Abstract
BACKGROUND
Albuminuria, defined as urine albumin/creatinine ratio (ACR) ≥30 mg/g, is a diagnostic component of chronic kidney disease (CKD). National estimates of ACR and CKD prevalence have been based on single random urine samples. Although 2 urine samples or a first morning void are known to produce different estimates of ACR, the impact of differing urine sampling schemes on nationally estimated rates of CKD is unknown.
METHODS
In 2009–2010, the National Health and Nutrition Examination Survey (NHANES) participants provided 2 untimed urine samples for sequential ACR measurement: an initial random urine collected in the NHANES mobile examination center and a subsequent first morning void collected at home. Rates of albuminuria were calculated in the overall population and broken down by demographics, diagnosed diabetes and hypertension status, and estimated glomerular filtration rate (eGFR).
RESULTS
Overall, 43.5% of adults with increased ACR (≥30 mg/g) in a random urine also had increased ACR in a first morning urine. This percentage was higher among individuals ≥50 years old (48.9%), males (53.3%), participants with diagnosed diabetes (56.3%) and hypertension (51.5%), and eGFR <60 mL/min/1.72m2 (56.9%). The use of confirmed increased ACR (defined as the presence of ACR ≥30 mg/g in both samples taken within 10 days) to define CKD resulted in a lower overall prevalence (11.6%) than first morning urine (12.7%) or random spot urine only (15.2%).
CONCLUSIONS
ACR measured on random urine samples appears to overestimate the prevalence of albuminuria compared to first morning urine collections.
More than 10% of US adults meet the diagnostic criteria for chronic kidney disease (CKD)4 (1), defined as reduced kidney function or the presence of kidney damage for at least 3 months regardless of kidney function. Kidney damage is ascertained by increased urinary markers, such as albuminuria, or by abnormal urinary sediment, abnormal imaging studies, or kidney biopsy (2). Albuminuria is defined by a urinary albumin-to-creatinine ratio (ACR) ≥30 mg/g, is often considered an early marker of glomerular kidney diseases, and is associated with an increased risk of death independent of the level of kidney function (3). In the United States, previous studies have estimated the population prevalence of increased ACR as 7.8% on the basis of a single random urine sample (4).
Owing to the high within-individual variation of ACR (5, 6), clinical recommendations for diagnosing CKD when glomerular filtration rate (GFR) is within reference intervals require that at least 2 urinary samples collected within 3–6 months contain ≥30 mg albumin per g creatinine in the absence of clinical or laboratory evidence of urinary tract infection (7). Although a 24-h urine collection is considered the gold standard for detecting increased albumin excretion, this method is laborious and prone to collection error and therefore impractical for surveillance. ACR measured in a spot morning urine collection correlates well with the timed excretion rate in cross-sectional studies and is a strong predictor of kidney disease progression in longitudinal studies (8, 9). ACR measurements in random untimed urine samples generally show a higher within-person biological CV than is found for first or second morning samples (5). However, comparisons between studies are difficult due to lack of standardized methodology.
In the United States, persistence of albuminuria, on the basis of a repeat examination at the National Health and Nutrition Examination Survey (NHANES) Mobile Examination Center (10), was previously estimated at approximately 63% in a subsample of 1988–1994 adult participants. It is not known if changes in the demographics and disease characteristics of the US population since the early 1990s have influenced this estimate. In NHANES 2009–2010, 2 urine samples were collected for ACR determination in participants aged 6 years and older, including an initial random spot urine at the Mobile Examination Center and a subsequent first morning void at home. This report presents distributions of random and first morning urine measures, measures of agreement between the 2 urine collections, and highlights related to methodological issues and differences in national prevalence estimates for CKD.
Methods
NHANES is a cross-sectional survey of the health and nutritional status of the US civilian, noninstitutionalized population conducted by the National Center for Health Statistics, CDC (11, 12). Participants were randomly selected through a complex, multistage cluster sampling probability design. NHANES collects data through interviews in participants’ homes and conducts medical examinations and laboratory assessments in the Mobile Examination Center, where survey participants are randomly assigned to either a morning or afternoon/evening examination session. For this report, we analyzed NHANES data from the survey period 2009–2010 on participants who were 20 years or older and also had 1 subsequent measurement of urinary albumin, creatinine, and ACR (n = 5247) (11–13). Participation in both the interview and physical examination was 77.3% (13). Approximately 89% of adults who had a random urine ACR measurement during the Mobile Examination Center also completed the first morning void home urine collection.
URINE SAMPLE COLLECTION
During the Mobile Examination Center examination session, participants were asked to provide an initial random spot urine sample (12). In addition, participants were provided with a kit for a home urine collection (14–16) and asked to collect a first morning void urine sample in their homes and mail it to the NHANES laboratory within 10 days of the initial Mobile Examination Center exam. The home sample collection kit included a Styrofoam shipping container, refrigerant gel pack pouch (Polar Pack®), a 60-mL plastic sample cup with screw-on lid, Ziploc® plastic bag with absorbent pad, temperature monitoring strip, and instructions on how to collect and mail the urine sample to the testing laboratory. The contents of the home urine collection kit were explained to the participants by the Mobile Examination Center examiner, who provided verbal, written, and visual instructions to reinforce the procedure. A self-addressed, stamped shipping container with a US Postal Service Priority Mail shipping label to the analytical laboratory was also provided (14).
The initial random urine collection at the Mobile Examination Center is referred to henceforth as the random sample, and the first morning void home urine collection is the first morning sample.
LABORATORY MEASUREMENTS AND DEFINITIONS
To measure urine albumin, we used a fluorescence immunoassay as described by Chavers et al. (17). The assay involves a solid-phase, noncompetitive, double-antibody reaction. Urine-sample albumin antigen reacts with albumin antibody that is covalently attached to polyacrylamide beads. The resulting solid-phase antibody complex is then reacted with fluoresceinlabeled antibody. Unattached fluorescent antibody and other proteins are removed by centrifugation. We measured the fluorescence of the stable solid-phase double-antibody complex with a Turner Digital 450 Fluorometer (Sequoia-Turner Corp.) with excitation wavelength 485 nm and emission wavelength 525 nm. More details on the urine albumin assay method are available (15). The interrun assay CV for urine albumin was 4.4%–12.0% for a mean concentration range of 1.9 –16.3 mg/L.
To measure urine creatinine, we used a coupled end-point enzymatic method in which urine creatinine is converted to creatine by creatininase. Creatine is then converted to sarcosine and urea via creatinase. Sarcosine oxidase converts sarcosine to glycine and hydrogen peroxide, and the hydrogen peroxide reacts with a chromophore HTIB (2,4,6-triiodo-3-hydroxybenzoic acid) by the use of peroxidase to produce a quinone imine chromogen product measured at 546 nm (secondary wavelength at 700 nm). We performed the urine creatinine method on a Roche/Hitachi Modular P analyzer (Roche Diagnostics). More details on the urine creatinine assay method are available (16). The interrun assay CV for urine creatinine was 1.4%–4.4% for a mean concentration range of 18.5–97.1 mg/dL.
We estimated urinary albumin excretion by computing the ACR in units of milligrams per gram. ACR was considered increased if ACR was ≥30 mg/g. Confirmed albuminuria was defined by ACR ≥30 mg/g in both random and first morning samples. Increased ACR was also defined by sex-specific values as ≥35 mg/g for women and ≥25 mg/g for men in separate analyses (18).
Diagnosed diabetes was defined as a positive response to the question, “Have you ever been told by a doctor or other health care professional that you had diabetes?”
Blood pressure was measured during the NHANES physical examination in the mobile examination center. Three blood pressure measurements were taken by a physician after a 5-min rest following the NHANES standard protocol, and the mean of the measurements was used for the analysis (19).
Hypertension was defined by either a positive response to 1 of the following questions: “Have you ever been told by a doctor or other healthcare professional that you had hypertension, also called high blood pressure?” or “Are you now taking prescribed medication for hypertension?,” or by measured systolic blood pressure > 140 mmHg and /or diastolic blood pressure >90 mmHg. A few participants (approximately 2%) had only 1 or 2 blood pressure measurements. The mean of 2 measurements or the single measurement was used for these participants.
To obtain estimated GFR (eGFR) based on serum creatinine, we used the Modification of Diet in Renal Disease (MDRD) and Chronic Kidney Disease Epidemiology Collaboration (CKD-Epi) equations (20, 21). eGFR is based on a single measurement of serum creatinine.
STATISTICAL ANALYSIS
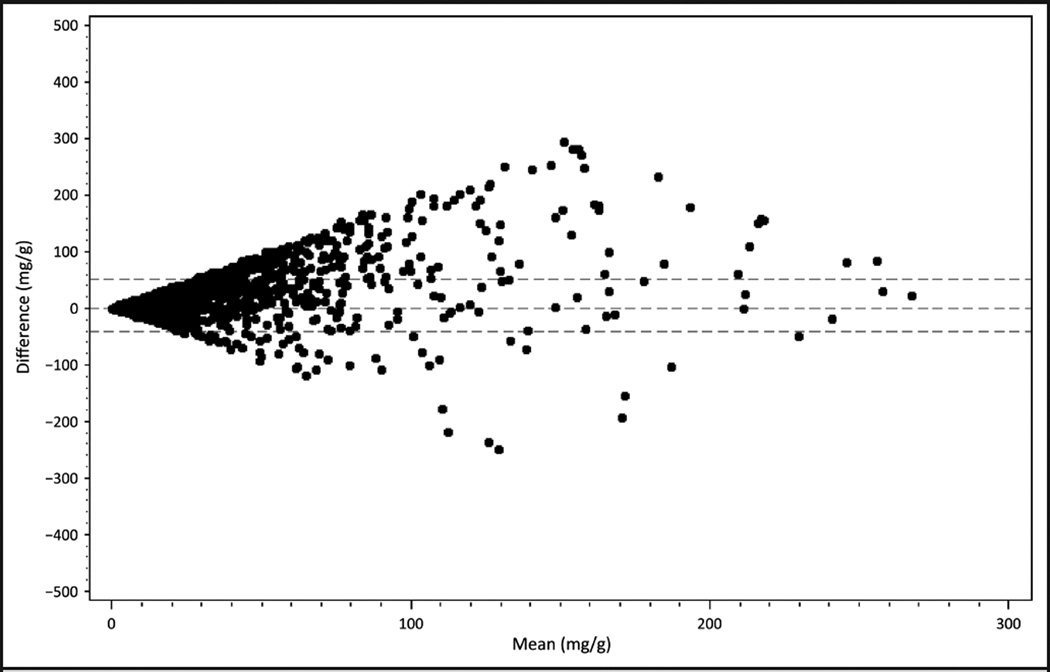
The distributions of urinary albumin, creatinine, and ACR were estimated for the random and first morning samples. Bland–Altman plot was used to look at the relationship between the paired differences of random ACR and first morning ACR with the mean of the 2. To improve the fit of the graph, participants with ACR ≥300 (n = 128) were excluded from the plot (22, 23).
The percentages of participants with increased ACR according to each sample and the percentages of confirmed increased ACR are presented in all participants and broken down by selected characteristics. Percent confirmed increased ACR was defined as increased ACR in first morning urine among participants with increased ACR in random urine.
To show the impact of the use of both urine collections for ACR measurement vs a single spot urine collection, we estimated the prevalence of CKD defined by eGFR <60 mL · min−1 · (1.73 m2)−1 or confirmed increased ACR and by eGFR <60 mL · min−1 · (1.73 m2)−1 or increased random ACR.
Concordance between the 2 measures was calculated by use of the κ statistic and observed agreement. The κ statistic was used as a quantitative measure of agreement for increased ACR status between the 2 urine collections, overall, and by demographic characteristics, diabetes, and hypertension. A κ coefficient of 1 indicates perfect agreement for increased ACR, whereas a κ coefficient of 0 indicates agreement equivalent to chance. Sample weights were used in the calculation of the κ coefficient. The percent agreement for increased ACR status was calculated on the basis of a 2 × 2 table as [(number concordant for increased ACR + number concordant for ACR within reference intervals)/total number] × 100. Both measures of concordance are based on 2 × 2 tables with ACR categories of <30 mg/g and ≥30 mg/g.
We used 2-sided t-tests to compare groups and considered P < 0.05 statistically significant. Sample weights were used to produce nationally representative estimates that accounted for the NHANES complex survey design, including its stratified multistage cluster sampling. We used SAS 9.2 for the Bland–Altman plot and κ analysis and SUDAAN 10 software (Research Triangle Institute) for the other statistical analyses.
Results
Clinical and demographic characteristics of the 5247 adults who completed both random and first morning collections and the 812 adults who provided a random urine sample only (i.e., did not mail the home urine collection) are shown in Table 1. Participants who completed only the random urine collection were younger (P = 0.001), more likely to be Hispanic or non-Hispanic black (P = 0.001), and less likely to have hypertension (P = 0.003) or decreased eGFR (P = 0.004) compared with adults who completed both urine collections. Of the participants who completed both urine collections, 77.4% were non-Hispanic whites, 44.9% were at least 50 years old, and 36.2% had hypertension.
Table 1.
Characteristics of adult participants (age ≥20 years) in NHANES 2009–2010 who had a random urine ACR measurement only, and those with ACR measurements in both random and first morning urine samples.a
| Random urine ACR only |
Both random and first morning urine ACR |
χ2P value |
|
|---|---|---|---|
| Number of participants | 812 | 5,247 | |
| Age, years | <0.001 | ||
| 20–49 | 70.3 (2.23) | 55.1 (1.46) | |
| ≥50 | 29.7 (2.23) | 44.9 (1.46) | |
| Male | 52.2 (1.83) | 47.8 (0.49) | 0.07 |
| Female | 48.8 (1.83) | 52.2 (0.49) | |
| Race/ethnicityb | 0.001 | ||
| Non-Hispanic white | 63.5 (4.96) | 74.4 (3.04) | |
| Non-Hispanic black | 17.0 (2.10) | 11.6 (1.00) | |
| Hispanic | 19.5 (4.59) | 14.0 (2.91) | |
| Diabetesc | 8.1 (1.70) | 8.6 (0.42) | 0.80 |
| Hypertensiond | 28.4 (1.94) | 36.2 (1.35) | 0.003 |
| eGFR,emL · min−1 · (1.73 m2)−1 | 0.004 | ||
| ≥90 | 63.0 (2.24) | 55.5 (1.39) | |
| 60–89 | 30.5 (2.29) | 39.0 (1.25) | |
| 30–59 | 5.3 (0.86) | 5.0 (0.39) |
Data are weighted percent ± SE.
“Other” not listed owing to small sample size (n = 298).
Defined by self-report of diagnosis.
Defined by self-report of diagnosis, self-reported anti-hypertensive treatment, or measured systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.
Defined by MDRD equation; <30 mL · min−1 · (1.73 m2)−1 not listed owing to small sample size (n = 33).
The means and distributions of albumin and creatinine are reported in Supplemental Table 1, which accompanies the online version of this article at http://www.clinchem.org/content/vol59/issue4. Median albumin concentration was 6.3 mg/L in the random sample and 5.6 mg/L in the first morning void. Median creatinine concentration was 104 mg/dL in the random sample and 117 mg/dL in the first morning void.
Means and distributions of ACR in the random and first morning urine samples are presented in the Table 2. The median ACR was 6.0 mg/g in the random sample and 4.5 mg/g in the first morning void. First morning ACR and random ACR were highly correlated (Pearson correlation coefficient r = 0.826, P < 0.001). Further, on the basis of linear regression models of the random ACR and first morning ACR, the R2 value was 0.683, indicating that close to 70% of the variation observed in the random ACR was explained by the first morning ACR. The analysis was repeated separately for males and females (see online Supplemental Table 2). Although similar patterns are observed for both males and females, the Pearson correlation for ACR and albumin was much higher in males (r = 0.956 and 0.859, respectively) compared with females (r = 0.612 and 0.672, respectively).
Table 2.
Means and distributions of ACR in the random and first morning urine samples collected within 10 days in adults ≥20 years old, NHANES 2009–2010 (n = 5247).a
| Random sample |
First morning sample |
|
|---|---|---|
| ACR, mg/g | ||
| Mean | 23.7 | 15.9 |
| 95th percentile | 46.5 | 28.2 |
| 75th percentile | 10.6 | 6.9 |
| 50th percentile | 6.0 | 4.5 |
| 25th percentile | 3.9 | 3.2 |
| 5th percentile | 2.4 | 2.1 |
Pearson correlation between random and first morning ACR, 0.826v (P <0.001).
From the Bland–Altman plot presented in Fig. 1, the larger the mean of the random and first morning samples, the greater the differences of those values from zero. The horizontal lines indicate the 95% confidence bands around zero on the graph. First morning ACR and random ACR were highly correlated (Pearson r = 0.826; P < 0.001). Further, on the basis of linear regression models of the random ACR and first morning ACR, the R2 value was 0.683, indicating that close to 70% of the variation observed in the random ACR was explained by the first morning ACR.
Fig. 1. Bland–Altman plot of random and first morning ACR for adult participants with ACR <300 (n = 5119), NHANES 2009–2010.
Horizontal dashed lines on either side of zero represent the 95% CIs for the difference between random and first morning ACR.
The proportion of participants with albuminuria in each of the 2 urine collections is presented in Table 3 according to demographic and clinical characteristics. The percent of participants with increased ACR was highest for the random urine alone (overall 7.7%), followed by the first morning urine (overall 4.7%). Only 3.5% of all participants had increased ACR in both the random and first morning samples. Among participants with increased ACR in the random urine, 43.5% of participants had confirmed increased ACR in the first morning urine, with a higher agreement in those 50 years and older, men, Hispanics, and participants with diabetes, hypertension, or decreased eGFR. When sex-specific definitions for increased ACR were used, similar patterns were found (see online Supplemental Table 3). However, confirmed increased ACR was higher among males (53.8%) compared with females (36.4%), even when sex-specific cutpoints were used (see online Supplemental Table 3).
Table 3.
Albuminuria† in random urine and first morning void urine samples, adults with both urine samples available for the NHANES 2009–2010.a
| Increased ACR in random sample |
Increased ACR in first morning sample |
Increased ACR in random and first morning sample |
Confirmed increased ACR in first morning sample among those with increased ACR in random sample |
|
|---|---|---|---|---|
| n | 5247 | 5247 | 5247 | 556 |
| Overall | 7.7 (6.4–9.1) | 4.7 (3.8–5.6) | 3.6 (2.6–4.5) | 43.5 (35.8–51.2) |
| Age 20–49 years | 4.7 (3.9–5.6) | 2.8 (2.0–3.6) | 1.6 (1.0–2.2) | 32.8 (22.4–43.3) |
| Age ≥50 years | 11.4 (8.9–13.9) | 7.1 (5.7–8.5) | 6.0 (4.4–7.6) | 48.9 (41.2–56.7) |
| Male | 7.2 (5.8–8.6) | 4.6 (3.6–5.5) | 4.0 (3.0–5.0) | 53.1 (41.4–64.9) |
| Female | 8.2 (6.4–10.0) | 4.9 (3.5–6.2) | 3.2 (2.0–4.3) | 35.8 (27.5–44.0) |
| Hispanic | 9.8 (7.2–12.3) | 5.8 (4.1–7.5) | 5.1 (3.4–6.8) | 49.0 (42.1–55.8) |
| Non-Hispanic white | 7.0 (5.3–8.7) | 4.2 (3.0–5.4) | 3.0 (1.9–4.2) | 41.0 (29.0–52.9) |
| Non-Hispanic black | 9.9 (7.1–12.7) | 6.2 (4.2–8.2) | 4.7 (3.0–6.5) | 44.50 (35.3–53.7) |
| Diabetesb | 24.1 (19.9–28.3) | 15.7 (12.3–19.0) | 15.5 (11.9–19.2) | 56.3 (46.7–65.8) |
| No diabetes | 6.2 (4.9–7.5) | 3.7 (2.9–4.5) | 2.5 (1.8–3.3) | 38.9 (30.2–47.6) |
| Hypertensionc | 13.7 (11.2–16.3) | 8.4 (6.8–10.0) | 7.8 (6.2–9.4) | 51.5 (43.7–59.3) |
| No hypertension | 5.1 (3.9–6.2) | 3.1 (2.2–4.0) | 1.7 (0.9–2.5) | 29.5 (19.7–39.4) |
| eGFR,dmL · min−1 · (1.73 m2)−1 | ||||
| ≥90 | 5.5 (4.3–6.7) | 3.4 (2.5–4.4) | 2.0 (1.2–2.9) | 35.0 (24.9–45.1) |
| 60–89 | 8.1 (5.8–10.4) | 4.3 (3.0–5.6) | 3.6 (2.2–5.0) | 41.7 (28.1–55.3) |
| 30–59 | 23.2 (16.6–29.8) | 16.0 (8.9–23.1) | 15.2 (9.1–21.2) | 56.9 (41.1–72.7) |
Data are % (95% CI). Increased ACR defined as ≥30 mg/g. Confirmed albuminuria is defined by the presence of ACR ≥30 mg/g in 2 samples taken within 10 days.
Diagnosed by self-report of diagnosis.
Defined by self-report of diagnosis, self-reported anti-hypertensive treatment, or measured systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.
Defined by MDRD equation; <30 mL · min−1 · (1.73 m2)−1 not listed owing to small sample size (n = 50).
Concordance of ACR category (increased or not) between the 2 samples was estimated by κ and percent agreement. The κ agreement for albuminuria status between the 2 samples was moderate in the overall population (0.51), and higher in males and in those with diabetes or hypertension (Table 4). The observed agreement for ACR status between the 2 measures was 94.3%, with lowest proportion among adults with diabetes and eGFR between 30 and 59 mL · min−1 · (1.73 m2)−1 (87.4%, 95% CI 84.6 –90.1) and highest proportion in those 20–49 years old (95.6%, 95% CI 94. 9–96.3) and men (95.9%, 95% CI 94.6 –97.1).
Table 4.
Concordance using κ statistics and observed agreement between the random and first morning urine samples for albuminuria in adult participants in NHANES 2009–2010 (n = 5247).a
| Variable | κ | Agreement, % (95% CI) |
|---|---|---|
| Overall | 0.51 | 94.3 (93.2–95.3) |
| Age 20–49 years | 0.39 | 95.6 (94.9–96.3) |
| Age ≥50 years | 0.57 | 92.7 (90.7–94.6) |
| Male | 0.63 | 95.9 (94.6–97.1) |
| Female | 0.41 | 92.8 (91.5–94.1) |
| Hispanic | 0.58 | 94.0 (92.5–95.5) |
| Non-Hispanic white | 0.48 | 94.5 (93.1–95.9) |
| Non-Hispanic black | 0.51 | 92.7 (90.3–95.2) |
| Diabetesb | 0.61 | 87.4 (84.6–90.1) |
| No diabetes | 0.46 | 94.9 (94.0–96.0) |
| Hypertensionc | 0.61 | 92.3 (90.1–94.5) |
| No hypertension | 0.38 | 95.4 (94.6–96.2) |
| eGFR,dmL · min−1 · (1.73 m2)−1 | ||
| ≥90 | 0.41 | 94.9 (94.0–95.8) |
| 60–89 | 0.51 | 94.4 (92.5–96.2) |
| 30–59 | 0.60 | 87.2 (81.9–92.6) |
Percent agreement defined as observed agreement based on agreement of increased ACR and non-increased ACR [# concordant (on diagonal)/total #] × 100. κ defined as (observed agreement − expected agreement)/(1 − expected agreement).
Diagnosed by self-report of diagnosis.
Defined by self-report of diagnosis, self-reported anti-hypertensive treatment, or measured systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.
Defined by MDRD equation; <30 mL · min−1 · (1.73 m2)−1 not listed owing to small sample size (n = 50).
When CKD was defined by a confirmed increased ACR or the MDRD eGFR <60 mL · min−1 · (1.73 m2)−1, the age-standardized prevalence of CKD was 11.6% (95% CI 9.9 –13.3). This was lower than the prevalence determined from increased ACR in the first morning urine (12.7%, 95% CI 11.0 –14.3) or increased ACR in the random urine measurement (15.2%, 95% CI 13.3–17.1) (Table 5). Similar results were seen with the CKD-Epi equation (see online Supplemental Table 3). The prevalence of CKD by a confirmed increased ACR or the CKD-Epi eGFR <60 mL · min−1 · (1.73 m2)−1 was 13.2% (95% CI 11.5– 14.9), whereas the prevalence based on the first morning urine was 12.2% (95% CI 10.5–13.9) and that based on the random urine measurement was 15.7% (95% CI 13.9 –17.6).
Table 5.
Age-standardized prevalence (95% CI) of CKD in adult participants in NHANES 2009–2010 who had repeat ACR measurements (n = 5247).a
| Variable | CKD based on random ACR |
CKD based on first morning ACR |
CKD based on confirmed increased ACR |
|---|---|---|---|
| All | 15.2 (13.3–17.1) | 12.7 (11.0–14.3) | 11.6 (9.9–13.3) |
| Age 20–49 years | 8.7 (7.1–10.3) | 6.7 (5.1–8.4) | 5.6 (4.0–7.3) |
| Age ≥50 years | 24.0 (21.4–26.7) | 20.8 (18.8–22.9) | 19.6 (17.5–21.8) |
| Male | 13.2 (11.3–15.1) | 11.0 (9.1–12.9) | 10.4 (8.4–12.5) |
| Female | 17.0 (14.6–19.4) | 14.3 (12.3–16.0) | 12.6 (10.9–14.4) |
| Hispanic | 16.3 (13.3–19.3) | 11.9 (9.8–14.1) | 11.1 (8.8–12.5) |
| Non-Hispanic white | 13.9 (11.7–16.1) | 11.6 (9.9–13.4) | 10.7 (8.9–12.5) |
| Non-Hispanic black | 21.1 (18.1–24.1) | 18.1 (15.5–20.7) | 16.4 (14.3–18.6) |
| Diabetesb | 29.6 (22.7–36.5) | 23.4 (17.2–29.6) | 22.7 (16.5–28.8) |
| No diabetes | 13.5 (11.7–15.2) | 11.28 (9.9–12.1) | 10.2 (8.8–11.7) |
| Hypertensionc | 20.1 (16.5–23.6) | 16.51 (13.1–19.9) | 15.79 (12.5–19.1) |
| No hypertension | 11.19 (8.9–13.4) | 9.27 (7.17–11.5) | 8.02 (5.9–10.2) |
CKD was defined by eGFR <60 mL · min−1 · (1.73 m2)−1 (MDRD equation), ACR ≥30 mg/g in the random sample only, ACR ≥30 mg/g in the first morning sample, or confirmed increased ACR in both samples. Results are age standardized to the 2000 US Census standard population using the following age groups and corresponding weights (20–44 years, 0.511356; 45–64 years, 0.311417; ≥65 years, 0.177227), except for age-group estimates.
Diagnosed by self-report of diagnosis.
Defined by self-report of diagnosis, self-reported anti-hypertensive treatment, or measured systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.
Discussion
The occurrence of increased ACR among NHANES 2009–2010 adult participants was significantly different when results from random urine collections were compared to results from first morning samples. Overall, we observed fewer increased ACR values in the first morning void. Estimates of prevalence of albuminuria are higher when measured from random urine than from first morning voids. Although both measurements were performed in laboratories by the use of the same analytic methods, different methodologies were used for the collection of samples. These results indicate that ACR values were primarily affected by preanalytic factors, such as when (i.e., random or first morning) and how the urine was collected during the day and shipped to the laboratory. Although regulatory agencies develop and maintain best practices by requesting that research and clinical laboratories maintain control over preanalytical, analytical, and postanalytical processes, the sample collected for urine albumin is not standardized (5, 24, 25).
Albuminuria is important in identifying people with CKD in the clinical and public health settings. Increased urine albumin is the diagnostic criterion for approximately half of the people identified with CKD in the United States. First morning urine is generally accepted as a more reliable indication of albumin excretion, since it corresponds closely with 24-h urine collection. A 24-h urine collection is often considered the gold standard for detecting increased ACR. Studies comparing a first morning void to a 24-h urine collection to detect increased ACR found high sensitivity (>94%) and specificity (98%) (26–32). Studies that compared a random urine to a 24-h urine collection to detect increased ACR found a wider range of sensitivities (48%–93%)((26, 27, 28, 30, 32). However, these studies had small sample sizes (26, 27), included only individuals with diabetes or kidney disease (26, 28), or were not representative of the general US population (29, 33).
Preanalytic factors, specifically whether the urine was collected from a random urine or first morning void, may have an important bearing on identification of people with CKD based on urine albumin. The use of either confirmed increased ACR or eGFR to define CKD lowers the estimated prevalence of CKD among adults from 15.2% with a random urine or 12.7% with first morning urine to 11.6%.
Several factors suggest caution when analyzing the ACR measurements. First, diurnal fluctuation in albumin excretion has been established to range between 20% and 50%, (5, 33), with the lowest excretion during nocturnal recumbence and in the morning, and higher excretion during daytime owing primarily to orthostasis and higher GFR (34). Indeed, the proportion of people in whom increased ACR was confirmed in the NHANES sample was much lower than the 63% persistence estimated among 1241 participants in NHANES III (1988 –1994) (10). However, both urine samples in NHANES III were random collections.
In NHANES III, both urine samples were collected at the Mobile Examination Center and therefore handled in uniform fashion, whereas in NHANES 2009–2010 only the random sample was collected at the Mobile Examination Center unit; the first morning void was collected at home and subsequently mailed to the NHANES laboratory. The different collection, handling, and storage conditions before laboratory analysis might have affected the consistency of the results across the 2 collections. Previous studies have indicated that albumin is stable in urine stored at 4 °C for up to 4 weeks (35); however, factors such as long storage at room temperature and bacterial contamination might alter the albumin immunoassay results. In an effort to reduce these potential factors in NHANES 2009–2010, the participants received detailed instructions for the home urine collection on how to collect and ship the sample and were provided with refrigerant packs and a temperature strip to detect high temperatures during shipping. The laboratory did not perform the albumin assay when the temperature strip reading was above 54 °C.
In clinical practice, patients often are asked initially to provide a random urine sample to screen for chronic kidney disease. If increased ACR is detected, these patients are typically advised to provide a first morning urine sample for confirmation of the diagnosis. However, there is little agreement across clinical professional organizations on the preferred method for diagnosis (5). These results show that groups with a higher prevalence of chronic kidney disease (i.e., older adults, adults with diabetes or hypertension) were also more likely to have persistently increased ACR. It is important to confirm persistence of albuminuria in diagnosing CKD for both public health and clinical purposes. Although both the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (7) and the American Diabetes Association (36) recommend screening for albuminuria in all patients at risk for kidney disease, inconsistency in measurement of ACR is also evident at the national level. According to US Renal Data System reports, the likelihood of a serum creatinine test in patients at risk for CKD is 5–13 times higher than that of a urinary albumin test across demographic categories (37). Only 1 in 3 patients with diabetes was tested for albuminuria in 2009, whereas 87% received a serum creatinine test. Among Medicare patients diagnosed with both diabetes and hypertension, the probability of being tested for albuminuria was 35%, and for serum creatinine, 93% (37). A survey conducted among general practitioners in 9 European countries found that 45%–77% of patients with an initially increased ACR received repeat testing, with large variations described in the type of samples tested and reported measurement units (38). Data on the use of persistence of albuminuria to diagnose CKD in clinical practice are not available for the United States.
In addition to the limitations in the data mentioned previously, it is also important to remember that NHANES is a survey of the noninstitutionalized population. Therefore, individuals living in nursing homes are not included. These individuals tend to be older and are more likely to have chronic conditions, both of which may increase the prevalence of increased ACR. However, NHANES is a nationally representative sample of the noninstitutionalized population, and current national estimates of the prevalence of CKD are based on data from NHANES (1).
Correct assessment of albumin excretion is a key step in the early detection and management of CKD. These results support previous studies highlighting the differences in prevalence rates of albuminuria on the basis of when a urine sample is collected. Clinicians should be aware of these differences and be careful to repeat positive random urine albumin results on a first morning void sample to avoid over-diagnosing patients with CKD based on albuminuria. Public health researchers need to be aware of these observations when using surveys to estimate increased ACR and chronic kidney disease. Increased ACR based on single random urine will likely overestimate the prevalence of both albuminuria and CKD in the population.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Role of Sponsor: No sponsor was declared.
Footnotes
Nonstandard abbreviations: CKD, chronic kidney disease; ACR, urinary albuminto-creatinine ratio; GFR, glomerular filtration rate; NHANES, National Health and Nutrition Examination Survey; HTIB, 2,4,6-triiodo-3-hydroxybenzoic acid; eGFR, estimated GFR; MDRD, Modification of Diet in Renal Disease; KDOQI, Kidney Disease Outcomes Quality Initiative.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: No authors declared any potential conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta (GA): CDC; 2010. [Google Scholar]
- 2.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39:s1–s266. [PubMed] [Google Scholar]
- 3.O’Hare AM, Hailpern SM, Pavkov ME, Rios-Burrows N, Gupta I, Maynard C, et al. Prognostic implications of the urinary albumin to creatinine ratio in veterans of different ages with diabetes. Arch Intern Med. 2010;170:930–936. doi: 10.1001/archinternmed.2010.129. [DOI] [PubMed] [Google Scholar]
- 4.Jones CA, Francis ME, Eberhardt MS, Chavers B, Coresh J, Engelgau M, et al. Microalbuminuria in the U.S. population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39:445–459. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- 5.Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55:24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- 6.Pugliese G, Solini A, Fondelli C, Trevisan R, Vedovato M, Nicolucci A, et al. Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the renal insufficiency and cardiovascular events (RIACE) study. Nephrol Dial Transplant. 2011;26:3950–3954. doi: 10.1093/ndt/gfr140. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation. K/DOQI clinical practice guidelines and clinical recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49:S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg JM, Chang BS, Matarese RA, Garella S. Use of a single voided urine sample to estimate quantitative proteinuria. N Engl J Med. 1983;309:1543–1546. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]
- 9.Heerspink HJL, Gansevoort RT, Brenner BM, Cooper ME, Parving HH, Shahinfar S, de Zeeuw D. Comparison of different measures of urinary protein excretion for the prediction of renal events. J Am Soc Nephrol. 2010;21:1355–1360. doi: 10.1681/ASN.2010010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult U.S. population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 11.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey: NHANES 2009–2010. [Accessed February 2013]; http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/nhanes09_10.htm.
- 12.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey: survey questionnaires, examination components and laboratory components 2009–2010. [Accessed February 2013];Blood and urine collection. http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/questexam09_10.htm. See “Laboratory components.”.
- 13.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey Response Rates and CPS Totals, 2009–2010. [Accessed April 2012]; http://www.cdc.gov/nchs/nhanes/response_rates_cps.htm.
- 14.CDC, National Center for Health Statistics. NHANES 2009–2010, home urine collection manual. [Accessed April 2012]; http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/homeurine.pdf.
- 15.CDC, National Center for Health Statistics. NHANES 2009–2010 laboratory manual: albumin. [Accessed April 2012]; http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/alb_cr_f_met_albumin.pdf.
- 16.CDC, National Center for Health Statistics. NHANES 2009–2010 laboratory manual: creatinine. [Accessed April 2012]; http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/alb_cr_f_met_creatinine.pdf.
- 17.Chavers BM, Simonson J, Michael AF. A solid-phase fluorescent immunoassay for the measurement of human urinary albumin. Kidney Int. 1984;25:576–578. doi: 10.1038/ki.1984.57. [DOI] [PubMed] [Google Scholar]
- 18.Chadban S, Howell M, Twigg S, Thomas M, Jermus G, Cass A, Campbell D, Nicholls K, Tong A, Mangos G, Stack A, MacIsaac RJ, Girgis S, Colagiuri R, Colagiuri S, Craig J. Assessment of kidney function in type 2 diabetes. Nephrology. 2010;15:S146–S161. doi: 10.1111/j.1440-1797.2010.01239.x. [DOI] [PubMed] [Google Scholar]
- 19.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey 2009–2010. Examination documentation. [Accessed April 2012];Blood pressure collection. http://www.cdc.gov/nchs/nhanes/nhanes2009–2010/bpx_f.Htm.
- 20.Levey AS, Busch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Statistician. 1983;32:307–317. [Google Scholar]
- 23.Dewitte K, Fierens C, Stöckl D, Thienport LM. Application of the Bland-Altman plot for interpretation of method comparison studies: a clinical investigation of its practice. Clin Chem. 2002;48:799–801. [PubMed] [Google Scholar]
- 24.NIDDK Best Practices for Sample Storage: a report from the workshop on urine biospecimen handling best practices for sample storage: urine as a paradigm. [Accessed March 2012]; http://www3.NIDDK.NIH.GOV/fund/other/best_practices_for_sample_storage.pdf.
- 25.Miller WG, Bruns DE. Laboratory issues in measuring and reporting urine albumin. Nephrol Dial Transplant. 2009;24:717–718. doi: 10.1093/ndt/gfp022. [DOI] [PubMed] [Google Scholar]
- 26.Claudi T, Cooper JG. Comparison of urinary albumin excretion rate in overnight urine and albumin creatinine ratio in spot urine in diabetic patients in general practice. Scand J Prim Health Care. 2001;19:247–248. doi: 10.1080/02813430152706774. [DOI] [PubMed] [Google Scholar]
- 27.Gansevoort RT, Verhave JC, Hillege HL, Burgerhof JG, Bakker SJ, de Zeeuw D, et al. The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int Suppl. 2005:S28–S35. doi: 10.1111/j.1523-1755.2005.09408.x. [DOI] [PubMed] [Google Scholar]
- 28.Howey JE, Browning MC, Fraser CG. Biologic variation of urinary albumin: consequences for analysis, specimen collection, interpretation of results, and screening programs. Am J Kidney Dis. 1989;13:35–37. doi: 10.1016/s0272-6386(89)80112-x. [DOI] [PubMed] [Google Scholar]
- 29.Ng WY, Lui KF, Thai AC. Evaluation of a rapid screening test for microalbuminuria with a spot measurement of urine albumin-creatinine ratio. Ann Acad Med (Singapore) 2000;29:62–65. [PubMed] [Google Scholar]
- 30.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20:436–443. doi: 10.1681/ASN.2008030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gatling W, Knight C, Mullee MA, Hill RD. Microalbuminuria in diabetes: a population study of the prevalence and an assessment of three screening tests. Diabet Med. 1988;5:343–347. doi: 10.1111/j.1464-5491.1988.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 32.Marshall SM. Screening for microalbuminuria: which measurement? Diabet Med. 1991;8:706–711. doi: 10.1111/j.1464-5491.1991.tb01688.x. [DOI] [PubMed] [Google Scholar]
- 33.Van Acker BAC, Strommer MKJ, Gosselink MAHE, Koomen GC, Koopman MG, Arisz L. Urinary protein excretion in normal individuals: diurnal changes, influences of orthostasis and relationship to the renin-angiotensin system. Contrib Nephrol. 1993;101:143–150. doi: 10.1159/000422123. [DOI] [PubMed] [Google Scholar]
- 34.Hansen HP, Hovind P, Jensen BR, Parving HH. Diurnal variations of glomerular filtration rate and albuminuria in diabetic nephropathy. Kidney Int. 2002;61:163–168. doi: 10.1046/j.1523-1755.2002.00092.x. [DOI] [PubMed] [Google Scholar]
- 35.Klasen IS, Reichert LJM, de Kat Angelino CM, Wetzels JFM. Quantitative determination of low and high molecular weight proteins in human urine: influence of temperature and storage time. Clin Chem. 1999;45:430–432. [PubMed] [Google Scholar]
- 36.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2012;(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Renal Data System, USRDS 2011 Annual Data Report: atlas of chronic kidney disease and end stage renal disease in the United States. Bethesda (MD): NIH, National Institute of Diabetes, Digestive and Kidney Diseases; 2011. [Google Scholar]
- 38.Aakre KM, Thue G, Subramaniam-Haavik S, Bukve T, Morris H, Muller M, et al. Postanalytical external quality assessment of urine albumin in primary health care: an international survey. Clin Chem. 2008;54:1630–1636. doi: 10.1373/clinchem.2007.100917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.