Abstract
Recent evidence suggests that the behavioral benefits associated with voluntary wheel running in rodents may be due to modulation of glutamatergic transmission in the hippocampus, a brain region implicated in learning and memory. However, the expression of the n-Methyl-d-Aspartate glutamate receptor subunits (GluNs) in the hippocampus in response to chronic sustained voluntary wheel running has not yet been investigated. Further, the developmental effects during young and mature adulthood on wheel running output and GluN expression in hippocampal subregions has not been determined, and therefore is the main focus of this investigation. Eight-week-old and sixteen-week-old male Wistar rats were housed in home cages with free access to running wheels and running output was monitored for four weeks. Wheel access was terminated and tissue from the dorsal and ventral hippocampi were processed for Western blot analysis of GluN subunit expression. Young adult runners demonstrated an escalation in running output but this behavior was not evident in mature adult runners. In parallel, young adult runners demonstrated a significant increase in total GluN (1 and 2A) subunit expression in the dorsal hippocampus, and an opposing effect in the ventral hippocampus compared to age-matched sedentary controls; these changes in total protein expression were not associated with significant alterations in the phosphorylation of the GluN subunits. In contrast, mature adult runners demonstrated a reduction in total GluN2A expression in the dorsal hippocampus, without producing alterations in the ventral hippocampus compared to age-matched sedentary controls. In conclusion, differential running activity-mediated modulation of GluN subunit expression in the hippocampal subregions was revealed to be associated with developmental effects on running activity, which may contribute to altered hippocampal synaptic activity and behavioral outcomes in young and mature adult subjects.
Keywords: exercise, hippocampus, NMDA receptor, aging, dorsal, ventral
Introduction
Physical activity has long been touted as a critical component of a long and high-quality life. Individuals who routinely exercise (via sustained physical activity) benefit from a longer life expectancy (Haapanen-Niemi et al., 2000; Savela et al., 2010; Autenrieth et al., 2011; Wen et al., 2011; Lee et al., 2014) and show improvements in cognitive function (for reviews, see (Hillman et al., 2008; Erickson and Kramer, 2009; Smith et al., 2013)). Specifically, studies have highlighted increases in hippocampal volume (Colcombe and Kramer, 2003; Colcombe et al., 2003; Colcombe et al., 2006; Pajonk et al., 2010; Varma et al., 2014) and hippocampal functioning (Colcombe and Kramer, 2003; Pajonk et al., 2010; Chang et al., 2012; Loprinzi and Kane, 2015) in both young adult and mature adult populations. Further, these findings in humans have been replicated in a large volume of preclinical studies, supporting the connection between physical activity and cognitive capacities, and prompting inquiry into its molecular and cellular underpinnings (for review of the overlapping studies, see (Voss et al., 2013)). The most often cited molecular mechanism in the hippocampus underlying the structural and functional improvement in exercising animals is adult neurogenesis and expression of brain derived neurotrophic factor (BDNF) (van Praag et al., 2005; Yau and Gil-Mohapel, 2014) with striking increases in cell proliferation and associated cognitive performance (van Praag et al., 1999; van Praag et al., 2005; Van der Borght et al., 2007; Wu et al., 2008; Siette et al., 2013; Speisman et al., 2013; Gibbons et al., 2014; Merkley et al., 2014). Interestingly, these pro-neurogenic effects are also observed in models of environmental enrichment (reviewed in (Bekinschtein et al., 2011)), a housing condition which often included access to an exercise wheel. However, the functional molecular components of hippocampal cognition, glutamatergic receptors, have been less investigated in both voluntary exercise and environmental enrichment. With regards to environmental enrichment, there is an increase in glutamatergic receptor expression (Tang et al., 2001; Andin et al., 2007), but evidence suggests that voluntary exercise is the strongest contributing factor to the molecular changes observed in environmentally enriched animals (Ehninger and Kempermann, 2003; Kobilo et al., 2011). While one study in mice implicated the glutamatergic signaling system as the potential source of physical activity’s positive modulation of hippocampal volume and function (Biedermann et al., 2012), the mechanisms associated with neuronal plasticity underlying the enhanced hippocampal function in the context of sustained physical activity have not been explicitly determined. Furthermore, the effects of exercise on the expression of plasticity-associated proteins in the hippocampus during young and mature adulthood have not been examined.
One molecular component that is associated with neuronal synaptic plasticity and is critical to hippocampal function is the glutamatergic ionotropic N-methyl-D-aspartate receptor (GluN). In the hippocampus, GluN receptor activation is essential for long-term potentiation (LTP) (Bashir et al., 1993; Shipton and Paulsen, 2014), the primary cellular property believed to underlie hippocampal learning and memory (Bliss and Collingridge, 1993). GluNs are heteromeric tetramers which are comprised of two GluN1 obligatory subunits and any combination of two GluN2A-D or GluN3A-B (Paoletti et al., 2013). As an obligatory subunit, GluN1 can serve as a suitable indicator of total GluN expression. The composition of the remaining subunits and their phosphorylation states can imply specific membrane localization and functionality, and thereby facilitate learning and memory (Paoletti et al., 2013). There has been considerable inquiry into how exercise via voluntary wheel running can independently influence GluN expression and function in the hippocampus. For example, prolonged wheel running (with resistance) in 12-week old rats has been implicated in increasing GluN subunit GluN2A and GluN2B mRNA expression (Molteni et al., 2002) and receptor functionality (Farmer et al., 2004; Dietrich et al., 2005; Vasuta et al., 2007). However, it is unknown whether such effects on GluN subunits by exercise are higher during young adulthood (6–10 weeks of age; a developmental timeframe associated with higher neurogenesis, higher density of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (GluAs) and BDNF protein levels in the hippocampus) compared with mature adulthood (16–20 weeks of age; (Gulve et al., 1993; Coutinho et al., 2006; Lang et al., 2009; Carreton et al., 2012)). Furthermore, this molecular-level regulation, evaluated thus far nearly exclusively in the dorsal hippocampus (DH) of young adult rodents, could be one potential mechanistic underpinning of exercise’s role in the enhancement of hippocampal-sensitive cognition (Voss et al., 2013). Given the mechanistic distinction and role of DH in spatial memory (for review see (Hartley et al., 2014)) and temporal memory (for review see (Eichenbaum, 2014)) and ventral hippocampus (VH) in emotional regulation and anxiety-like behaviors ((Moser et al., 1995; Kjelstrup et al., 2002; Bannerman et al., 2004; Pothuizen et al., 2004; Pentkowski et al., 2006); for review see (Miller and Hen, 2015)), it is essential to understand the effects of exercise on these anatomically defined regions of the hippocampus.
The current study therefore investigated the effect of prolonged voluntary exercise on glutamatergic receptor expression in distinct hippocampal subregions in animals of two disparate ages during adulthood. We hypothesize that, expression of GluNs will be increased in young adult runners and that this activity-related effect will be attenuated in mature adult runners compared with their age-matched sedentary controls. Additionally, we speculate that alterations in GluN expression would be greater in the DH due to the critical role of GluNs in DH cognitive function. However, as the role of GluNs in VH emotional functionality has not been extensively evaluated, it is possible that the two distinct regions be effected similarly subsequent to physical activity.
Materials and Methods
Experimental procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85–23, revised 1996) and approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Animals
Adult male Wistar rats (Charles River), were housed in a temperature-controlled (22°C) vivarium on a 12 h/12 h light/dark cycle (lights on at 8:00 P.M.) with ad libitum access to food and water. At either 8 weeks of age (young adult) or 16 weeks (mature adults), rats assigned to the voluntary exercise group were moved into individual housing with ad libitum access to a running wheel (Nalgene activity wheels 34.5 cm diameter x 9.7 cm wide with magnetic switches connected to a PC for monitoring). The total number of revolutions was recorded in 10 minute bins and summed for each 24 h period for four (VitalView, Minimitter Inc.).
Tissue Collection
Following cessation of voluntary exercise (or age-matched for sedentary controls), within one hour of removal from running wheel cages, rats were briefly anesthetized with isoflourane, then rapidly decapitated and the brain was immediately removed. The brain was cut along the mid-sagital axis and right hemisphere and was quickly frozen in dry ice-cooled isopentane and stored at −80°C until further processing. Dorsal (−3.14 to −4.30 mm from bregma) and ventral (−5.30 to −6.1 mm from bregma as identified in (Paxinos and Watson, 2007)) hippocampal tissue punches were collected from 500μm thick sections and stored at −80°C until further processing (Figure 3Ai and 3Bi).
Figure 3. GluN Expression in the Dorsal and Ventral Hippocampus of Young Adult and Mature Adult Runners.
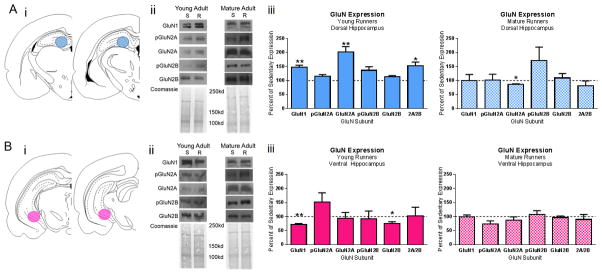
Dorsal (A) and ventral (B) hippocampus tissue was collected from young and mature adult runners. i) Schematic representations of dorsal (AP −3.14 mm to −4.30 mm from bregma) and ventral hippocampus (AP −5.3 mm to −6.1 mm from bregma) sections adapted from (Paxinos and Watson, 2007). Tissue punches were collected from 500 μm thick sections of young adult and mature adult sedentary and running rats. Blue circles represent site of dorsal tissue collection and pink circles represent site of ventral tissue collection via tissue punch. ii) Representative western blots and associated coomassie staining in sedentary (S) and running (R) animals. iii) Summarized data for GluN subunit expression as percent of sedentary age-match controls. Asterisks denote significant differences between age groups of runners within hippocampal subregion. *p≤0.05, **p≤0.01
Western Blot Analysis
Procedures optimized for measuring neuronal levels of both phosphoproteins and total proteins was performed as previously described (Kim et al., 2014; Galinato et al., 2015; Navarro and Mandyam, 2015; Staples et al., 2015). Tissue was homogenized on ice by sonication in buffer (320 mM sucrose, 5 mM HEPES, 1 mM EGTA, 1 mM EDTA, 1% SDS, with Protease Inhibitor Cocktail and Phosphatase Inhibitor Cocktails II and III diluted 1:100; Sigma), heated at 100 degrees C for five minutes, and stored at −80 degrees C until determination of protein concentration by a detergent-compatible Lowry method (Bio-Rad, Hercules, CA). Samples were mixed (1:1) with a Laemmli sample buffer containing β-mercaptoethanol. Each sample containing protein from one animal was run (20 μg per lane) on 8% SDS-PAGE gels (Bio-Rad) and transferred to polyvinylidene fluoride membranes (PVDF pore size 0.2 μm). Blots were blocked with 2.5% (for phosphoproteins) or 5% milk (w/v) in TBST (25 mM Tris-HCl (pH 7.4), 150 mM NaCl and 0.1% Tween 20 (v/v)) for one hour at room temperature and were incubated with the primary antibody for 16–20 h at 4 °C. Primary antibodies and dilutions are listed in Table 1. Blots were then washed three times for 5 min in TBST, and then incubated for 1 h at room temperature with horseradish peroxide–conjugated goat antibody to rabbit in TBST (for dilutions see Table 1). After another three washes for 5 min with TBST, immunoreactivity was detected using SuperSignal West Dura chemiluminescence detection reagent (Thermo Scientific) and collected using HyBlot CL Autoradiography film (Denville Scientific) and a Kodak film processor. Blots were then stained Coomassie Blue to normalize to the quantity of protein loaded in each lane (Welinder and Ekblad, 2011). Densitometry was performed using ImageStudio software (Li-Cor Biosciences). X-ray films were digitally scanned at 600dpi resolution, then bands of interest were selected in identically sized selection boxes within the imaging program which included a 3 pixel extended rectangle for assessment of the background signal. The average signal of the pixels in the “background” region (between the exterior border of the region of interest selection box and the additional 3 pixel border) was then subtracted from the signal value calculated for the band of interest. This was repeated for the coomassie stained lane corresponding to the band of interest, and the signal value of the band of interest following subtraction of the background calculation was then expressed as a ratio of the corresponding coomassie signal (following background subtraction). This ratio of expression for each band was then expressed as a percent of the age- and hippocampal subregion specific sedentary controls on the same blot.
Table 1.
Primary and Secondary Antibody Dilutions for Western Blot Analysis of GluN Subunits.
| Protein of Interest |
Reported kD |
Blocking | 1* Vendor | 1* Catalog Number |
1* Host | 1* Dilution |
2* Vendor | 2* Catalog Number |
2* Host | 2* Dilution |
Observed kD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GluN1 | 130 | 5% Milk | Santa Cruz Biotechnology | 1467 | Goat | 1:100 | Santa Cruz Biotechnology | 2020 | Donkey | 1:1000 | 125 |
| pGluN2A | 180 | 2.5% Milk | PhosphoSolutions | P1514-1325 | Rabbit | 1:200 | Bio-Rad | 170-6515 | Goat | 1:2000 | 200 |
| GluN2A | 178 | 5% Milk | Santa Cruz Biotechnology | 9056 | Rabbit | 1:200 | Bio-Rad | 170-6515 | Goat | 1:2000 | 200 |
| pGluN2B | 190 | 2.5% Milk | Cell Signaling | 4208S | Rabbit | 1:200 | Bio-Rad | 170-6515 | Goat | 1:2000 | 200 |
| GluN2B | 178 | 5% Milk | Santa Cruz Biotechnology | 9057 | Rabbit | 1:200 | Bio-Rad | 170-6515 | Goat | 1:2000 | 200 |
Statistical analysis
Body weight was monitored weekly for all subjects beginning at onset of voluntary exercise (age-matched for sedentary controls). Weight gained during the duration of the experiment was analyzed by a Two-Way ANOVA with age and physical activity the between-subject independent variables and weight gained the dependent variable.
Running output was monitored in one hour bins and analyzed as daily output over a period of 4 weeks. Daily running activity was statistically assessed utilizing a mixed-model Two-Way ANOVA with age-group as the between-subject and day of activity as the within-subject independent factors and number of wheel revolutions the dependent factors. Post-hoc comparisons (Fisher’s LSD) were performed comparing young adult and mature adult wheel activity on each day, as the daily average activity output within each age group to the day one average activity. Change in running activity was assessed as the percent change of wheel revolutions between the first three and last three activity days and was statistically analyzed by two-sided t-test. For circadian activity analysis, wheel revolutions per hour were analyzed on the first and last day of running activity for each age as Day 1 vs Day 28 activity within age groups (performed as a Repeated Measures Two-Way ANOVA with activity day and hour of day as the independent factors and wheel revolutions as the dependent factor).
The effects of exercise (analyzed within age groups and hippocampal subregions) on GluN subunit expression were analyzed using two-sided unpaired t-tests. A Pearson correlation was performed between running output on the last day of wheel access, the independent variable, and relative GluN subunit expression, the dependent variable, within each age and hippocampal subregion.
Data presented are expressed as mean ± SEM. Values of p ≤ 0.05 were considered statistically significant. Graphs and statistical analysis were generated using GraphPad Prism 6.0 software.
Results
Weight Gain during Voluntary Running in Young and Mature Adult Rats
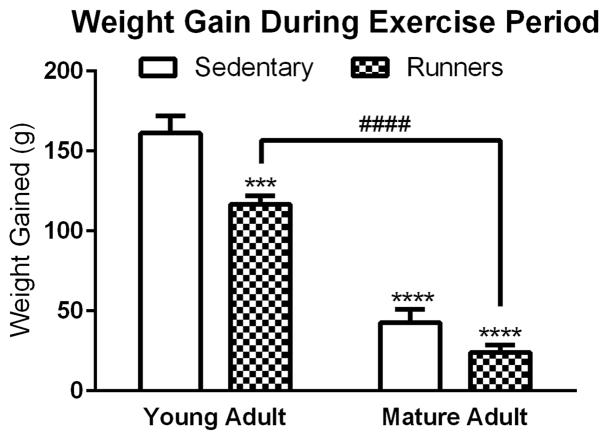
Rats were weighed once weekly during the exercise period (or time matched for sedentary controls); the average amount of weight gained by each group is presented in Figure 1. Two-way ANOVA analysis with age and activity as the independent factors and weight change as the dependent factor demonstrated a significant main effect of age [F(1, 23)=205.71, p<0.0001] and a significant main effect of activity [F(1, 23)=18.60, p=0.0003], with both factors resulting in reduced weight gains. The interaction of the two factors (age x activity) was not significant [F(1, 23)=3.08, p=0.0926]; therefore, post-hoc analysis was not conducted for this comparison.
Figure 1. Weight Gain during the Exercise Period.
The weight gained by each animal was monitored during the exercise period (Weight on final day of running – weight on first day of running). Running and age were significant factors in the amount of weight gained by each animal. Asterisk denotes a significant difference between sedentary young adult rats and other groups, hatch denotes a significant difference between young adult and mature adult runners. ***p≤0.001, ****p≤0.0001, ####p≤0.0001.
Running Output in Young and Mature Adult Rats
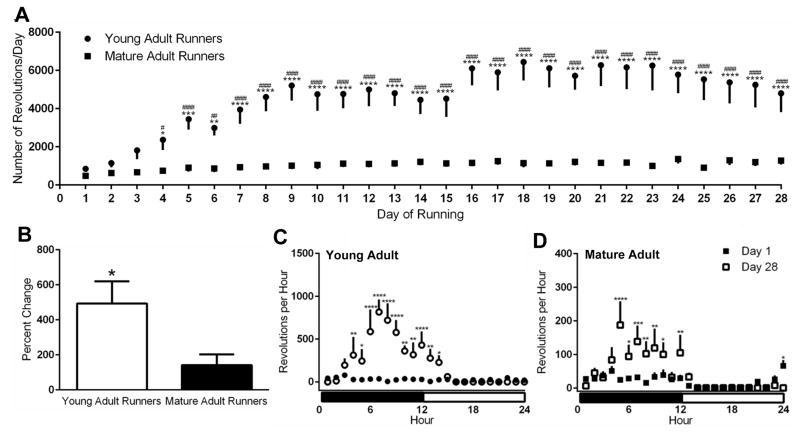
Running output as revolutions per hour and day was monitored for the duration of the exercise period (Figure 2). Mixed model two-way ANOVA analysis, with age as the between-subjects independent factor, day of running the within-subject independent factor, and average wheel revolutions per day as the dependent factor, demonstrated a significant main effect of age [F(1, 13)=19.58, p=0.0007], significant main effect of time [F(27, 351)=5.83, p<0.0001], and a significant interaction of the two factors (age x time) [F(27, 351)=3.66, p<0.0001]. Subsequent post-hoc comparisons demonstrated the young adult runners demonstrating greater activity levels that their mature adult counterparts (p<0.05; days 4–28; Figure 2A). Additionally, compared to day 1, the amount of running measured in young adult rats was significantly increased by day 4 (p<0.05) and remained higher for the duration of the activity period (p values); this pattern of increase in activity was not observed in the mature adult runners. Young adult runners exhibited a nearly 500% increase in activity (calculated as [(Average running days 26, 27, and 28)- (Average running days 1, 2, and 3)]/(Average running days 1, 2, and 3)*100) while mature adult runners exhibited a significantly smaller increase (141.5% increase over the average of day 1–3) over the duration of the running period (p=0.05; Figure 2B).
Figure 2. Running Activity in Young Adult and Mature Adult Rats.
A) Daily running activity measured as number of wheel revolutions per day for young adult and mature adult rats. Asterisk denotes a significant difference between young adult and mature adult animals on a given running day, hatch denotes a significant difference of running activity between a given day of activity and the first day of wheel access. B) Percent change in running activity in young adult and mature adult rats from the first day of running to the last day of running. C) Daily wheel running patterns of young adult rats on day one (filled circle) and day 28 (open circle) of wheel access. Asterisk denotes a significant difference in activity between groups at a given time of day. D) Daily wheel running patterns of mature adult rats on day one (filled square) and day 28 (open square) of wheel access. Asterisk denotes a significant difference in activity between groups at a given time of day. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, #p<0.05, ##p<0.01, ###p<0.001, ####p<0.0001
Circadian Patterns of Running Output in Young and Mature Adult Runners
Further analyses were performed with regards to the pattern of running during the first and last 24-hour period in young adult and mature adult runners (Figures 2C and 2D). Repeated measures ANOVA in the young adult animals demonstrated a main effect of hour of day [F(23, 184)=6.428, p<0.0001], a main effect of running day [F(1, 8)=12.67, p=0.0074], and a significant interaction of the two factors (hour x day) [F(23, 184)=6.717, p<0.0001]. Post-hoc analysis revealed no significant difference in running behavior at any time of day on the first day of running activity, but increased running during the dark phase of the 12h/12h light cycle as compared to the light phase on the last day of running activity (Figure 2C). Similarly, repeated measures ANOVA in the mature adult animals demonstrated a main effect of hour of day [F(23, 138)=5.050, p<0.0001], a main effect of running day [F(1, 6)=8.241, p=0.0284], and a significant interaction of the two factors (hour x day) [F(23, 138)=6.717, p<0.0001] and post-hoc analysis demonstrated significant preference for running during the dark cycle on the last day of activity (Figure 2D). Repeated measure Two-Way ANOVA of running output on the final day of activity between young and mature adult rats demonstrated a significant main effect of age [F(1, 14)=8.295, p=0.012], hour of day [F(23, 322)=6.783, p<0.0001], and a significant interaction of the two factors (age x hour) [F(23, 322)=3.451, p<0.0001]. Post-hoc analysis revealed significant increases in running behavior in young animals during the dark phase (hours 6–12) as compared to mature adult runners.
GluN Expression Following Voluntary Exercise in Young and Mature Adult Rats
Animals were euthanized following the completion of the running period, and their hippocampal tissue was processed for western blot analysis (Figure 3Ai and Bi). The expression of select GluN subunits, in relation to age-matched sedentary controls, is presented in Figure 3. Two-sided unpaired t-tests for each GluN subunit and phosphorylation site revealed significant differences between sedentary and exercising young adults in GluN1 DH (increase; t=3.313, df=13, p=0.0056) and VH (decrease; t=4.182, df=13, p=0.0011), GluN2A in the DH (increase; t=3.685, df=13, p=0.0027), and the ratio of 2A/2B in DH (t=2.685, df=13, p=0.0187).
Similar to young adult animals, mature adult rats were euthanized following the completion of the running period, and their hippocampal tissue was processed for western blot analysis as previously described. Two-sided unpaired t-tests for each GluN subunit and phosphorylation site revealed a significant reduction in GluN2A in the DH (t=2.365, df= 10, p=0.0369). However, there was no other significant difference in expression of other GluN subunits in the DH or VH of mature running animals as compared to age- and region- matched sedentary controls.
Relationship between GluN Expression and running output in young and mature adult rats
Pearson’s correlations of various metrics of running output and relative subunit expression revealed a significant negative correlation between running activity and GluN2A expression in the VH of young adults in relation to the last day of running (r = −0.76, R2 = 0.58, p = 0.03) and total running (r = −0.75, R2 = 0.56, p = 0.03), while running activity in mature adults was positively associated with GluN2A expression in the VH (last week of running: r = 0.86, R2 = 0.73, p = 0.03; last day of running: r = 0.88, R2 = 0.78, p = 0.02) (Table 2). Other relationships were not statistically significant.
Table 2.
Running Activity and GluN Expression in Young Adult and Mature Adult Dorsal and Ventral Hippocampus.
| Young Adult | Mature Adult | |
|---|---|---|
| GluN2A | GluN2A | |
| Total Running (Sum) | DH R2=0.23, p=0.22 VH R2=0.56, r = −0.76; p = 0.03* |
DH R2<0.01, p = 0.97 VH R2=0.59, p = 0.07 |
| Percent Change in Running | DH R2=0.18, p = 0.30 VH R2=0.23, p = 0.22 |
DH R2=0.25, p = 0.31 VH R2=0.14, p = 0.47 |
| Last Week of Running (Sum) | DH R2=0.40, p=0.09 VH R2=0.51, r = −0.71; p = 0.05 |
DH R2 = 0.03, p = 0.74 VH R2=0.73, r = 0.86; p = 0.03* |
| Last Day of Running | DH R2=0.40, p = 0.09 VH R2=0.59, r = −0.75; p = 0.03* |
DH R2=0.08, p = 0.58 VH R2=0.78, r = 0.88; p = 0.02* |
Discussion
The primary goal of this work was to identify the role of exercise in modulation of GluN expression in distinct hippocampal subregions in young adult animals and determine if this modulation differs in mature adult animals. By allowing rats to begin running at two different stages of adulthood, we are able to report two major findings: 1) there are striking behavioral (running output) differences between young adult and mature adult runners, 2) running-induced changes in GluN subunit expression was subregionally distinct within the hippocampus in young adult and mature adult runners.
Broadly speaking, young adult animals escalated their running activity and showed significantly more running output than mature adult animals, and this was reflective of the magnitude of GluN expression change in the DH. From a translational perspective, this difference in running behavior is potentially an important observation, as much of our understanding of how running influences molecular and cellular components of learning is derived from analysis of young adult and aged animals. Specifically, we found that young adult animals, over the 28 day wheel access period, increased their activity while mature adult animals maintained a low-level of voluntary exercise. This discrepancy has been observed previously in middle-aged and aged animals (6 month and 24 months of age), with the animals of advanced age running 1/10th the distance per day of the younger animals (McCullough et al., 2013). Similarly, it is observed that rats run significantly more at a younger age (4 months old) than they did as older rats (6–10 months of age) when allowed to freely exercise (Gulve et al., 1993). Taken together, the current findings in young adult and mature adult rats add to the previous studies conducted in middle-aged and aged rats that running output decreases with age, and this effect is observed at a time point during adult development, much earlier than previously investigated. A minor limitation in the current study is the lack of behavioral explanation for the age-based discrepancy in running behavior during adulthood. For example, peripheral physiological constraints such as reduced musculature efficiency (Hepple et al., 2003), increased propensity towards fatigue (Norton et al., 2001), and reduced blood oxygenation and utilization (Poole and Ferreira, 2007) could contribute to the effects.
With regards to the microanalysis of wheel running in young and mature adult animals, we found that rats in both groups on day one of wheel access do not have a preference for the day or night cycle for running activity. By day 2 of wheel access, both young adult and mature adult runners had established a preference for physical activity during the dark phase of the 24 hour cycle, suggesting that the temporal pattern of diurnal wheel running activity was similar in both groups (Klante et al., 1999).
Secondly, our findings demonstrate that young adult rats were more sensitive to the modulating effects of running on GluN expression when compared to mature adults. This finding is important because, the most widely investigated subunits of the GluN receptors are GluN2A and GluN2B due in large part to their extensive distribution in the adult hippocampus and their pivotal roles in hippocampal dependent learning and memory (Paoletti et al., 2013). With regards to hippocampal LTP, the GluN2A subunit is thought to be responsible for a larger influx of calcium upon receptor activation (potentially facilitating the induction of LTP) while GluN2B containing receptors are believed to moderate the intracellular signaling cascades (potentially facilitating the persistence of LTP) (Erreger et al., 2005). Therefore, the ratio of GluN2A to GluN2B expression can be indicative of the propensity towards LTP induction (Yashiro and Philpot, 2008). Further, the phosphorylation of these subunits (Glun2A at tyrosine 1325 and GluN2B at tyrosine 1472), both translationally modified by Src or Fyn kinase (Wang et al., 2014), can impact the functionality of the receptor. Increased phosphorylation of GluN2A at tyrosine 1325 is implicated in increasing the calcium conductance of GluN2A-containing receptors (Taniguchi et al., 2009) while phosphorylation of Glun2B at tyrosine 1472 implies increases in GluN2B-containing receptors at the synaptic membrane (Goebel-Goody et al., 2009). Taken together, the expression patterns of GluN subunits can be indicative of the total number of receptors, cellular localization, or functionality, of the receptors. Our findings demonstrate that there was a distinct difference in GluN modulation due to wheel running by age during adulthood in the DH, with young adult animals having increases in GluN1, GluN2A, and the ratio of GluN2A to GluN2B subunit expression, while mature adult animals exhibit a reduction of GluN2A in response to running. The changes in GluN subunit expression in DH of young adult runners are likely to be associated with previously reported increased cognitive capacities in the hippocampus (O’Callaghan et al., 2007; Griffin et al., 2009; Berchtold et al., 2010; Li et al., 2013; Siette et al., 2013; Speisman et al., 2013). In the VH, GluN expression was also differentially modulated in young adult runners (reduced expression of GluN1 and 2B) compared with mature adult runners (no change), providing further evidence that the effects of running on GluN subunit expression are affected by developmental effects during adulthood. Furthermore, linear regression analysis indicated that exercise output predicts GluN2A expression in the VH in young and mature adults, where higher output predicted lower GluN2A expression in young adults, whereas higher output predicted higher GluN2A expression in mature adults. These results suggest that while adaptations in GluN2A receptor expression are produced by long-term running activity, they are significantly varied in young adult versus mature adult animals. Taken together, given that the hippocampus subregions are associated with distinct functional roles, with the DH being implicated in cognitive functions (such as spatial navigation and contextual memories) and the VH responsible for the emotional regulation (negative affect and anxiety-like responses; (for review see (Fanselow and Dong (2010))), it will be important to determine the functional significance and direct linkage of the current findings on the varied GluN subunit expression in the DH and VH in young adult and mature adult runners.
These findings, while intriguing, must be presented with a caveat. We must acknowledge that this enhanced GluN sensitivity to exercise observed in young adult animals is likely due, at least in part, to the reduction in the amount of activity measured in the mature adult animals. There is possibly a threshold of minimal physical activity which must be achieved in order for the GluN modulating effects of exercise to be observable, and the mature adult animals did not breach this threshold. Therefore, future studies to address this would require a forced exercise paradigm, which was avoided in the present work to avoid the potential for stress effects subsequent to forced activity. Additionally, we examined hippocampal homogenate from the entirety of the DH and the VH. As the hippocampus is further subdivided into 3 functionally distinct regions (the cornu amonis 3 [CA3], cornu amonis 1 [CA1], and the dentate gyrus [DG]), there may be a loss of effect by not evaluating the regions separately.
In summary, our findings here reveal developmental effects of age during adulthood- and region- specific modulation of hippocampal GluN receptors following extended access to voluntary wheel running. Future studies stemming from this work should include investigations into the emotional and behavioral correlates of these findings to better attribute the molecular changes in receptor expression to a quantifiable and translational output.
Highlights.
Young adult runners escalate running activity
Age is a factor in the hippocampal GluN response to voluntary wheel running
GluN response is subregionally distinct within the hippocampus
Acknowledgments
The authors would like to thank Ms. McKenzie Fannon for her careful and thoughtful review and critique of this manuscript, Eva Zamora-Martinez and Jaqueline Quigly for assistance with animal handling, Atoosa Ghofranian and Jacob Garrett for assistance with tissue processing, as well as acknowledge NIH, AA020098, AA06420 and DA034140 (to CDM), and T32AA00747 (to MCS) for funding this work. This is manuscript number 29085 from The Scripps Research Institute.
Footnotes
Conflict of Interest
The authors claim no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andin J, Hallbeck M, Mohammed AH, Marcusson J. Influence of environmental enrichment on steady-state mRNA levels for EAAC1, AMPA1 and NMDA2A receptor subunits in rat hippocampus. Brain research. 2007;1174:18–27. doi: 10.1016/j.brainres.2007.06.101. [DOI] [PubMed] [Google Scholar]
- Autenrieth CS, Baumert J, Baumeister SE, Fischer B, Peters A, Doring A, Thorand B. Association between domains of physical activity and all-cause, cardiovascular and cancer mortality. European journal of epidemiology. 2011;26:91–99. doi: 10.1007/s10654-010-9517-6. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience and biobehavioral reviews. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Oomen CA, Saksida LM, Bussey TJ. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Seminars in cell & developmental biology. 2011;22:536–542. doi: 10.1016/j.semcdb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–597. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann S, Fuss J, Zheng L, Sartorius A, Falfan-Melgoza C, Demirakca T, Gass P, Ende G, Weber-Fahr W. In vivo voxel based morphometry: detection of increased hippocampal volume and decreased glutamate levels in exercising mice. NeuroImage. 2012;61:1206–1212. doi: 10.1016/j.neuroimage.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Carreton O, Giralt A, Torres-Peraza JF, Brito V, Lucas JJ, Gines S, Canals JM, Alberch J. Age-dependent decline of motor neocortex but not hippocampal performance in heterozygous BDNF mice correlates with a decrease of cortical PSD-95 but an increase of hippocampal TrkB levels. Experimental neurology. 2012;237:335–345. doi: 10.1016/j.expneurol.2012.06.033. [DOI] [PubMed] [Google Scholar]
- Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: a meta-analysis. Brain research. 2012;1453:87–101. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Coutinho AE, Fediuc S, Campbell JE, Riddell MC. Metabolic effects of voluntary wheel running in young and old Syrian golden hamsters. Physiology & behavior. 2006;87:360–367. doi: 10.1016/j.physbeh.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Mantese CE, Porciuncula LO, Ghisleni G, Vinade L, Souza DO, Portela LV. Exercise affects glutamate receptors in postsynaptic densities from cortical mice brain. Brain research. 2005;1065:20–25. doi: 10.1016/j.brainres.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cerebral cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Time cells in the hippocampus: a new dimension for mapping memories. Nature reviews Neuroscience. 2014;15:732–744. doi: 10.1038/nrn3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. British journal of sports medicine. 2009;43:22–24. doi: 10.1136/bjsm.2008.052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. The Journal of physiology. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Galinato MH, Orio L, Mandyam CD. Methamphetamine differentially affects BDNF and cell death factors in anatomically defined regions of the hippocampus. Neuroscience. 2015;286:97–108. doi: 10.1016/j.neuroscience.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons TE, Pence BD, Petr G, Ossyra JM, Mach HC, Bhattacharya TK, Perez S, Martin SA, McCusker RH, Kelley KW, Rhodes JS, Johnson RW, Woods JA. Voluntary wheel running, but not a diet containing (−)-epigallocatechin-3-gallate and beta-alanine, improves learning, memory and hippocampal neurogenesis in aged mice. Behavioural brain research. 2014;272c:131–140. doi: 10.1016/j.bbr.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009;19:973–980. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Gulve EA, Rodnick KJ, Henriksen EJ, Holloszy JO. Effects of wheel running on glucose transporter (GLUT4) concentration in skeletal muscle of young adult and old rats. Mechanisms of ageing and development. 1993;67:187–200. doi: 10.1016/0047-6374(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Haapanen-Niemi N, Miilunpalo S, Pasanen M, Vuori I, Oja P, Malmberg J. Body mass index, physical inactivity and low level of physical fitness as determinants of all-cause and cardiovascular disease mortality--16 y follow-up of middle-aged and elderly men and women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000;24:1465–1474. doi: 10.1038/sj.ijo.0801426. [DOI] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, O’Keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014;369:20120510. doi: 10.1098/rstb.2012.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT, Hagen JL, Krause DJ, Jackson CC. Aerobic power declines with aging in rat skeletal muscles perfused at matched convective O2 delivery. Journal of applied physiology (Bethesda, Md : 1985) 2003;94:744–751. doi: 10.1152/japplphysiol.00737.2002. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain structure & function. 2014 doi: 10.1007/s00429-014-0755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klante G, Secci K, Masson-Pevet M, Pevet P, Vivien-Roels B, Steinlechner S, Wollnik F. Interstrain differences in activity pattern, pineal function, and SCN melatonin receptor density of rats. The American journal of physiology. 1999;276:R1078–1086. doi: 10.1152/ajpregu.1999.276.4.R1078. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learning & memory. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang BT, Yan Y, Dempsey RJ, Vemuganti R. Impaired neurogenesis in adult type-2 diabetic rats. Brain research. 2009;1258:25–33. doi: 10.1016/j.brainres.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. Journal of the American College of Cardiology. 2014;64:472–481. doi: 10.1016/j.jacc.2014.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liang A, Guan F, Fan R, Chi L, Yang B. Regular treadmill running improves spatial learning and memory performance in young mice through increased hippocampal neurogenesis and decreased stress. Brain research. 2013;1531:1–8. doi: 10.1016/j.brainres.2013.07.041. [DOI] [PubMed] [Google Scholar]
- Loprinzi PD, Kane CJ. Exercise and Cognitive Function: A Randomized Controlled Trial Examining Acute Exercise and Free-Living Physical Activity and Sedentary Effects. Mayo Clinic proceedings. 2015 doi: 10.1016/j.mayocp.2014.12.023. [DOI] [PubMed] [Google Scholar]
- McCullough MJ, Gyorkos AM, Spitsbergen JM. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience. 2013;240:258–268. doi: 10.1016/j.neuroscience.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkley CM, Jian C, Mosa A, Tan YF, Wojtowicz JM. Homeostatic regulation of adult hippocampal neurogenesis in aging rats: long-term effects of early exercise. Frontiers in neuroscience. 2014;8:174. doi: 10.3389/fnins.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Current opinion in neurobiology. 2015;30c:51–58. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. The European journal of neuroscience. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro AI, Mandyam CD. Protracted abstinence from chronic ethanol exposure alters the structure of neurons and expression of oligodendrocytes and myelin in the medial prefrontal cortex. Neuroscience. 2015;293:35–44. doi: 10.1016/j.neuroscience.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton MW, Mejia W, McCarter RJ. Age, fatigue, and excitation-contraction coupling in masseter muscles of rats. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56:B58–65. doi: 10.1093/gerona/56.2.b58. [DOI] [PubMed] [Google Scholar]
- O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behavioural brain research. 2007;176:362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Kierer A, Muller S, Oest M, Meyer T, Backens M, Schneider-Axmann T, Thornton AE, Honer WG, Falkai P. Hippocampal plasticity in response to exercise in schizophrenia. Archives of general psychiatry. 2010;67:133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature reviews Neuroscience. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. 6. Amsterdam; Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. The European journal of neuroscience. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Poole DC, Ferreira LF. Oxygen exchange in muscle of young and old rats: muscle-vascular-pulmonary coupling. Experimental physiology. 2007;92:341–346. doi: 10.1113/expphysiol.2006.036764. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Zhang WN, Jongen-Relo AL, Feldon J, Yee BK. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory. The European journal of neuroscience. 2004;19:705–712. doi: 10.1111/j.0953-816x.2004.03170.x. [DOI] [PubMed] [Google Scholar]
- Savela S, Koistinen P, Tilvis RS, Strandberg AY, Pitkala KH, Salomaa VV, Miettinen TA, Strandberg TE. Leisure-time physical activity, cardiovascular risk factors and mortality during a 34-year follow-up in men. European journal of epidemiology. 2010;25:619–625. doi: 10.1007/s10654-010-9483-z. [DOI] [PubMed] [Google Scholar]
- Shipton OA, Paulsen O. GluN2A and GluN2B subunit-containing NMDA receptors in hippocampal plasticity. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014;369:20130163. doi: 10.1098/rstb.2013.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siette J, Westbrook RF, Cotman C, Sidhu K, Zhu W, Sachdev P, Valenzuela MJ. Age-specific effects of voluntary exercise on memory and the older brain. Biological psychiatry. 2013;73:435–442. doi: 10.1016/j.biopsych.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Potter GG, McLaren ME, Blumenthal JA. Impact of aerobic exercise on neurobehavioral outcomes. Mental health and physical activity. 2013;6:139–153. doi: 10.1016/j.mhpa.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain, behavior, and immunity. 2013;28:25–43. doi: 10.1016/j.bbi.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples MC, Kim A, Mandyam CD. Dendritic remodeling of hippocampal neurons is associated with altered NMDA receptor expression in alcohol dependent rats. Molecular and cellular neurosciences. 2015 doi: 10.1016/j.mcn.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Wang H, Feng R, Kyin M, Tsien JZ. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001;41:779–790. doi: 10.1016/s0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Nakazawa T, Tanimura A, Kiyama Y, Tezuka T, Watabe AM, Katayama N, Yokoyama K, Inoue T, Izumi-Nakaseko H, Kakuta S, Sudo K, Iwakura Y, Umemori H, Inoue T, Murphy NP, Hashimoto K, Kano M, Manabe T, Yamamoto T. Involvement of NMDAR2A tyrosine phosphorylation in depression-related behaviour. The EMBO journal. 2009;28:3717–3729. doi: 10.1038/emboj.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Borght K, Havekes R, Bos T, Eggen BJ, Van der Zee EA. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behavioral neuroscience. 2007;121:324–334. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VR, Chuang Y, Harris GC, Tan EJ, Carlson MC. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2014 doi: 10.1002/hipo.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasuta C, Caunt C, James R, Samadi S, Schibuk E, Kannangara T, Titterness AK, Christie BR. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippocampus. 2007;17:1201–1208. doi: 10.1002/hipo.20349. [DOI] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends in cognitive sciences. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Guo ML, Jin DZ, Xue B, Fibuch EE, Mao LM. Roles of subunit phosphorylation in regulating glutamate receptor function. European journal of pharmacology. 2014;728:183–187. doi: 10.1016/j.ejphar.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welinder C, Ekblad L. Coomassie staining as loading control in Western blot analysis. Journal of proteome research. 2011;10:1416–1419. doi: 10.1021/pr1011476. [DOI] [PubMed] [Google Scholar]
- Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- Wu CW, Chang YT, Yu L, Chen HI, Jen CJ, Wu SY, Lo CP, Kuo YM. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. Journal of applied physiology (Bethesda, Md : 1985) 2008;105:1585–1594. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau SY, Gil-Mohapel J. Physical exercise-induced adult neurogenesis: a good strategy to prevent cognitive decline in neurodegenerative diseases? 2014;2014:403120. doi: 10.1155/2014/403120. [DOI] [PMC free article] [PubMed] [Google Scholar]