Abstract
In Port-au-Prince, Haiti, the status of insecticide resistance has not recently been evaluated for Aedes aegypti (L) and Aedes albopictus (Skuse) populations. No prophylactics exist for dengue, so prevention is only through vector control methods. An earthquake occurred in Haiti on January 12, 2010, with a magnitude of 7.0 Mw that devastated the area. Dengue became a major concern for the humanitarian relief workers that entered the country. Bottle bioassays were conducted in the field on adult mosquitoes reared from larvae collected from the grounds of the U.S. Embassy and from an adjacent neighborhood in eastern Port-au-Prince, Haiti. At the CDC, Fort Collins, CO, bioassays, molecular, and biochemical assays were performed on mosquitoes reared from field-collected eggs. A small percentage of the population was able to survive the diagnostic dose in bioassays run in Haiti. Mosquitoes tested at the CDC demonstrated no phenotypic resistance. A variety of factors could be responsible for the discrepancies between the field and lab data, but temperature and larval nutrition are probably most important. Knowledge of localized resistance and underlying mechanisms helps in making rational decisions in selection of appropriate and effective insecticides in the event of a dengue outbreak.
Keywords: Aedes aegypti, Aedes albopictus, kdr resistance, Haiti
INTRODUCTION
Insecticide resistance has long been a concern in public health programs aimed at preventing disease through the judicious use of insecticides. In the United States, mosquito resistance was first detected in Aedes nigromaculis (Ludlow), which demonstrated resistance to DDT in 1950 (Bohart and Murray 1950). The first reported case of resistance in Aedes aegypti (L) occurred in Puerto Rico in 1960 to DDT (Fox et al. 1960). By 1964, resistance had been reported in Ae. aegypti to BHC/cyclodienes, malathion, and DDT in Florida, Surinam, Barbados, Lesser Antilles, and the Virgin Islands (Burton 1964, Flynn et al. 1964, Entwhistle 1964, Klassen and Brown 1964). Since then, 267 cases of resistance to 24 insecticides from 123 different locations in 41 countries have been reported for Ae. aegypti alone to the Arthropod Pesticide Resistance Database (APRD) maintained by Michigan State University (http://www.pesticideresistance.org/). In addition, 29 cases of resistance to six insecticides in 15 sites from 14 countries for Aedes albopictus (Skuse) have been reported to APRD. Surveillance for resistance in local populations needs to be ongoing as changes in insecticide use can lead to changes in selective pressure for development and maintenance of resistance. Furthermore, knowledge of localized resistance and underlying mechanisms helps in making rational decisions in the selection of appropriate and effective insecticides (Brogdon and McAllister 1998).
The status of insecticide resistance in Ae. aegypti and Ae. albopictus is of particular concern in areas where dengue virus and chikungunya virus infections occur. In recent years, both viruses have been active with dengue outbreaks occurring in the Caribbean region (Tomashek et al. 2009), and widespread outbreaks of chikungunya in Africa, the Indian Ocean region, Asia (Burt et al. 2011), and in Italy (Moro et al. 2010), highlighting the risk of introduction of that virus into the western hemisphere. In the absence of vaccines, vector control and/or personal protection education remain the mainstays for the prevention of infection with these viruses. Organized programs and personal protection strategies rely on the use of insecticides to varying degrees. Vector control programs may incorporate space spraying, impregnated materials like nets and sheeting, indoor residual spraying, and larviciding as part of their overall strategy. Personal protection strategies may include repellents and treated clothing as well as sanitation.
Vector-borne disease priorities in Haiti focus on malaria and lymphatic filariasis. No specific programs measuring the burden of dengue currently exist even though the virus was documented in Haiti in 2000 (http://www.paho.org/english/dd/ais/cp_214.htm) and is presently known from the Dominican Republic, which shares the island of Hispaniola with Haiti. (http://www.paho.org/English/AD/DPC/CD/dengue-cases-2008.htm)
In Haiti, control for malaria relies on impregnated nets and curtains. Some space spraying using malathion is used to target culicine vectors of filariasis and to some extent anopheline mosquitoes. Both malaria and filariasis are found throughout the country with a higher incidence of filariasis in the north and malaria being endemic in areas with lower elevation (less than 300m) such as the Artibonite Valley and the southeast (Beau de Rochars et al. 2004, CDC http://emergency.cdc.gov/disasters/earthquakes/haiti/malaria_pre-decision_brief.asp). Although dengue is present on the island, surveillance for it is rare, and reliable information on transmission foci is unavailable.
After an earthquake on January 12, 2010, with an epicenter near the town of Léogáne, approximately 25 km west of Port-au-Prince, Haiti’s capital, a large influx of humanitarian relief workers entered the country. Dengue, while not of high concern among Haitians, became a concern for the foreign relief workers, many coming from places where dengue is not endemic.
Both Ae. aegypti and Ae. albopictus were collected in Port-au-Prince, Haiti. Since dengue is not a priority disease in Haiti, little or no organized use of insecticides targeting these species has occurred. As a result, investigation into the status of resistance in either species has largely been ignored in Haiti. However, some of the same methods and chemicals that are used for malaria control have been used for Ae. aegypti control, namely pyrethroid-impregnated materials, residual sprays and space sprays, using ultra low volume (ULV) spray equipment with either pyrethroids, organophosphates, or carbamates. Also, a large number of insecticide-treated materials were distributed to persons displaced by the earthquake. The purpose of this study was to investigate the status of resistance in Ae. aegypti and Ae. albopictus in order to guide knowledge of the best choice of chemical for use in the event of a dengue outbreak.
MATERIALS AND METHODS
Mosquitoes
Mosquito larvae were collected from the grounds of the U.S. Embassy (Site A) and an adjacent neighborhood (Site B) in eastern Port-au-Prince, Haiti from May 24 to 26, 2010. Larvae were collected from natural habitats and held until they emerged as adults. Larvae were kept in 12×6×2 white plastic pans (Bioquip, Rancho Dominguez, CA, U.S.A.) with plexiglas sheets for lids. Pans were kept in a shaded area outdoors under ambient conditions (May average temp 28° C, average min temp 23° C, average max temp 33° C). Pupae were collected daily and placed in cups in 12×12 cages (Bioquip, Rancho Dominguez, CA, U.S.A.). Adults were supplied a 5% sugar solution. Adults were tested at three to five days post-eclosion.
Eggs were collected from May 24 to June 3, 2010 using black plastic cups filled ¾ full with water and containing a strip of seed germination paper around the inside of the cup. Papers were removed every other day and stored in plastic bags until returned to the Centers for Disease Control and Prevention in Fort Collins, CO. Eggs were hatched by placement in a vacuum chamber for 1 h. Larvae were reared as described above with the exception that they were fed ground fish food (Tetra, Cincinnati, OH) and kept in environmental chambers at 88% RH, 27° C with a 14:10 light:dark cycle.
Bioassays and biochemical assays were also run on mosquitoes from a susceptible colony maintained at the CDC, Fort Collins, CO. Colony mosquitoes were hatched and maintained as described above.
Bioassay
No known history of insecticide treatment was available for the Port-au-Prince area to guide choosing a particular compound for evaluation. Therefore, permethrin, deltamethrin, and malathion were assessed as representatives of common insecticides used in vector control programs. These compounds were also chosen as representatives of the pyrethroid and organophosphate classes of insecticides, respectively.
The protocol for the CDC bottle bioassay was followed (Brogdon and McAllister 1998). Briefly, 15 µg permethrin (CAS# 52645-53-1), 10 µg deltamethrin (CAS# 52918-63-5), or 50 µg malathion (CAS# 121-75-5), dissolved in acetone (CAS# 67-64-1), were applied to 250 ml glass bottles. All insecticides were purchased as technical grade material from ChemService (Westchester, PA, U.S.A.). Four bottles were treated for each compound and one untreated control was used (acetone only). After bottles were prepared, 25-35 mosquitoes were introduced. Mortality was considered to have occurred if the mosquito could not stand or fly. Counts of the numbers of affected mosquitoes were made at T=0, 5, 10, 15, and every 15 min thereafter until all mosquitoes were dead or two h had elapsed. A Weibel distribution curve was calculated for colony mosquitoes and compared to results from field-collected mosquitoes.
Biochemical assays
Assays to measure increased levels of detoxifying enzymes, protein, and the presence of the altered target site for organophosphates were conducted according to published protocols. Detoxifying enzymes measured were alpha (α) and beta (β) esterases, oxidases, and glutathione-S-transferase (GST). Individual mosquitoes were ground with a pestle in 2.0 ml microtubes containing 100 µl of potassium phosphate buffer (12.5 mmole/litre K2HPO4 and 37.9 mmol/litre KH2PO4) (CAS# 16788-51-1 and 7778-77-0, respectively) adjusted to pH 7.2. The homogenate was then diluted with an additional 1,900 μl of buffer. All assays were run in triplicate on each mosquito. Only mosquitoes collected as eggs were used for the enzyme assays. Unless otherwise noted all chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO).
Oxidases were indirectly measured using a heme-peroxidase assay using the substrate 3,3’,5,5’-tetra-methybenzidine (TMBZ) (CAS# 64285-73-0). A working solution of TMBZ was prepared by dissolving 50 mg of TMBZ in 25 ml absolute methanol (CAS# 67-56-1) and adding 75 ml of 0.25 M sodium acetate buffer (pH 5.0; pH adjusted with acetic acid)(CAS#64-19-7). The TMBZ substrate (200μl) was added to 100 μl of mosquito homogenate, followed by 25 μl of 3% hydrogen peroxide (CAS# 7722-84-1). Absorbance values of plates were read at a 620 nm wavelength after a 10-min incubation period using a SpectraMax M3 plate reader (Molecular Devices, Sunnvale, CA, U.S.A.) (Brogdon et al. 1997).
For α and β non-specific esterases, 100 μl of a 3.0 mmol/litre solution of α- or β-napthyl acetate (CAS# 830-81-9 and 1523-11-1, respectively) (56 mg α- or β-napthyl acetate dissolved in 20 ml of acetone, then diluted with 80 ml of pH 7.2 potassium phosphate buffer) was added to each well. Following a 20-min incubation period, 100 μl of 0-dianisidine (CAS# 14263-94-6) (100 mg 0-dianisidine in 100 ml water) was added to each well. After 4 min, the absorbance value for each well was determined at λ=540 nm (Vulule et al. 1999).
Glutathione-S-transferases (GST) were measured using 1-chloro-2,4-dinitrobenezene (CDNB) (CAS# 97-00-7) (20 mg CDNB dissolved in 20 ml acetone and 90 ml KPO4 buffer added) and reduced glutathione (CAS# 70-18-8)(61 mg glutathione in 100 ml KPO4 buffer). Briefly, 100 μl of the reduced glutathione was added to 100 μl of mosquito sample followed by 100 μl of CDNB. Absorbances were read at T=0 and T=5m with 340 nm as the wavelength (Brogdon and Barber 1990).
A competitive assay that measures insensitivity of acetylcholine-esterase (ACHE) was used to detect presence of the altered target site mechanism that confers organophosphate and carbamate resistance. This competitive assay incorporated propoxur (CAS# 114-26-1)(ChemService, West Chester, PA, U.S.A.) with the substrate acetylthiocholine iodide (ATCH) (CAS# 1866-15-5)(75 mg ATCH, 21 mg propoxur, 10 ml acetone, 90 ml KPO4 buffer). One hundred μl of the substrate was added to 100 μl of mosquito sample followed by 100 μl of 5,5’ Dithio-bis-2-nitrobenzoic acid (CAS# 69-78-3)(13 mg in 100 ml KPO4 buffer). Absorbances were read at T=0 min and at T=24 h with a wavelength of 414 nm.
The protein assay was performed using 20 μl of mosquito homogenate to which 80 μl of KPO4 buffer was added. The assay used is based on the Bradford method of protein quantification in which 200 μl of Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad, Hercules, CA, U.S.A.) was added and the absorbance was read at λ=620 nm (Brogdon and Dickinson 1983). A standard curve using bovine serum albumin (CAS# 9048-46-8) was calculated to quantitate the total protein present in each mosquito.
Molecular assay
Molecular assays to look for mutations in the para sodium channel gene, domain II associated with resistance to pyrethroids, were used on Ae. aegypti (Garcia et al. 2009). Mutations looked for included the Ile 1016, Gly 1016, Val 1011 and Met 1011. Primers and protocols were those described previously (Garcia et al., 2009, Saavedra-Rodriguez et al. 2007). Mosquito genomic DNA was extracted using DNAzol (Molecular Research Center Inc., OH). Individual mosquitoes were homogenized in 200 μl of DNAzol using a Kontes pellet pestle cordless motor with disposable pestles. The homogenate was incubated at room temperature for 5 min and then centrifuged for 1 min at 10,000 rpm to remove insoluble tissue. The resulting viscous supernatant was transferred to a new 1.5 ml microtube. The DNA was precipitated from the homogenate by adding 100 μl of 100% ethanol (CAS# 64-17-5). The samples were mixed by inversion to ensure a homogenous solution and incubated at room temperature for 3 min. The samples were centrifuged for 2 min at 14,000 rpm or until a visible pellet could be seen. The DNA precipitate was washed twice with 1 ml of 75% ethanol, allowed to air dry for 5-15 s, and then re-suspended in 50 μl of H20.
PCR was performed in a 25 μl volume in Low-Profile 96-Well unskirted white PCR Plates. (Bio-Rad Laboratories, Hercules, CA). Each reaction contained 12.5 μl of 2× IQ™ SYBR® Green Supermix (Bio-Rad Laboratories) (final concentrations = 50 mM KCl, 20 mM Tris-HCl, pH 8.4, 0.2 mM of each dNTP, 0.625 units iTaq® DNA polymerase, 3 mM MgCl2, 1× SYBR Green I, 10 nM fluorescein), 25 pm of each primer, ~100 ng of template DNA, and sterile filtered ddH2O water added to make a final 25 μl volume. The melting curve PCR reaction was performed on an Eppendorf Mastercycler ep realplex4 S. The thermal cycling conditions were modified to the following: (1) 95° C for 4 min (first denature); (2) 95° C for 10 s (denature in cycle); (3) 60° C for 10 s (anneal); (4) 72° C for 30 s (extension); (5) cycle to step (2) 39 times; (6) 95° C for 10 s; and (7) ramp from 65° C to 95° C at a rate of 0.2° C/10 s (melting curve).
The melting curve assay was used to detect four different mutations in codons 1011 and 1016 of para in Ae. aegypti. Each of the four single nucleotide polymorphisms (SNP) loci had three different genotypes. The Iso1011/Iso1011 homozygote has a single peak at 80° C, the Val1011/Iso1011 heterozygote has two peaks at 80° C and 85° C, and the Val1011/Val1011 homozygote has a single peak at 85° C. A single peak at 72° C indicates a Met1011/Met1011 homozygote, while two peaks at 77° C and 82° C indicate a Met1011/Iso1011 heterozygote. An Iso1016/1016 homozygote has a single peak at 80° C, the Iso1016/Val1016 heterozygote has two peaks at 80° C and 88° C, and the Val1016/Val1016 has a single peak at 88° C. Two peaks at 79° C and 84° C indicated a Gly1016/Val1016 heterozygote, while a single peak at 79° C indicated a Val1016/Val1016 homozygote. Frequencies of the Val1011, Met1011, Iso1016, and Gly1016 alleles were calculated using the following equation:
RESULTS
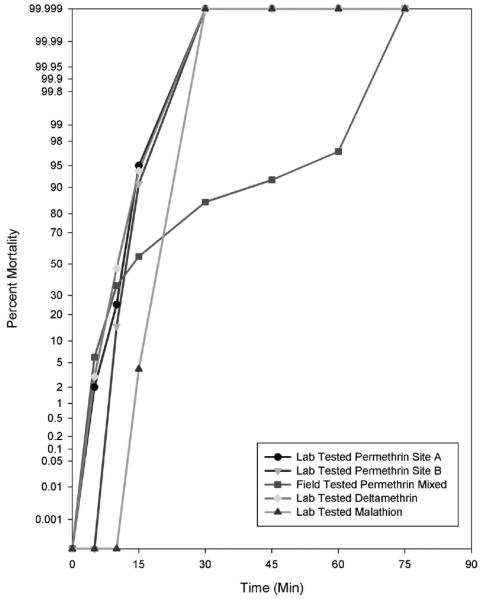
The bioassay run in Haiti from Sites A and B indicated that a low level of individuals (15%) from a mixed population of adults reared from larvae were able to able to survive a permethrin exposure for 45 min beyond the threshold time for resistance (Figure 1). Larvae collected from these sites produced few adults (N=78). In order to have enough mosquitoes for comparison all adult mosquitoes were combined for the field assays. Adults in this assay were 66% Ae. aegypti and 34% Ae. albopictus, but the survivors were mostly Ae. aegypti: ten individuals at T=30 min, five individuals at T=45 min, one individual at T=45 min, and all were dead at T=75 min. A single Ae. albopictus survived until T=45 min. However, subsequent tests performed in the laboratory on adults reared from eggs showed no phenotypic resistance to permethrin from site B, and no resistance to permethrin, deltamethrin, or malathion from site A on either species. Site A egg papers produced only Ae. aegypti while site B had a mix of Ae. aegypti and Ae. albopictus.
Figure 1.
Phenotypic expression of insecticide resistance in Aedes aegypti and Aedes albopictus using the CDC bottle bioassay.
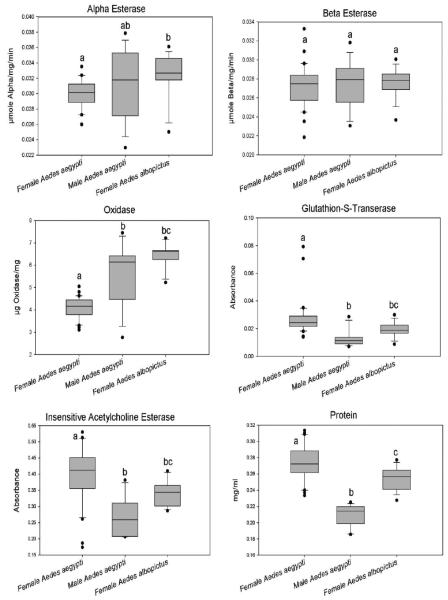
Results of biochemical assays are presented in figure 2. There were not enough male Ae. albopictus to run biochemical tests. Mosquitoes from sites A and B were not statistically different in their expression of enzymes and were therefore added together to provide more individuals to evaluate for differences between species and sexes. Values for protein and β-esterases were normally distributed and analyzed using a one-way ANOVA comparing means and pairwise multiple comparison procedures followed the Holm-Sidak method. Values for α-esterases, oxidases, GST, and insensitive ACHE were not normally distributed; therefore, Kruskal-Wallis one-way analysis of variance on ranks was used and pairwise multiple comparison procedures used Dunn’s method.
Figure 2.
Box plots of enzyme levels measured in Aedes aegypti and Aedes albopictus from Port-au-Prince, Haiti. Identical letters denote no significant difference P<0.05.
Size of mosquitoes was significantly different (P<0.05) between female Ae. aegypti (mean 0.274 mg, SD 0.0224) and female Ae. albopictus (mean 0.255mg, SD 0.0139). There was also a significant difference (P <0.05) between the size of male and female Ae. aegypti. (mean 0.209 mg, SD 0.0127 and 0.274 mg, SD 0.0224, respectively). These size differences were taken into account when comparing enzyme levels.
No significant differences (P >0.05) between the species or male/female Ae. aegypti for β-esterases was detected. There were significant differences between sex and species for oxidases with female Ae. aegypti expressing the least amount of oxidases 4.099 μg oxidase/mg. Differences in α-esterases were detected between female Ae. aegypti (0.0303 μmol α-esterase/mg/min) and Ae. albopictus (0.0327 α-esterase/mg/min). No difference was found between male Ae. aegypti (0.0318 α-esterase/mg/min) and females of either species. The same pattern of differences was found for oxidases, GST, and insensitive ACHE. Male and female Ae. aegypti were significantly different and female Ae. aegypti were different from female Ae. albopictus, but male Ae. aegypti were not different from female Ae. albopictus.
Table 1 shows the frequency of knockdown resistance (kdr) SNP mutations from Ae. aegypti and Ae. albopictus. A low frequency of Ile1016 was detected in Ae. aegypti from both sites (frequency 0.114) but not in Ae. albopictus. No Gly1016 was detected in either species. The frequencies of Val1011 and Met 1011 were high in both species and in Ae. aegypti from both sites (range 0.886-1.0).
Table 1.
Frequency of SNP mutations associated with kdr resistance in Aedes aegypti and Aedes albopictus from Port-au-Prince, Haiti. Val/Val is the wild type for 1016 and Ile/Ile is the wild type for 1011.
| Ile 1016 | Ile/Ile (AA) |
Ile/Val (AG) |
Val/Val (GG) |
N | Frequency of mutation |
95% CI |
| Aedes aegypti | ||||||
| Site A | 1 | 5 | 33 | 39 | 0.104 | 0.033 |
| Site B | 3 | 2 | 22 | 27 | 0.146 | 0.055 |
| Overall Frequency | 4 | 7 | 55 | 66 | 0.114 | 0.027 |
| Aedes albopictus | ||||||
| Site B | 0 | 0 | 12 | 12 | 0.000 | NA |
|
| ||||||
| Gly 1016 | Gly/Gly (GG) |
Gly/Val (GT) |
Val/Val (TT) | N | Frequency of mutation |
95% CI |
| Aedes aegypti | ||||||
| Site A | 0 | 0 | 39 | 39 | 0.000 | NA |
| Site B | 0 | 0 | 27 | 27 | 0.000 | NA |
| Overall Frequency | 0 | 0 | 66 | 66 | 0.000 | NA |
| Aedes albopictus | ||||||
| Site B | 0 | 0 | 12 | 12 | 0.000 | NA |
|
| ||||||
| Val 1011 | Val/Val (GG) |
Val/Ile (GA) |
Ile/Ile (AA) | N | Frequency of mutation |
95% CI |
| Aedes aegypti | ||||||
| Site A | 33 | 6 | 0 | 39 | 0.923 | 0.290 |
| Site B | 21 | 6 | 0 | 27 | 0.889 | 0.335 |
| Overall Frequency | 54 | 12 | 0 | 66 | 0.909 | 0.219 |
| Aedes albopictus | ||||||
| Site B | 12 | 0 | 0 | 12 | 1.000 | 0.566 |
|
| ||||||
| Met 1011 | Met/Met (GG) |
Met/Ile (GA) |
Ile/Ile (AA) |
N | Frequency of mutation |
95% CI |
| Aedes aegypti | ||||||
| Site A | 31 | 9 | 0 | 39 | 0.910 | 0.286 |
| Site B | 20 | 6 | 1 | 27 | 0.852 | 0.321 |
| Overall Frequency | 51 | 15 | 1 | 66 | 0.886 | 0.214 |
| Aedes albopictus | ||||||
| Site B | 8 | 4 | 0 | 12 | 0.833 | 0.471 |
DISCUSSION
Phenotypic resistance to permethrin was expressed only in assays run in the field in Ae. aegypti. This resistance was not reproduced in bioassays run in the laboratory on mosquitoes reared at a constant temperature. The average temperature in Port-au-Prince, Haiti, in May is much hotter and varies more than that maintained in the laboratory at a constant 27° C. The average minimum temperature is 24° C, average maximum temperature is 33° C, and the overall average is 30° C. According to Yu (2008), the factors known to affect intrinsic toxicity of insecticides are age, sex, population density, illumination, food supply, and rearing temperature. The last two factors were different between tests done in the lab and in the field. Mosquitoes tested in the field were kept under the same conditions wild mosquitoes would endure, including their larval diet. Water from their collection sites was used with no additional supplementation, whereas mosquitoes tested in the laboratory were reared under constant temperature with food supplementation. The increased nutrition available to the lab-reared mosquitoes likely contributed to their robustness, allowing them to overcome exposure to insecticides.
As Yu (2008) points out, it is well known that insecticide toxicity is affected by temperature. Pyrethroids are antagonized by high temperatures while organophosphates are potentiated by heat. Temperature and/or diet alone may account for the discrepancy in results although other variables may not be ruled out.
Overall levels of known resistance enzymes were not elevated to levels expected to confer resistance although variations between species and sexes were detected. The three classes of enzymes associated with insecticide resistance are composed of many individual enzymes with functions other than insecticide metabolism (Yu 2008). However, within a class, multiple enzymes may affect insecticides. For example, at least 230 enzymes putatively contribute to metabolic resistance in Anopheles gambiae Giles (David et al. 2005). An array of 235 enzyme genes comprising esterases, oxidases, and GST was constructed for Ae. aegypti by Strode et al. (2008). The authors found several genes in two families, CYP6 P-450’s and Epsilon GSTs, associated with resistance. Their results do not definitively imply that these enzymes actually confer resistance, just that they are overexpressed in resistant individuals. Furthermore, there is some evidence that resistance due to enzymes may also occur through mutations in an individual enzyme making it better at detoxifying a particular insecticide or class of insecticides (Li et al. 2007).
The tests performed in this study to measure enzyme activity are general in nature and can only be used to implicate an enzyme class. It cannot be ruled out that high levels of enzymes having nothing to do with resistance may be detected, even using detoxification chips (David et al. 2005, Strode et al. 2008). Had resistance been detected, additional bioassays with compounds that inhibit enzymes would still be needed to confirm biochemical test results. Chip assays to date are much more expensive and results are difficult to interpret.
Presence of Val and Met mutations are equivocal. They occur in Haiti with high frequency yet bioassays indicate little to no phenotypic expression of resistance, at least at lower temperatures. At higher temperatures resistant individuals were detected but at a low frequency, 16%. This does not correlate to the presence of Met in all individuals tested nor does it correlate to the level of Val present (almost 90%). There are many studies on kdr resistance in anophelines showing that the presence of kdr alleles does, or alternatively, does not show expression of phenotypic resistance. Since these studies rarely list the temperature at which the assay was actually run, these conflicting results may be confounded by temperature effects of the bioassay conditions. Alternatively, the Val and Met mutations may not express resistance in the adult stage or may not actually confer resistance on their own. Work by Hardstone et al. (2009) indicated that epistasis or the nonadditive interaction between different loci which contribute to a phenotype was important in the expression of resistance in Culex pipiens quinquefasciatus Say. The two loci explored were for kdr and cytochrome P450 monogxygenase mediated detoxification and they found all combinations (heterozygous and homozygous) of the two loci were multiplicative. In our study, we did not see increased levels of monooxygenases in Ae. aegypti, the species that showed some phenotypic resistance during assays in the field. On the other hand, Ae. albopictus, the species with higher monooxygenases and the Val and Met mutations, did not show phenotypic expression of resistance to permethrin suggesting epistasis if occurring is antagonistic in this species.
The current focus in Haiti is the prevention and control of malaria and lymphatic filariasis. The focus needs to be expanded and encompass evaluating the burden of dengue in Haiti, as the virus is endemic to the area. If a significant dengue outbreak were ever to occur in Haiti, vector control and personal protection would be the only safeguards against the virus. Therefore, resistance evaluation needs to be ongoing in local Ae. aegypti and Ae. albopictus populations, even though little to no resistance was detected in the limited area of Port-au-Prince sampled. Knowing when resistance develops and what mechanisms are present is essential to aiding public health entities in making rational decisions as to which insecticide to use or what control method to employ.
REFERENCES CITED
- Beau De Rochars MVE, Milord MD, Jean Y. St., Disormeaux AM, Dorvil JJ, Lafontant JG, Addiss DG, Streit TG. Geographic distribution of lymphatic filariasis in Haiti. Am. J. Trop. Med. Hyg. 2004;71:598–601. [PubMed] [Google Scholar]
- Bohart RM, Murray WD. DDT resistance in Aedes nigromaculis larvae. Proc. Calif. Mosq. Contr. Assoc. 1950;18:22–23. [Google Scholar]
- Brogdon WG, Barber AM. Microplate assay of glutathione S-transferase activity for resistance detection in single-mosquito triturates. Comp. Biochem. Physiol. 1990;96B:339–342. doi: 10.1016/0305-0491(90)90385-7. [DOI] [PubMed] [Google Scholar]
- Brogdon WG, Dickinson CM. A microassay system for measuring esterase activity and protein concentration in small samples and in high-pressure liquid chromatography eluate fractions. Analyt. Biochem. 1983;131:499–503. doi: 10.1016/0003-2697(83)90204-x. [DOI] [PubMed] [Google Scholar]
- Brogdon WG, McAllister JC. Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J. Am. Mosq. Contr. Assoc. 1998;14:159–164. [PubMed] [Google Scholar]
- Brogdon WG, McAllister JC. Insecticide resistance and vector control. Emerge. Infect. Diseases. 1998;4:605–613. doi: 10.3201/eid0404.980410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogdon WG, McAllister JC, Vulule J. Heme peroxidase activity measured in single mosquitoes identifies individuals expressing an elevated oxidase for insecticide resistance. J. Am. Mosq. Cont. Assoc. 1997;13:233–237. [PubMed] [Google Scholar]
- Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2011 doi: 10.1016/S0140-6736(11)60281-X. published online Nov. 18, 2011. DOI 10.1016/S0140-6736(11)-X. [DOI] [PubMed] [Google Scholar]
- Burton GJ. Results of insecticide resistance tests against Aedes aegypti adults and larvae in British Guiana. Mosq. News. 1964;24:200–202. [Google Scholar]
- David JP, Strode C, Vontas J, Nikou D, Vaughan A, Pignatelli PM, Louis C, Hemingway J, Ranson H. The Anopheles gambiae detoxification chip: A highly specific microarray to study metabolic-based insecticide resistance. Proc. Natl. Acad. Sci USA. 2005;102:4080–4084. doi: 10.1073/pnas.0409348102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entwhistle PF. The distribution of mirid species and of resistant mirids in Nigeria. Proc. Conf. Mirid Pests Cacao, W. Afr. Cacao Res. Inst., Ibadan. 1964:9–17. [Google Scholar]
- Flynn AD, Schoof HF, Morlan HB, Porter JE. Susceptibility of 17 strains of Aedes aegypti (L.) from Puerto Rico and the Virgin Islands to DDT, dieldrin, and malathion. Mosq. News. 1964;24:118–123. [Google Scholar]
- Fox I, Boike AH, Garcia-Moll I. Notes on rock hole breeding and resistance of Aedes aegypti in Puerto Rico. Am. J. Trop. Med. Hyg. 1960;9:425–429. doi: 10.4269/ajtmh.1960.9.425. [DOI] [PubMed] [Google Scholar]
- Garcia GP, Flores AE, Fernandez-Salas I, Saavedra-Rodrıguez K, Reyes-Solis G, Lozano-Fuentes S, Bond JG, Casas-Martınez M, Ramsey JM, Garcıa-Rejon J, Domınguez-Galera M, Ranson H, Hemingway J, Eisen L, Black WC., IV Recent rapid rise of a permethrin knock down resistance allele in Aedes aegypti in Mexico. PLoS Negl. Trop. Dis. 2009;3:e531. doi: 10.1371/journal.pntd.0000531. doi:10.1371/journal.pntd.0000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardstone MC, Leichter CA, Scott JG. Multiplicative interaction between the two major mechanisms of permethrin resistance, kdr and cytochrome P450-monooxygenase detoxification, in mosquitoes. J. Evol. Biol. 2009;22:416–423. doi: 10.1111/j.1420-9101.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- Klassen W, Brown AWA. Genetics of insecticide-resistance and several visible mutants in Aedes aegypti. Can. J. Genet. Cytol. 1964;6:61–73. doi: 10.1139/g64-009. [DOI] [PubMed] [Google Scholar]
- Li X, Schuler MA, Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- Moro ML, Gagliotti C, Silvi G, Angelini R, Sambri V, Rezza G, Massimiliani E, Mattivi A, Grilli E, Finarelli AC, Spataro N, Pierro AM, Seyler T, Macini P. Chikungunya virus in North-Eastern Italy: A seroprevalence survey. Am. J. Trop. Med. Hyg. 2010;82:508–511. doi: 10.4269/ajtmh.2010.09-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K, Urdaneta-Marquez L, Rajatileka S, Moulton M, Flores AE, Fernandez-Salas I, Bisset J, Rodriguez M, Mccall PJ, Donnelly MJ, Ranson H, Hemingway J, Black WC., IV A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Molec. Biol. 2007;16:785–798. doi: 10.1111/j.1365-2583.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- Strode C, Wondji CS, David JP, Hawkes NJ, Lumjuan N, Nelson DR, Dranee DR, Karunaratne SHPP, Hemingway J, Black WC, IV, Ranson H. Genomic analysis of detoxification genes in the mosquito Aedes aegypti. Insect Biochem. Molec. Biol. 2008;38:113–123. doi: 10.1016/j.ibmb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Tomashek KM, Rivera A, Muñoz-Jordan JL, Hunsperger E, Santiago L, Padro O, Garcia E, Sun W. Description of a large island-wide outbreak of dengue in Puerto Rico, 2007. Am. J. Trop. Med. Hyg. 2009;81:467–474. [PubMed] [Google Scholar]
- Vulule JM, Beach RF, Atieli FK, McAllister JC, Brogdon WG, Roberts JM, Mwangi RW, Hawley WA. Elevated oxidase and esterase levels associated with permethrin tolerance in Anopheles gambiae from Kenyan villages using permethrin-impregnated nets. Med. Vet. Entomol. 1999;13:239–244. doi: 10.1046/j.1365-2915.1999.00177.x. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Dengue and dengue hemorrhagic fever. 2009 WHO Fact Sheet No. 117 [accessed November 15, 2011]. http://www.who.int/mediacentre/factsheets/fs117/en/
- Yu S. The Toxicology and Biochemistry of Insecticides. CRC Press; Boca Raton, FL: 2008. p. 296. [Google Scholar]