Abstract
Attention‐deficit/hyperactivity disorder (ADHD) is increasingly understood as a disorder of spontaneous brain‐network interactions. The default mode network (DMN), implicated in ADHD‐linked behaviors including mind‐wandering and attentional fluctuations, has been shown to exhibit abnormal spontaneous functional connectivity (FC) within‐network and with other networks (salience, dorsal attention and frontoparietal) in ADHD. Although the cerebellum has been implicated in the pathophysiology of ADHD, it remains unknown whether cerebellar areas of the DMN (CerDMN) exhibit altered FC with cortical networks in ADHD. Here, 23 adults with ADHD and 23 age‐, IQ‐, and sex‐matched controls underwent resting state fMRI. The mean time series of CerDMN areas was extracted, and FC with the whole brain was calculated. Whole‐brain between‐group differences in FC were assessed. Additionally, relationships between inattention and individual differences in FC were assessed for between‐group interactions. In ADHD, CerDMN areas showed positive FC (in contrast to average FC in the negative direction in controls) with widespread regions of salience, dorsal attention and sensorimotor networks. ADHD individuals also exhibited higher FC (more positive correlation) of CerDMN areas with frontoparietal and visual network regions. Within the control group, but not in ADHD, participants with higher inattention had higher FC between CerDMN and regions in the visual and dorsal attention networks. This work provides novel evidence of impaired CerDMN coupling with cortical networks in ADHD and highlights a role of cerebro‐cerebellar interactions in cognitive function. These data provide support for the potential targeting of CerDMN areas for therapeutic interventions in ADHD. Hum Brain Mapp 36:3373–3386, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: cerebellum, attention‐deficit/hyperactivity disorder, default mode network, functional connectivity, inattention, resting state
INTRODUCTION
Attention‐deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder that can persist into adulthood and is characterized by inattention, hyperactivity and impulsivity. It affects both children and adults and is associated with distress, disability and morbidity across the life span [Spencer et al., 2007].
A growing literature shows that communication abnormalities among and within neural networks may underlie ADHD [Posner et al., 2014]. Resting state functional MRI (rs‐fMRI), can effectively identify such network abnormalities. In rs‐fMRI experiments, subjects are awake and are asked to simply rest while lying in the MRI scanner, so brain activity can be considered “spontaneous” rather than stimulus‐ or task‐driven [Fox and Raichle, 2007]. Networks with specific spatial patterns, defined by functional connectivity (FC; inter‐regional correlations in activity), have been identified consistently within subjects, across populations, and across brain states, suggesting that communication in these networks largely reflects an “intrinsic” aspect of brain function [Buckner et al., 2013]. Some well‐defined networks, including the default mode network (DMN), salience network, dorsal attention network (DAN), and frontoparietal network (FPN), are situated in association cortices and are thought to subserve higher order cognitive functions. Other networks, such as visual and sensorimotor, encompass sensory/motor regions related to processing environmental inputs and performing actions [Yeo et al., 2011].
ADHD is increasingly understood as a disorder of the aforementioned brain networks, with emphasis often placed on the DMN and its interactions with other networks hypothesized to underlie attentional dysfunctions [Castellanos and Proal, 2012; Sonuga‐Barke and Castellanos, 2007]. The DMN is of interest in the context of attentional deficits because it is normally deactivated when attention is engaged with the external environment [Shulman et al., 1997] but is activated during both attentional lapses [Weissman et al., 2006] and spontaneous mind‐wandering [Christoff et al., 2009; Kucyi et al., 2013]. In the healthy brain at rest, the DMN typically exhibits anticorrelated activity with the DAN, FPN and salience network [Chai et al., 2012; Fox et al., 2005; Keller et al., 2013; Kucyi et al., 2012]. Multiple rs‐fMRI studies have demonstrated that individuals with ADHD, relative to healthy subjects, exhibit (a) decreased within‐DMN FC [particularly between the medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC)/precuneus (PCu)] [Castellanos et al., 2008; Fair et al., 2010; Mattfeld et al., 2014] (with exceptions, however, [Barber et al., 2015]) and (b) reduced or absent anticorrelation between DMN and other association networks (DAN, FPN, salience) [Castellanos et al., 2008; Hoekzema et al., 2014; Mattfeld et al., 2014; Sun et al., 2012]. In a large rs‐fMRI study of ADHD and control subjects (aged 7.2–21.8 years), the ADHD subjects showed “lags” in development of FC within the DMN (delayed increased FC between PCC and mPFC) and of DMN FC with the DAN, FPN and salience network (delayed increased anticorrelation) [Sripada et al., 2014].
Notably, the focus of much research on ADHD and the brain has been on cognitively oriented cortical regions such as the dorsolateral prefrontal and anterior cingulate cortices. However, the cerebellum, traditionally considered a motor structure, is increasingly recognized as an important structure in cognition and in ADHD pathophysiology [Buckner, 2013; Durston et al., 2011; Strick et al., 2009]. The cerebellum is structurally connected with prefrontal and striatal circuits implicated in ADHD [Bostan et al., 2013]. Structural neuroimaging studies have revealed reduced volumes of the cerebellum or its subregions in ADHD that have been shown to correlate with attentional problems and clinical outcomes [Castellanos et al., 2002; Mackie et al., 2007; Makris et al., in press; Stoodley, 2014]. Additionally, fMRI studies have revealed decreased cerebellar activation in ADHD during performance of a number of cognitive tasks [Suskauer et al., 2008; Valera et al., 2005, 2010b]. However, the precise role of the cerebellum in ADHD pathophysiology remains unknown.
Although some rs‐fMRI studies implicate abnormal cerebro‐cerebellar FC as a feature of ADHD [Cao et al., 2009; Fair et al., 2012; Tian et al., 2006], others have excluded the cerebellum from their analyses [Sripada et al., 2014]. Recently, based on rs‐fMRI, the cerebellum has been divided into subregions that are coupled with specific cortical networks [Buckner et al., 2011; Habas et al., 2009; O'Reilly et al., 2010]. In a large sample of healthy subjects (n=1,000), lateral cerebellar areas including crus I/II were shown to be functionally connected with the DMN [Buckner et al., 2011], and neurostimulation of this region was shown to modulate FC specifically with and between cortical DMN areas [Halko et al., 2014]. It remains unknown whether intrinsic cerebellar DMN (CerDMN) FC with cortical networks is altered in ADHD. Understanding CerDMN FC in ADHD is critical to provide a more complete account of DMN dysfunction in ADHD. More generally, the CerDMN is an accessible target for noninvasive neuromodulation that may improve attentional function. Central DMN hub regions (PCC and mPFC) are deep and difficult to directly target but can be modulated with CerDMN stimulation [Halko et al., 2014].
We tested whether resting CerDMN FC with cortical networks is abnormal in adults with ADHD. We hypothesized that in ADHD the CerDMN would have decreased positive FC (lower correlation) with the cortical DMN and reduced or absent negative FC with the DAN, FPN, and salience network. Previous studies have linked inattention and related factors, including mind‐wandering and reaction time (RT) variability, with individual differences in FC within‐DMN and between DMN and anticorrelated networks [Andrews‐Hanna et al., 2010a; Barber et al., 2015; Gordon et al., 2014; Kelly et al., 2008; Kucyi and Davis, 2014]. Therefore, we also predicted that CerDMN FC with these cortical networks would be associated with inattention.
MATERIALS AND METHODS
Participants
Subjects were recruited via previous studies of ADHD conducted at MGH, internet postings and emails/letters sent to individuals signed up to be informed about volunteer opportunities. Adults with ADHD and healthy controls (HC) provided written informed consent for study participation. Subject demographics and characteristics are summarized in Table 1. A Chi‐square test revealed no group differences in sex (Chi‐square = 0.36, P = 0.55). Procedures were approved by the Partners Human Research Institutional Review Board. Subject exclusion criteria were: current use of psychotropic medications (other than short‐acting psychostimulants), full scale IQ < 80, a current DSM‐IV Axis I mood, psychotic or anxiety disorder (excluding simple phobias), any neurological disorder, any major sensorimotor handicaps, and current alcohol or substance abuse/dependence or a chronic history of abuse/dependence as defined by review of the Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID). Any ADHD subjects who were currently taking psychostimulants (N = 11) were asked to refrain from taking them 24 h prior to testing. Six other subjects had taken psychostimulants in the past and seven were psychostimulant naive. Subjects were right‐handed, except for two left‐handed ADHD subjects.
Table 1.
Subject demographics and characteristics, including head motion (mean relative head displacement) during the resting state fMRI scan
| ADHD (N = 23) | Control (N = 23) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 24.3 | 3.9 | 24.2 | 2.9 |
| IQ estimate | 119.9 | 14.0 | 119.9 | 11.9 |
| Sex (F/M) | 13/10 | 15/8 | ||
| Head motion (mm) | 0.091 (range: 0.03–0.25) | 0.073 | 0.066 (range: 0.02–0.27) | 0.063 |
Diagnostic and Cognitive Assessment
All participants underwent the Structured Clinical Interview for DSM‐IV (SCID; [First et al., 2012]) consistent with previous studies (e.g., [Valera et al., 2010a]). To assess ADHD, a module derived from the Schedule for Affective Disorders and Schizophrenia for School Age Children was used [Kaufman et al., 1997]. This module systematically acquires retrospective information on all DSM‐IV ADHD symptoms, domains of impairment and age at onset. Previous work has shown that retrospective childhood diagnoses of ADHD can be made in a reliable and valid manner using this method [Biederman et al., 1990; Faraone et al., 2000]. ADHD participants met DSM‐IV criteria for ADHD with childhood onset and persistence into adulthood. We included one ADHD participant who had an age‐of‐onset of 11 years. This decision was based on studies supporting the validity of ADHD in subjects with onset of symptoms later than the 7‐year cutoff [Faraone et al., 2006]. A board‐certified child and adult psychiatrist resolved any diagnostic uncertainties. Participants also completed the Adult ADHD Self‐Report Scale (ASRS) [Kessler et al., 2005] to obtain a measure of inattention for use in our neuroimaging analysis. Vocabulary and matrix reasoning from the Wechsler Abbreviated Scale of Intelligence [Wechsler, 1999] were used to calculate full‐scale IQ, for which there was no significant difference between ADHD and HC. The subtypes of the ADHD subjects were as follows: 10 inattentive, 1 hyperactive, and 12 combined type.
MRI Acquisition
Resting state fMRI and structural MRIs were acquired on a Siemens Tim Trio 3‐Tesla scanner. Instructions prior to the resting state scan were as follows: “Remain as still as possible. Keep eyes open, you can blink normally, but it is important to remain as still as possible.” The T2*‐weighted fMRI scan took 10 min 8 s (TR = 3.34 s; TE = 30 ms; flip angle = 90°; FoV = 200 mm; 60 slice, interleaved acquisition; voxel size: 2.5 × 2.5 × 2.5 mm). The structural MRI used for coregistration was a T1‐weighted MEMPRAGE sagittal scan (TR = 2.54 s; TE = 1.64/3.5/5.36/7.22 ms; TI: 1.2s; flip angle = 7°; FoV = 256 mm; 176 slices; voxel size: 1.0 × 1.0 ×1.0 mm).
Data Preprocessing
Resting state fMRI data were preprocessed with previously reported procedures [Kucyi and Davis, 2014; Kucyi et al., 2013, 2014] using FSL v5.0.7 [Jenkinson et al., 2012], MATLAB 8.0.0.783 (Mathworks), and the fMRISTAT toolbox [Worsley et al., 2002]. Using FSL's FEAT, the following were first performed: motion correction (MCFLIRT), brain extraction (BET) and linear registration (FLIRT) with the T1 structural scan (6 DOF) and the MNI 152 2mm3 standard brain (12 DOF). During further preprocessing, fMRI data remained in the subjects' native space. The T1 image was segmented into partial volume maps for gray matter, white matter (WM), and cerebrospinal fluid (CSF) using FSL's FAST. These WM and CSF maps were linearly transformed to fMRI space and were thresholded to retain voxels with the highest values (i.e., greatest tissue‐type probability) within volumes of 198 cm3 and 20 cm3, respectively [Chai et al., 2012]. We then followed the aCompCor approach, which avoids issues associated with the commonly performed global signal regression approach [Murphy et al., 2009], captures multiple aspects of physiological and scanner‐related noise, and allows detection of anticorrelations between resting state networks [Behzadi et al., 2007; Chai et al., 2012]. Furthermore, aCompCor may address the problem of motion artifact at an acceptable level, as there is no additional benefit to data quality of “scrubbing” problematic volumes after aCompCor is performed [Muschelli et al., 2014] [but see [Pruim et al., 2015] for an alternative approach]. Principal components analysis was conducted on the fMRI data within the thresholded WM and CSF volumes, separately. The time series representing the top five WM principal components, top five CSF principal components [Chai et al., 2012], and six motion parameters obtained with MCFLIRT were then regressed out of the fMRI data. The data were then spatially smoothed (6 mm full‐width at half‐maximum kernel) and temporally filtered (0.01–0.1 Hz).
Cerebellar Seed Definition
The CerDMN seed region was defined in standard MNI152 2mm3 space using the map provided by Buckner et al. [2011] based on data from 1,000 healthy adults (http://www.freesurfer.net/fswiki/CerebellumParcellation_Buckner2011). The CerDMN included lateral clusters in the right and left cerebellum (spanning areas within Crus I and II) as well as a medial cluster (spanning areas within the vermis) (Fig. 1A) for a total of 2,320 voxels (18,560 mm3; ∼10% total cerebellar volume). The seed was registered from MNI152 to native fMRI space using the previously computed linear transform. The average time course across all voxels within the CerDMN was then calculated.
Figure 1.
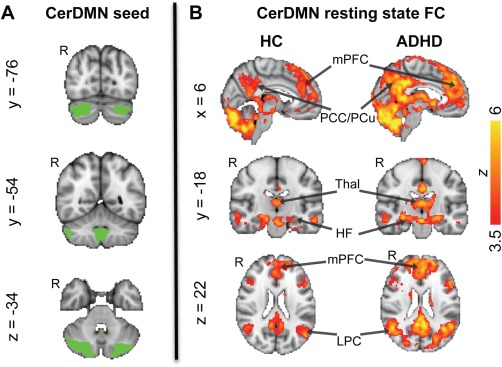
CerDMN seed and its FC in the HC and ADHD groups. (A) The CerDMN seed (based on [Buckner et al., 2011]) from which the resting state fMRI time series were extracted. B) Voxels showing significant positive FC with the CerDMN in the HC and ADHD groups [FWE‐corrected Z > 2.3 (threshold increased to 3.5 for display purposes); cluster‐based P < 0.05]. HF, hippocampal formation; LPC, lateral parietal cortex; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; PCu, precuneus; Thal, thalamus. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Statistical Analyses
In first‐level (within‐subject) general linear model (GLM) analyses using FSL's FEAT, the CerDMN time course was entered as a regressor to derive a whole‐brain map of Contrast of Parameter Estimate (COPE) values reflecting FC with the seed region. The resulting COPE maps were transformed to standard MNI152 space using the combined 6 and 12 DOF transformation matrices and submitted to a second‐level (group‐level) mixed effects GLM (FLAME 1 + 2) with four contrasts to identify: (a) positive and negative FC within the HC group; (b) positive and negative FC within the ADHD group; (c) HC > ADHD FC; and (d) ADHD > HC FC. Group‐level statistical maps were thresholded with significance set at FWE‐corrected Z>2.3 and cluster‐based P<0.05. To account for a potential influence of handedness, we repeated the group differences analysis excluding the 2 left‐handed ADHD patients in our sample.
In additional group‐level analyses (using identical GLM and thresholding approaches as above), within‐group demeaned ASRS inattention scores were entered as a regressor to identify regions where CerDMN FC is associated with inattention in HC and in ADHD. Furthermore, a two‐group with continuous covariate interaction analysis was conducted with inattention scores as a regressor (mean across both groups subtracted out), and statistical contrasts were set up to identify significant group differences in CerDMN FC relationships with inattention.
Voxels that were identified as significant in group‐level contrasts were classified as belonging to 1 of 7 cortical networks [DMN, salience, frontoparietal, DAN, sensorimotor, visual, and limbic]. To quantify volumes overlapping with each network, the significant‐level parametric maps were multiplied by the 7 cortical networks (“tight mask”) provided by Yeo et al. [2011], based on clustering of resting state FC data from 1,000 healthy adults (http://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation_Yeo2011).
Supplementary Analyses
Despite performance of the described preprocessing approaches to minimize the impact of non‐neuronal noise on results, systematic group differences in head motion or signal‐to‐noise could impact analysis outcomes [Power et al., 2015; Van Dijk et al., 2012]. Mean relative head displacement has been shown to be particularly problematic for FC estimates [Power et al., 2015]. We, therefore, calculated mean relative displacement (with MCFLIRT in FSL) [Jenkinson et al., 2002] for each subject and conducted a two‐tailed t‐test to compare ADHD versus HC group values. To further control for individual differences in head movement, we reconducted the GLM analysis of ADHD versus HC CerDMN FC with inclusion of mean relative displacement values as a regressor of no interest. Additionally, we conducted two‐tailed t‐tests to compare group average values for the six motion parameters obtained with MCFLIRT (x, y, z, pitch, roll, yaw).
Notably, the CerDMN seed region in the main analysis was a large volume that included areas within both cerebellar hemispheres, which did not allow us to determine whether CerDMN subregions (or right versus left hemisphere regions) contributed to results more than others. Areas within CerDMN have lateralized intrinsic FC [Wang et al., 2013], so it is possible that right and left regions contributed differently. We, therefore, repeated the procedures described above for the GLM analysis of ADHD versus HC FC using two small, spherical (6 mm diameter) seeds in the right (xyz = 29, −78, ‐32; Supporting Information Fig. S1A) and left CerDMN (xyz = −32, −79, −31; Supporting Information Fig. S1B), both located in Crus I. Small regions surrounding these coordinates were previously shown to exhibit FC specifically with cortical DMN areas [Buckner et al., 2011].
RESULTS
Inattention
There was no overlap in ASRS inattention scores between groups (HC range: 0–12; ADHD range: 14–35). A two‐tailed independent samples t‐test revealed a significant group difference in ASRS inattention (mean ± SD: HC 5.1 ± 3.8; ADHD 24.6 ± 5.1, P < 0.00001).
Group Differences in Functional Connectivity of Cerebellar Default Network Areas
Within each group, the CerDMN exhibited the expected positive FC with cortical and subcortical regions of the DMN, including mPFC, PCC/PCu, lateral parietal cortex/posterior temporoparietal junction, middle frontal gyrus, subgenual anterior cingulate cortex, lateral temporal cortex, temporal pole, parahippocampal gyrus, hippocampal formation, thalamus, and caudate nucleus (Fig. 1B). No regions showed significant negative FC with the CerDMN within either group. There were no regions showing greater CerDMN FC in the HC compared to ADHD group. However, the ADHD group showed higher CerDMN FC with many regions, including bilateral insula (anterior, middle, and posterior portions), bilateral midcingulate cortex (MCC), bilateral retrosplenial cortex (Rsp), bilateral putamen, bilateral precentral and postcentral gyri, right superior temporal gyrus, bilateral lateral occipital cortex (superior and inferior aspects), bilateral fusiform and lingual gyri, bilateral cuneus, bilateral frontal eye fields (FEF), bilateral superior parietal lobule (SPL), and left cerebellum (lobules V/VI) (Fig. 2A). When excluding the 2 left‐handed ADHD patients in our sample, very similar group differences were obtained (ADHD > HC parametric maps with versus without left‐handed patients excluded were had a voxelwise correlation of r = 0.99).
Figure 2.
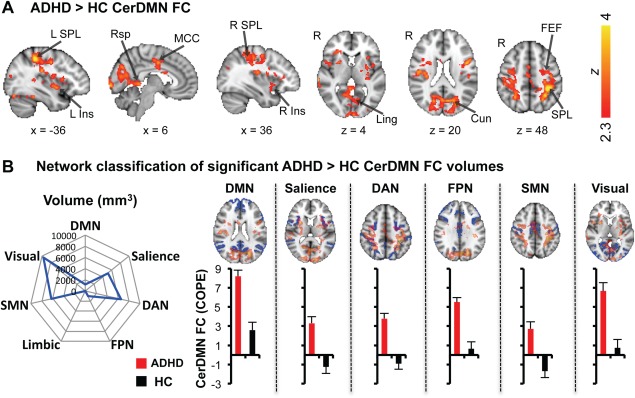
Regions showing higher CerDMN resting FC in ADHD patients and their classification by network. (A) Voxels showing significantly greater FC in ADHD compared to HC (FWE‐corrected Z > 2.3; cluster‐based P < 0.05). (B) Polar plot (left) shows voxels from (A) quantified in terms of volume (mm3) that overlaps with each of seven networks defined by Yeo et al. [2011]. Brain images (right, top) show networks in blue and their overlap with ADHD > HC CerDMN FC maps (transparent red/yellow). Bar plots (right, bottom) show mean FC within each group across voxels in each network that overlapped with ADHD > HC CerDMN FC maps (ADHD, red; HC, black). Error bars depict standard error of the mean. COPE, contrast of parameter estimate; Cun, cuneus; DAN, dorsal attention network; DMN, default mode network; Ins, insula; FEF, frontal eye fields; FPN, frontoparietal network; Ling, lingual gyrus; MCC, mid‐cingulate cortex; Rsp, retrosplenial cortex; SMN, sensorimotor network; SPL, superior parietal lobule. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The cortical volumes exhibiting greater CerDMN FC in ADHD compared to HC were classified into the 7 Yeo et al [2011] defined networks [network: volume, percentage of total volume in network]: [DMN: 1,216 mm3, 0.9%], [Salience: 5,120 mm3, 8.6%], [DAN: 6,568 mm3, 11.3%], [FPN: 1,000 mm3, 1.2%], [Sensorimotor: 6,288 mm3, 9.2%], [Visual: 9,632 mm3, 14.7%], and [Limbic: 0 mm3, 0.0%] (Fig. 2B). Within the salience, DAN and sensorimotor network volumes, there was an average of FC in the negative direction in HC but an average of FC in the positive direction in ADHD. Within the DMN, FPN, and visual network volumes, the ADHD group had greater positive FC on average than the HC group (Fig. 2B).
Relationship Between Inattention and Functional Connectivity of Cerebellar Default Network Areas
Within the ADHD group there were no regions showing a significant association between inattention and CerDMN FC. However within the HC group, there was a positive association between inattention and CerDMN FC with left SPL, left sensorimotor cortex, left posterior insula, left Heschl's gyrus (auditory cortex), and left lateral occipital complex (inferior). In other words, controls with greater inattention scores showed greater connectivity between the CerDMN and regions largely within DAN and visual networks. To assess the different relationships observed between groups, we analyzed the group interaction. This analysis revealed that the relationship was greater in HC compared to ADHD between inattention and CerDMN FC with the bilateral SPL, right postcentral gyrus, right lateral occipital complex (superior), and bilateral fusiform gyrus/lateral occipital complex (inferior) (Fig. 3A). Those volumes exhibiting a stronger CerDMN FC relationship with inattention in HC compared to ADHD could be classified into networks as follows: [DMN: 8 mm3, 0.006%], [Salience: 0 mm3, 0.0%], [DAN: 824 mm3, 1.4%], [FPN: 160 mm3, 0.2%], [Sensorimotor: 384 mm3, 0.6%], [Visual: 1,400 mm3, 2.1%], [Limbic: 0 mm3, 0.0%] (Fig. 3B). Plots of FC versus inattention for volumes from the visual network and DAN, the two networks with the greatest volumes contributing to the group interaction, show that relationships were positive in the HC group but trended in the negative direction in the ADHD group (Fig. 3B).
Figure 3.

Differences between ADHD and HC groups in the relationship between ASRS inattention and resting CerDMN FC. (A) Voxels showing a stronger inattention‐CerDMN FC association in HC compared to ADHD (FWE‐corrected Z > 2.3; cluster‐based P < 0.05). (B) Polar plot (left) shows voxels from (A) quantified in terms of volume (mm3) that overlaps with each of seven networks defined by Yeo et al. [2011]. Scatter plots (right) show, in each group, individual inattention scores versus mean CerDMN FC across voxels from (A) that overlap with the labeled networks (HC, black; ADHD, red). ASRS, Adult ADHD Self‐Report Scale; COPE, Contrast Of Parameter Estimate; DAN, dorsal attention network; DMN, default mode network; FPN, frontoparietal network; Fus, fusiform gyrus; LOC, lateral occipital complex; SMN, sensorimotor network; SPL, superior parietal lobule. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Group Differences Related to Inattention
To illustrate regions that both exhibited a group difference in CerDMN FC and a group interaction in the relationship between inattention and CerDMN FC, we overlaid the maps from the two analyses on one another (Fig. 4). This revealed that areas in bilateral SPL (part of the DAN) and areas within the visual network exhibited greater CerDMN FC in ADHD compared to HC, as well as a weaker relationship between inattention and CerDMN FC in ADHD compared to HC.
Figure 4.
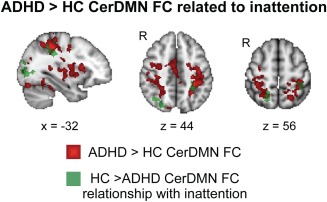
Overlap of voxels showing increased CerDMN FC in ADHD compared to HC (red) and a weaker association of inattention with CerDMN FC in ADHD compared to HC (green). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Controlling for Head Motion
There was no significant group difference in mean relative head displacement (mean ± SD: ADHD 0.091 ± 0.073; HC 0.066 ± 0.063; P = 0.25). When controlling for mean relative displacement in the group differences GLM analysis, virtually the same map of regions exhibiting ADHD > HC CerDMN appeared as significant (r = 0.99 correlation between maps with versus without controlling for motion). Additionally, there were no significant group differences in average values for any of the six motion parameters (x, y, z, pitch, roll, and yaw) (P > 0.35 in all cases).
Group Differences with Lateralized Cerebellar Seeds
When reconducting CerDMN FC group difference analyses with right and left Crus I subregions of the CerDMN as seeds, similar results were obtained compared to when using the whole CerDMN seed. For both right and left seeds, there were no regions showing greater FC in HC compared to ADHD. Similar to results from the main analysis, ADHD compared to HC had greater FC with regions comprising several networks (DAN, salience, DMN, visual, sensorimotor, FPN). Qualitatively, more clusters were significant for the left compared to right CerDMN seed (Fig. 5). However, differences in effect magnitudes for right compared to left CerDMN seeds were small, and at the cluster‐uncorrected level, group difference results for right and left seeds showed a very high degree of overlap.
Figure 5.
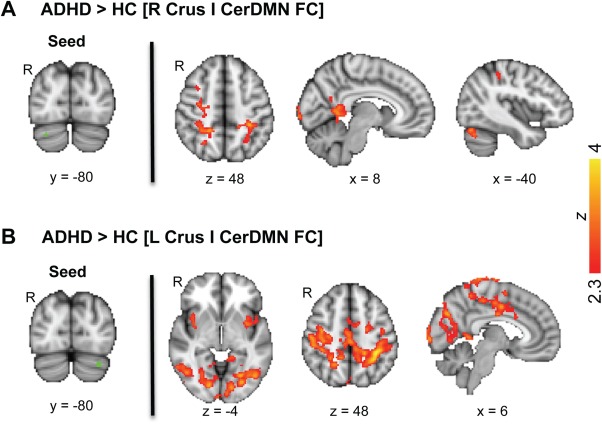
Regions showing higher resting FC with right and left CerDMN subregions in ADHD patients compared to HC. (A) Right CerDMN Crus I seed region (left) and voxels showing significantly greater FC in ADHD compared to HC (right; FWE‐corrected Z > 2.3; cluster‐based P < 0.05). (B) Left CerDMN Crus I seed region (left) and voxels showing significantly greater FC in ADHD compared to HC (right; FWE‐corrected Z > 2.3; cluster‐based P < 0.05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
We capitalized on advances in understanding the cerebellum's role in cognition to reveal insights into neural network mechanisms of ADHD. We showed that in ADHD the cerebellar component of the DMN exhibits disrupted resting state FC with several brain networks spanning sensory and association areas of the cerebral cortex. In ADHD, the CerDMN showed positive FC (in contrast to average FC in the negative direction found in controls) with widespread regions of salience, dorsal attention and sensorimotor networks. ADHD individuals also exhibited greater FC (a more positive correlation) of the CerDMN with frontoparietal and visual network regions. Furthermore, healthy subjects with higher inattention scores had greater FC of the CerDMN with areas of the visual network and the DAN. This suggests that normal‐range “ADHD‐like” traits (inattention) are linked with “ADHD‐like” cerebro‐cerebellar network organization. Taken together, these results show that cerebro‐cerebellar circuits implicated in attention are reorganized in ADHD.
Cerebellum and ADHD
Neuroimaging evidence indicates that ADHD is characterized by patterns of abnormal connectivity in brain networks relevant to cognition, emotion and sensorimotor functions and with regions spanning the cerebral cortex, subcortex, and cerebellum [Castellanos and Proal, 2012]. Although the role of the cerebellum, particularly as it relates to cognition, is not usually emphasized in ADHD, previous studies have revealed cerebellar structural abnormalities and decreased activations during cognitive task performance [Ivanov et al., 2014; Makris et al., in press; Stoodley, 2014; Valera et al., 2007, 2010b].
Prominent contemporary theories of neural dysfunction in ADHD are grounded in an understanding of networks across the cortex that exhibit abnormal within‐ and between‐network interactions [Castellanos and Proal, 2012; Sonuga‐Barke and Castellanos, 2007]. While previous studies have identified resting cerebellar FC abnormalities in ADHD [Cao et al., 2009; Fair et al., 2012; Tian et al., 2006], our work is unique in that we capitalized on the development of a functional atlas of cerebellar subregions linked to well‐defined cortical networks [Buckner et al., 2011] to integrate the role of the cerebellum into existing theories of ADHD and the DMN. Much of the cerebellum displays FC with association cortices involved in attention and higher order cognition [Buckner, 2013], so cerebro‐cerebellar communication changes should be expected in ADHD. Our results confirm a link of cerebro‐cerebellar connectivity with ADHD, and particularly for inattention, suggesting a potential new target for intervention in ADHD including transcranial magnetic stimulation (TMS) of the lateral cerebellum, which has been shown to impact attentional performance [Arasanz et al., 2012].
Inattention and the DMN
Based on evidence of DMN engagement during attentional fluctuations and lapses, Sonuga‐Barke and Castellanos [Sonuga‐Barke and Castellanos, 2007] proposed the “default mode interference hypothesis.” They suggested that DMN dysfunction characterizes ADHD and may be linked with behaviors related to inattention, including increased RT variability during sustained attention task performance and instances of spontaneous mind‐wandering away from the external environment. The hypothesis is supported by distinct reports of DMN abnormalities [Posner et al., 2014], increased RT variability [Castellanos et al., 2005; Klein et al., 2006] and a higher frequency of mind‐wandering [Franklin et al., in press; Seli et al., 2015] with ADHD or ADHD‐like symptoms. However, with a few exceptions [Barber et al., 2015; Fassbender et al., 2009], rarely have studies integrated these neural and behavioral aspects to assess their direct links in ADHD.
Notably, within‐DMN resting state FC has been linked with individual differences in inattention [Gordon et al., 2014] and the tendency to mind‐wander [Andrews‐Hanna et al., 2010a; Doucet et al., 2012; Kucyi and Davis, 2014; O'Callaghan et al., 2015] (both of which are associated with ADHD) in healthy individuals. The association of these behaviors with DMN FC in ADHD remains unclear. One possibility is that previous studies showing reduced within‐DMN FC between PCC/PCu and mPFC in ADHD [Castellanos et al., 2008; Fair et al., 2010; Mattfeld et al., 2014] are reflective of attentional dysfunction, particularly as this reduced FC has been found in those who persist with ADHD into adulthood but not in those who remit [Mattfeld et al., 2014]. Studies of individual variability in resting state FC have revealed that both inattention [Gordon et al., 2014] and forms of mind‐wandering [Doucet et al., 2012; Kucyi and Davis, 2014] are linked with lower within‐DMN FC, whereas rumination (an inability to shift attention away from a given train of thought) is linked with enhanced within‐DMN (PCC‐mPFC) FC [Kucyi et al., 2014; Zhu et al., 2012].
We found evidence of increased CerDMN FC with a few regions in the DMN (e.g., retrosplenial cortex) in ADHD, but the behavioral significance of this finding remains unknown as this FC was not linked with inattention. This finding goes against our initial hypothesis of reduced CerDMN FC with cortical DMN regions in ADHD, but we note that the result is still largely consistent with previous findings of reduced mPFC‐PCC FC. We found that only a very small proportion of the DMN (0.90%, with little overlap with mPFC or PCC) showed enhanced FC with the CerDMN. The DMN is believed to be composed of several subsystems [Andrews‐Hanna et al., 2010b], so it is possible that some within‐DMN FC is enhanced but some is reduced in ADHD. A recent study revealed hyperconnectivity between cortical DMN areas in children with ADHD [Barber et al., 2015], supporting the notion that ADHD may not be characterized by uniformly decreased within‐DMN FC.
DMN Interactions with Anticorrelated Networks
The initial finding that the DMN exhibits anticorrelated spontaneous activity with the FPN, DAN and salience network [Fox et al., 2005] was met with controversy due to fMRI methodological issues [Murphy et al., 2009], but neurophysiological evidence [Keller et al., 2013] and fMRI methodological advances [Chai et al., 2012] give support for the existence of such an anticorrelation. Importantly, anticorrelated spontaneous activity fluctuations suggest that these networks may normally interact with one another and that disruption in the interaction could affect cognition and behavior. In traumatic brain injury for example, it has been shown that damage to WM tracts of the salience network is associated with abnormal DMN function [Bonnelle et al., 2012] and impaired DMN‐salience network FC during task performance [Jilka et al., 2014]. Furthering evidence for interactions between anticorrelated networks, it was shown that TMS to an FPN node altered DMN FC [Chen et al., 2013]. At the level of behaviors relevant to ADHD, individual differences in RT variability [Kelly et al., 2008] and trial‐to‐trial fluctuations in mind‐wandering [Mittner et al., 2014] have been associated with strength of DMN FC with anticorrelated networks. Additionally, the balance of DMN versus DAN activity has been shown to subserve intra‐individual fluctuations in RT variability during sustained attention [Esterman et al., 2013; Esterman et al., 2014].
Our results extend existing literature on interactions between the DMN and anticorrelated networks, showing a role of the CerDMN, which is typically neglected. Broadly, our results of impaired coupling of the CerDMN with the DAN, FPN and salience network are consistent with previous rs‐fMRI ADHD studies that showed similar results for cortical nodes of these networks [Castellanos et al., 2008; Hoekzema et al., 2014; Mattfeld et al., 2014; Sripada et al., 2014; Sun et al., 2012]. The negative FC with the CerDMN with these networks in HCs was not as strong (not significant at the whole‐brain FWE‐corrected level) as typically reported for cortical DMN nodes, but the general pattern of our findings was similar to previous cortical FC findings. Furthermore, our findings demonstrate that in healthy individuals, less anticorrelation between the CerDMN and areas of the DAN is associated with greater inattention. This result can be reconciled with the finding of greater RT variability (potentially representing inattention) associated with a weaker anticorrelation of the (cortical) DMN with networks associated with externally oriented attention in healthy subjects [Kelly et al., 2008]. As such, the role of the cerebellum in attention and internetwork interactions should be given increased focus in future studies.
DMN Interactions with Motor and Sensory Networks
Our findings of higher FC of the CerDMN with the sensorimotor and visual networks in ADHD were unexpected, but several studies suggest that these networks have altered structure and function in ADHD [Castellanos and Proal, 2012]. In previous rs‐fMRI studies, efficiency of FC for occipital areas was altered in ADHD [Wang et al., 2009], and altered SMN FC was found in two ADHD subtypes [Fair et al., 2012]. Furthermore, greater anticorrelated spontaneous DMN‐occipital FC was shown to be associated with greater attentional control in typically developing but not ADHD children [Barber et al., 2015], compatible with our findings of greater CerDMN‐occipital FC associated with inattention in HCs but not adults with ADHD.
Visual network abnormalities in ADHD could reflect failure to ignore irrelevant stimuli. This network functions closely with the DAN in the control of visual attention [Corbetta and Shulman, 2002]. As we found that CerDMN FC with regions within both DAN and visual networks was both enhanced in ADHD and linked with normal‐range inattention, interactions among these networks may play a role in suppressing or enhancing the attentional capture of irrelevant visual stimuli.
Limitations
Our sample size was adequate to detect FC group differences and relationships with inattention in healthy subjects. However, it is possible that limited statistical power prevented us from identifying relationships of FC with inattention in ADHD. Because of small sample sizes for each ADHD subtype (inattentive, hyperactive, and combined) in our study, we also were unable to parse potentially varied contributions of subject subtype on the FC findings. The IQ of our sample, though well matched between subjects with ADHD and HCs, was higher than average. As such, additional work would be required to determine whether these findings would be similar for groups with different IQs. Additionally, while ADHD subjects refrained from taking psychostimulants for 24 h prior to scanning, medication status differences between subjects could have affected our intrinsic FC results [Mueller et al., 2014; Ramaekers et al., 2013]. Some evidence suggests that psychostimulants tend to normalize abnormalities in brain structure and function in ADHD [Spencer et al., 2013], so it is possible that inclusion of medicated patients reduced our ability to detect group differences in FC. Further studies with larger sample sizes would be required to delineate the effects of psychostimulant use on intrinsic FC in ADHD.
Future Directions
In our study, we took a highly focused approach to study spontaneous CerDMN FC in ADHD. Our findings may motivate future investigations that further explore the mechanisms of abnormal cerebro‐cerebellar interactions in ADHD. Advanced rs‐fMRI analysis methods, such as graph theory and dynamic FC have recently been applied to give insight to global‐level spontaneous network abnormalities in ADHD [Di Martino et al., 2013; Ou et al., 2014; Wang et al., 2009]. Furthermore, multivariate approaches combining structural and functional changes have given insight into the pathophysiology of ADHD [Kessler et al., 2014] and could be further applied to understanding cerebro‐cerebellar network abnormalities and their effects on behavior. Additionally, it was recently shown that targeted noninvasive CerDMN stimulation with TMS leads to changes in FC with and within cortical networks [Halko et al., 2014] supporting further investigation of the effects of CerDMN neurostimulation on inattention and related behaviors. Finally, both the DMN and cerebellum exhibit abnormalities in a wide range of psychiatric and neurological disorders [Villanueva, 2012; Whitfield‐Gabrieli and Ford, 2012], so investigation of CerDMN FC and its potential targeting in populations beyond ADHD is warranted.
ACKNOWLEDGMENTS
The authors thank Mike Esterman for comments and discussion, and Clay Riley and Zhi Li for technical support and assistance with data acquisition. Drs. Kucyi, Hove and Van Dijk reported no biomedical financial interests or potential conflicts of interest. Dr. Valera has received travel support and/or honoraria from Galenea, Eli Lilly, Shire Pharmaceuticals, and divisions of Ortho‐McNeil Janssen Pharmaceuticals (McNeil Pediatrics and Janssen Pharmaceuticals), Remedica Medical Education and Publishing, MGH Psychiatry Academy for a tuition‐funded CME course and consulting, Reed Exhibitions and Veritas Institute. Dr. Joseph Biederman is currently receiving research support from the following sources: The Department of Defense, Food & Drug Administration, Ironshore, Lundbeck, Magceutics Inc., Merck, PamLab, Pfizer, Shire Pharmaceuticals Inc., SPRITES, Sunovion, Vaya Pharma/Enzymotec, and NIH. In 2015, Dr. Joseph Biederman has a US Patent Application pending (Provisional Number #61/233,686) through MGH corporate licensing, on a method to prevent stimulant abuse. In 2014, Dr. Joseph Biederman received honoraria from the MGH Psychiatry Academy for tuition‐funded CME courses. He received research support from AACAP, Alcobra, Forest Research Institute, and Shire Pharmaceuticals Inc. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Ingenix, Prophase, Shire, Bracket Global, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH. In 2013, Dr. Joseph Biederman received an honorarium from the MGH Psychiatry Academy for a tuition‐funded CME course. He received research support from APSARD, ElMindA, McNeil, and Shire. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Shire and Sunovion; these royalties were paid to the Department of Psychiatry at MGH. In 2012, Dr. Joseph Biederman received an honorarium from the MGH Psychiatry Academy and The Children's Hospital of Southwest Florida/Lee Memorial Health System for tuition‐funded CME courses. In previous years, Dr. Joseph Biederman received research support, consultation fees, or speaker's fees for/from the following additional sources: Abbott, Alza, AstraZeneca, Boston University, Bristol Myers Squibb, Cambridge University Press, Celltech, Cephalon, Cipher Pharmaceuticals Inc., Eli Lilly and Co., Esai, Fundacion Areces (Spain), Forest, Fundación Dr.Manuel Camelo A.C., Glaxo, Gliatech, Hastings Center, Janssen, Juste Pharmaceutical Spain, McNeil, Medice Pharmaceuticals (Germany), Merck, MGH Psychiatry Academy, MMC Pediatric, NARSAD, NIDA, New River, NICHD, NIMH, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, Phase V Communications, Physicians Academy, The Prechter Foundation, Quantia Communications, Reed Exhibitions, Shionogi Pharma Inc, Shire, the Spanish Child Psychiatry Association, The Stanley Foundation, UCB Pharma Inc., Veritas, and Wyeth.
This work was performed at Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital.
REFERENCES
- Andrews‐Hanna JR, Reidler JS, Huang C, Buckner RL (2010a): Evidence for the default network's role in spontaneous cognition. J Neurophysiol 104:322−335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010b): Functional‐anatomic fractionation of the brain's default network. Neuron 65:550−562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasanz CP, Staines WR, Schweizer TA (2012): Isolating a cerebellar contribution to rapid visual attention using transcranial magnetic stimulation. Front Behav Neurosci 6:55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Jacobson LA, Wexler JL, Nebel MB, Caffo BS, Pekar JJ, Mostofsky SH (2015): Connectivity supporting attention in children with attention deficit hyperactivity disorder. Neuroimage Clin 7:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT (2007): A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Knee D, Munir K (1990): Retrospective assessment of DSM‐III attention deficit disorder in nonreferred individuals. J Clin Psychiatry 51:102−106. [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ (2012): Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci USA 109:4690−4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL (2013): Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci 17:241−254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL (2013): The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80:807−815. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011): The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322−2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BT (2013): Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 16:832−837. [DOI] [PubMed] [Google Scholar]
- Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, Zuo X, Zang Y, Wang Y (2009): Abnormal resting‐state functional connectivity patterns of the putamen in medication‐naive children with attention deficit hyperactivity disorder. Brain Res 1303:195–206. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Proal E (2012): Large‐scale brain systems in ADHD: Beyond the prefrontal‐striatal model. Trends Cogn Sci 16:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL (2002): Developmental trajectories of brain volume abnormalities in children and adolescents with attention‐deficit/hyperactivity disorder. JAMA 288:17408. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga‐Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR (2005): Varieties of attention‐deficit/hyperactivity disorder‐related intra‐individual variability. Biol Psychiatry 57:1416−1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, et al. (2008): Cingulate‐precuneus interactions: a new locus of dysfunction in adult attention‐deficit/hyperactivity disorder. Biol Psychiatry 63:332−337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield‐Gabrieli S (2012): Anticorrelations in resting state networks without global signal regression. Neuroimage 59:1420−1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, Glover GH, Deisseroth K, Etkin A (2013): Causal interactions between fronto‐parietal central executive and default‐mode networks in humans. Proc Natl Acad Sci USA 110:19944−19949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW (2009): Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA 106:8719−8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Zuo XN, Kelly C, Grzadzinski R, Mennes M, Schvarcz A, Rodman J, Lord C, Castellanos FX, Milham MP (2013): Shared and distinct intrinsic functional network centrality in autism and attention‐deficit/hyperactivity disorder. Biol Psychiatry 74:623−632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G, Naveau M, Petit L, Zago L, Crivello F, Jobard G, Delcroix N, Mellet E, Tzourio‐Mazoyer N Mazoyer B, Joliot M (2012): Patterns of hemodynamic low‐frequency oscillations in the brain are modulated by the nature of free thought during rest. NeuroImage 59:3194–200. [DOI] [PubMed] [Google Scholar]
- Durston S, van Belle J, de Zeeuw P (2011): Differentiating frontostriatal and fronto‐cerebellar circuits in attention‐deficit/hyperactivity disorder. Biol Psychiatry 69:1178−1184. [DOI] [PubMed] [Google Scholar]
- Esterman M, Noonan SK, Rosenberg M, Degutis J (2013): In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cereb Cortex 23:2712−2723. [DOI] [PubMed] [Google Scholar]
- Esterman M, Rosenberg MD, Noonan SK (2014): Intrinsic fluctuations in sustained attention and distractor processing. J Neurosci 34:1724−1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT (2010): Atypical default network connectivity in youth with attention‐deficit/hyperactivity disorder. Biol Psychiatry 68:1084−1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangar US, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang YF, Mostofsky S, Castellanos FX, Milham MP (2012): Distinct neural signatures detected for ADHD subtypes after controlling for micro‐movements in resting state functional connectivity MRI data. Front Syst Neurosci 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Feighner JA, Monuteaux MC (2000): Assessing symptoms of attention deficit hyperactivity disorder in children and adults: which is more valid ? J Consult Clin Psychol 68:830−842. [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer T, Mick E, Murray K, Petty C, Adamson JJ, Monuteaux MC (2006): Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry 163:1720−1729; quiz 1859. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB (2009): A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res 1273:114−128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB ( 2012): Structured Clinical Interview for DSM‐IV® Axis I Disorders (SCID‐I), Clinician Version, Administration Booklet. New York: American Psychiatric Publishing.
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van EDC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin MS, Mrazek MD, Anderson CL, Johnston C, Smallwood J, Kingstone A, Schooler JW: Tracking Distraction: The Relationship Between Mind‐Wandering, Meta‐Awareness, and ADHD Symptomatology. J Atten Disord (in press). [DOI] [PubMed]
- Gordon EM, Breeden AL, Bean SE, Vaidya CJ (2014): Working memory‐related changes in functional connectivity persist beyond task disengagement. Hum Brain Mapp 35:1004−1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD (2009): Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586−8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual‐Leone A (2014): Intermittent theta‐burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci 34:12049−12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos‐Quiroga JA, Richarte Fernandez V, Bosch R, Soliva JC, Rovira M, Bulbena A, Tobena A Casas M, et al. (2014): An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Hum Brain Mapp 35:1261−1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I, Murrough JW, Bansal R, Hao X, Peterson BS (2014): Cerebellar morphology and the effects of stimulant medications in youths with attention deficit‐hyperactivity disorder. Neuropsychopharmacology 39:718−726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): Fsl. NeuroImage 62:782−790. [DOI] [PubMed] [Google Scholar]
- Jilka SR, Scott G, Ham T, Pickering A, Bonnelle V, Braga RM, Leech R, Sharp DJ (2014): Damage to the salience network and interactions with the default mode network. J Neurosci 34:10798−10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997): Schedule for affective disorders and schizophrenia for School‐age Children‐present and lifetime version (K‐SADS‐PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980−988. [DOI] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, Lado FA, Kelly C, Milham M, Mehta AD (2013): Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J Neurosci 33:6333−6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2008): Competition between functional brain networks mediates behavioral variability. Neuroimage 39:527−537. [DOI] [PubMed] [Google Scholar]
- Kessler D, Angstadt M, Welsh RC, Sripada C (2014): Modality‐spanning deficits in attention‐deficit/hyperactivity disorder in functional networks, gray matter, and white matter. J Neurosci 34:16555−16566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, Howes MJ, Jin R, Secnik K Spencer T, et al. (2005): The world health organization adult ADHD Self‐report scale (ASRS): A short screening scale for use in the general population. Psychol Med 35:245−256. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M (2006): Intra‐subject variability in attention‐deficit hyperactivity disorder. Biol Psychiatry 60:1088−1097. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Davis KD (2014): Dynamic functional connectivity of the default mode network tracks daydreaming. NeuroImage 100:471–480. C: [DOI] [PubMed] [Google Scholar]
- Kucyi A, Hodaie M, Davis KD (2012): Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience‐ and attention‐related brain networks. J Neurophysiol 108:3382−3392. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Salomons TV, Davis KD (2013): Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci USA 110:18692−18697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Moayedi M, Weissman‐Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD (2014): Enhanced medial prefrontal‐default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci 34:3969−3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd Sharp WS, Giedd JN Rapoport JL (2007): Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry 164:647−655. [DOI] [PubMed] [Google Scholar]
- Makris N, Liang L, Biederman J, Valera EM, Brown AB, Petty C, Spencer TJ, Faraone SV, Seidman LJ: Toward Defining the Neural Substrates of ADHD: A Controlled Structural MRI Study in Medication‐Naive Adults. J Atten Disord (in press). [DOI] [PMC free article] [PubMed]
- Mattfeld AT, Gabrieli JD, Biederman J, Spencer T, Brown A, Kotte A, Kagan E, Whitfield‐Gabrieli S (2014): Brain differences between persistent and remitted attention deficit hyperactivity disorder. Brain 137:2423−2428. [DOI] [PubMed] [Google Scholar]
- Mittner M, Boekel W, Tucker AM, Turner BM, Heathcote A, Forstmann BU (2014): When the brain takes a break: A model‐based analysis of mind wandering. J Neurosci 34:16286−16295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Costa A, Keeser D, Pogarell O, Berman A, Coates U, Reiser MF, Riedel M, Moller HJ Ettinger U, et al. (2014): The effects of methylphenidate on whole brain intrinsic functional connectivity. Hum Brain Mapp 35:5379−5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009): The impact of global signal regression on resting state correlations: Are anti‐correlated networks introduced? Neuroimage 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH (2014): Reduction of motion‐related artifacts in resting state fMRI using aCompCor. NeuroImage 96:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C, Shine JM, Lewis SJ, Andrews‐Hanna JR, Irish M (2015): Shaped by our thoughts—A new task to assess spontaneous cognition and its associated neural correlates in the default network. Brain Cogn 93:1–10. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen‐Berg H (2010): Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex 20:953−965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J, Lian Z, Xie L, Li X, Wang P, Hao Y, Zhu D, Jiang R, Wang Y Chen Y, et al. (2014): Atomic dynamic functional interaction patterns for characterization of ADHD. Hum Brain Mapp 35:5262−5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Park C, Wang Z (2014): Connecting the dots: A review of resting connectivity MRI studies in attention‐deficit/hyperactivity disorder. Neuropsychol Rev 24:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE (2015): Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 105:536–551. C: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, Buitelaar JK, Beckmann CF (2015): Evaluation of ICA‐AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage 112:278−287. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Evers EA, Theunissen EL, Kuypers KP, Goulas A, Stiers P (2013): Methylphenidate reduces functional connectivity of nucleus accumbens in brain reward circuit. Psychopharmacology (Berl) 229:219−226. [DOI] [PubMed] [Google Scholar]
- Seli P, Smallwood J, Cheyne JA, Smilek D (2015): On the relation of mind wandering and ADHD symptomatology Psychon Bull Rev 22:629–636. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE (1997): Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9:648−663. [DOI] [PubMed] [Google Scholar]
- Sonuga‐Barke EJ, Castellanos FX (2007): Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev 31:977−986. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Mick E (2007): Attention‐deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol 32:631−642. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J (2013): Effect of psychostimulants on brain structure and function in ADHD: A qualitative literature review of magnetic resonance imaging‐based neuroimaging studies. J Clin Psychiatry 74:902−917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Kessler D, Angstadt M (2014): Lag in maturation of the brain's intrinsic functional architecture in attention‐deficit/hyperactivity disorder. Proc Natl Acad Sci USA 111:14259−14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ (2014): Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front Syst Neurosci 8:92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA (2009): Cerebellum and nonmotor function. Annu Rev Neurosci 32:413−434. [DOI] [PubMed] [Google Scholar]
- Sun L, Cao Q, Long X, Sui M, Cao X, Zhu C, Zuo X, An L, Song Y Zang Y, et al. (2012): Abnormal functional connectivity between the anterior cingulate and the default mode network in drug‐naive boys with attention deficit hyperactivity disorder. Psychiatry Res 201:120−127. [DOI] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Fotedar S, Blankner JG, Pekar JJ, Denckla MB, Mostofsky SH (2008): Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: Differences in activation associated with response inhibition but not habitual motor response. J Cogn Neurosci 20:478−493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S Peng M, et al. (2006): Altered resting‐state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett 400:39–43. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ (2005): Functional neuroanatomy of working memory in adults with attention‐deficit/hyperactivity disorder. Biol Psychiatry 57:439−447. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ (2007): Meta‐analysis of structural imaging findings in attention‐deficit/hyperactivity disorder. Biol Psychiatry 61:1361−1369. [DOI] [PubMed] [Google Scholar]
- Valera EM, Brown A, Biederman J, Faraone SV, Makris N, Monuteaux MC, Whitfield‐Gabrieli S, Vitulano M, Schiller M, Seidman LJ (2010a): Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am J Psychiatry 167:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, Biederman J, Seidman LJ (2010b): Neural substrates of impaired sensorimotor timing in adult attention‐deficit/hyperactivity disorder. Biol Psychiatry 68:359−367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59:431−438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva R (2012): The cerebellum and neuropsychiatric disorders. Psychiatry Res 198:527−532. [DOI] [PubMed] [Google Scholar]
- Wang D, Buckner RL, Liu H (2013): Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J Neurophysiol 109:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu C, He Y, Zang Y, Cao Q, Zhang H, Zhong Q, Wang Y (2009): Altered small‐world brain functional networks in children with attention‐deficit/hyperactivity disorder. Hum Brain Mapp 30:638−649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D ( 1999): Wechsler Abbreviated Scale of Intelligence. San Antonio TX: Psychological Corporation. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG (2006): The neural bases of momentary lapses in attention. Nat Neurosci 9:971−978. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Ford JM (2012): Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC (2002): A general statistical analysis for fMRI data. NeuroImage 15:1–15. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L Polimeni JR, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125−1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, Yao S (2012): Evidence of a dissociation pattern in resting‐state default mode network connectivity in first‐episode, treatment‐naive major depression patients. Biol Psychiatry 71:611−617. [DOI] [PubMed] [Google Scholar]


