Abstract
Aim
To determine if the drug doses and administration schedules of carboplatin and gemcitabine combination affect antitumor effects.
Materials and Methods
The inhibition of cell viability was measured by MTT assay. Median effect analysis was conducted to determine the cytotoxicity activity of carboplatin and gemcitabine combination. Cell cycle changes were analyzed by flow cytometry.
Results
Synergism was observed when the bladder cancer cell line 5637 cells were treated with gemcitabine followed by carboplatin or concurrent carboplatin/gemcitabine. In contrast, moderate antagonism was observed when cells were treated with carboplatin followed by gemcitabine. Cell cycle analysis showed that the combined effect of these two drugs was cell cycle disturbance.
Conclusions
Different doses and administration schedules affect the anti-tumor effect of carboplatin/gemcitabine combination that may have clinical significance in the treatment for bladder cancer.
Keywords: Bladder cancer, chemotherapy, synergism, cell cycle
The platinum/gemcitabine combination and MVAC (methotrexate, vinblastine, adriamycin and cisplatin) are the two first-line regimens with similar anti-tumor efficacy in the treatment of metastatic bladder transitional cell carcinoma (TCC) (1). Because of the significant toxicity associated with MVAC, the combination of platinum/gemcitabine has largely replaced MVAC and become the first-line chemotherapy regimen for bladder cancer. Carboplatin is a second-generation platinum analog. Compared with the first-generation cisplatin, carboplatin has milder toxicity profile with less nephrotoxicity and neurotoxicity. This is especially appealing in bladder cancer as many of these patients have renal insufficiency and cardiovascular co-morbidities that precludes them from using cisplatin. Cisplatin or carboplatin in combination with gemcitabine has also been widely used in many other cancer types (2–5). Gemcitabine may provide a survival benefit in some platinum-resistant cancer cases because of the different mechanisms of action (6). Several studies have analyzed the anti-tumor activity of carboplatin/gemcitabine combination with contradicting results (7–10). This study performed a systemic analysis to determine how the doses and administration schedules of carboplatin/gemcitabine combination affect its anti-tumor effects and correlate with the underlying mechanisms in a bladder cancer cell line.
Materials and Methods
Chemicals
Gemcitabine (GEMZAR®) was obtained from Eli Lilly (Indianapolis, IN, USA), carboplatin (CARBOplatin®, 10 mg/ml) from Hospira (Lake Forest, IL, USA). Thiazolyl Blue Tetrazolium Bromide (MTT) and Propidium iodide (PI) was obtained from Sigma (Saint Louis, MO, USA). RPMI-1640 Medium was obtained from ATCC (Manassas, VA, USA). A human urinary bladder TCC cell line 5637 was obtained from ATCC (Manassas, VA, USA). The cells were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 µM L-glutamine and 1% penicillin/streptomycin.
Growth inhibition tests
The MTT assay was performed to determine the growth inhibition (11). In brief, 5637 cells at 4000 cells/well were seeded in 96-well plates. After overnight culture, cells were treated with carboplatin and/or gemcitabine. When cells were treated with carboplatin alone, they were treated for 4 h to mimic the in vivo half-life of carboplatin of 1.3–6 h (12,13). The following combinations were tested: (i) 4 h gemcitabine followed by 4 h carboplatin; (ii) 0.5 h carboplatin followed by 3.5 h carboplatin plus gemcitabine; (iii) 4 h carboplatin followed by 4 h gemcitabine. In Group ii, the treatment schedule was designed to mimic the clinical administration of carboplatin followed by gemcitabine infusion in patients. After these treatments, the cells were washed and cultured with complete medium at 37°C for 68 h in a humidified atmosphere containing 5% CO2. After treatment with MTT, the absorption was measured at 570 nm and 690 nm using a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The percentage of viable cells was calculated using the formula (14): % living cells = (sample ext. − blank ext.) / (control ext.− blank ext.) where ext.= extinction of the purple color measured at 570 nm − that measured at 690 nm. The IC50 (the concentration required for 50% inhibition) was calculated using the GraphPad Prism 5 program (GraphPad Software Inc., San Diego, CA, USA). Each drug or combination was tested at least in triplicate.
Median effect analysis
This method proposed by Chou and Talalay was used to determine the nature (synergism, additivity and antagonism) of drug and drug interaction (15,16). The drugs were combined in the same concentration ratio based on their corresponding IC50s (carboplatin : gemcitabine = 289.30 µM: 0.086 µM = 3364:1). This method, termed as the combination index (CI) equation, allows quantitative determinations of drug interactions at increasing levels of cell kill (Figure 1 and Tables I and II). The CI value allows the classification of the anti-tumor activity of the drug combination (Table I). Dm is the antilog of x-intercept, meaning the concentration of carboplatin, gemcitabine, or in combination needed to induce 50% of cell killing. Fa is the fraction of cell death induced by drug treatment. It ranges from 0–1, with the Fa value of 0 meaning no cell killing and the value of 1 representing 100% of cell killing. The 5637 cells were treated with serial dilutions of each drug alone or with carboplatin/gemcitabine combination at a fixed ratio of 3364:1. Five dilutions ranging from one fourth of the IC50 to four times of the IC50 concentrations (serial dilution factor = 2) of each drug, in combination plus a control were tested in three independent experiments with triplicate samples.
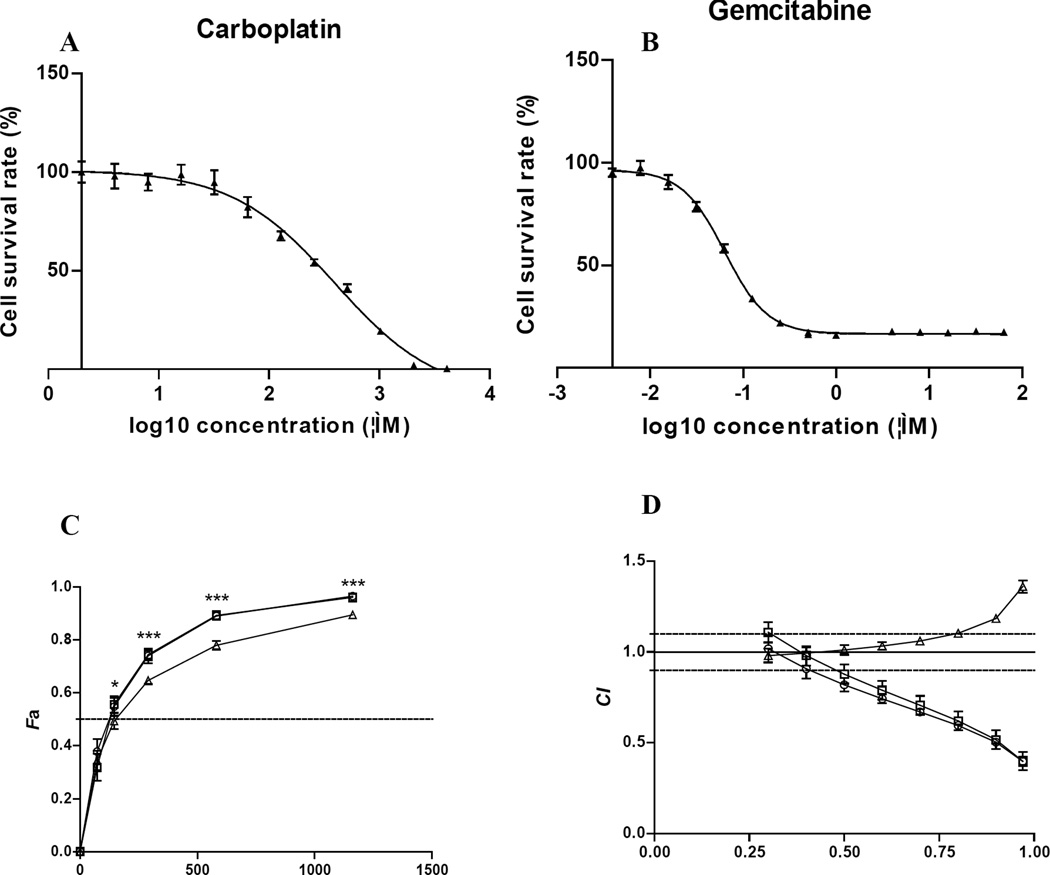
Figure 1.
The anti-tumor activity of carboplatin, gemcitabine or in combination. A and B: Dose-dependent cytotoxic effects of 5637 cells exposed to carboplatin (A) or gemcitabine (B). C. Dose-effect curves for the carboplatin/gemcitabine combinations. 5637 cells were treated with: gemcitabine for 4 h followed by carboplatin for 4 h (square); carboplatin for 0.5 h followed by carboplatin plus gemcitabine for 3.5 h (circle); carboplatin for 4 h followed by gemcitabine for 4 h (triangle). Data are means ± SD of three independent experiments (each with samples in triplicate). *p<0.05, ***p<0.001. D. CI Plot. CI values are plotted as a function of the fractional inhibition (Fa) from 0.10 to 0.97. The CI values of <0.9 (below the lower dash line), =0.9–1.1, and <1.1 (above the upper dash line) represent synergism, additivity and antagonism, respectively.
Table I.
The classification of the combination index (CI) and anti-tumor activity. The CI value of between 0.9 and 1.1 represent additivity. The CI value less than 0.9 represents synergism while the value above 1.1 means antagonism.
| Synergism | Antagonism | ||
|---|---|---|---|
| CI value | Synergy category | CI value | Antagonism category |
| 0.85–0.9 | Slight synergy | 1.1–1.2 | Slight antagonism |
| 0.7–0.85 | Moderate synergy | 1.2–1.45 | Moderate antagonism |
| 0.3–0.7 | Synergy | 1.45–3.3 | antagonism |
| 0.1–0.3 | Strong synergy | 3.3–10 | Strong antagonism |
| <0.1 | Very strong synergy | >10 | Very strong antagonism |
Table II.
The dose-effect relationship parameters and mean CI values of carboplatin, gemcitabine or in combination.
| Drug combination | Dose-effect parameters | CI values | ||||
|---|---|---|---|---|---|---|
| Dm (µM) | r | IC50 | IC75 | IC90 | IC95 | |
| Carbo 4 h | 290.48±20.4 | 0.990 | — | — | — | — |
| Gem 4 h | 0.085±0.002 | 0.993 | — | — | — | — |
| Gem 4h before carbo 4 h | 127.16±7.61 | 0.999 | 0.88±0.06 Syn |
0.66±0.05 Syn |
0.52±0.05 Syn |
0.44±0.04 Syn |
| Carbo 0.5 h before carbo + gem 3.5 h | 119.03±5.42 | 0.994 | 0.82±0.04 Syn |
0.63±0.01 Syn |
0.50±0.01 Syn |
0.44±0.02 Syn |
| Carbo 4 h before gem 4 h | 148.08±3.39 | 0.997 | 1.02±0.03 Ant |
1.08±0.01 Ant |
1.17±0.01 Ant |
1.26±0.02 Ant |
Carbo: carboplatin; Gem: gemcitabine; Syn: synergism; Ant: antagonism.
Cell cycle analysis
Flow cytometry analysis was used to analyze cell cycle distribution. After culture overnight, cells in 60 mm culture dishes were treated with culture medium containing 289.3 µM (IC50) of carboplatin and 0.086 µM (IC50) of gemcitabine, or in combination for 4 h. The drug administration schedules for the combinations were as followed: (i) 4 h gemcitabine followed by 4 h carboplatin; (ii) 0.5 h carboplatin followed by 3.5 h carboplatin plus gemcitabine. The time point when drug was added was considered to be 0 h. Three dishes from each group were collected at 24, 48 and 72 h, and fixed with 70% ethanol in −20°C for 2 h. The cells were treated with 100 µg/ml RNase A at 37°C for 30 m to eliminate RNA and stained with 50 µg/ml propidium iodide at 4°C for 30 m. DNA histogram data was analyzed with a Stratedigm S1000 (Stratedigm Corporation, San Jose, CA, USA) and the list mode files were analyzed with WinMDI version 2.9.
Statistical analysis
The data from MTT assay were expressed as mean ± standard deviation (SD). The IC50 values of carboplatin and gemcitabine and linear regression analyses were computed by GraphPad Prism 5 program (GraphPad Software Inc., San Diego, CA, USA). Dose-effect curve parameters, CI values, Fa-CI plot (plot representing CI versus Fa, the fraction affected by a particular dose) were calculated by CompuSyn program (Compusyn Inc, Paramus, NJ, USA). A p-value of <0.05 was denoted a statistically significant difference.
Results
Dose- and schedule-dependent cytotoxic effects of carboplatin/gemcitabine combination
The cytotoxic activities of carboplatin and gemcitabine were first determined individually on 5637 cells. As expected, there was a dose-dependent cell killing effect. IC50 values ± SD were 289.3 ± 2.90 µM for carboplatin (Figure 1A) and 0.086 ± 0.008 µM for gemcitabine (Figure 1B).
The cytotoxicity of carboplatin/gemcitabine combination on 5637 cells was then evaluated using the CI method (16). Table II shows the dose-effect curve parameters (Dm and r) of the two drugs either as single agents or in combination, as well as mean CI values of combinations in different treatment doses and schedules. The r values of 0.95 or above indicated good conformity of the dose-effect data with respect to the median-effect principle (16). The r values for all of the experiments were 0.99 or higher. Figure 1 C showed dose-effect relationship of the two drugs combinations. The X axis represents the drug combination concentrations (µM); the Y axis (Fa) represents the ratio of the combination effect on inhibition of cell growth with Fa of 1.0 representing 100% cell killing. The horizontal dash line represents Fa of 0.5, or 50% cell killing. The antitumor activity of treatment with gemcitabine followed by carboplatin, or concurrent carboplatin and gemcitabine treatment was significantly better than that of carboplatin followed by gemcitabine. Figure 1 D showed plots of the combination indices for the interaction between the two drugs as a function of the treatment schedule. When 5637 cells were treated with gemcitabine for 4 h followed by carboplatin for 4 h, the Dm value was 127.16 ± 7.61 µM (Table II), slightly less than the calculated IC50 of the combination at 145.28 µM [(289.3 + 0.086) ÷ 2]. That corresponded to the CI value of 0.88 ± 0.06, or slight synergism (Table I). Treatment of cells with carboplatin for 0.5 h followed by two drug combination for an additional 3.5 h had the Dm value of 119.03 ± 5.42 µM, corresponding to the CI value of 0.82 ± 0.04, or moderate synergism. However, these two CI values were not statistically significant (p=0.19). As the concentration of carboplatin and gemcitabine combination increased, the CI value decreased (Table II). At the IC95 concentration, the CI values were 0.44 ± 0.04 and 0.44 ± 0.02, respectively, indicating synergism. On the other hand, when 5637 cells were treated with carboplatin for 4 h followed by gemcitabine for 4 h, the Dm value was 148.08 ± 3.39 µM, close to the calculated IC50 of 145.28 µM, which corresponded to the CI value of 1.02 ± 0.03, or nearly additive (Table II). This was less effective than the other two treatment schedules (p=0.017 and p=0.002, respectively). As the concentration of carboplatin and gemcitabine combination increased, the combined cytotoxic effects fell into moderate antagonism at IC95, much less effective than the treatments of gemcitabine followed by carboplatin or carboplatin followed by concurrent carboplatin/gemcitabine treatment (p<0.001) (Figure 1C).
Cell cycle analysis
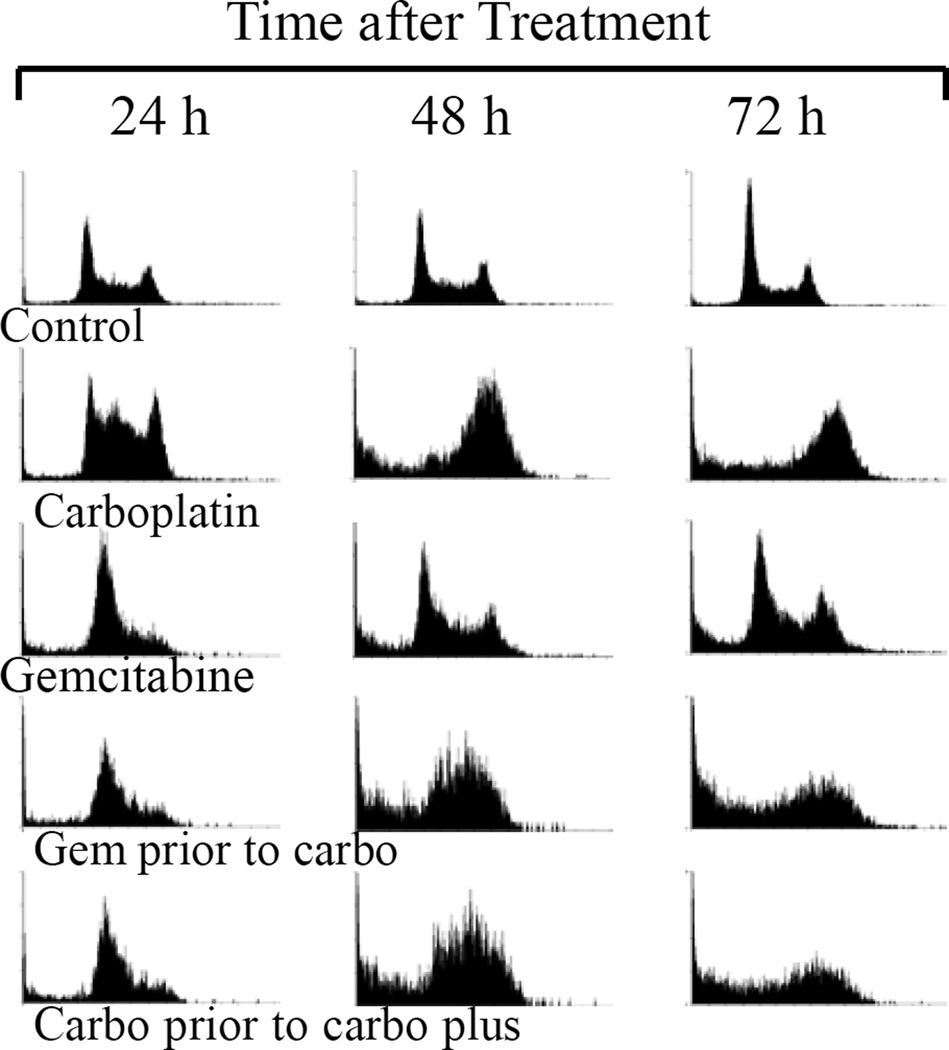
It was next determined how carboplatin/gemcitabine combination changed cell cycle distribution, and affected the anti-tumor activity (Figure 2, Table III). The cell cycle distribution of untreated control cells did not change significantly except that the S phase slightly decreased at 72 h, possibly related to cell confluence and/or nutrition depletion. More carboplatin-treated cells were arrested at the S phase after 24 h of exposure (53.4%), and at the G2/M phase at the later time points (56.3% at 48 h and 52.6% at 72 h). The portion of cells with subdiploid DNA content (apoptosis) increased significantly at the 48 h and 72 h time points (15.3% and 19.5%, respectively). Gemcitabine-treated cells were arrested at the G0/G1 and early S phases (59.2%), with a parallel decrease of the population in G2/M phase at 24 h. At 48 and 72 h, more cells treated with gemcitabine had the subdiploid DNA content (15.1% and 15.9%, respectively) with the corresponding decrease of cells at G0/G1 phase, but the proportions of cells at the S and G2/M phases returned to those of the control cells. The cell cycle distribution patterns of cells treated with carboplatin and gemcitabine combinations, in either order, showed the combined effects of these two medications. At 24 h after treatment, more cells were at the G0/G1 and early S phases (58.4% for cells treated with gemcitabine before carboplatin versus 56.6% for cells treated with carboplatin before carboplatin plus gemcitabine), similar to the cells treated with gemcitabine alone. At 48 h, more cells were arrested at the S phase (48.97% versus 52.44%, respectively). At 72 h, more cells were arrested at the late S and G2/M phases (38.1% versus 33.7%, respectively), similar to the cells treated with carboplatin alone. More cells were at the sub-G0/G1 portion (34.3% versus 35.1%, respectively), suggesting synergistic or additive effect of the combination.
Figure 2.
Characteristic DNA histograms of 5637 cells treated with carboplatin, gemcitabine or in combination. The progressive cell cycle changes were observed after 24, 48 and 72 h of treatment, as compared with the untreated controls. Content of DNA is represented on the X axis; number of cells counted is represented on the Y axis.
Table III.
Cell cycle distribution determined by flow cytometry analysis. 5637 cells were treated with carboplatin, gemcitabine or in combinations. The time points when drugs were added were considered to be 0 h. Cells were harvested at the indicated times (24, 48 and 72 h); At least 10000 events were analyzed for each sample. The data as mean ± SD were from triplicate experiments.
| Phase of the cell cycle (%) |
Control | Carboplatin | Gemcitabine | Gem prior to carbo |
Carbo prior to carbo plus gem |
|
|---|---|---|---|---|---|---|
| Sub-G1 | ||||||
| 24 h | 2.36 ± 0.32 | 4.16 ± 0.38 | 8.68 ± 1.28 | 11.36 ± 1.05 | 12.42 ± 2.07 | |
| 48 h | 3.45 ± 0.97 | 15.26 ± 2.34 | 15.08 ± 0.82 | 24.98 ± 4.19 | 22.45 ± 0.27 | |
| 72 h | 4.96 ± 1.49 | 19.47 ± 1.44 | 15.90 ± 1.86 | 34.25 ± 4.29 | 35.12 ± 2.20 | |
| G0/G1 | ||||||
| 24 h | 38.72 ±2.30 | 18.54 ± 0.07 | 19.51 ± 1.93 | 18.37 ± 1.23 | 18.95 ± 0.51 | |
| 48 h | 36.35 ± 1.21 | 5.51 ± 0.55 | 29.44 ± 0.85 | 7.27 ± 0.38 | 7.58 ± 0.61 | |
| 72 h | 47.24 ± 3.45 | 6.53 ± 1.64 | 31.49 ± 1.40 | 7.50 ± 0.74 | 7.08 ± 0.66 | |
| S | ||||||
| 24 h | 36.32 ± 1.13 | 53.41 ± 0.90 | 59.20 ± 2.20 | 58.38 ± 0.87 | 56.55 ± 0.35 | |
| 48 h | 36.18 ± 0.16 | 23.13 ± 1.89 | 40.43 ± 1.45 | 48.97 ± 5.80 | 52.44 ± 160 | |
| 72 h | 26.77 ± 2.04 | 21.53 ± 1.25 | 29.97 ± 0.56 | 20.37 ± 1.27 | 24.31 ± 2.53 | |
| G2/M | ||||||
| 24 h | 23.13 ± 1.72 | 24.14 ± 0.53 | 12.97 ±1.27 | 12.16 ± 0.86 | 12.44 ± 2.21 | |
| 48 h | 24.00 ± 0.58 | 56.34 ± 1.24 | 15.36 ± 1.74 | 18.96 ±2.51 | 17.75 ± 1.46 | |
| 72 h | 21.25 ± 0.22 | 52.63 ± 1.82 | 22.93 ± 0.52 | 38.05 ± 4.00 | 33.67 ± 1.51 | |
Discussion
This study found that, when 5637 cells were treated with carboplatin for 4 h followed by gemcitabine, the cytotoxic activity fell into additivity to antagonism range depending on the drug concentration relative to the IC50. On the other hand, when cancer cells were treated with gemcitabine followed by carboplatin, or carboplatin and gemcitabine simultaneously, additive to synergistic effects were observed. These findings are different from a previous study showing more synergistic effects when non-small cell lung cancer cells were treated with carboplatin followed by gemcitabine (7), but are consistent with another study showing that simultaneous treatment with carboplatin and gemcitabine exerted synergistic anti-tumor effect in canine osteosarcoma cell lines (10). These differences may be secondary to the fact that different cell lines were used in different studies. The current authors believe that the findings of the current study are more consistent with the underlying cell-killing mechanisms of these two drugs. As an alkylating agent, carboplatin kills cells mainly through induction of DNA adducts (17). Its cell killing is not cell cycle-specific. Gemcitabine is a nucleoside analog in which the hydrogen on the 2' carbon of deoxycytidine is replaced by a fluorine atom. During DNA replication, gemcitabine triphosphate replaces dCTP and is incorporated into the DNA strands that terminates DNA replication (18,19). Gemcitabine exhibits cell cycle specificity in that it primarily kills cells undergoing DNA synthesis (S-phase). It is hypothesized that treatment of carboplatin prior to gemcitabine induces DNA damage and arrests cell cycle that decreases the incorporation of gemcitabine triphosphate into DNA, and mitigates the cytotoxic effects of gemcitabine. The dose-dependent antagonism is also observed. This suggests that, as more cells are arrested in their cell cycle with increased carboplatin concentration, less cytotoxicity with gemcitabine is observed. Treatment with carboplatin for 4 h is needed as it allows cells enough time to respond to DNA damage induced by carboplatin. No antagonism was seen when cells were treated with carboplatin for 0.5 h followed by carboplatin/gemcitabine concurrent treatment.
On the other hand, when cells were treated with gemcitabine followed by carboplatin or simultaneously with these two drugs, synergistic effects were observed (Table II and Figure 1). Several mechanisms may contribute to the enhanced cytotoxicity of this combination. DNA structural changes by incorporation of gemcitabine favor the binding of cisplatin (20), and gemcitabine inhibits DNA repair of Pt-DNA adducts (21). Furthermore, platinum inhibits ribonucleotide reductase (22), and further enhances the incorporation of gemcitabine triphosphate into DNA. Nucleotide excision repair is the major pathway responsible for the removal of platinum-DNA adducts. Gemcitabine might reduce the effectiveness of nucleotide excision repair through its inhibition of ribonucleotide reductase (10).
The cell cycle analysis of the current study showed that, around the concentration of IC50, cell cycle disturbance of carboplatin/gemcitabine combination was the combined effects of each individual drug. Treatment of gemcitabine arrested the cells at G1 and early S phase at 24 h because of the termination of DNA replication. Treatment of carboplatin leads to the S phase arrest at an early time point (24 h), associated with reduced expression of cyclin E and cyclin B (23), and then G2/M phase blockage at 48 and 72 h, associated with CDC25C phosphorylation (24). The G2/M checkpoint allows for the repair of DNA damage occurred late in the S or G2 phase of cell cycle before mitosis. Because of the double insults, more cells underwent apoptosis (24,25), as it was observed that more cells were at the sub-G0/G1 portion (Figure 2, Table III). The concentrations of these two drugs used in this study were at the corresponding IC50s. These findings were consistent with the current analysis with the CI method that showed near additive or slightly synergistic effect at the IC50 concentrations (Figure 1 and Table II).
The current findings may have significant clinical implication in the treatment for bladder cancer. The carboplatin-DNA adduct formation is essentially the same as that of cisplatin, even though a higher concentration of carboplatin is needed (17). Cisplatin is more commonly used in the treatment of bladder cancer. However, unlike carboplatin that is administered within one hour, cisplatin is given intravenously over several hours. If cisplatin is given before gemcitabine, arrest of cell cycle by cisplatin may affect the cytotoxic effect of gemcitabine. Further randomized clinical trials are needed to address this issue.
In conclusion, this study systemically analyzed the effects of doses and administration schedules of carboplatin/gemcitabine combination on the cytotoxic effects on a bladder cancer cell line. The analysis suggests that administration of gemcitabine before carboplatin or administration of these two drugs simultaneously is more effective than the schedule of carboplatin followed by gemcitabine.
Acknowledgements
We are grateful to Professor Ting-Chao Chou for providing the CompuSyn software. This publication is based upon work supported in part by the Career Development Award-II from the Office of Research and Development, Department of Veteran Affairs (PI: Pan), and by a K12 award (Grant Number UL1 RR024146) from the National Center for Research Resources (NCRR).
References
- 1.von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM, Kerbrat P, Sanchez Rovira P, Wersall P, Cleall SP, Roychowdhury DF, Tomlin I, Visseren-Grul CM, Conte PF. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 2.Linardou H, Aravantinos G, Efstathiou E, Kalofonos C, Anagnostopoulos A, Deliveliotis C, Bafaloukos D, Athanasios Dimopoulos M, Bamias A. Gemcitabine and carboplatin combination as first-line treatment in elderly patients and those unfit for cisplatin-based chemotherapy with advanced with bladder carcinoma: phase II study of the hellenic cooperative oncology group. Urology. 2004;2004;64:479–484. doi: 10.1016/j.urology.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Bajetta E, Stani SC, De Candis D, Zaffaroni N, Zilembo N, Cortinovis D, Aglione S, Mariani L, Formisano B, Bidoli P. Preclinical and clinical evaluation of four gemcitabine plus carboplatin schedules as front-line treatment for stage IV non-small-cell lung cancer. European Society for Medical Oncology. 2003;2003;14:242–247. doi: 10.1093/annonc/mdg060. [DOI] [PubMed] [Google Scholar]
- 4.Hudson E, Lester JF. Gemcitabine and carboplatin in the treatment of transitional cell carcinoma of the urothelium: a single centre experience and review of the literature. Eur J Cancer Care. 2010;2010;19:324–328. doi: 10.1111/j.1365-2354.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 6.Soga N, Kise H, Arima K, Sugimura Y. Third-line gemcitabine monotherapy for platinum-resistant advanced urothelial cancer. Int J Clin Oncol. 2010;2010;15:376–381. doi: 10.1007/s10147-010-0071-8. [DOI] [PubMed] [Google Scholar]
- 7.Bajetta E, Stani SC, De Candis D, Zaffaroni N, Zilembo N, Cortinovis D, Aglione S, Mariani L, Formisano B, Bidoli P. Preclinical and clinical evaluation of four gemcitabine plus carboplatin schedules as front-line treatment for stage IV non-small-cell lung cancer. Ann Oncol. 2003;2003;14:242–247. doi: 10.1093/annonc/mdg060. [DOI] [PubMed] [Google Scholar]
- 8.Edelman MJ, Quam H, Mullins B. Interactions of gemcitabine, carboplatin and paclitaxel in molecularly defined non-small-cell lung cancer cell lines. Cancer Chemother Pharmacol. 2001;2001;48:141–144. doi: 10.1007/s002800000273. [DOI] [PubMed] [Google Scholar]
- 9.Smith JA, Brown J, Martin MC, Ramondetta LM, Wolf JK. An in vitro study of the inhibitory activity of gemcitabine and platinum agents in human endometrial carcinoma cell lines. Gynecol Oncol. 2004;2004;92:314–319. doi: 10.1016/j.ygyno.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 10.McMahon MB, Bear MD, Kulp SK, Pennell ML, London CA. Biological activity of gemcitabine against canine osteosarcoma cell lines in vitro. Am J Vet Res. 2010;2010;71:799–808. doi: 10.2460/ajvr.71.7.799. [DOI] [PubMed] [Google Scholar]
- 11.Chen SZ, Jiang M, Zhen YS. HERG K+ channel expression-related chemosensitivity in cancer cells and its modulation by erythromycin. Cancer Chemother Pharmacol. 2005;2005;56:212–220. doi: 10.1007/s00280-004-0960-5. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara K, Yamauchi H, Suzuki S, Ishikawa H, Tanaka Y, Fujiwara M, Kohno I. The platelet-sparing effect of paclitaxel is not related to changes in the pharmacokinetics of carboplatin. Cancer Chemother Pharmacol. 2001;2001;47:22–26. doi: 10.1007/s002800000212. [DOI] [PubMed] [Google Scholar]
- 13.Sharma H, Thatcher N, Baer J, Zaki A, Smith A, McAucliffe CA, Crowther D, Owens S, Fox BW. Blood Clearance of Radioactively Labelled cis-Diammine 1,1-cyclobutane Dicarboxylate Platinum(II) (CBDCA) in Cancer Patients. Cancer Chemother Pharmacol. 1983;1983;11:5–7. doi: 10.1007/BF00257407. [DOI] [PubMed] [Google Scholar]
- 14.Ono Y, Nonomura N, Harada Y, Fukui T, Tokizane T, Sato E, Nakayama M, Nishimura K, Takahara S, Okuyama A. Loss of p73 Induction in a Cisplatin-Resistant Bladder Cancer Cell Line. Molecular Urology. 2001;2001;5:25–30. doi: 10.1089/109153601750124267. [DOI] [PubMed] [Google Scholar]
- 15.Chou T-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Research. 2010;2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 16.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 17.Knox RJ, Friedlos F, Lydall DA, Roberts JJ. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986;1986;46:1972–1979. [PubMed] [Google Scholar]
- 18.Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2',2'-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991;1991;51:6110–6117. [PubMed] [Google Scholar]
- 19.Ross DD, Cuddy DP. Molecular effects of 2',2'-difluorodeoxycytidine (Gemcitabine) on DNA replication in intact HL-60 cells. Biochem Pharmacol. 1994;1994;48:1619–1630. doi: 10.1016/0006-2952(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 20.van Moorsel CJ, Pinedo HM, Veerman G, Bergman AM, Kuiper CM, Vermorken JB, van der Vijgh WJ, Peters GJ. Mechanisms of synergism between cisplatin and gemcitabine in ovarian and non-small-cell lung cancer cell lines. British Journal of Cancer. 1999;1999;80:981–990. doi: 10.1038/sj.bjc.6690452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L-YLL, Liu L, et al. Gemcitabine suppresses the repair of cisplatin adducts in plasmid DNA by extracts of cisplatin-resistant human colon carcinoma cells. Proc AACR. 1995;1995;36:257a. [Google Scholar]
- 22.Smith SL, Douglas KT. Stereoselective, strong inhibition of ribonucleotide reductase from E. Coli by cisplatin. Biochem Biophys Res Commun. 1989;1989;162:715–723. doi: 10.1016/0006-291x(89)92369-3. [DOI] [PubMed] [Google Scholar]
- 23.Cruet-Hennequart S, Villalan S, Kaczmarczyk A, O'Meara E, Sokol AM, Carty MP. Characterization of the effects of cisplatin and carboplatin on cell cycle progression and DNA damage response activation in DNA polymerase eta-deficient human cells. Cell Cycl. 2009;2009;8:3043–3054. [PubMed] [Google Scholar]
- 24.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nature Reviews. 2005;2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 25.Bartek J, Lukas J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Current Opinion in Cell Biology. 2001;2001;13:738–747. doi: 10.1016/s0955-0674(00)00280-5. [DOI] [PubMed] [Google Scholar]