Abstract
Background and Purpose
Obstructive sleep apnea (OSA) subjects show brain injury in sites that control autonomic, cognitive, and mood functions that are deficient in the condition. The processes contributing to injury may include altered blood-brain barrier (BBB) actions. Our aim was to examine BBB function, based on diffusion-weighted pseudo-continuous arterial spin labeling (DW-pCASL) procedures, in OSA compared to controls.
Methods
We performed DW-pCASL imaging in 9 OSA and 9 controls on a 3.0-Tesla MRI scanner. Global mean gray and white matter arterial transient time (ATT, an index of large artery integrity), water exchange rate across the BBB (Kw, BBB function), DW-pCASL ratio, and cerebral blood flow (CBF) values were compared between OSA and control subjects.
Results
Global mean gray and white matter ATT (OSA vs controls; gray matter, 1.691±0.120 vs 1.658±0.109 sec, p=0.49; white matter, 1.700±0.115 vs 1.650±0.114 sec, p=0.44), and CBF values (gray matter, 57.4±15.8 vs 58.2±10.7 ml/100g/min, p=0.67; white matter, 24.2±7.0 vs 24.6±6.7 ml/100g/min, p=0.91) did not differ significantly, but global gray and white matter Kw (gray matter, 158.0±28.9 vs 220.8±40.6 min−1, p=0.002; white matter, 177.5±57.2 vs 261.1±51.0 min−1, p=0.006), and DW-pCASL ratio (gray matter, 0.727±0.076 vs 0.823±0.069, p=0.011; white matter, 0.722±0.144 vs 0.888±0.100, p=0.004) values were significantly reduced in OSA over controls.
Conclusions
OSA subjects show compromised BBB function, but intact large artery integrity. The BBB alterations may introduce neural damage contributing to abnormal functions in OSA, and suggest a need to repair BBB function with strategies commonly used in other fields.
Keywords: Magnetic resonance imaging, Pseudo-continuous arterial spin labeling, Arterial transient time, Autonomic control
Introduction
Obstructive sleep apnea (OSA) subjects show significant brain damage, particularly in sites that control autonomic, cognitive, emotional, and breathing functions,1–3 which are deficient in the condition. Symptoms associated with injury to these brain areas are linked to higher morbidity, mortality, and decreased quality of life in the syndrome.4–6 However, the underlying pathological processes contributing to damage in these regions are unclear. Compromised blood-brain barrier (BBB) function is a potential mechanism of brain injury, as hypothesized recently in a review article,7 together with the substantial blood pressure, intermittent hypoxia and hypercarbic changes encountered in the syndrome; the altered BBB function may induce or exacerbate injury over time in OSA subjects.
The BBB is located in the brain capillaries, and restricts the diffusion and exchange of microscopic and large molecules to protect brain tissue from harmful substances, such as bacteria, antigens, or chemicals.8 Altered BBB function has been linked to significant brain damage in such conditions as meningitis, epilepsy, multiple sclerosis, and Alzheimer’s disease.9–12 Obstructive sleep apnea subjects are accompanied by hypoxic/ischemic episodes, and the majority of subjects are hypertensive; both conditions are known to alter BBB function.13–15 However, reports of BBB changes in OSA subjects are lacking, although a recent study hypothesized alterations in BBB due to intermittent hypoxia in OSA subjects.7
Pseudo-continuous arterial spin labeling (pCASL) has been used to evaluate reduced regional cerebral blood flow (CBF) in OSA.16 In this study, a novel two-stage non-invasive diffusion-weighted (DW)-pCASL procedure was implemented to assess water exchange across the BBB, without use of radioactive tracers or contrast agents.17 This technique differentiates labeled blood in the microvascular compartment (capillaries) and tissue with fast and slow diffusion coefficients, respectively; the ratio of these two signals indicates water exchange rate across the BBB.18 For comparison, large artery integrity was concurrently assessed by measuring the arterial transient time (ATT, an index of large artery integrity) using DW-pCASL procedures.17,19 We hypothesized that the water exchange rate across the BBB is altered, while large artery function is intact in OSA compared to controls.
Methods
Subjects
Nine OSA [age, 46.7±10.5 years; body-mass-index (BMI), 24.7±3.3 kg/m2; 5 female; apnea-hypopnea-index (AHI, n = 6), 23.8±10.2 events/hour] and 9 healthy control subjects (age, 38.8±6.8 years; BMI, 23.4±2.9 kg/m2; 4 female) were studied. Although all patients included here were diagnosed with OSA, we could not retrieve AHI values for 3 OSA subjects from their clinical records. All OSA subjects were recently-diagnosed via overnight polysomnography with at-least mild severity (AHI ≥ 5),20 treatment-naïve, and recruited from the sleep disorders laboratory at the UCLA Medical Center. All OSA subjects were drug free, and were not taking any cardiovascular-altering (e.g., β-blockers, α-agonists, angiotension-converting enzyme inhibitors, and vasodilators) or mood altering drugs, (e.g., serotonin reuptake inhibitors). Other than the OSA condition, OSA subjects were healthy, without any history of stroke, heart failure, and diagnosed brain abnormalities. Control subjects were healthy, without neurologic or psychiatric disorders, and were recruited from the UCLA Medical Center and West Los Angeles region. Both OSA and control subjects had no metallic implants or devices that might interfere with the MRI scanner environment, and body weights were less than 150 kg (a scanner limitation). All OSA and control subjects provided written informed consent before the study, and the protocol was approved by the Institutional Review Board at UCLA.
Magnetic Resonance Imaging
Brain imaging studies were performed on a 3.0-Tesla MRI scanner (Magnetom Tim-Trio; Siemens, Erlangen, Germany). High-resolution T1-weighted imaging data were collected using a magnetization-prepared rapid acquisition gradient echo (MPRAGE) pulse sequence in the sagittal plane [repetition time (TR)=2200 ms; echo time (TE)=2.34 ms; inversion-time=900 ms; flip-angle (FA)=9°; matrix-size=320×320; field-of-view (FOV)=230×230 mm; slice-thickness=0.9 mm; slices=192]. Proton-density and T2-weighted images were collected using a dual-echo turbo spin-echo pulse sequence in the axial plane (TR=10,000 ms; TE1, 2=17, 134 ms; FA=130°; matrix-size=256×256; FOV=230×230 mm; slice-thickness=3.5 mm). Arterial spin labeling imaging was performed using a pCASL pulse sequence in the axial plane (TR=4000 ms, TE=11 ms, FA=90°, bandwidth=3004 Hz/pixel, label-offset=90 mm, post-labeling-delay (PLD)=1200 ms, 64×64 matrix size, 230×230 mm FOV, 3.5 mm slice thickness, 20% distance factor, 38 axial slices, and 100 acquisitions). Two background suppressed DW-pCASL scans were collected with different imaging protocols for examination of ATT (TR=3500 ms; TE=43 ms; bandwidth=3004 Hz/pixel; label-offset=90 mm; PLD=800 ms; matrix-size=64×64; FOV=230×230 mm; slice-thickness=7.0 mm; distance-factor=20%; 17 axial slices; repeats=40; b =0 and 10 s/mm2) and calculation of water exchange rates across the BBB (TR=4300 ms; TE=47 ms; PLD=1500 ms; repeats=80; b=0 and 50 s/mm2), respectively.
Data Processing and Analysis
High-resolution T1-weighted, PD- and T2-weighted images of all subjects were used to assess any visible brain tissue injury including tumors, cysts, or any other major lesion. DW-pCASL and pCASL images of OSA and control subjects were examined for any head-motion related or other imaging artifacts using the MRIcroN software before further processing. All subjects included in this study did not show any major visible brain injury, head-motion, or other imaging artifacts.
Calculation of ATT, Kw, DW-pCASL ratio, and CBF values
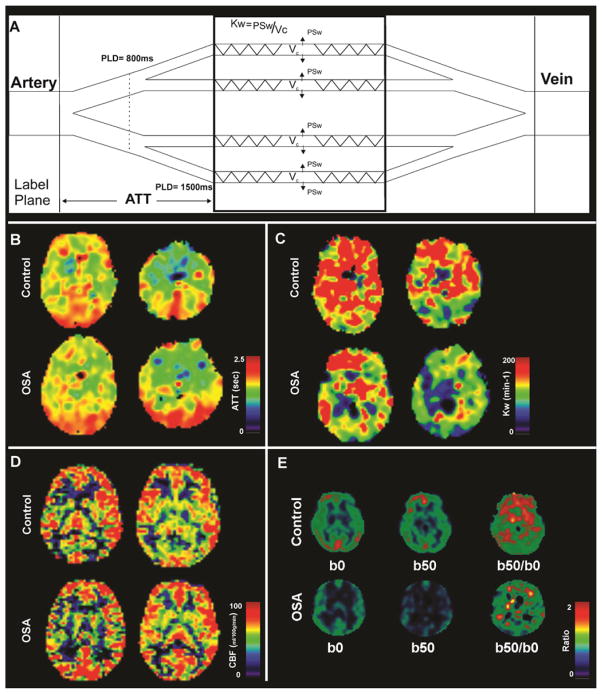
Using DW-pCASL data corrected for head motion, ATT maps (unit, sec) were calculated using the flow-encoding ASL regime by calculating the ratio of DW-pCASL signals with b of 0 and 10 s/mm2.19 With known ATT, Kw values (Kw = PSw/Vc; PSw = capillary permeability surface area product of water, Vc = distribution volume of water tracer in capillary space; unit, min−1), indices of BBB function (water exchange rate across the BBB) were also estimated by calculating the ratio of DW-pCASL signals with b of 0 and 50 s/mm2 at the long delay of 1500 ms (Figure 1A).17 Using global gray and white matter brain masks of individual subjects, gray and white matter ATT, Kw, and DW-pCASL ratio values were calculated from corresponding maps for each OSA and control subject.
Figure 1.
Diagram of ATT and Kw measures, ATT, Kw, CBF maps, and DW-pCASL images and ratio maps from one control and one OSA subject. Figure (A) shows a diagram for the proposed two-stage ATT and Kw measurements. In stage one, a shorter PLD of 800 ms and a lower b value 10 s/mm2 are used to differentiate labeled water in large and small arteries in order to estimate ATT; In stage two, a longer PLD of 1500 ms and a higher b value 50 s/mm2 are used to differentiate labeled water in capillaries and brain tissue in order to estimate Kw (details see St Lawrence et al MRM 2012). Multiple brain sites in OSA subject show comparable ATT (B) and CBF (D) values, and reduced Kw (C) and DW-pCASL ratio (E) values compared to control subject (sites with hot vs. cool color).
Whole-brain CBF maps were calculated using MATLAB-based custom software. Labeled and non-labeled ASL brain volumes were realigned, perfusion images were calculated with simple subtraction, and were used to calculate CBF maps (unit, ml/100g/min), based on a modified single-compartment ASL perfusion model. We averaged the non-labeled EPI scans and CBF maps across the series to derive mean EPI scans and CBF maps for individual subjects. Using the mean EPI scans, gray and white matter masks were generated, and were used to calculate average gray and white matter CBF values from mean CBF maps.
Statistical Analysis
Data analyses were performed using the IBM statistical package for the social sciences software (IBM SPSS, v22). Demographic and biophysical variables between groups were assessed with independent samples t-tests and Chi-square. We examined normality of ATT, Kw, CBF, and DW-pCASL ratio values using Kolmogorov-Smirnov tests, and found that the data were not normally distributed. Therefore, global mean gray and white matter ATT, Kw, CBF, and DW-pCASL ratio values were compared between OSA and control subjects using Mann Whitney U tests. We used a p-value of 0.05 to establish statistical significance.
Results
No significant differences in age (OSA vs controls; 46.7±10.5 vs 38.8±6.8 years, p=0.08) or gender (p=0.64) appeared between OSA and control subjects. Body-mass-index also did not differ significantly between groups (24.7±3.3 vs 23.4±2.9 kg/m2, p=0.38).
Mean global gray and white matter ATT, Kw, CBF, and DW-pCASL ratio values of OSA and control subjects are summarized in Table 1. Global gray and white matter ATT and CBF values did not differ significantly between groups, while the global gray and white matter Kw and DW-pCASL ratio (b50/b0) values were significantly reduced in OSA over control subjects. A set of ATT (Figure 1B), Kw (Figure 1C), and CBF maps (Figure 1D), and DW-pCASL images (b50 and b0) and their ratio maps (Figure 1E) from an OSA and a control subject are displayed, showing comparable global ATT and CBF values, but global reduction in Kw and the DW-pCASL ratio values. The Kw value indicates BBB integrity to water exchange and the DW-pCASL ratio indicates the fraction of labeled water exchanged into tissue, which is directly linked to Kw values.
Table 1.
Global mean gray and white matter ATT, Kw, CBF, and DW-pCASL ratio values of OSA and control subjects.
| Variables | Tissue types | OSA (n = 9; Mean ±SD) | Controls (n = 9; Mean ± SD) | p values |
|---|---|---|---|---|
| ATT (unit, s) | Gray matter | 1.691±0.120 | 1.658±0.109 | 0.49 |
| White matter | 1.700±0.115 | 1.650±0.114 | 0.44 | |
| Kw (unit, min−1) | Gray matter | 158.0±28.9 | 220.8±40.6 | 0.002 |
| White matter | 177.5±57.2 | 261.1±51.0 | 0.006 | |
| CBF (unit, ml/100g/min) | Gray matter | 57.4±15.8 | 58.2±10.7 | 0.67 |
| White matter | 24.2±7.0 | 24.6±6.7 | 0.91 | |
| DW-pCASL (b50/b0) ratio | Gray matter | 0.727±0.076 | 0.823±0.069 | 0.011 |
| White matter | 0.722±0.144 | 0.888±0.100 | 0.004 |
OSA = Obstructive sleep apnea, SD = Standard deviation, ATT = Arterial transient time, CBF = Cerebral blood flow.
Discussion
Overview
OSA subjects showed intact large artery integrity, but reduced water exchange rate across the BBB. Reduced water exchange and potentially compromised BBB function in OSA subjects may accelerate neurodegenerative processes in the condition. Our previous studies, based on various structural MRI procedures, show regional brain injury at multiple sites;1,21–23 compromised BBB function may contribute to at-least a portion of that damage in these areas. These findings suggest a potential therapeutic target for BBB repair in OSA subjects.
BBB function
The BBB provides a separation of circulating blood from the brain’s extracellular fluid in the central nervous system (CNS). Capillaries of the CNS form the BBB via tight junctions between endothelial cells that selectively restrict the passage of solutes, and particularly inhibit the diffusion of microscopic objects (e.g., bacteria and antigens), and large or hydrophilic molecules. The BBB also actively promotes the transport of metabolic products (e.g., glucose) into the CNS, and thus provides important neuroprotection for cellular energy, an essential component for neural structures undergoing excessive excitation during hypoxic periods.
Effect of hypoxia on BBB
Compromised BBB function in OSA subjects may result in secondary injuries to hypoxemia, since subjects experience successive hypoxic/ischemic episodes with each apneic event during sleep. Each apneic event is accompanied by multiple challenges, including increased blood CO2 levels, increased O2 demands, decreased arterial O2 partial pressure, large transient elevation of arterial pressure, and post-event hyperventilation. Enhanced intra-thoracic pressure swings in OSA during apneic episodes result in increased systolic left ventricular transmural pressure, which, in turn, leads to increased left ventricular diastolic filling pressures and frequent episodes of O2 desaturation;24 such hypoxic/ischemic actions are injurious, and can alter the brain and vasculature, and thus, BBB function in this condition. Similar hypoxia-induced BBB disruption has been documented in other disease states,14,15 but the findings here provide the first evidence-based report assessing BBB integrity in OSA, although speculated earlier in a review.7
BBB integrity and Kw, CBF, ATT, and DW-pCASL ratio values
A significant reduction in Kw values, an indicator of BBB integrity, appeared in OSA subjects. Kw values are a ratio of capillary permeability surface area product of water (PSw) by capillary volume (Vc). The relationship between changes in Kw and BBB permeability remains to be clarified. Nevertheless, a recent validation study in animal models demonstrated that mannitol-induced BBB breakdown reduces Kw values,25 suggesting that reduced Kw is linked to increased BBB permeability. Kw values may decrease due to a reduction in PSw or an increase in Vc values. The PSw is influenced by alterations in aquaporin channels, which allow selectively movement of water in and out of the cells. If aquaporin’s function is impaired or compromised, PSw would be decreased, and Kw values would be reduced. Since OSA is accompanied by hypoxia/ischemia, such a possibility exists; hypoxia significantly reduces water aquaporin channels, as demonstrated by various studies.26,27 However, the possibility of changes in Vc is less likely, since no alterations in global gray and white matter CBF values were observed here, but regional CBF changes are noted in OSA subjects.16 It is worth mentioning that Vc may increase in other neurological conditions involving disrupted BBB where the capillary and interstitial space are connected, leading to further decreased Kw.
DW-pCASL ratio values were significantly reduced, and ATT values were comparable to controls in OSA subjects. DW-PCASL ratio values represent BBB integrity, and reduced values indicate impaired BBB function. However, ATT values, indices of large artery integrity, did not differ significantly between groups. These findings of no changes in ATT values suggest that large arteries are intact in OSA subjects.
Altered BBB function and brain injury
Enhanced BBB permeability is associated with an increased incidence of brain tissue infection and injury in various conditions, including traumatic brain injury, stroke, cardiac arrest, multiple sclerosis, Alzheimer’s disease, chronic hypo-perfusion, mild cognitive impairment, cortical dysplasia, autoimmune encephalomyelitis, and hypertension.13,28–36 Ischemia and hypoxia always accompany OSA, conditions that are known to introduce BBB deficits,28 and ischemia/hypoxia-induced BBB changes can contribute to regional brain tissue changes. Given the association between reduced Kw and increased BBB permeability demonstrated in animal models,25 our findings are consistent with other neurological conditions.13,28–36
Structural brain injury and functional deficits in OSA
Autonomic, cognitive, memory, affective, and breathing abnormalities are common in OSA subjects.37–41 In addition to these symptoms of neural injury, brain studies show tissue changes, using various MRI and MRS procedures, in OSA subjects. Studies by our group and others have shown gray and white matter injury, as well as brain metabolic abnormalities in multiple brain areas in OSA patients. Gray matter injury appears in the anterior and posterior cingulate and insular cortices, mammillary bodies, hippocampus, bilateral caudate and thalamus, cerebellar cortex and deep nuclei, putamen, and frontal, parietal, and temporal regions,42,43 and metabolic changes appear in the hippocampus,44 insular cortices,23 parietal-occipital cortex,45 centrum semiovale,46 and frontal and mid-temporal areas.47 White matter injury also appears in the corpus callosum, cingulum bundle, fornix, internal capsule, cerebellum and peduncles, and cortico-spinal tract,1,43 and frontal white matter regions showed abnormal brain metabolites.48 These targeted neural sites play major roles in control of hallmark OSA symptoms, including sympathetic and parasympathetic deficits,49 short-term memory loss,50 executive decision making abnormalities,51 depression,52 and breathing deficits.53
Possibility for BBB function repair and functional recovery
Several effective interventions can repair BBB function, and improve brain performance. Both pharmacologic and non-pharmacologic methods are used in acute (stroke, traumatic brain injury, and multiple sclerosis)30,31,54 and chronic onset (Alzheimer’s disease, chronic hypo-perfusion, mild cognitive impairment, cortical dysplasia, and autoimmune encephalomyelitis)32–36 for BBB function repair, and similar interventions may be useful to repair BBB function in the OSA condition. Such repair, by overcoming brain injury, will benefit autonomic, cognitive, and breathing deficits, and may dramatically improve the morbidity, mortality, and quality of life in OSA.
Limitations
Multiple limitations of this study should be acknowledged. These limitations include small sample sizes and calculation of global gray and white matter ATT, Kw, and DW-pCASL ratio values. We included a small number of subjects in this study, and thus, findings need to be replicated with larger sample sizes. However, global gray and white matter Kw and DW-pCASL ratio values showed significant differences with this limited number of subjects, which indicate large effect sizes between groups. We calculated global gray and white matter ATT, Kw, and DW-pCASL ratio values, and compared those values between OSA and control subjects. Regional comparisons of such values across the brain would be more valuable, and regional alterations in BBB function may correspond more precisely to previously-shown regional structural injury in the condition.
Conclusions
OSA subjects showed significantly lower Kw values over control subjects, with ATT values comparable to controls, suggesting compromised BBB function, with intact integrity of large arteries. The impaired BBB function in OSA may contribute to neural tissue injury in areas that control autonomic, cognitive, and affective functions which are abnormal in the condition. The findings suggest a need to repair BBB function in OSA, with strategies commonly-used in other fields both in conditions with acute (e.g., stroke, traumatic brain injury, cardiac arrest, and multiple sclerosis) and chronic onset (e.g., Alzheimer’s disease, chronic hypoperfusion, mild cognitive impairment, cortical dysplasia, and autoimmune encephalomyelitis) to protect neural tissue in the syndrome.
Acknowledgments
We thank Ms. Karen Harada and Ms. Kelly Hickey for assistance with data collection. This research was supported by National Instututes of Health R01 HL-113251 and R01 NR-015038. Dr. Wang was also supported by National Instututes of Health R01-NS081077 and R01-EB014922.
Abbreviations
- OSA
Obstructive sleep apnea
- BBB
Blood-brain barrier
- DW-pCASL
Diffusion-weighted pseudo-continuous arterial spin labeling
- Kw
Water exchange rate across the BBB
- ATT
Arterial transient time
- TR
Repetition time
- TE
Echo time
- FOV
Field-of-view
- FA
Flip angle
- CBF
Cerebral blood flow
- CNS
Central nervous system
References
- 1.Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90:2043–52. doi: 10.1002/jnr.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joo EY, Jeon S, Kim ST, Lee JM, Hong SB. Localized cortical thinning in patients with obstructive sleep apnea syndrome. Sleep. 2013;36:1153–62. doi: 10.5665/sleep.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54:787–93. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asghari A, Mohammadi F, Kamrava SK, Jalessi M, Farhadi M. Evaluation of quality of life in patients with obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2013;270:1131–6. doi: 10.1007/s00405-012-2157-6. [DOI] [PubMed] [Google Scholar]
- 5.Lavie P, Lavie L. Cardiovascular morbidity and mortality in obstructive sleep apnea. Curr Pharm Des. 2008;14:3466–73. doi: 10.2174/138161208786549317. [DOI] [PubMed] [Google Scholar]
- 6.Sampaio R, Pereira MG, Winck JC. Psychological morbidity, illness representations, and quality of life in female and male patients with obstructive sleep apnea syndrome. Psychol Health Med. 2012;17:136–49. doi: 10.1080/13548506.2011.579986. [DOI] [PubMed] [Google Scholar]
- 7.Lim DC, Pack AI. Obstructive sleep apnea and cognitive impairment: Addressing the blood-brain barrier. Sleep Med Rev. 2014;18:35–48. doi: 10.1016/j.smrv.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segal M, Zlokovic BV. The blood-brain barrier, amino acids and peptides. Springer; 1990. p. 212. [Google Scholar]
- 9.Beam TR, Jr, Allen JC. Blood, brain, and cerebrospinal fluid concentrations of several antibiotics in rabbits with intact and inflamed meninges. Antimicrob Agents Chemother. 1977;12:710–6. doi: 10.1128/aac.12.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in alzheimer’s disease. Acta Neuropathol. 2009;118:103–13. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornford EM, Oldendorf WH. Epilepsy and the blood-brain barrier. Adv Neurol. 1986;44:787–812. [PubMed] [Google Scholar]
- 12.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–9. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 13.Mayhan WG. Disruption of blood-brain barrier during acute hypertension in adult and aged rats. Am J Physiol. 1990;258:H1735–8. doi: 10.1152/ajpheart.1990.258.6.H1735. [DOI] [PubMed] [Google Scholar]
- 14.Mayhan WG, Faraci FM, Heistad DD. Disruption of the blood-brain barrier in cerebrum and brain stem during acute hypertension. Am J Physiol. 1986;251:H1171–5. doi: 10.1152/ajpheart.1986.251.6.H1171. [DOI] [PubMed] [Google Scholar]
- 15.Zehendner CM, Librizzi L, Hedrich J, et al. Moderate hypoxia followed by reoxygenation results in blood-brain barrier breakdown via oxidative stress-dependent tight-junction protein disruption. PLoS One. 2013;8:e82823. doi: 10.1371/journal.pone.0082823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav SK, Kumar R, Macey PM, et al. Regional cerebral blood flow alterations in obstructive sleep apnea. Neurosci Lett. 2013;555:159–64. doi: 10.1016/j.neulet.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St Lawrence KS, Owen D, Wang DJ. A two-stage approach for measuring vascular water exchange and arterial transit time by diffusion-weighted perfusion MRI. Magn Reson Med. 2012;67:1275–84. doi: 10.1002/mrm.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Fernandez-Seara MA, Wang S, St Lawrence KS. When perfusion meets diffusion: In vivo measurement of water permeability in human brain. J Cereb Blood Flow Metab. 2007;27:839–49. doi: 10.1038/sj.jcbfm.9600398. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Alsop DC, Song HK, et al. Arterial transit time imaging with flow encoding arterial spin tagging (FEAST) Magn Reson Med. 2003;50:599–607. doi: 10.1002/mrm.10559. [DOI] [PubMed] [Google Scholar]
- 20.Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The report of an american academy of sleep medicine task force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 21.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- 22.Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett. 2005;375:123–8. doi: 10.1016/j.neulet.2004.10.091. [DOI] [PubMed] [Google Scholar]
- 23.Yadav SK, Kumar R, Macey PM, Woo MA, Yan-Go FL, Harper RM. Insular cortex metabolite changes in obstructive sleep apnea. Sleep. 2014;37:951–8. doi: 10.5665/sleep.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley TD. Right and left ventricular functional impairment and sleep apnea. Clin Chest Med. 1992;13:459–79. [PubMed] [Google Scholar]
- 25.Tiwari YV, Shen Q, Jiang Z, et al. Measuring blood-brain-barrier permeability using diffusion-weighted arterial spin labeling (DW-ASL): Corroboration with ktrans and evan’s blue measurements. Proc Intl Soc Mag Reson Med. 2015;23:792. [Google Scholar]
- 26.Baronio D, Martinez D, Fiori CZ, et al. Altered aquaporins in the brains of mice submitted to intermittent hypoxia model of sleep apnea. Respir Physiol Neurobiol. 2013;185:217–21. doi: 10.1016/j.resp.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Kawedia JD, Yang F, Sartor MA, Gozal D, Czyzyk-Krzeska M, Menon AG. Hypoxia and hypoxia mimetics decrease aquaporin 5 (aqp5) expression through both hypoxia inducible factor-1alpha and proteasome-mediated pathways. PLoS One. 2013;8:e57541. doi: 10.1371/journal.pone.0057541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–19. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Pop V, Sorensen DW, Kamper JE, et al. Early brain injury alters the blood-brain barrier phenotype in parallel with beta-amyloid and cognitive changes in adulthood. J Cereb Blood Flow Metab. 2013;33:205–14. doi: 10.1038/jcbfm.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Cui X, Zacharek A, Chopp M. Increasing ang1/tie2 expression by simvastatin treatment induces vascular stabilization and neuroblast migration after stroke. J Cell Mol Med. 2009;13:1348–57. doi: 10.1111/j.1582-4934.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ifergan I, Wosik K, Cayrol R, et al. Statins reduce human blood-brain barrier permeability and restrict leukocyte migration: Relevance to multiple sclerosis. Ann Neurol. 2006;60:45–55. doi: 10.1002/ana.20875. [DOI] [PubMed] [Google Scholar]
- 32.Ahishali B, Kaya M, Orhan N, et al. Effects of levetiracetam on blood-brain barrier disturbances following hyperthermia-induced seizures in rats with cortical dysplasia. Life Sci. 2010;87:609–19. doi: 10.1016/j.lfs.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Gawryluk JW, Wagener JF, Ghribi O, Geiger JD. Caffeine blocks disruption of blood brain barrier in a rabbit model of alzheimer’s disease. J Neuroinflammation. 2008;5:12. doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurses C, Orhan N, Ahishali B, et al. Topiramate reduces blood-brain barrier disruption and inhibits seizure activity in hyperthermia-induced seizures in rats with cortical dysplasia. Brain Res. 2013;1494:91–100. doi: 10.1016/j.brainres.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 35.Ueno Y, Zhang N, Miyamoto N, Tanaka R, Hattori N, Urabe T. Edaravone attenuates white matter lesions through endothelial protection in a rat chronic hypoperfusion model. Neuroscience. 2009;162:317–27. doi: 10.1016/j.neuroscience.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 36.Pfeiffer F, Schafer J, Lyck R, et al. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122:601–14. doi: 10.1007/s00401-011-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asghari A, Mohammadi F, Kamrava SK, Tavakoli S, Farhadi M. Severity of depression and anxiety in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2012;269:2549–53. doi: 10.1007/s00405-012-1942-6. [DOI] [PubMed] [Google Scholar]
- 38.Belozeroff V, Berry RB, Khoo MC. Model-based assessment of autonomic control in obstructive sleep apnea syndrome. Sleep. 2003;26:65–73. doi: 10.1093/sleep/26.1.65. [DOI] [PubMed] [Google Scholar]
- 39.Felver-Gant JC, Bruce AS, Zimmerman M, Sweet LH, Millman RP, Aloia MS. Working memory in obstructive sleep apnea: Construct validity and treatment effects. J Clin Sleep Med. 2007;3:589–94. [PMC free article] [PubMed] [Google Scholar]
- 40.Saunamaki T, Himanen SL, Polo O, Jehkonen M. Executive dysfunction and learning effect after continuous positive airway pressure treatment in patients with obstructive sleep apnea syndrome. Eur Neurol. 2010;63:215–20. doi: 10.1159/000278301. [DOI] [PubMed] [Google Scholar]
- 41.Svanborg E, Larsson H. Development of nocturnal respiratory disturbance in untreated patients with obstructive sleep apnea syndrome. Chest. 1993;104:340–3. doi: 10.1378/chest.104.2.340. [DOI] [PubMed] [Google Scholar]
- 42.Kumar R, Birrer BV, Macey PM, et al. Reduced mammillary body volume in patients with obstructive sleep apnea. Neurosci Lett. 2008;438:330–4. doi: 10.1016/j.neulet.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 43.Kumar R, Farahvar S, Ogren JA, et al. Brain putamen volume changes in newly-diagnosed patients with obstructive sleep apnea. Neuroimage Clin. 2014;4:383–91. doi: 10.1016/j.nicl.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halbower AC, Degaonkar M, Barker PB, et al. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Med. 2006;3:e301. doi: 10.1371/journal.pmed.0030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonon C, Vetrugno R, Lodi R, et al. Proton magnetic resonance spectroscopy study of brain metabolism in obstructive sleep apnoea syndrome before and after continuous positive airway pressure treatment. Sleep. 2007;30:305–11. doi: 10.1093/sleep/30.3.305. [DOI] [PubMed] [Google Scholar]
- 46.Kamba M, Inoue Y, Higami S, Suto Y. Age-related changes in cerebral lactate metabolism in sleep-disordered breathing. Neurobiol Aging. 2003;24:753–60. doi: 10.1016/s0197-4580(02)00191-4. [DOI] [PubMed] [Google Scholar]
- 47.Sarchielli P, Presciutti O, Alberti A, et al. A 1h magnetic resonance spectroscopy study in patients with obstructive sleep apnea. Eur J Neurol. 2008;15:1058–64. doi: 10.1111/j.1468-1331.2008.02244.x. [DOI] [PubMed] [Google Scholar]
- 48.Kamba M, Inoue Y, Higami S, Suto Y, Ogawa T, Chen W. Cerebral metabolic impairment in patients with obstructive sleep apnoea: An independent association of obstructive sleep apnoea with white matter change. J Neurol Neurosurg Psychiatry. 2001;71:334–9. doi: 10.1136/jnnp.71.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oppenheimer SM, Kedem G, Martin WM. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clin Auton Res. 1996;6:131–40. doi: 10.1007/BF02281899. [DOI] [PubMed] [Google Scholar]
- 50.Thoresen C, Jensen J, Sigvartsen NP, et al. Arousal modulates activity in the medial temporal lobe during a short-term relational memory task. Front Hum Neurosci. 2011;5:177. doi: 10.3389/fnhum.2011.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Z, Taylor WD, Styner M, Steffens DC, Krishnan KR, MacFall JR. Hippocampus shape analysis and late-life depression. PLoS One. 2008;3:e1837. doi: 10.1371/journal.pone.0001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grasso MG, Lubich S, Guidi L, Rinnenburger D, Paolucci S. Cerebellar deficit and respiratory impairment: A strong association in multiple sclerosis? Acta Neurol Scand. 2000;101:98–103. doi: 10.1034/j.1600-0404.2000.101002098.x. [DOI] [PubMed] [Google Scholar]
- 54.Yang D, Knight RA, Han Y, et al. Vascular recovery promoted by atorvastatin and simvastatin after experimental intracerebral hemorrhage: Magnetic resonance imaging and histological study. J Neurosurg. 2011;114:1135–42. doi: 10.3171/2010.7.JNS10163. [DOI] [PMC free article] [PubMed] [Google Scholar]