Abstract
Treatment of individuals exposed to potentially lethal doses of radiation is of paramount concern to health professionals and government agencies. We evaluated the efficacy of filgrastim to increase survival of nonhuman primates (NHP) exposed to an approximate mid-lethal dose (LD50/60) (7.50 Gy) of LINAC-derived photon radiation. Prior to total-body irradiation (TBI), nonhuman primates were randomized to either a control (n =22) or filgrastim-treated (n =24) cohorts. Filgrastim (10 μg/kg/d) was administered beginning 1 day after TBI and continued daily until the absolute neutrophil count (ANC) was >1,000/μL for 3 consecutive days. All nonhuman primates received medical management as per protocol. The primary end point was all cause overall mortality over the 60 day in-life study. Secondary end points included mean survival time of decedents and all hematologic-related parameters. Filgrastim significantly (P < 0.004) reduced 60 day overall mortality [20.8% (5/24)] compared to the controls [59.1% (13/22)]. Filgrastim significantly decreased the duration of neutropenia, but did not affect the absolute neutrophil count nadir. Febrile neutropenia (ANC <500/μL and body temperature ≥103°F) was experienced by 90.9% (20/22) of controls compared to 79.2% (19/24) of filgrastim-treated animals (P = 0.418). Survival was significantly increased by 38.3% over controls. Filgrastim, administered at this dose and schedule, effectively mitigated the lethality of the hematopoietic subsyndrome of the acute radiation syndrome.
Introduction
World events over the past decade have highlighted the threat of nuclear terrorism. This threat is exacerbated by the noted deficiencies in available medical countermeasures (MCM) for mitigating the morbidity and/or mortality consequent to the hematopoietic (H) or gastrointestinal (GI) subsyndromes of the acute radiation syndrome (ARS). Currently, there are no Food and Drug Administration (FDA)-approved medical countermeasures for the treatment of people exposed to potentially lethal doses of radiation within either the H-ARS or GI-ARS ranges. Treatment strategies for exposed individuals have been the subject of several international conferences during the past two decades (1–7). Although there is a consensus for treating patients exposed to ionizing radiation, there is no FDA-approved drug for this indication (1, 4, 8). A number of candidates for medical countermeasures are available. Granulocyte colony stimulating factor (G-CSF) Neupogen® (filgrastim), granulocyte macrophage colony stimulating factor (GM-CSF) Leukine® (sargramostim), and pegylated G-CSF Neulasta® (pegfilgrastim) are FDA approved and have been shown to enhance recovery of neutrophils and reduce the incidence of febrile neutropenia (FN), antibiotic support and risk of infection consequent to cytotoxic therapy or myeloablative conditioning for stem cell transplant (9–16). A recent review and meta-analysis comparing prophylactic use of G-CSF in solid tumor and malignant lymphoma patients concluded that G-CSF reduced the risk of infection and early deaths, including infection-related mortality (16). Current guidelines from the American Society of Clinical Oncology recommend the prompt administration of G-CSF or pegylated G-CSF in the management of patients exposed to lethal total-body radiotherapy (17). Similar European guidelines were issued for use of G-CSF (18). Furthermore, there are a substantial number of studies that consistently show the efficacy of G-CSF to enhance recovery of granulopoiesis and survival subsequent to myelosuppressive or lethal high doses of total-body irradiation (TBI) (19–28). However, only a single report using a canine model established the treatment efficacy of supportive care and G-CSF across the lethal H-ARS (27).
In an effort to broaden the indication for currently approved drugs to treat chemotherapy-induced neutropenia or thrombocytopenia and allow new medical countermeasures to be approved to treat potentially lethally irradiated personnel, the FDA has established the “Animal Rule” (21 CFR 314.600) (29). The Animal Rule enumerates criteria whereby the FDA would approve a drug or biologic for ARS indication based on animal efficacy data when efficacy studies in humans are unethical or not feasible. The FDA may grant marketing approval based on adequate and well-controlled animal studies that demonstrate the candidate drug is likely to reduce serious morbidity or mortality in humans.
Determining the efficacy of candidate MCM requires well-characterized animal models capable of assessing survival as the primary clinically relevant end point in support of FDA regulatory approval. Clinically, there are two components to effective treatment regimens for mitigating the myelosuppressive effects of cytotoxic therapy. While lineage-specific human growth factors can stimulate recovery of critical cells, the use of antibiotics, fluids, blood products, analgesics, antipyretics, anti-diarrheals, antiemetics, antiulceratives, proton pump inhibitors and nutrition is the standard of care for patients exposed to myelosuppressive doses of chemotherapy or conditioning for stem cell transplant. This will not change for personnel exposed to potentially lethal doses of radiation. Medical management was used extensively at accidents at Chernobyl in the former USSR and Tokaimura, Japan (30–33). There is a reliable database demonstrating that medical management alone can significantly enhance survival of animals exposed to lethal doses of radiation (27, 34–37). We previously determined the effect of medical management on the lethal dose-response relationship for the H-ARS in nonhuman primates and herein, used the estimated LD50/60 for NHP administered medical management to assess the efficacy of filgrastim (38).
The FDA requires that the MCM must show a significant increase in survival as the primary clinically relevant parameter to proceed along the path for eventual FDA approval and licensure. We evaluated the efficacy of filgrastim (Neupogen) plus medical management in a lethal, NHP model of H-ARS in a blinded and randomized good laboratory practice (GLP)-compliant study.
Materials and Methods
Test and Control Articles
The test article, Neupogen (filgrastim) (Amgen Inc., Thousand Oaks, CA) and control article, dextrose 5% in water (D5W) (Baxter, Deerfield, IL), were purchased commercially and stored from 2–8°C and from 20–30°C, upon receipt, respectively.
Animals
A total of 46 (38 males/8 females) rhesus macaques, Macaca Mulatta, Chinese substrain, 4.0–6.5 kg body weight, were used. Animal holding rooms were maintained at 70–80°F with 30–70% relative humidity, using at least 10 air changes per hour of 100% conditioned fresh air. A 12 h light/dark cycle was maintained. The animals were housed in individual stainless steel cages at the AAALAC accredited testing facility. Animals were provided commercial certified primate biscuits ad libitum supplemented with fresh fruit (i.e., apples, bananas, pears) and primate treats. The animals had unlimited access to water that had been subjected to deionization, carbon and sand filtration and UV light disinfection, and were provided environmental enrichment and dietary supplements (i.e., a multivitamin). All animals were pre-acclimated to a supine restraint device prior to irradiation.
Radiation Exposure
Nonhuman primates, restrained and awake, were exposed to 6 MV LINAC-derived photons (2 MV average energy, Varian Model EX-21, Palo Alto, CA) at a dose rate of 0.80 Gy/min to a total midline tissue dose of 7.50 Gy. The total dose was divided into anterior (AP) and posterior (PA) exposure. Initial dosimetric measurements were made using Lucite, water-filled phantoms that approximated the diameter of nonhuman primates used in the study. In-life radiation exposure was monitored by ionization chambers (CNMC model 22D, Nashville, TN). Nonhuman primates were administered Ondansetron (1–2 mg/kg i.m.), 45–90 min prior to TBI and within 30–45 min post-TBI. The NHP were observed throughout the irradiation procedure by in-room cameras.
Rationale for Dose of TBI
The irradiation dose, an approximate LD50/60 was 7.50 Gy (712, 793; 95% CI), estimated from a previous, contemporary study that determined the dose-response relationship for the H-ARS in NHP exposed to lethal doses of 6 MV photon irradiation and administered medical management (Fig. 1). The overall mortality observed in the current study was an LD59/60in the control cohort and an LD21/60 in the Neupogen cohort.
Fig. 1.
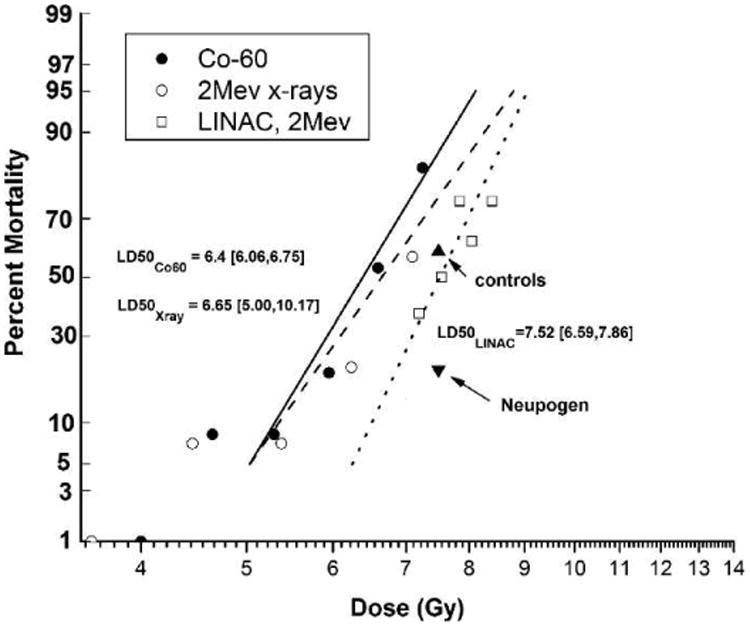
Dose-response relationship for total-body irradiated (TBI) rhesus macaques. The 60 day lethality, dose-response relationship (DRR) for the hematopoietic subsyndrome of the acute radiation syndrome (ARS) in rhesus macaques, presented as log dose (Gy) of TBI versus probit percentage lethality. Two historical data sets showing the dose-response relationship (DRR) and calculated LD50/30 values (95% CI) of rhesus macaques exposed to TBI from 60Co γ radiation or 2 MeV X radiation without the administration of medical management are presented (46, 47). A third data set where medical management was administered to rhesus macaques shows the dose-response relationship and calculated LD50/60 values (95% CI) after exposure to TBI from 6 MV LINAC-derived photons at a dose rate of 0.80 Gy/min (36). In the current study, NHP were exposed to 6 MV LINAC-derived photons at a dose rate of 0.80 Gy/min, n = 24 in the Neupogen cohort and n = 22 in the control cohort, and received medical management as described in the Materials and Methods section.
Animal Cage Side Observations
Veterinarians performed cage-side observations. Nonhuman primates activity, posture, stool consistency, vomit, hemorrhage, alopecia, respiratory and seizure activity were graded and recorded twice daily, at least 6 h apart. The veterinarians were blinded to the Neupogen or the control D5W randomization assignments, antibiotic administration and complete blood counts (CBC).
Animal Clinical Observations
Nonhuman primates were anesthetized with ketamine HCl, (10 mg/kg, i.m., KetaSet®, Fort Dodge, IA) or a combination of ketamine and Xylazine (1 mg/kg, i.m., AnaSed®, Ben Venue Laboratories, Bedford, OH), and clinical parameters such as body weight, body temperature, dehydration status, presence of mouth ulcers and observation of blood in the stool were assessed. A blood sample was obtained on study days (SD) 0-25, 28, 30, 32, 35, 39, 42, 45, 49, 53, 56 and 60 or more often if medically indicated for CBC analyses. An automated cell counter (Beckman Coulter Ac·T diff™) analyzed the CBC, and manual white blood cell (WBC) differentials were performed on a Wright-Giemsa-stained blood film. White blood cell counts were corrected when nucleated red blood cells were ≥10/100 WBC.
Medical Management
Medical management was provided to all NHP as indicated by specified criteria. This included hydration fluids, antibiotics, analgesics, anti-diarrheals, antipyretics, anti-emetics, anti-ulceratives, nutritional support and blood transfusions.
Analgesics
Buprenorphine HCl (Hospira, Lake Forest, IL) (0.01 mg/kg up to 0.02 mg/kg, i.m., BID) was administered to all NHP from study days 5–35 and whenever mouth ulcers or bloody stools were observed.
Anti-ulcerative
Sucralfate (Carafate®, Axcan Scandipharm Inc., Birmingham, AL and Nostrum Laboratories Inc., Edison, NJ) [1 g/day, per os (PO), BID] was administered from study days 5–35 or if bloody stool was observed.
Anti-diarrheals
Following the observation of diarrhea, Loperamide Hydrochloride (Imodium, McNeil) (0.1-0.2 mg/kg PO, BID) was administered. If diarrhea persisted for 3 successive days during Imodium treatment or if watery stool were observed, diphenoxylate hydrochloride (Lomotil, Pfizer Inc., 0.1 mg/kg, PO, BID) was administered for 3 days. If diarrhea persisted after 3 days, Imodium treatment was re-administered.
Antibiotics
Antibiotics were initiated when the absolute neutrophil count (ANC) was <500 cells/μL and were continued until the NHP maintained an ANC >500 cells/μL for 48 h. The primary antibiotic was enrofloxacin (Baytril® Bayer HealthCare LLC, Shawnee Mission, KS) 5 ± 0.25 mg/kg i.m. or intravenous (i.v.), BID or 10 ± 0.5 mg/kg i.m. or i.v., once daily (QD). Additionally, gentamicin sulfate (GentaMax®, Phoenix Scientific, Inc.) (5 mg/kg, i.m. or i.v., QD) was administered in combination with Baytril when the body temperature was ≥103°F and was continued for 24 h. If FN persisted ceftriaxone (Rocephin®, Roche Laboratories Inc., Nutley, NJ) was administered after gentamicin sulfate was discontinued or if microbial resistance was demonstrated to enrofloxacin or gentamicin. Primaxin® (Merck & Co. Inc., Whitehouse Station, NJ) was administered when microbial resistance was demonstrated to enrofloxacin, gentamicin and ceftriaxone.
Antipyretic
Carprofen (Rimadyl®, Pfizer Inc.) (2.2 mg/kg BID or 4.4 mg/kg QD, i.m., i.v. or PO) was administered when a body temperature of ≥104°F was observed and was continued for 48 h after the first day the temperature was < 104°F.
Nutritional support
On all days post-irradiation, NHP received fresh fruit, soft food and bottles containing diluted fruit juice or oral rehydration solution (Prang™, Bio-Serv®). Animals with weight loss ≥10% of their baseline body weight received Bio-Serv certified Rhesus Liquidiets at 15 ml/kg by oral gastric gavage (OG): 7 ml/kg was administered if the NHP was also receiving OG treated-water for hydration.
Blood product support
Whole blood, anti-coagulated with 10% citrate phosphate dextrose with adenine (CPD-A) (Sigma-Aldrich, St. Louis, MO) was obtained from healthy, male NHP, body weight ≥7.0 kg. Blood was filtered through a 70 μm cell strainer (BD Biosciences, Chicago, IL) and irradiated to 2.50 Gy (Gammacell® Elite 1000, Best Theratonics, Ottawa, ON, Canada) prior to use. Transfusions were administered at 7–14 ml/kg, i.v, using an 18 μm blood filter (Hemo Nate® Filter, Utah Medical Products, Inc., Midvale, UT) if there was a decrease of ≥5% in hematocrit (HCT) resulting in an HCT ≤25% over 24 h, HCT <20% or if there were obvious signs of uncontrolled hemorrhage.
Fluid support
Hydration fluid was provided based on a grading system delineated as mild, moderate or severe dehydration. Mild: presence of tacky mucus membranes or a skin tent time (STT) or capillary refill time (CRT) ≥2 but <3 s. Mildly dehydrated animals received a bolus of lactated Ringer's solution (LRS) (10–15 mL/kg) by slow i.v. push (5–10 min) and treated-water (10–15 mL/kg) by OG. Moderate: NHP displaying any of the mild criteria plus dry mucous membranes, >3% increase in HCT from the day before (not transfusion related), sunken eyes or skin tent time or capillary refill time ≥3 s. Moderately dehydrated animals received a bolus of LRS (20–30 mL/kg) over 15–20 min by slow i.v. push and treated-water (7–10 mL/kg by OG). Severe: NHP displaying any of the mild and/or moderate criteria plus pale mucous membranes, >5% increase in HCT from the day before (not transfusion related), pulse >180 beats per minute, cold extremities, lethargy or rapid breathing. Severely dehydrated animals received fluids as described for moderate dehydration with the addition of a slow i.v. infusion (15 ± 5 mL/kg/h) administered over 2–4 h. Animals were placed in a restraint device and allowed to awaken. If necessary, midazolam HCl (Bedford Laboratories™) (0.2 mg/kg, i.m.) may have been administered to calm the NHP while restrained.
Euthanasia
Veterinarians, who were blinded to the Neupogen or the control D5W randomization assignments, antibiotic administration and CBC, adhered to a specific set of criteria, as outlined below when determining if euthanasia was the appropriate action prior to the end of the study (day 60). Any NHP that was recumbent in the cage or had decreased or absent responsiveness to touch, or hemorrhage from the GI tract to be in excess of 20% of the estimated blood volume in any 24 h period or that experienced unrelieved pain was euthanized. Any NHP that experienced any combination of the following observations were euthanized: respiratory distress, decreased food and water intake, reluctance to move for >24 h, or severe dehydration not reversed by hydration therapy. Nonhuman primates were euthanized using U.S. Drug Enforcement Agency (DEA) Class III euthanasia solution (Euthasol®, Virbac AH Inc., 0.27 ml/kg, i.v.). Expiration was confirmed by lack of pulse and respiration.
Experimental Design
Randomization of treatment groups and study blinding
Nonhuman primates in cohorts of 6 to 10 were randomized to the Neupogen or the control D5W group by the study statistician. Both the Neupogen and the control D5W groups contained 4 females. The treatment assignments were provided to a select group of laboratory personnel who were responsible for preparing either the control article or the diluted Neupogen for injection. All other study personnel were blinded to the test and control article randomization assignments. Additionally, the veterinarians who performed cage-side observations and evaluation of moribund NHP for euthanasia purposes were also blinded to the NHP's antibiotic administration history and CBC.
Neupogen dose and route of administration
The route and schedule of Neupogen administration was based on the currently licensed indication of Neupogen for the treatment of cancer patients receiving myelosuppressive chemotherapy or bone marrow transplant. The dose used herein reflects previous pharmacokinetic (PK) and pharmacodynamic (PD) analysis of filgrastim in rhesus monkey and was considered to be bioequivalent to the dose proposed for humans (5 μg/kg/d) (39).
Dose administration
Neupogen (10 μg/kg/d) or the control D5W were administered subcutaneously (sc), at 23 ± 3 h after TBI and then daily until the ANC ≥1,000/μL for 3 consecutive days or if at any time the ANC was ≥10,000/μL. At any point following discontinuation of dosing, if the ANC was <500/μL, daily injections were reinitiated the same day and continued until the ANC was ≥1,000/μL for 3 consecutive days.
Specimen collection
Blood specimens with a target volume of 0.5 mL were collected into EDTA-treated tubes (BD Vacutainer®, Franklin Lakes, NJ) for hematology evaluations by puncture of the saphenous, anti-cubical or femoral vein following anesthesia. Blood was collected aseptically for microbial analysis, inoculated (1 mL each) into aerobic and anaerobic culture vials (BD BACTEC™, Franklin Lakes, NJ) on the first study day FN (ANC <500 cells/μL and body temperature ≥103°F) was observed or on any day the body temperature was ≥105°F. Additional blood cultures were performed if FN either persisted and previous blood culture results were negative or if FN re-occurred following body temperatures <103°F. The maximum blood volume collected for hematology and/or microbiology over the entire 60 day study average was approximately 23 mL. This was within the Institutional Animal Care and Use Committee guidelines of 10 mL/kg of body weight/month.
Determination of endogenous platelet recovery
Only animals that demonstrate a platelet count ≥20,000 platelets/μL prior to death or to end of the study, according to the criteria listed below are included when determining the mean ± SEM duration of thrombocytopenia and time to recovery to a platelet count ≥20,000 platelets/μL.
If an animal is transfusion independent, recovery from thrombocytopenia is the first day of 2 consecutive days the platelet count ≥20,000 platelets/μL. When a transfusion(s) is administered, the animal must survive for 5 days post-transfusion to be evaluated for recovery from thrombocytopenia. At this time, the following guidelines apply to determine endogenous recovery from thrombocytopenia: the platelet count <20,000 platelets/μL prior to transfusion and remains ≥20,000 platelets/μL on day 3 through day 5 post-transfusion, then day 3 post-transfusion will be considered the day of endogenous platelet recovery to ≥20,000 platelets/μL. However, if multiple transfusions are administered prior to recovery from thrombocytopenia, the final transfusion will be used as a starting point when determining when recovery from thrombocytopenia occurred. In the event that the first transfusion occurs on or after the second consecutive day that the platelet count ≥20,000 platelets/μL and remains ≥20,000 platelets/μL on day 1 through day 3 post-transfusion, recovery to platelet count ≥20,000 platelets/μL is considered to be the first day the platelet count is ≥20,000 platelets/μL regardless of the transfusion.
Necropsy, microbiology and histopathology
A necropsy was performed on all animals, and gross observations of the animal and major organs were noted. Microbial analyses were performed on blood and tissue from the lung, liver, spleen and kidney. Tissue from the heart, lung, liver, spleen, kidney, large intestine, small intestine, thymus, mesenteric lymph nodes, skin, and bone marrow were collected and fixed in formalin, embedded in paraffin, cut, mounted and stained with H&E.
Statistical Design
The primary end point of the statistical analysis was to determine and analyze all-cause survival measured at 60 days post TBI. The primary analysis was conducted on the intention-to-treat population using a χ2 test of a one-tailed null hypothesis with a 5% significance level. All statistical analyses were performed using Statistical Analysis Software [SAS (R)] version 9.2, while NHP randomizations used version 9.1. An interim analysis for efficacy and futility was conducted based on the Lan-DeMets version of the O'Brien-Fleming boundary to provide an overall one-sided P = 0.05 test. Futility was to be assessed informally based on conditional power (40, 41).
Secondary end points used summary descriptive statistics for all randomized NHP by treatment group and radiation dose. Dichotomous outcomes were examined using Fischer's exact or χ2 tests. Continuous outcomes were compared among treatment groups using Student's t tests or Mann-Whitney U tests. Survival time was estimated for each treatment using the Kaplan-Meier product limit method and a log-rank test was also performed. Survival curves were compared using the Cox proportional hazards regression model.
Results
Neupogen Administration Increases Survival
The primary objective of this study was to demonstrate whether Neupogen administered within 24 h after lethal TBI significantly increased survival of NHP exposed to TBI with 7.50 Gy [712, 793, 95% CI] an approximate LD50/60. The overall mortality observed in the current study was an LD59/60 for the control cohort and an LD21/60 for the Neupogen cohort (Fig. 1). Neupogen, administered as described herein, significantly reduced overall mortality by 38.3% (Fig. 2) (P < 0.004).
Fig. 2.
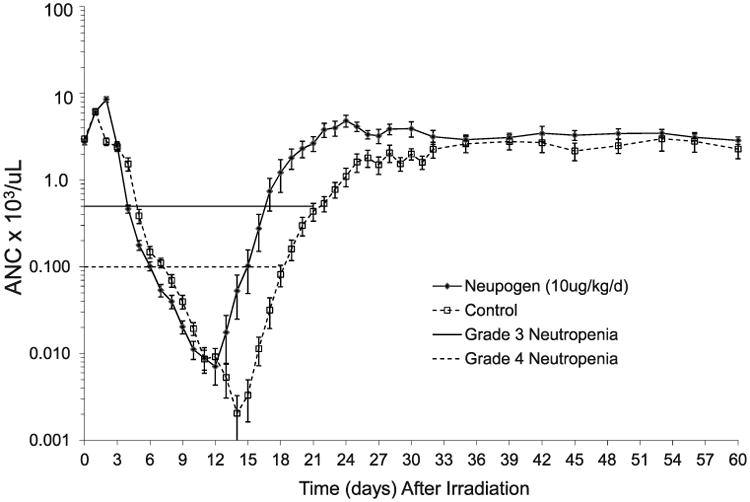
Kaplan-Meier survival curve in rhesus macaques after total-body irradiation. Rhesus macaques were exposed to 7.50 Gy TBI with 6 MV LINAC photons (2 MV average energy) at a dose rate of 0.80 Gy/min. The TBI was delivered as 50% in the anterior (AP), then at 50% in the posterior (PA) directions. Nonhuman primates (n = 46) were observed for 60 days post TBI for cage-side and clinical observations and protocol euthanasia criteria for all-cause mortality. Neupogen administered at 10 μg/kg/d or control-article administered at 10 μL/kg/d, SC, starting day 1 post TBI and continued daily until the ANC >1,000 cells/μL for 3 consecutive days. Lethality in the Neupogen treated cohort was 20.8% (5/24) and occurred on day 8 (n = 1), day 11 (n = 3) and day 19 (n = 1). Lethality in the control cohort was 59.1% (13/22) and occurred on day 11 (n = 1), day 12 (n =1), day 15 (n = 1), day 17 (n = 3), day 18 (n = 2), day 19 (n = 2), day 31 (n = 1), day 36 (n = 1) and day 43 (n = 1).
Secondary Objectives
Secondary objectives evaluated included indices of hematopoietic recovery, mean survival time (MST, days) of decedents, incidence of FN and infection, number of whole blood transfusions, body weights and temperatures, and incidence and severity of diarrhea.
Neutrophil-Related Parameters
The 7.50 Gy exposure reduced the ANC in both cohorts from pre-irradiation values to <500 cells/μL within 5 days post TBI (Fig. 3). The ANC in both the control and Neupogen-treated cohorts decreased further to <100 cells/μL by 6.5 days ± 0.03 and 7.1 days ± 0.4, respectively (Table 1). The mean nadir by day post TBI was not significantly different between the Neupogen (5.0 cells/μL ± 2.0) and the control cohort (1.5 cells/μL ± 1.0) (P = 0.115) (Table 1). Absolute neutropenia (ANC = 0 cells/μL) was experienced by 82% (18/22) of all control-treated animals and 78% (7/9) of those that survived to day 60. Absolute neutropenia was observed in 58% (14/24) of all Neupogen-treated animals and in 63% (12/19) of the survivors.
Fig. 3.
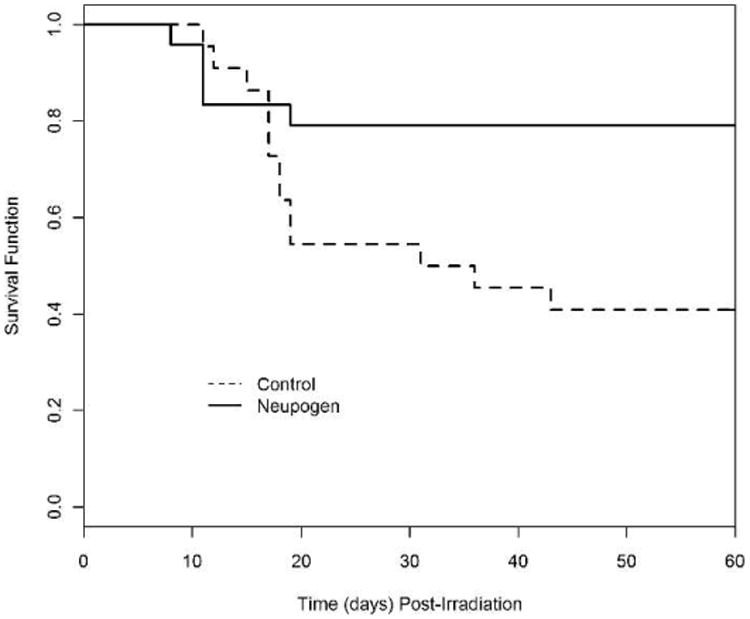
Mean absolute neutrophil counts in rhesus macaques after total-body irradiation. Animals were exposed to total-body irradiation (TBI) at 7.50 Gy with 6 MV LINAC-derived photons at a dose rate of 0.80 Gy/min and were administered medical management. Shown are the mean (±SEM) absolute neutrophil count (ANC) × 103 cells/μL in the peripheral blood of rhesus macaques administered Neupogen (n = 24) or 5% dextrose in water (n = 22) as a function of time post TBI.
Table 1. Neutrophil-Related Parameters for Rhesus Macaques after Exposure to 7.50 Gy TBI by 6 MV LINAC-Derived Photons at a Dose Rate of 0.8 0 Gy/min and Administration of Neupogen or Control Article.
| First day ANC <500 cells/μL or 100 cells/μL | Last day (d) ANC <500 cells/μL or 100 cells/μL | Duration(days)ANC <500 c ells/μL or 100 cells/μL | Recovery to ANC ≥1000 cells/μL | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Article (n) | <500 cells/μL | <100 cells/μL | <500 cells/μL | <100 cells/μL | <500 cells/μL | ≥100 cells/μL | ≥1,000 cells/μL | ANC Nadir (cells/μL) | |
| Neupogen (n = 24) | Mean ± SEM | 4.3 ± 0.1 | 6.5 ± 0.3 | 17.6 ± 0.5a | 15.6 ± 0.5 | 14.3 ± 0.5a | 10.4 ± 0.6a | 19.7 ± 0.6a | 5.0 ± 2.0 |
| P value | Not done | Not done | Not done | Not done | <0.0001 | 0.009 | <0.0001 | 0.115 | |
| Median | NA | NA | NA | NA | 14.0 | 10.0 | 20 | 0 | |
| Range | Day 3–5 | Day 4–9 | Day 13–22a | Day 12–19a | 9–19 days | 4–14 days | 15–25 days | NA | |
| Control (n = 22) | Mean ± SEM | 4.9 ± 0.2 | 7.1 ± 0.4 | 21.8 ± 0.7b | 18.4 ± 0.4b | 18.6 ± 0.8b | 12.3 ± 0.6b | 25.8 ± 0.9b | 1.5 ± 1.0 |
| Median | NA | NA | NA | NA | 18.0 | 12.0 | 24.5 | 0 | |
| Range | Day 3–6 | Day 5–10 | Day 19–26b | Day 16–22b | 14–22 days | 8–15 days | 22–32 days | NA | |
Notes. The mean, SEM, median and range (where applicable) are reported. The absolute neutrophil count (ANC) nadir, duration and the first and final day that grade 3 or 4 neutropenia [ANC <500 cells/μL or <100 cells/μL, respectively] for Neupogen and control cohorts, and ANC recovery ≥1000 cells/μL occurred are shown. The durations last day of grade 3 or 4 neutropenia do not include data from decedent animals unless recovery occurred to that level, e.g., ANC ≥500 cells/μL prior to death. The duration of neutropenia was estimated as the number of days that a subject has an observed or an extrapolated ANC below 500 cells/μL. If an animal's ANC was ≥500 cells/μL for a single day but <500 cells/μL on the day preceding and following this day, it was considered a day of severe neutropenia when determining the animal's duration of ANC <500 cells/μL. The time to recovery was estimated as the number of days from study day 1 until the first 2 consecutive observed or extrapolated ANC after the nadir was ≥1,000 cells/μL. The nadir was the lowest observed or extrapolated ANC/μL observed at least 2 days after irradiation.
n = 19.
n =12.
The stimulation of granulopoiesis by Neupogen administration in lethally irradiated NHP resulted in a significant reduction in the duration of neutropenia (ANC <500 cells/μL or <100 cells/μL) and the time to recovery of ANC >1,000 cells/μL. Neupogen reduced the duration of neutropenia (ANC <500 cells/μL) from 18.6 days ± 0.8 in the control cohort to 14.3 days ± 0.5 (P < 0.0001), and the duration of neutropenia (ANC <100 cells/μL) from 12.3 days ± 0.6 in the control cohort to 10.4 days ± 0.6 (P = 0.009) (Table 1). The time of recovery to an ANC >1,000 cells/μL was also significantly reduced from 25.8 days ± 0.9 in the control cohort to 19.7 days ± 0.6 in the Neupogen cohort (P < 0.0001) (Table 1).
Platelet-Related Parameters
Total body irradiation at 7.50 Gy induced a severe decrease in platelet levels for both control and Neupogen-treated cohorts. The mean nadir by day for the study occurred at approximately 11 days post TBI for both cohorts and reflects the effect of transfusions administered to one control animal and two Neupogen-treated animals on day 10 (Fig 4). The platelet nadir of each animal in a cohort, when averaged, was 0.50 ± 0.2 × 103 platelets/μL for the control cohort and 0.9 ± 0.3 × 103 platelets/μL in the Neupogen-treated cohort, and it occurred in the majority of animals prior to the administration of a transfusion(s) (P = 0.134) (Table 2). Absolute thrombocytopenia (0 platelets/μL) was observed in 14/22 (range 0—4 × 103 platelets/μL) for control-treated animals and 10/24 (range 0—6 × 103 platelets/μL) for Neupogen-treated animals. The first day the platelet count deceased to <20,000 platelets/μL was not different between the Neupogen- or control-treated cohorts (9.3 days ± 0.2 to 9.7 days ± 0.2, respectively) (Table 2). The duration of thrombocytopenia (platelets <20,000 platelets/μL) between the Neupogen- and control-treated cohorts was 12.6 days ± 1.4 to 17.1 days ± 2.1 (P = 0.077), respectively (Table 2). The Neupogen-treated cohort required on average 22.0 days ± 1.4 to recover to platelet counts ≥20,000 platelets/μL, whereas the control-treated cohort required 26.9 days ± 2.2 (P = 0.062) (Table 2). Because a platelet count in an animal may be elevated as a result of a whole blood transfusion, several rules were applied when determining thrombocytopenia and true (endogenous) recovery to a platelet count ≥20,000 platelets/μL versus platelets received by transfusion. The duration of thrombocytopenia when defined as a platelet count <50,000 platelets/μL was not influenced by transfusions, and was 16.2 days ± 1.2 relative to 20.4 days ± 2.9 for the Neupogen- and control-treated cohorts, respectively (statistics not performed). Recovery to a platelet count ≥50,000 platelets/μL by day 60 was never attained in one animal from each treatment cohort.
Fig. 4.
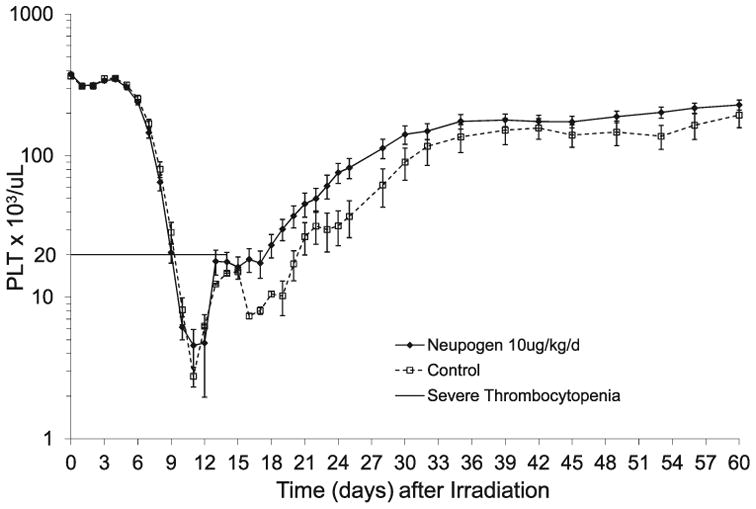
Mean platelet counts in rhesus macaques after total-body irradiation. Animals were exposed to total-body irradiation (TBI) at 7.50 Gy from LINAC-derived photons at a dose rate of 0.80 Gy/min with administration of medical management as defined in the Materials and Methods section. Shown are the mean (±SEM) platelet counts × 103 platelets/μL in the peripheral blood of rhesus macaques administered Neupogen (n = 24) or 5% dextrose in water (n = 22) as a function of time post TBI. Note that the effect of platelet transfusions has elevated both the platelet nadir and recovery displayed.
Table 2. Platelet-Related Parameters for Rhesus Macaques after Exposure to 7.50 Gy TBI and Administration of Neupogen or Control.
| Article | First day platelet count <20,000 platelets/μL | Durationa <20,000 platelets/μL | Nadirb (platelet count×103/μL) | Recovery to platelet count ≥20,000 platelets/μL | No.transfusionsb (54 mL) | First day transfusion occurred | |
|---|---|---|---|---|---|---|---|
| Neupogen | Mean ± SEM | 9.3 ± 0.2 | 12.6 ± 1.4c | 0.9 ± 0.3 | 22.0 ± 1.4c | 1.8 ± 0.3 | 10.8 ± 0.9 |
| P value | Not done | 0.077 | 0.134 | 0.062 | Not done | Not done | |
| Median | 9.0 | 10 | 1 | 21 | 1.3 | 12 | |
| Range | Day 8–11 | Day 6–33 | 0–6 | Day 15–42 | 0.5–5.5 | Day 10–18 | |
| Control | Mean ± SEM | 9.7 ± 0.2 | 17.1 ± 2.1d | 0.5 ± 0.2 | 26.9 ± 2.2d | 2.4 ± 0. 3 | 11.8 ± 0.6 |
| Median | 10 | 14 | 0 | 24 | 2.0 | 12 | |
| Range | Day 8–11 | Day 11–33 | 0–4 | Day 20–44 | 1–6.5 | Day 10–14 |
Notes. Animals were exposed to total body irradiation (TBI) from 6 MV LINAC-derived photons at a dose rate of 0.80 Gy/min. The platelet count (PLT) mean, SEM, median and range of the first day of thrombocytopenia (platelet count <20,000 platelets/μL) is observed in an animal in each treatment cohort, duration of thrombocytopenia, platelet nadir, day recovery from thrombocytopenia occurs, number of whole blood transfusions administered (54 mL) and first day a transfusion is observed in an animal in each radiation cohort is displayed.
Note that durations do not include data from decedent animals unless recovery occurred to that level, e.g., platelet count ≥20,000 platelets/μL prior to death.
The platelet nadir and number of transfusions includes both survivors and non-survivors.
n = 19.
n = 11.
Transfusion Requirements
The time to the administration of the first transfusion ranges between day 10 and day 18. In the surviving animals (28/46), all but one animal (Neupogen treated) received a minimum of one transfusion. The mean value for the time interval between the day of TBI and the first transfusion for all animals that required whole blood transfusions (n = 42/46) is 12.4 days. On average, the Neupogen-treated animals required fewer transfusions (1.8 ± 0.3) than the control-treated cohort (2.4 ± 0.3) during this period. The range of the number of transfusions was similar for both the Neupogen-treated cohort (0.5–5.5) and the control-treated cohort (1.0–6.5).
Microbiology: Peripheral Blood Culture in Association with FN
A positive blood culture was noted in 58% (14/24) of the Neupogen cohort and 86% (19/22) in the control cohort. The majority of the blood cultures grew gram-positive organisms (94%). Two NHP receiving control article (2/22, 9%) and 4 NHP administered Neupogen (4/24, 17%) did not require a blood culture. Fifty-two percent (68/131) of all peripheral blood-derived culture results were negative for the presence of bacteria (Table 3). Seventy percent (72%, 33/46) of all NHP had at least one positive blood culture during the study. Of the animals that required blood culture evaluation, 14 Neupogen-treated NHP (14/20, 70%) and 19 control-article treated NHP (19/20, 95%) had at least one culture that was positive for bacteria. Thirteen Neupogen-treated NHP and 14 control-article treated NHP had at least one organism identified that was resistant to either enrofloxacin (Baytril), gentamicin sulfate (GentaMax), and/or ceftriaxone (Rocephin). All identified organisms were sensitive to imipenem and cilastatin (Primaxin).
Table 3. Summary of Blood Culture Results in Rhesus Macaques after exposure to 6 MV Photon TBI at 7.50 Gy and Administration of Neupogen or Control Article.
| Peripheral blood cultures pe rformed | Peripheral blood cultures ne gative for bact eria | Number o f NHP having at least one bacteria-positive blood culture | Cultures positive for gram-negative bacteria | Cultures positive for gram-positive bacteria | Cultures positive for gram-negative and gram-positive bacteria | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Total | 135 | 68 | 52 | 33/46 | 72 | 7/63 | 11 | 59/63 | 94 | 3/63 | 5 | |
| Article | ||||||||||||
| –Neupogen n = 24 | 60/131 | 46 | 36/60 | 60 | 14/24 | 58 | 1/7 | 14 | 23/63 | 37 | 3/3 | 100 |
| –Control n = 22 | 71/131 | 54 | 32/71 | 45 | 19/22 | 86 | 6/7 | 86 | 36/63 | 57 | 0/3 | 0 |
Notes. Animals (n = 46) were total-body irradiated to 7.50 Gy by LINAC-derived photons and were administered either Neupogen or control (5% dextrose in water). Animals were assessed for the presence of gram-positive (+) and/or gram-negative (−) bacteria in the peripheral blood. Blood cultures were obtained per protocol guidelines.
Antibiotic Administration
Antibiotics were administered prophylactically, e.g., when the ANC <500 cells/μL, because it was anticipated that the ANC would continue to decrease to values <100 cells/μL within several days and that the ANC would proceed to absolute values or remain at <100 cells/μL for 5–7 days. The ANC in all NHP decreased from an ANC <500 cells/μL to an ANC <100 cells/μL within the next 2.3 days. The duration of the ANC <100 cells/μL was 12.3 days and 10.4 days for the control and Neupogen-treated cohorts, respectively (Table 1). This is grade 4 neutropenia and the NHP is at greatest risk for infection and sepsis. Furthermore, these values support the validity of administering primary antibiotic prophylaxis relative to recommendations of the Infectious Diseases Society of America (IDSA) and the National Comprehensive Cancer Network (42, 43).
Febrile Neutropenia and Antibiotic Requirements
Seventy-nine percent (79.1%) (19/24) of Neupogen-treated animals experienced FN, whereas 90.0% (20/22) control-treated NHP experienced FN (P = 0.418). The first day of FN for control and Neupogen-treated NHP was 11.7 days ± 0.8 and 10.7 days ± 0.7, respectively (Table 4). The duration of FN was 6.2 days ±1.5 and 3.8 days ± 0.8 for the control and Neupogen-treated cohorts, respectively (Table 4). Neupogen treatment reduced the duration of FN by 2.4 days and antibiotic requirements by 4.8 days. All animals (45/46) experienced a core body temperature ≥ 103.0°F at some point during the study, with the exception of a control-treated animal that died on day 11.
Table 4. Febrile Neutropenia and Antibiotic Requirements after TBI to 7.50 Gy in Rhesus Macaques Administered Neupogent® or Control Article: Incidence and Duration.
| Article | First day FN | Duration (day) FN | Days on antibiotics | Days CBT ≥103°F |
|---|---|---|---|---|
| Neupogen | 10.7 ± 0.7 | 3.8 ± 0.8 | 17.5 ± 1.0a | 7.6 ± 1.2 |
| Control | 11.7 ± 0.8 | 6.2 ± 1.5 | 22.3 ± 1.8b | 7.2 ± 1.1 |
n = 19.
n = 9.
Notes. Animals (n = 46) were exposed to total-body irradiation from 6 MV LINAC-derived photons at a dose rate of 0.80 Gy/min. Febrile neutropenia (FN) is defined as the ANC <500 cells/μL and the core body temperature (CBT) ≥103°F. The mean of the first day of the occurrence of FN for each irradiation dose is shown. The duration for FN and the numbers of days an animal required antibiotic administration was determined for the survivors only. Four animals (16.7%) administered Neupogen and 1 control animal (4.5%) did not experience FN. One animal in each treatment cohort succumbed prior to experiencing FN. The mean numbers of days an animal's CBT ≥103°F is shown for all animals regardless of survival. The SEM is reported for each parameter.
Diarrhea
The incidence and severity of diarrhea was equivalent in both the control and Neupogen-treated cohorts. All of the control cohort animals experienced grade 2 or 3 diarrhea (loose and/or watery, or bloody diarrhea, respectively). Nearly the same percentage of animals experienced grade 2 diarrhea in the Neupogen and control cohorts (79.2%, n = 19 and 77.3%, n = 17, respectively).
Euthanasia and Necropsy
The majority of animals euthanized prior to the end of study in both the control and Neupogen-treated cohorts qualified for euthanasia based on abnormal appearance and decreased activity. Additionally, a few animals in each treatment group had progressive tissue necrosis and were humanely euthanized. Necropsy was performed on all animals. Those that succumbed prior to the end of the study from both treatment cohorts were found to have bacterial emboli, focal hemorrhage, sometimes with necrosis, moderate focal inflammation and bone marrow aplasia. In general, in both experimental groups, animals that survived until the end of the experimental period had the least number of pathological alterations. There were few differences between the untreated control and the Neupogen-treated animals that survived to 60 days or more. Bacterial growth (predominantly gram-positive organisms) in tissue samples was observed in 32% (7/22) of the controls compared to 17% (4/24) in the Neupogen-treated animals. The microbiological data supports the conclusion that the Neupogen-treated NHP had fewer systemic infections than did the control article treated group.
Discussion
The efficacy of Neupogen in enhancing the recovery of neutrophils in NHP models of sublethal radiation-induced myelosuppression has been substantial and consistent. Our laboratory has investigated the efficacy of Neupogen in mitigating myelosuppression induced by TBI of several different qualities, e.g., 60Co γ radiation, 250 kVp X radiation, mixed γ-neutron radiation and 6 MV LINAC-derived photons (23, 25, 27, 44, 45). Neupogen, administered at 20 h and continued daily post TBI in concert with medical management, enhanced recovery of neutrophils that reduced the degree and duration of severe neutropenia and the incidence and duration of febrile neutropenia relative to medical management alone. Current studies have focused on enhancing survival from radiation-induced lethality as the primary clinically relevant parameter. We used TBI that induced 60% lethality and demonstrated that Neupogen significantly enhanced survival in addition to all of the neutrophil-related parameters. The current focus is placed on defining a path for approval of Neupogen under the criteria of the FDA “Animal Rule” to treat lethally irradiated personnel consequent to a nuclear terrorist event. Pivotal studies are designed to be good laboratory practice compliant, blinded, randomized and focused on a minimum 30% survival versus control for significant efficacy.
It was therefore concluded that Neupogen, when administered as described herein, significantly reduced 60 day overall mortality 38.3% in rhesus macaques that were administered medical management. Overall mortality was reduced from 59.1% in the control cohort to 20.8% in the Neupogen-treated cohort. The preclinical efficacy of Neupogen to ameliorate radiation-induced myelosuppression has been demonstrated in rodents, canines and NHP (19–28). However, a statistically designed, good laboratory practice compliant, randomized and blinded study to demonstrate efficacy in a NHP model of mid-lethal TBI plus administration of medical management within the H-ARS had not been performed. This study compared TBI plus medical management in the control cohort to TBI, with medical management and Neupogen administration in the treatment cohort.
We contend that medical management will be the standard of care for personnel exposed to myelosuppressive and potentially lethal doses of radiation and therefore is an essential component of a successful protocol for use of an MCM. We established the relationship between medical management and lethality over the dose range responsible for the H-ARS in the NHP. Two studies reported the LD50/30 for NHP exposed to TBI with radiation quality comparable to the 6 MV photons used herein: one unpublished (data provided to T. MacVittie) and the other published, showed the LD50/30 for NHP without use of medical management to be 6.45 Gy (60Co γ irradiation) or 6.65 Gy (2 MeV X ray), respectively (46, 47). Farese et al. showed that medical management alone resulted in an LD50/60 of 7.52 Gy (38).
A noted advantage provided by medical management is the improved survival time post TBI, thereby providing additional time for Neupogen to affect neutrophil production. The increased mean survival time of decedents receiving medical management herein is 19 days. Our only comparison to the mean survival time of decedents exposed to TBI at the approximate LD50/30 for NHP that did not receive the benefit of medical management is derived from the literature (48–54). The average mean survival time of decedents was 14.3 days for NHP not receiving such care and that were exposed to an approximate LD50/30 dose of TBI (38).
The most important and readily available measure of susceptibility to infection is the level of the ANC over time after irradiation. The relationship between the degree and duration of neutropenia and mortality is well documented (55–60). As the ANC decreases to <500/μL, the susceptibility to infection increases and the frequency and severity of infection are inversely proportional to the ANC. The risk of severe infection and bacteremia are greatest when the ANC <∼100 cells/μL. The prophylactic use of antibiotics herein is based on the fact that all lethally irradiated NHP reached an ANC <500 cells/μL within 5 days post TBI. The ANC continued to decrease within the subsequent 2 days and reached an ANC <100 cells/μL by 7 days post TBI. The duration of ANC <100 cells/μL for control NHP was 12.3 days. These parameters support the use of prophylactic antibiotics as recommended by the IDSA guidelines, and others (42, 43, 57–59). They suggest that antibiotic prophylaxis should only be considered in afebrile patients at the highest risk for infection, e.g., profound neutropenia (ANC <100 cells/μL) with an expected duration of 7 days. Herein, the administration of Neupogen stimulated increased production of neutrophils within the critical, clinically manageable period of time post lethal TBI. The duration of ANC <100 cells/μL or <500 cells/μL was reduced from 12.3 days and 18.6 days in the control cohort to 10.4 days and 14.3 days consequent to Neupogen administration, respectively.
These results are in concordance with the proposed mechanism of action for Neupogen. A sufficient number of Neupogen-responsive hematopoietic progenitor cells (HPC) survived the 7.50 Gy TBI. Neupogen enhanced regeneration of the HPC pool, stimulated production of neutrophils and decreased the bone marrow transit time, resulting in an estimated increase of greater than three divisions in the neutrophil maturation and expansion process (61, 62). Of interest to this mechanism of action, Engel et al., in analysis of human granulocyte kinetics, suggested that approximately 43% of post-mitotoic neutrophil precursors emerge from the bone marrow into the circulation (63). Mackey and colleagues report that approximately 55% of cells entering the post-mitotic neutrophil compartment die before being released into the circulation (64). It was estimated that this fraction of neutrophils that was released from the marrow compartment into the circulation increased to 100% survival under maximum G-CSF stimulation (63–65). The rapid maturation and decreased transition time coupled with increased mitotic activity in HPC and mitotic pools, along with diminished apoptosis supports an increased production of neutrophils in the Neupogen-treated high dose, lethally irradiated NHP.
Medical management is in effect, the best medical countermeasures available to date. It increases survival and MST of decedents consequent to lethal doses of TBI and it does not have to be administered as shown here until days 5–12 post TBI. Neupogen, when administered at 24 h post TBI at an approximate LD50/60 significantly increased survival within the 60 day in-life phase of this study. The use of Neupogen in combination with a standard protocol of medical management is the best example of combined medical countermeasures activity to ameliorate the lethal effects of TBI within the H-ARS. Neupogen, administered daily, continued to stimulate granulopoiesis in combination with medical management and significantly increased survival beyond that shown for medical management alone.
Acknowledgments
The authors would like to thank David Cassatt, Bert Maidment and Erica Brittain, for their advice and support in the planning and design of this study. We would also like to acknowledge and thank the following individuals for their work on this study: Alexander Bennett, Michael Flynn, Kim Hankey, Adam Higgins, Emylee McFarland, Kyle O'Donnell, Ashley Wilson, Amanda Ward, UMB Veterinary Resources, UMB Radiation Oncology, UMB Pathology Associates, Jeffery Parker and Maritza Patton. This study was sponsored by NIAID Contract #HHSN266200500043C.
References
- 1.Biological effects of radiation injury. Miamisburg (OH): AlphaMed Press; 1997. [Google Scholar]
- 2.Ricks RC, Fry SA, editors. The medical basis for radiation accident preparedness II: Clinical experience and follow-up since 1979. New York: Elsevier Science Inc.; 1990. [Google Scholar]
- 3.Browne D, Weiss JF, MacVittie TJ, Pillai MV, editors. Proceedings of the first consensus development conference on the treatment of radiation injuries. New York: Plenum Press; 1990. Treatment of radiation injuries. [Google Scholar]
- 4.MacVittie TJ, Weiss JF, Browne D, editors. Proceedings of 2nd consensus development conference on the treatment of radiation injuries. Tarrytown: Pergamon: Elsevier Science Inc.; 1996. Advances in the treatment of radiation injuries. [Google Scholar]
- 5.Ricks RC, Berger ME, O'Hara F, editors. The medical basis for radiation accident preparedness IV: Clinical care of victims. Washington, DC; Parthenon Publishers; 2002. [Google Scholar]
- 6.Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, et al. Literature review and global consensus on management of acute radiation syndrome affecting nonhematopoietic organ systems. Disaster Med Pub Health Prep. 2011;5:183–201. doi: 10.1001/dmp.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, et al. First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Public Health Prep. 2011;5:202–12. doi: 10.1001/dmp.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, et al. Medical management of the acute radiation syndrome: recommendations of the strategic national stockpile radiation working group. Ann Int Med. 2004;140(12):1037–51. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 9.Sheridan WP, Morstyn G, Wolf M, Dodds A, Lusk J, Maher D, et al. Granulocyte colony-stimulating factor and neutrophil recovery after high-dose chemotherapy and autologous bone marrow transplantation. Lancet. 1989;2:891–5. doi: 10.1016/s0140-6736(89)91552-3. [DOI] [PubMed] [Google Scholar]
- 10.Monroy RL, Skelly RR, MacVittie TJ, Davis TA, Sauber JJ, Clark SC, et al. The effect of recombinant GM-CSF on the recovery of monkeys transplanted with autologous bone marrow. Blood. 1987;70:1696–9. [PubMed] [Google Scholar]
- 11.Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, et al. Reduction by granulocyte-colony stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Eng J Med. 1991;325:164–70. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 12.Nienhuis AW, Donahue RE, Karlsson S, Clark SC, Agricola B, Antinoff N, et al. Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) shortens the period of neutropenia after autologous bone marrow transplantation in a primate model. J Clin Invest. 1987;80:573–77. doi: 10.1172/JCI113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-metHuG-CSF): the first 10 years. Blood. 1996;88:1907–29. [PubMed] [Google Scholar]
- 14.Bishop MR, Tarantolo SR, Geller RB, Lynch JC, Bierman PJ, Pavletic ZS, et al. A randomized, double-blind trial of filgrastim (granulocyte colony-stimulating factor) versus placebo following allogeneic blood stem cell transplantation. Blood. 2000;96(1):80–5. [PubMed] [Google Scholar]
- 15.MacVittie TJ, Monroy RL. Potential improvement in the management of seriously irradiated person. In: Ricks RC, Fry SA, editors. The medical basis for radiation accident preparedness II: Clinical experience and follow-up since 1979. New York: Elsevier; 1990. pp. 121–147. [Google Scholar]
- 16.Kuderer N, Dale DC, Crawford J, Lyman G. Impact of primary prophylaxis with granulosyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158–67. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 17.Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187–205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 18.Aapro MS, Cameron DA, Pettengell R, Bohlius J, Crawford J, Ellis M, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidents of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42(15):2433–53. doi: 10.1016/j.ejca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi Y, Okabe T, Urabe A, Suzukci N, Takaku F. Human granulocyte colony stimulating factor produced by Escherichia coli shortens the period of granulocytopenia induced by irradiation in mice. Japan J Cancer Res. 1987;78:763–8. [PubMed] [Google Scholar]
- 20.Schuening FG, Storb R, Goehle S, Graham TC, Appelbaum FR, Hackman R, et al. Effect of recombinant human granulocyte colony-stimulating factor on hematopoiesis of normal dogs and on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 1989;74:1308–13. [PubMed] [Google Scholar]
- 21.Patchen ML, MacVittie TJ, Solberg BD, Souza LM. Therapeutic administration of recombinant human granulocyte colony stimulating factor accelerated hemopoietic regeneration and enhances survival in a murine model of radiation-induced myelosuppression. Int J Cell Clon. 1990;8:107–22. doi: 10.1002/stem.5530080204. [DOI] [PubMed] [Google Scholar]
- 22.Fushiki M, Ono K, Sasai K, Shitamoto Y, Tsutsui K, Nishidai T, et al. Effect of recombinant human granulocyte colony stimulating factor on granulocytopenia in mice induced by irradiation. Int J Radiat Oncol Biol Phys. 1990;18:353–7. doi: 10.1016/0360-3016(90)90100-x. [DOI] [PubMed] [Google Scholar]
- 23.MacVittie TJ, Monroy RL, Patchen ML, Souza LM. Therapeutic use of recombinant human G-CSF in a canine model of sublethal and lethal whole-body irradiation. Int J Radiat Biol. 1990;57:723–36. doi: 10.1080/09553009014550891. [DOI] [PubMed] [Google Scholar]
- 24.Tanikawa S, Nose M, Yoshiro A, Tsuneoka K, Shikita M, Nara N. Effects of recombinant human granuloctye colony-stimulating factor on the hematologic recovery and survival of irradiated mice. Blood. 1990;76:445–9. [PubMed] [Google Scholar]
- 25.Farese AM, Hunt P, Grab LB, MacVittie TJ. Combined administration of recombinant human megakaryocyte growth and development factor and granulocyte colony-stimulating factor enhances multilineage hematopoietic reconstitution in nonhuman primates after radiation-induced marrow aplasia. J Clin Invest. 1996;97:2145–51. doi: 10.1172/JCI118652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neelis KJ, Hartong SCC, Egeland T, Thomas GR, Eaton DL, Wagemaker G. The efficacy of single-dose administration of thrombopoietin with coadministration of either granulocyte/macrophage or granulocyte colony-stimulating factor in myelosup-pressed rhesus monkeys. Blood. 1997;90:2565–73. [PubMed] [Google Scholar]
- 27.MacVittie TJ, Farese AM, Jackson WI. Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys. 2005;89(5):546–55. doi: 10.1097/01.hp.0000173143.69659.5b. [DOI] [PubMed] [Google Scholar]
- 28.Hosoi Y, Kurishita A, Ono T, Sakamoto K. Effect of recombinant human granulocyte colony-stimulating factor on survival in lethally irradiated mice. Acta Oncol. 2011;31(1):59–63. doi: 10.3109/02841869209088267. [DOI] [PubMed] [Google Scholar]
- 29.Crawford LM. New drug and biological drug products; Evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Federal Register. 2002 May 31;67(105):37988–37998. 21 CFR parts 314 and 601, FDA, HHS; ACTION: Final Rule. [PubMed] [Google Scholar]
- 30.Baranov AE, Selidovkin GD, Butturini A, Gale RP. Hematopoietic recovery after 10-Gy acute total body radiation. Blood. 1994;83(2):596–99. [PubMed] [Google Scholar]
- 31.Konchalovski MV, Baranov AE, Kolganov AV. Multiple organ involvement and failure: selected Russian radiation accident cases re-visited. Br J Radiol Suppl. 2005;27:26–29. [Google Scholar]
- 32.Uozaki H, Fukayama M, Nakagawa K, Ishikawa T, Misawa S, Doi M, et al. The pathology of multi-organ involvement: two autopsy cases from the Tokai-mura criticality accident. Br J Radiol Suppl. 2005;27:16–6. [Google Scholar]
- 33.Asano S. Multi-organ involvement: lessons from the experience of one victim of the Tokai-mura criticality accident. Br J Radiol Suppl. 2005;27:9–12. [Google Scholar]
- 34.Jackson DP, Sorensen DK, Cronkite EP, Bond VP, Fliedner TM. Effectiveness of transfusions of fresh and lyophiloized platelets in controlling bleeding due to thrombocytopenia. J Clin Invest. 1959;38:1689–97. doi: 10.1172/JCI103947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacVittie TJ, Monroy R, Vigneulle RM, Zeman GH, Jackson WE. The relative biological effectiveness of mixed fission-neutron-gamma radiation on the hematopoietic syndrome in the canine: Effect of therapy on survival. Radiat Res. 1991;128:S29–36. [PubMed] [Google Scholar]
- 36.Perman V, Cronkite EP, Bond VP, Sorensen DK. The regenerative ability of hemopoietic tissue following lethal x-irradiation in dogs. Blood. 1962;19:724–37. [PubMed] [Google Scholar]
- 37.Sorensen DK, Bond VP, Cronkite EP, Perman V. An effective therapeutic regimen for the hemopoietic phase of the actue radiation syndrome in dogs. Radiat Res. 1960;13:669–85. [Google Scholar]
- 38.Farese AM, Cohen MV, Katz BP, Smith CP, Jackson W, III, Cohen DM, et al. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys. 2012;103:367–82. doi: 10.1097/HP.0b013e31825f75a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farese AM, Cohen MV, Stead RB, Jackson W, III, MacVittie TJ. Peg-filgrastim, administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose, radiation-induced myelosuppression in rhesus macaques. Radiat Res. 2012;178:403–14. doi: 10.1667/RR2900.1. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 41.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63. [Google Scholar]
- 42.Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–51. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 43.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology – v.1: Fever and neutropenia: 2005; Version 1. 2005 [Google Scholar]
- 44.Farese AM, Kirschner KF, Patchen ML, Zsebo KM, MacVittie TJ. The effect of recombinant canine stem cell factor and/or recombinant canine granulocyte colony stimulating factor on marrow aplasia recovery in lethally irradiated canines. Experi Hematol. 1993;21:1169. [Google Scholar]
- 45.MacVittie TJ, Monroy RL, Farese AM, Patchen ML, Seiler FR, Williams D. Cytokine therapy in canine and primate models of radiation-induced marrow aplasia. Behring Inst Mitt. 1991;90:1–13. [PubMed] [Google Scholar]
- 46.Dalrymple GV, Lindsay IR, Ghidoni JJ. The effect of 2-Mev whole-body irradiation on primates. Radiat Res. 1965;25:377–400. [PubMed] [Google Scholar]
- 47.Eltringham JR. Recovery of the rhesus monkey from acute radiation exposure as evaluated by the split dose technique: preliminary results. Abstracts of the fifteenth annual meeting of the radiation research society, San Juan, Puerto Rico May 7-11, 1967 (Ab-4) Radiat Res. 1967;31:533. [Google Scholar]
- 48.Eldred E, Trowbridge WV. Radiation sickness in the monkey. Radiology. 1954;62:65–73. doi: 10.1148/62.1.65. [DOI] [PubMed] [Google Scholar]
- 49.Stanley RE, Seigneur LJ, Strike TA. SR66-23. Armed Forces Radiobiology Research Institute; Bethesda, MD: 1966. The acute mortality response of monkeys (Macaca mulatta) to mixed gamma-neutron radiations and 250 kVp X rays. [Google Scholar]
- 50.Wise D, Turbyfill CL. SR68-17; DTIC: AD695424. Armed Forces Radiobiology Research Institute; Bethesda (MD): 1968. The acute mortality response of monkeys (Macaca mulatta) to pulsed mixed gamma-neutron radiation. [Google Scholar]
- 51.Henschke UK, Morton JL. Mortality of Rhesus monkeys after single total body irradiation. Am J Roentgenol. 1957;77(5):889–909. [PubMed] [Google Scholar]
- 52.Haigh MV, Paterson E. Effects of a single session of whole body irradiation in the rhesus monkey. Br J Radiol. 1956;29:148–7. doi: 10.1259/0007-1285-29-339-148. [DOI] [PubMed] [Google Scholar]
- 53.Schlumberger HG, Vazquez JJ. Pathology of total body irradiation in the monkey. Am J Pathol. 1954;30:1013–47. [PMC free article] [PubMed] [Google Scholar]
- 54.Dalrymple GV, Lindsay IR, Ghidoni JJ. The effect of 2-Mev whole-body x-irradiation on primates. Radiat Res. 1965;25:377–400. [PubMed] [Google Scholar]
- 55.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Int Med. 1966;64:328–40. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 56.Hughes WT, Armstrong D, Bodey GP, Feld R, Mandell GL, Meyers JD, et al. Guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. J Infect Dis. 1990;161:381–96. doi: 10.1093/infdis/161.3.381. [DOI] [PubMed] [Google Scholar]
- 57.Hughes WT. Use of antimicrobial agents for treatment of infection in the neutropenic immunocompromised patient. In: Ricks R, Berger ME, O'Hara FM Jr, editors. The medical basis for radiation-accident preparedness The clinical care of victims. Washington, DC: The arthenon Publishing Group; 2002. pp. 117–29. [Google Scholar]
- 58.Schimpff SC. Infections in radiation accidents. In: Browne D, Weiss JF, MacVittie TJ, Pillai MV, editors. Treatment of radiation injuries. New York: Plenum Press; 1990. pp. 75–85. [Google Scholar]
- 59.Gafter-Gvili A, Fraser A, Mical P, Leibovici L. Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Int Med. 2005;142(12):979–95. doi: 10.7326/0003-4819-142-12_part_1-200506210-00008. [DOI] [PubMed] [Google Scholar]
- 60.Timmer-Bonte JN, de Boo TM, Smit HJ, Biesma B, Wilschut FA, Cheragwandi SA, et al. Prevention of chemotherapy-induced febrile neutropenia by prophylatic antibiotics plus or minus granulocyte colony-stimulating factor in small cell lung cancer: a Dutch Randomized Phase III Study. J Clin Oncol. 2005;23:7974–84. doi: 10.1200/JCO.2004.00.7955. [DOI] [PubMed] [Google Scholar]
- 61.Lord BI, Woolford LB, Molineux G. Kinetics of neutrophil production in normal and neutropenic animals during the response to filgrastim (r-metHu G-CSF) or filgrastim SD/01 (Peg-r-metHu G-CSF) Clin Cancer Res. 2001;7:2085–90. [PubMed] [Google Scholar]
- 62.Lord BI, Bronchud MH, Owens S, Chang J, Howell A, Souza L, et al. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci U S A. 1989;86:9499–503. doi: 10.1073/pnas.86.23.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engel C, Scholz M, Loeffler M. A computational model of human granulopoiesis to stimulate the hamatotoxic effects of multicycle polychemotherapy. Blood. 2004;104(8):2323–31. doi: 10.1182/blood-2004-01-0306. [DOI] [PubMed] [Google Scholar]
- 64.Mackey MC, Aprikyan AAG, Dale DC. The rate of apoptosis in post mitotic neutrophil precursors of normal and neutropenic humans. Cell Prolif. 2003;36:27–34. doi: 10.1046/j.1365-2184.2003.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–61. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]


