Abstract
Introduction
A serological and entomological investigation was performed to monitor for potential Bunyamwera (BUN) serogroup virus activity in Montana.
Results
To facilitate the serological investigation, sera were collected from 104 sheep in 2013 and 2014 and assayed by plaque reduction neutralization test using all six BUN serogroup viruses known to occur in the United States: Cache Valley virus (CVV), Lokern virus (LOKV), Main Drain virus (MDV), Northway virus, Potosi virus and Tensaw virus. BUN serogroup virus-specific antibodies were detected in 41 (39%) sheep. Of these, three were seropositive for MDV, one was seropositive for CVV, one was seropositive for LOKV and 36 had antibodies to an undetermined BUN serogroup virus. Additionally, 30,606 Culicoides sonorensis were collected in 2013 using Centers for Disease Control and Prevention (CDC) light traps and assayed for cytopathic virus by virus isolation in African Green Monkey kidney (Vero) cells. All midges were negative. Almost one-third of the midges were further tested by reverse transcription-polymerase chain reaction using BUN serogroup virus-reactive primers and all were negative.
Conclusions
We provide evidence of BUN serogroup virus infection in sheep but not C. sonorensis in Montana in 2013-2014. This study also provides the first evidence of CVV, MDV and LOKV activity in Montana.
Keywords: Bunyamwera serogroup, Orthobunyavirus, Cache Valley virus, Sheep, Surveillance, Culicoides
Introduction
All viruses in the Bunyamwera (BUN) serogroup (genus Orthobunyavirus, family Bunyaviridae) are maintained in transmission cycles involving haematophagous arthropods and vertebrate hosts (Schmaljohn and Nichol 2007). Six BUN serogroup viruses occur in the USA: Cache Valley virus (CVV), Lokern virus (LOKV), Main Drain virus (MDV), Northway virus (NORV), Potosi virus (POTV) and Tensaw virus (TENV) (Calisher and others 1986, Francy and others 1990). CVV infections in sheep can result in embryonic and fetal death, stillbirths and congenital malformations (Edwards and others 1989, Chung and others 1990a, b). MDV induces severe musculoskeletal and nervous system malformations in ovine fetuses infected in utero by injection into the amniotic vesicle and is a cause of equine encephalomyelitis but has not been associated with naturally occurring disease in sheep (Emmons and others 1983, Edwards and others 1997). LOKV, POTV, NORV and TENV are not recognised ovine pathogens; however, their ability to cause disease has not been widely investigated. Because most veterinary diagnostic laboratories do not routinely test for BUN serogroup viruses, information on their ability to cause disease and data on their true disease incidence and seroprevalence in livestock are limited.
Most viruses in the BUN serogroup are transmitted primarily by mosquitoes (Calisher and others 1986). Several species of biting midges in the genus Culicoides also play an important role in BUN serogroup virus transmission. LOKV and MDV have been repeatedly isolated from Culicoides species, and Culicoides variipennis is a competent vector of MDV (Mellor and others 1974, Calisher and others 1986). Culicoides species are also important vectors of several medically and veterinary important orthobunyaviruses in the Simbu serogroup (e.g. Akabane virus, Oropouche virus and Schmallenberg virus (SBV)) (Mellor and others 2000, Rasmussen and others 2012, Veronesi and others 2013). For instance, field studies in Denmark (Rasmussen and others 2012), Italy (Goffredo and others 2013) and Belgium (De Regge and others 2012) have implicated biting midges of the C. obsoletus group as relevant vectors of SBV and Culicoides sonorensis was demonstrated to be a suitable model vector species for SBV transmission (Veronesi and others 2013). C. sonorensis is an abundant species in Montana (Johnson, unpublished data), produced in the wet, manure-contaminated soil surrounding stock ponds and other lentic water sources on farms and ranches.
There is no recent information on the seroprevalence of BUN serogroup viruses in livestock in Montana, nor are there any recent data of the significance of Culicoides species in BUN serogroup virus transmission in this region. Therefore, the overall goal of this study was to perform a serological and entomological investigation to determine the seroprevalence of BUN serogroup viruses in sheep and the prevalence of these viruses in C. sonorensis temporally and spatially associated with sheep in Montana.
Materials and methods
Sera collections
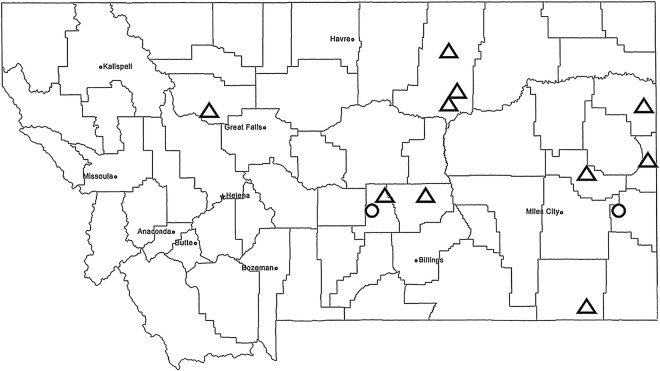
Sera were collected from sheep from a ranch in south central Montana in 2013 and a ranch in eastern Montana in 2014 (Fig 1). Several ranchers were contacted in the study area where Culicoides were collected regarding participation in the study. Some declined and others were unable to participate because of other commitments.
FIG 1:
Locations of Culicoides sampling sites (Δ) and premises for sheep sera collections (Ο)
Plaque reduction neutralisation tests
Plaque reduction neutralisation tests (PRNTs) were performed using CVV (strain CVV-478), LOKV (strain FMS 4332), MDV (strain BFS 5015), NORV (strain 0234), POTV (strain BeAr7272) and TENV (strain A9-171b). CVV-478 was originally isolated from mosquitoes collected in Mexico (Farfan-Ale and others 2009, Blitvich and others 2012). All other viruses were obtained from the World Arbovirus Reference Collection at the University of Texas Medical Branch in Galveston, Texas. PRNTs were performed using all BUN serogroup viruses that occur in the USA (as opposed to only those associated with disease) because antibodies to one BUN serogroup virus often cross-react with other viruses in this serogroup due to their close antigenic relatedness (Hunt and Calisher 1979); thus, the exclusion of one or more viruses may result in serological misdiagnosis. PRNTs were performed in six-well plates containing confluent monolayers of African Green Monkey kidney (Vero) cells following published protocols (Beaty and others 1995). Initially, all sera were screened at a single dilution of 1:20. All sera that tested positive for BUN serogroup-specific antibodies were further diluted and tested by PRNT in order to identify the viruses responsible for these infections. Titres were expressed as the reciprocal of highest serum dilutions yielding ≥90 per cent reduction in the number of plaques (PRNT90). For aetiological diagnosis, the PRNT90 antibody titre to the respective virus was required to be at least four-fold greater than that to the other viruses tested.
Arthropod collections
Arthropods were collected at 10 trap sites in eight Montana counties from May to August 2013 (Fig 1). Five arthropod collection sites were adjacent to or within 5 km of summer-pastured sheep. Collections were made using Centers for Disease Control and Prevention (CDC) light traps placed near the edges of stock ponds or other lentic water sources. Traps were baited with dry ice in the late afternoon, and arthropods were collected early the following morning and transported to the laboratory on dry ice. C. sonorensis were identified according to morphological characteristics and sorted into pools of up to 50 according to date and study site.
Homogenisations
C. sonorensis were placed in polypropylene, round-bottom 5 ml tubes with 1.8 ml phosphate-buffered saline (pH 7.4) supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin and 2.5 μg/ml fungizone. Four 4.5 mm diameter copper-clad steel beads (BB-calibre airgun shot) were added to each tube, and midge pools were homogenised by vortexing for 60 seconds. Homogenates were centrifuged (3000 rpm, 10 minutes, 4°C) and supernatants were collected.
Virus isolation in cell culture
An aliquot (100 μl) of each homogenate was added to 0.5 ml of maintenance medium which consisted of Dulbecco's modified Eagle medium (DMEM; Invitrogen, Carlsbad, California, USA) supplemented with 2 per cent fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin and 2.5 μg/ml fungizone. Supernatants were filtered using a 0.22 μm filter and inoculated onto subconfluent monolayers of Vero cells in 6-well plates. Cells were incubated for one hour at room temperature on an orbital shaker and then 5 ml of maintenance medium was added to each well. Cells were incubated at 37°C in the presence of 5 per cent CO2 for 14 days and monitored regularly for cytopathic effect.
Reverse transcription PCR
Total RNA was extracted from homogenates using the QIAamp viral RNA extraction kit (QIAGEN, Valencia, California, USA) and analysed by RT-PCR using primers BCS82C and BCS332V, which are specific to orthobunyaviruses in the BUN and California serogroups (including CVV, MDV, POTV and TENV (Kuno and others 1996) and LOKV and NORV (B.J. Blitvich, unpublished data)). Complementary DNAs were generated using Superscript III reverse transcriptase (Invitrogen), and PCRs were performed using Taq polymerase (Invitrogen).
Results
BUN serogroup virus-specific antibodies were identified in 41 (39.4 per cent) sheep (Table 1). Of these, three were seropositive for MDV, one was seropositive for CVV, one was seropositive for LOKV and 36 had antibodies to an undetermined BUN serogroup virus. Sixteen sheep that had antibodies to an undetermined BUN serogroup virus had low PRNT90 titres to MDV and no detectable antibody titres to any of the other viruses. Antibodies to NORV, POTV and TENV were not identified in any sheep.
TABLE 1:
PRNT data for sheep with Bunyamwera serogroup virus-specific antibodies
| PRNT90 titre |
|||||||
|---|---|---|---|---|---|---|---|
| Serum ID | CVV | LOKV | MDV | NORV | POTV | TENV | PRNT diagnosis |
| A03 | *– | – | 40 | – | – | – | †Und |
| A13 | – | – | 40 | – | – | – | Und |
| A15 | – | – | 20 | – | – | – | Und |
| A17 | – | – | 20 | – | – | – | Und |
| A21 | – | – | 20 | – | – | – | Und |
| A25 | ≥1280 | 160 | 40 | 160 | – | 20 | CVV |
| A30 | – | – | 20 | – | – | – | Und |
| A34 | – | – | 20 | – | – | – | Und |
| A35 | – | – | – | –– | 20 | – | Und |
| A36 | – | – | 40 | – | – | – | Und |
| A37 | – | – | 40 | – | – | – | Und |
| A39 | – | 20 | – | – | – | – | Und |
| A41 | – | – | 20 | – | – | – | Und |
| A42 | – | – | 80 | – | – | – | MDV |
| A44 | – | 40 | – | – | – | – | Und |
| A45 | – | – | 80 | – | – | – | MDV |
| A49 | – | – | 20 | – | – | – | Und |
| A53 | – | – | 20 | – | – | – | Und |
| A60 | – | – | – | 40 | – | – | Und |
| B01 | – | 20 | – | – | – | – | Und |
| B05 | – | – | 40 | – | – | – | Und |
| B07 | – | – | 20 | – | – | – | Und |
| B13 | 640 | 320 | 160 | 40 | 20 | 160 | Und |
| B14 | – | 80 | – | – | – | 20 | LOKV |
| B17 | – | – | 20 | – | – | – | Und |
| B18 | – | – | 20 | 20 | 20 | – | Und |
| B19 | – | – | – | – | 20 | – | Und |
| B20 | – | – | 80 | 20 | 20 | – | MDV |
| B21 | – | 20 | – | – | – | – | Und |
| B22 | – | – | – | 20 | – | – | Und |
| B24 | 40 | 20 | 20 | 80 | 20 | 40 | Und |
| B25 | – | – | – | – | 20 | – | Und |
| B29 | – | – | – | – | 20 | – | Und |
| B30 | – | 20 | – | – | – | – | Und |
| B31 | – | – | – | – | 20 | – | Und |
| B32 | – | – | – | 20 | – | – | Und |
| B35 | – | 20 | 20 | – | – | – | Und |
| B36 | – | – | 20 | – | – | – | Und |
| B37 | – | – | 20 | – | 20 | – | Und |
| B38 | – | 20 | 20 | – | 20 | – | Und |
| B39 | – | – | – | – | 20 | – | Und |
*<20
†Undetermined Bunyamwera serogroup virus
CVV, Cache Valley virus; LOKV, Lokern virus; MDV, Main Drain virus; NORV, Northway virus; POTV, Potosi virus; PRNT, plaque reduction neutralisation test; TENV, Tensaw virus
A total of 30,606 C. sonorensis were collected and sorted into 629 pools. All were homogenised and an aliquot of each homogenate was tested by virus isolation in Vero cells. All homogenates were negative for cytopathic virus. A subset of homogenates (n=200) was further tested by RT-PCR using primers specific to BUN and California serogroup viruses. All homogenates were negative.
Discussion
BUN serogroup virus-specific antibodies were identified in 41 (39.4 per cent) sheep. At least three viruses were responsible for these infections: CVV, LOKV and MDV. None of these viruses have been previously reported in Montana. CVV has been detected throughout much of North America including North and South Dakota which border Montana to the east, and Alberta and Saskatchewan which border Montana to the north (Iversen and others 1979, Calisher and others 1986, Pabbaraju and others 2009) but there are no published data demonstrating the occurrence of this virus in Montana. Previously, LOKV activity had only been reported in California, Colorado, New Mexico, Texas and Utah while MDV is known to occur in all of the aforementioned states as well as Arizona (Crane and others 1983, Calisher and others 1986, Kramer and others 1990). Our findings suggest that the geographic distribution of CVV, MDV and LOKV is wider than previously reported.
Because our data indicate that BUN serogroup viruses commonly infect sheep in Montana, it is likely that some of the sheep analysed in this study had been exposed to more than one BUN serogroup virus. However, information on the antibody responses in vertebrates sequentially infected with BUN serogroup viruses is limited and research should be performed to address this issue. There is currently only one report that describes the antibody responses in vertebrates experimentally inoculated with different BUN serogroup viruses. The study was performed using white-tailed deer that were first inoculated with CVV or POTV then sequentially challenged with the alternate virus five to seven months later (Blackmore and Grimstad 1998).
As already noted, 36 sheep had antibodies to an undetermined BUN serogroup virus. Of these, 16 had low PRNT90 titres to MDV and no detectable antibody titres to any of the other viruses. This could indicate that the sheep had been infected with MDV several years ago and, because neutralising antibody levels steadily decline over time (Gibbs and others 2005), only trace amounts of MDV-specific antibodies remained. Alternatively, the sheep could have been infected with a novel BUN serogroup virus that is more closely related to MDV than it is to the other viruses included in the PRNTs. In this regard, the three sheep seropositive for MDV had MDV PRNT90 titres that could be considered low (all titres were 80); thus, we cannot dismiss the possibility that these sheep had instead been infected with an unrecognised MDV-like virus and that the MDV PRNT90 titres are a consequence of serological cross-reactivity. Likewise, the sheep seropositive for LOKV had a LOKV PRNT90 titre of 80 and, therefore, an unknown LOKV-like virus may have been responsible for this infection.
BUN serogroup viruses were not isolated from any C. sonorensis collected in this study. Our rationale for testing the midges by virus isolation in cell culture is because this technique is not restricted to the isolation of BUN serogroup viruses; other arthropod-transmitted viruses can also be detected. However, viral RNA is more stable than infectious viral particles and therefore a subset of C. sonorensis was further tested by RT-PCR using BUN serogroup-reactive primers. Another reason why we also decided to perform RT-PCRs is because this technique has the potential to detect novel BUN serogroup viruses that lack the capacity to replicate in Vero cells. However, as already noted, viral RNA was not detected in any midges.
In summary, we provide evidence of BUN serogroup virus infection in sheep but not C. sonorensis in Montana. At least three viruses were responsible for these infections: CVV, LOKV and MDV. This study provides the first evidence of CVV, MDV and LOKV activity in Montana. Additional research is needed to determine the impact of these viruses on ovine health.
Acknowledgments
The authors thank the undergraduate researchers at Aaniiih Nakoda College, Carroll College, Little Big Horn College and Salish Kootenai College who performed the trapping efforts in Montana, Marni Rolston for processing Culicoides collections, and Hayes Goosey and Lisa Surber for collecting sheep sera. The authors also thank Robert Tesh for providing isolates of LOKV, MDV, POTV, NORV and TENV. Financial support was provided by Boehringer Ingelheim, the Iowa Livestock Health Advisory Committee, the National Institute of General Medical Sciences of the National Institutes of Health (award number P20GM103474) and the Howard Hughes Medical Institute (award number 52007534).
Footnotes
Contributors: GDJ and DGH coordinated the collection of sera and midges, CSB and ZNC performed the PRNTs, PI tested midges by virus isolation and RT-PCR, PJP and LCB participated in the design and coordination of the study, and BJB conceived of the study, participated in its design and coordination and drafted the manuscript. All authors read and approved the final manuscript.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- Beaty B. J., Calisher C. H., Shope R. E. (1995) Arboviruses. In Diagnostic Procedures for Viral and Rickettsial Diseases. Ed Lennette E. Washington, DC: American Public Health Association; 189–212 [Google Scholar]
- Blackmore C. G., Grimstad P. R. (1998) Cache Valley and Potosi viruses (Bunyaviridae) in white-tailed deer (Odocoileus virginianus): experimental infections and antibody prevalence in natural populations. The American Journal of Tropical Medicine and Hygiene 59, 704–709 [DOI] [PubMed] [Google Scholar]
- Blitvich B. J., Lorono-Pino M. A., Garcia-Rejon J. E., Farfan-Ale J. A., Dorman K. S. (2012) Nucleotide sequencing and serologic analysis of Cache Valley virus isolates from the Yucatan Peninsula of Mexico. Virus Genes 45, 176–180 [DOI] [PubMed] [Google Scholar]
- Calisher C. H., Francy D. B., Smith G. C., Muth D. J., Lazuick J. S., Karabatsos N., Jakob W. L., Mclean R. G. (1986) Distribution of Bunyamwera serogroup viruses in North America, 1956–1984. The American Journal of Tropical Medicine and Hygiene 35, 429–443 [DOI] [PubMed] [Google Scholar]
- Chung S. I., Livingston C. W. Jr., Edwards J. F., Crandell R. W., Shope R. E., Shelton M. J., Collisson E. W. (1990a) Evidence that Cache Valley virus induces congenital malformations in sheep. Veterinary Microbiology 21, 297–307 [DOI] [PubMed] [Google Scholar]
- Chung S. I., Livingston C. W. Jr., Edwards J. F., Gauer B. B., Collisson E. W. (1990b) Congenital malformations in sheep resulting from in utero inoculation of Cache Valley virus. American Journal of Veterinary Research 51, 1645–1648 [PubMed] [Google Scholar]
- Crane G. T., Elbel R. E., Francy D. B., Calisher C. H. (1983) Arboviruses from western Utah, USA, 1967–1976. Journal of Medical Entomology 20, 294–300 [DOI] [PubMed] [Google Scholar]
- De Regge N., Deblauwe I., De Deken R., Vantieghem P., Madder M., Geysen D., Smeets F., Losson B., Van Den Berg T., Cay A. B. (2012) Detection of Schmallenberg virus in different Culicoides spp. by real-time RT-PCR. Transboundary and Emerging Diseases 59, 471–475 [DOI] [PubMed] [Google Scholar]
- Edwards J. F., Karabatsos N., Collisson E. W., De La Concha Bermejillo A. (1997) Ovine fetal malformations induced by in utero inoculation with Main Drain, San Angelo, and LaCrosse viruses. The American Journal of Tropical Medicine and Hygiene 56, 171–176 [DOI] [PubMed] [Google Scholar]
- Edwards J. F., Livingston C. W., Chung S. I., Collisson E. C. (1989) Ovine arthrogryposis and central nervous system malformations associated with in utero Cache Valley virus infection: spontaneous disease. Veterinary Pathology 26, 33–39 [DOI] [PubMed] [Google Scholar]
- Emmons R. W., Woodie J. D., Laub R. L., Oshiro L. S. (1983) Main Drain virus as a cause of equine encephalomyelitis. Journal of the American Veterinary Medical Association 183, 555–558 [PubMed] [Google Scholar]
- Farfan-Ale J. A., Loroño-Pino M. A., Garcia-Rejon J. E., Hovav E., Powers A. M., Lin M., Dorman K. S., Platt K., Bartholomay L. C., Soto V., Beaty B. J., Lanciotti R. S., Blitvich B. J. (2009) Detection of RNA from a novel West Nile-like virus and high prevalence of an insect-specific flavivirus in mosquitoes in the Yucatan Peninsula of Mexico. The American Journal of Tropical Medicine and Hygiene 80, 85–95 [PMC free article] [PubMed] [Google Scholar]
- Francy D. B., Karabatsos N., Wesson D. M., Moore C. G. Jr, Lazuick J. S., Niebylski M. L., Tsai T. F., Craig G. B. Jr (1990) A new arbovirus from Aedes albopictus, an Asian mosquito established in the United States. Science 250, 1738–1740 [DOI] [PubMed] [Google Scholar]
- Gibbs S. E., Hoffman D. M., Stark L. M., Marlenee N. L., Blitvich B. J., Beaty B. J., Stallknecht D. E. (2005) Persistence of antibodies to West Nile virus in naturally infected rock pigeons (Columba livia). Clinical and Diagnostic Laboratory Immunology 12, 665–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffredo M., Monaco F., Capelli G., Quaglia M., Federici V., Catalani M., Montarsi F., Polci A., Pinoni C., Calistri P., Savini G. (2013) Schmallenberg virus in Italy: a retrospective survey in Culicoides stored during the bluetongue Italian surveillance program. Preventive Veterinary Medicine 111, 230–236 [DOI] [PubMed] [Google Scholar]
- Hunt A. R., Calisher C. H. (1979) Relationships of Bunyamwera group viruses by neutralization. The American Journal of Tropical Medicine and Hygiene 28, 740–749 [PubMed] [Google Scholar]
- Iversen J. O., Wagner R. J., Leung M. K., Hayles L. B., Mclintock J. R. (1979) Cache Valley virus: isolations from mosquitoes in Saskatchewan, 1972–1974. Canadian Journal Of Microbiology 25, 760–764 [DOI] [PubMed] [Google Scholar]
- Kramer W. L., Jones R. H., Holbrook F. R., Walton T. E., Calisher C. H. (1990) Isolation of arboviruses from Culicoides midges (Diptera: Ceratopogonidae) in Colorado during an epizootic of vesicular stomatitis New Jersey. Journal of Medical Entomology 27, 487–493 [DOI] [PubMed] [Google Scholar]
- Kuno G., Mitchell C. J., Chang G. J., Smith G. C. (1996) Detecting bunyaviruses of the Bunyamwera and California serogroups by a PCR technique. Journal of Clinical Microbiology 34, 1184–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor P. S., Boorman J., Baylis M. (2000) Culicoides biting midges: their role as arbovirus vectors. Annual Review of Entomology 45, 307–340 [DOI] [PubMed] [Google Scholar]
- Mellor P. S., Boorman J., Loke R. (1974) The multiplication of main drain virus in two species of Culicoides (Diptera, Ceratopogonidae). Archiv fur die gesamte Virusforschung 46, 105–110 [DOI] [PubMed] [Google Scholar]
- Pabbaraju K., Ho K. C., Wong S., Fox J. D., Kaplen B., Tyler S., Drebot M., Tilley P. A. (2009) Surveillance of mosquito-borne viruses in Alberta using reverse transcription polymerase chain reaction with generic primers. Journal of Medical Entomology 46, 640–648 [DOI] [PubMed] [Google Scholar]
- Rasmussen L. D., Kristensen B., Kirkeby C., Rasmussen T. B., Belsham G. J., Bødker R., Bøtner A. (2012) Culicoides as vectors of Schmallenberg virus. Emerging Infectious Diseases 18, 1204–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn C. S., Nichol S. T. (2007) Bunyaviridae. In Fields Virology. 5th edn Ed Knipe D. M. Philadelphia: Lippincott Williams and Wilkins; 1741–1789 [Google Scholar]
- Veronesi E., Henstock M., Gubbins S., Batten C., Manley R., Barber J., Hoffmann B., Beer M., Attoui H., Mertens P. P., Carpenter S. (2013) Implicating Culicoides biting midges as vectors of Schmallenberg virus using semi-quantitative RT-PCR. PLoS ONE 8, e57747. [DOI] [PMC free article] [PubMed] [Google Scholar]