Abstract
Background
Knowledge of the number of persons with chronic hepatitis C virus (HCV) infection in the United States is critical for public health and policy planning.
Objective
To estimate the prevalence of chronic HCV infection between 2003 and 2010 and to identify factors associated with this condition.
Design
Nationally representative household survey.
Setting
U.S. noninstitutionalized civilian population.
Participants
30 074 NHANES (National Health and Nutrition Examination Survey) participants between 2003 and 2010.
Measurements
Interviews to ascertain demographic characteristics and possible risks and exposures for HCV infection. Serum samples from participants aged 6 years or older were tested for antibody to HCV; if results were positive or indeterminate, the samples were tested for HCV RNA, which indicates current chronic infection.
Results
Based on 273 participants who tested positive for HCV RNA, the estimated prevalence of HCV infection was 1.0% (95% CI, 0.8% to 1.2%), corresponding to 2.7 million chronically infected persons (CI, 2.2 to 3.2 million persons) in the U.S. noninstitutionalized civilian population. Infected persons were more likely to be aged 40 to 59 years, male, and non-Hispanic black and to have less education and lower family income. Factors significantly associated with chronic HCV infection were illicit drug use (including injection drugs) and receipt of a blood transfusion before 1992; 49% of persons with HCV infection did not report either risk factor.
Limitation
Incarcerated and homeless persons were not surveyed.
Conclusion
This analysis estimated that approximately 2.7 million U.S. residents in the population sampled by NHANES have chronic HCV infection, about 500 000 fewer than estimated in a similar analysis between 1999 and 2002. These data underscore the urgency of identifying the millions of persons who remain infected and linking them to appropriate care and treatment.
Primary Funding Source
None.
Hepatitis C virus (HCV) infection is a treatable but underrecognized and underdiagnosed disease. An estimated 130 to 170 million persons, 2% to 3% of the world’s population, are living with HCV infection, and almost 500 000 persons die of HCV-related conditions each year (primarily decompensated cirrhosis and liver cancer) (1). In the United States, previous estimates have consistently indicated that approximately 3 million or more persons have chronic HCV infection.
An analysis of 21 241 serum specimens from participants in NHANES (National Health and Nutrition Examination Survey), which provides nationally representative statistics on the health of the U.S. noninstitutionalized civilian population, indicated that 2.7 million persons (95% CI, 2.4 to 3.0 million persons) had chronic HCV infection between 1988 and 1994 (2). A similar analysis of 15 079 NHANES specimens between 1999 and 2002 estimated that 3.2 million persons (95% CI, 2.7 to 3.9 million persons) had chronic HCV infection (3). These estimates do not include cases of chronic HCV not captured by NHANES, notably among homeless persons and persons who were incarcerated during the survey (4).
The Institute of Medicine recently concluded that it is essential to know the dimensions and direction of this epidemic, which has major implications for health burden and costs for the United States (5). Current treatment can cure HCV in a substantial proportion of persons who complete therapy, thereby decreasing the risk for hepatocellular carcinoma and all-cause mortality. However, many persons infected with HCV remain untested and unaware of their infection, are unknown to the health care system, and are not captured in case-based surveillance because they are typically asymptomatic (6). Deaths among persons with HCV infection have superseded deaths in those with HIV infection (7).
Surveillance for antibody to HCV (anti-HCV) and HCV RNA has been part of NHANES since the 1980s, although RNA testing for NHANES III was done retrospectively. Surveillance through such a large national survey presents the best measurement of the prevalence of anti-HCV and chronic HCV infection in the general U.S. population. Accordingly, using methods similar to analyses from 20 and 10 years ago (2, 3), we analyzed data from participants in NHANES between 2003 and 2010 to estimate the prevalence of HCV infection and to determine risk factors and exposures associated with chronic infection.
METHODS
Survey Design
The National Health and Nutrition Examination Survey, conducted by the Centers for Disease Control and Prevention’s National Center for Health Statistics, collects nationally representative data on the health and nutritional status of the U.S. noninstitutionalized civilian population. This survey uses a complex, stratified, multistage probability sampling design and collects information from approximately 5000 persons annually using standardized interviews, physical examinations, and tests of biological samples. Participants were interviewed in their homes using the interviewer-administered Computer-Assisted Personal Interviewing system to ascertain demographic characteristics and in the Mobile Examination Center to ascertain possible risks and exposures for HCV infection.
Context
Chronic hepatitis C virus (HCV) infection is an important public health issue. Using data from a U.S. household survey conducted between 2003 and 2010, the authors compared the estimated prevalence of chronic HCV infection and risk factors for infection with those from earlier periods.
Contribution
The estimated prevalence of chronic HCV infection in the United States has decreased. Risk factors are essentially unchanged from previous periods and were reported by only about one half of infected persons.
Caution
Homeless and incarcerated persons were not surveyed.
Implication
The burden of chronic HCV infection in the United States is substantial. National data on prevalence are useful for the design of programs for HCV screening, linkage to care, and treatment.
—The Editors
Persons aged 16 years or older and emancipated minors were interviewed directly; an adult proxy provided information for participants younger than 16 years and for persons unable to answer the questions themselves. All participants provided written informed consent. More detailed information on survey design for NHANES, including approval from the National Center for Health Statistics Institutional Review Board (Hyattsville, Maryland), is available from the survey documentation at www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
Laboratory Testing
Qualitative determination of anti-HCV in blood serum or plasma was measured using direct solid-phase enzyme immunoassay with an anti-HCV screening chemiluminescence immunoassay (VITROS Anti-HCV Immunodiagnostic System, Ortho Clinical Diagnostics, Rochester, New York). Screening reactive specimens were then tested using a confirmatory recombinant immunoblot assay (RIBA) (RIBA HCV 3.0 Strip Immunoblot Assay, Chiron, Emeryville, California), an in vitro qualitative immunoassay for the detection of anti-HCV in human serum or plasma. Samples with positive results on RIBA testing were reported as confirmed positive for anti-HCV, those with results that were negative were reported as negative for anti-HCV, and those with indeterminate results were reported as indeterminate.
In clinical practice, it is most important to identify persons who are currently infected; however, for surveillance purposes, we are interested in having a reliable measure of both those who are currently infected and those who were ever infected. Although the sensitivity and specificity of anti-HCV tests have improved over time for at-risk populations, estimating the true prevalence in a low-risk, low-prevalence population, such as that sampled in NHANES, requires a confirmatory test, such as RIBA, to eliminate false-positive results from our estimates of persons ever infected. Serum samples that were confirmed positive or indeterminate for anti-HCV were further tested for HCV RNA using an in vitro nucleic acid amplification test for the quantitation of HCV RNA in human serum or plasma. We used the COBAS AMPLICOR HCV Test, version 2.0 (Roche Diagnostics, Indianapolis, Indiana), on the COBAS AMPLICOR Analyzer (Roche Diagnostics) for samples from 2005 to 2010 and the COBAS AmpliPrep/ TaqMan HCV Test, version 2 (Roche Diagnostics), on the COBAS TaqMan 48 Analyzer (Roche Diagnostics) for samples from 2003 to 2004.
We considered persons to have chronic HCV infection if results of their test for anti-HCV were confirmed positive or indeterminate and results of their test for HCV RNA were positive. Our comparison group comprised persons who tested negative for anti-HCV; these participants were considered to be never infected with HCV. Those who tested positive for anti-HCV but negative for HCV RNA (resolved infections; n = 90) and those who had no serum available for RNA testing (n = 51) were not included in our analyses, except for estimation of overall anti-HCV prevalence, because we wanted to focus on chronic HCV infection.
Statistical Analysis
SAS-Callable SUDAAN, Release 10.0 (Research Triangle Institute, Research Triangle Park, North Carolina) (8), a statistical package designed to analyze complex survey data, was used for analysis. Estimates were weighted to represent the total U.S. noninstitutionalized civilian population and to account for oversampling and nonresponse to the household interview and physical examination. Two-year sample weights (WTMEC2yr) were further adjusted to account for the fact that not all examination participants were tested for anti-HCV, not all participants who tested positive or indeterminate for anti-HCV had samples available for HCV RNA testing, and multiple years of data were used. A P value less than 0.05 was considered statistically significant.
We analyzed demographic characteristics (age at interview, sex, race/ethnicity, birthplace, education, and income), potential risk factors or exposures (receipt of blood or a blood product before 1992, any past injection drug use, and number of lifetime sexual partners), and a proxy for sexual risk (antibodies to herpes simplex virus type 2) (9, 10). Although sex is not a usual method of transmission of HCV infection (11), number of sexual partners and presence of herpes simplex virus type 2 antibodies are included because these are indices of increased likelihood of being infected with HCV and have been used in previous analyses. We restricted most analyses to persons aged 20 years or older because only 2 persons aged 6 to 19 years had evidence of chronic HCV infection (that is, tested positive for HCV RNA) and because data on drug use and sexual behaviors among those younger than 20 years are not available from NHANES public-use data files. Ages included in our reporting of risks and exposures reflect age eligibility for a particular question or laboratory test rather than a focus on particular age groups.
We used bivariate analyses to estimate demographic characteristics of persons with chronic HCV infection and those who were never infected and to estimate the prevalence of potential risk factors or exposures among population subgroups. Chi-square tests were used for statistical comparisons between subgroups. Simple (unadjusted) and multivariate logistic regression analyses were used to identify factors associated with chronic HCV infection. We performed separate logistic analyses for persons aged 20 to 59 years and those aged 60 years or older because NHANES does not query those older than 59 years about sexual behaviors or drug use.
We retained 3 potential confounders—age at interview, sex, and race/ethnicity—in all multivariate models. Variables that were associated with HCV infection in previous NHANES analyses (2, 3) were included in multivariate models to identify factors independently associated with chronic HCV infection. Variables not included in the initial multivariate model building were added to the model individually to assess possible additional confounders. We excluded observations with missing covariate data from the corresponding analyses. We did not include markers for sexual behavior in the multivariate model for persons aged 20 to 59 years because sexual transmission of HCV has been documented primarily among HIV-infected men who have sex with men and rarely among monogamous heterosexual couples (11).
Role of the Funding Source
No external funding was received for this study.
RESULTS
Of the 43 898 persons aged 6 years or older sampled in NHANES between 2003 and 2010, a total of 34 039 (77.5%) were interviewed and 32 791 (96.3% of those interviewed) were examined. Serum samples were available for anti-HCV testing for 30 074 persons (91.7% of those examined). Of these, 386 tested positive for anti-HCV and, of those who had additional serum available for HCV RNA testing (approximately 86%), 273 tested positive for HCV RNA.
Among the 30 114 persons aged 20 years or older sampled in NHANES between 2003 and 2010, a total of 22 173 (73.6%) were interviewed and 21 281 (96.0% of those interviewed) were examined. Serum samples were available for anti-HCV testing for 20 042 persons (94.2% of those examined). Of these, 381 tested positive for anti-HCV; of those who had additional serum available for HCV RNA testing (approximately 87%), 271 tested positive for HCV RNA.
The estimated prevalence of anti-HCV among persons aged 6 years or older was 1.3% (95% CI, 1.2% to 1.5%), corresponding to approximately 3.6 million persons (CI, 3.0 to 4.2 million persons) with past or current HCV infection in the general U.S. population. The estimated prevalence of HCV RNA among those aged 6 years or older was 1.0% (CI, 0.8% to 1.2%), corresponding to approximately 2.7 million persons (CI, 2.2 to 3.2 million persons) with chronic (current) HCV infection in the general U.S. population (Figure).
Figure. Estimated prevalence of anti-HCV and HCV RNA in persons aged ≥6 y, according to NHANES III (1988–1994), NHANES 1999–2002, and NHANES 2003–2010.
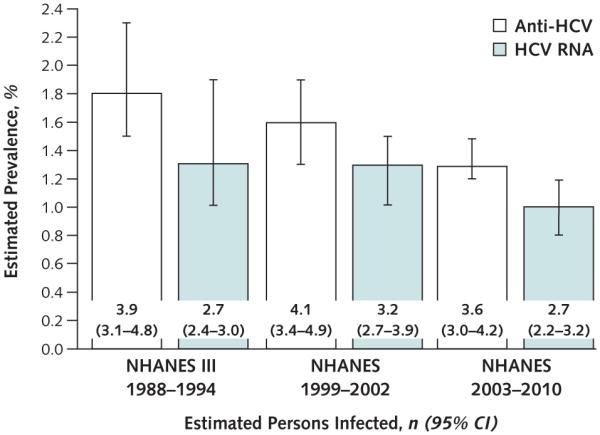
Estimated persons infected are reported in millions. Anti-HCV = antibody to HCV; HCV = hepatitis C virus; NHANES = National Health and Nutrition Examination Survey.
Demographic and Risk Characteristics of Persons With Chronic Infection
Compared with persons who were never infected with HCV, those with chronic HCV infection were more likely to be aged 40 to 59 years, male, of non-Hispanic black race/ethnicity, and born in the United States. They were also less likely to have education past high school or family income at least twice the poverty level (Table 1). Of the estimated 2.68 million chronically infected persons aged 20 years or older (CI, 2.18 to 3.19 million persons), we estimated that 1.09 million (CI, 780 000 to 1.40 million persons) were aged 40 to 49 years, 1.03 million (CI, 770 000 to 1.28 million persons) were aged 50 to 59 years, 1.71 million (CI, 1.29 to 2.13 million persons) were male, 1.64 million (CI, 1.19 to 2.10 million persons) were non-Hispanic white, 2.52 million (CI, 2.03 to 3.02 million persons) were born in the United States, 1.04 million (CI, 740 000 to 1.33 million persons) had education beyond high school, and 1.11 million (CI, 760 000 to 1.46 million persons) had family income at least twice the poverty level. Among those born between 1945 and 1965, the estimated prevalence was 2.6% (CI, 2.1% to 3.2%), corresponding to an estimated 2.16 million persons (CI, 1.70 to 2.61 million persons) with chronic HCV infection.
Table 1.
Demographic Characteristics by HCV Status for Participants Aged ≥20 y: NHANES 2003–2010 (n = 19 901)
| Characteristic | HCV Status |
P Value* | |||
|---|---|---|---|---|---|
| Anti-HCV–Negative |
HCV RNA–Positive |
||||
| Participants, n | Proportion (95% CI) | Participants, n | Proportion (95% CI) | ||
| Age at interview | <0.001 | ||||
|
| |||||
| 20–29 y | 3501 | 19.3 (18.3–20.4) | 5 | 1.2†‡ (0.3-5.2) | |
|
| |||||
| 30–39 y | 3320 | 19.0 (18.1–19.9) | 28 | 10.1 (6.4–15.6) | |
|
| |||||
| 40–49 y | 3284 | 20.4 (19.5–21.4) | 89 | 40.7 (34.0–47.8) | |
|
| |||||
| 50–59 y | 2746 | 17.5 (16.7–18.4) | 93 | 38.3 (31.6–45.4) | |
|
| |||||
| ≥60 y | 6779 | 23.8 (22.5–25.1) | 56 | 9.7 (6.8–13.6) | |
| Sex | <0.001 | ||||
| Male | 9469 | 47.8 (47.2–48.5) | 165 | 63.6 (56.1–70.6) | |
|
| |||||
| Female | 10 161 | 52.2 (51.5–52.8) | 106 | 36.4 (29.4–43.9) | |
|
| |||||
| Race/ethnicity | <0.001 | ||||
| Hispanic | 5184 | 12.5 (10.4–14.8) | 47 | 9.6 (5.9–15.3) | |
|
| |||||
| Non-Hispanic white | 9819 | 70.3 (66.9–73.6) | 112 | 61.3 (52.4–69.5) | |
|
| |||||
| Non-Hispanic black | 3734 | 11.2 (9.6–13.0) | 103 | 25.3 (19.2–32.6) | |
|
| |||||
| Other (including multiple races) | 893 | 6.0 (5.1–7.0) | 9 | 3.8†‡ (1.8-8.0) | |
|
| |||||
| Birthplace | <0.001 | ||||
| United States | 14 819 | 83.4 (81.0–85.5) | 253 | 94.1 (89.2–96.9) | |
|
| |||||
| Other | 4805 | 16.6 (14.5–19.0) | 18 | 5.9‡ (3.1-10.8) | |
|
| |||||
| Highest education level | <0.001 | ||||
| Less than high school | 5690 | 18.7 (17.5–20.0) | 105 | 29.6 (24.0–36.0) | |
|
| |||||
| High school/GED | 4684 | 24.7 (23.6–25.9) | 79 | 31.7 (24.2–40.2) | |
|
| |||||
| More than high school | 9228 | 56.6 (54.8–58.4) | 86 | 38.7 (30.8–47.3) | |
| Family income | <0.001 | ||||
|
| |||||
| ≥2.0 times poverty level | 9744 | 66.7 (64.9–68.5) | 84 | 43.7 (35.7–52.0) | |
|
| |||||
| 1.0–1.9 times poverty level | 4901 | 20.4 (19.3–21.6) | 78 | 27.2 (21.2–34.3) | |
|
| |||||
| Below poverty level | 3551 | 12.9 (11.9–13.9) | 93 | 29.1 (21.8–37.7) | |
|
| |||||
| Total | 19 630 | 98.7 (98.5–98.9) | 271 | 1.3 (1.1–1.5) | – |
Anti-HCV = antibody to HCV; HCV = hepatitis C virus; NHANES = National Health and Nutrition Examination Survey.
P value for chi-square test of difference in characteristic by HCV status.
Estimate may be unstable and should be interpreted with caution because it is based on <10 HCV RNA–positive persons.
Estimate may be unstable and should be interpreted with caution because the relative SE is >30%.
Persons aged 20 to 59 years with chronic HCV infection were more likely to have received a blood transfusion before 1992, ever injected illicit drugs, or had 10 or more lifetime sexual partners than those aged 20 to 59 years who never had HCV infection; however, only 50.8% reported a risk related to illicit injection drug use or transfusion. Persons aged 60 years or older with chronic HCV infection were more likely to have received a blood transfusion before 1992 than those of the same age who never had HCV infection.
Among persons aged 20 to 49 years, those with chronic HCV infection were more likely to be positive for antibody to herpes simplex virus type 2 than those who were never infected with HCV (Table 2). Because only 3 persons with chronic HCV infection tested positive for antibody to HIV, we were unable to produce reliable estimates for this variable. Of the estimated 2.68 million chronically infected persons, 250 000 (CI, 120 000 to 380 000 persons) aged 20 to 59 years and 130 000 (CI, 70 000 to 190 000 persons) aged 60 years or older were estimated to have received a blood transfusion before 1992, a total of 1.04 million (CI, 760 000 to 1.32 million persons) were estimated to have ever injected illicit drugs, and 550 000 (CI, 340 000 to 770 000 persons) who reported no drug- or transfusion-related risk were estimated to have had at least 10 lifetime sexual partners. An estimated 1.19 million chronically infected persons aged 20 to 59 years (CI, 870 000 to 1.52 million persons) did not report illicit injection drug use or a transfusion-related risk.
Table 2.
Potential Risk Factors and Exposures by HCV Status: NHANES 2003–2010*
| Potential Risk Factor or Exposure | HCV Status |
P Value† | |||
|---|---|---|---|---|---|
| Anti-HCV–Negative |
HCV RNA–Positive |
||||
| Participants, n | Proportion (95% CI) | Participants, n | Proportion (95% CI) | ||
| Blood transfusion before 1992 | |||||
| Participants aged 20–59 y at interview | 0.027 | ||||
|
| |||||
| Yes | 486 | 4.2 (3.7–4.8) | 24 | 10.5 (6.1–17.5) | |
|
| |||||
| No | 12 241 | 95.8 (95.2–96.3) | 187 | 89.5 (82.5–93.9) | |
| Participants aged ≥60 y at interview | <0.001 | ||||
|
| |||||
| Yes | 894 | 14.8 (13.7–15.9) | 21 | 52.6 (37.9–66.9) | |
|
| |||||
| No | 5644 | 85.2 (84.1–86.3) | 32 | 47.4 (33.1–62.1) | |
| Lifetime drug use‡ | <0.001 | ||||
|
| |||||
| None or marijuana only | 9248 | 79.4 (78.0–80.8) | 51 | 26.5 (19.7–34.6) | |
|
| |||||
| Only noninjection drugs | 2070 | 19.0 (17.7–20.4) | 42 | 22.0 (16.2–29.2) | |
|
| |||||
| Injection drugs | 158 | 1.6 (1.3–2.0) | 89 | 51.5 (43.6–59.3) | |
|
| |||||
| Lifetime sexual partners‡ | <0.001 | ||||
| 0–1 | 2286 | 18.8 (17.5–20.3) | 10 | 3.7§ (1.7–7.8) | |
|
| |||||
| 2–9 | 5337 | 47.6 (46.3–48.9) | 56 | 28.8 (21.5–37.5) | |
|
| |||||
| 10–19 | 1823 | 16.5 (15.5–17.5) | 33 | 17.3 (12.4–23.5) | |
|
| |||||
| 20–49 | 1388 | 12.7 (11.8–13.6) | 51 | 30.3 (23.6–38.0) | |
|
| |||||
| ≥50 | 522 | 4.4 (3.9–4.9) | 31 | 19.9 (13.2–28.8) | |
| Antibody to herpes simplex virus type 2∥ | <0.001 | ||||
|
| |||||
| Positive | 2161 | 18.3 (17.2–19.5) | 63 | 52.8 (41.8–63.6) | |
|
| |||||
| Negative | 7800 | 81.7 (80.5–82.8) | 57 | 47.2 (36.4–58.2) | |
Anti-HCV = antibody to HCV; HCV = hepatitis C virus; NHANES = National Health and Nutrition Examination Survey.
Sample sizes for each variable vary depending on age eligibility for a particular question or laboratory test.
P value for chi-square test of difference in the prevalence of the factor or exposure by HCV status.
Participants aged 20 to 59 y at interview.
Estimate may be unstable and should be interpreted with caution because the relative SE is >30%.
Participants aged 20 to 49 y at interview.
Factors Associated With Chronic HCV Infection
Simple (unadjusted) logistic regression models for persons aged 20 to 59 years found that age 40 to 59 years, male sex, non-Hispanic black race/ethnicity, U.S. birth, high school education or less, and family income less than twice the poverty level were all statistically significantly associated with chronic HCV infection. Having ever used illicit drugs other than marijuana (including injection drugs), receipt of a blood transfusion before 1992, and having at least 10 lifetime sexual partners were also statistically significantly associated with chronic HCV infection. In the final multivariate logistic model, only receipt of a blood transfusion before 1992 did not remain statistically significantly associated with chronic HCV infection (Table 3).
Table 3.
Adjusted Odds Ratios for the Presence of HCV RNA in Participants Aged 20 to 59 y: NHANES 2003–2010 (n = 10 941)
| Characteristic | Participants Tested, n* |
HCV RNA–Positive Participants, n* |
Final Multivariate Logistic Model |
|
|---|---|---|---|---|
| Adjusted Odds Ratio (95% CI) | P Value | |||
| Age at interview | ||||
|
| ||||
| 20–39 y | 5733 | 28 | 1.0 (reference) | – |
|
| ||||
| 40–49 y | 2834 | 72 | 6.0 (3.2–11.1) | <0.001 |
|
| ||||
| 50–59 y | 2374 | 74 | 9.5 (5.3–16.8) | <0.001 |
|
| ||||
| Sex | ||||
| Male | 5292 | 109 | 1.6 (1.1-2.4)† | 0.021 |
|
| ||||
| Female | 5649 | 65 | 1.0 (reference) | – |
|
| ||||
| Race/ethnicity | ||||
| Non-Hispanic black | 2195 | 60 | 1.6 (1.1-2.3)† | 0.018 |
|
| ||||
| All others | 8746 | 114 | 1.0 (reference) | – |
|
| ||||
| Birthplace | ||||
| United States | 8326 | 164 | 1.0 (reference) | – |
|
| ||||
| Other | 2615 | 10 | 0.4 (0.2-0.9)† | 0.027 |
|
| ||||
| Highest education level | ||||
| Less than high school/GED | 5110 | 117 | 2.0 (1.2-3.3)† | 0.007 |
|
| ||||
| High school or more | 5831 | 57 | 1.0 (reference) | – |
|
| ||||
| Family income | ||||
| <2.0 times poverty level | 4937 | 121 | 3.7 (2.6–5.3) | <0.001 |
|
| ||||
| ≥2.0 times poverty level | 6004 | 53 | 1.0 (reference) | – |
|
| ||||
| Lifetime drug use | ||||
| None or marijuana only | 8706 | 49 | 1.0 (reference) | – |
|
| ||||
| Other (including injection drugs) | 2235 | 125 | 8.7 (5.9–12.8) | <0.001 |
HCV = hepatitis C virus; NHANES = National Health and Nutrition Examination Survey.
Sample sizes from final model.
Estimate may be unstable and should be interpreted with caution because the relative SE is >30%.
Simple logistic models for persons aged 60 years or older found that age 60 to 69 years, non-Hispanic black race/ethnicity, and receipt of a blood transfusion before 1992 were statistically significantly associated with chronic HCV infection. Male sex was also statistically significantly associated with chronic HCV infection when added to the final main-effects multivariate logistic model (Table 4). Because male sex was statistically significantly associated with chronic HCV infection in the multivariate model but not its simple logistic model, first-order interactions between sex and the other 3 independent variables in the final model were investigated by adding each interaction term separately to the final main-effects model; none was found to be statistically significant. Of note, NHANES does not assess drug use or sexual behaviors in persons aged 60 years or older, which accounts for the difference in factors examined for the 2 age groups (persons aged 20 to 59 years and ≥60 years).
Table 4.
Adjusted Odds Ratios for the Presence of HCV RNA in Participants Aged ≥60 y: NHANES 2003–2010 (n = 6591)
| Characteristic | Participants Tested, n* |
HCV RNA–Positive Participants, n* |
Final Multivariate Logistic Model |
|
|---|---|---|---|---|
| Adjusted Odds Ratio (95% CI) | P Value | |||
| Age at interview | ||||
|
| ||||
| 60–69 y | 3035 | 35 | 2.0 (1.1–3.8)† | 0.034 |
|
| ||||
| ≥70 y | 3556 | 18 | 1.0 (reference) | – |
| Sex | ||||
|
| ||||
| Male | 3297 | 32 | 2.7 (1.4–5.4)† | 0.005 |
|
| ||||
| Female | 3294 | 21 | 1.0 (reference) | – |
| Race/ethnicity | ||||
|
| ||||
| Non-Hispanic black | 1129 | 28 | 10.0 (4.9–20.1) | <0.001 |
|
| ||||
| All others | 5462 | 25 | 1.0 (reference) | – |
| Blood transfusion before 1992 | ||||
|
| ||||
| Yes | 915 | 21 | 8.5 (4.5–16.3) | <0.001 |
| No | 5676 | 32 | 1.0 (reference) | – |
HCV = hepatitis C virus; NHANES = National Health and Nutrition Examination Survey.
Sample sizes from final model.
Estimate may be unstable and should be interpreted with caution because the relative SE is >30%.
DISCUSSION
Using NHANES data from 2003 to 2010, we estimated that 1.3% of persons in the general U.S. population, or about 3.6 million (CI, 3.0 to 4.2 million persons), had anti-HCV indicative of past or current infection with HCV and that 1.0% of persons in the general population, or about 2.7 million (CI, 2.2 to 3.2 million persons), have chronic HCV infection (that is, are HCV RNA–positive). Our analysis of recent survey data may suggest a decrease in NHANES-estimated anti-HCV prevalence from 1.8% between 1988 and 1994 (2) and 1.6% between 1999 and 2002 (3). As previously assessed using NHANES, approximately 3.9 million persons (CI, 3.1 to 4.8 million persons) between 1988 and 1994 (2) and 4.1 million (CI, 3.4 to 4.9 million persons) between 1999 and 2002 were ever infected with HCV (anti-HCV–positive) (3). Our findings also suggest a possible decrease in chronic HCV infection from 1.3%, or 3.2 million persons (CI, 2.7 to 3.9 million persons), between 1999 and 2002 (3) (Figure). However, because of overlap in the 95% CIs of the survey estimates of persons who ever had HCV infection or chronic infection, any differences may represent the variability around estimates computed from a sample of the civilian noninstitutionalized U.S. population.
Although increased successful treatment of HCV infection might also cause a decrease in prevalence estimates since the last survey, all available current information indicates that no more than one half of persons with chronic HCV infection have been tested for anti-HCV; many who are anti-HCV–positive do not receive medical care or confirmatory HCV RNA testing; and, if positive, few to date have received antiviral therapy and achieved sustained virologic response indicative of cure (6, 12–14). Thus, differences in prevalence due to successful treatment are unlikely.
From 1999 to 2007, HCV-associated deaths as captured in vital records increased by 50% to 15 106 in 2007 (7), and the annual number of deaths among HCV-infected persons is probably much higher when other HCV-associated deaths not captured in vital records and deaths from other causes are considered. Using the NHANES III Linked Mortality File, El-Kamary and colleagues (15) estimated that 31 163 persons with chronic HCV infection in the general population died from all causes annually between 1988 and 2006; they considered this an underestimate. A recently presented analysis supports the idea that HCV is grossly underrecorded on death certificates, even when the main cause of death is liver-related (16). Thus, mortality in HCV-infected persons seems to be much higher than suggested by the 16 000 to 17 000 death certificates with HCV noted on them. In addition, a recent study (17) reported substantial increases in hospitalizations for HCV.
An increase in mortality among HCV-infected persons could help explain the decrease in prevalence of chronic HCV infection. Our finding of equivalent decreases in persons who ever had HCV infection (anti-HCV–positive persons) and those who had chronic (current) infection (persons with detectable HCV RNA) supports the suggestion that mortality among chronically infected persons has increased. We would not expect decreases in both if treatment were substantially affecting prevalence, because successful treatment should result in a decrease in the number of persons who are HCV RNA–positive but not in the number of persons who are anti-HCV–positive.
Demographic characteristics and risk factors or exposures associated with chronic HCV infection in our analysis of NHANES data from 2003 to 2010 did not differ substantially from those previously found to be associated with ever having had infection with HCV (2, 3). As in the previous NHANES analyses, injection drug use and receipt of a blood transfusion before 1992 remain important risk factors for HCV infection. Of interest, although simple (unadjusted results) logistic regression models for persons aged 20 to 59 years found receipt of a blood transfusion before 1992 to be statistically significantly associated with chronic HCV infection, blood transfusion before 1992 was not found to be statistically significantly associated with chronic infection in the final multivariate logistic model for those aged 20 to 59 years. This is probably due to the fact that age and sex are more strongly associated with chronic HCV infection and that these factors were also statistically significantly associated with receipt of a transfusion before 1992 (P < 0.001 and P = 0.005, respectively).
Because only about one half of persons aged 20 to 59 years with current HCV infection reported having at least 1 of these risk factors, risk-based screening alone is an incomplete approach to identifying chronically infected persons. Our analysis found that the prevalence of chronic HCV infection among persons in the general population born between 1945 and 1965 (2.6%) is 6-fold greater than that of other adults, representing 81% of all persons chronically infected with HCV. For this reason, the Centers for Disease Control and Prevention recently augmented existing risk-based policies for HCV testing to recommend a 1-time HCV test for all persons born during this period (18). With implementation of this strategy, approximately 800 000 persons currently unaware of their infection could be identified and linked to appropriate care and treatment, potentially averting approximately 120 000 deaths (19).
A major limitation of NHANES is that it does not include homeless and incarcerated persons, who are probably at higher risk for HCV infection. Accordingly, considerations of the prevalence and effect of chronic HCV infection in the United States should supplement data from NHANES with those from populations with a higher risk for and prevalence of HCV infection, such as institutionalized (incarcerated) and homeless persons. Having only 273 HCV RNA–positive participants imposed limitations on our analysis: It was not possible to provide statistically valid estimates by age, or by sex and age within race/ethnicity, to perform a trend analysis or to produce an odds ratio for injection drug use separate from use of drugs other than marijuana. For this reason, we also did not perform an analysis of prevalence by birth year, especially because this effect has already been well-documented (3).
Because only 90 participants were anti-HCV–positive and HCV RNA–negative, analyzing them as a separate subgroup was not possible. The fact that NHANES does not query persons aged 60 years or older on drug use or sexual behavior restricted our ability to investigate these risk behaviors to those aged 20 to 59 years. In addition, the unavoidable missing data on self-reported risk factors may limit our ability to generalize our findings to the entire population sampled by NHANES. We were unable to estimate the prevalence of HIV infection among those who had chronic HCV infection because only 3 participants with chronic HCV infection were also positive for HIV antibodies. Finally, because NHANES participants are allowed to opt out of HIV testing, the participants who were positive for HIV antibodies are almost certainly not representative of such persons even in the NHANES population.
The availability of new, direct-acting antiviral medications with higher success rates (20) creates an enormous opportunity to prevent morbidity and mortality among persons with chronic HCV infection. Additional antiviral regimens that do not require interferon and ribavirin are anticipated soon (21), potentially improving patient adherence and health outcomes. However, the health impact of advances in HCV therapy can be realized only when HCV-infected persons are tested, identified, and linked to appropriate care and treatment (22). Our study and others have found that persons with chronic HCV infection are frequently poor and less educated, factors that could pose barriers to receipt of these costly novel HCV treatments. Improving HCV testing, care, and treatment will require a comprehensive set of implementation activities, including changes in health policies, reimbursement for care and treatment, community education, and provider training. In the future, health surveys and surveillance systems may need to monitor the burden of disease and the effect of strategies for HCV testing, care, and treatment.
In summary, we estimate that approximately 2.7 million persons in the civilian noninstitutionalized U.S. population sampled by NHANES continue to have chronic HCV infection. If HCV infections among high-risk populations not sampled by NHANES are taken into account, our estimated prevalence of chronic infection is conservative. An important public health implication is that our analysis suggests decreases in prevalence that probably reflect increasing mortality from HCV-related conditions. That these deaths largely occur in the age group born between 1945 and 1965 (7) (the “Baby Boom” generation) underscores the urgency of addressing this underappreciated national epidemic partly through implementation of the recent Centers for Disease Control and Prevention recommendation for 1-time screening for HCV infection and referral to appropriate care and treatment for persons in this birth cohort (18).
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Potential Conflicts of Interest: None disclosed. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M13-1133.
Reproducible Research Statement: Study protocol: Available at www.cdc.gov/nchs/nhanes/about_nhanes.htm. Statistical code: Available from Ms. Denniston (e-mail, mmd1@cdc.gov or mdennist@gmail.com). Data set: Available at www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
Current author addresses and author contributions are available at www.annals.org.
Author Contributions: Conception and design: M.M. Denniston, R.B. Jiles, J. Drobeniuc, M. Klevens, S.D. Holmberg.
Analysis and interpretation of the data: M.M. Denniston, R.B. Jiles, J. Drobeniuc, R.M. Klevens, G.M. McQuillan, S.D. Holmberg.
Drafting of the article: M.M. Denniston, J. Drobeniuc, S.D. Holmberg. Critical revision of the article for important intellectual content: M.M. Denniston, R.B. Jiles, J. Drobeniuc, J.W. Ward, G.M. McQuillan, S.D. Holmberg.
Final approval of the article: M.M. Denniston, J. Drobeniuc, R.M. Klevens, J.W. Ward, G.M. McQuillan.
Provision of study materials or patients: G.M. McQuillan.
Statistical expertise: M.M. Denniston.
Obtaining of funding: J.W. Ward, S.D. Holmberg.
Administrative, technical, or logistic support: R.B. Jiles, J. Drobeniuc, R.M. Klevens, G.M. McQuillan, S.D. Holmberg.
Collection and assembly of data: J. Drobeniuc, G.M. McQuillan.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [PMID: 23245604] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–62. doi: 10.1056/NEJM199908193410802. [PMID: 10451460] [DOI] [PubMed] [Google Scholar]
- 3.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [PMID: 16702586] [DOI] [PubMed] [Google Scholar]
- 4.Pyenson B, Fitch K, Iwasaki K. Consequences of Hepatitis C Virus (HCV): Costs of a Baby Boomer Epidemic of Liver Disease. Milliman; New York: 2009. [Google Scholar]
- 5.Institute of Medicine . A National Strategy for Prevention and Control of Hepatitis B and C. National Academies Pr; Washington, DC: 2010. Hepatitis and Liver Cancer. [PubMed] [Google Scholar]
- 6.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–61. doi: 10.1056/NEJMp1302973. [PMID: 23675657] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [PMID: 22351712] [DOI] [PubMed] [Google Scholar]
- 8.Research Triangle Institute . SUDAAN Language Manual, Release 10.0. Research Triangle Institute; Research Triangle Park, NC: 2008. [Google Scholar]
- 9.Wald A, Langenberg AG, Link K, Izu AE, Ashley R, Warren T, et al. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA. 2001;285:3100–6. doi: 10.1001/jama.285.24.3100. [PMID: 11427138] [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb SL, Douglas JM, Jr, Foster M, Schmid DS, Newman DR, Baron AE, et al. Project RESPECT Study Group Incidence of herpes simplex virus type 2 infection in 5 sexually transmitted disease (STD) clinics and the effect of HIV/STD risk-reduction counseling. J Infect Dis. 2004;190:1059–67. doi: 10.1086/423323. [PMID: 15319854] [DOI] [PubMed] [Google Scholar]
- 11.Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010;52:1497–505. doi: 10.1002/hep.23808. [PMID: 20635398] [DOI] [PubMed] [Google Scholar]
- 12.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55:1652–61. doi: 10.1002/hep.25556. [PMID: 22213025] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spradling PR, Rupp L, Moorman AC, Lu M, Teshale EH, Gordon SC, et al. Chronic Hepatitis Cohort Study Investigators. Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55:1047–55. doi: 10.1093/cid/cis616. [PMID: 22875876] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North CS, Hong BA, Adewuyi SA, Pollio DE, Jain MK, Devereaux R, et al. Hepatitis C treatment and SVR: the gap between clinical trials and real-world treatment aspirations. Gen Hosp Psychiatry. 2013;35:122–8. doi: 10.1016/j.genhosppsych.2012.11.002. [PMID: 23219917] [DOI] [PubMed] [Google Scholar]
- 15.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53:150–7. doi: 10.1093/cid/cir306. [PMID: 21665867] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahajan R, Xing J, Liu SJ, Ly KN, Moorman AC, Rupp L, et al. Mortality among persons in care with hepatitis C virus infection—Chronic Hepatitis Cohort Study (CHeCS), 2006-2010; Presented at IDWeek 2013; San Francisco, California. Oct 2–6, 2013. [Abstract] Abstract no. 1774. [DOI] [PubMed] [Google Scholar]
- 17.Sie L, Gatto NM, Bancroft E. Hospitalizations due to hepatitis C in Los Angeles County, 2007-2009: case characteristics and factors associated with mortality. J Viral Hepat. 2013;20:628–37. doi: 10.1111/jvh.12086. [PMID: 23910647] [DOI] [PubMed] [Google Scholar]
- 18.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, et al. Centers for Disease Control and Prevention Recommendations for the identifi of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PMID: 22895429] [PubMed] [Google Scholar]
- 19.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Ward JW. Hepatitis C virus testing of persons born during 1945-1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med. 2012;157:817–22. doi: 10.7326/0003-4819-157-9-201211060-00529. [PMID: 22910836] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox AN, Jacobson IM. Recent successes and noteworthy future prospects in the treatment of chronic hepatitis C. Clin Infect Dis. 2012;55(Suppl 1):S16–24. doi: 10.1093/cid/cis391. [PMID: 22715209] [DOI] [PubMed] [Google Scholar]
- 21.Poordad F, Lawitz E, Kowdley KV, Cohen DE, Podsadecki T, Siggelkow S, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368:45–53. doi: 10.1056/NEJMoa1208809. [PMID: 23281975] [DOI] [PubMed] [Google Scholar]
- 22.Grant WC, Jhaveri RR, McHutchison JG, Schulman KA, Kauf TL. Trends in health care resource use for hepatitis C virus infection in the United States. Hepatology. 2005;42:1406–13. doi: 10.1002/hep.20941. [PMID: 16317670] [DOI] [PubMed] [Google Scholar]


