Abstract
Attraction of the mosquitoes Aedes aegypti and Ae. albopictus to plant infusions was evaluated by using a modified sticky-screen bioassay that improved the resolution of mosquito responses to odorants. Under bioassay conditions, solid-phase microextraction-gas chromatographic analyses of the volatile marker chemical indole showed that odorants diffused from bioassay cups, forming a concentration gradient. Infusions were prepared by separately fermenting senescent leaves of eight plant species in well water. Plant infusions were evaluated over an 8-fold range of leaf biomass and/or a 28d fermentation period. The responses of gravid females of both mosquito species varied with the plant species and biomass of plant materials used to make infusions, and with the length of the fermentation period. Infusions made from senescent bamboo (Arundinaria gigantea) and white oak (Quercus alba) leaves were significantly attractive to both mosquitoes. In general, infusions prepared by using low biomass of plant material over a 7–14d fermentation period were most attractive to Ae. aegypti. In contrast, Ae. albopictus was attracted to infusions made using a wider range of plant biomass and over a longer fermentation period. Both mosquito species were more attracted to a non-sterile white oak leaf infusion than to white oak leaf infusion that was prepared using sterilized plant material and water, thus suggesting a role for microbial activity in the production of odorants that mediate the oviposition response of gravid mosquitoes.
Keywords: Aedes aegypti, Aedes albopictus, oviposition, attractants, plant infusion, fermentation, microbe-insect interactions
Introduction
Aedes aegypti L. and Ae. albopictus (Skuse) are the principal vectors of dengue and dengue hemorrhagic fever viruses on a global basis (Gubler 2002). Immature stages of both mosquito species inhabit human-made containers placed in residential landscapes. Gravid females lay eggs in water-filled containers. The oviposition behavior of these mosquitoes is mediated by visual, olfactory, tactile, or chemo-tactile cues associated with their container habitats (Bentley and Day 1989; Millar et al. 1992; Navarro et al. 2003; Ponnusamy et al. 2008). Chemical cues that mediate oviposition behavior originate from organic infusions in containers as metabolic products of microbial origin (Benzon and Apperson 1988; Isoe and Millar 1995; Sumba et al. 2004; Sant’Ana et al. 2006; Ponnusamy et al. 2008, 2010)
A variety of plant species and plant-associated materials have been used to produce organic infusions for investigating the oviposition behavior of mosquitoes or for monitoring oviposition activity in the field. Infusions made from a variety of grasses (Reiter et al. 1991; Chadee et al. 1993; Rawlins et al. 1998; Polson et al. 2002; Sant’ Ana et al. 2006) and from oak leaves (Quercus spp.) (Szumlas et al. 1996; Trexler et al. 1998) have been used in oviposition traps (ovitraps) for monitoring the egg-laying activity of container-inhabiting Aedes mosquitoes in the field. Other organic materials, such as sod and pelletized plant-based animal feeds, have been fermented to create infusions that were attractive to gravid Aedes (Lampman and Novak 1996a; Ritchie 2001) and Culex mosquitoes (Lampman and Novak 1996b). Gravid females of other mosquitoes, such as Culex nigripalpus (Theobald) (Ritchie 1984), Cx. quinquefasciatus (Say) (Isoe et al. 1995b; Mboera et al. 2000), and Cx. tarsalis Coq. (Isoe and Millar 1995) are attracted to hay and grass infusions. Ovitraps baited with organic infusions have been used to monitor arbovirus vectors during disease outbreaks or to collect mosquitoes for arbovirus testing (Tsai et al. 1989; Savage et al. 1993; Nasci et al. 2002; Polson et al. 2002).
Notably, there are no standard methods for producing infusions. Tightly closed plastic garbage bags (Reiter et al. 1991; Chadee et al. 1993; Trexler et al. 1998), fiberglass tubs (Isoe et al. 1995a), open containers (Rawlins et al. 1998), and closed plastic containers (Sant’ Ana et al. 2006) have been used to make infusions from plant materials. Moreover, the attractiveness of infusions to gravid mosquitoes changes over the fermentation time (Isoe et al. 1995b; Sant’ Ana et al. 2006; Ponnusamy et al. 2010) and with the species and amounts of plant materials fermented (Ponnusamy et al. 2010). Maw (1970) concluded that these changes were because of variations in bacterial activity, and Reiter (1986) recommended that infusions should be prepared by a standard protocol because rapid changes in the microbial population and chemical composition could change the attractiveness of infusions with time.
There also appears to be a lack of standard methods of evaluating and interpreting the biological activity of organic infusions. Most commonly, infusions are evaluated based on the numbers of eggs laid in containers holding test and control media. However, this measure represents an endpoint of the biological activity of various cues, and it fails to separate the effects of attractants and contact chemo-stimulants on the oviposition response (Benzon and Apperson 1988; Isoe et al. 1995a).
The objectives of our research were to: (1) refine a sticky-screen bioassay used previously (Trexler et al. 1998) for measuring the attraction of Aedes mosquitoes to plant infusions; (2) compare several methods of preparing infusions that were attractive to gravid Ae. aegypti and Ae. albopictus; (3) validate that females can be guided within our 2-choice bioassay cages by an odor gradient emanating from the cup holding plant infusion; (4) evaluate effects of plant species, plant biomass, and fermentation time of infusions on the responses of gravid mosquitoes; and (5) determine the significance of microbial activity to the production of attractive infusions.
Methods and Materials
Origin and Maintenance of Mosquito Colonies
Aedes aegypti and Ae. albopictus colonies were established from eggs collected in New Orleans, LA, USA in 2003. At 6–8 mo intervals, adults were added to each mosquito colony to sustain genetic diversity. Larvae of both species were reared as described by Trexler et al. (2003). Mosquito colonies were maintained in separate insectaries at ~28°C, ~75% RH, and a photoperiod of 14:10, L:D, including two twilight periods (60 min each). Eggs for maintenance of mosquito colonies were obtained from females that were blood-fed on a human forearm. Gravid females for oviposition bioassays were produced by feeding 7–14-d-old females on a human forearm for 4–5 d prior to the setup of an experiment. The protocol for feeding was in compliance with IRB Guidelines of North Carolina State University.
Preparation of Plant Infusions
We hypothesized that production of odorants that attract gravid mosquitoes would be influenced by many factors, including the type and amount of fermented organic material, the amount of headspace available, and duration of the fermentation. Initially, the ratio of plant biomass to well water (33.6 g per 4 L = 1X infusion) described by Trexler et al. (1998) was used to make white oak leaf (WOL) infusion in black plastic trash can liners (14 μm thickness, No. 386014, Central Polybag, Corp., Linden, NJ, USA). We compared three fermentation methods in which: (1) the plastic bag was left open; (2) the plastic bag was tightly sealed with no headspace above the infusion; or (3) the bag was tightly sealed, but ballooned to create a headspace above the infusion equal to approximately 50% of the capacity of the bag. Methods 1 and 3 were intended to achieve aerobic decomposition, while method 2 was intended to accomplish facultative anaerobic fermentation of plant materials. After 1, 2, and 4 wk fermentation periods, each type of infusion was tested for attractive odorants against gravid Ae. aegypti in a sticky-screen bioassay (Trexler et al. 1998) modified as described above.
The potential chemical reactivity of the plastic trash bags was of concern. Therefore, we tested used Teflon bags (TFM Modified PTFE homopolymer inert bag, No. P-00021-2, 63.5 μm thickness, Big Science, Inc., Huntersville, NC, USA) as an alternative container for producing plant infusions. Infusions were made in tightly clamped Teflon bags, leaving a headspace equivalent to ~50% of the bag volume for the entire fermentation period. Plant infusions were prepared by using senescent leaves of bamboo (Arundinaria gigantea), white oak (Quercus alba), live oak (Quercus virginiana), pecan (Carya illinoensis), hackberry (Celtis occidentalis), red maple (Acer rubrum), redtop panicgrass (Panicum rigidulum), and harvested Bermuda grass (Cynodon dactylon) that were obtained from multiple sites in Raleigh, NC and New Orleans, LA. For each plant infusion, there were 6–8 replicate bioassays per trial with three trials completed on different dates.
Optimization of Sticky-screen Attractant Bioassay
In preliminary experiments, the original and a modification of the sticky-screen bioassay method of Trexler et al. (1998) were evaluated using white oak leaf (WOL) (Quercus alba) infusion (Trexler et al. 1998) and gravid Ae. aegypti and Ae. albopictus. The original method involved placing a screen coated with insect glue (Tanglefoot®, Tangle Foot Co., Grand Rapids, MI, USA) directly on top of the bioassay cup (120 ml, polypropylene, No.13–711–57, Fisher Scientific) that was spray-painted flat black on the exterior. Gravid females attracted to volatiles and attempting to enter the test cup were trapped on the sticky-screen. However, some females were routinely found free in the cage, indicating that the capture efficiency of this design needed improvement. Therefore, we modified the sticky-screen method by placing the screen approximately 4.2 cm below the cup rim on top of a clear polycarbonate ring (4.2 cm diam. x 3.2 cm high) that was cut from a fluorescent tube lamp guard (No. 1743564, Copper Lighting, Peachtree City, GA, USA); this ring held the sticky-screen just above the surface of the water. In each bioassay, two black cups (test and control) were placed randomly in diagonal corners of a reach-in cage (30 × 30 × 30 cm, Lucite® CP acrylic, Lucite International, Inc.) fitted with a stockinette sleeve (No. 081620, Albahealth LLC, Rockwood, TN, USA). Cages were placed on shelves in a room where environmental condition were the same as in the insectary. Ten gravid females were transferred into each cage, and after a 24 or 48 h bioassay, females trapped on each sticky screen were counted. For the original or modified bioassay, there were 6 replicate cages per trial with three to four trials completed on different dates.
Reach-in Cage Bioassay of Plant Infusions
The modified sticky screen bioassay described above proved to be superior to the conventional method for evaluating plant infusions in reach-in cages. Consequently, the modified method was used to bioassay plant infusions. In setting up a bioassay, the contents of the Teflon bag were mixed vigorously prior to removing infusion. The 1X plant infusions were diluted to 50% with well water before the test cup was filled (30 ml) and well water (30 ml) was placed in the control cup. The 1X Bermuda hay infusion was evaluated undiluted (100% infusion) and diluted to 50% and 10% with well water.
Evaluation of Odorant Diffusion Patterns
To examine diffusion patterns of odorants in bioassay cages, indole was used as a marker chemical to quantify volatiles in and around a bioassay cage. Specifically, we were interested in measuring the concentrations of indole inside the bioassay cage at various distances from the test cup and above the cage at two time points: immediately after setting up the bioassay (0 h) and 24 h later (24 h). Two cups, a test cup, and a control cup were placed diagonally at the corners inside a standard bioassay cage. The test cup contained 30 ml of distilled water to which 90 μl of a 10 μg/μl indole (CAS No. 120–72–9, Sigma Aldrich Co) solution in dichloromethane were added. The control cup contained 30 ml of distilled water. The headspace was sampled simultaneously at various positions inside and outside the bioassay cage with solid phase microextraction (SPME) (DVB/CAR/PDMS, Supelco) for 10 min. Prior to placement, SPME fibers were conditioned at 250°C for 20 min in a gas chromatograph (GC) inlet (Agilent) purged with helium. Volatiles were captured at five positions inside the bioassay cage (at the surface of the sticky screen inside the test cup, in the middle at the lip of the test cup, 10 cm above the test cup, in the middle of the cage, and at the surface of the sticky screen inside the control cup) and at one position outside the cage, one cm above the center of the cage. These experiments were replicated three times.
Indole captured on SPME fibers at both time points (0 h and 24 h) was analyzed by using an Agilent 7890A GC with a flame ionization detector (FID), equipped with a DB-5 (30 m x 0.25 mm × 0.25 μm) column (J&W Scientific) and a 2-m deactivated guard column (Alltech) using helium as a carrier gas. The injector and the detector were kept at 260°C and 280°C, respectively. The oven was held at 45°C for one min, then heated to 180°C at 10°C/min, and finally heated at 20°C/min to 280°C and held at the final temperature for 5 min. Samples were injected splitless with an inlet purge time of 0.75 min. Quantification of samples was based on external calibration using a dilution series of indole in the range of 0.1–100 ng of compound injected in the GC.
Comparison of Sterile and Non-sterile Infusions
We evaluated the attraction of gravid females to non-sterile and sterile WOL infusions based on the hypothesis that non-sterile infusions would be significantly more attractive to gravid mosquitoes. In behavioral assays, we compared two-week old WOL (0.5X) infusion (4.2 g leaves/l water) that was produced using sterilized oak leaves and well water (autoclaved for 45 min at 120°C) against unsterilized infusion (unsterilized leaves and well water). After autoclaving, well water (1 L) and WOL (4.2 g) were combined in sterile glass jars (2 L) fitted with threaded plastic lids. Similarly, non-sterile infusion was produced in sterile jars but with leaves and water that were not sterilized. Jars were held at 28°C for 2 wk before infusions were tested in modified sticky-screen bioassays. Test cups filled with sterile WOL infusion (30 ml, 50% dilution) were evaluated against control cups containing non-sterile WOL infusion (30 ml, 50% dilution). After a 48-h bioassay, females trapped on screens in test and control cups in each cage were counted separately.
Walk-in Cage Bioassay of Plant Infusions
Based on results from small cage experiments, one-wk-old bamboo leaf (BL) (1X) and 2-wk-old WOL (0.5X) infusions were evaluated against gravid Ae. aegypti and Ae. albopictus in a two-choice sticky screen bioassay in a walk-in cage (4 × 4 × 2 m height) (Lumite, Inc., Gainesville, GA, USA) that was set up indoors using a wooden frame. Environmental conditions in the walk-in cage were variable, with mean daily temperature and humidity ranging from 24–27°C and 66–83%, respectively. Humidity was provided by a household humidifier (Cool-Moisture Humidifier, No. 564B, Kaz Inc., Southborough, MA, USA). A light regimen of 14:10, L:D was used with the photophase provided by 4 twin bulb (35 W) fluorescent fixtures placed at each corner of the cage. At the beginning and end of the dark period, partial light (1 h) was provided by an incandescent bulb (25 W) placed in the center of the cage. To eliminate external light that penetrated into the cage, it was covered with white cotton bed sheets, which were then covered with black plastic sheeting.
Each infusion was evaluated four times on separate dates. With each replicate trial, the positions of treatment and control containers were exchanged. Number 10 tin cans (17 cm high x 15 cm diam., 4 L nominal) painted black inside and out with Rustoleum® were used as bioassay containers. Infusion was added to one can, and the same volume of well water was added to the control can. The volume of infusion was 0.5 L or 1.23 L, either undiluted 1x infusion (100% infusion) or 1X infusion diluted with an equal volume of well water (50% infusion). Sticky screens (15 cm diam.) then were placed on top of clear polypropylene cups (7.5 cm high x 6 cm diam.) inside each can approximately 15 cm from the lip. At approximately noon, the test and a control container were placed one m apart in the middle of the cage, and 30 gravid mosquitoes were released in the cage. After 24 h, the bioassay was terminated and females trapped on each screen were counted.
Statistical Analyses
The Oviposition Activity Index (OAI), described by Kramer and Mulla (1979), was used to evaluate the response of gravid mosquitoes to plant infusions. The OAI standardizes the data by converting the number of females trapped on the sticky screen in the test cup to a proportion after correcting for the number of females trapped on the screen in the control cup. The OAI ranges from −1 to +1, with 0 indicating neutral response. Within each experiment, an OAI was calculated for each replicate as follows:
where Nt is the number of females trapped on the screen in the test container and Nc is the number of females trapped on the screen in the control container. The SAS procedure PROC UNIVARIATE (SAS for Windows ver. 9.1, SAS Institute, Cary, NC, USA) was used to generate an approximate t statistic which was used to test the hypothesis that the mean OAI for each treatment is significantly greater (attraction) or less (repulsion) than 0 at α ≤ 0.05.
Results
Optimized Sticky-screen Bioassay
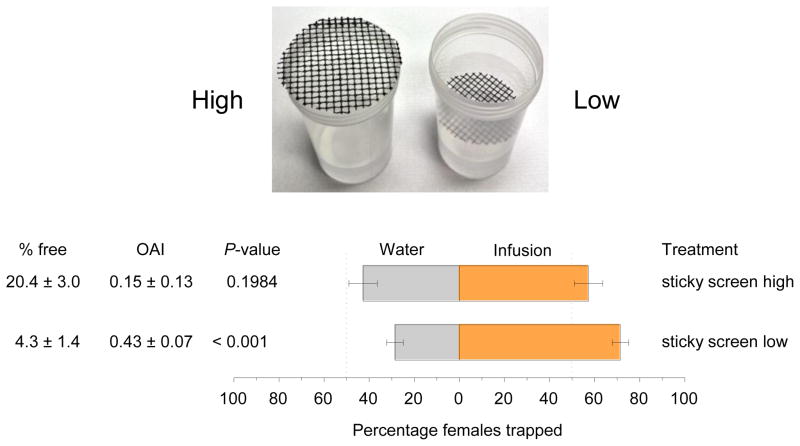
Modifying the sticky-screen bioassay by placing the glue-coated screen inside the cup increased the number of gravid females trapped in the test cup containing WOL infusion (Fig. 1). Additionally, fewer free females remained in cages in which the sticky-screen was placed inside of the bioassay cup than in cages with the screen on top of the bioassay cup (Fig. 1).
Fig. 1.
High and low placement of glue-coated screens in bioassay cups used in modified and original sticky-screen bioassay, respectively. Clear cups are shown here to demonstrate the placement of the screens, but for bioassays the cups were spray-painted flat black on the exterior. Sticky-screen bioassay results for 0.5× (4.2 g leaves/l water) white oak leaf infusion showing higher capture of Aedes aegypti in the modified assay. There were 6 replicate cages per trial and three trials completed on different dates for both the modified and original bioassays.
Fermentation Methods for Plant Infusions
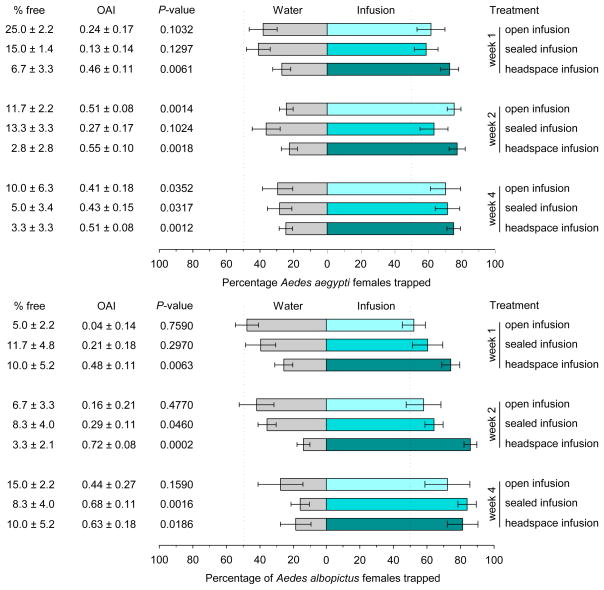
Generally, 7-d-old WOL infusions were less attractive to gravid Aedes than 14- and 28-d-old infusions (Fig. 2). For both mosquito species, infusions prepared in closed bags with a headspace above the infusion elicited consistently higher responses from gravid females than infusions prepared in open bags or closed bags without a headspace (Fig. 2).
Fig. 2.
Evaluation of fermentation procedures and infusion age on attraction of gravid Aedes aegypti and Ae. albopictus to 0.5× (4.2 g leaves/l water) white oak leaf infusion. There were 6 replicate bioassay cages per infusion age within each fermentation procedure.
Evaluation of Odorant Diffusion Patterns
Under “still air” bioassay conditions, the amount of indole detected using 10 min of headspace sampling with SPME was highest at the position nearest to its source and declined toward the center of the cage as well as upward (Table 1). Only trace amounts of indole were detected in the center of the cage, in the control cup, and above the cage. The pattern was the same at the two time points indicating that the bioassay arrangement maintained an odor gradient for at least 24 h
Table 1.
Amount of indole detected in 10-min SPME collections inside and above the bioassay cagea
| Amount of indole (ng)
|
||||
|---|---|---|---|---|
| Sampling position | 0 h | 24 h | ||
|
|
||||
| Meanb | SE | Meanb | SE | |
| Test cup, at the screen | 1.27 | 0.67 | 0.91 | 0.26 |
| Test cup, at cup lip | 0.43 | 0.16 | 0.49 | 0.14 |
| Test cup, 10 cm above | 0.12 | 0.01 | 0.20 | 0.07 |
| Cage center | LODc | LOD | ||
| Control cup, at the screen | LOD | LOD | ||
| Outside the cage, 1 cm above in the center | LOD | LOD | ||
The test cup contained 30 μg/ml of indole. Headspace samples were collected immediately after setting up the experiment (0 h) and 24 h later. Samples were analyzed using GC-FID. Quantification was based on external calibration.
n = 3.
Amounts below the limit of detection (0.10 ng) are indicated by ‘LOD’
Response to Plant Infusions: White Oak
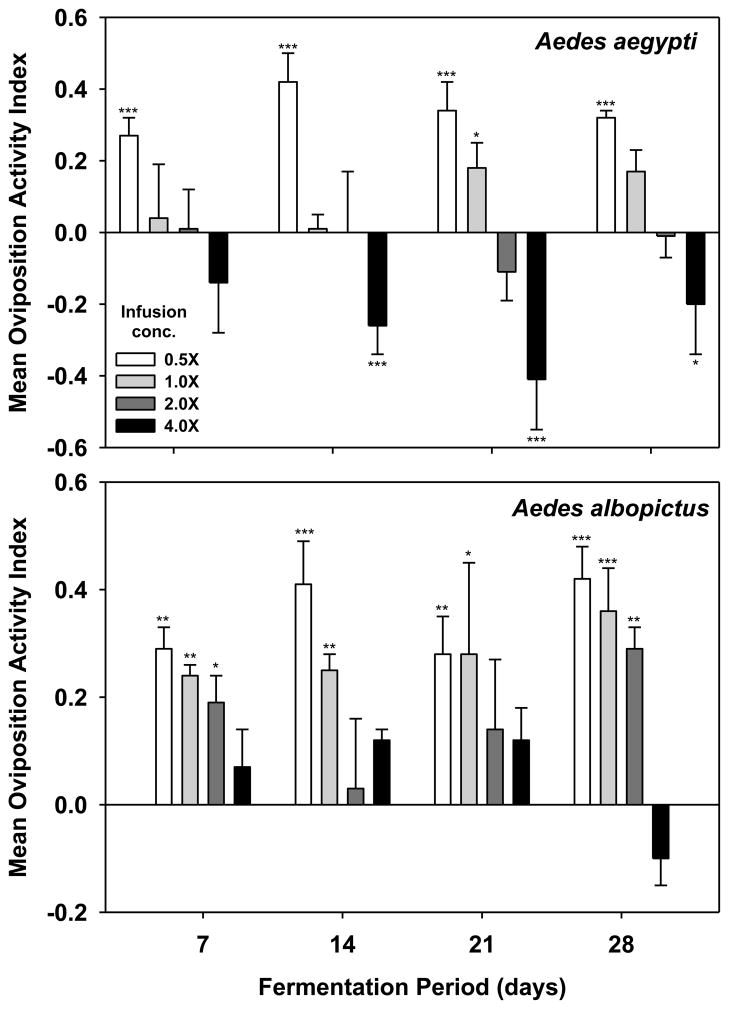
In general, the number of gravid Ae. aegypti and Ae. albopictus caught on the sticky screen decreased as leaf biomass increased, but this effect was more pronounced for Ae. aegypti (Fig. 3). Gravid Ae. aegypti were attracted to low biomass infusions (0.5× = 4.2 g leaves/L well water) across the entire 28-d fermentation period. The highest OAI (0.42) was achieved with 0.5X biomass infusions after 14 days of fermentation. In contrast, with the exception of the first week, Ae. aegypti females were repelled by high biomass (4X) infusions over the 28 day fermentation period (Fig. 3).
Fig. 3.
Effects of leaf biomass and fermentation duration on attraction of gravid mosquitoes to white oak leaf infusions. For each infusion age, there were 6 replicate cages per trial and three trials completed on different dates. Student s t-test of the hypothesis that mean OAI for each treatment is significantly greater (attraction) or less (repulsion) than 0. ***P ≤ 0.001, **0.01 ≤ P > 0.001, *0.05 ≤ P > 0.01. 1X infusion = 8.4 g leaves per L water
In comparison, Ae. albopictus was attracted to WOL infusions over a broader range of leaf biomass and fermentation times (Fig. 3). The highest mean OAI for Ae. albopictus was achieved using 0.5X biomass infusions after 14 and 28 days of fermentation, but the 1X biomass infusions also elicited responses from Ae. albopictus across 28 days of fermentation. Aedes albopictus was not significantly attracted to 4X biomass infusion (Fig. 3).
Bamboo
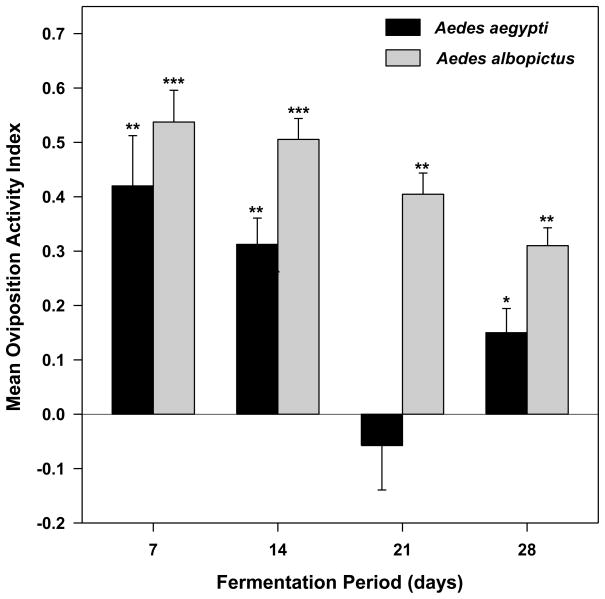
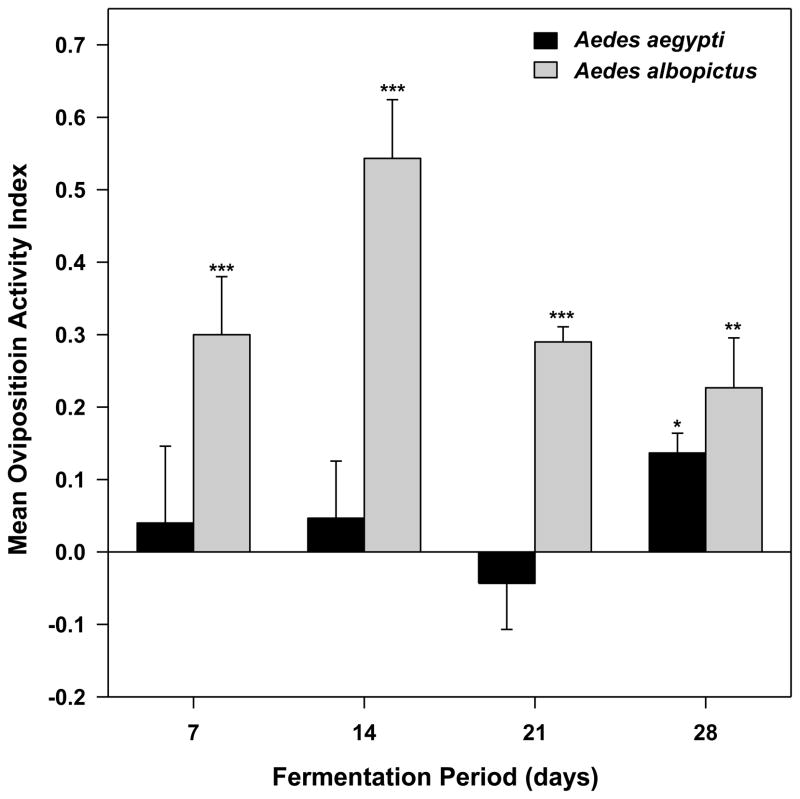
The largest response from both mosquito species to 1X biomass infusions was to bamboo infusion that had been fermented for 7 days (Fig. 4). Attraction of Ae. aegypti decreased with longer fermentations. The responses of gravid Ae. aegypti were significantly greater than 0 after 7, 14, or 28 days of fermentation; however, the OAI was not significantly different from 0 after 21 days of fermentation (P = 0.288). Aedes albopictus females were attracted to 1X biomass infusion at each bioassay interval over the 28-d time course of fermentation (Fig. 4).
Fig. 4.
Effects of infusion age on attraction of gravid Aedes mosquitoes to 1X bamboo leaf infusion. For each infusion age, there were 6 replicate cages per trial and four trials completed on different dates. Student s t-test of the hypothesis that mean OAI for each treatment is significantly greater (attraction) or less (repulsion) than 0. ***P ≤ 0.001, **0.01 ≤ P > 0.001, *0.05 ≤ P > 0.01. 1X infusion = 8.4 g leaves per L water
Hackberry
Aedes aegypti was attracted to a 1X biomass infusion made from hackberry tree leaves only at the 28 day time point (Fig. 5). For other fermentation time points, mean OAI values were < 0.15 and not significantly different from zero (P > 0.05). In contrast, Ae. albopictus responded to 1X biomass infusion at each bioassay interval in the 28 day fermentation period (Fig. 5) and responded most strongly to infusion that had been fermented for 14 days (Fig. 5).
Fig. 5.
Effects of infusion age on attraction of gravid Aedes mosquitoes to 1× (8.4 g leaves/L water) hackberry leaf infusion. For each infusion age, there were 6 replicate cages per trial and three trials completed on different dates. Student s t-test of the hypothesis that mean OAI for each treatment is significantly greater (attraction) or less (repulsion) than 0. ***P ≤ 0.001, **0.01 ≤ P > 0.001, *0.05 ≤ P > 0.01.
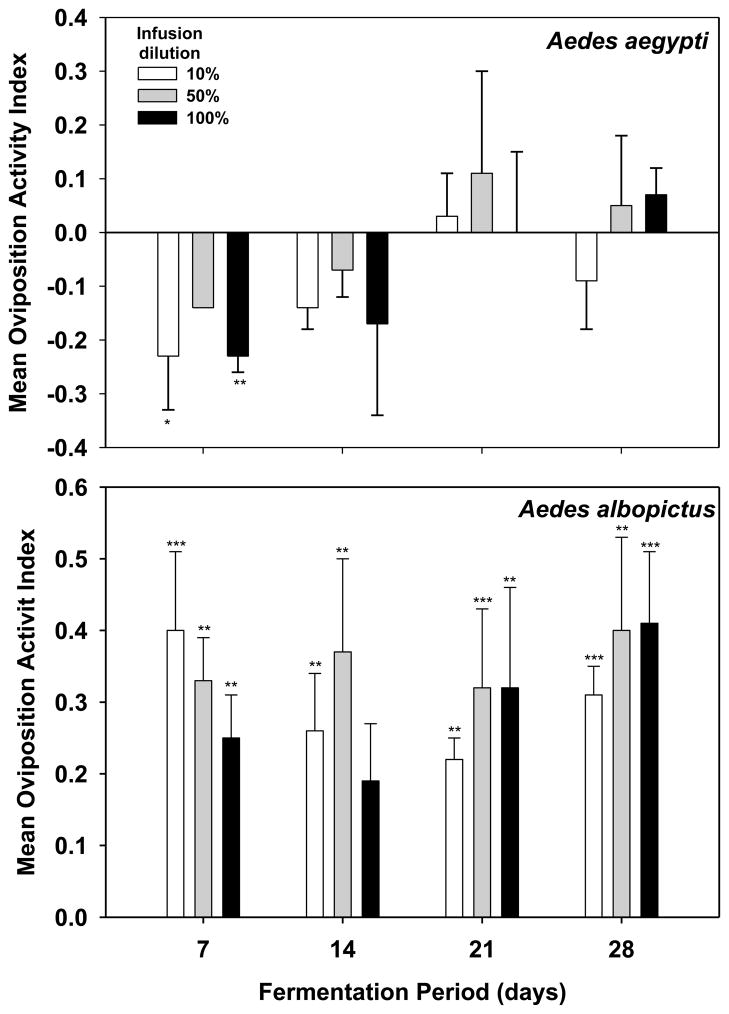
Bermuda Hay
In general, Ae. aegypti exhibited repellent or neutral responses to 1X Bermuda hay infusions and two dilutions of this infusion, regardless of the age of the infusion (Fig. 6). In contrast, Ae. albopictus was attracted to all dilutions and ages of Bermuda hay infusion, except for the undiluted infusion after 14 days of fermentation (Fig. 6).
Fig. 6.
Effects of infusion concentration and fermentation duration on attraction of gravid mosquitoes to 1X Bermuda hay infusion. Student s t-test of the hypothesis that mean OAI for each treatment is significantly greater (attraction) or less (repulsion) than 0. ***P ≤ 0.001, **0.01 ≤ P > 0.001, *0.05 ≤ P > 0.01. 1X infusion = 8.4 g leaves per L water
Live Oak, Red Maple, Pecan, and Panicgrass
A live oak infusion at 1X biomass was not attractive to Ae. aegypti or Ae. albopictus in any of the bioassays completed during the 28 day period of fermentation. For both species, mean OAI values were ≤ 0.15 and not significantly different from zero (P > 0.05). Similarly, OAI values derived from responses of both mosquito species to 1X biomass infusions prepared from red maple leaves, pecan leaves, and panicgrass were not significantly different from zero.
Responses to Non-sterile and Sterile WOL Infusions
Non-sterile WOL infusion was significantly more attractive to Ae. aegypti and Ae. albopictus than was sterile infusion (Table 2). The mean OAI for both mosquito species was highly significant, reflecting the high level of attraction of these mosquitoes to the non-sterile infusions and the possible role of microbial fermentation (Table 2).
Table 2.
Response of gravid mosquitoes to non-sterile and sterile white oak leaf infusions in sticky-screen bioassays
| Species | Mean total no. females (SE) captured per trial*
|
Mean OAI** (SE) | P ≥ |t|*** | |
|---|---|---|---|---|
| Non-sterile Infusion | Sterile Infusion | |||
| Aedes aegypti | 41.6 (1.6) | 16.3 (0.4) | 0.43 (0.05) | < 0.001 |
| Aedes albopictus | 45.6 (0.4) | 13.0 (0.4) | 0.55 (0.03) | < 0.001 |
In each of 3 experimental trials, there were 6 replicate cages, each containing 10 gravid females
OAI = Oviposition Activity Index.
Student s t test of the hypothesis that mean OAI for each treatment is significantly greater (attraction) or less (repulsion) than 0.
Walk-in Cage Boassays
Of the plant infusions that were tested, BL and WOL infusions elicited the strongest oviposition responses and were further evaluated in walk-in cage bioassays. Both mosquito species exhibited significant, albeit variable, responses to the dilutions and volumes of both plant infusions (Table 3). The responses of Ae. aegypti generally were more consistent to undiluted infusions of both plant species. Aedes albopictus was highly attracted to undiluted infusions of both plant species, but exhibited the strongest response to BL infusion (Table 3).
Table 3.
Effects of volume and dilution on attraction of gravid Aedes mosquitoes to bamboo leaf (1X) and white oak leaf (0.5X) infusions in walk-in cages using the sticky-screen bioassay procedure. Extracts were prepared as 1× (33.6 g/4 l well water) or 0.5X (8.4 g/l well water) infusions, and were used either at 100% or after dilution with an equal volume of well water (50%).
| Infusion | Volume (ml) | Dilution (%) | Mean Oviposition Activity Index (± SE)* | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Ae. aegypti | P > |t|** | Ae. albopictus | P > |t|** | |||
|
|
||||||
| Bamboo leaf | 500 | 50 | −0.12 (0.13) | 0.4287 | 0.44 (0.09) | 0.0159 |
| 100 | 0.34 (0.01) | < 0.001 | 0.72 (0.13) | 0.0125 | ||
| 1230 | 50 | 0.36 (0.09) | 0.0310 | 0.63 (0.14) | 0.0193 | |
| 100 | 0.35 (0.11) | 0.0557 | 0.50 (0.13) | 0.0305 | ||
| White oak leaf | 500 | 50 | 0.47 (0.08) | 0.0116 | 0.08 (0.11) | 0.5486 |
| 100 | 0.32 (0.13) | 0.0869 | 0.56 (0.04) | < 0.001 | ||
| 1230 | 50 | 0.22 (0.04) | 0.0125 | 0.16 (0.06) | 0.0718 | |
| 100 | 0.41 (0.10) | 0.0276 | 0.32 (0.15) | 0.1151 | ||
OAI (Oviposition Activity Index) is the mean of 4 trials.
Student s t test of the hypothesis that mean OAI for each treatment is significantly greater (attraction) or less (repulsion) than 0.
Discussion
Our investigation is the first comprehensive analysis of methods used to produce and optimize organic infusions as potential of oviposition attractants for Ae. aegypti and Ae. albopictus. With indole as a marker odorant, we determined that volatiles from test containers placed inside bioassay cages would provide females with differential cues to guide them to attractive infusions. In contrast to our present research with WOL infusion, Trexler et al. (1998) reported that WOL infusions mediated oviposition of Ae. triseriatus (Say) and Ae. albopictus through contact with nonvolatile arrestants or oviposition stimulants rather than through odorants. However, we found that lowering the sticky screen deeper into the bioassay cup significantly increased the ability of females to differentiate between treatments, presumably by creating more headspace or through a yet unknown interaction of olfactory and visual cues at close-range as the female enters the infusion container.
The importance of microbial metabolic activity in breaking down organic matter and producing semiochemicals that mediate the oviposition responses was suggested by the lack of significant attraction of Ae. aegypti and Ae. albopictus to a sterile WOL infusion. However, it should be noted that the leaves used to construct the sterile WOL infusion were autoclaved. It is possible that high temperature would have affected the chemistry of the resulting infusion, altering the response of gravid mosquitoes by chemical changes rather than biological changes. Recently, however, we showed that microbe-associated contact chemical cues in the infusion elicited Ae. aegypti females to oviposit more when microbes were present than when they were filtered out (Ponnusamy et al. 2008). Similarly, Culex laid fewer eggs in response to Bermuda grass infusion that had been filtered to remove microorganisms (Isoe and Millar 1995).
The importance of microbial metabolism is also suggested by our finding that infusions prepared in closed Teflon bags with some headspace were more attractive to gravid mosquitoes than infusions produced in open bags or closed bags without headspace. In closed bags with headspace, microbial breakdown of organic materials likely occurred through facultative anaerobic fermentation. In contrast, Sant’ Ana and coworkers (2006) found that fermentation of Panicum maximum grass in a sealed plastic container produced infusions that were more active in eliciting an oviposition response from Aedes (Stegomyia) mosquitoes than infusions prepared in an open containers.
Even under uniform fermentation conditions, there were large batch-to-batch variations in the response of gravid mosquitoes to some infusions. We attribute this variation to differences in the quality and amounts of attractive odorants released from infusions. Since we used senescent plant materials that were collected from the ground to make infusions, it is likely there were differences in the leaf condition as well as differences in the species composition of the microbial community associated with plant materials. Significant variation in the abundance and species composition of microbial populations is known to occur on the surfaces of leaves from the same trees (Brunel et al. 1994; Yang et al. 2001; Lambais et al. 2006). Such differences would be expected to result in variations in microbial populations in our experimental plant infusions and in corresponding differences in the amounts and kinds of odorants that were produced. Notably, infusions made from live oak, red maple, pecan, and panicgrass leaves were not attractive to Ae. aegypti or Ae. albopictus. These results reinforce the critical importance of the plant species used to produce infusions. The implication of our findings is that plant infusions can be an inconsistent source of attractive odorants, and that each batch of infusion should be evaluated to verify that it is behaviorally active before it is used in the field. Ideally development of a mosquito monitoring or surveillance system would involve controlled release of specific chemically defined oviposition cues.
In sticky-screen bioassays in reach-in cages, infusions were differentially attractive to each mosquito species depending on the plant species, biomass used and length of fermentation period. In general, Ae. aegypti exhibited higher attraction to low biomass infusions produced over short fermentation times from just a few plant species. In contrast, Ae. albopictus was attracted to infusions that were produced using a broader range of plants species, biomass, and fermentation periods. For example, Ae. aegypti was either repelled or exhibited a neutral response to infusions made from Bermuda hay, while Ae. albopictus was significantly attracted to Bermuda hay infusions regardless of the age of the infusion. Walk-in bioassay cage results were concordant with results of reach-in cages. In walk-in cage bioassays, both mosquito species were significantly attracted to WOL and BL infusions. Generally, Ae. aegypti was attracted to diluted and low volume infusions. Surprisingly, Ae. albopictus was attracted to 1X WOL infusion over a narrower range of infusion volumes compared to Ae. aegypti.
In previous investigations, infusions made by fermenting a variety of grass and hay species have been reported to be active towards gravid Ae. aegypti in laboratory and field bioassays (Reiter et al. 1991; Chadee et al. 1993; Rawlins et al. 1998; Polson et al. 2002; Sant’ Ana et al. 2006). In these investigations, the response of mosquitoes was evaluated by using an end point; the numbers of eggs deposited in test containers holding infusions compared to control containers holding water. Higher numbers of eggs deposited in test containers was interpreted to indicate that more gravid mosquitoes were attracted to the test infusion. With an olfactometer, Hazard et al. (1967) showed that gravid Ae. aegypti were not attracted to any of the volatile chemicals released from arrested females at the surface of the water, and stimulated them to oviposit. Similar negative results of olfactometer experiments involving hay infusion were reported by Allan and Kline (1995) for gravid Ae. aegypti. Isoe et al. (1995a) and Isoe and Millar (1995), in bioassays of gravid Culex mosquitoes, cautioned against using the numbers of eggs deposited as an endpoint for assessing attraction to plant infusions. They emphasized that differentiating oviposition responses mediated by olfactory attractants from nonvolatile oviposition stimulants cannot be made simply by counting eggs. Thus, the increased oviposition previously reported for Ae. aegypti in response to hay and grass infusions likely resulted from nonvolatile chemicals that arrested gravid females at the surface of the water and stimulated them to lay eggs, rather than from odorants that attracted gravid females from a distance. The intent of our study was to evaluate olfactory-mediated responses of gravid females to volatiles from plant infusions. It should be noted that attraction of gravid females to odorants that emanate from a plant infusion might not result in increased oviposition because volatile chemicals that attract females may not necessarily function also as oviposition stimulants. However, some species of cultivable bacteria in bamboo leaf infusion produce metabolites that attract gravid females and also stimulate them to lay eggs. With bioassay-guided fractionation of bacterial extracts, carboxylic acids and methyl esters associated with 14 species of bacteria cultured from bamboo leaf infusion were shown to be potent oviposition stimulants (Ponnusamy et al. 2008). A mix of these same 14 species of bacteria produces odorants that are highly attractive to gravid Ae. aegypti (Ponnusamy et al. 2010).
Optimally attractive infusions for Ae. aegypti and Ae. albopictus required fermentation periods of different lengths depending on the plant species. Similar results were reported by Isoe et al. (1995b) for response of Culex quinquefasciatus and Cx. tarsalis to Bermuda grass infusion. Culex quinquefasciatus continued to respond significantly to Bermuda grass infusions that were fermented over a longer period of time compared to Cx. tarsalis, which preferred infusions that were fermented over a shorter period. Sant’ Ana et al. (2006) found that Aedes (Stegomyia) mosquitoes exhibited the highest oviposition responses to 15–20-d-old infusions produced from the grass Panicum maximum. On average, ovitraps containing younger or older infusions received lower numbers of eggs. Ponnusamy et al. (2010) showed that the attraction of gravid Ae. aegypti and Ae. albopictus to WOL and BL infusions was significantly associated with changes in the abundance and diversity of bacterial species, which in turn was affected by plant species, leaf biomass and fermentation time.
Currently, we are characterizing the attraction and egg-laying response of gravid Ae. aegypti and Ae. albopictus to individual bacterial species cultured from BL and WOL infusions and characterizing semiochemicals that mediate attraction and oviposition. An understanding of the bioactivity of bacterial species may explain why Ae. aegypti and Ae. albopictus are differentially attracted to the two types of infusions. Pickett et al. (2010) emphasized the importance of investigating the chemical ecology of disease vectors with the aim of developing efficient tools for surveillance and control. In this regard, the optimized infusions, specific bioactive microbes isolated from them, and specific compounds that attract and stimulate females to oviposit will be used in mosquito and virus surveillance, mass trapping, and other intervention approaches to reduce the human burden of Aedes-transmitted arboviral diseases.
Acknowledgments
Support for our research was provided by the NIH, NIAID through cooperative agreement U01-AI-58303-03 and the Blanton J. Whitmire Endowment at North Carolina State University.
Contributor Information
Loganathan Ponnusamy, Department of Entomology North Carolina State University Raleigh, NC 27695, USA.
Ning Xu, Department of Entomology North Carolina State University Raleigh, NC 27695, USA.
Katalin Böröczky, Department of Entomology North Carolina State University Raleigh, NC 27695, USA.
Dawn M. Wesson, Department of Tropical Medicine Tulane University New Orleans, LA 70112, USA
Luma Abu Ayyash, Department of Entomology North Carolina State University Raleigh, NC 27695, USA.
Coby Schal, Department of Entomology North Carolina State University Raleigh, NC 27695, USA.
Charles S. Apperson, Department of Entomology North Carolina State University Raleigh, NC 27695, USA.
References
- ALLAN SA, KLINE DL. Evaluation of organic infusions and synthetic compounds mediating oviposition in Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J Chem Ecol. 1995;21:1847–1860. doi: 10.1007/BF02033681. [DOI] [PubMed] [Google Scholar]
- BENTLEY MD, DAY JF. Chemical ecology and behavioral aspects of mosquito oviposition. Annu Rev Entomol. 1989;34:401–421. doi: 10.1146/annurev.en.34.010189.002153. [DOI] [PubMed] [Google Scholar]
- BENZON GL, APPERSON CS. Reexamination of chemically mediated oviposition behavior in Aedes aegypti (L.) (Diptera: Culicidae) J Med Entomol. 1988;25:158–164. doi: 10.1093/jmedent/25.3.158. [DOI] [PubMed] [Google Scholar]
- BRUNEL B, PERISSOL C, FERNANDEZ M, BOEUFGRAS JM, LE PETIT J. Occurrence of Bacillus species on evergreen oak leaves. FEMS Microbiol Ecol. 1994;14:331–342. [Google Scholar]
- CHADEE DD, LAKKAN A, RAMDATH WR, PERSAD RC. Oviposition response of Aedes aegypti mosquitoes to different concentrations of hay infusion in Trinidad, West Indies. J Am Mosq Control Assoc. 1993;9:346–348. [PubMed] [Google Scholar]
- GUBLER DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- HAZARD EI, MAYER MS, SAVAGE KE. Attraction and oviposition stimulation of gravid female mosquitoes by bacteria isolated from hay infusion. Mosq News. 1967;27:133–136. [Google Scholar]
- ISOE J, MILLAR JG. Characterization of factors mediating oviposition site choice by Culex tarsalis. J Am Mosq Control Assoc. 1995;11:21–28. [PubMed] [Google Scholar]
- ISOE J, MILLAR JG, BEEHLER JW. Bioassays for Culex (Diptera: Culicidae) mosquito oviposition attractants and stimulants. J Med Entomol. 1995a;32:475–483. doi: 10.1093/jmedent/32.4.475. [DOI] [PubMed] [Google Scholar]
- ISOE J, MILLAR JG, BEEHLER JW, MULLA MS. Oviposition responses of Culex tarsalis and Culex quinquefasciatus to aged Bermuda grass infusions. J Am Mosq Control Assoc. 1995b;11:39–44. [PubMed] [Google Scholar]
- KRAMER WL, MULLA MS. Oviposition attractants and repellents of mosquitoes: oviposition responses of Culex mosquitoes to organic infusions. Environ Entomol. 1979;8:1111–1117. [Google Scholar]
- LAMBAIS MR, CROWLEY DE, CURY JC, BULL RC, RODRIGUES RR. Bacterial diversity in tree canopies of the Atlantic forest. Science. 2006;312:1917. doi: 10.1126/science.1124696. [DOI] [PubMed] [Google Scholar]
- LAMPMAN RL, NOVAK RJ. Ovipositional preferences of Culex pipiens and Culex restuans in Illinois. J Am Mosq Control Assoc. 1996a;12:23–32. [PubMed] [Google Scholar]
- LAMPMAN RL, NOVAK RJ. Attraction of Aedes albopictus adults to sod infusion. J Am Mosq Control Assoc. 1996b;12:119–124. [PubMed] [Google Scholar]
- MAW MG. Capric acid as a larvicide and an oviposition stimulant for mosquitoes. Nature (Lond) 1970;227:1154–1155. doi: 10.1038/2271154a0. [DOI] [PubMed] [Google Scholar]
- MBOERA LEG, TAKKEN W, MDIRA KY, PICKETT JA. Sampling gravid Culex quinquefasciatus (Diptera: Culicidae) in Tanzania with traps baited with synthetic oviposition pheromone and grass infusions. J Med Entomol. 2000;37:172–176. doi: 10.1603/0022-2585-37.1.172. [DOI] [PubMed] [Google Scholar]
- MILLAR JG, CHANEY JD, MULLA MS. Identification of oviposition attractants for Culex quinquefasciatus from fermented Bermuda grass infusions. J Am Mosq Control Assoc. 1992;8:11–17. [PubMed] [Google Scholar]
- NASCI RS, KOMAR N, MARFIN AA, LUDWIG GV, KRAMER LD, DANIELS TJ, FALCO RC, CAMPBELL SR, BROOKES K, GOTTFRIED KL, BURKHALTER KL, ASPEN SE, KERST AJ, LANCIOTTI RS, MOORE CG. Detection of West Nile virus-infected mosquitoes and seropositive juvenile birds in the vicinity of virus-positive dead birds. Am J Trop Med Hyg. 2002;67:492–496. doi: 10.4269/ajtmh.2002.67.492. [DOI] [PubMed] [Google Scholar]
- NAVARRO DMAF, DE OLIVEIRA PES, POTTING RPJ, BRITO AC, FITAL SJF, GOULART SANT’ ANA AE. The potential attractant or repellent effects of different water types on oviposition in Aedes aegypti L. (Diptera: Culicidae) J Appl Entomol. 2003;127:46–50. [Google Scholar]
- PICKETT JA, BIRKETT MA, DEWHIRST SY, LOGAN JG, OMOLO MO, TORTO B, PELLETIER J, ZAINULABEUDDIN S, LEAL WS. Chemical ecology of animal and human pathogen vectors in a changing global climate. J Chem Ecol. 2010;36:113–121. doi: 10.1007/s10886-010-9739-9. [DOI] [PubMed] [Google Scholar]
- POLSON KA, CURTIS C, SENG CM, OLSON JG, CHANTHA M, RAWLINS SC. The use of ovitraps baited with hay infusion for surveillance of Aedes aegypti in Cambodia. Dengue Bull. 2002;26:178–184. [Google Scholar]
- PONNUSAMY L, WESSON DM, ARELLANO C, SCHAL C, APPERSON CS. Species composition of bacterial communities influences attraction of mosquitoes to experimental plant infusions. Microb Ecol. 2010;59:158–173. doi: 10.1007/s00248-009-9565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONNUSAMY L, XU N, NOJIMA S, WESSON DM, SCHAL C, APPERSON CS. Identification of bacteria and bacteria-associated chemical cues that mediate oviposition site preferences by Aedes aegypti. Proc Natl Acad Sci USA. 2008;105:9262–9267. doi: 10.1073/pnas.0802505105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAWLINS SC, MARTINEZ R, WILTSHIRE S, LEGALL G. A comparison of surveillance systems for the dengue vector Aedes aegypti in Port of Spain, Trinidad. J Am Mosq Control Assoc. 1998;14:131–136. [PubMed] [Google Scholar]
- REITER P. A standardized procedure for the quantitative surveillance of certain Culex mosquitoes by egg raft collection. J Am Mosq Control Assoc. 1986;2:219–221. [PubMed] [Google Scholar]
- REITER P, AMADOR MA, COLON N. Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J Am Mosq Control Assoc. 1991;7:52–55. [PubMed] [Google Scholar]
- RITCHIE SA. Hay infusion and isopropyl alcohol baited CDC light trap: a simple effective trap for gravid Culex mosquitoes. Mosq News. 1984;44:404–407. [Google Scholar]
- RITCHIE SA. Effect of some animal feeds and oviposition substrates on Aedes oviposition in ovitraps in Cairns, Australia. J Am Mosq Control Assoc. 2001;17:206–208. [PubMed] [Google Scholar]
- SANT’ ANA AL, ROQUE RA, EIRAS AE. Characteristics of grass Infusions as oviposition attractants to Aedes (Stegomyia) (Diptera: Culicidae) J Med Entomol. 2006;43:214–220. doi: 10.1603/0022-2585(2006)043[0214:cogiao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- SAVAGE HM, SMITH GC, MOORE CG, MITCHELL CJ, TOWNSEND M, MARFIN AA. Entomological investigations of an epidemic of St. Louis encephalitis in Pine Bluff, Arkansas, 1991. Am J Trop Med Hyg. 1993;49:38–45. doi: 10.4269/ajtmh.1993.49.38. [DOI] [PubMed] [Google Scholar]
- SUMBA LA, GUDA TO, DENG AL, HASSANALI A, BEIER JC, KNOLS BGJ. Mediation of oviposition site selection in the African malaria mosquito Anopheles gambiae (Diptera: Culicidae) by semiochemicals of microbial origin. Int J Trop Insect Sci. 2004;24:260–265. [Google Scholar]
- SZUMLAS DE, APPERSON CS, POWELL EE. Seasonal occurrence and abundance of Aedes triseriatus and other mosquitoes in a La Crosse virus-endemic area of western North Carolina. J Am Mosq Control Assoc. 1996;12:184–193. [PubMed] [Google Scholar]
- TSAI TF, SMITH GC, HAPP CM, KIRK LJ, JAKOB WL, BOLIN RA, FRANCY DB, LAMPERT KJ. Surveillance of St. Louis encephalitis virus vectors in Grand Junction, Colorado in 1987. J Am Mosq Contr Assoc. 1989;5:161–165. [PubMed] [Google Scholar]
- TREXLER JD, APPERSON CS, SCHAL C. Laboratory and field evaluations of oviposition responses of Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) to oak leaf infusions. J Med Entomol. 1998;35:967–976. doi: 10.1093/jmedent/35.6.967. [DOI] [PubMed] [Google Scholar]
- TREXLER JD, APPERSON CS, ZUREK L, GEMENO C, SCHAL C, KAUFMAN M, WALKER E, WATSON DW, WALLACE L. Role of bacteria in mediating the oviposition responses of Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2003;40:841–848. doi: 10.1603/0022-2585-40.6.841. [DOI] [PubMed] [Google Scholar]
- YANG CH, CROWLEY DE, BORNEMAN J, KEEN NT. Microbial phyllosphere populations are more complex than previously realized. Proc Natl Acad Sci USA. 2001;98:3889–3894. doi: 10.1073/pnas.051633898. [DOI] [PMC free article] [PubMed] [Google Scholar]