Abstract
Male circumcision for HIV-1 prevention will require high uptake among at-risk populations. 318 HIV-1 serodiscordant couples in Kampala, Uganda [155 (48.7%) with HIV-1 uninfected male partners] were interviewed about male circumcision for HIV-1 prevention. 77.1% of men and 89.6% of women were aware that circumcision reduces men’s risk for HIV-1 acquisition. Almost all understood the partial protective efficacy of circumcision for HIV-1 acquisition and lack of reduced HIV-1 transmission from circumcising HIV-1 infected men. Among couples with uncircumcised HIV-1 negative men (n = 92), 53.3% of men and 88.1% of female partners expressed interest in male circumcision. Previous discussion within the couple about circumcision for HIV-1 prevention was significantly associated with interest in the procedure. HIV-1 serodiscordant couples in Uganda demonstrated a high level of understanding of the partial protective effect of male circumcision for HIV-1 prevention, but only half of HIV-1 uninfected uncircumcised men expressed interest in the procedure.
Keywords: Male circumcision, HIV-1 prevention, Knowledge and attitudes, HIV-1 discordant couples, Uganda
Introduction
Three randomized clinical trials, from South Africa, Kenya, and Uganda, recently demonstrated that male circumcision reduces the risk of HIV-1 acquisition by ~50% among adult heterosexual men [1–3]. In response to this compelling evidence, WHO/UNAIDS recommended male circumcision be part of a comprehensive HIV-1 prevention package in countries with high HIV-1 prevalence where circumcision is not commonly practiced [4]. Programs to roll-out male circumcision have subsequently been initiated in several African countries [5]. Recently, UNAIDS/WHO/SACEMA summarized mathematical modeling of expected impact of male circumcision on HIV-1 incidence [6] which indicates the utility of targeting high-risk men, including HIV-1 seronegative men in serodiscordant couples (i.e., in which one partner was HIV-1 seropositive and the other partner was HIV-1 seronegative), a population at high risk for HIV-1 transmission [7, 8].
Challenges related to development of national policies, need for provider training, lack of appropriate surgical facilities, and cultural and religious beliefs have been encountered in the roll-out of male circumcision as an HIV-1 prevention strategy. Moreover, there are little data concerning dissemination of knowledge and community understanding of the trial results, particularly of the concept of partial protection from HIV-1, and the relationship between knowledge of trial results and potential uptake of male circumcision services. Finally, another clinical trial recently demonstrated that circumcision of HIV-1 infected men did not decrease the risk of HIV-1 transmission to their female partners [9]. Understanding of the difference between these results and the findings from the circumcision trials among HIV-1 uninfected men has not been assessed in at-risk populations.
It is important that plans for large-scale roll-out of male circumcision are informed by a thorough knowledge of factors which could influence uptake of this intervention in populations at high risk for HIV-1. We sought to examine knowledge, understanding, and attitudes regarding the effect of male circumcision in reducing HIV-1 susceptibility among Ugandan men and women within HIV-1 serodiscordant partnerships.
Methods
Study Population
Between May and August 2008, heterosexual couples attending a research clinic for HIV-1 serodiscordant couples in Kampala, Uganda, were recruited for this cross-sectional study. The population consisted of both couples who had been attending the clinic for ongoing longitudinal studies [10], as well as couples newly presenting to the clinic for serodiscordant couples counseling and evaluation for eligibility for longitudinal studies. Recruitment methods have been described previously [11] in brief, couples found to be HIV-1 serodiscordant were referred to the study clinic from HIV testing and care clinics from a radius of 120 km, representing primarily an urban population but also with semiurban and rural representation; participants were thus generally reflective of HIV-1 serodiscordant couples in the greater Kampala area. The study clinic provides primary care medical services to research participants, including provision or referral for antiretroviral therapy, but it is not otherwise a treatment center; it does not provide male circumcision services. Eligible couples attended the clinic together on the day of the interview, and both members were required to be at least 18 years of age and willing to provide written informed consent. The study was approved by the institutional review boards of University Hospitals Case Medical Center, University of Washington Human Subjects Review committee, and Makerere University.
Procedures
Members of consenting HIV-1 serodiscordant couples were interviewed separately by gender-matched research assistants using a standardized questionnaire, developed from items on the validated Uganda National Serosurvey Individual Questionnaire [12], by a literature review of relevant questions used previously by other investigators [13], and by expert review. The questionnaire was a 73-item instrument with 18 items on knowledge about male circumcision for HIV-1 prevention, 21 on attitudes about the male partner and their male children being circumcised, 12 on sources of HIV-1-related information, and the remainder on general characteristics of the partnership. The instrument was pretested on 32 couples, and had Cronbach’s alpha coefficient for internal reliability for the knowledge of effect of male circumcision on HIV-1 susceptibility of 0.699 for the pre-test instrument, and 0.692 for the final study instrument. HIV-1 serostatus results were abstracted from the participant’s medical records at the clinic, and men were examined to confirm circumcision status.
Analysis
The primary outcome measure was participant’s knowledge of the effect of male circumcision on HIV-1 susceptibility and infectiousness. Secondary outcome measures included correlates of willingness to circumcise for HIV-1 prevention, reasons for or against circumcision, barriers to access for those interested in undertaking male circumcision, acceptability of circumcision as an HIV-1 prevention strategy, and sources of information about HIV-1 prevention. Some questions used a 5-item Likert scale (e.g., definitely agree, agree, neutral, disagree, definitely disagree); these were recategorized into binary answers (e.g. definitely agree or agree vs. neutral, disagree or definitely disagree) for analysis.
The study design anticipated enrolling at least 200 HIV-1 serodiscordant couples during a 3-month period to enable detection of important differences (20% difference in prevalence) between HIV-1 infected versus HIV-1 uninfected men and circumcised versus uncircumcised men, with reasonable statistical power (≥80%), assuming a conservative prevalence of knowledge about male circumcision of 50%, and an alpha level of 0.05.
Data were double-entered in Epidata version 3.2, and statistical analyses were conducted in SAS version 9.1.3. Men and women were analyzed separately. Univariate and multivariable models were stratified by HIV-1 serostatus and used log-binomial regression or robust Poisson regression when generalized linear models failed to converge [14]. The univariate significance level of ≤0.2 was used as the cut-off for inclusion of variables into initial multivariable model. Final parsimonious multivariable models were selected by manual backward elimination. For sensitivity analyses, we compared knowledge and attitudes among couples who were already in follow-up for other studies at the research clinic (and thus who may have already had extensive HIV-1 prevention counseling) to those who were new clinic attendees, and by excluding participants from traditionally circumcising ethnic groups in Uganda. The results did not substantially differ and thus are not reported separately.
Results
General Characteristics
During the study period, 580 heterosexual HIV-1 serodiscordant couples attended the clinic, of whom 320 (55.2%) were enrolled. The principal barrier to study participation was time constraints, such as need to return to home or work or to complete procedures for another study occurring at clinic. In two of the 320 couples, the HIV-1 negative partners had seroconverted to HIV-1 prior to the interview date and data from these couples were excluded, yielding a final sample size of 318 couples.
Of 318 couples, 163 (51.3%) were couples in which the male partner was HIV-1 seropositive and 155 (48.7%) were couples in which the male partner was HIV-1 seronegative. The median age was 37 years for men and 31 years for women and did not differ by HIV-1 serostatus (Table 1). Most couples were married and cohabiting; the median length of partnership was 6.5 years. Nearly 50% of the study population was Baganda, the predominant ethnic group in the Kampala region; the traditionally circumcising Gishu tribe comprised only 2% of the study population. Muslim men were less likely to be HIV-1 infected, likely in part reflecting the high prevalence of circumcision among Muslim men (53/57, 93%).
Table 1.
General characteristics stratified by gender
| Characteristics | Males (n = 318) | Females (n = 318) | ||
|---|---|---|---|---|
| Positive (n = 163) (%) |
Negative (n = 155) (%) |
Positive (n = 155) (%) |
Negative (n = 163) (%) |
|
| Confirmed male circumcision statusa | N/A | N/A | ||
| Circumcised | 34 (21.0) | 65 (41.9) | ||
| Uncircumcised | 124 (76.5) | 89 (57.4) | ||
| Partially circumcised (glans partially covered) | 4 (2.6) | 1 (0.7) | ||
| Age [median years, IQR] | 38 [32–43] | 36 [30–40] | 30 [25–35] | 31 [25–38] |
| Length of partnership [median years; IQR] | 7 [3–14] | 5.75 [3–9] | 6 [3–10] | 7 [3–15] |
| Status of partnership | ||||
| Married and cohabiting | 158 (96.93) | 144 (92.9) | 140 (90.3) | 156 (95.7) |
| Not cohabiting | 5 (3.07) | 11 (7.1) | 15 (9.7) | 7 (4.3) |
| Duration known to be HIV-1 serodiscordant with partner [median years; IQR] | 1 [0.33–2] | 1 [0.42–2] | 1 [0.33–1.92] | 1 [0.25–2] |
| Prior discussion with partner about male circumcision | ||||
| Yes | 36 (22.1) | 41 (26.4) | 45 (29) | 39 (23.9) |
| No | 127 (77.9) | 114 (73.6) | 110 (71) | 124 (76.1) |
| Place of residence | ||||
| Urban | 87 (53.4) | 82 (52.9) | 80 (51.6) | 87 (53.4) |
| Semi urban | 52 (31.9) | 46 (29.6) | 50 (32.3) | 26 (15.9) |
| Rural | 24 (14.7) | 27 (17.4) | 25 (16.1) | 50 (30.7) |
| Education level attained | ||||
| Primary and below | 83 (50.9) | 64 (41.3) | 98 (63.2) | 104 (63.8) |
| Secondary and above | 80 (49.1) | 91 (58.7) | 57 (36.8) | 59 (36.2) |
| Employment | ||||
| Salaried | 39 (23.9) | 38 (24.5) | 17 (11.0) | 14 (8.6) |
| Self employed | 120 (73.6) | 113 (72.9) | 50 (32.2) | 59 (36.2) |
| Unemployed/domestic work | 4 (2.5) | 4 (2.6) | 88 (56.8) | 90 (55.2) |
| Religion | ||||
| Catholics | 63 (38.7) | 46 (29.7) | 58 (37.3) | 63 (38.7) |
| Anglicans | 65 (39.9) | 44 (28.4) | 32 (20.7) | 52 (31.9) |
| Muslims | 16 (9.8) | 41 (26.4) | 32 (20.7) | 18 (11.0) |
| Evangelicals/others | 19 (11.7) | 24 (15.5) | 33 (21.3) | 30 (18.4) |
| Ethnicity | ||||
| Baganda | 81 (49.7) | 75 (48.4) | 85 (54.8) | 86 (52.76) |
| Non-Baganda | 82 (50.3) | 80 (51.6) | 70 (45.2) | 77 (47.24) |
| Visit type | ||||
| New at clinic (first time attendee) | 41(25.2) | 44 (28.4) | 44 (28.4) | 41 (25.2) |
| Old at clinic (in follow-up) | 122 (74.8) | 111 (71.6) | 111 (71.6) | 122 (74.8) |
| Has radio at home | ||||
| Yes | 145 (89.0) | 140 (90.3) | 119 (76.8) | 141 (86.5) |
| No | 18 (11.0) | 15 (9.7) | 36 (23.2) | 22 (13.5) |
| Has TV at home | ||||
| Yes | 66 (40.5) | 50 (32.3) | 50 (32.3) | 57 (35) |
| No | 97 (59.5) | 105 (67.7) | 105 (67.7) | 106 (65) |
IQR interquartile range
One male did not consent to be examined
Circumcision Status
Among the male participants, 99 (31.2%) were circumcised, 5 (1.6%) were partially circumcised (i.e., with glans partially covered), 213 (67.2%) were uncircumcised, and 1 declined examination to verify circumcision status, but was included in the analyses based on self-reported circumcision status. HIV-1 uninfected men were more likely to be circumcised than HIV-1 infected men (41.9 vs. 21.0%, χ2 = 16.18; p < 0.001). Overall, there was excellent agreement between examination-confirmed and self-reported circumcision status: 95 of 99 (95.9%) circumcised men and 212 of 213 (99.5%) uncircumcised men correctly reported their circumcision status (kappa statistic = 0.96, 95% confidence interval (CI) 0.93–1.00). Similarly, most women knew the circumcision status of their male partners: 197 of 213 (92.5%) with uncircumcised partners and 95 of 99 (95.9%) with circumcised partners correctly identified the circumcision status of their male partners (kappa statistic = 0.86, 95% CI 0.80–0.92).
Knowledge of Male Circumcision for HIV-1 Prevention
Knowledge that male circumcision was partially protective against HIV-1 as a prevention strategy was high (Table 2). Overall, 77.1% (245/318) of men and 89.6% (285/318) of women were knowledgeable that circumcision reduces HIV-1 risk for HIV-1 uninfected men, and over 95% (305 men/310 women) were knowledgeable that circumcision does not offer HIV-1 negative men complete protection from HIV-1. More than 90% of men (292/318) and women (305/318) understood that circumcision of HIV-1 infected man does not offer protection to their HIV-1 uninfected female partners. There were no significant differences in knowledge by participant’s HIV-1 serostatus. Approximately one quarter of men (77/318) and women (84/318) reported prior discussion with their partner about male circumcision as a possible HIV-1 prevention strategy.
Table 2.
Knowledge of the effect of male circumcision on HIV-1 susceptibility stratified by gender
| Characteristics | Males | χ2 a |
p value |
Females | χ2 a |
p value |
||
|---|---|---|---|---|---|---|---|---|
| HIV negative (n = 155) (%) |
HIV positive (n = 163) (%) |
HIV positive (n = 155) (%) |
HIV negative (n = 163) (%) |
|||||
| Circumcising an HIV negative man reduces his chance of getting HIV | ||||||||
| Definitely agree/agree | 123 (79.3) | 122 (74.9) | 0.91 | 0.3 | 139 (89.7) | 146 (89.6) | 0.001 | 0.9 |
| Definitely disagree/disagree/not sure | 32 (20.7) | 41 (25.1) | 16 (10.3) | 17 (10.4) | ||||
| Circumcising an HIV negative man completely removes his chance of getting HIV | ||||||||
| Definitely agree/agree | 5 (3.2) | 8 (4.9) | 0.57 | 0.4 | 4 (2.6) | 4 (2.4) | 0.005 | 0.9 |
| Definitely disagree/disagree/not sure | 150 (96.8) | 155 (95.1) | 151 (97.4) | 159 (97.6) | ||||
| Circumcision of a man with HIV does not protect his female partner from getting HIV | ||||||||
| Definitely agree/agree | 141 (91.0) | 151 (92.6) | 0.29 | 0.6 | 150 (96.8) | 155 (95.1) | 0.057 | 0.4 |
| Definitely disagree/disagree/not sure | 14 (9.0) | 12 (7.3) | 5 (3.2) | 8 (4.9) | ||||
Chi-square or fisher’s test of differences in knowledge about male circumcision by HIV-1 serostatus for males and females
Sources of Information about HIV-1 Prevention
The majority (>80%) of men (285/318) and women (262/318) reported to have heard in the previous 1 year that circumcision reduces the risk of HIV-1 for HIV-1 uninfected men. The most common source of health-related information was radio (82.4% of men and 72.3% of women) and health care workers (47.2% of men and 88.4% of women). Other important sources of information (mentioned by >20%) were education seminars, peers, TV, and newspapers.
Attitudes towards Male Circumcision
Overall, >90% (282/318) of men and (304/318) of women reported that they perceived male circumcision as an acceptable HIV-1 prevention strategy. Over 50% of uncircumcised HIV-1 seronegative men (53%, 49/92) expressed willingness to undergo circumcision, with a higher proportion (88%) of their HIV-1 seropositive female partners expressing an interest in their male partner being circumcised (74/84, accounting for women who did not correctly identify their partners’ circumcision status). A lower proportion of serodiscordant couples with an HIV-1 seropositive, uncircumcised male partner reported an interest in male circumcision: 39.2% (51/130) of uncircumcised HIV-1 seropositive men and 69.2% (83/120) of their HIV-1 seronegative female partners.
Uncircumcised HIV-1 seronegative men who had prior discussion about male circumcision with their female partners were significantly more likely to be interested in circumcision than those who had not discussed the procedure [unadjusted prevalence ratio (UPR) = 1.76, 95% CI 1.27–2.45, χ2 = 11.9; p = 0.0007] (Table 3). In addition, those who reported knowledge of the effect of circumcision on HIV-1 susceptibility (vs. those who were not aware of the effect of circumcision) were more likely to be interested in undergoing circumcision [UPR = 3.13, 95% CI 1.51–6.50, χ2 = 9.42; p = 0.002]. Notably, age, education, religion, ethnicity, place of residence, current employment status, and perceived individual risk for acquiring HIV-1 were not significantly related to interest in male circumcision. In multivariable analysis, the probability of expressing interest in circumcision was 50% higher for men who were knowledgeable that circumcision reduces men’s risk for HIV-1 acquisition [adjusted prevalence ratio (APR) = 1.50, 95% CI 1.22–1.83] compared to those who were not, and 23% higher for men who had discussed circumcision with their partners [APR = 1.23, 95% CI 1.01–1.50] compared to those who had not discussed the procedure. Addition of age, ethnicity, religion, place of residence, employment, education, and individual perceived risk of HIV-1 acquisition to the multivariable model did not have substantial effects on the estimates.
Table 3.
Correlates of willingness to circumcise among self-reported uncircumcised HIV-1 seronegative males (n = 92)
| Characteristics | Willing to circumcise (n = 49) (%) |
Not willing to circumcise (n = 43) (%) |
Unadjusted prevalence ratios (95% CI) |
χ2 | p value | APR (95% CI) | p value |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| < 30 years | 14 (28.6) | 12 (27.9) | 1.15 (0.68, 1.95) | 0.29 | 0.5 | ||
| 30–39 years | 21(42.8) | 15 (34.9) | 1.25 (0.78, 2.00) | 0.86 | 0.3 | ||
| ≥40 years | 14 (28.6) | 16 (37.2) | Reference | ||||
| Duration of partnership | |||||||
| < 5 years | 21 (42.8) | 19 (44.2) | 1.17 (0.66, 2.07) | 0.28 | 0.6 | ||
| 5–9 years | 19 (38.8) | 13 (30.2) | 1.32 (0.75, 2. 32) | 0.93 | 0.3 | ||
| ≥10 years | 9 (18.4) | 11 (25.6) | Reference | ||||
| Place of residence | |||||||
| Urban | 37 (75.5) | 28 (65.1) | Reference | ||||
| Rural | 12 (24.5) | 15 (34.9) | 0.78 (0.49, 1.25) | 1.06 | 0.3 | ||
| Education level reached | |||||||
| Primary and below | 19 (38.8) | 23 (53.5) | Reference | ||||
| Secondary and above | 30 (61.2) | 20 (46.5) | 1.32 (0.89, 1.98) | 1.89 | 0.2 | ||
| Employment | |||||||
| Salaried | 14 (28.6) | 7 (16.3) | 1.33 (0.91, 1.96) | 2.15 | 0.1 | ||
| Self employed/manual work | 34 (69.4) | 34 (79.1) | Reference | ||||
| Domestic work/unemployed | 1 (2) | 2 (4.6) | 0.67 (0.13, 3.36) | 0.24 | 0.6 | ||
| Knowledge that male circumcision reduces men’s risk for HIV-1 acquisition | |||||||
| Yes | 43 (87.8) | 21 (48.8) | 3.13 (1.51, 6.50) | 9.42 | 0.002 | 1.50 (1.22, 1.83) | 0.001 |
| No | 6 (12.2) | 22 (51.2) | Reference | Reference | |||
| Prior discussion about male circumcision with female partner | |||||||
| Yes | 14 (28.6) | 3 (7) | 1.76 (1.27, 2.45) | 11.9 | 0.0007 | 1.23 (1.01, 1.50) | 0.04 |
| No | 35 (71.4) | 40 (93) | Reference | Reference | |||
| Perceived risk of HIV-1 acquisition from partner | |||||||
| No chance at all | 15 (30.6) | 13 (30.2) | Reference | ||||
| Little chance | 8 (16.3) | 11 (25.6) | 0.79 (0.42, 1.48) | 0.56 | |||
| Moderate chance | 6 (12.2) | 6 (14) | 0.93 (0.48, 1.81) | 0.04 | |||
| Strong chance | 20 (40.8) | 13 (30.2) | 1.13 (0.73, 1.76) | 0.3 | |||
| Religion | |||||||
| Catholics | 16 (32.7) | 21 (48.8) | Reference | ||||
| Anglicans | 20 (40.8) | 14 (32.6) | 1.36 (0.85, 2.16) | 1.69 | |||
| Evangelicals | 13 (26.5) | 8 (18.6) | 1.43 (0.87, 2.36) | 1.99 | |||
| Ethnicity | |||||||
| Baganda | 17 (34.7) | 22 (51.2) | 0.72 (0.48, 1.10) | 2.33 | |||
| Non-Baganda | 32 (65.3) | 21 (48.8) | Reference | ||||
Addition of age, ethnicity, religion, place of residence, education reached, employment, perceived risk of HIV-1 acquisition did not substantially affect estimates from the multivariate model
APR adjusted prevalence ratios; 95% CI 95% confidence interval; χ2 Wald chi-square
Motivation and Barriers to Circumcision
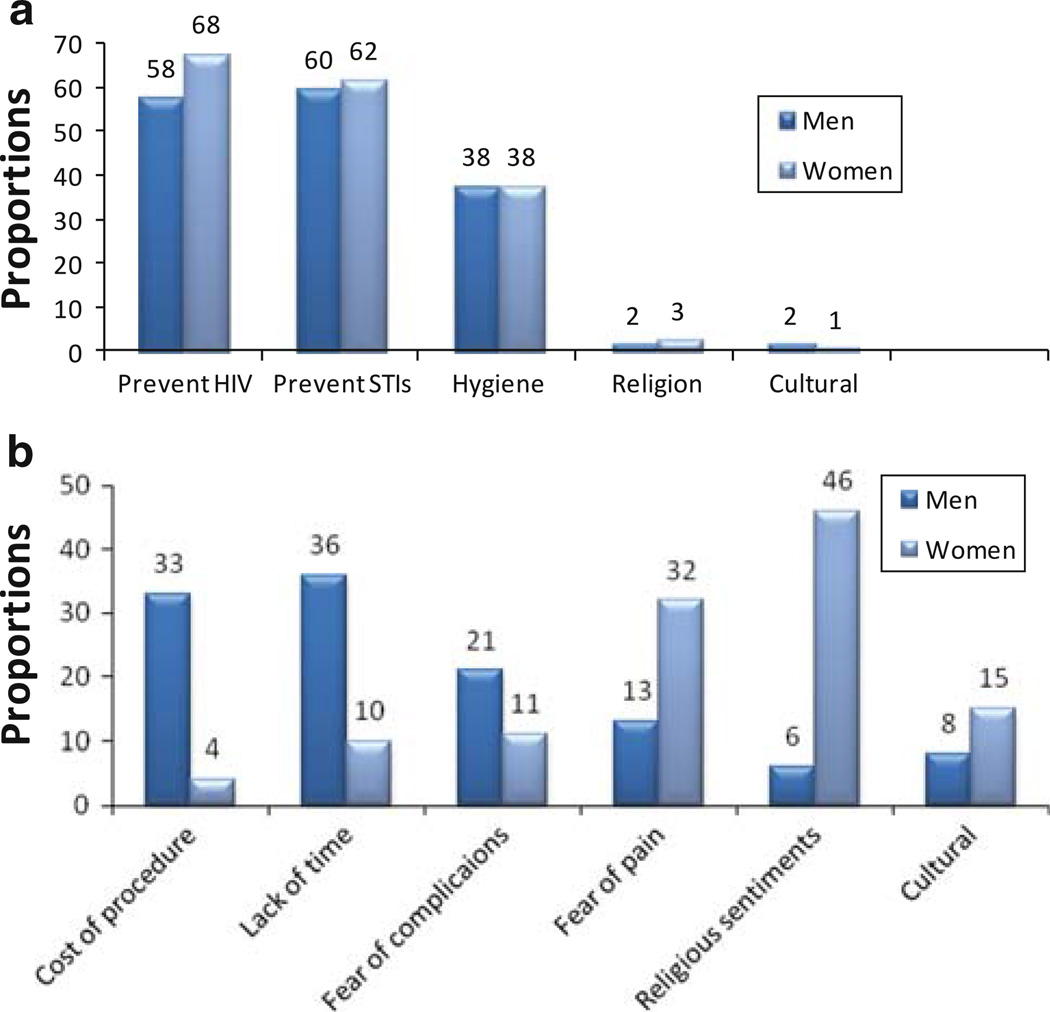
Motivators and impediments for male circumcision were determined among couples with uncircumcised HIV-1 seronegative males (n = 92). The main motivations for circumcision were medical reasons, including prevention of HIV-1, prevention of other sexually transmitted infections, and hygienic benefits (Fig. 1a). Circumcision for religious and cultural reasons was not commonly mentioned. Cost of the procedure, time away from work, and fear of possible surgical complications were the most commonly mentioned impediments for not having yet undertaken circumcision among HIV-1 seronegative men who were interested in the procedure (Fig. 1b). A high proportion of HIV-1 seropositive women mentioned religious sentiments (48%) and fear of pain (32%) as important barriers to circumcision for their HIV-1 seronegative male partners, compared to 5 and 15%, respectively, of their male partners. Only 26 of 49 (53.1%) HIV-1 seronegative uncircumcised men who were interested in becoming circumcised reported knowledge of a health unit where circumcision could be performed.
Fig. 1.
a Motivation factors for undertaking circumcision, and b factors hindering uptake of circumcision in couples with HIV-1 uninfected male partners
Among HIV-1 seronegative men who preferred to remain uncircumcised (n = 43), commonly mentioned barriers were fear of pain (26%), belief that circumcision was not helpful in reducing the chance of HIV acquisition (21%), religious sentiments (15%), cultural sentiments (11%), fear of complications (11%), and lack of time (6%).
Discussion
Heterosexual HIV-1 serodiscordant couples attending a research clinic in Kampala were highly knowledgeable about male circumcision for HIV-1 prevention, including that circumcision partially reduces men’s HIV-1 susceptibility and that adult circumcision of an HIV-1 infected male partner does not reduce a women’s risk of acquiring HIV-1. HIV-1 serostatus was not related to knowledge about the partial protective effect of male circumcision against HIV-1 infection. Importantly, among couples with uncircumcised HIV-1 seronegative men, male circumcision was highly acceptable as an HIV-1 prevention strategy, with ~50% of HIV-1 uninfected uncircumcised interested in the procedure.
This study is among the first to explore knowledge and attitudes concerning male circumcision among HIV-1 at-risk populations, including HIV-1 serodiscordant couples for whom this could be an important new prevention strategy, since the completion of the three randomized trials of circumcision for HIV-1 prevention in men [1–3]. Notably, our study was conducted prior to development of a national policy on male circumcision for Uganda [15], and our results may primarily reflect knowledge gained through popular media. Although knowledge may be insufficient to lead to uptake of male circumcision by at-risk HIV-1 negative men, it is an important component in determining interest and willingness to be circumcised [16].
HIV-1 uninfected, uncircumcised men in serodiscordant partnerships are a priority for roll-out of male circumcision in high HIV-1 prevalence settings [6]. An encouraging finding was that almost half of HIV-1 uninfected, uncircumcised men in this sample of serodiscordant couples in Kampala expressed interest in circumcision for HIV-1 prevention and almost 90% of their HIV-1 seropositive female partners. Moreover, interest in circumcision among HIV-1 uninfected, uncircumcised men was associated with prior discussion about circumcision for HIV-1 prevention with their partners. Our results suggest that engaging women in discussion about male circumcision, in addition to increasing awareness of the benefits of circumcision for reducing men’s HIV-1 susceptibility, could be important for increasing willingness of HIV-1 seronegative men to be circumcised. Importantly, these findings reinforce that counseling about male circumcision for HIV-1 prevention should become part of couples HIV-1 counseling and testing, particularly among HIV-1 serodiscordant partnerships in which the male partner is HIV-1 seronegative.
Our findings are consistent with the expectation that both HIV-1 seronegative and seropositive men would be interested in male circumcision [17, 18]. Because of concerns of increased risk of HIV-1 transmission if circumcised HIV-1 infected men resume sexual activity before wound healing [9], it is imperative that pre- and post-circumcision counseling messages emphasize the dangers of early resumption of sexual activity before complete healing of the surgical wound. It is also important that efforts are made to engage women in the discussions about risks and benefits of circumcision, and the need for post-operative abstinence until complete wound healing.
Our results are supportive of the efforts in Uganda and other countries with high HIV-1 prevalence that are initiating male circumcision programs for HIV-1 prevention. Most of Uganda is composed of traditionally non-circumcising populations. Thus, the modest interest in circumcision to prevent HIV-1 among HIV-1 seronegative uncircumcised men in serodiscordant partnerships is encouraging, especially before a formal public campaign to promote circumcision in Uganda. This finding also highlights an opportunity for targeted counseling messaging in order to increase willingness of HIV-1 uninfected uncircumcised men in serodiscordant partnerships to undertake circumcision. The primary motivators for interest in circumcision were health benefits, including HIV-1 prevention, prevention of sexually transmitted infections, and promotion of genital hygiene. In our study population, less than one quarter of men had salaried employment (Table 1), and the principal impediments to uptake of male circumcision program included the cost of procedure and potential loss of income when away from work during the post-operative period. These findings suggest key messaging strategies for promoting circumcision rollout in Uganda.
Limitations of this study include cross-sectional design and non-probabilistic sampling. Almost 75% of the study population were research-experienced and may not be representative of HIV-1 serodiscordant couples, particularly those who are unaware of their HIV-1 discordant status or less informed about HIV-1 prevention. Additional studies among newly-identified serodiscordant couples would be useful. However, we did not see significant differences in knowledge and attitudes between participants who were in follow-up and those who were new clinic attendees. Our results can serve as a benchmark for program implementers for future evaluations as roll out of male circumcision for HIV-1 prevention progresses.
Acknowledgments
The authors acknowledge the dedication of the two research assistants, Kijjambu Godfrey and Namanda Sylvia, Grace Svilar, and Alice Cantini, and the staff of Partners Clinic Bukoto. We thank Drs. Shelley Francis and Ajay Sethi for their contributions to questionnaire design and results interpretation. We are most grateful to the study participants. This project was supported by the AIDS International Training and Research Program (AITRP) at Case Western Reserve University, Grant #D43-TW000011, funded by the Fogarty International Center of the National Institutes of Health and through the Partners in Prevention HSV/HIV Transmission Study (Bill & Melinda Gates Foundation grant #26469, to the University of Washington). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Kenneth K. Mugwanya, Email: kmugwanya@gmail.com, Infectious Diseases Institute, College of Health Sciences, Makerere University, P.O. Box 22418, Kampala, Uganda; Department of Epidemiology and Biostatistics, School of Medicine, Case Western Reserve University, Cleveland, USA.
Jared M. Baeten, Departments of Global Health, Medicine, and Epidemiology, University of Washington, Seattle, USA
Edith Nakku-Joloba, School of Public Health, College of Health Sciences, Makerere University, Kampala, Uganda.
Elly Katabira, Infectious Diseases Institute, College of Health Sciences, Makerere University, P.O. Box 22418, Kampala, Uganda.
Connie Celum, Departments of Global Health, Medicine, and Epidemiology, University of Washington, Seattle, USA.
Daniel Tisch, Department of Epidemiology and Biostatistics, School of Medicine, Case Western Reserve University, Cleveland, USA.
Christopher Whalen, Department of Epidemiology and Biostatistics, College of Public Health, University of Georgia, Athens, USA.
References
- 1.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou Jl, Sitta Rm, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 trial. PLoS Med. 2005;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.WHO/UNAIDS. Recommendations from expert consultation on male circumcision for HIV prevention. [Accessed 11 Sep 2009]; http://www.who.int/mediacentre/news/releases/2007/pr10/en/index.html. [Google Scholar]
- 5.Weiss HA, Halperin D, Bailey RC, Hayes RJ, Schmid G, Hankins CA. Male circumcision for HIV prevention: from evidence to action? AIDS. 2008;22(5):567–574. doi: 10.1097/QAD.0b013e3282f3f406. [DOI] [PubMed] [Google Scholar]
- 6.UNAIDS/WHO/SACEMA Expert Group on Modelling the Impact and Cost of Male Circumcision for HIV Prevention. Male circumcision for HIV prevention in high HIV prevalence settings: what can mathematical modelling contribute to informed decision making? PLoS Med. 2009;6(9):e1000109. doi: 10.1371/journal.pmed.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371(9631):2183–2191. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 8.Hugonnet S, Mosha F, Todd J, et al. Incidence of HIV infection in stable sexual partnerships: a retrospective cohort study of 1802 couples in Mwanza Region, Tanzania. J Acquir Immune Defic Syndr. 2002;30(1):73–80. doi: 10.1097/00042560-200205010-00010. [DOI] [PubMed] [Google Scholar]
- 9.Wawer MJ, Makumbi F, Kigozi G, et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009;374(9685):229–237. doi: 10.1016/S0140-6736(09)60998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362(5):427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingappa JR, Lambdin B, Bukusi EA, et al. Regional differences in prevalence of HIV-1 discordance in Africa and enrollment of HIV-1 discordant couples into an HIV-1 prevention trial. PLoS One. 2008;3(1):e1411. doi: 10.1371/journal.pone.0001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MOH. Uganda HIV/AIDS sero-behavioural survey, 2004–2005. [Accessed 20 Mar 2008]; http://www.aidsuganda.org/texbits/the%20uganda%20hiv-aids%20status%20report%202005.final%20version.pdf. [Google Scholar]
- 13.Westercamp N, Bailey RC. Acceptability of male circumcision for prevention of HIV/AIDS in sub-Saharan Africa: a review. AIDS Behav. 2007;11(3):341–355. doi: 10.1007/s10461-006-9169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen MR, Deddens JA. A comparison of two methods for estimating prevalence ratios. BMC Med Res Methodol. 2008;8:9. doi: 10.1186/1471-2288-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. Country experiences in the scale-up of male circumcision in the Eastern and Southern Africa Region: two years and counting. [Accessed 11 Nov 2009];2009 http://www.malecircumcision.org/publications/documents/Country_experiences_in_scale-up_in_Eastern_and_Southern_Africa_06.09.09.pdf.
- 16.Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 17.Muula AS. Male circumcision to prevent HIV transmission and acquisition: what else do we need to know? AIDS Behav. 2007;11(3):357–363. doi: 10.1007/s10461-007-9211-1. [DOI] [PubMed] [Google Scholar]
- 18.Sawires SR, Dworkin SL, Fiamma As, Peacock D, Szekeres G, Coates TJ. Male circumcision and HIV/AIDS: challenges and opportunities. Lancet. 2007;369(9562):708–713. doi: 10.1016/S0140-6736(07)60323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]