Abstract
Background
In 2010, a 13-valent pneumococcal conjugate vaccine (PCV13) replaced a 7-valent vaccine (PCV7) that contained all PCV7 serotypes plus 6 additional serotypes (PCV6+). We conducted annual surveys from 2008–2012 to determine the effect of PCV13 on colonization by pneumococcal serotypes.
Methods
We obtained nasopharyngeal swabs for pneumococcal identification and serotyping from residents of all ages at 8 rural villages and children age <60 months at 2 urban clinics. We conducted interviews/medical records review for all participants.
Results
A total of 18,207 nasopharyngeal swabs (rural=16,098; urban=2,109) were collected. From 2008–2012, 84% of rural and 90% of urban children age <5 years were age-appropriately vaccinated with a PCV. Overall pneumococcal colonization prevalence remained stable among rural (66%) and urban (35%) children age <5 years, and adults age ≥18 years (14%). Colonization by PCV6+ serotypes declined significantly among rural children age <5 years, urban children age <5, and adults age ≥18 over the course of the study (25%–5%, 22%–9%, 22%–6%, respectively).
Conclusions
PCV13 was rapidly introduced into the Alaska childhood immunization schedule and reduced colonization by PCV6+ serotypes among children. Unvaccinated adults also experienced comparable reductions in vaccine serotype colonization indicating substantial indirect protection from PCV13.
Keywords: Streptococcus pneumoniae, Conjugate vaccine, Nasopharyngeal colonization, Alaska Native people, Alaska
BACKGROUND
Alaska Native (AN) children suffer from high rates of invasive pneumococcal disease (IPD) compared to other children in Alaska and children in the general United States (US) population [1–4]. Nasopharyngeal colonization by Streptococcus pneumoniae is a prerequisite for IPD [5]. A 7-valent pneumococcal conjugate vaccine (PCV7) was introduced into the Alaska childhood immunization schedule in early 2001. Subsequently, colonization and rates of IPD by vaccine-related pneumococcal serotypes decreased dramatically among children receiving PCV7 and among unvaccinated adults [6, 7]. However, the overall community-wide prevalence of pneumococcal colonization remained unchanged because of increased colonization by non- PCV7 serotypes, especially serotype 19A [8]. Correspondingly, rates of IPD caused by non12 vaccine serotypes increased, thereby diminishing the overall beneficial impact of PCV7 [3]. The increase in non-PCV7 colonization observed in the AN population was similar to other U.S. studies, but the increase in non-vaccine type IPD rates was greater than that seen for the overall US population. [6, 9–13].
The Food and Drug Administration (FDA) approved a 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar-13®, Pfizer) for use in the US in 2010 that contained the 7 serotypes included in PCV7 (4, 6B, 9V, 14, 18C, 19F, 23F) plus 6 additional serotypes (1, 3, 5, 6A, 7F, 19A). The FDA licensure of PCV13 was based on immunogenicity trials that demonstrated that vaccination with PCV13 generated an antibody response similar to that of PCV7 [14, 15]. However, the impact of PCV13 on pneumococcal colonization, pneumonia and invasive disease had not been directly demonstrated.
In 2008, anticipating the availability of PCV13, we started conducting annual cross2 sectional pneumococcal colonization surveys in urban and rural Alaskan communities. In this paper, we used data from those surveys to describe changes in the prevalence of pneumococcal serotypes colonizing the nasopharynx following universal vaccination of children with PCV13.
METHODS
The study population consisted of a convenience sample of residents of all ages from 8 rural villages (population range: 209–721 residents/village) located in the Yukon Kuskokwim (YK), Norton Sound, and Bristol Bay regions of Alaska, and children age <60 months presenting to 2 urban pediatrics clinics in Anchorage, Alaska (population: 291,826) [16]. Village participants were excluded if they did not reside in the village for >3 weeks. Children from the Anchorage pediatrics clinics were excluded if they lived outside the Anchorage metropolitan area or if another child from the same household had already been recruited into the study.
Alaskan children were offered PCV7 through March 2010. Starting April 1, 2010, the State of Alaska stopped using PCV7 and began vaccinating all children with PCV13 according to ACIP guidelines [14]. However, a small proportion of children age <5 years at two YK villages received PCV13 starting in January 2009 as part of a different Pfizer-sponsored open-label clinical trial [17]. The PCV13 and PCV7 schedules were the same: a 3-dose primary series (2, 4, 6 months of age) and a booster dose at 12–15 months. For children who had partially completed the PCV7 series, their subsequent doses were PCV13. A supplemental vaccination program was enacted to provide children age 14–59 months with a single dose of PCV13 if they had not received any prior PCV13.
For data collection, research staff traveled to each village/clinic annually (at the same time of year for each site) to enroll participants from 2008–2012. Demographic information and antibiotic use history were obtained by interviewing participants and confirmed by medical records review; participants’ vaccination status was determined by medical records review alone. A swab specimen was obtained from the posterior nasopharynx of each participant by using a nylon fiber flocked swab (Copan Diagnostics, Corona, CA) and placed immediately into culture medium containing skim milk-tryptone-glucose-glycerin (STGG) for transportation on ice packs to the laboratory in Anchorage for analysis [18].
Standard laboratory methods used to identify and serotype S. pneumoniae isolates have been previously described [19]. Briefly, 50 μl of the STGG specimens were plated onto trypticase soy agar supplemented with 5% sheep blood containing 10μg/ml of gentamicin (BAP) and incubated at 37°C in 5% CO2. After 18–24 hours of incubation, pneumococci were identified by colony morphology, susceptibility to optochin, and bile solubility. We did not systematically test for colonization with multiple serotypes. However, if two morphologically distinct colonies were identified, each colony was subcultured onto separate BAP and assigned a different colony number. From 2008–2010, pneumococcal isolates were serotyped by latex agglutination and confirmed by the Quellung reaction. Starting in 2011, a different algorithm for serotyping pneumococcal isolates was implemented that involved first using a series of 6 sequential multiplex polymerase chain reaction (PCR) assays with primers for a total of 42 serogroups/serotypes [19]. Primers representing the most prevalent serotypes from the previous year were grouped together so that the most common serotypes would be detected in the first 3 PCR reactions; once a serotype was identified, the remaining reactions were not performed. The first PCR reaction included primers for the cps gene locus; isolates negative for cps were classified as nontypeable or serotype 38 and confirmed as such by the Quellung reaction. Isolates whose serotype could not be resolved by PCR underwent testing by latex agglutination and Quellung.
For statistical analyses, Pearson’s chi square test was used to assess changes in the frequencies of specific serotypes between two periods by 3 age groups: <5 years, 5–17 years, ≥18 years. We defined period 1 as the 2 years when all children received PCV7 (2008–2009) and period 2 as the 2 years when all children received PCV13 (2011–2012). Isolates collected during 2010 when the immunization schedule transitioned from PCV7 to PCV13 or from children at the YK villages who received PCV13 during 2009 were not included in this analysis. Because of the large number of individual serotypes tested, a Bonferroni correction was used to account for multiple comparisons and a p-value of <0.002 was considered statistically significant. For all other statistical tests conducted, the traditional value of 0.05 was used to report statistical significance. The Cochran Armitage test was used to evaluate trends across all 5 study years. We determined the vaccine effectiveness (VE) for reducing colonization by one of the 6 additional serotypes included in PCV13 (PCV6+ serotype) among rural children age <5 years old by using this formula [20]:
where P1 = proportion of non-vaccinated rural children colonized with a PCV13 serotype in 2010, and P2 = proportion of vaccinated rural children colonized with a PCV13 serotype during 2011 or 2012.
This study was approved by the Centers for Disease Control and Prevention (CDC) and Alaska Area Institutional Review Boards. Additional approval was obtained from the Alaska Native Tribal Health Consortium and the participating regional tribal health organizations. Written informed consent for participation was obtained from all adults and legal guardians of children. Written assent was obtained from children age 7–17 years.
RESULTS
Demographics
During 2008–2012, 18,207 nasopharyngeal swabs were collected (mean: 3,641 participants/year). Of those swabs, 16,098 were from the 8 rural sites (range: 2,422–3,459 participants/year) and 2,109 were from the 2 urban pediatrics clinics (range: 300–455 participants/year) (Table 1). The overall participation rate by residents in the 8 villages over the 5-year study period was 74.5% and ranged from 57.9%–91.8%/village/year. Among rural participants, 14% were age <5 years, 34% were age 5–17 years, and 52% were age ≥18 years; all urban participants were age <5 years. Among those <5 years old, rural children were older than urban children (median age: 2.5 years versus 2.1 years, p < 0.0001), were more likely to live in an overcrowded home with >2 household members/room (38% versus 4%, p < 0.0001), and were less likely to have in-home piped water service (52% versus 100%, p < 0.0001). Almost all (99%) rural participants were AN compared to 51% of urban participants. Other commonly represented races in the urban study population were White (36%), Asian/Pacific Islander (7%), and African American (4%). Fifty percent of rural children <5 years old and 29% of urban children received antibiotics <90 days prior to study enrollment; those proportions were similar prior to and after PCV13 introduction.
Table 1.
Characteristics of rural and urban participants — Alaska, 2008–2012
| Characteristics | Geographic location | |
|---|---|---|
| Rural, No., % | Urban, No., % | |
| Total No. nasopharyngeal swabs | 16098 | 2109 |
| Mean number persons/family householda (range) (standard deviation) | 6.6 (2, 19) (2.8) | 4.5 (2, 18)(1.8) |
| Mean number rooms in family homesa,b (range) (standard deviation) | 4.1 (1, 9)(1.4) | 5.4 (1, 15)(1.9) |
| % of overcrowded homesc | 38% | 4% |
| % of homes with running water | 52% | 100% d |
| Age group | ||
| <5 years | 2201 (14%) | 2109 (100%) |
| 5–17 years | 5561 (34%) | |
| ≥18 years | 8336 (52%) | |
| Male sex | 8220 (51%) | 1132 (54%) |
| Race | ||
| AI/AN | 15953 (99%) | 1098 (51%) |
| Asian/PI | 9 (0.1%) | 146 (7%) |
| White | 12 (0.1%) | 770 (36%) |
| African American | 3 (<0.1%) | 92 (4%) |
| Other | 90 (0.6%) | 32 (1%) |
| Unknown | 31 (0.2%) | 28 (1%) |
| Pneumococcal colonization | ||
| <5 years | 1445 (66%) | 745 (35%) |
| 5–17 years | 2665 (48%) | None |
| ≥18 years | 1183 (14%) | None |
| 2 morphologically distinct colonies | 225 (1%) | 22 (1%) |
| Among children <5 years of age | (n = 2201) | (n = 2109) |
| Antibiotic use <90 days | 50% (1094) | 29% (605) |
| ≥1 dose of a PCVe | 2139 (97%) | 2079 (99%) |
| Age-appropriately vaccinatede,f | 84% (1811/2152) | 90% (1884/2100) |
| ≥4 PCV dosesg | 80% (504/632) | 90% (497/550) |
Abbreviations: AI/AN, American Indian/Alaska Native; No., number; NP, nasopharyngeal; PCV, pneumococcal conjugate vaccine; PI, Pacific Islander
Family household defined as households with ≥ 1 child < 5 years of age.
Living rooms excluding bathrooms and closets
Overcrowded defined as >2 household members/living room
From US Census 2010.
Among participants aged 6 weeks–5 years
According to Advisory Committee on Immunization Practices recommendations for PCV
Among participants aged 19–35 months
Vaccine coverage
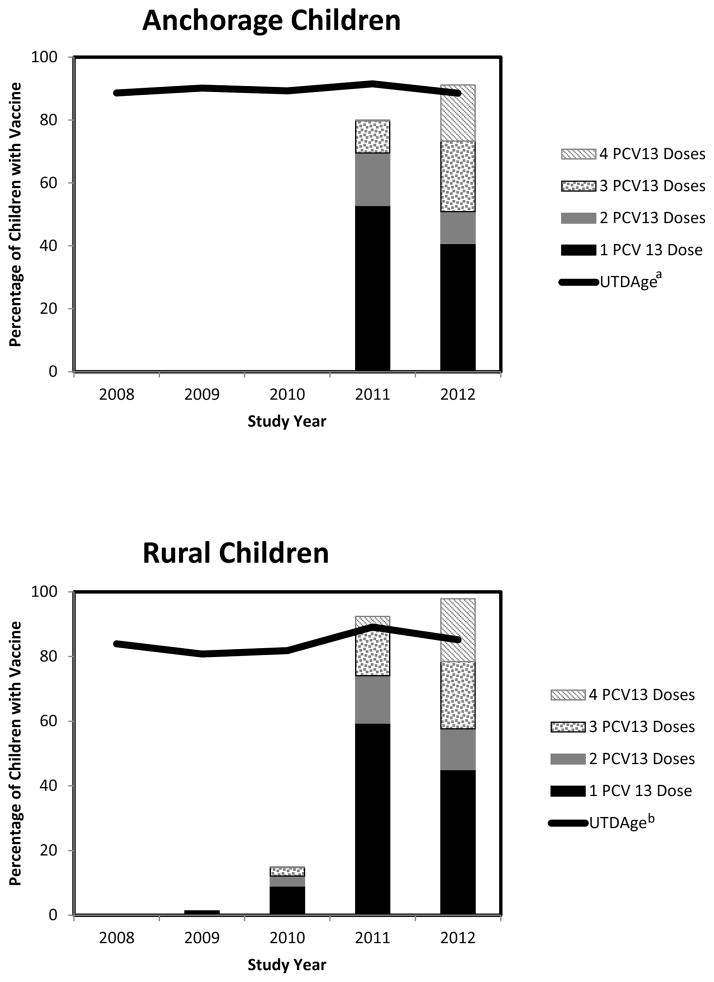
Children were defined as age-appropriately vaccinated with PCV by using an algorithm based on ACIP guidelines (Table 2). Among children age <5 years, 81%–89% (range/year) of rural children and 89%–92% (range/year) of urban children were age-appropriately vaccinated with a PCV during the 5-year study period (Figure 1). During January, 2009–April, 2010, only 2% (6/289) of study participants age <5 years in the YK had received ≥1 dose of PCV13. At the other study sites in 2010, 100% of urban and 30% of rural study participants were recruited before PCV13 was introduced into the vaccine schedule. In 2011, 92% of rural children (range: 76%–100%/village) and 80% of urban children had received ≥1 dose of PCV13 and that proportion increased to 98% (range: 95%–100%/ village) and 91%, respectively, by 2012. Among rural children age 15–59 months, 47% and 32% received a supplemental dose of PCV13 in 2011 and 2012, respectively. For urban children of the same age, 42% and 31% received a supplemental dose of PCV13 in 2011 and 2012, respectively.
Table 2.
Algorithm used to determine whether child had received age-appropriate number of pneumococcal conjugate vaccine (PCV) doses at time of submitting nasopharyngeal swaba
| If child age at time of culture date (months): | Then age-appropriately vaccinated if received: |
|---|---|
| 3 | 1 PCV dose |
| 4 | ≥1 PCV doses |
| 5 | 2 PCV doses |
| 6 | ≥2 PCV doses |
| 7–12 | 3 PCV doses |
| 12–15 | ≥3 PCV doses, if first dose at age ≤6 months ≥2 PCV doses, if first dose at age 7–12 months ≥1 PCV doses, if first dose at age ≥12 months |
| 16–23 | 4 PCV doses, if first dose at age ≤6 months and 1 dose was received at ≥12 months ≥3 PCV doses, if first dose at age 7–12 months and 1 dose was received at ≥12 months ≥2 doses PCV doses at ≥1 year of age |
| ≥24 | 4 PCV doses, if first dose at age ≤6 months and 1 dose was received at ≥12 months ≥3 PCV doses, if first dose at age 7–11 months and 1 dose was received at ≥12 months ≥2 PCV doses at ≥1 years of age ≥1 PCV doses at ≥2 years of age |
Based on the Advisory Committee on Immunization Practices recommendations.
Figure 1.
Pneumococcal conjugate vaccination (PCV) status among urban and rural children aged <5 years — Alaska, 2008–2012
Abbreviations: PCV13, 13-valent pneumococcal conjugate vaccine; UTDAge, vaccination status up-to-date for age with PCV7 or PCV13 according to Advisory Committee on Immunization Practices recommendations
aAmong urban children receiving 1 dose of PCV13 in 2011 and 2012, 60% and 63% respectively of were aged >15 months and received PCV13 as a supplemental dose after completing an age-appropriate vaccination schedule with PCV7
bAmong rural children receiving 1 dose of PCV13 in 2010, 2011 and 2012, 9%, 59% and 54% respectively of were aged >15 months and received PCV13 as a supplemental dose after completing an age-appropriate vaccination schedule with PCV7
From 2008–2012, 80% of rural children (range: 73%–86%/year) and 90% of urban children (range 86%–95%/year) age 19–35 months received ≥4 doses of a PCV. By comparison, data from the National Immunization Survey (NIS) in 2011 for children age 19–35 months estimated that 86% (95% confidence interval [CI]: 76%–96%) of AN children, 78% (95% CI: 72%–84%) of Alaskan children (all races), and 84% (95% CI: 83%–85%) of children in the general US population had received ≥4 doses of a PCV [21].
Trends in serotypes colonizing the nasopharynx
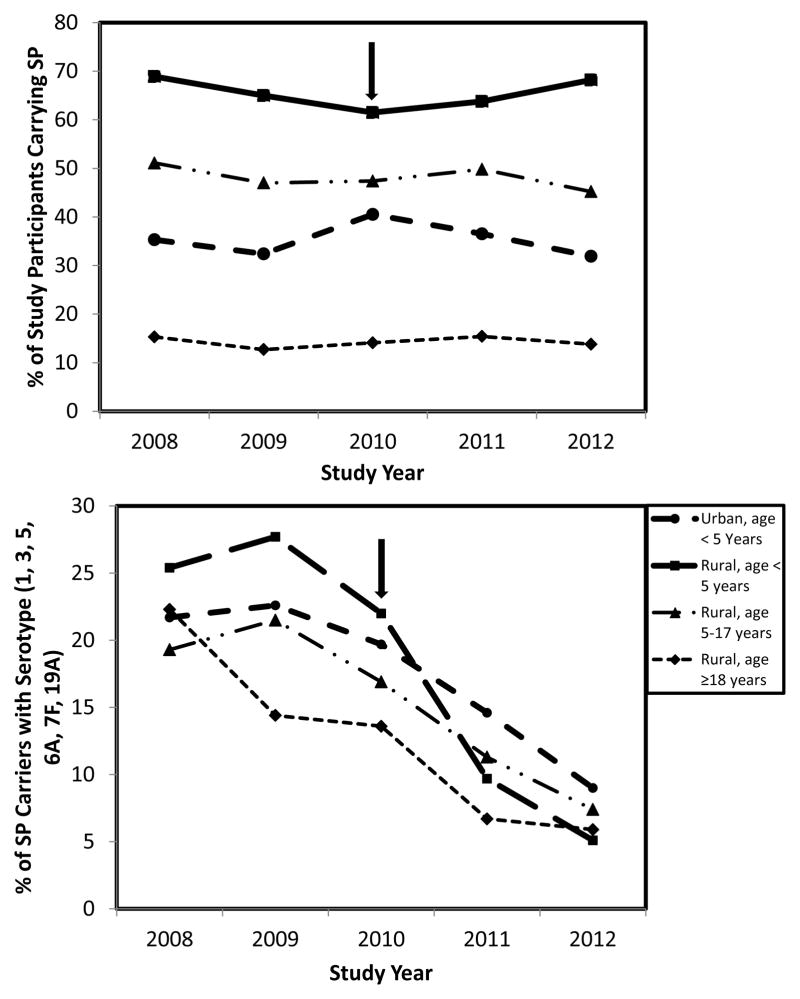
The proportion of participants colonized with pneumococci remained stable during the study period, although it differed by age and geographic location (Figure 2). The average colonization prevalence from 2008–2012 was 66% (range: 62%–69%/year) for rural children age <5 years, 35% (range: 32%–40%/year) for urban children age <5 years, and 14% (range: 13%–15%/year) for adults age ≥18 years. Between period 1 and period 2, there were no significant differences in pneumococcal colonization prevalence between urban AN children and non-AN children age <5 years (data not shown).
Figure 2.
Prevalence of nasopharyngeal colonization by S. pneumoniae (SP) by age and geographic location — Alaska, 2008–2012
Note: Arrow indicates the yearPCV13 became available in Alaska
aPrevalence shown only for the 6 new serotypes included in the 13-valent pneumococcal conjugate vaccine because the prevalence of the other 7vaccine serotypes were low and did not decline during the study period.
A total of 36 different pneumococcal serotypes were identified and 9% of isolates were nontypeable (Table 3). The prevalence of PCV13 serotypes declined significantly in all age groups from period 1 to period 2, largely because of a decrease in colonization by PCV6+ serotypes which comprised >90% of vaccine-related isolates. The prevalence of colonization by PCV6+ serotypes during the study period was similar each year between urban AN children and urban non-AN children age < 5years (data not shown). Serotype 19A was the most prevalent PCV13 serotype in period 1; it decreased significantly in all age groups, especially among children <5 years old (19% of all isolates in period 1 to 7% in period 2). VE of PCV13 in reducing colonization among rural children age <5 years by a PCV6+ serotype was 65%.
Table 3.
S. pneumoniae serotypes colonizing the nasopharynx for 3 different age groups before and after introduction of the 13-valent pneumococcal conjugate vaccine (PCV13) — Alaska, 2008–2012a,b,c
| Serotype | <5 Years of Age | 5–17 Years of Age | ≥18 Years of Age | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Period 1f (n = 78 0) | Period 2 (n = 93 8) | Period 1 (n = 1009) | Period 2 (n = 108 2) | Period 1 (n = 411) | Period 2 (n = 521) | |||
| PCV7 types | 19F | 11 (1) | 9 (1) | 3 (.3) | 52 (5) *↑ | 3 (1) | 10 (2) | |
| 4, 6B, 9V, 14, 18C, or 23F d,e | 2 (.3) | 1 (.1) | 4 (.4) | 2 (.2) | 4 (1) | 0 (0) | ||
|
| ||||||||
| Total PCV7 | 13 (2) | 10 (1) | 7 (1) | 54 (5) *↑ | 7 (2) | 10 (2) | ||
|
| ||||||||
| PCV6+ types | 19A | 143 (18) | 61 (7) *↓ | 87 (9) | 39 (4) *↓ | 33 (8) | 13 (3) *↓ | |
| 3 | 21 (3) | 18 (2) | 79 (8) | 35 (3) *↓ | 29 (7) | 13 (3) *↓ | ||
| 7F | 23 (3) | 1 (.1) *↓ | 36 (4) | 21 (2) | 11 (3) | 5 (1) | ||
| 6A | 11 (1) | 4 (.4) | 8 (.8) | 8 (.7) | 2 (.5) | 2 (.4) | ||
| 1, 5 d | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
|
| ||||||||
| Total PCV6+ | 198 (25) | 83 (9) *↓ | 207 (21) | 102 (9) *↓ | 74 (18) | 33 (6) *↓ | ||
|
| ||||||||
| Total PCV13 | 211 (27) | 93 (10) | 214 (21) | 156 (14) *↓ | 81 (20) | 43 (8) *↓ | ||
|
| ||||||||
| 6C | 67 (9) | 72 (8) | 93 (9) | 77 (7) | 21 (5) | 31 (6) | ||
| Non-PCV13 types | 16F | 39 (5) | 78 (8) | 82 (8) | 82 (8) | 31 (8) | 32 (6) | |
| 23B | 64 (8) | 63 (7) | 66 (7) | 61 (6) | 20 (5) | 20 (4) | ||
| 11A | 28 (4) | 77 (8) *↑ | 25 (2) | 60 (6) *↑ | 9 (2) | 28 (5) | ||
| 35F | 29 (4) | 32 (3) | 54 (5) | 48 (4) | 26 (6) | 29 (6) | ||
| 35B | 15 (2) | 77 (8) *↑ | 7 (1) | 71 (7) *↑ | 10 (2) | 19 (4) | ||
| 15A | 48 (6) | 40 (4) | 49 (5) | 34 (3) | 13 (3) | 13 (3) | ||
| 33F | 22 (3) | 53 (6) | 21 (2) | 52 (5) *↑ | 9 (2) | 24 (5) | ||
| 22F | 56 (7) | 27 (3) *↓ | 42 (4) | 24 (2) | 15 (4) | 5 (1) | ||
| 21 | 11 (1) | 50 (5) *↑ | 16 (2) | 59 (5) *↑ | 7 (2) | 22 (4) | ||
| 15B | 40 (5) | 62 (7) | 20 (2) | 20 (2) | 6 (1) | 9 (2) | ||
| 37 | 3 (.4) | 10 (1) | 88 (9) | 66 (6) | 19 (5) | 20 (4) | ||
| 23A | 30 (4) | 25 (3) | 29 (3) | 30 (3) | 12 (3) | 13 (3) | ||
| 10A | 22 (3) | 25 (3) | 28 (3) | 26 (2) | 7 (2) | 10 (2) | ||
| 31 | 7 (1) | 19 (2) | 18 (2) | 33 (3) | 5 (1) | 21 (4) | ||
| 8 | 1 (.1) | 11 (1) | 3 (.3) | 49 (5) *↑ | 4 (1) | 32 (6) *↑ | ||
| 9N | 7 (1) | 14 (1) | 31 (3) | 16 (1) | 15 (4) | 15 (3) | ||
| 34 | 8 (1) | 12 (1) | 20 (2) | 29 (3) | 11 (3) | 10 (2) | ||
| 17F | 7 (1) | 14 (1) | 10 (1) | 18 (2) | 1 (.2) | 25 (5) *↑ | ||
| 7C | 5 (1) | 17 (2) | 4 (.4) | 21 (2) *↑ | 1 (.2) | 23 (4) *↑ | ||
|
| ||||||||
| Non Typeable | 43 (6) | 60 (6) | 105 (10) | 88 (8) | 75 (18) | 59 (11) | ||
Note:
statistically significant at P <0.002;
denotes increase in prevalence between periods;
denotes decrease in prevalence between periods
Table lists serotypes included in PCV13 and the 20 most common non-vaccine serotypes that represent 95% of pneumococcal isolates.
The 10 most prevalent serotypes for each period are shaded.
Period 1, 2008 –2009; period 2, 2011–2012
Following number of isolates identified by serotype in all years and all age groups: serotype 4 (n = 2); 6B (n = 3); 9V (n = 0); 14 (n = 1); 18C (n = 5); and 23F (n = 4).
Statistical tests not done to compare prevalence between periods.
Children at the Yukon Kuskokwim villages who received PCV13 during 2009 were excluded
Colonization by a PCV7 serotype was low and did not increase during the study period, except for those age 5–17 years. Serotype 19F accounted for the majority of PCV7 serotypes. Among those age 5–17 years in particular, the proportion colonized by serotype 19F increased significantly from 0.3% of all pneumococcal isolates in period 1 to 5% in period 2. Of the 52 participants age 5–17 years colonized by serotype 19F in period 2, 49 (94%) of them lived in 3 villages situated in the YK region. Among children age 5–7 years, 99% had received ≥3 doses of a PCV during period 1 compared with 98% during period 2 (children age >7 years were excluded from analysis during period 1 because PCV7 was not available prior to 2001).
Trends in the proportion colonized by a PCV6+ serotype were assessed among adults living in a household with or without a PCV13-vaccinated child age <5 years, and among children age <5 years who did and did not receive ≥1 dose of PCV13 (Table 4). The prevalence of PCV6+ colonization declined by 79% among rural adults with children <5 years of age in their household during the 5-year study period (from 27% in 2008 to 6% in 2012; P<0.0001 for trend). During that same period, adults living in households without children age <5 years also experienced a 63% reduction in colonization by PCV6+ serotypes (from 17% in 2008 to 6% in 2012; P=0.007 for trend). The prevalence of PCV6+ colonization decreased by 75% from 2010– 2012 among rural children who received ≥1 dose of PCV13 (from 24% to 6%; P=0.0002 for trend). PCV6+ colonization did not decline significantly among rural children who did not receive any PCV13 doses.
Table 4.
Trends in nasopharyngeal colonization by vaccine-type pneumococci over time for adults according to presence of children in their household, and for children according to PCV13 vaccination status — Alaska, 2008–2012a
| Study Year | Prevalence of nasopharyngeal colonization by PCV13-type pneumococci among… | |||||||
|---|---|---|---|---|---|---|---|---|
| Adults with children aged <5 years in household | Adults without children aged <5years in household | Children aged <5 years receiving ≥1 dose PCV13b | Children aged <5 years receiving 0doses PCV13b | |||||
| Colonized, | Total, | Colonized, | Total, | Colonized, | Total, | Colonized, | Total, | |
| No., % | No. | No., % | No. | No., % | No. | No., % | No. | |
| 2008 | 28 (27) | 104 | 14 (17) | 84 | ||||
| 2009 | 21 (17) | 126 | 11 (11) | 97 | ||||
| 2010 | 16 (13) | 124 | 18 (14) | 127 | 9 (24) | 38 | 89 (21) | 428 |
| 2011 | 11 (7) | 151 | 7 (6) | 117 | 42 (11) | 398 | 12 (17) | 72 |
| 2012 | 8 (6) | 140 | 7 (6) | 113 | 26 (6) | 432 | 3 (11) | 27 |
| P-valuec | <0.0001 | 0.007 | 0.0002 | 0.16 | ||||
Analysis limited to the 6 additional serotypes included in PCV13 because the prevalence of the other 7 serotypes included in the 7-valent pneumococcal conjugate vaccine was low during the study period.
Data not shown for 2008 and 2009 because PCV13 was not introduced into vaccination schedule for children in Alaska until 2010.
Calculated by using a chi-squared test for trend
DISCUSSION
Widespread vaccination with PCV7 led to decreased colonization and, subsequently, lower IPD rates caused by vaccine-serotypes. Alaska Natives remained at higher risk for IPD compared to non-Native Alaskans because of an increase in disease caused by non-PCV7 serotypes [6]. By 2009, the 6 additional serotypes included in PCV13 accounted for 55% of all IPD cases in Alaska according to CDC. The proportion of Alaskan children age-appropriately vaccinated was high and comparable with children in the general US population. Vaccinating children with PCV13 resulted in decreased colonization by vaccine serotypes directly among children and indirectly among unvaccinated adults during 2011–2012.
This study demonstrates that widespread use of PCV13 can provide indirect protection to non-vaccinated adults. Children have the highest prevalence of pneumococcal colonization and commonly transmit S. pneumoniae to adult household members [7, 22–24]. Vaccinating children provides indirect protection to adults by interrupting this chain of transmission. In our study, rural adults did not receive PCV13, yet they experienced a 4-fold reduction in colonization by a PCV6+ serotype. Moreover, the reduction in colonization by a PCV6+ serotype among adults who lived in households with children aged <5 years was similar to adults who did not (74% versus 76%). These results imply that the prevalence of adult pneumococcal colonization in rural Alaska depends more on community-level interactions between children and adults than household-level interactions. Thus, vaccinating children with PCV13 appears to have interrupted community-wide pneumococcal transmission to adults. We were unable to determine whether the magnitude of the indirect vaccine effect is the result of attributes unique to AN communities (e.g., small populations or more extensive contact with others outside the household) because this study did not include an urban adult comparison group.
Within the study population, there were substantial differences in the prevalence of pneumococcal colonization by geography and age. Rural children age <5 years were predominantly AN and were twice as likely to be colonized by Streptococcus pneumoniae compared with urban children. However, overall pneumococcal colonization among urban AN children age <5 years was similar to urban non-AN children in our study and to pediatric populations in other studies [9, 11]. Therefore, the increased risk for colonization among AN children in Alaska likely reflects social and environmental risk factors associated with living in rural Alaska. Children living in rural Alaska are more likely to experience household crowding and lack of access to in-home piped water. These conditions have been demonstrated to increase the risk for respiratory tract infections and IPD in AN people [8, 25]. By comparison, access to in-home piped water in Anchorage is similar to other urban communities in the US. Further, the disparity in colonization between rural and urban children is likely an underestimate. Antibiotics eradicate susceptible pneumococci and result in lower overall pneumococcal colonization [26, 27]. A higher proportion of rural children had received antibiotics <90 before study enrollment, which could have suppressed the prevalence of pneumococcal colonization to a greater extent in rural children than in urban children. In addition, the prevalence of pneumococcal colonization among rural children age <5 years was four-fold higher than rural adults age >18 years. These findings are consistent with the documented age-dependent risk for pneumococcal colonization that peaks in early childhood [28].
Trends in vaccine-related serotypes 19F and 19A are noteworthy. Serotype 19F represented the majority of PCV7 serotypes colonizing the nasopharynx in period 1. These finding corroborate previous findings that PCV7 was least effective in reducing colonization by serotype 19F [8]. PCV13 does not appear to reduce serotype 19F colonization more than PCV7 since its prevalence remained unchanged for those aged <5 years. Furthermore, there was a resurgence of colonization by serotype 19F among participants age 5–17 years during period 2. Decreasing vaccine effectiveness with increasing age cannot explain this observation for two reasons. First, the proportion of participants age 5–17 years during period 1 who were colonized with serotype 19F was substantially lower compared with period 2 even though a similar proportion of children were vaccinated during both periods. Additionally, the resurgence of serotype 19F should have been observed in all geographic regions if it resulted from reduced vaccine effectiveness, not just in the 3 YK region villages. The reasons for this regional increase in serotype 19F colonization are unclear. In this study, serotype 19A was the predominant serotype in period 1, and its prevalence decreased substantially by period 2. It is not clear if the prevalence of serotype 19A colonization has plateaued or will continue to decline.
This study had certain limitations. First, only 2 years of data were available for analysis after PCV13 introduction, so we cannot exclude the possibility that the observed changes resulted from natural year-to-year variations in serotype distributions unrelated to the vaccine. However, the decline in prevalence of vaccine-related serotypes are consistent with expectations based on observations following PCV7 introduction [6, 9, 11, 29]. Additionally, there were no changes in key risk factors for pneumococcal colonization (e.g., antibiotic use <90 days of swab collection) in the study populations between the two periods. Therefore, widespread vaccination with PCV13 is the most plausible explanation for the results observed in this study. Second, study participants were voluntarily recruited and do not represent a random sample. Among the rural participants, this concern is mitigated by the fact that most (60%–92%) residents in each village enrolled in the study annually, and the study villages are representative of most AN villages. Because living and environmental conditions in Anchorage are similar to other urban communities in the US, the pneumococcal colonization among urban children in our study should be comparable with results from carriage studies conducted in other clinic based populations. Finally, other risk factors for pneumococcal colonization, such as day care attendance or underlying medical conditions, were not accounted for and could have confounded the results. Although it is unclear how any of these factors would have resulted in the serotype-specific changes noted in our study.
This study is among the first to describe the changing epidemiology of pneumococcal colonization following universal vaccination with PCV13. Additional years of data should be collected to understand the full efficacy of the vaccine and to determine whether the reduced prevalence of PCV6+ serotypes will be sustained. The increase in colonization by non-vaccine serotypes is of concern because this might result in increased rates of IPD. Therefore, ongoing IPD surveillance is necessary in order to monitor the impact of emerging serotypes.
Summary.
Nasopharyngeal colonization by pneumococcal serotypes was assessed before and after introduction of a 13-valent pneumococcal conjugate vaccine in Alaska during 2008–2012. Colonization by vaccine-type pneumococci decreased rapidly after vaccine introduction among vaccinated children and unvaccinated adults.
Acknowledgments
We thank the study participants and staff in the 2 Anchorage pediatrics clinics and clinics in each of the 8 rural villages. We thank the Alaska Native Tribal Health Consortium, Yukon Kuskokwim Health Corporation, Bristol Bay Area Health Corporation, Norton Sound Health Corporation, and Southcentral Foundation for their support of this research. We thank members of CDC’s Arctic Investigations Program for their statistical expertise (Lisa Bulkow), entering and managing the data (Richard Baum, Jennie Lee, Tony Kretz, Debbie Parks, Rita Tangiegak), conducting the laboratory analysis (Carolynn DeByle, Marcella Harker-Jones, Karen Miernyk, Julie Morris, Alisa Reasonover, Lyn Zanis), and recruiting and interviewing participants, and reviewing medical records and collecting nasopharyngeal specimen (Helen Peters, Kim Boy Hummel, Sassa Kitka, Lisa Rea, Gail Thompson, Michele Toomey, Greg Raczniak).
Financial support
This work was supported by an Investigator Originated Project grant from Pfizer pharmaceuticals and through in-kind support from CDC.
Footnotes
Note
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Previous presentation of data
Preliminary data from this study were presented in part at the 5th International Meeting on Indigenous Child Health in Portland, OR on April 19, 2013.
Conflict of interest
Rosalyn Singleton and Jay Wenger conducted research on the 13-valent pneumococcal conjugate vaccine sponsored by Pfizer during 2009–2011. None declared by the other coauthors.
References
- 1.Davidson M, Schraer CD, Parkinson AJ, et al. Invasive pneumococcal disease in an Alaska native population, 1980 through 1986. JAMA. 1989;261:715–8. [PubMed] [Google Scholar]
- 2.Rudolph KM, Parkinson AJ, Reasonover AL, Bulkow LR, Parks DJ, Butler JC. Serotype distribution and antimicrobial resistance patterns of invasive isolates of Streptococcus pneumoniae: Alaska, 1991–1998. J Infect Dis. 2000;182:490–6. doi: 10.1086/315716. [DOI] [PubMed] [Google Scholar]
- 3.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 4.Davidson M, Parkinson AJ, Bulkow LR, Fitzgerald MA, Peters HV, Parks DJ. The epidemiology of invasive pneumococcal disease in Alaska, 1986–1990--ethnic differences and opportunities for prevention. J Infect Dis. 1994;170:368–76. doi: 10.1093/infdis/170.2.368. [DOI] [PubMed] [Google Scholar]
- 5.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–54. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 6.Hennessy TW, Singleton RJ, Bulkow LR, et al. Impact of heptavalent pneumococcal conjugate vaccine on invasive disease, antimicrobial resistance and colonization in Alaska Natives: progress towards elimination of a health disparity. Vaccine. 2005;23:5464–73. doi: 10.1016/j.vaccine.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 7.Hammitt LL, Bruden DL, Butler JC, et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis. 2006;193:1487–94. doi: 10.1086/503805. [DOI] [PubMed] [Google Scholar]
- 8.Wenger JD, Zulz T, Bruden D, et al. Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J. 2010;29:251–6. doi: 10.1097/INF.0b013e3181bdbed5. [DOI] [PubMed] [Google Scholar]
- 9.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics. 2005;116:e408–13. doi: 10.1542/peds.2004-2338. [DOI] [PubMed] [Google Scholar]
- 10.Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–51. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 11.Ghaffar F, Barton T, Lozano J, et al. Effect of the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae in the first 2 years of life. Clin Infect Dis. 2004;39:930–8. doi: 10.1086/423379. [DOI] [PubMed] [Google Scholar]
- 12.Byington CL, Samore MH, Stoddard GJ, et al. Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups. Clin Infect Dis. 2005;41:21–9. doi: 10.1086/430604. [DOI] [PubMed] [Google Scholar]
- 13.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to nonpneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196:1346–54. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease C and Prevention. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children - Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59:258–61. [PubMed] [Google Scholar]
- 15.Food and Drug Administration. Prevnar 13: clinical review of new product license application. Rockville, MD: Food and Drug Administration; 2010. [Google Scholar]
- 16.Department of Labor and Workforce Development. [Accessed October 3 2013];Census and Geographic Information. Available at: http://laborstats.alaska.gov/census/
- 17.Singleton R, Wenger J, Klejka JA, et al. The 13-Valent Pneumococcal Conjugate Vaccine for Invasive Pneumococcal Disease in Alaska Native Children: Results of a Clinical Trial. Pediatr Infect Dis J. 2012 doi: 10.1097/INF.0b013e3182748ada. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien KL, Bronsdon MA, Dagan R, et al. Evaluation of a medium (STGG) for transport and optimal recovery of Streptococcus pneumoniae from nasopharyngeal secretions collected during field studies. J Clin Microbiol. 2001;39:1021–4. doi: 10.1128/JCM.39.3.1021-1024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miernyk K, Debyle C, Harker-Jones M, et al. Serotyping of Streptococcus pneumoniae isolates from nasopharyngeal samples: use of an algorithm combining microbiologic, serologic, and sequential multiplex PCR techniques. J Clin Microbiol. 2011;49:3209–14. doi: 10.1128/JCM.00610-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orenstein WA, Bernier RH, Dondero TJ, et al. Field evaluation of vaccine efficacy. Bulletin of the World Health Organization. 1985;63:1055–68. [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) [Accessed September 28 2012];Statistics and Surveillance: Vaccination Coverage in the US. Available at: http://wwwdev.cdc.gov/vaccines/stats-surv/imzcoverage.htm#nis.
- 22.Hussain M, Melegaro A, Pebody RG, et al. A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiology and infection. 2005;133:891–8. doi: 10.1017/S0950268805004012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshino K, Watanabe H, Sugita R, et al. High rate of transmission of penicillin-resistant Streptococcus pneumoniae between parents and children. J Clin Microbiol. 2002;40:4357–9. doi: 10.1128/JCM.40.11.4357-4359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Givon-Lavi N, Fraser D, Dagan R. Vaccination of day-care center attendees reduces carriage of Streptococcus pneumoniae among their younger siblings. Pediatr Infect Dis J. 2003;22:524–32. doi: 10.1097/01.inf.0000069760.65826.f2. [DOI] [PubMed] [Google Scholar]
- 25.Hennessy TW, Ritter T, Holman RC, et al. The relationship between in-home water service and the risk of respiratory tract, skin, and gastrointestinal tract infections among rural Alaska natives. Am J Public Health. 2008;98:2072–8. doi: 10.2105/AJPH.2007.115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipsitch M. Measuring and interpreting associations between antibiotic use and penicillin resistance in Streptococcus pneumoniae. Clin Infect Dis. 2001;32:1044–54. doi: 10.1086/319604. [DOI] [PubMed] [Google Scholar]
- 27.Schrag SJ, Pena C, Fernandez J, et al. Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial. JAMA. 2001;286:49–56. doi: 10.1001/jama.286.1.49. [DOI] [PubMed] [Google Scholar]
- 28.Muhlemann K, Matter HC, Tauber MG, Bodmer T Sentinel Working G. Nationwide surveillance of nasopharyngeal Streptococcus pneumoniae isolates from children with respiratory infection, Switzerland, 1998–1999. J Infect Dis. 2003;187:589–96. doi: 10.1086/367994. [DOI] [PubMed] [Google Scholar]
- 29.Moore MR, Hyde TB, Hennessy TW, et al. Impact of a conjugate vaccine on communitywide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J Infect Dis. 2004;190:2031–8. doi: 10.1086/425422. [DOI] [PubMed] [Google Scholar]