may be an animal model for HAND [33, 34]. It shows reduced spatial learning at 5 months of age. At 7–9 months, neuroinflammation and synaptic loss occur, associated with increased expression of AA-metabolizing cytosolic cPLA2 IVA, secretory sPLA2 IIA and COX-2 [19] and changes in fatty acid composition [35].
We treated 9-month old HIV-1 Tg and wildtype rats with 10 mg/kg/day ASA in drinking water for 42 days, using ASA-free water as a control. This ASA regimen is equivalent to a low therapeutic dose of 100 mg in human (for a 70 kg subject) [36–38]. We used ELISA assays to measure brain concentrations of PGE2, thromboxane TXB2, leukotriene LTB4, lipoxin LXA4, 15-epi-LXA4, 15-hydroxyeicosatetraenoic acid (HETE), 8-isoprostane and resolvin D1.
Materials and Methods
Chemicals
ASA was purchased from Sigma-Aldrich (Saint Louis, MO). Hexane and isopropanol (Reagent Grade) were obtained from Fisher Scientific (Pittsburgh, PA). Ultra-pure water was purchased from KD Medical (Columbia, MD).
Animals
The experiments were conducted under an approved NICHD animal protocol (12–027) in accordance with the NIH Guidelines on the Care and Use of Laboratory Animals. Age-matched male HIV-1 Tg or Fischer 344/NHsd wildtype rats (9 months-old), purchased from Harlan Laboratories (Madison, WI) were housed in an animal facility under a 12 h/12 h light–dark cycle with ad libitum access to water and an identical Teklad global 18% protein 2018S diet. The diet was 2018S (sterilized) for controls and 2918 (irradiated 2018S) for HIV-1 Tg rats (Teklad Harlan, Madison, WI). The diets were processed in identical ways, except that the 2018S diet was further gamma irradiated to minimize the risk of infection in the HIV-1 Tg colony. The diet contained (as % of total fatty acid) 16.7% saturated, 21.8% monounsaturated, 54.8% linoleic acid, 6.2% α-linolenic acid, 0.03% AA, 0.02% eicosapentaenoic (EPA, 20:5 n−3) and 0.06% docosahexaenoic acid (DHA, 22:6 n−3) [35]. After 42 days on the diet, the rats were anesthetized with Nembutal (40 mg/kg, i.p.), and subjected to head-focused microwave irradiation at 5.5 kW for 3.4 sec (Cober Electronics, Stamford, CT). The brain was immediately removed, placed on dry ice, and stored at −80 °C.
ASA treatment
Before going on the diet, rats were separated into four different groups (n = 12): untreated wildtype, untreated HIV-1 Tg, ASA-treated wildtype, and ASA-treated HIV-1 Tg. Untreated groups received regular drinking water. The ASA-treated groups received ASA in water (10 mg/kg/day) for 42 days. Fresh ASA drinking water was prepared and provided every two days, as was control drinking water. Rat weight and water intake were monitored weekly. According to an interspecies conversion factor based on body surface, the 10 mg/kg/day dose used here was equivalent to a dose of 100 mg for a 70 kg person [36].
Sample preparation
Extraction of oxygenated metabolites of AA and DHA was performed according to the Radin method [39]. Half-brains were homogenized in a glass Tenbroeck homogenizer in hexane-isopropanol (3:2 v:v, 18 ml/g brain). The homogenate was transferred to a glass centrifuge tube and the homogenizer was washed twice with 4 volumes of hexane-isopropanol solution. The pooled homogenate was centrifuged at 1500 rpm for 5 min at room temperature, and the organic supernatant was collected. The pellet was re-extracted twice in 5 ml hexane-isopropanol. The pooled extracts were dried under N2 at 45 °C, resuspended in 3 ml hexane-isopropanol and stored at −80 °C.
Measurement of brain eicosanoids and docosanoids by enzyme immunoassay
To perform an enzyme immunoassay, 1 ml of the sample in hexane-isopropanol was dried under N2 and resuspended in 500 µl buffer. Concentrations of eicosanoids or docosanoids were determined with commercially available ELISA kits in accordance with the manufacturer’s instructions. PGE2 and 15-epi-LXA4 kits were obtained from Oxford Biochemical (Oxford, MI) and TXB2, LTB4, 15-HETE, LXA4, 8-isoprostane and Resolvin D1 kits were from Cayman Chemicals (Ann Arbor, MI).
Statistical analyses
All data are expressed as mean ± SEM (n = 10–12 per group). In some groups, an outlier was identified using Grubbs’ test, and removed from the data set (as indicated in the figure legend). For 8-isoprostane quantification, n = 10 for controls and n = 11 for HIV-1 Tg rats due to sample shortage. A two-way ANOVA was performed to identify global effects of genotype and treatment (GraphPad Prism 5.0, GraphPad Software, La Jolla, CA), and was followed by Least Significant Difference (LSD) post-hoc tests for multiple comparison. The level of significance was set at p < 0.05.
Results
Body weight
There was a significant main effect of genotype on body weight. HIV-1 Tg rats weighed significantly less than wildtype controls (−20%, p < 0.001). No effect of ASA treatment was observed.
Brain Metabolites
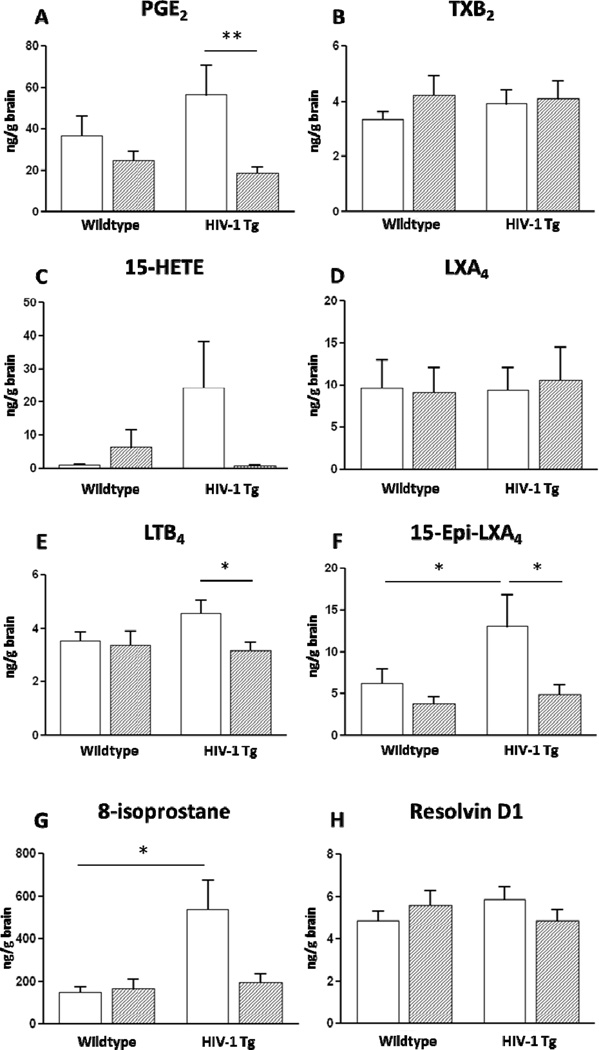
The brain concentrations of AA-derived metabolites and Resolvin D1 in HIV-1 Tg and wildtype rats are shown Figure 1.
Figure 1.
Effects of HIV-1 genotype and low dose aspirin (ASA) on concentrations of PGE2, TXB2, 15-HETE, LXA4, LTB4, 15-epi-LXA4, 8-isoprostane, and Resolvin D1 in microwaved rat brain. Data are means ± SEM (n=10–12) and were analyzed using a two-way ANOVA followed by LSD post-hoc test. * p < 0.05, ** p < 0.01. Some outliers were identified using the Grubbs’ test, therefore n = 11 for PGE2: wildtype ASA and HIV-1 ASA, LXA4: wildtype water and HIV-1 ASA, LTB4: HIV-1 ASA, epi-LXA4: wildtype water, wildtype ASA and HIV-1 ASA, Resolvin D1: HIV-1 ASA. Variability of the 15-HETE assay was high, with outliers removed from the four groups.
PGE2 – A two-way ANOVA showed a significant effect of ASA on brain PGE2 levels (p = 0.009), but no effect of genotype. Post-hoc analysis by an LSD test indicated that the brain PGE2 concentration was significantly lowered by ASA treatment in HIV-1 Tg rats, compared to nontreated HIV-1 Tg rats (−66%, p = 0.006).
TXB2 – There was no effect of genotype or ASA treatment on brain TXB2 concentration.
15-HETE – There was no significant effect of genotype or ASA treatment on brain 15-HETE concentration.
12-HETE – 12 HETE levels in most samples were below the limit of detection (196 pg/mL).
Statistical comparisons therefore were not performed.
LXA4 – There was no effect of genotype or ASA treatment on brain LXA4 concentration.
LTB4 – Brain LTB4 concentration did not significantly differ between untreated HIV-1 Tg and wildtype rats. However, a post-hoc test showed that LTB4 concentration was significantly lower in HIV-1 Tg rats that received ASA, compared to untreated animals (−29%, p = 0.03).
15-epi-LXA4 – A two-way ANOVA showed a significant effect of ASA on brain 15-epi-LXA4 levels (p = 0.02), but no effect of genotype. Post-hoc analysis by LSD test showed that 15-epi-LXA4 concentration was significantly (2.1. fold) higher in HIV-1 Tg compared to wildtype rats (p = 0.04). This increase was prevented by ASA treatment in HIV-1 Tg rats, compared to non-treated (−62%, p = 0.01).
8-Isoprostane – Two-way ANOVA showed a significant effect of HIV-1 Tg genotype (p = 0.01) on 8-isoprostane concentration, as well as a significant effect of ASA treatment (p = 0.04). There also was a significant HIV-1 genotype × ASA interaction (p = 0.02). Subsequent post-hoc tests indicated that the levels of 8-isoprostane in untreated HIV-1 Tg rats were significantly increased (p = 0.02) compared to levels in the wildtype rats. ASA treatment resulted in a lower 8-isoprostane level in HIV-1 Tg compared to wildtype, which approached statistical significance (p = 0.06).
Resolvin D1 – We found no significant effect of genotype or ASA treatment on brain resolvin D1 concentration.
Discussion
Whole brain 15-epi-LXA4 and 8-isoprostane concentrations were higher in 9 month-old HIV-Tg rats than in wildtype controls that had been administered drinking water for an additional 42 days. In contrast, treatment with low-equivalent dose ASA for 42 days significantly reduced the increases in 15-epi-LXA4 and 8-isoprostane concentrations seen in the HIV-1 Tg compared to wildtype rats. Chronic ASA compared with aspirin also reduced brain PGE2 and LTB4 concentrations in HIV-1 Tg rats, but not in wildtype controls. In comparison, ASA treatment did not change the level of any other measured AA-derived eicosanoid in wildtype rats.
ASA directly inhibits COX-1 and limits COX-2 activity via acetylation, and therefore reduces the production of AA-derived prostaglandins [13, 24–26]. COX-1 and COX-2 catalyze the production of PGH2 from AA, which subsequently is converted to PGE2 by PG synthase or to TXB2 by TX synthase [40]. Our results showed a significant global effect of ASA on brain PGE2 concentration, particularly in HIV-1 Tg rats, in which ASA treatment reduced PGE2 concentration by 66%. This difference may correspond to upregulation of COX-2 in the brain HIV-1 Tg rats, whereas COX-1 is unchanged [19]. It suggests that ASA is more effective in a system where inflammatory processes are already activated. The ability of low-dose ASA to reduce the overproduction of brain PGE2 confirms that low dose ASA has a central effect when administered peripherally, and agrees with prior preclinical and clinical evidence of a central action [27–32]. ASA crosses the blood-brain barrier and can directly target central PG synthesis, although the effective dose reaching the brain might increase if barrier integrity is altered by pathology [41, 42].
TXB2 is measured as a marker for unstable TXA2, which has potent vasoconstrictor function and facilitates platelet aggregation. ASA treatment inhibits TXA2 synthesis in blood at doses as low as 40 mg/day in humans [43, 44], and is recommended in the prevention of cardiovascular complications. In this study, however, the brain TXB2 level was not reduced by ASA.
AA can be converted to 15 (S)-HETE by 15-LOX, which is elevated in brain from 9-month HIV-1 Tg compared to wildtype rats [19]. Although its role in brain remains to be explored, 15-HETE has been detected in different brain regions [45] and shown to trigger constriction in piglet cerebral arterioles [46]. In this study, however, the concentration of 15 (S)-HETE was unchanged by the HIV-1 genotype or by ASA treatment. The synthesis of the stereoisomer 15 (R)-HETE is triggered by ASA via acetylation of COX-2 [25]. 15 (R)-HETE was not measured in this study because an ELISA assay method is not available to specifically determine its level.
Lower levels of 15-HETE were detected compared to levels of PGE2. This might be explained by the use of commercial kits and possible difference in extraction efficiency depending on the metabolites. Additionally, negative interference may have affected detection of 15-HETE in the assay. Negative interference is associated with the composition of the sample and can arise from cross-reactants, heterophilic antibodies, or endogenous interferers [47]. Since our extraction process for lipid mediators removed proteins, the negative interference could have been caused by reduced binding of 15-HETE or lowered binding affinity of 15-HETE to its specific antibodies by interfering substances.
5-LOX is involved in the synthesis of LXA4 from 15-HETE, or via the production of LTA4 from 5-HETE [10]. LXA4 is a mediator in the resolution of inflammation in various animal models, including stroke [48, 49], and also was increased in cultured HIV-1 infected monocytes and astroglia [50]. The brain LXA4 level was not impacted by the HIV-1 genotype or by ASA treatment in the present study.
The brain 15-epi-LXA4 concentration was significantly higher in untreated HIV-1 Tg rats than in wildtype controls, but this effect was absent following ASA treatment. 15-epi-LXA4 is an ASA-triggered anti-inflammatory AA-derived mediator that is synthesized from 15 (R)-HETE by acetylated COX-2 [12, 13]. Increased 15-epi-LXA4 concentration may reflect the brain response to increased pro-inflammatory eicosanoid production in HIV-1 Tg rats.
Brain LTB4 was lowered by ASA in HIV-1 Tg rats. Derived from AA conversion by 5-LOX. LTB4 is involved in pro-inflammatory signaling by promoting vascular permeability and leukocyte adhesion and activation [11]. LTB4 also has been implicated in chronic inflammatory diseases such as asthma [51], rheumatoid arthritis [52] and irritable bowel disease [52].
The concentration of the PGF2-like compound 8-isoprostane was significantly higher in HIV-1 Tg compared to wildtype brain, but the elevated 8-isoprostane level was reduced to a control level by ASA, suggesting that low-dose ASA reduced oxidative stress in the pathological brain. 8-Isoprostane is produced by non-enzymatic peroxidation of AA by free radicals and has been used as a marker of oxidative stress [53, 54]. Accumulation of free radicals and redox imbalance were observed in brain tissue from HIV-1 infected patients who died with dementia [55, 56]. The 8-isoprostane level also was increased by the HIV-1 tat protein in microglial cell culture [57]. F2-isoprostanes have been reported in CSF of patients with Alzheimer and other neuroinflammatory diseases [54, 58], and might be examined in CSF of HIV-1 patients.
Changes in brain AA-derived eicosanoid concentrations occurred despite the reported lack of change in brain esterified and unesterified AA concentrations in the HIV-1 Tg rat model [35]. These results are not inconsistent, since eicosanoid levels are 3 to 4 orders of magnitude lower than their AA substrate concentration, about 12000 nmol/g (25000 times the concentration of the most abundant eicosanoid detected, 8-isoprostane. Furthermore, a 3.6 fold increase in 8-isoprostane concentration in the HIV-1 Tg group would result in a < 0.5% change in brain AA concentration, which would not be detected using the gas-chromatography method [35].
Resolvin D1 is DHA-derived metabolite that is a precursor to 10(R), 17(S) hydroxyl-DHA, which has potent anti-inflammatory properties in brain [59, 60]. Neither ASA nor genotype affected the concentration of resolvin D1. According to the manufacturer, the kit that we used is selective for 17 (S)-resolvin D1 isomer. However, ASA can trigger the synthesis of protective 17-(R)-resolvin D1 in response to inflammatory stress [26] and thus it would be important in the future to quantify this isomer using LC-MS-MS.
A limitation of this study is that we used Fisher 344/NHsd controls from which the HIV-1 line was derived, instead of littermate controls. We did this to be consistent with our previous study on HIV-1 Tg rats [35]. We cannot rule out possible confounding effects of genetic drift in the Fisher 344/NHsd line, but we consider this unlikely. At an extreme, the limitation may affect our interpretation of whether HIV-1 Tg rats have higher eicosanoid concentrations than control littermates specifically, but it would not alter our conclusions with regard to ASA, which significantly reduced several eicosanoid concentrations in the HIV-1 Tg rats.
In summary, chronic low dose human equivalent ASA blocked the increases in AA-derived LTB4 and 8-isoprostane concentrations that were produced in 9-month old HIV-1 Tg compared with wildtype rats, which show demonstrable pathology, behavioral changes and upregulated expression of AA metabolizing enzymes, including cPLA2, sPLA2 and COX-2. ASA also reduced 15 (S)-HETE and PGE2 concentrations in HIV-1 Tg rats. Thus, treatment with low dose ASA may dampen brain inflammation associated with upregulated brain AA metabolism and potentially contributing to the presence and progression of HAND. ASA in HIV-1 patients is well tolerated, and chronic low dose ASA is recommended for reducing cardiovascular events in HIV-1 patients [61, 62], although only one in five HIV-1 patients are actually taking ASA [61, 62]. In this regard, other nonsteroidal inhibitors of COX-2 have been shown to be beneficial on peripheral markers of disease severity in HIV-1 patients [63–65].
Even treated HIV-1 patients can develop neurocognitive disorders.
We studied a transgenic rat model of HIV-1, free of infectious Gag or Pol genes.
The model had high brain 15-epi-lipoxin A4 and 8-isoprostane concentrations.
These and other brain changes were prevented by chronic low-dose aspirin.
Low-dose aspirin enters brain and might be used to treat HIV-1 neuroinflammation.
Acknowledgements
The authors thank Lisa Chang and Mei Chen for skillful technical assistance. The research was supported by the Intramural Research Program of the National Institute on Aging.
Abbreviations
- AA
arachidonic acid
- ART
antiretroviral therapy
- ASA
acetylsalicylic acid
- CNS
central nervous system
- COX
cyclooxygenase
- CSF
cerebrospinal fluid
- DHA
docosahexaenoic acid
- HAND
HIV-1 associated neurocognitive disorders
- HETE
hydroxyeicosatetraenoic acid
- HIV
human immunodeficiency virus
- LOX
lipoxygenase
- LT
leukotriene
- LX
lipoxin
- PG
prostaglandin
- PLA
phospholipase
- TX
thromboxane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 2.Letendre S, Ellis RJ. Neurologic complications of HIV disease and their treatments. Top HIV Med. 2006;14:21–26. [PubMed] [Google Scholar]
- 3.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature reviews. Immunology. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 4.Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, Schifitto G, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Epstein LG, Marder K. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 6.Dhasmana DJ, Dheda K, Ravn P, Wilkinson RJ, Meintjes G. Immune reconstitution inflammatory syndrome in HIV-infected patients receiving antiretroviral therapy : pathogenesis, clinical manifestations and management. Drugs. 2008;68:191–208. doi: 10.2165/00003495-200868020-00004. [DOI] [PubMed] [Google Scholar]
- 7.Heyes MP, Ellis RJ, Ryan L, Childers ME, Grant I, Wolfson T, Archibald S, Jernigan TL. Elevated cerebrospinal fluid quinolinic acid levels are associated with region-specific cerebral volume loss in HIV infection. Brain : a journal of neurology. 2001;124:1033–1042. doi: 10.1093/brain/124.5.1033. [DOI] [PubMed] [Google Scholar]
- 8.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. Journal of acquired immune deficiency syndromes. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller WE, Pergande G, Ushijima H, Schleger C, Kelve M, Perovic S. Neurotoxicity in rat cortical cells caused by N-methyl-D-aspartate (NMDA) and gp120 of HIV-1: induction and pharmacological intervention. Prog Mol Subcell Biol. 1996;16:44–57. doi: 10.1007/978-3-642-79850-4_3. [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell-cell interactions or a therapeutic opportunity? Prostaglandins. 1997;53:107–137. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- 11.Bray MA, Cunningham FM, Ford-Hutchinson AW, Smith MJ. Leukotriene B4: a mediator of vascular permeability. Br J Pharmacol. 1981;72:483–486. doi: 10.1111/j.1476-5381.1981.tb11000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: an update and role in anti-inflammation and pro-resolution. Prostaglandins & other lipid mediators. 2002;68–69:433–455. doi: 10.1016/s0090-6980(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez M, Bayon Y, Sanchez Crespo M, Nieto ML. Signaling mechanisms involved in the activation of arachidonic acid metabolism in human astrocytoma cells by tumor necrosis factor-alpha: phosphorylation of cytosolic phospholipase A2 and transactivation of cyclooxygenase-2. Journal of neurochemistry. 1999;73:1641–1649. doi: 10.1046/j.1471-4159.1999.0731641.x. [DOI] [PubMed] [Google Scholar]
- 15.Mollace V, Colasanti M, Rodino P, Lauro GM, Nistico G. HIV coating gp 120 glycoprotein-dependent prostaglandin E2 release by human cultured astrocytoma cells is regulated by nitric oxide formation. Biochem Biophys Res Commun. 1994;203:87–92. doi: 10.1006/bbrc.1994.2152. [DOI] [PubMed] [Google Scholar]
- 16.Flora G, Pu H, Hennig B, Toborek M. Cyclooxygenase-2 is involved in HIV-1 Tat-induced inflammatory responses in the brain. Neuromolecular Med. 2006;8:337–352. doi: 10.1385/NMM:8:3:337. [DOI] [PubMed] [Google Scholar]
- 17.Maccarrone M, Bari M, Corasaniti MT, Nistico R, Bagetta G, Finazzi-Agro A. HIV-1 coat glycoprotein gp120 induces apoptosis in rat brain neocortex by deranging the arachidonate cascade in favor of prostanoids. Journal of neurochemistry. 2000;75:196–203. doi: 10.1046/j.1471-4159.2000.0750196.x. [DOI] [PubMed] [Google Scholar]
- 18.Bagetta G, Corasaniti MT, Paoletti AM, Berliocchi L, Nistico R, Giammarioli AM, Malorni W, Finazzi-Agro A. HIV-1 gp120-induced apoptosis in the rat neocortex involves enhanced expression of cyclo-oxygenase type 2 (COX-2) Biochem Biophys Res Commun. 1998;244:819–824. doi: 10.1006/bbrc.1998.8321. [DOI] [PubMed] [Google Scholar]
- 19.Rao JS, Kim HW, Kellom M, Greenstein D, Chen M, Kraft AD, Harry GJ, Rapoport SI, Basselin M. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in brain of HIV-1 transgenic rats. J Neuroinflammation. 2011;8:101. doi: 10.1186/1742-2094-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Pereira CF, Boven LA, Middel J, Verhoef J, Nottet HS. Induction of cyclooxygenase-2 expression during HIV-1-infected monocyte-derived macrophage and human brain microvascular endothelial cell interactions. J Leukoc Biol. 2000;68:423–428. [PubMed] [Google Scholar]
- 21.Moses GS, Jensen MD, Lue LF, Walker DG, Sun AY, Simonyi A, Sun GY. Secretory PLA2-IIA: a new inflammatory factor for Alzheimer's disease. J Neuroinflammation. 2006;3:28. doi: 10.1186/1742-2094-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JC, Simonyi A, Sun AY, Sun GY. Phospholipases A2 and neural membrane dynamics: implications for Alzheimer's disease. Journal of neurochemistry. 2011;116:813–819. doi: 10.1111/j.1471-4159.2010.07033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhry U, Zhuang H, Dore S. Microsomal prostaglandin E synthase-2: cellular distribution and expression in Alzheimer's disease. Exp Neurol. 2010;223:359–365. doi: 10.1016/j.expneurol.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattacharyya DK, Lecomte M, Dunn J, Morgans DJ, Smith WL. Selective inhibition of prostaglandin endoperoxide synthase-1 (cyclooxygenase-1) by valerylsalicylic acid. Archives of biochemistry and biophysics. 1995;317:19–24. doi: 10.1006/abbi.1995.1130. [DOI] [PubMed] [Google Scholar]
- 25.Lecomte M, Laneuville O, Ji C, DeWitt DL, Smith WL. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. The Journal of biological chemistry. 1994;269:13207–13215. [PubMed] [Google Scholar]
- 26.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. The Journal of experimental medicine. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senol N, Ceyhan BM, Ersoy IH, Senol A, Acarturk G, Sutcu R. Aspirin increases NMDA receptor subunit 2A concentrations in rat hippocampus. J Recept Signal Transduct Res. 2012;32:17–21. doi: 10.3109/10799893.2011.641975. [DOI] [PubMed] [Google Scholar]
- 28.Maharaj H, Maharaj DS, Saravanan KS, Mohanakumar KP, Daya S. Aspirin curtails the acetaminophen-induced rise in brain norepinephrine levels. Metab Brain Dis. 2004;19:71–77. doi: 10.1023/b:mebr.0000027418.33772.8b. [DOI] [PubMed] [Google Scholar]
- 29.Kurata K, Nishida N, Tsukuda R, Suzuki T, Sato S, Tokuriki M. Frequency selectivity on aspirin-induced hearing loss in rats with auditory stimulus-induced conditioned suppression. J Vet Med Sci. 1997;59:879–884. doi: 10.1292/jvms.59.879. [DOI] [PubMed] [Google Scholar]
- 30.Stolk P, Souverein PC, Wilting I, Leufkens HG, Klein DF, Rapoport SI, Heerdink ER. Is aspirin useful in patients on lithium? A pharmacoepidemiological study related to bipolar disorder. Prostaglandins Leukot Essent Fatty Acids. 2010;82:9–14. doi: 10.1016/j.plefa.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:520–527. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- 32.Ketterer MW, Brymer J, Rhoads K, Kraft P, Lovallo WR. Is aspirin, as used for antithrombosis, an emotion-modulating agent? J Psychosom Res. 1996;40:53–58. doi: 10.1016/0022-3999(95)00524-2. [DOI] [PubMed] [Google Scholar]
- 33.Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O'Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lashomb AL, Vigorito M, Chang SL. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. Journal of neurovirology. 2009;15:14–24. doi: 10.1080/13550280802232996. [DOI] [PubMed] [Google Scholar]
- 35.Taha AY, Basselin M, Ramadan E, Modi HR, Rapoport SI, Cheon Y. Altered lipid concentrations of liver, heart and plasma but not brain in HIV-1 transgenic rats. Prostaglandins Leukot Essent Fatty Acids. 2012;87:91–101. doi: 10.1016/j.plefa.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voisin EM, Ruthsatz M, Collins JM, Hoyle PC. Extrapolation of animal toxicity to humans: interspecies comparisons in drug development. Regulatory toxicology and pharmacology : RTP. 1990;12:107–116. doi: 10.1016/s0273-2300(05)80052-2. [DOI] [PubMed] [Google Scholar]
- 37.Casado-Arroyo R, Gargallo C, Lanas Arbeloa A. Balancing the risk and benefits of lowdose aspirin in clinical practice. Best practice & research. Clinical gastroenterology. 2012;26:173–184. doi: 10.1016/j.bpg.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 38.Patrono C. Low-dose aspirin in primary prevention: cardioprotection, chemoprevention, both, or neither? European heart journal. 2013;34:3403–3411. doi: 10.1093/eurheartj/eht058. [DOI] [PubMed] [Google Scholar]
- 39.Radin NS. Extraction of tissue lipids with a solvent of low toxicity. Methods in enzymology. 1981;72:5–7. doi: 10.1016/s0076-6879(81)72003-2. [DOI] [PubMed] [Google Scholar]
- 40.Aid S, Bosetti F. Targeting cyclooxygenases-1 and-2 in neuroinflammation: Therapeutic implications. Biochimie. 2011;93:46–51. doi: 10.1016/j.biochi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parepally JM, Mandula H, Smith QR. Brain uptake of nonsteroidal anti-inflammatory drugs: ibuprofen, flurbiprofen, and indomethacin. Pharmaceutical research. 2006;23:873–881. doi: 10.1007/s11095-006-9905-5. [DOI] [PubMed] [Google Scholar]
- 42.Ajmone-Cat AM, Bernado A, Greco A, Minghetti L. Non-steroidal antiinflammatory drugs and brain inflammation: effects on microglial functiona. Pharaceuticals. 2010;6:1949–1965. doi: 10.3390/ph3061949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. The Journal of clinical investigation. 1982;69:1366–1372. doi: 10.1172/JCI110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tohgi H, Konno S, Tamura K, Kimura B, Kawano K. Effects of low-to-high doses of aspirin on platelet aggregability and metabolites of thromboxane A2 and prostacyclin. Stroke; a journal of cerebral circulation. 1992;23:1400–1403. doi: 10.1161/01.str.23.10.1400. [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto T, Lindgren JA, Hokfelt T, Samuelsson B. Regional distribution of leukotriene and mono-hydroxyeicosatetraenoic acid production in the rat brain. Highest leukotriene C4 formation in the hypothalamus. FEBS Lett. 1987;216:123–127. doi: 10.1016/0014-5793(87)80769-x. [DOI] [PubMed] [Google Scholar]
- 46.Lovelady GK, Mirro R, Armstead WM, Busija DW, Leffler CW. Effect of 15-HETE on cerebral arterioles of newborn pigs. Prostaglandins. 1988;36:507–513. doi: 10.1016/0090-6980(88)90046-9. [DOI] [PubMed] [Google Scholar]
- 47.Rauch P, Zellmer A, Specht C, Sperling D. Interference in immunoassays. Recognize and avoid (translation from German, http://www.oxfordbiosystems.com/Portals/0/PDF_2/lw-IV-05-englisch.pdf) Labowelt. 2005;6:1–7. [Google Scholar]
- 48.Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005;73:163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Wu Y, Ye XH, Guo PP, Xu SP, Wang J, Yuan SY, Yao SL, Shang Y. Neuroprotective effect of lipoxin A4 methyl ester in a rat model of permanent focal cerebral ischemia. Journal of molecular neuroscience : MN. 2010;42:226–234. doi: 10.1007/s12031-010-9355-8. [DOI] [PubMed] [Google Scholar]
- 50.Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ, et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. The Journal of experimental medicine. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hallstrand TS, Henderson WR., Jr An update on the role of leukotrienes in asthma. Current opinion in allergy and clinical immunology. 2010;10:60–66. doi: 10.1097/ACI.0b013e32833489c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathis S, Jala VR, Haribabu B. Role of leukotriene B4 receptors in rheumatoid arthritis. Autoimmunity reviews. 2007;7:12–17. doi: 10.1016/j.autrev.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 55.Turchan J, Pocernich CB, Gairola C, Chauhan A, Schifitto G, Butterfield DA, Buch S, Narayan O, Sinai A, Geiger J, Berger JR, Elford H, Nath A. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60:307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- 56.Uzasci L, Nath A, Cotter R. Oxidative stress and the HIV-infected brain proteome. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:1167–1180. doi: 10.1007/s11481-013-9444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicolini A, Ajmone-Cat MA, Bernardo A, Levi G, Minghetti L. Human immunodeficiency virus type-1 Tat protein induces nuclear factor (NF)-kappaB activation and oxidative stress in microglial cultures by independent mechanisms. Journal of neurochemistry. 2001;79:713–716. doi: 10.1046/j.1471-4159.2001.00568.x. [DOI] [PubMed] [Google Scholar]
- 58.Montine TJ, Montine KS, McMahan W, Markesbery WR, Quinn JF, Morrow JD. F2-isoprostanes in Alzheimer and other neurodegenerative diseases. Antioxid Redox Signal. 2005;7:269–275. doi: 10.1089/ars.2005.7.269. [DOI] [PubMed] [Google Scholar]
- 59.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. The Journal of biological chemistry. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 60.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annual review of pathology. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tornero C, Ventura A, Mafe M. Aspirin is indicated for primary prevention of cardiovascular events in HIV-infected patients. Journal of acquired immune deficiency syndromes. 2010;54:560. doi: 10.1097/QAI.0b013e3181d913fd. [DOI] [PubMed] [Google Scholar]
- 62.InfecBurkholder GAJ, Tamhane AR, Salinas JL, Mugavero MJ, Raper JL, Westfall AO, Saag MS, Willig JH. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55:1550–1557. doi: 10.1093/cid/cis752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansson CC, Bryn T, Aandahl EM, Areklett MA, Aukrust P, Tasken K, Froland SS. Treatment with type-2 selective and non-selective cyclooxygenase inhibitors improves T-cell proliferation in HIV-infected patients on highly active antiretroviral therapy. AIDS. 2004;18:951–952. doi: 10.1097/00002030-200404090-00015. [DOI] [PubMed] [Google Scholar]
- 64.Clemente MI, Alvarez S, Serramia MJ, Turriziani O, Genebat M, Leal M, Fresno M, Munoz-Fernandez MA. Non-steroidal anti-inflammatory drugs increase the antiretroviral activity of nucleoside reverse transcriptase inhibitors in HIV type-1-infected T-lymphocytes: role of multidrug resistance protein 4. Antiviral therapy. 2009;14:1101–1111. doi: 10.3851/IMP1468. [DOI] [PubMed] [Google Scholar]
- 65.Kvale D, Ormaasen V, Kran AM, Johansson CC, Aukrust P, Aandahl EM, Froland SS, Tasken K. Immune modulatory effects of cyclooxygenase type 2 inhibitors in HIV patients on combination antiretroviral treatment. AIDS. 2006;20:813–820. doi: 10.1097/01.aids.0000218544.54586.f1. [DOI] [PubMed] [Google Scholar]