Abstract
Purpose
Acquired resistance to erlotinib in patients with EGFR-mutant non-small cell lung cancer can result from aberrant activation of alternative receptor tyrosine kinases, such as the HGF-driven c-MET receptor. We sought to determine whether inhibition of AKT signaling could augment erlotinib activity and abrogate HGF-mediated resistance.
Methods
The effects of MK-2206, a selective AKT inhibitor, were evaluated in combination with erlotinib on a large panel of 13 lung cancer cell lines containing different EGFR or KRAS abnormalities. The activity of the combination was assessed using proliferation assays, flow cytometry and immunoblotting. The MEK inhibitor PD0325901 was used to determine the role of the MAP kinase pathway in erlotinib resistance.
Results
The combination of MK-2206 and erlotinib resulted in synergistic growth inhibition independent of EGFR mutation status. In cell lines where HGF blocked the anti-proliferative and cytotoxic effects of erlotinib, MK-2206 could restore cell cycle arrest, but MEK inhibition was required for erlotinib-dependent apoptosis. Both AKT and MEK inhibition contributed to cell death independent of erlotinib in the T790M-containing H1975 and the EGFR-WT cell lines tested.
Conclusions
These findings illustrate the potential advantages and challenges of combined signal transduction inhibition as a generalized strategy to circumvent acquired erlotinib resistance.
Keywords: AKT, HGF, EGFR mutations, Erlotinib, NSCLC
Introduction
The epidermal growth factor receptor (EGFR) regulates key intracellular pathways responsible for cell proliferation and survival and is frequently overexpressed in non-small cell lung cancer (NSCLC). Agents blocking the function of EGFR tyrosine kinase activity, including erlotinib, have demonstrated a modest clinical benefit after first- or second-line chemotherapy (Shepherd et al. 2005; Thatcher et al. 2005). A more robust and dramatic response has been observed following treatment with EGFR tyrosine kinase inhibitors (TKIs) in patients whose tumors possess somatic mutations in EGFR leading to constitutive activation of the receptor and its downstream pathways primarily including PI3K/AKT and MEK/ERK (Amann et al. 2005; Hirsch et al. 2006; Lynch et al. 2004; Mukohara et al. 2005). In most lung cancers with activating EGFR mutations, the induction of signaling pathways appears under the sole regulation of EGFR (Weinstein 2002).
Acquired resistance to EGFR TKIs occurs primarily through the development of secondary mutations (T790M) in EGFR or the activation of parallel downstream pathways via alternative receptor tyrosine kinases (RTKs; Bean et al. 2007; Engelman et al. 2007; Kobayashi et al. 2005; Niederst and Engelman 2013; Pao et al. 2005). For example, MET amplification or expression of the MET receptor ligand hepatocyte growth factor (HGF) has been implicated in erlotinib resistance by maintaining the signaling of the PI3K/AKT and MEK/ERK pathways (Engelman et al. 2007; Wang et al. 2012; Yano et al. 2008, 2011). Multiple studies have sought to directly target the PI3K/AKT and MEK/ERK pathways to overcome their aberrant reactivation resulting from mutational events or alternative RTK signaling in the EGFR TKI-resistant setting (Donev et al. 2011; Li et al. 2011; Sano et al. 2013; Sos et al. 2009). However, engagement of negative feedback loops may curtail the effectiveness of targeting downstream pathways. Suppression of either PI3K/AKT or MEK/ERK may induce signaling in the parallel pathway or increase upstream RTK signaling, reducing the impact of targeted therapy (Niederst and Engelman 2013). Effective combination therapies in the EGFR TKI-resistant setting would therefore be of greatest utility if they could re-establish EGFR dependency while mitigating alternative signaling salvage pathways.
MK-2206 is a highly selective first-in-class allosteric inhibitor of AKT 1/2/3 (Barnett et al. 2005). As an allosteric inhibitor, MK-2206 retains AKT in an inactive conformation, preventing membrane localization, and subsequent activation. Phase I data on MK-2206 have shown it to be well tolerated, with pharmacodynamic and pharmacokinetic data indicating substantial AKT inhibition at a dose of 60 mg QOD 22 (Molife et al. 2014; Yap et al. 2011). Early studies conducted with MK-2206 suggest that it may enhance anti-tumor activity of EGFR-targeted therapeutics including EGFR TKIs and the monoclonal antibody cetuximab (Hirai et al. 2010; Iida et al. 2013; Meng et al. 2010). Here, we describe the effects of AKT inhibition as a strategy to augment erlotinib activity and overcome HGF-mediated EGFR TKI resistance.
Materials and methods
Cell culture and reagents
The NSCLC cell lines HCC827, H358, H1666, H460, H1975, H1650, A549, H727, H1703, Calu-1, A427 and H1355 were purchased from American Type Culture Collection (Manassas, VA, USA). PC-9 cells were kindly provided by Dr. Reen Wu (University of California, Davis, CA, USA). All cell lines were maintained in RPMI supplemented with 10 % FBS (JR Scientific, Woodland, CA, USA), 1× penicillin/streptomycin/L-glutamine, and 1× MEM vitamin solution (Invitrogen, Carlsbad, CA, USA). Cell line authentication for H1975, HCC827, PC-9, H1666, and H358 was performed by the University of Arizona Genetics Core on 2/3/14 comparing the autosomal STR profiles with reference databases. EGFR and/or KRAS mutations were confirmed separately for all cell lines by DNA sequencing. The AKT inhibitor MK-2206 was provided by MERCK Inc., and erlotinib was provided by OSI Pharmaceuticals. Both agents were diluted in DMSO to a concentration of 10 mM. Hepatocyte growth factor (HGF) was purchased from Peprotech (Rocky Hill, NJ, USA) and reconstituted in 0.1 % BSA to a concentration of 10 μg/mL. The c-Met inhibitor PHA665752 and MEK inhibitor PD0325901 were purchased from Tocris Bioscience (R&D systems, Minneapolis, MN, USA), reconstituted to 100 mM in DMSO and 25 mM EtOH, respectively, and used at the listed concentrations. After reconstitution and/or dilution to stock concentrations, agents were stored at −20 °C until use.
Proliferation assay
Cell lines were plated at 1,000–5,000 cells/well in 96-well plates in the presence of media and were allowed to attach overnight prior to treatment. Plating density was determined based upon doubling time of each cell line. All samples were performed in triplicate. For single-agent and drug interaction studies, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Thiazolyl blue; Sigma-Aldritch, St. Louis, MO, USA) assays were performed to assess growth following three days of treatment. Cells were incubated with 5 mg/mL MTT for 3.5 h at 37 °C. The supernatant was removed, cells were lysed in 100 % DMSO for 15 min, and absorbance was measured at 550 nm with reference background at 690 nm on a Benchmark plus photometer (Bio-Rad, Camarillo, CA, USA). For drug interaction studies, ten of the cell lines within our panel were treated with 1:1 fixed ratios of both agents at 0.5, 2.5, and 5μM (with the exception of HCC827 where the IC50 for erlotinib is ~0.05 μM). The selected dose range for each agent was sub-IC50 for most cell lines in order to limit possible non-targeted effects observed at higher doses. Following 72 h of treatment, cells were incubated with MTT as already described. For combination studies the Cell Titer-Fluor Cell Viability Assay (Promega, Madison, WI) was performed according to manufacturer’s specifications. Fluorescence was measured at 380–400 nmEx/500Em on a Tecan Safire fluorescent microplate reader with Magellan data analysis software (Tecan, San Jose, CA, USA).
Flow cytometry
Cells (7.5 × 105 cells) were seeded into 100-mm dishes and allowed to adhere overnight. Cells were then treated for 24 h prior to trypsinization and prepared for cell cycle analysis as previously described by Chinn et al. (2011). Briefly, cells were re-suspended in 0.5 mL PBS (pH7.4) then fixed in a final concentration of 75 % ethanol and stored at −20 °C. Cells were then centrifuged, re-suspended in PBS with DNase-free RNase (Fermentas, Glen Burnie, MD, USA), and incubated at 37 °C for 45 min. Propidium iodide staining was followed. Cellular DNA content was measured by a Becton–Dickinson FACScan and CellQuest software (BD Biosciences, San Jose, CA, USA). Cell cycle analysis was performed and sub-G1 population was calculated using ModFit LT (BD Biosciences) and Flowing Software (Turku Centre for Biotechnology, Turku, Finland), respectively. Data are reported for triplicate experiments.
Immunoblotting
Cell lysates were prepared by washing once with cold PBS followed by lysis with a modified RIPA buffer containing 25 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM NaF, 1 % NP-40, 10 % glycerol, 2 mM Na3VO4, and 1X EDTA-free protease inhibitor cock-tail tablets (Roche) as previously described by Chinn et al. (2011). Protein concentration was determined using the Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL, USA) according to the manufacturer’s protocol. SDS-PAGE was performed with 10–25 μg of protein loaded for each sample. Protein was transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA) and probed overnight at 4 °C with the following primary antibodies: phospho-AKT (Ser473) (#4060), AKT (#9272), phospho-ERK1/2 (Thr202/Tyr204) (#4370), ERK1/2 (#4696), EGFR (C74B9, #2646), BIM (#2819), cleaved caspase-3 (#9661; Cell Signaling Technology, Danvers, MA, USA), phospho-EGFR (Tyr1068; #44788G; Invitrogen, Carlsbad, CA, USA), PARP-1 (#sc-8007; Santa Cruz Biotechnology, Santa Cruz, CA, USA), β-actin (#A2228; Sigma-Aldrich, St. Louis, MO, USA). Blots were then incubated for 1 h at room temperature with the HRP-conjugated secondary antibodies, anti-mouse IgG (W4021) and anti-rabbit IgG (W4011; Promega, Madison, WI, USA), and visualized by chemiluminescence with Amersham ECL (GE Healthcare, Buckinghamshire, UK).
Statistical analysis
All plots were generated using GraphPad Prism 5 version 5.03 for Windows (GraphPad Software, San Diego, CA, USA). Using median-effect analysis as originally described by Chou and Talalay, combination indexes (CI) were formally generated with CalcuSyn software (Biosoft, Cambridge, UK) to determine whether the combination treatments were synergistic (CI < 1; Chou and Talalay 1984). A 50 and 75 % effective dose (ED) was reported for triplicate experiments as these coincide with the actual dose effects observed by the assay for most cell lines.
Results
Combination treatment with MK-2206 and erlotinib synergistically inhibits cell proliferation
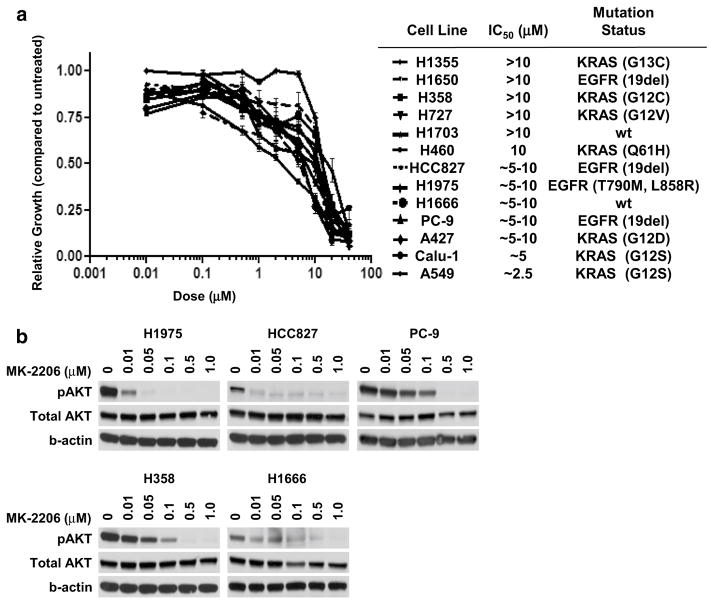
We assessed the anti-proliferative activity of MK-2206 in a panel of 13 NSCLC cell lines comprised of four EGFR-mutant cell lines (HCC827, H1975, H1650, and PC9), seven KRAS-mutant cell lines (A549, H460, Calu-1, H727, H358, A427, and H1355), and two EGFR and KRAS wild-type cell lines (H1703 and H1666). As a single-agent, treatment with MK-2206 resulted in growth inhibition for all cell lines within our panel with an IC50 ranging from 2.5–20 μM (Fig. 1b). There was no discernible difference in treatment response to single-agent MK-2206 in the three population groups examined. As shown in Fig. 1b, MK-2206 potently inhibited phospho-AKT (S473) in a dose-dependent manner achieving almost complete inhibition between 0.1 and 0.5 μM.
Fig. 1.
a Growth curves of single-agent MK-2206 in NSCLC cell lines. Cells were treated for 72 h prior to MTT. Legend is in order of sensitivity as indicated by IC50. Mutation status indicates the presence of an EGFR or KRAS mutation, with the specific substitution in parentheses; wt indicates no known abnormalities in either EGFR or KRAS. b Immunoblot of phospho-AKT (S473) following 3 h of treatment with MK-2206 at the indicated dose
To test the effects of MK-2206 on erlotinib activity, we examined how addition of MK-2206 would shift the dose curve for erlotinib within our cell line panel. Using a sub-IC50 fixed dose of MK-2206 (0.5 μM) effective in attenuating AKT phosphorylation, the combination showed greater anti-proliferative activity than single-agent erlotinib (Online Resource 1) for most of the cell lines tested. We next performed drug interaction studies on a subset of the cell lines according to methods described by Chou and Talalay (see Materials and methods). CI values were generated based upon median-effect plots of single-agent MK-2206, single-agent erlotinib, and the combination of both agents (Online Resource 2). CI < 1 is considered synergistic, >1 is antagonistic, and =1 is additive. Shown in Table 1 are the CI values measured at two different dose–effect levels: at ED50 (effective dose of 50 % response) and ED75 (effective dose of 75 % response). Of the ten cell lines examined, almost all showed a synergistic interaction between erlotinib and MK-2206 over the dose range tested, including the T790M-positive EGFR-mutant H1975 cell line, which is poorly responsive to erlotinib. These results suggest that AKT inhibition may enhance the activity of erlotinib in both EGFR-mutant and wild-type cells.
Table 1.
Combination index (CI) at the ED50 and ED75 of erlotinib plus MK-2206 for NSCLC cell lines
| Cell line | Mutation (EGFR or KRAS) | CI at ED50 | CI at ED75 |
|---|---|---|---|
| H1355 | KRAS | 0.761 | 4.242 |
| H1650 | EGFR | 2.458 | 36.260 |
| H358 | KRAS | 0.077 | 0.039 |
| H460 | KRAS | 0.432 | 1.354 |
| HCC827 | EGFR | 0.347 | 0.186 |
| H1975 | EGFR | 0.346 | 0.565 |
| H1666 | 0.161 | 0.018 | |
| A427 | KRAS | 0.490 | 0.835 |
| Calu-1 | KRAS | 0.296 | 0.163 |
| A549 | KRAS | 0.425 | 0.504 |
Listed in order of sensitivity to MK-2206 from least to greatest sensitivity. CI<1 indicates synergistic interactions between the two agents. CI curves for each cell line can be found in Online Resource 2
MK-2206 partially restores erlotinib sensitivity following treatment with HGF in EGFR wild-type and mutant NSCLC cell lines
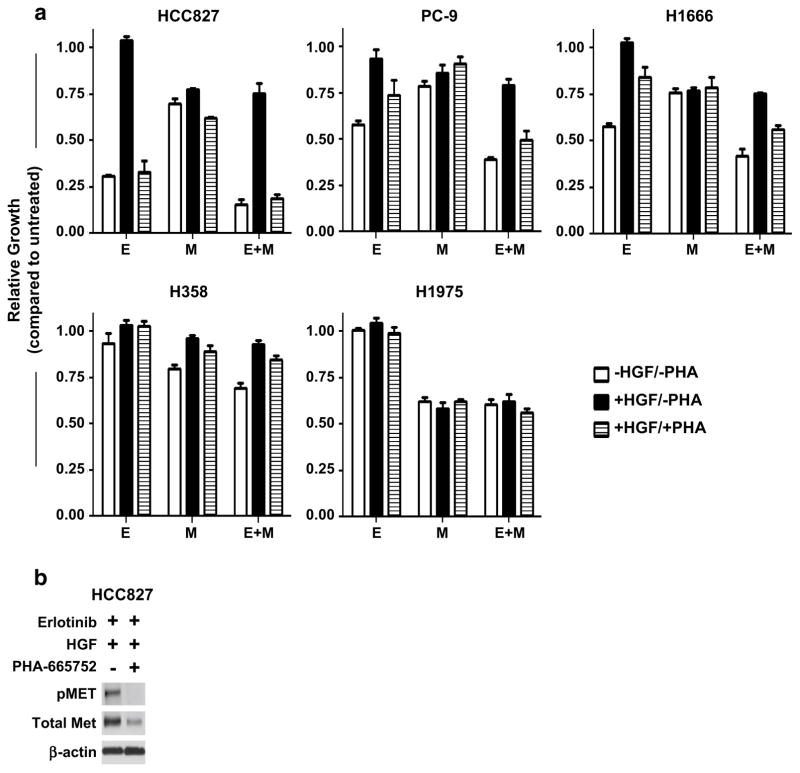
Activation of the MET receptor has been shown to bypass inhibition of EGFR in erlotinib-sensitive cell lines and patient tumors, leading to acquired resistance to EGFR TKI monotherapy. This effect has largely been associated with continued maintenance of downstream signaling pathways including PI3K/AKT. We therefore sought to determine whether direct inhibition of AKT by MK-2206 was sufficient to restore erlotinib sensitivity in cells rendered resistant due to MET activation. Of the cell lines in our panel, we selected five for continued study: two EGFR-mt (HCC827, PC-9) containing an exon 19 deletion, one KRAS-mt (H358), one EGFR/KRAS-wt (H1666), and one EGFR-mt (L858R/T790M) erlotinib-resistant cell line H1975. In order to minimize inhibiting non-specific targets, doses of each agent were limited to the dose needed to ablate EGFR phosphorylation. For the EGFR-mutant cell lines HCC827 and PC-9, we found complete inhibition of EGFR was observed by 50 nM, whereas for the EGFR-wt cell lines a ten-fold higher dose was required (data not shown). In order to develop models of resistance, cells were treated with human HGF to stimulate MET signaling in the presence of erlotinib. As shown in Fig. 2a, the HCC827, PC-9, and H1666 cell lines became insensitive to erlotinib upon addition of HGF. This effect was largely reversed by subsequent treatment with the MET inhibitor PHA-665752, at a dose that abrogated HGF-induced pMET expression (Fig. 2b). Neither H358 nor H1975 showed much sensitivity to erlotinib at this dose level (see Online Resource 3 for activity of erlotinib in H358 and H1975 using higher dosing). Single-agent treatment with PHA-665752 had no effect on cell proliferation absent both HGF and erlotinib (data not shown); this would suggest that, in our models, targeting MET outside of erlotinib resistance has little utility overall for disrupting cell growth and survival.
Fig. 2.
a Growth response of NSCLC cell lines following 72 h of treatment with erlotinib (E) at 0.05 μM (HCC827/PC-9) or 0.5 μM, MK-2206 (M) at 0.5 μM, HGF at 50 ng/mL, and/or PHA-665752 (PHA) at 0.5 μM using the CellTiter-Fluor Cell Viability Assay. Data are graphed as % growth relative to untreated cells. White columns have no added HGF or PHA; black columns are supplemented with HGF; hatched columns are supplemented with both HGF and PHA. b Immunoblot of the HCC827 cell line following 24 h of treatment with erlotinib, HGF, and/or PHA-665752 at similar dose levels
Using MK-2206 to inhibit AKT in combination with erlotinib was sufficient to partially restore erlotinib sensitivity in the resistant cell lines. Significantly enhanced growth inhibition was observed following MK-2206 co-treatment compared with erlotinib alone in HGF-stimulated cells (HCC827: 25 vs. −4 %; PC-9: 21 vs. 6 %; H1666: 25 vs. −3 %; H358: 7 vs. −3 %). This effect was similar to the level of inhibition observed for MK-2206 alone. HGF stimulation had a limited effect on MK-2206-mediated growth kinetics with the exception of the KRAS-mutant H358. Taken together, it appears that HGF-induced erlotinib resistance is not solely dependent upon the AKT pathway, and treatment with the MET inhibitor is still required to fully restore erlotinib sensitivity. However, including MK-2206 in the combination of erlotinib plus PHA-665752 was still superior to the double combination (HCC827: 81 vs. 67 %; PC-9: 51 vs. 26 %; H1666: 44 vs. 16 %; H358: 15 vs. −3 %). These results would suggest that neither EGFR nor MET inhibition is sufficient to completely alter or eliminate AKT signaling, thus providing a therapeutic window for MK-2206 to further disrupt cell growth in the combination treatments.
MK-2206 restores cytostatic but not apoptotic activity in erlotinib-treated cells under HGF-mediated resistance
To explore the anti-proliferative effects observed in our treatment combinations, we examined the cell cycling profiles of four of the cell lines by flow cytometry (a representative sample is seen in Online Resource 4). As the low dose of erlotinib (0.5 μM) had little effect on proliferation in the H358 and H1975 cell lines, we utilized the higher dose (2.5 μM) for those cell lines in cell cycling experiments. After 24 h, both erlotinib and MK-2206 induced a partial G1 arrest in the four cell lines tested, with a substantial reduction in the S-phase population, although the effect of erlotinib was still minimal in the T790 M-positive H1975 line (Online Resource 5). In three of the four cell lines (H1666, H1975, and H358), the combination of erlotinib and MK-2206 resulted in a greater G1 arrest with a concomitant reduction in S-phase and/or G2 cells when compared with single-agent treatment. In the fourth cell line (HCC827), sub-G1 DNA content, an indicator of apoptosis, was substantially increased by the combination (37.9 vs. 22.8 %), an effect that was also noted in the H358 cell line (10.1 vs. 4.1 %) (Online Resource 4, detailed in Online Resource 6). As expected based upon the proliferation studies, HGF strikingly reversed the cytostatic and cytotoxic effect of erlotinib in all cell lines. MK-2206 largely restored erlotinib cytostatic activity; however, this effect did not appear to restore the cytotoxic effects observed absent HGF-induced resistance. In this setting, it therefore appears that AKT inhibition principally targets cell cycling and proliferation rather than survival.
Combined AKT and MEK inhibition is effective in HGF-mediated resistance models
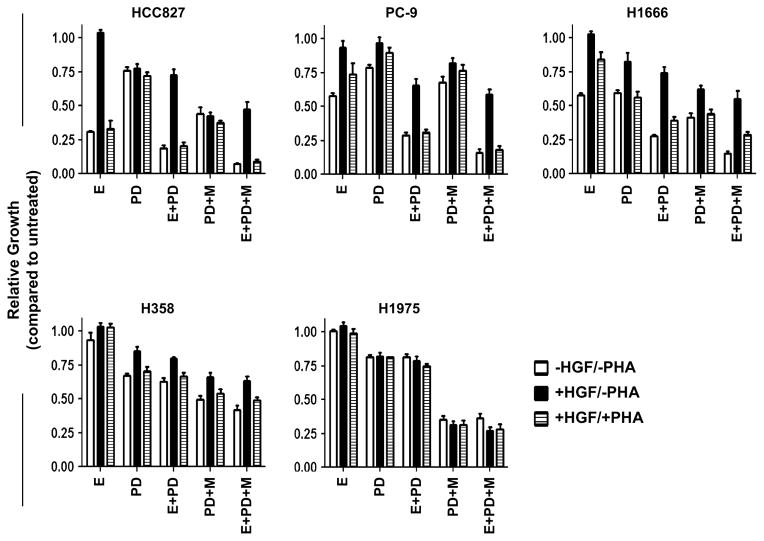
While MK-2206 appears to restore the cytostatic capabilities of erlotinib when treated with HGF, it is not sufficient to restore cytotoxicity. Persistent activation of the MEK/ERK signaling network maintained by the HGF/MET pathway represents one possible avenue by which cell viability may be maintained in the presence of EGFR inhibition. As shown in Fig. 3, single-agent treatment with the MEK inhibitor PD0325901 showed modest anti-proliferative activity in all cell lines tested. The dose chosen for PD0325901 (0.5 μM) was sufficient to completely abrogate ERK1/2 phosphorylation for all cell lines (data not shown). Notably, the addition of HGF could negatively modulate the growth inhibitory effects of PD0325901 treatment in the PC-9, H1666, and H358 cell lines (PC-9: 21–3 %; H1666: 41–18 %; H358: 33–15 %), although HGF had little effect on PD0325901 activity in the erlotinib-sensitive HCC827 and erlotinib-resistant H1975 cell lines. This contrasts with the response observed with MK-2206, where HGF-induced modulation of MK-2206 treatment was confined to the KRAS-mutant H358 cell line. In combination, PD0325901 and erlotinib showed a synergistic effect (similar to the combination treatment of erlotinib and MK-2206) on proliferation. In HGF-stimulated cells, combined PD0325901 and erlotinib treatment marginally outperformed erlotinib, in all tested cell lines, and PD0325901, in PC9 only (Fig. 3).
Fig. 3.
Growth response of NSCLC cell lines models of HGF-dependent erlotinib resistance following treatment with erlotinib (E)* (0.5 μM), MK-2206 (M) (0.5 μM), PD0325901 (PD) (0.5 μM), PHA665752 (PHA) (0.5 μM), and HGF (50 ng/mL) as single-agents or in combination using the CellTiter-Fluor Cell Viability Assay. All cells were treated for 72 h prior to analysis. Data are graphed as % growth relative to untreated cells. White columns have no added HGF or PHA; black columns are supplemented with HGF; hatched columns are supplemented with both HGF and PHA. *HCC827 and PC-9 cells were treated with lower doses of erlotinib (0.05 μM)
Examining combined AKT and MEK inhibition, we observed apparent synergism between MK-2206 and PD0325901 as concurrent treatment showed increased anti-proliferative activity in all cell lines when compared to single-agent treatments of MK-2206 and PD0325901 (HCC827: 56 vs. 30 and 24 %; PC-9: 33 vs. 21 and 21 %; H1666: 59 vs. 24 and 41 %; H358: 50 vs. 20 and 33 %; H1975: 65 vs. 38 and 19 %). Combined EGFR/AKT/MEK inhibition further reduced cell proliferation in all cell lines except the erlotinib-resistant H1975, more so than the double combinations of MK-2206 plus erlotinib or PD0325901 plus erlotinib. Given the efficacy of the double and triple combinations, it appears that erlotinib as a single-agent was not able to completely abrogate downstream signaling through these two pathways at the does given. Although HGF stimulation reduced the efficacy of the triple combination, combined AKT and MEK inhibition with erlotinib was still superior to erlotinib alone under the influence of HGF (HCC827: 53 vs. −4 %; PC-9: 41 vs. 6 %; H1666: 45 vs. −3 %; H358: 37 vs. −3 %; H1975: 73 vs. −5 %). This was observed even in the presence of the MET inhibitor PHA-665752 (HCC827: 91 vs. 67 %; PC-9: 82 vs. 26 %; H1666: 71 vs. 16 %; H358: 51 vs. −3 %; H1975: 72 vs. 1 %).
Inhibition with MK-2206 and PD0325901 is required to abrogate activity of AKT and ERK in EGFR wild-type and erlotinib-resistant cells
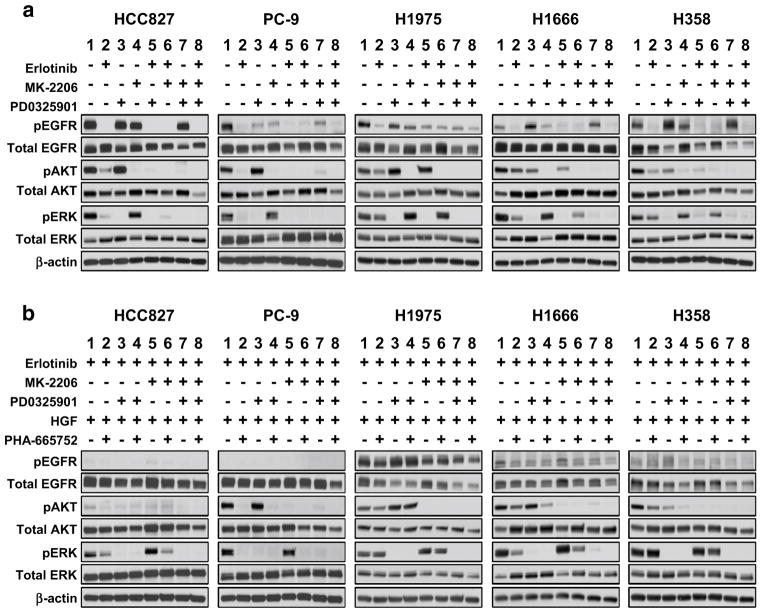
Additional analysis was conducted to determine the molecular changes leading to growth inhibition in the treatment combinations. For the EGFR-mutant cell lines HCC827 and PC-9, both pAKT and pERK were largely dependent upon EGFR signaling as treatment with erlotinib was sufficient to abrogate greater than 90 % of the activity of AKT and ERK (Fig. 4a), whereas erlotinib was less effective in the EGFR wild-type cell lines. While both single-agent MK-2206 and PD0325901 treatments successfully inhibited their targets in all cell lines, we also found that MEK inhibition had an inhibitory effect on pAKT in the EGFR-WT cells. Conversely, MEK inhibition appeared to induce pAKT in the EGFR-mutant PC-9 and H1975 lines. MEK and AKT inhibition also resulted in modulation of EGFR phosphorylation, with distinctive patterns in each cell line. While erlotinib, at the doses used, could effectively diminish EGFR phosphorylation, in most cases this did not translate into complete down-regulation of pAKT and/or pERK. In the EGFR-WT cell lines, maximal ablation of both of these signaling intermediaries was only achieved in the triple combination (Fig. 4a, lane 8).
Fig. 4.
Immunoblotting analysis of phospho-EGFR, AKT, and ERK following 24 h of treatment in NSCLC cell lines. a Cells were treated with erlotinib* (0.5 μM), MK-2206 (0.5 μM), and PD0325901 (0.5 μM) as single-agents and in combination. b Cells were treated with erlotinib, MK-2206, PD0325901 with or without HGF (50 ng/mL) and PHA665752 (0.5 μM). *HCC827 and PC-9 cells were treated with lower doses of erlotinib (0.05 μM)
MET pathway activation leading to erlotinib resistance has primarily been associated with the maintenance of signaling downstream of EGFR. As shown in Fig. 4b (lane 1), HGF stimulation protects pAKT and pERK expression from erlotinib treatment. This effect is largely reversed in the presence of the MET inhibitor PHA-665752. The H1975 cell line showed limited effects from either erlotinib treatment or HGF stimulation, likely arising from the presence of the T790M mutation and the continued signaling of pEGFR to downstream pathways. In this event, only the combined treatment with both MK-2206 and PD0325901 was sufficient to abrogate AKT and ERK signaling. Notably, in the EGFR-mutant HCC827 which shows high baseline expression of pMET, erlotinib-ablated pMET and substantially reduced total MET protein levels suggesting that, under normal conditions, MET is primarily regulated by EGFR in these cells (Online Resource 7). MET signaling only becomes uncoupled from the activity of erlotinib upon stimulation with HGF. Both AKT and MEK appear to play a role in MET regulation as simultaneous treatment with MK-2206 and PD0325901 partially reduce MET phosphorylation compared with an untreated sample irrespective of erlotinib.
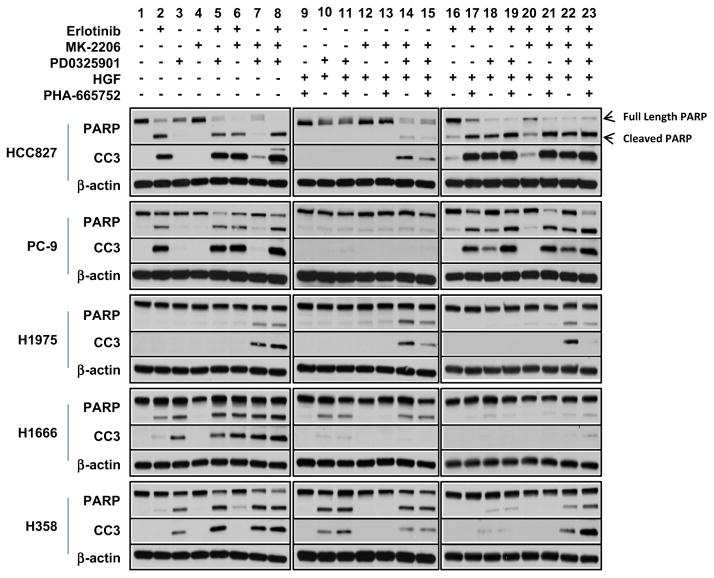
Combined AKT and MEK pathway inhibition induces apoptosis in HGF-treated erlotinib-resistant cell line models
Using PARP cleavage and cleaved caspase-3 (CC3) as surrogates for apoptosis, we examined the differential accumulation of each cleavage product to determine the likelihood that growth inhibition was resulting from a cytotoxic response to treatment. As expected in the EGFR-mutant erlotinib-sensitive cell lines HCC827 and PC-9, exposure to erlotinib showed demonstrable accumulation of both cleaved PARP and caspase-3 after 24 h, whereas the EGFR-WT cells showed only a modest increase in apoptosis (Fig. 5). In further contrast, the EGFR-WT H1666 and H358 showed accumulation of cleavage products following single-agent treatment with the MEK inhibitor not observed in the EGFR-mutant HCC827 and PC-9 lines (Fig. 5, lane 3). None of the cell lines tested showed any induction of apoptosis following treatment with single-agent MK-2206 (Fig. 5, lane 4), although MK-2206 enhanced erlotinib-induced apoptosis. Notably, the erlotinib-resistant EGFR-T790M-positive H1975 was not affected by receptor modulation, showing an induction of PARP cleavage and CC3 only when treated with combined AKT and MEK inhibition.
Fig. 5.
Immunoblotting analysis of PARP cleavage and cleavage caspase-3 (CC3) following 24 h of treatment with erlotinib* (0.5 μM), MK-2206 (0.5 μM), PD0325901 (0.5 μM) as single-agents and in combination with NSCLC cell lines with or without HGF (50 ng/mL) and PHA665752 (0.5 μM). *HCC827 and PC-9 cells were treated with lower doses of erlotinib (0.05 μM)
Following exposure to HGF, erlotinib as a single-agent was no longer sufficient to induce apoptosis to the same extent as in the absence of HGF (compare Fig. 5, lane 16–lane 2). However, in the EGFR-mutant HCC827 and PC-9 cells, inhibition of MEK signaling in combination with erlotinib was able to override this effect, restoring apoptosis even in the presence of HGF (Fig. 5, lane 18). With that said, upstream pathway inhibition of EGFR signaling was required for the preponderance of cell death observed as dual inhibition of AKT and MEK in the absence of erlotinib, and was not sufficient to induce a similar amount of apoptosis (Fig. 5, lane 7). This would suggest that additional factors, apart from AKT and MEK, are necessary for survival downstream of EGFR.
The EGFR-WT and H1975 cells were more susceptible to the combination of AKT and MEK inhibition (Fig. 5, lane 7), even in the presence of HGF (lane 14). Examining the overall effect of treatment on the HCC827 and PC-9 cells, apoptosis appears primarily associated with EGFR phosphorylation status, although ERK is involved in survival relating to HGF-mediated erlotinib resistance.
Discussion
There is an unmet clinical need to develop new therapies for NSCLC patients who have benefited from an EGFR TKI, but subsequently progressed. We therefore sought to determine whether AKT inhibition, using the allosteric AKT inhibitor MK-2206, could enhance activity of the EGFR TKI erlotinib and/or restore erlotinib sensitivity in the context of acquired resistance mediated by the MET receptor pathway. EGFR TKI resistance resulting from activation of MET, via MET amplification or elevated HGF expression, has been well documented (Engelman and Janne 2008; Gadgeel and Wozniak 2013). Directly targeting downstream signal transduction factors such as AKT in lieu of or in combination with EGFR TKIs could therefore prove a viable strategy for cancer therapeutics if the following conditions are met: (1) cells are sufficiently dependent upon identifiable downstream signaling intermediaries, such as the PI3K-AKT, or MEK-ERK pathways, (2) agents sufficiently active against signaling pathway targets are available, and (3) such combination treatments can be tolerated by the patient. In this work, we focused on erlotinib-sensitive and erlotinib-resistant models specifically mediated through MET receptor activation.
Prior studies have shown anticancer activity of MK-2206 across multiple tumor types as well as synergy with established chemotherapeutic agents in NSCLC (Agarwal et al. 2014; Almhanna et al. 2013; Hirai et al. 2010; Konopleva et al. 2014; Rashmi et al. 2014). Additional work has indicated synergy and enhanced cytotoxicity when MK-2206 is combined with EGFR inhibitors including erlotinib, gefitinib, and cetuximab in the context of a limited number of NSCLC, breast, and glioma cell lines (Cheng et al. 2012; Hirai et al. 2010; Iida et al. 2013). This is consistent with our observations among a larger cohort of NSCLC cell lines showing synergistic growth inhibition with concurrent erlotinib and MK-2206. Immunoblotting demonstrated that doses of erlotinib that effectively abrogated EGFR phosphorylation could reduce, but not completely ablate, AKT phosphorylation. Consistent with this, others have shown that inhibition by EGFR TKIs may modulate the frequency of downstream signaling molecules and thereby reduce overall steady-state levels, but within the specific context of a single cell, residual activity may still be present (Albeck et al. 2013). One likely mechanism of synergy therefore is the ability of MK-2206 to effectively eliminate residual AKT signaling in an erlotinib-independent manner.
To simulate acquired resistance to erlotinib, we exposed erlotinib-responsive lines to HGF, the ligand for the MET receptor. HGF expression has been shown to mediate resistance to both reversible and irreversible EGFR TKIs as well the monoclonal antibody cetuximab in NSCLC cells (Nanjo et al. 2013; Yamada et al. 2010, 2012; Yano et al. 2008). Under baseline conditions, many of the NSCLC cell lines in our study express MET, but lack receptor phosphorylation. Upon the addition of exogenous HGF, activated MET signaling substantially diminished the efficacy of erlotinib, dramatically curtailing both cytotoxicity in EGFR-mutant cell lines and cytostatic effects in EGFR-WT lines. Using this model system, we then explored whether the AKT inhibitor MK-2206 could restore activity of erlotinib under HGF-stimulated conditions. In prior studies, PI3K inhibitors have been shown to overcome resistance to EGFR TKIs induced by HGF, highlighting a role for sustained AKT inhibition in reversing erlotinib sensitivity to HGF (Donev et al. 2011; Sano et al. 2013). In HGF-stimulated cells, the combination of erlotinib plus MK-2206 outperformed erlotinib alone, although the degree of growth inhibition achieved was comparable to that of single-agent MK-2206. However, MK-2206 co-treatment could override HGF-mediated cell cycle progression, restoring the cytostatic activity of erlotinib. These data suggest that HGF-dependent erlotinib resistance is, with respect to cell cycle progression, derived from continued activation of the AKT pathway. However, we did not observe a concomitant increase in apoptosis markers, implying that AKT inhibition is insufficient to overcome HGF-mediated resistance in cells that would otherwise undergo apoptosis following treatment with erlotinib.
In light of these findings, we sought to determine the role of MEK-ERK signaling in this system. The importance of ERK signaling in HGF-MET signaling has been highlighted previously in hepatocellular carcinoma and lung cancer (Ma et al. 2007; Menakongka and Suthiphongchai 2010; Nagai et al. 2011). We investigated whether targeted inhibition of both MEK and AKT could mitigate the pro-survival effects of HGF in erlotinib-sensitive cells (Buonato and Lazzara 2014; Ercan et al. 2012). In contrast to MK-2206, the MEK inhibitor PD0325901 was better able to restore erlotinib-induced cytotoxicity under HGF-mediated resistance.
In EGFR-mutant, erlotinib-responsive cell lines, erlotinib substantially reduced both MEK and AKT phosphorylation. Treatment with combined MEK and AKT inhibition could not fully recapitulate the activity of single-agent erlotinib, suggesting that additional signaling intermediaries beyond AKT and MEK are engaged by mutant-activated EGFR. However, extensive growth inhibition and apoptosis were observed following treatment with MK-2206 plus PD0325901 in the H1975 line (EGFR L858R/T790M). This line was little impacted by erlotinib or through modulation of MET receptor activity (i.e. by HGF or deactivation by the Met inhibitor PHA-665752), but was responsive to combined AKT/MEK ablation. Furthermore, the EGFR-WT lines H1666 and H358, which underwent cell cycle arrest following erlotinib or MK-2206 treatment, exhibited extensive apoptosis when treated with the MEK inhibitor, further enhanced when in combination with the AKT inhibitor.
Overall, these results are consistent with previous findings that observed differential effects between PI3K and AKT inhibition on apoptosis in EGFR TKI sensitive cell lines (Faber et al. 2009). Where PI3K inhibition led to a down-regulation of the pro-survival BCL-2 family member MCL-1 which led to apoptosis, AKT inhibition failed to decrease MCL-1 levels and only negligibly affected cell survival. While MK-2206 and erlotinib could synergistically inhibit cell cycle progression, it is clear that, in the cell lines examined here, survival dependency is not singly reliant on AKT signaling. It has been previously noted that while the PI3K/AKT pathway is important in EGFR-dependent tumors and cell lines, activation of the PI3K pathway does not specifically imply dependency upon AKT signaling (Vasudevan et al. 2009). At the same time, inhibition of the MEK pathway downstream of the EGFR signaling cascade has been shown to induce expression of the pro-apoptotic BH3-only BCL2 family member BIM leading to cell death (Gong et al. 2007). As observed in our study, it does appear that MK-2206 cooperates with the MEK inhibitor PD0325901 to increase both growth inhibition and cell death. Supporting this observation, co-treatment with MK-2206 and the MEK inhibitor, AZD6244, in a limited number of lung cancer cell lines, was found to enhance the accumulation of BIM resulting in an increase in cell death compared with the MEK inhibitor alone (Meng et al. 2010).
In summary, we demonstrate that the combination of erlotinib plus the AKT inhibitor MK-2206 results in synergistic growth suppression in NSCLC cell lines. In cells rendered resistant to erlotinib by treatment with the MET receptor ligand HGF, MK-2206 could restore cytostatic effects but not completely restore cytotoxic effects of erlotinib. Instead, erlotinib-mediated cytotoxicity in NSCLC lines appeared to be preferentially dependent on MEK signaling. A phase II National Cancer Institute-sponsored clinical trial of erlotinib plus MK-2206 in advanced NSCLC patients who had progressed on erlotinib after previous benefit has been initiated (PHII-108, NCI 8698).
Supplementary Material
Acknowledgments
Grant support came from the Bonnie J. Addario Lung Cancer Foundation. We greatly appreciate OSI Pharmaceuticals and Merck Inc. for supplying erlotinib and MK-2206, respectively.
Abbreviations
- NSCLC
Non-small cell lung cancer
- EGFR
Epidermal growth factor
- HGF
Hepatocyte growth factor
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00432-014-1855-4) contains supplementary material, which is available to authorized users.
Ethical standard This manuscript does not contain clinical studies or patient data.
Conflict of interest Dr. Gandara is a consultant to Merck. Dr. Gandara and Dr. Lara are consultants to Genentech. Dr. Mack has received funding from Merck.
References
- Agarwal E, Chaudhuri A, Leiphrakpam PD, Haferbier KL, Brattain MG, Chowdhury S. Akt inhibitor MK-2206 promotes anti-tumor activity and cell death by modulation of AIF and Ezrin in colorectal cancer. BMC Cancer. 2014;14:145. doi: 10.1186/1471-2407-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeck JG, Mills GB, Brugge JS. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Mol Cell. 2013;49:249–261. doi: 10.1016/j.molcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almhanna K, et al. MK-2206, an Akt inhibitor, enhances carboplatinum/paclitaxel efficacy in gastric cancer cell lines. Cancer Biol Ther. 2013;14:932–936. doi: 10.4161/cbt.25939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann J, et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226–235. [PubMed] [Google Scholar]
- Barnett SF, et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem J. 2005;385:399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean J, et al. MET amplification occurs with or without T790M mutations in EGFR-mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonato JM, Lazzara MJ. ERK1/2 blockade prevents epithelial-mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Cancer Res. 2014;74:309–319. doi: 10.1158/0008-5472.CAN-12-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, et al. MK-2206, a novel allosteric inhibitor of Akt, synergizes with gefitinib against malignant glioma via modulating both autophagy and apoptosis. Mol Cancer Ther. 2012;11:154–164. doi: 10.1158/1535-7163.MCT-11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn DC, Holland WS, Yoon JM, Zwerdling T, Mack PC. Anti-tumor activity of the HSP90 inhibitor SNX-2112 in pediatric cancer cell lines. Pediatr Blood Cancer. 2011 doi: 10.1002/pbc.23270. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Donev IS, et al. Transient PI3K inhibition induces apoptosis and overcomes HGF-mediated resistance to EGFR TKIs in EGFR-mutant lung cancer. Clin Cancer Res. 2011;17:2260–2269. doi: 10.1158/1078-0432.CCR-10-1993. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Ercan D, et al. Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov. 2012;2:934–947. doi: 10.1158/2159-8290.CD-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber AC, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci USA. 2009;106:19503–19508. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgeel SM, Wozniak A. Preclinical rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non-small-cell lung cancer. Clin Lung Cancer. 2013;14:322–332. doi: 10.1016/j.cllc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, Pao W. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- Hirsch FR, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer Journal of clinical oncology: official journal of the American Society of. Clin Oncol. 2006;24:5034–5042. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- Iida M, Brand TM, Campbell DA, Starr MM, Luthar N, Traynor AM, Wheeler DL. Targeting AKT with the allosteric AKT inhibitor MK-2206 in non-small cell lung cancer cells with acquired resistance to cetuximab. Cancer Biol Ther. 2013;14:481–491. doi: 10.4161/cbt.24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- Konopleva MY, et al. Preclinical and early clinical evaluation of the oral AKT inhibitor, MK-2206, for the treatment of acute myelogenous leukemia. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Schmid-Bindert G, Wang D, Zhao Y, Yang X, Su B, Zhou C. Blocking the PI3K/AKT and MEK/ERK signaling pathways can overcome gefitinib-resistance in non-small cell lung cancer cell lines. Adv Med Sci. 2011;56:275–284. doi: 10.2478/v10039-011-0043-x. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Ma PC, Tretiakova MS, Nallasura V, Jagadeeswaran R, Husain AN, Salgia R. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: implications for tumour invasion. Br J Cancer. 2007;97:368–377. doi: 10.1038/sj.bjc.6603884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menakongka A, Suthiphongchai T. Involvement of PI3K and ERK1/2 pathways in hepatocyte growth factor-induced cholangio-carcinoma cell invasion. World J Gastroenterol, WJG. 2010;16:713–722. doi: 10.3748/wjg.v16.i6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, et al. Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PloS one. 2010;5:e14124. doi: 10.1371/journal.pone.0014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molife LR, et al. Phase 1 trial of the oral AKT inhibitor MK-2206 plus carboplatin/paclitaxel, docetaxel, or erlotinib in patients with advanced solid tumors. J Hematol Oncol. 2014;7:1. doi: 10.1186/1756-8722-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukohara T, et al. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J Natl Cancer Inst. 2005;97:1185–1194. doi: 10.1093/jnci/dji238. [DOI] [PubMed] [Google Scholar]
- Nagai T, et al. Sorafenib inhibits the hepatocyte growth factor-mediated epithelial mesenchymal transition in hepatocellular carcinoma. Mol Cancer Ther. 2011;10:169–177. doi: 10.1158/1535-7163.MCT-10-0544. [DOI] [PubMed] [Google Scholar]
- Nanjo S, et al. Ability of the Met kinase inhibitor crizotinib and new generation EGFR inhibitors to overcome resistance to EGFR inhibitors. PLoS One. 2013;8:e84700. doi: 10.1371/journal.pone.0084700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederst MJ, Engelman JA. Bypass mechanisms of resistance to receptor tyrosine kinase inhibition in lung cancer. Science Signal. 2013;6:re:6. doi: 10.1126/scisignal.2004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashmi R, et al. AKT inhibitors promote cell death in cervical cancer through disruption of mTOR signaling and glucose uptake. PloS One. 2014;9:e92948. doi: 10.1371/journal.pone.0092948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, et al. The novel phosphoinositide 3-kinase-mammalian target of rapamycin inhibitor, BEZ235, circumvents erlotinib resistance of epidermal growth factor receptor mutant lung cancer cells triggered by hepatocyte growth factor International journal of cancer. J Int du Cancer. 2013;133:505–513. doi: 10.1002/ijc.28034. [DOI] [PubMed] [Google Scholar]
- Shepherd FA, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- Sos ML, et al. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci USA. 2009;106:18351–18356. doi: 10.1073/pnas.0907325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher N, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- Vasudevan KM, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in EGFR-mutant lung cancer. Clin Cancer Res. 2012;18:1663–1671. doi: 10.1158/1078-0432.CCR-11-1171. [DOI] [PubMed] [Google Scholar]
- Weinstein IB. Cancer. Addiction to oncogenes—the Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- Yamada T, et al. Hepatocyte growth factor reduces susceptibility to an irreversible epidermal growth factor receptor inhibitor in EGFR-T790M mutant lung cancer. Clin Cancer Res. 2010;16:174–183. doi: 10.1158/1078-0432.CCR-09-1204. [DOI] [PubMed] [Google Scholar]
- Yamada T, Takeuchi S, Kita K, Bando H, Nakamura T, Matsumoto K, Yano S. Hepatocyte growth factor induces resistance to anti-epidermal growth factor receptor antibody in lung cancer. J Thorac Oncol. 2012;7:272–280. doi: 10.1097/JTO.0b013e3182398e69. [DOI] [PubMed] [Google Scholar]
- Yano S, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- Yano S, et al. Hepatocyte growth factor expression in EGFR-mutant lung cancer with intrinsic and acquired resistance to tyrosine kinase inhibitors in a Japanese cohort. J Thorac Oncol. 2011;6:2011–2017. doi: 10.1097/JTO.0b013e31823ab0dd. [DOI] [PubMed] [Google Scholar]
- Yap TA, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.