Abstract
It is unknown whether racial differences exist in adjuvant chemotherapy initiation among women with similar oncotype DX (ODX) risk scores. We examined whether adjuvant chemotherapy initiation varied by race. Data come from the Phase III, Carolina Breast Cancer Study, a longitudinal, population-based study of North Carolina women diagnosed with breast cancer between 2008 and 2014. We used modified Poisson regression and report adjusted relative risk (aRR) and 95 % confidence intervals (95 %CI) to estimate the association between race and adjuvant chemotherapy initiation across ODX risk groups among women who received the test (n = 541). Among women who underwent ODX testing, 54.2, 37.5, and 8.3 % of women had tumors classified as low-, intermediate-, and high-risk groups, respectively. We observed no racial variation in adjuvant chemotherapy initiation. Increasing ODX risk score (aRR = 1.39, 95 %CI = 1.22, 1.58) and being married (aRR = 2.92, 95 %CI = 1.12, 7.60) were independently associated with an increased likelihood of adjuvant chemotherapy in the low-risk group. Among women in the intermediate-risk group, ODX risk score (aRR = 1.15, 95 %CI = 1.11, 1.20), younger age (aRR = 1.95, 95 %CI = 1.35, 2.81), larger tumor size (aRR = 1.70, 95 %CI = 1.22, 2.35), and higher income were independently associated with increased likelihood of adjuvant chemotherapy initiation. No racial differences were found in adjuvant chemotherapy initiation among women receiving ODX testing. As treatment decision-making becomes increasingly targeted with the use of genetic technologies, these results provide evidence that test results may drive treatment in a similar way across racial subgroups.
Keywords: Oncology, Breast cancer, Cancer, Disparities, Gene expression profiling
Background
Black women are less likely to develop, but more likely to die from, breast cancer than non-Black women [1]. This disparity likely arises from a complex array of biological, societal, and health system factors, including access to high-quality cancer care [2]. For example, Black women with breast cancer are less likely to receive guideline-concordant cancer treatment, with mixed evidence about adjuvant chemotherapy [3, 4] and endocrine therapy initiation and adherence [5]. While the vast majority of women with node-negative, hormone receptor-positive breast cancer are recommended to take endocrine therapy to reduce 10-year risk of recurrence, only about 15 % of these women are thought to reap added benefit from adjuvant chemotherapy in addition to endocrine therapy [6].
In 2004, Oncotype DX (ODX) (Genomic Health, Redwood City, CA) became commercially available as a tool for identifying which women with early-stage, estrogen receptor-positive (ER+) breast cancer are likely to benefit from adjuvant chemotherapy. ODX is a 21-tumor gene expression profiling panel that not only predicts 10-year distant recurrence, but also estimates the benefit of adjuvant chemotherapy [6, 7]. The test categorizes women as being at “low,” “intermediate,” or “high” risk of recurrence; low-risk women are predicted to derive no significant benefit from adjuvant chemotherapy, while high-risk women appear to have improved recurrence-free survival if they receive adjuvant chemotherapy in addition to endocrine therapy. Thus current guidelines recommend that women with low-ODX risk scores forgo adjuvant chemotherapy and that women with high-risk scores have adjuvant chemotherapy [8]. Within the intermediate group, there is less certainty regarding the benefit of adjuvant chemotherapy in terms of 10-year distant recurrence; instead adjuvant chemotherapy decision-making can be tailored to individual patients.
ODX has the potential to decrease racial disparities in treatment—especially among women in the low- and high-risk groups—because it provides an evidence-based tool to guide decisions. Alternatively, ODX testing could exacerbate treatment disparities if minority women have lower access to the test (and therefore fewer opportunities for informed decisions). To date, little is known about adjuvant chemotherapy decision-making in the presence of ODX risk information in a population-based study. The only published study to examine this question did not find racial disparities in the use of adjuvant chemotherapy among women with ODX; however findings may not be generalizable, as it was conducted within three urban, academic-affiliated hospitals [9].
Using a large, registry-based prospective cohort study, we examined racial disparities in adjuvant chemotherapy initiation among women receiving ODX. We described racial differences in ODX risk scores and elucidated whether adjuvant chemotherapy initiation varies by race within (1) the low- and high-risk groups, with clear guidelines for adjuvant chemotherapy use, and (2) the intermediate group, where the treatment decision in response to ODX results is less clear.
Methods
Data source
We used Carolina Breast Cancer Study, Phase III (CBCS-III) data (NCI-P50-CA58223). CBCS-III is a prospective cohort study of 2998 women with invasive breast cancer across 44 counties in North Carolina (NC). In collaboration with the NC Cancer Registry, women were enrolled using rapid case ascertainment. Notably, CBCS-III oversamples young and Black women, making it ideally suited for examining racial differences in breast cancer care. Between 2008 and 2013, patients were randomly sampled from four strata: Black women <50 years, Black women ≥50 years, non-Black women <50 years, and non-Black women ≥50 years [10]. For this study, we used data from baseline surveys, medical record abstractions, and pathology reports.
Sample
Inclusion criteria for this study included women with a single breast tumor that was ER+, stage I–II, HER2 negative, and who had ODX test results; we excluded women with undetermined tumor grade or missing data on tumor size, PR status, employment status or family income (Appendix 1 in ESM). Our final sample included 186 Black and 355 non-Black women.
Measures
Dependent variable
Our primary outcome was adjuvant chemotherapy initiation, defined as initiation following the primary surgery as determined from medical records.
Independent variable
Race was self-reported. We dichotomized race as non-Black (93.5 % White, 2.3 % Asian, and 4.2 % other) or Black, irrespective of ethnicity.
Covariates
Covariates included clinical (comorbidities, age at diagnosis), tumor (size, grade, ODX risk score, node status), treatment (lumpectomy/mastectomy), and socioeconomic characteristics. Age at diagnosis was dichotomized (<50 versus ≥50 years). We calculated the number of comorbidities recorded in patient medical records. We considered five comorbidities that are clinically likely to affect adjuvant chemotherapy decision-making: heart disease, hypertension, obesity, diabetes, and chronic obstructive pulmonary disease. Tumor and treatment characteristics were abstracted from the pathology and medical reports, respectively. Socioeconomic variables included marital status (married, living as married/other), education (less than high school/high school/college and more), current employment since diagnosis (yes/no), annual family income (<$15,000/$15,000–30,000/$30,000–50,000/>$50,000), and insurance type (insured/uninsured).
Analyses
Descriptive analyses used weighted linear regression for continuous variables and weighted Chi square tests for categorical variables. We compared the distribution of ODX risk scores in the sample overall and by race. We also graphed the kernel density of ODX risk scores by race. We compared sample characteristics by race and adjuvant chemotherapy initiation. Per our data-use agreement, data were not reported for cell sizes with <5 observations. Our primary analysis employed a modified Poisson regression to examine the association between race and adjuvant chemotherapy initiation. Modified Poisson regression estimates relative risk consistently and efficiently with binary outcomes, using sandwich standard errors [11, 12]. We addressed complex survey design through sample weights and design effects using Taylor series approximations. We accounted for clustered standard errors at the provider-level, and we conducted a complete case analysis (missing data, n = 37).
We used the Institute of Medicine (IOM) definition of health disparity to guide covariate inclusion. Specifically, the IOM model for disparities implies that race is a social construct [13, 14]. Thus, we did not include socioeconomic variables (marital status, education, current employment, family income, and insurance type) in our primary model, which measured the reduced form effect of race on adjuvant chemotherapy use. To examine the residual direct effect of race on adjuvant chemotherapy use, we also estimated a secondary model, including socioeconomic covariates.
A priori, we specified that we would stratify analyses by ODX risk category: low (risk score<18), intermediate (risk score 18–30), high (risk score >30), because the evidence-based guidelines for adjuvant chemotherapy differ across ODX risk groups. Because so few women in the sample were categorized with high-ODX scores, we lacked sufficient power to examine multivariate relationships between race and adjuvant chemotherapy initiation. Thus, we only present unadjusted racial differences in this group. Finally, we present six models including unadjusted, primary, and secondary models within low- and intermediate-risk group strata. Analyses were conducted using STATA (Stata-Corps, College Station, TX, USA). This research was approved by the University of North Carolina Institutional Review Board.
Results
Racial differences in ODX risk score
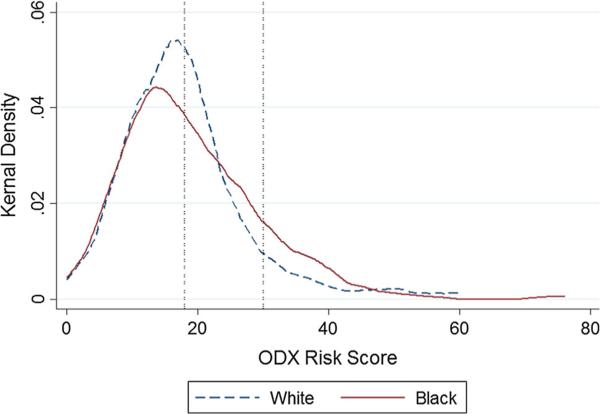
Overall, 54.2 % of women were in the low-, 37.5 % in the intermediate-, and 8.3 % in the high-risk groups (Table 1). There were no racial differences in the proportion of non-Black compared to Black women in the low-, intermediate-, and high-ODX risk groups (Table 1). Within ODX risk groups, mean ODX risk scores were similar among non-Black and Black women in the high- (41.6, 39.6, p = 0.85) and low-risk groups (11.2, 11.3, p = 0.42); however, Black women had a somewhat higher mean risk score within the intermediate-ODX risk group than non-Black women (23.5, 22.3, p = 0.04) (Table 1; Fig. 1).
Table 1.
Patient characteristics by race and ODX risk group
| n (weighted proportion) | ODX risk group |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (standard deviation) or % |
|||||||||
| Low (54.2 %) |
Intermediate (37.5 %) |
High (8.3 %) |
|||||||
| Non-Black 195 (54.8 %) | Black 93 (49.8 %) | p † | Non-Black 131 (37.4 %) | Black 69 (37.9 %) | p † | Non-Black 29 (7.7 %) | Black 24 (12.3 %) | p † | |
| Tumor characteristics | |||||||||
| ODX recurrence score | 11.2 (3.9) | 11.3 (6.6) | 0.85 | 22.3 (3.2) | 23.5 (5.9) | 0.04 | 41.6 (7.2) | 39.6 (13.1) | 0.42 |
| AJCC Stage 1 (v. 2) | 68.3 | 66.5 | 0.79 | 62.5 | 67.8 | 0.47 | 77.9 | 51.6 | 0.08 |
| Tumor size category | 0.64 | 0.60 | 0.16 | ||||||
| <2 cm | 75.3 | 72.3 | 70.8 | 74.1 | 77.9 | 57.0 | |||
| 2+ cm | 24.7 | 27.7 | 29.2 | 25.9 | 22.1 | 43.0 | |||
| Combined grade | 0.03 | 0.39 | 0.29 | ||||||
| 1 | 42.3 | 26.3 | 23.1 | 32.2 | * | * | |||
| 2 | 53.2 | 65.2 | 57.8 | 54.2 | * | 25.8 | |||
| 3 | 4.5 | 8.5 | 19.1 | 13.6 | 79.9 | 68.8 | |||
| PR positive | 96.7 | 97.3 | 0.79 | 89.9 | 72.7 | 0.003 | 67.8 | 55.9 | 0.44 |
| Treatment characteristics | |||||||||
| Chemo initiation | 5.9 | 7.2 | 0.69 | 46.2 | 42.7 | 0.66 | 76.5 | 94.6 | 0.11 |
| ET initiation | 92.4 | 93.1 | 0.84 | 97 | 94.4 | 0.41 | 92.6 | 84.9 | 0.42 |
| Radiation initiation | 67.4 | 70.2 | 0.68 | 66.9 | 70.6 | 0.62 | 57 | 59.1 | 0.89 |
| Lumpectomy (v. mastectomy) | 66.3 | 66.5 | 0.98 | 63.5 | 71.7 | 0.27 | 57 | 62.4 | 0.74 |
| Clinical characteristics | |||||||||
| Age at diagnosis | 57.4 (8.7) | 54.4 (15.6) | 0.002 | 56.4 (8.7) | 55.8 (15.7) | 0.66 | 57.7 (9.8) | 55.4 (17.3) | 0.22 |
| #Comorbidities | 0.7 (0.8) | 1.1 (1.5) | <0.001 | 0.5 (0.6) | 1.0 (1.4) | <0.001 | 0.7 (1.0) | 1.4 (1.2) | 0.02 |
| Diabetes | 7.4 | 20.7 | 0.003 | 7.7 | 20.3 | 0.01 | * | 40.9 | 0.01 |
| COPD | * | * | 0.62 | * | * | 0.56 | * | * | 0.59 |
| Obesity | 14 | 21.3 | 0.13 | 8.7 | 18.2 | 0.1 | * | 31.2 | 0.07 |
| Heart disease | 4.8 | * | 0.19 | * | * | 0.3 | * | * | >0.99 |
| Hypertension | 39.8 | 64.9 | <0.001 | 31.1 | 59.4 | <0.001 | 43.6 | 66.7 | 0.08 |
| Socioeconomic factors | |||||||||
| Annual family income | <0.001 | <0.001 | 0.02 | ||||||
| <$15K | 7.6 | 18.9 | 4.4 | 17.5 | * | * | |||
| $15-30K | 12.2 | 24.7 | 6.5 | 30.1 | 23.5 | 32.3 | |||
| $30-50K | 18.1 | 23.4 | 16.3 | 26.9 | 19.5 | * | |||
| >$50K | 62.0 | 33.0 | 72.8 | 25.5 | 57 | 37.6 | |||
| Insurance | |||||||||
| Private | 80.7 | 69.3 | 0.05 | 85.7 | 66 | 0.01 | 67.8 | 77.6 | 0.47 |
| Medicaid | 5.6 | 19.3 | <0.001 | 4 | 19.3 | <0.001 | * | 22.4 | 0.14 |
| Medicare | 32.5 | 24.4 | 0.19 | 31 | 28.2 | 0.73 | 43 | 42.4 | 0.97 |
| Uninsured | * | 10.6 | <0.001 | 3 | 9.4 | 0.07 | * | * | 0.01 |
| Married | 69.5 | 46.8 | <0.001 | 82.2 | 43.7 | <0.001 | 68.5 | 31.2 | 0.02 |
| Employed | 49.8 | 51.3 | 0.81 | 53.7 | 43.7 | 0.19 | 41.6 | 40.9 | 0.96 |
| Education | 0.008 | 0.1 | 0.81 | ||||||
| HS & Post-HS | 47.8 | 48.1 | 46.9 | 59.8 | 51.7 | 48.4 | |||
| College+ | 49.4 | 39.6 | 47.9 | 31.1 | 37.6 | 34.4 | |||
| <HS | 2.8 | 12.2 | 5.3 | 9.1 | * | * | |||
| Year of diagnosis | |||||||||
| 0.16 | 0.30 | 0.80 | |||||||
| 2008 | 6.1 | 13.6 | 15.4 | 9.1 | 23.5 | * | |||
| 2009 | 19.2 | 20.2 | 20.3 | 17.5 | * | * | |||
| 2010 | 27.3 | 17.8 | 20.1 | 24.8 | 27.5 | 22.6 | |||
| 2011 | 25.2 | 28.5 | 20.7 | 31.5 | 22.1 | 37.6 | |||
| 2012/2013 | 22.2 | 19.9 | 23.5 | 17.1 | * | * | |||
Data not reported for cell sizes <5
p value for weighted χ2 = 0.23. Because of small cell size, we combined women who were diagnosed in 2013 (n = 8) with 2012, and women who had tumor size >5 cm (n = 5) with 2-5 cm
Fig. 1.
Distribution of ODX risk scores by race, with reference lines indicating cut off points (at 18 and 30 scores) for the low-, intermediate-, and high-risk groups [6]
Within the low- and high-risk groups, tumor characteristics by race were similar; except in the low-risk group where Black women were more likely to have higher tumor grade than non-Black women (Table 1). In the intermediate-risk group, Black women had higher ODX scores and were more likely to have progesterone receptor-negative breast cancer than non-Black women. Treatment characteristics were similar between Black and non-Black women within all ODX risk groups. Comorbidities, especially diabetes and hypertension, were higher in Black than non-Black women across risk groups. Age at diagnosis was similar across racial groups in all risk groups; however Black women were slightly younger at diagnosis than non-Black in the low-risk group. Socioeconomic characteristics were lower among Black women than non-Black women regardless of risk group (Table 1).
In bivariate analyses, women who initiated chemotherapy had higher ODX risk scores, larger tumors, higher tumor grade, younger age at diagnosis, less heart disease and COPD, and less likely to have Medicare (Table 2). Among those with low-risk ODX scores, only 6.1 % received adjuvant chemotherapy. Conversely, among women with high-risk scores, 80.1 % initiated chemotherapy, given evidence-based recommendations to do so. In the intermediate-risk group, (45.7 %) of patients started chemotherapy (Table 2).
Table 2.
Sample characteristics by adjuvant chemotherapy initiation
| n | Mean (SD) or % |
||
|---|---|---|---|
| No. chemotherapy 373 | Chemotherapy 168 | p | |
| Tumor characteristics | |||
| ODX recurrence score | 14.4 (6.6) | 27.6 (11.4) | <0.001 |
| ODX risk group | |||
| Low | 93.9 | 6.1 | <0.001 |
| Intermediate | 54.3 | 45.7 | |
| High | 19.9 | 80.1 | |
| <2 cm tumor size (vs. 2 cm+) | 76.9 | 64.2 | 0.01 |
| Combined grade | <0.001 | ||
| 1 | 36.8 | 18.3 | |
| 2 | 55.2 | 44 | |
| 3 | 8 | 37.7 | |
| PR positive | 92.3 | 86.4 | 0.06 |
| Treatment characteristics | |||
| ET initiation | 93.1 | 96.1 | 0.25 |
| Radiation initiation | 68.4 | 62.4 | 0.24 |
| Lumpectomy | 65 | 68 | 0.03 |
| Clinical characteristics | |||
| Age at diagnosis | 58.0 (9.4) | 53.6 (11.3) | <0.001 |
| # Comorbidities | 0.7 (0.9) | 0.6 (0.9) | 0.40 |
| Diabetes | 9.4 | 10.3 | 0.78 |
| COPD | 2.5 | a | 0.12 |
| Obesity | 12.7 | 13.2 | 0.87 |
| Heart disease | 4.5 | a | 0.001 |
| Hypertension | 40.9 | 38.9 | 0.7 |
| Socioeconomic characteristics | |||
| Annual family income | 0.98 | ||
| <15K | 7.6 | 7.2 | |
| 15-30K | 13.3 | 13.0 | |
| 30-50K | 18.8 | 17.4 | |
| >50K | 60.4 | 62.4 | |
| Insurance | |||
| Private | 79.2 | 82 | 0.55 |
| Medicaid | 6.2 | 8.5 | 0.41 |
| Medicare | 35.2 | 23.9 | 0.05 |
| Uninsured | 2.6 | 4.0 | 0.41 |
| Married | 69.8 | 70.7 | 0.85 |
| Employed | 49.7 | 51.3 | 0.78 |
| Education | 0.93 | ||
| HS & Post-HS | 48.5 | 48.3 | |
| College+ | 46.5 | 45.7 | |
| <HS | 5.1 | 6.0 | |
| Year of diagnosis | |||
| Year | 0.07 | ||
| 2008 | 8.5 | 18.1 | |
| 2009 | 19.8 | 17.1 | |
| 2010 | 25.4 | 20.7 | |
| 2011 | 23.4 | 26.8 | |
| 2012/2013 | 23.0 | 17.2 | |
Data not reported for cell sizes <5. We combined women who were diagnosed in 2013 (n = 8) with 2012, and women who had tumor size >5cm (n = 5) with 2-5 cm
Racial differences in adjuvant chemotherapy initiation in risk groups: higher treatment certainty in guidelines
Low-risk group
No association between race and adjuvant chemotherapy uptake was observed within the low-risk group (Table 3; Appendix 2 in ESM). In this group, holding other factors constant, higher ODX scores were associated with an increased likelihood of chemotherapy initiation in both the primary and secondary models (primary aRR 1.35, 95 %CI = 1.17–1.55, p < 0.001; secondary aRR 1.39, 95 %CI = 1.22–1.58, p < 0.001). In the secondary model, being married was also independently associated with an increased likelihood of adjuvant chemotherapy uptake (secondary aRR 2.92, 95 %CI = 1.12–7.60, p < 0.028). Finally, having high- versus low-grade tumors (secondary aRR 3.57, 95 %CI = 1.08–11.76, p = 0.037) and having larger tumor size (secondary aRR 3.45, 95 %CI = 1.28–9.29, p = 0.014) were each independently associated with an increased likelihood of adjuvant chemotherapy initiation in the secondary model.
Table 3.
Adjusted risk ratios for the association between race and adjuvant chemotherapy initiation in the low- and intermediate-ODX risk groups
| Low risk group (N=285) |
Intermediate ODX risk group (N=199) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary model |
Secondary model |
Primary model |
Secondary model |
|||||||||
| aRR | 95 %CI | p > \t\ | aRR | 95 %CI | p > \t\ | aRR | 95 %CI | p > \t\ | aRR | 95 % CI | p > \t\ | |
| Black | 1.26 | 0.50-3.21 | 0.63 | 1.37 | 0.60-3.12 | 0.29 | 0.88 | 0.64-1.22 | 0.45 | 0.92 | 0.62-1.36 | 0.67 |
| ODX risk score | 1.35 | 1.17-1.55 | <0.001 | 1.39 | 1.22-1.58 | <0.001 | 1.15 | 1.11-1.19 | <0.001 | 1.15 | 1.11-1.20 | <0.001 |
| Node positive | 0.81 | 0.28-2.29 | 0.68 | 0.77 | 0.20-2.96 | 0.70 | 1.22 | 0.82-1.82 | 0.31 | 1.12 | 0.76-1.64 | 0.56 |
| Tumor size >2 cm | 2.53 | 0.99-6.43 | 0.052 | 3.45 | 1.28-9.29 | 0.014 | 1.51 | 1.12-2.035 | 0.007 | 1.7 | 1.22-2.35 | 0.002 |
| Grade | ||||||||||||
| 2 | ||||||||||||
| 3 | 1.88 | 0.52-6.83 | 0.33 | 3.57 | 1.08-11.76 | 0.037 | 1.09 | 0.72-1.63 | 0.69 | 1.16 | 0.77-1.75 | 0.49 |
| Mastectomy (v. Lumpectomy) | 1.65 | 0.70-3.92 | 0.25 | 1.61 | 0.69-3.76 | 0.27 | 0.89 | 0.64-1.23 | 0.48 | 0.84 | 0.61-1.17 | 0.30 |
| #Comorbidities | 1.3 | 0.76-2.24 | 0.34 | 1.3 | 0.73-2.33 | 0.37 | 0.87 | 0.67-1.13 | 0.29 | 0.85 | 0.65-1.12 | 0.24 |
| Family income | ||||||||||||
| $15-30K | 1.29 | 0.29-5.69 | 0.74 | 0.3 | 0.12-0.77 | 0.013 | ||||||
| $30-50K | 1.14 | 0.24-5.39 | 0.87 | 0.33 | 0.15-0.75 | 0.009 | ||||||
| >50K | 0.58 | 0.11-3.17 | 0.53 | 0.43 | 0.18-1.012 | 0.053 | ||||||
| Uninsured | 0.8 | 0.17-3.64 | 0.77 | 1.23 | 0.58-2.63 | 0.59 | ||||||
| Married | 2.92 | 1.12-7.60 | 0.028 | 1.22 | 0.78-1.93 | 0.38 | ||||||
| Employed | 0.68 | 0.20-2.26 | 0.53 | 1.31 | 0.94-1.83 | 0.108 | ||||||
| Education | ||||||||||||
| College+ | 2.86 | 0.92-8.9 | 0.07 | 0.84 | 0.59-1.18 | 0.31 | ||||||
| <HS | 0.7 | 0.059-8.32 | 0.78 | 0.74 | 0.26-2.13 | 0.57 | ||||||
| Year | ||||||||||||
| 2009 | 0.71 | 0.14-3.47 | 0.67 | 0.77 | 0.15-4.01 | 0.76 | 0.85 | 0.48-1.51 | 0.58 | 0.91 | 0.49-1.68 | 0.76 |
| 2010 | 1.7 | 0.41-7.17 | 0.47 | 1.78 | 0.36-8.81 | 0.481 | 0.72 | 0.36-1.45 | 0.35 | 0.76 | 0.36-1.58 | 0.45 |
| 2011 | 0.57 | 0.12-2.68 | 0.48 | 0.36 | 0.069-1.87 | 0.22 | 0.99 | 0.61-1.62 | 0.98 | 1.013 | 0.61-1.70 | 0.96 |
| 2012/2013* | 0.38 | 0.060-2.41 | 0.30 | 0.34 | 0.044-2.58 | 0.29 | 0.56 | 0.33-0.97 | 0.037 | 0.54 | 0.30-0.96 | 0.036 |
| Constant | <0.001 | <0.001-0.005 | <0.001 | <0.001 | <0.001-0.002 | <0.001 | 0.02 | 0.0054-0.068 | <0.001 | 0.031 | 0.0086-0.11 | <0.001 |
We combined women who were diagnosed in 2013 (n = 8) with 2012, and women who had tumor size >5 cm (n = 5) with 2-5 cm
High-risk group
No racial differences were found in adjuvant chemotherapy initiation among women in the high-risk group, though a non-significant increased likelihood of chemotherapy was found among Black women (unadjusted RR = 1.24, 95 % CI = 0.94–1.62, p = 0.12) (Appendix 2 in ESM). Small sample size precluded our ability to explore beyond the unadjusted model.
Racial differences in adjuvant chemotherapy initiation in the intermediate-risk group: lower treatment certainty in guidelines
No racial differences in adjuvant chemotherapy initiation were observed; however, several other factors independently associated with increased risk of chemotherapy initiation included (Table 3; Appendix 2 in ESM) higher ODX score (primary aRR = 1.15, 95 %CI = 1.11–1.19, p < 0.001; secondary aRR = 1.15, 95 %CI = 1.11–1.20, p < 0.001), younger age (primary aRR = 2.00, 95 %CI = 1.39–2.89, p < 0.001; secondary aRR = 1.95, 95 %CI = 1.35–2.81, p < 0.001), and tumor size ≥2 cm (primary aRR = 1.51, 95 %CI = 1.12–2.035, p = 0.007; secondary aRR = 1.70, 95 %CI = 1.22–2.35, p = 0.002). Being diagnosed in 2012 or 2013 was associated with lower risk of adjuvant chemotherapy initiation (primary aRR = 0.56, 95 %CI = 0.33–0.97, p < 0.037; secondary aRR = 0.54, 95 %CI = 0.30–0.96, p = 0.036). In the secondary model, the lowest income patients were independently more likely to initiate chemotherapy than patients with higher incomes.
Discussion
Overall, we found no racial disparities in adjuvant chemotherapy initiation across ODX risk groups, nor did we observe racial differences in mean ODX risk scores within the high- and low-risk groups. In the intermediate-risk group, Black women had slightly higher ODX risk scores than non-Black women. However, the magnitude of this difference was small, and likely not clinically meaningful, corroborating a previous study that reported no significant racial differences in mean ODX risk scores [9]. Unlike that study, we did not observe significant racial differences in the distribution of women into ODX risk groups [9].
In our study, only 8 % of women were classified with high-risk scores. This differs significantly from preliminary ODX validation studies, where 27 % of women with early-stage breast cancer were classified as high risk [6]. Notably, subsequent observational studies have found the proportion of high-risk scores to be similar to what we observed [9]. In a qualitative study, providers discussed being less likely to order ODX testing for more aggressive tumors, because they felt that they already had the necessary information to offer adjuvant chemotherapy [15]. This may explain why women with high-risk scores are less likely to receive ODX testing in real-world practices.
We did not find racial disparities in adjuvant chemotherapy initiation. There are several plausible explanations for this finding. First, we examined women with early-stage, ER+, HER2− breast cancer who received ODX testing. These women may already have had access to high-quality breast cancer care that would attenuate racial differences in chemotherapy initiation. Second, it is possible that ODX information mediates variation in the uptake of adjuvant chemotherapy by providing an objective tool to guide treatment decision-making. Third, the nonsignificant association between race and adjuvant chemotherapy initiation may result from small sample size, especially in multivariate models. Finally, studies of racial variation in breast cancer care have not consistently demonstrated racial disparities in adjuvant chemotherapy initiation. While some studies report underuse of adjuvant chemotherapy among minority and low-income women [3, 4, 16], others do not [17–19]. Furthermore, some studies suggest that racial disparities in adjuvant chemotherapy use occur not in initiation, but rather in delays [20–22], which was not evaluated in our study.
Despite guideline recommendations to forgo chemotherapy for low-ODX risk tumors, 6.1 % of women still initiated adjuvant chemotherapy. Within the low-risk group, higher ODX score was associated with an increased likelihood of chemotherapy initiation. This may reflect that ODX scores are being used along a continuum; if so, within risk groups, physicians may perceive some women to be at higher risk than others. In our secondary model, larger tumor size and higher tumor grade were each independently associated with increased risk of chemotherapy initiation, suggesting that such tumor characteristics play a role in adjuvant chemotherapy initiation, even in the presence of a low-ODX risk score. Finally, consistent with previous literature, being married was associated with an increased likelihood of chemotherapy initiation among women in the low-risk group, possibly due to more social support and resources to pursue additional treatments [23].
Interestingly, 19.9 % of patients in the high-risk group failed to initiate adjuvant chemotherapy despite guidelines. Small sample size limited our ability to investigate factors associated with adjuvant chemotherapy receipt in this group. Qualitative analyses suggest that women who forgo chemotherapy in this group typically do so because of patient preferences [15].
Within the intermediate group, almost half of women received chemotherapy. Race did not influence chemotherapy initiation; however, similar to the low-risk group, higher ODX score and larger tumors were associated with adjuvant chemotherapy initiation. Taken together, tumor characteristics appeared to play a major role in directing adjuvant chemotherapy decision-making within the intermediate-risk group. Younger age was also associated with higher risk of adjuvant chemotherapy initiation, which is consistent with studies demonstrating that younger women receive more aggressive treatment [15, 24]. This may be explained by better overall health, tolerability of adjuvant treatment, more aggressive tumors, or patient preferences. Interestingly, having an annual family income below $15,000 was associated with a higher likelihood of adjuvant chemotherapy initiation, potentially due to differential patient preferences or provider-level prescribing patterns. Finally, being diagnosed with breast cancer in 2012 or 2013 was associated with a lower likelihood of adjuvant chemotherapy use, perhaps indicating a change in practice patterns over time.
This study has several limitations. First, while CBCS-III is a large, longitudinal breast cancer cohort that oversamples Black women, we were underpowered to fully explore racial disparities in multivariable models, particularly in the high-risk group. Second, we were unable to account for patient preferences, which likely play a role in ordering ODX testing and adjuvant chemotherapy decision-making [25]. Third, we lacked data on organizational and provider characteristics that influence adjuvant chemotherapy use [9, 26, 27]. Moving forward, it may be possible to link organizational- and provider-level data to the CBCS-III dataset, facilitating such analyses. Finally, we conducted a complete case analysis under the assumption that data were missing at random. If this assumption is violated, then estimates may be biased. However, we did not observe significant differences by race, ODX risk score, or chemotherapy initiation between women with and without missing data.
Our study adds to the literature by investigating the uptake of adjuvant chemotherapy among women receiving ODX testing by race within a large prospective, population-based cohort study of breast cancer patients. Our findings suggest that racial disparities in adjuvant chemotherapy use do not exist among breast cancer patients receiving ODX testing. This is heartening, as more genetic technologies are incorporated into treatment decision-making. Future research should consider potential disparities at the point of access and incorporate organizational-, provider-, and patient-level data into studies that seek to understand racial variation in oncology treatment decision-making.
Supplementary Material
Acknowledgements
We thank Dr. Andrew Olshan for facilitating the use of the Carolina Breast Cancer Study, Phase III data and for supporting this work. We also thank Mary Beth Bell, the Project Director for the CBCS, and Chiu Kit Tse for her programming and data management support. This work was funded in part by the University of North Carolina, Lineberger Cancer Control Education Program (CCEP) (R25 CA57726), the University Cancer Research Fund of North Carolina and the National Cancer Institute (NCI) Specialized Program of Research Excellence (SPORE) in Breast Cancer (P50-CA58223); By Author: MCR: CCEP (R25CA57726); MW: Veterans Affairs Health Services Research and Development Senior Research Career Scientist (RCS 91-408); SBD: National Institutes of Health (NIH) Building Interdisciplinary Research Careers in Women's Health (BIRCWH) K12 Program and North Carolina Translational and Clinical Sciences Institute (UL1TR001111); MAD: Agency for Healthcare Research and Quality (AHRQ) K99 HS022189; KRH: NIH BIRCWH, 5K12HD001441-12; MAT and LAC: P50-CA58223; SBW: AHRQ Comparative Effectiveness Research Career Development Award, 1-K-12 HS019468-01 and American Cancer Society Mentored Research Scholar Award, MRSG-13-17-01-CPPB.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-015-3518-9) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflicts of interest Dr. Michaela Dinan has been consulting for Salix; this work is unrelated to the research presented in this paper. The authors declare that they have no conflict of interest.
References
- 1.Aizer AA, Chen MH, McCarthy EP, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120(10):1532–1539. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18(9):986–993. doi: 10.1634/theoncologist.2013-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23(27):6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 4.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 5.Roberts MC, Wheeler SB, Reeder-Hayes K. Racial/ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. Am J Public Health. 2015 Apr 23;:e1–e12. doi: 10.2105/AJPH.2014.302490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 7.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 8.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 9.Lund MJ, Mosunjac M, Davis KM, et al. 21-Gene recurrence scores: racial differences in testing, scores, treatment, and outcome. Cancer. 2012;118(3):788–796. doi: 10.1002/cncr.26180. [DOI] [PubMed] [Google Scholar]
- 10.McGee SA, Durham DD, Tse CK, Millikan RC. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol Biomark Prev. 2013;22(7):1227–1238. doi: 10.1158/1055-9965.EPI-12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 12.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661–670. doi: 10.1177/0962280211427759. [DOI] [PubMed] [Google Scholar]
- 13.Hebert PL, Sisk JE, Howell EA. When does a difference become a disparity? Conceptualizing racial and ethnic disparities in health. Health Aff (Millwood) 2008;27(2):374–382. doi: 10.1377/hlthaff.27.2.374. [DOI] [PubMed] [Google Scholar]
- 14.IOM . Institute of Medicine (US) committee on understanding and eliminating racial and ethnic disparities in health care. Washington, DC: 2014. [Google Scholar]
- 15.Roberts MC, Bryson A, Wheeler SB, et al. Barriers and facilitators for the use of oncotype DX use among oncologists: a qualitative study.. Academy health annual research meeting; Minneapolis. 2015. [Google Scholar]
- 16.Lund MJ, Brawley OP, Ward KC, et al. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109(3):545–557. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler SB, Carpenter WR, Peppercorn J, et al. Predictors of timing of adjuvant chemotherapy in older women with hormone receptor-negative, stages II-III breast cancer. Breast Cancer Res Treat. 2012;131(1):207–216. doi: 10.1007/s10549-011-1717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enewold L, Zhou J, McGlynn KA, et al. Racial variation in breast cancer treatment among Department of Defense beneficiaries. Cancer. 2012;118(3):812–820. doi: 10.1002/cncr.26346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du XL, Key CR, Osborne C. Community-based assessment of adjuvant hormone therapy in women with breast cancer, 1991–1997. Breast J. 2004;10(5):433–439. doi: 10.1111/j.1075-122X.2004.21357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hershman D, Weinberg M, Rosner Z, et al. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J Natl Cancer Inst. 2003;95(20):1545–1548. doi: 10.1093/jnci/djg073. [DOI] [PubMed] [Google Scholar]
- 21.Hershman DL, Wang X, McBride R, et al. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006;99(3):313–321. doi: 10.1007/s10549-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 22.Fedewa SA, Ward EM, Steward AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. J Clin Oncol. 2010;28(27):4135–4141. doi: 10.1200/JCO.2009.27.2427. [DOI] [PubMed] [Google Scholar]
- 23.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bremnes RM, Andersen K, Wist EA. Cancer patients, doctors and nurses vary in their willingness to undertake cancer chemotherapy. Eur J Cancer. 1995;31A(12):1955–1959. doi: 10.1016/0959-8049(95)00513-7. [DOI] [PubMed] [Google Scholar]
- 25.Hayman JA, Fairclough DL, Harris JR, Weeks JC. Patient preferences concerning the trade-off between the risks and benefits of routine radiation therapy after conservative surgery for early-stage breast cancer. J Clin Oncol. 1997;15(3):1252–1260. doi: 10.1200/JCO.1997.15.3.1252. [DOI] [PubMed] [Google Scholar]
- 26.Guth AA, Fineberg S, Fei K, Franco R, Bickell NA. Utilization of oncotype DX in an inner city population: race or place? Int J Breast Cancer. 2013;2013:653805. doi: 10.1155/2013/653805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30(18):2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.