Abstract
Background
Depression is a frequent comorbidity in HIV infection that has been associated with worse treatment outcomes and increased mortality. Recent studies suggest that increased innate immune activation and tryptophan catabolism are associated with higher risk of depression in HIV infection and other chronic inflammatory diseases, but the mechanisms leading to depression remain poorly understood.
Methods
The severity of depressive symptoms was assessed by Beck Depression Inventory or Center for Epidemiological Studies Depression Scale. Untargeted metabolomic profiling of plasma from 104 subjects (68 HIV-positive and 36 HIV-negative) across three independent cohorts was performed using liquid or gas chromatography followed by mass spectrometry. Cytokine profiling was by Bioplex array. Bioinformatic analysis was performed in Metaboanalyst and R.
Results
Decreased monoamine metabolites (phenylacetate, 4-hydroxyphenylacetate) and acylcarnitines (propionylcarnitine, isobutyrylcarnitine, isovalerylcarnitine, 2-methylbutyrylcarnitine) in plasma distinguished depressed subjects from controls in HIV-positive and HIV-negative cohorts, and these alterations correlated with the severity of depressive symptoms. In HIV-positive subjects, acylcarnitines and other markers of mitochondrial function correlated inversely with tryptophan catabolism, a marker of IFN responses, suggesting inter-relationships between inflammatory pathways, tryptophan catabolism, and metabolic alterations associated with depression. Altered metabolites mapped to pathways involved in monoamine metabolism, mitochondrial function, and inflammation, suggesting a model in which complex relationships between monoamine metabolism and mitochondrial bioenergetics contribute to biological mechanisms involved in depression that may be augmented by inflammation during HIV infection.
Conclusions
Integrated approaches targeting inflammation, monoamine metabolism, and mitochondrial pathways may be important for prevention and treatment of depression in people with and without HIV.
Keywords: HIV, depression, metabolomics, tryptophan catabolism, monoamines, acylcarnitines
Introduction
Mood disorders are common in HIV infection, with 20-60% of HIV patients having depressive symptoms or major depressive disorder (MDD) [1-5]. In addition to impairing quality of life, overall function, and well-being, depression is also associated with delayed initiation of antiretroviral therapy (ART), poor adherence, accelerated disease progression, and increased mortality [6-10]. Depression is also associated with high rates of alcohol and illicit drug abuse, which are associated with risk behaviors, higher HIV transmission rates, and greater burden of medical and mental health comorbidities. Although rates and severity of depression have fallen since the introduction of ART, depression remains an predictor of poor outcomes and increased mortality [3, 11-13].
MDD is a heterogeneous disorder frequently associated with anxiety, anhedonia, reduced locomotor activity, chronic fatigue, and loss of energy. The underlying pathophysiology remains poorly understood, but may include altered synthesis, catabolism, or uptake of monoamines (serotonin, dopamine, catecholamines, and trace amines), dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, stress responses, and mitochondrial dysfunction [14-17]. Recent studies suggest that inflammation, in particular chronic innate immune activation, may influence development of depressive symptoms via interactions with neurotransmitter and neuroendocrine systems [14, 15, 18, 19]. Pro-inflammatory mediators, and interferon (IFN) responses in HIV and other settings have been associated with increased tryptophan catabolism and decreased phenylalanine metabolism , which may affect serotonin and dopamine biosynthesis, respectively [15, 20-23]. Increased tryptophan catabolism has been linked to depression not only in HIV infection [24, 25], but also in post-partum depression [26] and cancer [27]. Decreased enzymatic conversion of phenylalanine to tyrosine, a rate-limiting step in dopamine biosynthesis, is another pathway that has been linked to depression [28, 29]. ART treatment improves but does not normalize these metabolite alterations in HIV infection [23, 30-32].
Successful diagnosis and treatment of depression in HIV-infected individuals has been limited by poor clinical recognition, delayed treatment, and variable treatment responses [5, 33]. Diagnosis of MDD in both HIV-positive and general populations is based on interviews, self-report scales, and checklists, and is often subjective and variable. The identification of reliable biomarkers of MDD is important to improve diagnosis and monitor therapeutic responses and to characterize depressive subtypes, provide mechanistic insights, and identify novel therapeutic targets [34]. Recent untargeted metabolomic studies in HIV-negative subjects have provided insights into possible biochemical mechanisms associated with depression and therapeutic responses to antidepressants [35-38]. However, these findings have not been examined in HIV cohorts. Here, we performed untargeted metabolomic profiling of 104 plasma samples across 3 independent cohorts to investigate metabolic pathways associated with MDD in both HIV-positive and negative subjects. We then examined inter-relationships between these metabolic abnormalities and inflammation markers in HIV-positive subjects.
Methods
Study subjects
Plasma samples from subjects with (45%) and without depression (55%) (n=104; 68 HIV-positive subjects and 36 HIV-negative subjects) were collected between 2002-2012. Subjects in the HIV-positive test cohort were from the National NeuroAIDS Tissue Consortium (NNTC) (Manhattan HIV Brain Bank, National Neurological AIDS Bank, California NeuroAIDS Tissue Network, Texas NeuroAIDS Research Center), and the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study. Subjects in the HIV-positive validation and HIV-negative cohorts were from the AIDS Linked to the Intravenous Experience (ALIVE) Study, a longitudinal cohort of current or former injection drug users [39]. Subjects were selected based on the following inclusion criteria. All subjects were enrolled with written informed consent and IRB approval at each study site. Inclusion criteria for the HIV-positive test cohort were between the ages of 35-60, advanced disease (nadir CD4<300 cells/ul), HIV plasma viral load <1500 copies/ml, and >1 year on ART. Inclusion criteria for the HIV-positive validation cohort were between the ages of 35-60 and HIV plasma viral load <10,000 copies/ml. The HIV-negative cohort was between the ages of 35-62. Exclusion criteria for all cohorts were body mass index (BMI) >40, severe hepatotoxicity (defined in accordance with the AIDS Clinical Trials Group criteria as grades 3 or 4) and moderate to severe renal insufficiency at time of sampling [40]. MDD diagnosis was determined by self-report. Depressive symptoms in the NNTC and CHARTER studies were assessed using the Beck Depression Inventory (BDI>14), a validated 21-item self-report scale that measures characteristic attitudes and symptoms of depression in psychiatrically diagnosed patients and normal populations [41, 42]. In the ALIVE study, symptoms were assessed using the Center for Epidemiological Studies Depression Scale (CES-D>16), a 20-item self-report scale designed to measure levels of depressive symptomatology in the general population [43]. These depression scales are generally comparable, and have been shown to correlate with each other [44, 45]. Substance abuse was determined by Psychiatric Research Interview for Substance and Mental Disorders (PRISM), Composite International Diagnostic Interview (CIDI) and urine toxicology (NNTC and CHARTER studies) or by self-report (ALIVE study). Matching for age, gender, race, HCV status, ART use, smoking, and current or former drug use was performed to achieve similar distributions of these covariates in groups defined by depression scale cut-offs. HIV-positive cohorts were also matched for stage of disease (current and nadir CD4 counts).
Quantification of soluble markers in plasma
Interferon (IFN)-α, IFN-γ, CXCL8, CXCL9, CXCL10, interleukin (IL)-1b, IL-6, IL-10, IL-12, TNF-α and CCL2 were measured using a Luminex multiplex assay (Bio-Plex System, Bio-Rad Laboratories).
Metabolomic profiling
Metabolomic profiling was performed by Metabolon (Durham, NC) using ultra high performance liquid chromatography and tandem mass spectrometry (UHLC/MS/MS2) and gas chromatography (GC)/MS. Plasma samples were extracted using the MicroLab STAR® system (Hamilton Company, Salt Lake City, Utah). LC/MS extracts were reconstituted in acidic or basic conditions and run on a Waters ACQUITY UPLC and a Thermo-Finnigan LTQ mass spectrometer, which consisted of an electrospray ionization (ESI) source and linear ion-trap (LIT) mass analyzer. GC/MS samples were run on 5% phenyl column and analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization. Data files were loaded into a relational database, and compounds were identified using Metabolon's proprietary peak integration software, which compares chromatographic and mass spectra properties to those from a library of purified standards or recurrent unknown entities (>1000 compounds).
Data processing, bioinformatics, and statistical analysis
Metabolite data was normalized by median centering. Missing values were imputed with the lower limit of detection for a given metabolite. Significantly altered metabolites were defined by a fold change (FC) >1.2, p-value <0.05 and false discovery rate (FDR) ≤0.1. Multiple hypothesis testing corrections were performed by calculating the local false discovery rates using fdrtool in R. Classification analysis including unsupervised hierarchical clustering, partial least squares discriminant analysis (PLS-DA), and Random Forest was performed in the Metaboanalyst web portal (http://www.metaboanalyst.ca). Pearson and Spearman correlations analysis were performed on log-transformed data in R. Metabolic pathways were extracted from the Kyoto Encyclopedia of Genes and Genomes (KEGG), Small Molecule Pathways Database (SMPDB), and PubChem. Biofunctions were extracted from Human Metabolome Database (HMDB) (www.hmdb.ca). Visualization of pathway mapping was performed in Cytoscape. Further information about bioinformatics and statistical analysis can be found in the Supplemental Methods.
Results
Study cohorts
Clinical characteristics of HIV-positive and negative cohorts are shown in Table 1. The median age of subjects across cohorts was 47 (IQR 43-51), 56% were male, 74% were African American, and the median BMI was 25.7 (22.7-28.2). Both HIV-positive and negative cohorts had a high prevalence of hepatitis C virus (HCV) co-infection (66%), smoking (79%), and alcohol (47%) and cocaine use (50%). Two subjects were being treated for diabetes, 6 for high cholesterol, and 24 for high blood pressure. HIV-positive subjects in the test cohort had late stage disease (nadir CD4<300 cells/ul) and were on ART (77% on protease inhibitors (PI), 22% on d-drugs associated with mitochondrial toxicity [e.g., stavudine and didanosine]) with suppressed plasma viral loads (<400 copies/ml). 58% of patients in the HIV-positive validation cohort were on ART (68% on PI). In the total cohort (all study subjects), 45% had a clinical diagnosis of MDD and BDI score ≥14 or CES-D score ≥16. 25% were on antidepressant medications.
Table 1.
Clinical and demographic characteristics of study cohorts
| HIV-positive test cohort (n=32) | HIV-positive validation cohort (n=36) | HIV-negative cohort (n=36) | |
|---|---|---|---|
| Age (years)* | 44 (33-59) | 48 (35-57) | 49 (36-62) |
| Gender (male) | 67% | 53% | 53% |
| Race (African American) | 60% | 86% | 78% |
| BMI* | 23.6 (21.9-30.1) | 25.3 (18.6-34.6) | 26.1 (19.4-39.9) |
| HCV seropositive | 73% | 67% | 64% |
| Smokers | NA | 81% | 78% |
| Alcohol use | 40% | 53% | 50% |
| Cocaine use | 47% | 53% | 56% |
| % on ART | 100% | 58% | NA |
| % on PI | 77% | 68% | NA |
| Current CD4 (cells/ul)* | 288 (16-1396) | 350 (64-992) | NA |
| Nadir CD4 (cells/ul)* | 54 (2-267) | 227 (6-856) | NA |
| Plasma HIV viral load (log copies/ml)* | 1.7 (1.7-3.2) | 2.8 (1.6-4.0) | NA |
| BDI* | 9 (0-35) | NA | NA |
| % with BDI ≥ 14 | 47% | NA | NA |
| CES-D* | NA | 11 (0-43) | 12 (0-36) |
| % with CES-D ≥ 16 | NA | 36% | 33% |
| Antidepressant use | 30% | 27% | 16% |
Median (IQR)
Abbreviations: BMI, body mass index; BDI, Beck Depression Inventory; CES-D, Center for Epidemiological Studies Depression Scale; NA, not available.
Characterization of the plasma metabolomes from HIV-positive and HIV-negative cohorts with and without depression
Untargeted metabolomic profiling of 104 plasma samples detected a total of 390, 457, and 445 metabolites in the HIV-positive test cohort, HIV-positive validation cohort, and HIV-negative cohort, respectively (Supplemental Figure 1). Preprocessing was performed to reduce noise by excluding xenobiotics, carbohydrates, and metabolites with >70% missing/imputed values. A total of 229 metabolites detected across cohorts met acceptability criteria and were further analyzed using bioinformatic tools (Supplementary Table 1). 32% (74/229) of metabolites mapped to biologically relevant pathways, including phenylalanine and tyrosine metabolites (metabolism of dopamine and other monoamines), tryptophan metabolites (serotonin synthesis), and markers of mitochondrial function (acylcarnitines, TCA components, branched chain amino acid metabolites (BCAA)) and oxidative stress (glutathione and purine metabolites).
Depressive symptoms are associated with alterations in monoamine metabolites and decreased acylcarnitines in HIV-positive and HIV-negative cohorts
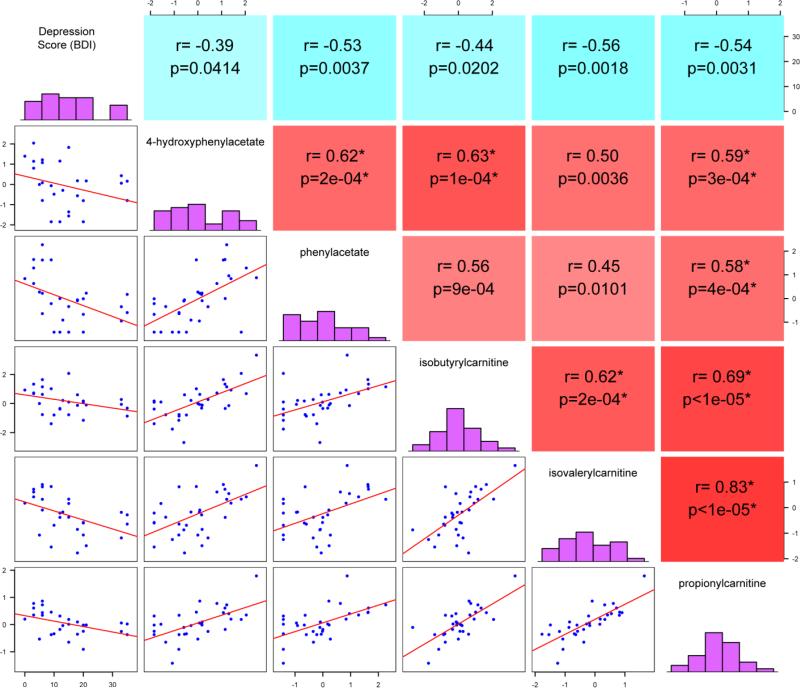
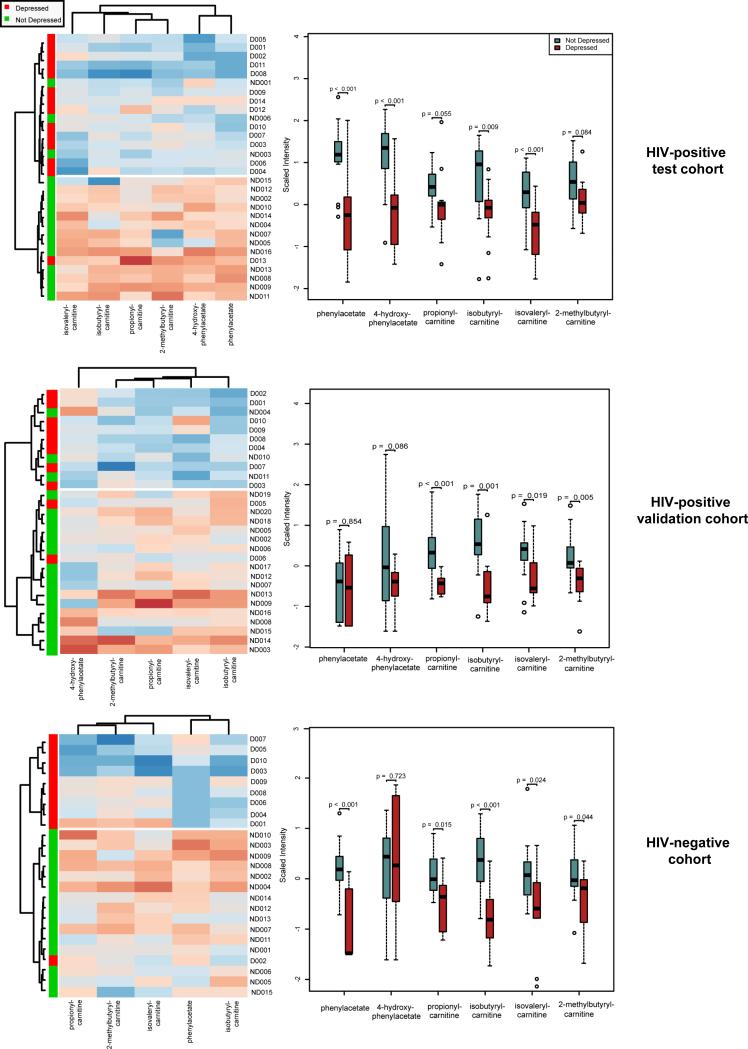
Next, we examined relationships between plasma metabolites and severity of depressive symptoms (BDI score) in the HIV-positive test cohort. Spearman correlation analysis identified 22 metabolites that correlated negatively with BDI scores (P<0.05, FDR<10%). Evaluating inter-relationships between these metabolites identified a cluster of 5 metabolites that correlated positively in pair-wise comparisons including metabolites related to phenylalanine/tyrosine catabolism (phenylacetate and 4-hydroxyphenylacetate, which are derived from degradation of the biogenic amines phenylethylamine and tyramine, respectively, by monoamine oxidase) and acylcarnitines (isovalerylcarnitine, isobutyrylcarnitine, and propionylcarnitine) (Figure 1). CES-D correlated negatively with isovalerylcarnitine, isobutyrylcarnitine, and propionylcarnitine in the HIV-positive validation cohort and negatively with phenylacetate and isobutyrylcarnitine in the HIV-negative cohort (p<0.05, Spearman correlation). Eighteen metabolites distinguished between HIV subjects on ART with versus without depressive symptoms (n=14 and n=16, respectively) in groups matched by age, gender, HCV status, drug use, and stage of disease (current and nadir CD4 count), derived from the HIV-positive test cohort (FC >1.2, P<0.05, FDR<10%, Supplemental Table 1). Unsupervised hierarchical clustering in heatmaps identified 6 metabolites that distinguished between subjects with versus without depressive symptoms with >80% predictive accuracy (Figure 2 and Supplemental Figure 2). Five of these metabolites were also altered in subjects with depressive symptoms in the HIV-positive validation cohort (n=10 with and n=20 without depressive symptoms; groups matched for age, gender, race, HCV status, % on ART, smoking, drug use, and stage of disease) and 5 were altered in subjects with depressive symptoms in the HIV–negative cohort (n=10 with and n=15 without depressive symptoms; groups matched for age, gender, race, HCV status, smoking, and drug use) (Figure 2, middle and bottom panels). Thus, these metabolite alterations were associated with depressive symptoms in HIV-positive and HIV-negative cohorts.
Figure 1. Inter-relationships between plasma metabolites and depression scores in HIV patients on ART.
Spearman correlation matrix visualization reveals inter-relationships between a set of 5 plasma metabolites and BDI scores in the HIV-positive test cohort (n=30). Comparisons were performed in a pair-wise manner and the correlation matrix was constructed in R. Diagonals display the histogram for each individual variable. Units displayed on the x-axis of the histogram represent depression scores (BDI) or metabolite concentrations (scaled intensities). Units on the y-axis represent numbers of subjects with values within the specified range for depression scale scores or metabolite concentrations. Panels to the right of the histogram show Spearman correlation coefficients and p-values. Red and blue represent positive and negative correlations, respectively.
Figure 2. Plasma metabolites distinguishing between subjects with and without depressive symptoms in HIV-positive and HIV-negative cohorts.
On the left, heatmaps with unsupervised hierarchical clustering of signature metabolites (n=6; FC>1.2, p<0.01, FDR <10%) altered across independent cohorts of HIV-positive and HIV-negative subjects with compared to without depressive symptoms. Red and blue indicate increased and decreased metabolite levels, respectively. Subjects with versus without depressive symptoms (n=14 and n=16, respectively) from the HIV-positive test cohort were matched by age, gender, HCV status, type of ART regimen (% on protease inhibitors), drug use, and stage of disease (current and nadir CD4 count). Subjects with versus without depressive symptoms in the HIV-positive validation cohort (n=10 and n=20, respectively) were matched for age, gender, race, HCV status, % on ART, smoking, drug use, and stage of disease). Subjects with versus without depressive symptoms in the HIV–negative cohort (n=10 with and n=15 without depressive symptoms) were matched for age, gender, race, HCV status, smoking, and drug use. Dendrograms show clusters of similar subjects (top) and metabolites (left). On the right, box plots of metabolites altered in subjects with compared to without depressive symptoms. Medians are represented by horizontal bars, boxes span the interquartile range (IQR), and whiskers extend to extreme data points within 1.5 times IQR. Outliers plotted as open circles lie outside 1.5 times the IQR. Blue and red represent subjects without and with depressive symptoms, respectively. P-values were calculated using Welch's t-tests.
Depressive symptoms in HIV-positive subjects are associated with augmented IFN responses and tryptophan catabolism
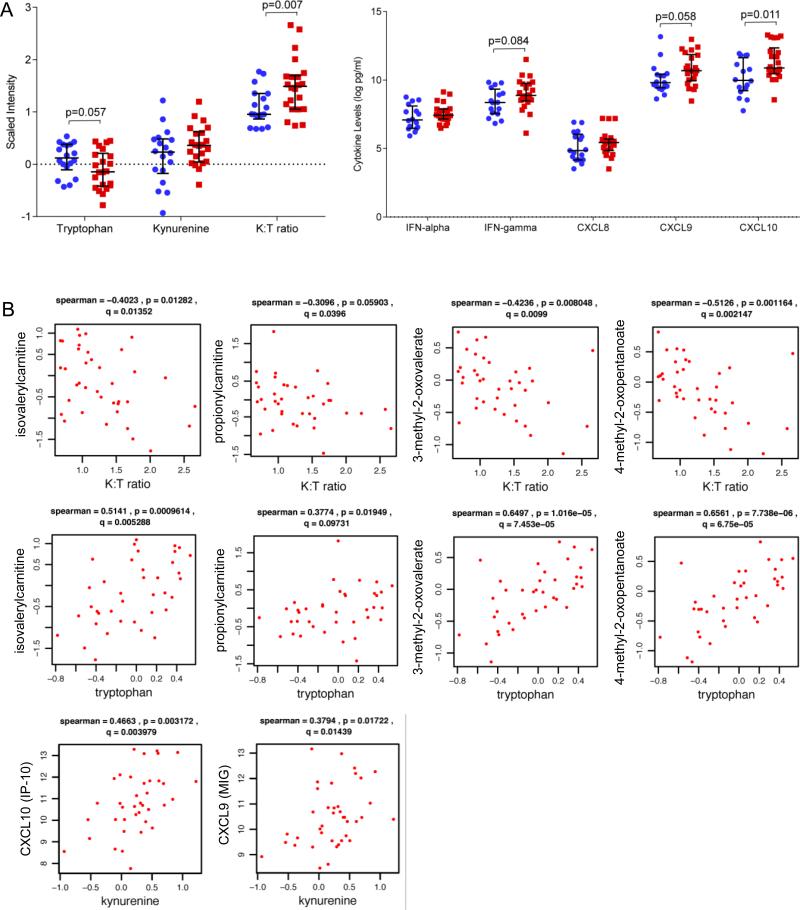
Accumulating evidence suggests that inflammation and tryptophan catabolism contribute to mechanisms involved in the pathophysiology of depression [15, 27]. Next, we examined alterations in inflammatory cytokines (IL-1-beta, IL-6, IL-10, IL-12, CCL2, CXCL8, CXCL9, CXCL10, IFN-alpha, and IFN-gamma), tryptophan and kynurenine levels, and kynurenine to tryptophan ratio (K:T ratio) in HIV-positive and -negative subjects with and without depressive symptoms across test (n=22) and validation cohorts (n=18). These analyses identified trends toward increased IFN-gamma, CXCL9, and CXCL10 in the HIV-positive test and validation cohorts in subjects with depressive symptoms (0.1<p<0.2). To minimize confounding effects of age on inflammation markers [46-48], subsequent analyses were limited to HIV subjects < age 50. Tryptophan and K:T ratio were altered across both test and validation cohorts (p<0.05, Supplemental Figure 3). Additionally, analyses of the merged HIV dataset (n=20 depressed and n=20 not depressed) identified increased IFN-gamma (p=0.084), CXCL9 (MIG; p=0.058)), and CXCL10 (IP-10; p=0.011) in HIV-positive subjects with compared to without depressive symptoms in groups matched by age, race, gender, stage of disease (current and nadir CD4), and HIV plasma viral load (p<0.05, Figure 3A). While there were trends toward similar increases in IFN-gamma, CXCL9, and CXCL10 in the HIV-negative cohort, these differences were not statistically significant. Evaluating inter-relationships between tryptophan catabolism and inflammation markers in the merged HIV dataset (n=40 subjects) identified strong associations between kynurenine and the IFN-gamma-induced chemokines CXCL9 and CXCL10 (p<0.05, FDR<10%). In contrast, tryptophan levels and K:T ratio were associated with metabolite alterations indicative of altered mitochondrial function (isovaleryl carnitine, propionylcarnitine, 3-methyl-3-oxovalerate, 4-methyl-2-oxopentanoate) (Figure 3B), including two acylcarnitine metabolites associated with depression across independent cohorts (Figures 1 and 2). These results suggest that augmented tryptophan catabolism, a marker of IFN responses, may influence biochemical alterations associated with depression.
Figure 3. Depressive symptoms are associated with augmented IFN responses and increased tryptophan catabolism in HIV patients.
A) Beeswarm plots of tryptophan catabolism metabolites and inflammation markers altered in HIV patients with (n=20, red) vs. without depressive symptoms (n=20, blue) from the merged HIV dataset (n=40, age<50 years old). HIV-positive subjects with versus without depressive symptoms were matched for age, race, gender, stage of disease (current and nadir CD4), HIV plasma viral load, and ART use. Medians are represented by horizontal bars and error bars represent IQR. P-values were calculated using Mann Whitney U test. B) Correlation plots of markers of tryptophan catabolism, IFN responses (IFN-gamma-induced chemokines CXCL9/MIG and CXCL10/IP-10), and metabolites associated with depressive symptoms in the merged HIV dataset (n=40). Spearman correlations were used to examine relationships between IFN-gamma-induced chemokines and metabolite levels. The correlation coefficient R, and p-value are shown above each plot.
Inter-relationships between plasma metabolites, depression scores, and tryptophan catabolism in HIV-positive cohorts
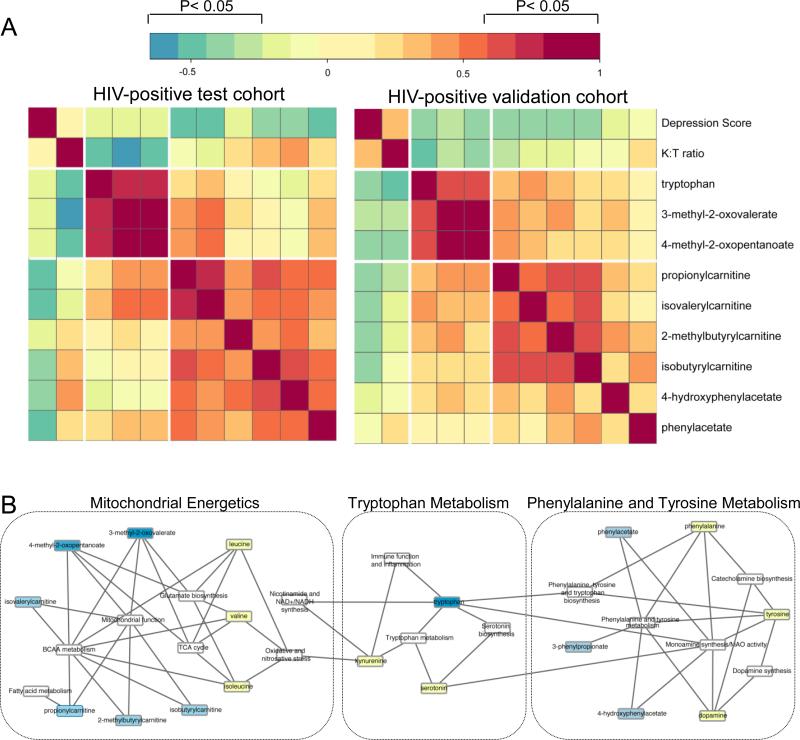
Next, we examined inter-relationships between plasma metabolites, depression scores, and tryptophan catabolism (tryptophan and K:T ratio) in the HIV-positive test and validation cohorts. Correlation analysis, matrix visualization, and hierarchical clustering identified two distinct metabolite clusters in both HIV cohorts (P<0.05, FDR<10%, Figure 4A). In cluster 1, tryptophan, 3-methyl-2-oxovalerate, and 4-methyl-2-oxopentanoate (BCAA catabolites associated with mitochondrial dysfunction) correlated negatively with K:T ratio. In cluster 2, acylcarnitines (propionylcarnitine, isovalerylcarnitine, isobutyrylcarnitine, 2-methylbutyrylcarnitine), phenylacetate, and 4-hydroxyphenylacetate correlated positively with tryptophan and BCAA catabolites, and negatively with severity of depression (depression scores [BDI or CES-D]). Unsupervised hierarchical clustering identified similar metabolite clusters in the HIV-negative cohort (Supplemental Figure 4). Mapping these inter-related metabolites to metabolic pathways (KEGG and SMPDB) and bio-functions (HMDB) identified alterations in pathways involved in monoamine metabolism (phenylalanine and tyrosine metabolism, tryptophan metabolism, serotonin biosynthesis, dopamine synthesis, and catecholamine synthesis), mitochondrial function and energy production (TCA cycle, Nicotinamide and NAD+ synthesis, fatty acid metabolism, BCAA metabolism), and immune function and inflammation (tryptophan metabolism). These results suggest that altered monoamine metabolism and subtle alterations in mitochondrial energetics may contribute to the pathogenesis of depression through mechanisms potentially augmented in HIV-positive individuals as a consequence of chronic inflammation.
Figure 4. Inter-relationships between plasma metabolites, depression scores, and markers of tryptophan catabolism in two HIV-positive cohorts.
A) The correlation matrix was constructed in R using the heatmap.2 function. Pearson correlations were used to evaluate relationships between metabolites (p<0.05, FDR≤0.1). Spearman correlations were used to examine relationships between metabolites, depression scores, and K:T ratio. Significant correlations had a −0.32> r >0.32, p<0.05, and FDR<10%. Red and blue indicate positive and negative correlations, respectively (see Color Key). B) Network model of metabolites and metabolic pathways altered in HIV patients with depression. Metabolites identified by fold change and correlation analysis (see A) were mapped to metabolite pathways, and interaction networks were generated in Cytoscape. Light blue nodes represent signature metabolites associated with depressive symptoms across independent cohorts. Dark blue nodes represent inter-related metabolites identified by correlation analysis. Yellow and white nodes represent other related metabolites and metabolic pathways, respectively.
Discussion
The pathophysiology of depression is complex. While current therapeutics primarily target monamine-related mechanisms (monoamine oxidase inhibitors, tricyclic antidepressants, selective serotonin reuptake inhibitors, noradrenaline reuptake inhibitors and serotonin and noradrenaline reuptake inhibitors), accumulating evidence suggests non-monoamine mechanisms including alterations in the HPA axis, inflammation, stress responses, and mitochondrial dysfunction may also contribute to MDD [49]. Here, we used an untargeted approach to characterize the plasma metabolome of three independent cohorts and identified a set of 6 metabolites altered in both HIV-positive and negative subjects with depression. This metabolite set (phenylacetate, 4-hydroxyphenylacetate, propionylcarnitine, isobutyrylcarnitine, isovalerylcarnitine, and 2-methylbutyrylcarnitine) correlated with the severity of depressive symptoms, and mapped to pathways involved in monoamine metabolism, mitochondrial function, and inflammation. Furthermore, correlation analysis identified inter-relationships between IFN responses (IFN-gamma, CXCL10 and CXCL9), kynurenine, and K:T ratio, and metabolites associated with mitochondrial function, suggesting that augmented IFN responses and increased tryptophan catabolism may influence mechanisms involved in the pathophysiology of MDD. Together, these results suggest that subtle alterations in the metabolism of monoamines (tryptophan [serotonin], phenylalanine [dopamine], and trace amines) and mitochondrial energetics may contribute to mechanisms involved in the development of MDD, and these processes may be influenced by inflammation during HIV infection.
Several lines of evidence suggest interactions between inflammation and depression, including increased risk of depressive symptoms in chronic inflammatory conditions (diabetes, infections, and cancer), increased inflammatory mediators in depressed versus not depressed subjects (pro-inflammatory cytokines and acute phase proteins), and development of depressive symptoms in patients undergoing cytokine therapy [14, 15, 18, 19, 28]. Factors that drive inflammation in these conditions are multifactorial and may include psychosocial stressors, increased gut permeability, alterations in the microbiome, and innate immune activation associated with chronic disease and/or infection (reviewed in [50]). In the current study, depression in HIV-infected subjects was associated with augmented IFN responses (IFN-gamma, CXCL9, CXCL10). Consistent with these findings, approximately 50% of cancer and HCV-infected or HIV/HCV-co-infected patients (also HIV/HCV co-infected) treated with IFN-alpha develop depressive symptoms, which can be reversed by antidepressants [51-54]. IFN-alpha is thought to contribute to the susceptibility to depression by altering serotonin metabolism, HPA function, and metabolic activity in the basal ganglia [27, 55, 56].
Consistent with previous studies [24, 27], depression was associated with decreased tryptophan and increased K:T ratio in two independent cohorts of HIV-infected individuals (with and without ART). These alterations correlated with other metabolic alterations associated with depression. Tryptophan is a precursor of serotonin and its bioavailability has been shown to determine the rate of serotonin synthesis in the brain [15]. Tryptophan can also be catabolized via the kynurenine pathway, forming kynurenine derivatives and nicotinamide adenine dinucleotide (NAD), a coenzyme important for energy metabolism [57]. Indoleamine 2,3-dioxygenase (IDO), a rate-limiting enzymes for tryptophan catabolism by the kynurenine pathway, is induced by inflammatory cytokines and its activity is enhanced in chronic inflammatory conditions such as atherosclerosis and obesity [58, 59]. In HIV infection, increased IDO activity predicts poor CD4+ T-cell recovery and increased mortality and has been linked to dysregulation of the Th1 to Treg balance, driving further inflammation [21, 25, 60-62]. While ART has been shown to reduce tryptophan catabolism and improve phenylalanine to tyrosine ratios in HIV-positive individuals, these metabolite alterations persist despite ART [22, 23, 30-32]. Furthermore, some kynurenine derivatives are neurotoxic (3-hydroxykynurenine and quinolinic acid) and have been shown to both agonize and antagonize the N-methyl-D-aspartate (NMDA) receptor, which plays an important role in regulating glutamatergic neurotransmission [63]. Thus, increased tryptophan catabolism via the kynurenine pathway may contribute to depression not only by effects on serotonin biosynthesis, but also by production of neurotoxic metabolites and possible effects on the glutamatergic system.
Another finding in HIV-positive and negative subjects with depression was decreased phenylacetate and 4-hydroxphenylacetate, products of phenylalanine/tyrosine catabolism derived from oxidation of phenylethylamine and tyramine through the action of MAO. However, we did not find significant differences in the Phe/Tyr ratio between subjects with and without depressive symptoms. Alterations in phenylalanine metabolism have been reported in older adults with depression [36] and depressed patients with heart failure [37]. Furthermore, decreased phenylacetate in urine was proposed as a biomarker of some forms of unipolar depression [64, 65]. Decreased dopamine (and its metabolites) and increased MAO activity have been reported in the basal ganglia of SIV-infected macaques and HIV patients with neurological disease [66-68]; these alterations also correlated with neuroinflammation and oxidative stress [68]. Further studies are required to better understand how HIV-associated inflammation may play a role in dysregulating this metabolic pathway.
Plasma metabolomics also revealed decreased acylcarnitines (propionylcarnitine, isobutyrylcarnitine, isovalerylcarnitine, and 2-methylbutyrylcarnitine) in HIV-positive and negative subjects with depression, a finding that correlated with severity of depression. Acylcarnitines are fatty acyl esters of L-carnitine that facilitate entry of long-chain fatty acids into the mitochondria for ATP synthesis. Acylcarnitine supplementation was shown to have beneficial effects in treatment of certain neurological diseases, and brain acylcarnitines have been shown to have neuroprotective effects, stabilizing membrane composition, modulating neurotransmission, protecting against excitotoxicity, and having antioxidant and anti-apototic functions (reviewed in [69]). Alterations in acylcarnitine levels are used to diagnose genetic defects in mitochondrial β-oxidation [70-72]. Several studies found evidence of altered mitochondrial function [73, 74] or decreased ATP production and alterations in respiratory chain enzymes in muscle [75] from patients with lifetime diagnosis of MDD. Furthermore, patients with mitochondrial disorders frequently have depressive symptoms, with one study reporting lifetime diagnosis of MDD in >50% of patients tested [17, 76, 77]. In the current study, acylcarnitines correlated with the BCAA catabolites 3-methyl-2-oxovalerate and 4-methyl-2-oxopentanoate. BCAA are catabolized in mitochondria and serve as an alternative energy source for metabolically active tissues [78, 79]. Furthermore, 4-methyl-2-oxopentanoate is often used to assess liver mitochondrial function and can influence glutamate synthesis in neurons [80, 81]. Several studies have implicated alterations in brain energetics in the pathogenesis of depression and effects of antidepressants [38, 82, 83]. Consistent with these observations, our results suggest a model in which subtle alterations in mitochondrial bioenergetics may contribute to the pathophysiology of depressive symptoms.
We acknowledge certain limitations of our study. While all depressed subjects had a clinical diagnosis of MDD, different depression scales were used in the HIV-positive test cohort (BDI) compared to the HIV-positive validation and HIV-negative cohorts (CES-D). HIV-positive and -negative subjects from the ALIVE study were current or past injection drug users and may not represent general populations. Samples were collected between 2002-2012 and therefore may not reflect HIV patients seen in the clinic today due to differences in ART regimens. Depression is often associated with high risk behaviors such as smoking, alcohol consumption, and drug abuse. Given potential confounding effects of these variables, these findings should be validated in cohorts without these covariates. Twenty five percent of subjects were on antidepressants. Although we cannot exclude the possibility that these medications may have contributed to metabolic alterations, subanalyses of HIV-positive and negative cohorts excluding subjects on antidepressants identified similar alterations to those reported for the total cohort. Finally, our study was cross-sectional and causality cannot be inferred. Future studies should be performed on subjects followed longitudinally, on and off ART, and before and after initiation of antidepressants.
In summary, metabolomics identified altered monoamine and acylcarnitine metabolites, along with increased inflammatory markers in HIV-positive and HIV-negative subjects with depression compared to non-depressed controls, suggesting inter-relationships between inflammation, altered monoamine metabolism (serotonin, dopamine, trace amines) and mitochondrial energetics may be involved in the biological mechanisms underlying depression. Integrated approaches targeting inflammation, monoamine metabolism, and mitochondrial pathways may be beneficial for prevention and treatment of depressive symptoms affecting quality of life and clinical outcomes in HIV-positive and HIV-negative populations suffering from MDD.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health (NIMH) grant RO1 MH097659 and National Institute on Drug Abuse (NIDA) grant R01 DA30985 to D.G. E.C. was supported in part by a fellowship from Canadian Institutes of Health Research (CIHR). NNTC and CHARTER sites were supported by NIMH and National Institute of Neurological Disorders and Stroke (NINDS) (grants U01MH083501, R24MH59724, U01MH083507, R24NS45491, U01MH083500, R24NS38841, U01MH083506, R24MH59745, U01MH083545, N01MH32002, and N01MH22005). The ALIVE study was supported by NIDA (grants U01 DA023832, R01 DA012568, and R01 DA004334). Core facilities were supported by the Harvard Center for AIDS Research grant (P30 AI060354) and Dana-Farber Cancer Institute/Harvard Cancer Center Research grant (P30 CA06516).
Footnotes
Conflicts of Interest: The authors declare that they have no competing interests.
EC performed the experiments and data analysis, drafted the manuscript, and prepared figures. VM performed bioinformatics and statistical analysis and prepared figures. SM, SM, and GK participated in study design, data analysis, and manuscript editing. DG conceived of the study, participated in its design, coordination, and data analysis, and helped write and edit the manuscript. All authors read, participated in editing the manuscript, and approved the final manuscript.
References
- 1.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 2.Moore J, Schuman P, Schoenbaum E, Boland B, Solomon L, Smith D. Severe adverse life events and depressive symptoms among women with, or at risk for, HIV infection in four cities in the United States of America. AIDS. 1999;13:2459–2468. doi: 10.1097/00002030-199912030-00018. [DOI] [PubMed] [Google Scholar]
- 3.Gold JA, Grill M, Peterson J, Pilcher C, Lee E, Hecht FM, et al. Longitudinal Characterization of Depression and Mood States Beginning in Primary HIV Infection. AIDS Behav. 2014;18:1124–1132. doi: 10.1007/s10461-013-0688-5. [DOI] [PubMed] [Google Scholar]
- 4.Do AN, Rosenberg ES, Sullivan PS, Beer L, Strine TW, Schulden JD, et al. Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS One. 2014;9:e92842. doi: 10.1371/journal.pone.0092842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5:163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- 6.Mayne TJ, Vittinghoff E, Chesney MA, Barrett DC, Coates TJ. Depressive affect and survival among gay and bisexual men infected with HIV. Arch Intern Med. 1996;156:2233–2238. [PubMed] [Google Scholar]
- 7.Leserman J, Jackson ED, Petitto JM, Golden RN, Silva SG, Perkins DO, et al. Progression to AIDS: the effects of stress, depressive symptoms, and social support. Psychosom Med. 1999;61:397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 9.Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA. 1993;270:2568–2573. [PubMed] [Google Scholar]
- 10.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58:181–187. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbie T, Mijch A, Ellen S, Hoy J, Hutchison C, Wright E, et al. Depression and neurocognitive performance in individuals with HIV/AIDS: 2-year follow-up. HIV Med. 2006;7:112–121. doi: 10.1111/j.1468-1293.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- 12.Mayeux R, Stern Y, Tang MX, Todak G, Marder K, Sano M, et al. Mortality risks in gay men with human immunodeficiency virus infection and cognitive impairment. Neurology. 1993;43:176–182. doi: 10.1212/wnl.43.1_part_1.176. [DOI] [PubMed] [Google Scholar]
- 13.Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner A, Boles RG. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:730–743. doi: 10.1016/j.pnpbp.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- 18.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anisman H, Hayley S. Inflammatory factors contribute to depression and its comorbid conditions. Sci Signal. 2012;5:pe45. doi: 10.1126/scisignal.2003579. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs D, Forsman A, Hagberg L, Larsson M, Norkrans G, Reibnegger G, et al. Immune activation and decreased tryptophan in patients with HIV-1 infection. J Interferon Res. 1990;10:599–603. doi: 10.1089/jir.1990.10.599. [DOI] [PubMed] [Google Scholar]
- 21.Huengsberg M, Winer JB, Gompels M, Round R, Ross J, Shahmanesh M. Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clin Chem. 1998;44:858–862. [PubMed] [Google Scholar]
- 22.Murray MF. Tryptophan depletion and HIV infection: a metabolic link to pathogenesis. Lancet Infect Dis. 2003;3:644–652. doi: 10.1016/s1473-3099(03)00773-4. [DOI] [PubMed] [Google Scholar]
- 23.Zangerle R, Kurz K, Neurauter G, Kitchen M, Sarcletti M, Fuchs D. Increased blood phenylalanine to tyrosine ratio in HIV-1 infection and correction following effective antiretroviral therapy. Brain Behav Immun. 2010;24:403–408. doi: 10.1016/j.bbi.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Martinez P, Tsai AC, Muzoora C, Kembabazi A, Weiser SD, Huang Y, et al. Reversal of the Kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr. 2014;65:456–462. doi: 10.1097/QAI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuchs D, Moller AA, Reibnegger G, Stockle E, Werner ER, Wachter H. Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. J Acquir Immune Defic Syndr. 1990;3:873–876. [PubMed] [Google Scholar]
- 26.Kohl C, Walch T, Huber R, Kemmler G, Neurauter G, Fuchs D, et al. Measurement of tryptophan, kynurenine and neopterin in women with and without postpartum blues. J Affect Disord. 2005;86:135–142. doi: 10.1016/j.jad.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 28.Sperner-Unterweger B, Kohl C, Fuchs D. Immune changes and neurotransmitters: possible interactions in depression? Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:268–276. doi: 10.1016/j.pnpbp.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Zangerle R, Widner B, Quirchmair G, Neurauter G, Sarcletti M, Fuchs D. Effective antiretroviral therapy reduces degradation of tryptophan in patients with HIV-1 infection. Clin Immunol. 2002;104:242–247. doi: 10.1006/clim.2002.5231. [DOI] [PubMed] [Google Scholar]
- 31.Gisslen M, Larsson M, Norkrans G, Fuchs D, Wachter H, Hagberg L. Tryptophan concentrations increase in cerebrospinal fluid and blood after zidovudine treatment in patients with HIV type 1 infection. AIDS Res Hum Retroviruses. 1994;10:947–951. doi: 10.1089/aid.1994.10.947. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs D, Gisslen M, Larsson M, Norkrans G, Hagberg L, Wachter H. Increase of tryptophan in serum and in cerebrospinal fluid of patients with HIV infection during zidovudine therapy. Adv Exp Med Biol. 1996;398:131–134. doi: 10.1007/978-1-4613-0381-7_21. [DOI] [PubMed] [Google Scholar]
- 33.Pence BW, O'Donnell JK, Gaynes BN. Falling through the cracks: the gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS. 2012;26:656–658. doi: 10.1097/QAD.0b013e3283519aae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopresti AL, Maker GL, Hood SD, Drummond PD. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:102–111. doi: 10.1016/j.pnpbp.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Kaddurah-Daouk R, Boyle SH, Matson W, Sharma S, Matson S, Zhu H, et al. Pretreatment metabotype as a predictor of response to sertraline or placebo in depressed outpatients: a proof of concept. Transl Psychiatry. 2011;1 doi: 10.1038/tp.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paige LA, Mitchell MW, Krishnan KR, Kaddurah-Daouk R, Steffens DC. A preliminary metabolomic analysis of older adults with and without depression. Int J Geriatr Psychiatry. 2007;22:418–423. doi: 10.1002/gps.1690. [DOI] [PubMed] [Google Scholar]
- 37.Steffens DC, Wei J, Krishnan KR, Karoly ED, Mitchell MW, O'Connor CM, Kaddurah-Daouk R. Metabolomic differences in heart failure patients with and without major depression. J Geriatr Psychiatry Neurol. 2010;23:138–146. doi: 10.1177/0891988709358592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaddurah-Daouk R, Bogdanov MB, Wikoff WR, Zhu H, Boyle SH, Churchill E, et al. Pharmacometabolomic mapping of early biochemical changes induced by sertraline and placebo. Transl Psychiatry. 2013;3:e223. doi: 10.1038/tp.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlahov D, Anthony JC, Munoz A, Margolick J, Nelson KE, Celentano DD, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 40.Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: Part I. Definition, disease stages, evaluation, treatment, and risk factors. Am Fam Physician. 2004;70:869–876. [PubMed] [Google Scholar]
- 41.Steer RA, Beck AT, Riskind JH, Brown G. Differentiation of depressive disorders from generalized anxiety by the Beck Depression Inventory. J Clin Psychol. 1986;42:475–478. doi: 10.1002/1097-4679(198605)42:3<475::aid-jclp2270420311>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 43.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 44.Fountoulakis KN, Bech P, Panagiotidis P, Siamouli M, Kantartzis S, Papadopoulou A, et al. Comparison of depressive indices: reliability, validity, relationship to anxiety and personality and the role of age and life events. J Affect Disord. 2007;97:187–195. doi: 10.1016/j.jad.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 46.Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longev Healthspan. 2013;2:8. doi: 10.1186/2046-2395-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 49.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 50.Berk M, Williams LJ, Jacka FN, O'Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 52.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raison CL, Woolwine BJ, Demetrashvili MF, Borisov AS, Weinreib R, Staab JP, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25:1163–1174. doi: 10.1111/j.1365-2036.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 54.Fumaz CR, Munoz-Moreno JA, Ballesteros AL, Paredes R, Ferrer MJ, Salas A, et al. Influence of the type of pegylated interferon on the onset of depressive and neuropsychiatric symptoms in HIV-HCV coinfected patients. AIDS Care. 2007;19:138–145. doi: 10.1080/09540120600645539. [DOI] [PubMed] [Google Scholar]
- 55.Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 56.Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, et al. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl) 2000;152:383–389. doi: 10.1007/s002130000549. [DOI] [PubMed] [Google Scholar]
- 57.van der Goot AT, Nollen EA. Tryptophan metabolism: entering the field of aging and age-related pathologies. Trends Mol Med. 2013;19:336–344. doi: 10.1016/j.molmed.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 59.Wirleitner B, Neurauter G, Schrocksnadel K, Frick B, Fuchs D. Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem. 2003;10:1581–1591. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- 60.Byakwaga H, Boum Y, 2nd, Huang Y, Muzoora C, Kembabazi A, Weiser SD, et al. The Kynurenine Pathway of Tryptophan Catabolism, CD4+ T-Cell Recovery, and Mortality Among HIV-Infected Ugandans Initiating Antiretroviral Therapy. J Infect Dis. 2014 doi: 10.1093/infdis/jiu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 64.Sabelli HC, Fawcett J, Gusovsky F, Javaid J, Edwards J, Jeffriess H. Urinary phenyl acetate: a diagnostic test for depression? Science. 1983;220:1187–1188. doi: 10.1126/science.6857245. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez-Sastre F, Mora J, Guillamat R, Queralto JM, Alvarez E, Udina C, Massana J. Urinary phenylacetic acid excretion in depressive patients. Acta Psychiatr Scand. 1988;78:208–210. doi: 10.1111/j.1600-0447.1988.tb06325.x. [DOI] [PubMed] [Google Scholar]
- 66.Scheller C, Sopper S, Jenuwein M, Neuen-Jacob E, Tatschner T, Grunblatt E, et al. Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. J Neurochem. 2005;95:377–387. doi: 10.1111/j.1471-4159.2005.03373.x. [DOI] [PubMed] [Google Scholar]
- 67.Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- 68.Meulendyke KA, Ubaida-Mohien C, Drewes JL, Liao Z, Gama L, Witwer KW, et al. Elevated Brain Monoamine Oxidase Activity in SIV- and HIV-associated Neurological Disease. J Infect Dis. 2014 doi: 10.1093/infdis/jiu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones LL, McDonald DA, Borum PR. Acylcarnitines: role in brain. Prog Lipid Res. 2010;49:61–75. doi: 10.1016/j.plipres.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Wanders RJ, Vreken P, den Boer ME, Wijburg FA, van Gennip AH, L IJ. Disorders of mitochondrial fatty acyl-CoA beta-oxidation. J Inherit Metab Dis. 1999;22:442–487. doi: 10.1023/a:1005504223140. [DOI] [PubMed] [Google Scholar]
- 71.Kler RS, Jackson S, Bartlett K, Bindoff LA, Eaton S, Pourfarzam M, et al. Quantitation of acyl-CoA and acylcarnitine esters accumulated during abnormal mitochondrial fatty acid oxidation. J Biol Chem. 1991;266:22932–22938. [PubMed] [Google Scholar]
- 72.Vreken P, van Lint AE, Bootsma AH, Overmars H, Wanders RJ, van Gennip AH. Quantitative plasma acylcarnitine analysis using electrospray tandem mass spectrometry for the diagnosis of organic acidaemias and fatty acid oxidation defects. J Inherit Metab Dis. 1999;22:302–306. doi: 10.1023/a:1005587617745. [DOI] [PubMed] [Google Scholar]
- 73.Suomalainen A, Majander A, Haltia M, Somer H, Lonnqvist J, Savontaus ML, Peltonen L. Multiple deletions of mitochondrial DNA in several tissues of a patient with severe retarded depression and familial progressive external ophthalmoplegia. J Clin Invest. 1992;90:61–66. doi: 10.1172/JCI115856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beasley CL, Pennington K, Behan A, Wait R, Dunn MJ, Cotter D. Proteomic analysis of the anterior cingulate cortex in the major psychiatric disorders: Evidence for disease-associated changes. Proteomics. 2006;6:3414–3425. doi: 10.1002/pmic.200500069. [DOI] [PubMed] [Google Scholar]
- 75.Gardner A, Johansson A, Wibom R, Nennesmo I, von Dobeln U, Hagenfeldt L, Hallstrom T. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord. 2003;76:55–68. doi: 10.1016/s0165-0327(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 76.Kato T. The other, forgotten genome: mitochondrial DNA and mental disorders. Mol Psychiatry. 2001;6:625–633. doi: 10.1038/sj.mp.4000926. [DOI] [PubMed] [Google Scholar]
- 77.Fattal O, Link J, Quinn K, Cohen BH, Franco K. Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS Spectr. 2007;12:429–438. doi: 10.1017/s1092852900015303. [DOI] [PubMed] [Google Scholar]
- 78.Michaletz PA, Cap L, Alpert E, Lauterburg BH. Assessment of mitochondrial function in vivo with a breath test utilizing alpha-ketoisocaproic acid. Hepatology. 1989;10:829–832. doi: 10.1002/hep.1840100513. [DOI] [PubMed] [Google Scholar]
- 79.Lauterburg BH, Liang D, Schwarzenbach FA, Breen KJ. Mitochondrial dysfunction in alcoholic patients as assessed by breath analysis. Hepatology. 1993;17:418–422. [PubMed] [Google Scholar]
- 80.Murin R, Hamprecht B. Metabolic and regulatory roles of leucine in neural cells. Neurochem Res. 2008;33:279–284. doi: 10.1007/s11064-007-9444-4. [DOI] [PubMed] [Google Scholar]
- 81.Daikhin Y, Yudkoff M. Compartmentation of brain glutamate metabolism in neurons and glia. J Nutr. 2000;130:1026S–1031S. doi: 10.1093/jn/130.4.1026S. [DOI] [PubMed] [Google Scholar]
- 82.Reus GZ, Stringari RB, Rezin GT, Fraga DB, Daufenbach JF, Scaini G, et al. Administration of memantine and imipramine alters mitochondrial respiratory chain and creatine kinase activities in rat brain. J Neural Transm. 2012;119:481–491. doi: 10.1007/s00702-011-0718-2. [DOI] [PubMed] [Google Scholar]
- 83.Scaini G, Santos PM, Benedet J, Rochi N, Gomes LM, Borges LS, et al. Evaluation of Krebs cycle enzymes in the brain of rats after chronic administration of antidepressants. Brain Res Bull. 2010;82:224–227. doi: 10.1016/j.brainresbull.2010.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.