Abstract
Methods
An ectoparasiticide containing spinosad was evaluated as an oral formulation for cats. Two European laboratory studies and a European multicentre field efficacy and safety study assessed the use of a chewable tablet formulation of spinosad at a dose range of 50–75 mg/kg for treatment and control of flea infestations on cats.
Results
The studies with experimentally infested cats consistently demonstrated persistent activity against Ctenocephalides felis with >98 per cent efficacy at four weeks post-treatment. In the field study with naturally infested client-owned cats from 18 clinics across Germany and Italy, two monthly doses of spinosad were administered; selamectin was the comparator product. Safety was evaluated in 226 cats, each receiving at least one dose of spinosad or selamectin; both products were well tolerated. 113 spinosad-treated cats and 71 selamectin-treated cats showed >97 per cent reductions in geometric mean flea counts within 14 days post-treatment and at 60 days post-treatment efficacy was >98 per cent in both groups. Analysis of success rates showed 96 per cent in spinosad-treated cats compared with 90.9 per cent in selamectin-treated cats at day 60. The spinosad tablets were successfully administered to over 98 percent of cats. These studies indicate that this formulation of spinosad is safe and efficacious for treatment and prevention of flea infestations in cats.
Keywords: Cats, Ectoparasites, Fleas
Introduction
Fleas remain far more common in cats than in dogs (Bond and others 2007, Farkas and others 2009, Bourdeau 2012); cats are more likely to exhibit the typical clinical signs of flea bite hypersensitivity or flea allergy dermatitis (FAD). (Bond and others 2007) As multipet households present a risk factor for the presence of household infestations of fleas (Farkas and others 2009), there is a continuing need to provide flea treatment solutions for pet owners that lead to the rapid eradication of fleas, are simple to administer and that can be administered to both cats and dogs in the household.
In an established flea population, rapid speed of kill of adult fleas can prevent egg-laying and, hence, prevent further environmental contamination. In canine studies reported by Blagburn and others, spinosad was shown to be highly effective in this respect, with 100 per cent of fleas (Ctenocephalides felis) dead or moribund four hours after its oral administration (Blagburn and others 2010). Rapid flea kill is also seen following spinosad treatment of cats, with signs of flea mortality within 30 minutes of treatment. Following treatment in the dose range 50–100 mg/kg, >92 per cent of fleas were dead within two hours of treatment and >96 per cent of fleas were dead at eight hours post-treatment (Snyder and others 2013). Further analysis of these data to extract the speed of kill within the dose range of 50–75 mg/kg demonstrated that 94 per cent of fleas were dead after two hours and >99 per cent after eight hours.
In cats affected by FAD, an adult flea infestation can rapidly result in severe dermatological signs due to pruritus exacerbated by self-trauma. Effective control of FAD relies on reducing the allergenic stimulus below the pruritic threshold for a particular animal and thereafter suppressing any subsequent challenge to maintain it below that level. Thus insecticides with a rapid kill and population reduction profile are likely to be useful in suppressing and preventing clinical signs of FAD.
Spinosad was introduced for flea control in dogs in Europe in 2011 in the form of a palatable tablet (Comfortis chewable tablets for dogs; Elanco Animal Health) and has demonstrated excellent efficacy and safety in the field (Franc and Bouhsira 2009, Wolken and others 2012). This paper summarises data gathered during the studies conducted to evaluate the use of oral spinosad (Comfortis chewable tablets for dogs and cats, Elanco Animal Health) for flea control in cats in Europe and supplements data from a US field study where efficacy and tolerance of spinosad in cats in the dose range 50–100 mg/kg were investigated (Paarlberg and others 2013).
Materials and methods
Two laboratory dose confirmation studies and one field efficacy and safety study are reported that were conducted as part of the data for submission for regulatory approval in Europe. These studies complied with good clinical practice (EMEA 2000) and other relevant guidelines (EMEA 2007, Marchiondo and others 2007), as well as appropriate ethical guidance and local regulations.
Laboratory studies
Study design
Both of the laboratory trials were masked, randomised, complete block parallel group studies with one group of cats treated orally with spinosad tablets and a second control group treated with placebo tablets containing no active ingredient. One study was conducted in France and the other in Germany.
Animals and infestations
Individually housed, adult domestic cats were used in both studies. In the French study, the control group comprised nine cats (four female and five male) and eight cats (four female and four male) in the treated group; in Germany, both groups comprised nine cats (control: seven female and two male; treated: three female and six male). Cats in the French study weighed between 2.4 and 8.9 kg and were aged between approximately 39 and 108 months and those in the German study weighed between 3.6 and 5.6 kg and were aged between approximately 12 and 105 months. The suitability of cats was established approximately one week before the study started, based on their retention of adult fleas 48 hours after an infestation with approximately 100 unfed C felis. These flea counts were also used to rank cats in the randomisation process. Further infestations each comprising 100 unfed C felis were performed on study day −1 and then weekly throughout the study (on study days 7, 14, 21 and 28). Surviving fleas were removed by combing and counted on study day 1, approximately 24 hours after dosing, and then 48 hours after each subsequent infestation (on study days 9, 16, 23 and 30).
Treatment
It is recommended that spinosad is administered with or within one hour of a meal in order to maximise absorption of the active ingredient. In both studies, food was withdrawn from all cats on the day before treatment and cats were each offered approximately 25 per cent of their daily food ration approximately 30 minutes before dosing. Cats in the treated groups were treated orally with flavoured tablets containing spinosad to achieve a dose of spinosad between 50 and 75 mg/kg based on bodyweights recorded on the day of dosing. Cats in the control groups were dosed with placebo tablets. After treatment, all cats were offered the remainder of their food.
Statistical analysis
The efficacy of spinosad against fleas was assessed for study days 1, 7, 14, 21 and 28. Efficacy, based on the reduction of flea counts attributable to treatment, was calculated using the following formula:
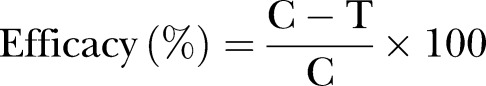 |
where ‘C’ is the geometric mean of the flea counts for the placebo control group and ‘T’ is the corresponding geometric mean flea count for the spinosad-treated group.  Logarithmic transformed C felis counts from all time points (study days 1, 7, 14, 21 and 28) were analysed with a repeated measures linear mixed model with fixed effects for treatment, time and treatment by time interaction. Non-parametric Wilcoxon rank sum exact tests together with Hodges-Lehmann estimates of the treatment difference with respect to flea burden were performed at each time point to validate the robustness of the parametric analyses.
Logarithmic transformed C felis counts from all time points (study days 1, 7, 14, 21 and 28) were analysed with a repeated measures linear mixed model with fixed effects for treatment, time and treatment by time interaction. Non-parametric Wilcoxon rank sum exact tests together with Hodges-Lehmann estimates of the treatment difference with respect to flea burden were performed at each time point to validate the robustness of the parametric analyses.
Field study
Study design
A field efficacy and safety study was conducted at multiple veterinary clinics in Germany and Italy to evaluate spinosad oral tablets compared with a positive control product, selamectin (Stronghold; Pfizer Animal Health).i The primary objectives of the study were to evaluate the clinical efficacy of the flavoured spinosad tablets at a dose of 50–75 mg/kg against natural flea infestations in cats that had been treated for two consecutive months compared with selamectin and to assess safety of the product based primarily on clinical observations, monitoring bodyweights and clinical chemistry parameters. Secondary objectives included the evaluation of treatment success, defined as a decrease of at least 90 per cent in the flea burden on a cat, the percentage of flea-free cats following treatment and the effect of treatment on FAD in cats.
The study employed a randomised complete block design and at each clinic a random allocation table was provided which provided for enrolment of cats in a 2:1 ratio (spinosad:selamectin) in order of presentation at the clinic. Within households all cats received the same treatment and blocks were formed with either three single-cat households or three multicat households. Data collection and recording was standardised across all sites. Masking was achieved by ensuring that personnel responsible for flea counts were unaware of treatment allocation.
The study required each cat to visit the clinic on four separate occasions as follows: visit 1 (study day 0) – enrolment and first treatment; visit 2 (study day 14) – flea counts; visit 3 (study day 30) – flea counts and second treatment; visit 4 (study day 60) – flea counts and study completion. Physical examinations and FAD scores were performed on each visit. A window of ±3 days was allowed for visits 2, 3 and 4.
Animals
Cats were eligible for enrolment if they were generally healthy, had a minimum of five fleas, resident in a household of up to a maximum of three cats, temperamentally suited and had not been recently treated with a product with residual activity against fleas. In households with more than one cat, all cats received treatment with the same product; however, only those cats within the household that met the enrolment criteria were included in the assessments of efficacy. The safety population included all cats treated with spinosad or selamectin. Cohabiting dogs were required to be treated with an appropriate flea control product for the duration of the study and were not permitted to mingle with the household cat(s). Owners were required to give their informed consent before the start of the study. No other products with activity against fleas, including environmental control products, were permitted to be used during the study and, additionally, cats with signs of FAD at the outset were not permitted to be treated with any form of corticosteroid for the duration of the study.
Treatments
Cats allocated to the spinosad group were treated with final formulation oral spinosad tablets that were administered once at the time of the first clinic visit on study day 0 and with a second dose approximately 30 days later. On each occasion each cat received one spinosad tablet in order to provide a dose of spinosad of between approximately 50 and 75 mg/kg bodyweight. Cats in the positive control group were treated topically with selamectin (Stronghold; Pfizer Animal Health) at a minimum dose rate of 6 mg/kg administered as per the label instructions on day 0 and with a second dose approximately 30 days later. On study day 0, the first treatment was administered at the clinic and cats were either fed before the clinic visit or were offered food at the clinic; where a cat refused to eat in the clinic or had not eaten before being taken to the clinic, the appropriate treatment was dispensed for the owner to administer at home. Most second treatments were administered by the client in their own home. Spinosad tablets were offered to cats according to the choice of the person administering the product by one or more of the following methods until the dose was administered: by free choice, for example, from the hand or floor or in a small amount of food, or by ‘pilling’. If a tablet or part of a tablet fell from the cat's mouth during administration, owners and clinic personnel were instructed to retrieve the tablet or part tablet and to re-administer using the same route. Cats treated with selamectin were also fed before treatment and were dosed in accordance with the manufacturer's instructions for the product.
Flea counts and FAD assessment
A standardised flea combing process was performed by trained study personnel using a separate flea comb for each cat. Cats were combed continually for a minimum of five minutes or until no more fleas were found for five minutes. Flea counts were performed on days 0, 14, 30 and 60 and only live viable fleas were counted. The clinical signs of FAD (pruritus, papules, erythema, alopecia, scaling and dermatitis/pyodermatitis) were scored on a scale of 0 to 3 (0=absent; 1=mild; 2=moderate; 3=severe) on study days 0, 14, 30 and 60 for each cat that had at least five fleas at the time of enrolment in the study.
Adverse events
Owners were instructed to observe their cat's behaviour during the entire study period and to give details of any abnormal signs by contacting the clinic and/or by reporting at the next scheduled visit. Additionally, where possible, owners were contacted by a member of the clinic staff by telephone within three days of both the first clinic visit (study day 0) and the study day 30 visit to confirm the actual date of dosing and also to enquire whether any abnormalities had been observed in their cat(s). Owner observations were also collected at the time of each clinic visit. Venous blood samples were collected at the time of the first clinic visit, before dosing and at the fourth visit (study day 60) for analysis of haematological and serum biochemistry parameters. The bodyweight of all cats was recorded at the first clinic visit, before dosing and again on the third (study day 30) and fourth (study day 60) visits.
Statistical analysis
For cats included in the evaluation of efficacy, effectiveness was calculated by comparing post-treatment geometric mean flea counts with baseline data collected at the time of the clinic visit before the first treatment. Further analysis on the log-transformed (flea count+1) data used a repeated measures mixed effects linear model with treatment group, time, time by treatment group and household type (single cat and multiple cats) as fixed effects in the model.
A non-inferiority analysis was performed by calculating the reduction in flea count for each treatment group using back-transformed least squares (LS) means with the following formula:
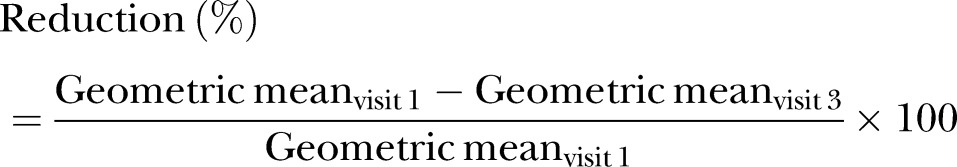 |
In order to declare the non-inferiority of spinosad in relation to selamectin, the evaluation required the following conditions to be met: (1) a statistically significant (P<0.05) decrease in LS mean flea count at visit 3 (study day 30) compared with visit 1 for each of the spinosad and selamectin groups; (2) a reduction of ≥90 per cent in flea count at visit 3, based on geometric means for both spinosad and selamectin.
For each cat, the percentage reduction was calculated as:
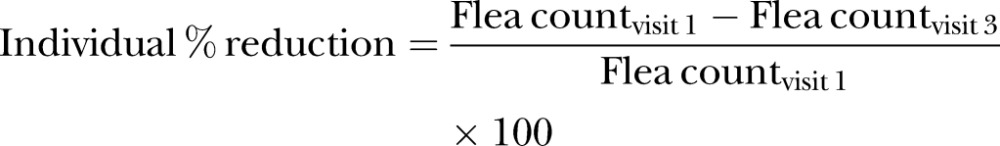 |
The difference in success rates was also evaluated to assess non-inferiority in case the percentage reduction in either group fell below the 90 per cent threshold.
Treatment was considered successful in any cat where the individual reduction was ≥90 per cent. The difference in the proportion of cats successfully treated between treatment groups, and the corresponding 95% CI was calculated.
The number and percentage of cats in each treatment group on which no fleas could be found (flea-free cats) was also calculated.
The analysis of FAD included those cats with a score of 1 (mild) or greater in one or more of the clinical signs of FAD at the time of clinic visit 1 (study day 0). Improvement in an individual cat for a particular sign was defined as a reduction of at least one in the score for the sign from visit 1 to visit 4. The percentage of cats showing an improvement in each clinical sign was calculated for both treatment groups. In addition to evaluating each clinical sign of FAD, a single metric of ‘overall improvement in FAD’ was produced using a weighted average of the percentage of cats that had shown an improvement in each sign. This metric was used to compare reduction in signs of FAD between the two treatment groups.
Results
Laboratory studies
In both the French and German studies, 100 per cent knockdown of fleas was achieved within 24 hours following treatment and no fleas remained on any cat treated with spinosad. By comparison, the geometric mean flea counts for the untreated control groups on study day 1 were 86.1 and 63.7, respectively; there were no flea-free cats in either untreated control group, which was indicative of the establishment of good infestations. Persistent efficacy, based on geometric mean flea counts, was >98 per cent for at least four weeks after treatment in both studies; on study day 28, efficacy against C. felis was 98.1 per cent in the French study and 98.9 per cent in the German study (Table 1).
TABLE 1:
Mean flea counts and percentage efficacy observed in cats treated with an oral tablet formulation of spinosad compared with placebo-treated controls 24 hours after dosing and 48 hours after each artificial infestation in laboratory dose confirmation studies
| Geometric and (arithmetic) mean flea counts and % efficacy* |
|||||||
|---|---|---|---|---|---|---|---|
| Study | Dose of spinosad (mg/kg) | Day: | 1 | 7 | 14 | 21 | 28 |
| 1. France | 50–75 | 0.0 (0.0) 100.0% |
0.0 (0.0) 100.0% |
0.3 (0.6) 99.7% |
0.4 (2.1) 99.5% |
1.5 (4.6) 98.1% |
|
| 2. Germany | 50–75 | 0.0 (0.0) 100.0% |
0.4 (0.6) 99.3% |
0.0 (0.0) 100.0% |
0.1 (0.1) 99.9% |
0.6 (0.9) 98.9% |
|
*% efficacy based on geometric means
In the French study, one cat was seen to regurgitate and then re-consume the material within the first hour after treatment with spinosad and a second cat vomited between 3 and 4 hours after treatment with spinosad and then again on the following day. No other clinically significant signs were seen in treated cats.
Field study
A total of 142 cats in 90 households were treated at least once with spinosad tablets within the dose range 50–75 mg/kg and 84 cats in 50 households were treated at least once with selamectin, and these 226 animals were considered to be the ‘safety population’. This population was geographically well distributed across Germany (10 clinics; 139 cats in 83 households) and Italy (8 clinics; 87 cats in 57 households). A total of 113 cats that received treatment with spinosad and 71 cats treated with selamectin were included in the evaluation of efficacy. The geometric mean flea count at day 0 for the spinosad-treated cats was 11.9 (range: 5–361) and for the selamectin group 10.7 (range: 5–207). Treatment with both spinosad and selamectin was highly effective in reducing mean flea counts from pretreatment levels; for cats treated with spinosad the reductions, based on geometric mean flea counts, were 97.4 per cent on study day 14 and 97.1 and 99.1 per cent on study days 30 and 60, respectively. The corresponding reductions for cats treated with selamectin were 97.4, 98.1 and 98.7 per cent (Table 2). The non-inferiority analysis confirmed that the efficacy of spinosad against fleas under field conditions was comparable with that of selamectin. Treatment success, defined as the percentage of cats with at least 90 per cent reduction in flea counts, was 96.0 per cent on day 60 for cats treated with spinosad compared with 90.9 per cent in the selamectin group.
TABLE 2:
Percentage reduction in flea counts observed in cats treated with a flavoured oral tablet formulation of spinosad at a dose of 50–75 mg/kg and cats treated with selamectin at a minimum dose rate of 6 mg/kg compared with pretreatment values on day 0, and the proportion of flea-free cats on each observation day in the multicentre European field study
| Reduction in flea count (%) based on geometric and (arithmetic) mean flea counts† |
|||||
|---|---|---|---|---|---|
|
(number and (%) of flea-free cat) |
|||||
| Treatment | Number of cats* | Geometric mean number of fleas on day 0 | Day 14 (visit 2) | Day 30 (visit 3) | Day 60 (visit 4) |
| Spinosad | 113 | 11.9 | 97.4 (96.2) | 97.1 (89.9) | 99.1 (98.7) |
| (86 (76.8)) | (90 (79.6)) | (91 (91.0)) | |||
| Selamectin (Stronghold) | 71 | 10.7 | 97.4 (96.0) | 98.1 (98.1) | 98.7 (98.2) |
| (57 (80.3)) | (56 (78.9)) | (58 (87.9)) | |||
*Number of cats treated with spinosad at 50–75 mg/kg or selamectin at least once and included in the evaluation of efficacy for day 0
†All post-treatment geometric mean flea counts were shown to be significantly different from pretreatment counts in both groups (P<0.001); there were no statistically significant differences in the counts between the groups on any day
An improvement in the clinical signs of FAD was seen in both groups over the course of the study (Table 3). For each of the six clinical signs of FAD that were recorded, a greater percentage of cats in the spinosad treatment group showed signs of improvement than in those treated with selamectin. Using the weighted average metric to assess the overall improvement in FAD in each treatment group, cats treated with spinosad demonstrated a 96.2 per cent overall improvement compared with 89.6 per cent in cats treated with selamectin.
TABLE 3:
Percentage improvement in clinical signs of flea allergy dermatitis (FAD) in the multicentre European field study
| Clinical sign |
||||||
|---|---|---|---|---|---|---|
| Treatment | Pruritus | Papules | Erythema | Alopecia | Scaling | Dermatitis/pyodermatitis |
| Spinosad | ||||||
| No. of cats with score >0 at day 0)* | 71 | 25 | 30 | 26 | 41 | 20 |
| No. of cats with improved score at day 60)† | 68 | 25 | 29 | 26 | 38 | 19 |
| Improvement (%)‡ | 95.8 | 100.0 | 96.7 | 100.0 | 92.7 | 95.0 |
| Selamectin (stronghold) | ||||||
| No. of cats with score >0 at day 0)* | 53 | 25 | 22 | 11 | 27 | 16 |
| No. of cats with improved score at day 60)† | 48 | 23 | 21 | 9 | 22 | 15 |
| Improvement (%)‡ | 90.6 | 92.0 | 95.5 | 81.8 | 81.5 | 93.8 |
*For each clinical sign, the number of cats that had a score >0 for that sign at the first clinic visit (study day 0) that were included in the evaluation of efficacy and were within the overall FAD population
†For each clinical sign, the number of cats that had a score >0 for that sign at the first clinic visit (study day 0) and that had an improved score for that sign at the fourth clinic visit (study day 60)
‡For each clinical sign, the percentage of those cats that had a score >0 for that sign at the first clinic visit (study day 0) and that had an improved score for that sign at the fourth clinic visit (study day 60)
Within the population of cats that were offered spinosad tablets in the field study, 38.3 per cent of animals accepted the tablet freely or in food and 60.0 per cent of cats received the product by ‘pilling’ and, overall, more than 98 per cent of the tablets were administered successfully.
Over the two-month treatment period, the most common abnormal signs observed by the cat owners were emesis, diarrhoea and rhinitis. Over the entire study period (60 days), the average monthly rate of vomiting in the safety population (142 cats) was 4.55 per cent in cats treated with spinosad and 1.19 per cent in cats treated with selamectin. Diarrhoea occurred in 1.42 per cent of the cats treated with spinosad and in 0.60 per cent of those cats treated with selamectin. These signs were mild and transient in both treatment groups. There were no clinically relevant changes in group mean clinical pathology parameters within or between either the spinosad-treated or selamectin-treated groups and bodyweight changes were comparable taking into account the age structure of the populations.
Discussion and conclusions
Effective flea control can only be achieved by removing adult fleas from the infested host, removing immature flea life cycle stages from the host's home environment and, finally, by preventing reinfestation. It has been common practice to use separate products to treat the on-host and environmental infestations; however, the evolution of adulticide products that have a very rapid speed of kill has meant that it is possible to significantly reduce or stop the production and deposition of viable flea eggs into the environment. As described by Chin et al, when treated animals move through infested environments newly emerged fleas infest the cat or dog and are rapidly killed before producing eggs and, over time, this has the effect of clearing the environmental infestation, thereby preventing reinfestation (Chin and others 2005, Dryden 2009). In this context, spinosad has proved highly effective in reducing flea egg output (Blagburn and others 2010) and in a simulated home environment study designed to assess prevention of establishment of a flea population where no live fleas were observed on treated cats throughout the study period (Snyder and others 2013).
The laboratory studies demonstrated that spinosad tablets are highly effective in controlling an established on-host flea population and in preventing recurrence over a period of one month after treatment. In the European field trial, monthly administered spinosad and selamectin were both seen to be highly effective at 30 and 60 days after treatment commenced. In the North American field study with an extended duration including three monthly treatments, those cats treated with spinosad at 50–75 mg/kg showed comparable results with those reported in Europe with efficacy based on geometric mean flea counts of >98 per cent on both study days 30 and 90 and more than 77 and 92 per cent of cats flea free at those time points, respectively. The efficacy of selamectin at the same time points was more variable: 88.8 and 97.7 per cent, with 29.4 and 64.7 per cent of cats flea free at the same time points, respectively. This variation may be due to geographical differences in flea strains and their susceptibility to selamectin or to the differences in flea burdens (Paarlberg and others 2013).
Selamectin is known to exhibit both ovicidal and larvicidal activity (McTier and others 2000). In contrast, when administered orally, spinosad is not known to have efficacy against other stages of the flea life cycle. Blagburn and others demonstrated that the developmental success of eggs and larvae exposed to debris (flea faeces, skin debris) from treated and untreated dogs was similar, concluding that there is no presence of spinosad in the sebum and skin debris following oral administration (Blagburn and others 2010). In the field study results reported here, there was no additional benefit associated with the use of a product with ovicidal and larvicidal activity combined with adulticidal activity.
It may appear counterintuitive to suggest that a product that requires oral ingestion to exhibit efficacy against fleas in cats could be effective in controlling FAD. The goal of flea treatment in relation to FAD must be to kill adult fleas as rapidly as possible. This reduces potential feeding time, which in turn reduces exposure to flea saliva (antigen) and thereby reduces the allergen load below the FAD threshold for a particular individual. A reduction and/or resolution of the clinical signs of FAD may then be anticipated. This hypothesis is borne out by the results of the field study reported here where an improvement of >95 per cent in the signs of FAD was observed in the spinosad-treated cats by day 60.
Administration of tablets to cats has traditionally been seen as challenging, often requiring restraint of the animal and can be associated with reduced owner compliance (Thombre 2004). The data from the field study show that over a third of cats freely accepted the flavoured spinosad tablet or ate it with food and acceptance by free choice increased when the tablets were offered in the cat's home environment, although some owners and clinic staff elected to administer the tablet by pilling and did not use any other method. Overall acceptance of the tablets was recorded as >98 per cent, indicating that owners successfully chose an administration method that was most appropriate to themselves and their cat.
There are several advantages to orally administered products, including that efficacy is not affected by bathing or shampooing and there is no active ingredient present on the surface of the animal that could potentially be transferred to the owner or to children. Additionally, the risk of animals removing some of the active ingredient during grooming and thus affecting the duration and level of efficacy is eliminated. The risk of run-off associated with topical products is absent. The most common sign of abnormal health was emesis (4.9 per cent), followed by diarrhoea (1.2 per cent) and rhinitis (1.2 per cent) in spinosad-treated animals. Gastrointestinal signs were identified as the most commonly observed adverse events when using oral spinosad tablets in dogs (5.6 per cent after initial dose) (NOAH, 2013). Emesis has been observed following oral administration of other licensed parasiticides with emesis rates of 14–16 per cent (Plue and others 1992).
In these studies, spinosad tablets in cats were shown to be safe and effective with residual activity for up to four weeks in the laboratory studies. In the field study, treatment with both orally administered flavoured spinosad tablets and topically applied selamectin for two consecutive months was highly effective in providing flea control in client-owned cats. The number of flea-free cats at each time point was similar for both products; however, the number and percentage of cats showing improvement in the clinical signs of FAD was greater in the group treated with spinosad than in those treated with selamectin.
In conclusion, spinosad oral tablets for cats have been demonstrated to be an effective and safe method to treat and prevent natural flea infestations in cats and offer an alternative to topically applied insecticides.
Acknowledgments
The authors express their gratitude to all personnel involved in the independent laboratory studies and to the cat owners and staff of veterinary clinics taking part in the field investigations. Members of the Elanco team, particularly Dr Dan Snyder, are thanked for their participation and support.
Footnotes
Funding: These studies as reported herein were funded by Elanco Animal Health. The paper has been written in accordance with the ARRIVE guidelines.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Pfizer Animal Health became Zoetis 2013.
References
- Blagburn B. L., Young D. R., Moran C., Meyer J. A., Leigh-Heffron A., Paarlberg T., Zimmermann A. G., Mowrey D., Wiseman S., Snyder D. E. (2010) Effects of orally administered spinosad (Comfortis) in dogs on adult and immature stages of the cat flea (Ctenocephalides felis). Veterinary Parasitology 168, 312–317 [DOI] [PubMed] [Google Scholar]
- Bond R., Riddle A., Mottram L., Beugnet F., Stevenson R. (2007) Survey of flea infestation in dogs and cats in the United Kingdom during 2005. The Veterinary Record 160, 503–506 [DOI] [PubMed] [Google Scholar]
- Bourdeau P. (2012) Topical anti-parasitic therapy: a few myths dispelled. Companion Animal 17, 42–44 [Google Scholar]
- Chin A., Lunn P., Dryden M. W. (2005) Persistent flea infestations in dogs and cats controlled with monthly topical applications of fipronil and methoprene. Australian Veterinary Practitioner 35, 89–96 [Google Scholar]
- Dryden M. W. (2009) Flea and tick control in the 21st century: challenges and opportunities. Veterinary Dermatology 20, 435–440 [DOI] [PubMed] [Google Scholar]
- EMEA (2000) VICH Topic GL9 (GCP): Guideline on Good Clinical Practices. CVMP/VICH/595/98-Final, London.
- EMEA (2007) Committee for Medicinal Products for Veterinary Use (CVMP): Guideline for the testing and evaluation of the efficacy of antiparasitic substances for the treatment and prevention of tick and flea infestations in dogs and cats. EMEA/CVMP/EWP/005/2000-Rev.2, London.
- Farkas R., Gyurkovszky M., Solymosi N., Beugnet F. (2009) Prevalence of flea infestation in dogs and cats in Hungary combined with a survey of owner awareness. Medical and Veterinary Entomology 23, 187–194 [DOI] [PubMed] [Google Scholar]
- Franc M., Bouhsira E. (2009) Evaluation of speed and duration of efficacy of spinosad tablets for treatment and control of Ctenocephalides canis (Siphonaptera: Pulicidae) infestations in dogs. Parasite 16, 125–128 [DOI] [PubMed] [Google Scholar]
- Marchiondo A. A., Holdsworth P. A., Green P., Blagburn B. L., Jacobs D. E. (2007) World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guidelines for evaluating the efficacy of parasiticides for the treatment, prevention and control of flea and tick infestation on dogs and cats. Veterinary Parasitology 145, 332–344 [DOI] [PubMed] [Google Scholar]
- Mctier T. L., Shanks D. J., Jernigan A. D., Rowan T. G., Jones R. L., Murphy M. G., Wang C., Smith D. G., Holbert M. S., Blagburn B. L. (2000) Evaluation of the effects of selamectin against adult and immature stages of fleas (Ctenocephalides felis felis) on dogs and cats. Veterinary Parasitology 91, 201–212 [DOI] [PubMed] [Google Scholar]
- NOAH Compendium of Data Sheets for Animal Medicines (2013) Noah: Enfield, 357
- Paarlberg T. E., Wiseman S., Trout C. M., Kee E. A., Snyder D. E. (2013) Safety and efficacy of spinosad chewable tablets for treatment of flea infestations of cats. Journal of the American Veterinary Medical Association 242, 1092–1098 [DOI] [PubMed] [Google Scholar]
- Plue R. E., Jernigan A. D., Acre K. E., Coleman M. W., Currin S. T., Ellis A. J., Lange R. L., Weiner D. R. (1992) Field efficacy, safety, and acceptability of ivermectin plus pyrantel in growing and adult dogs. Heartworm Symposium ‘92, 27–29 March 1992 Austin TX, USA: American Heartworm Society [Google Scholar]
- Snyder D. E., Meyer K. A., Wiseman S., Trout C. M., Young D. R. (2013) Speed of kill efficacy and efficacy of flavored spinosad tablets administered orally to cats in a simulated home environment for the treatment and prevention of cat flea (Ctenocephalides felis) infestations. Veterinary Parasitology 2013;196:492–496 [DOI] [PubMed] [Google Scholar]
- Thombre A. G. (2004) Oral delivery of medications to companion animals: palatability considerations. Advanced Drug Delivery Reviews 56, 1399–1413 [DOI] [PubMed] [Google Scholar]
- Wolken S., Franc M., Bouhsira E., Wiseman S., Hayes B., Schnitzler B., Jacobs D. E. (2012) Evaluation of spinosad for the oral treatment and control of flea infestations on dogs in Europe. The Veterinary Record 170, 99. [DOI] [PubMed] [Google Scholar]


