Abstract
HIV-1 infection depends on effective viral entry mediated by the interaction of its envelope (Env) glycoprotein with specific cell surface receptors. Protective antiviral antibodies generated by passive or active immunization must prevent these interactions. Because the HIV-1 Env is highly variable, attention has also focused on blocking the HIV-1 primary cell receptor CD4. We therefore analyzed the in vivo protective efficacy of three potent neutralizing monoclonal antibodies (mAbs) to HIV-1 Env compared to an antibody against the CD4 receptor. Protection was assessed after mucosal challenge of rhesus macaques with simian/HIV (SHIV). Despite its comparable or greater neutralization potency in vitro, the anti-CD4 antibody did not provide effective protection in vivo, whereas the HIV-1–specific mAbs VRC01, 10E8, and PG9, targeting the CD4 binding site, membrane-proximal, and V1V2 glycan Env regions, respectively, conferred complete protection, albeit at different relative potencies. These findings demonstrate the protective efficacy of broadly neutralizing antibodies directed to the HIV-1 Env and suggest that targeting the HIV-1 Env is preferable to the cell surface receptor CD4 for the prevention of HIV-1 transmission.
INTRODUCTION
Neutralizing antibodies confer protective immunity against many viral pathogens, but eliciting such antibodies against HIV-1 has proven elusive. During the first 20 years of HIV-1 research, only a few broadly neutralizing monoclonal antibodies (mAbs) against HIV-1 were defined, each with limited breadth and potency, and in some cases displaying autoreactivity [reviewed in (1, 2)]. Despite their limited breadth against diverse HIV-1 strains, several of these mAbs were able to block infection of macaques by simian/HIV (SHIV) (3–7). More recently, it was recognized that there exists a continuum of HIV-1–infected subjects that generate cross-reactive serum neutralizing antibody responses (8–14). Further analysis of these subjects led to the isolation of mAbs that were exceptionally potent and broadly reactive. These mAbs are directed to four highly conserved structural regions on the viral spike: the CD4 binding site (CD4bs), variable region 1 and 2 (V1V2) glycopeptide, outer domain glycans, and the membrane-proximal external region (MPER) [reviewed in (15, 16)]. Among CD4bs mAbs, VRC01 neutralizes more than 90% of the circulating HIV-1 strains and is representative of a large class of antibodies that target this site (17, 18). PG9 represents one of a growing number of mAbs directed to HIV-1 envelope (Env) glycans (11, 19–21) and recognizes a conserved motif, including two glycans and a V1V2 peptide strand found on diverse viruses (22, 23). A variety of mAbs directed to a conserved MPER structure have also been isolated (24–28), and the recently identified 10E8 demonstrates a combination of high potency and minimal autoreactivity not seen in other such mAbs to date (29).
Although the number of broadly neutralizing mAbs to conserved epitopes on the HIV-1 Env has increased, the high genetic diversity of Env has prompted continued efforts to block HIV-1 infection by targeting the invariant cellular receptors of HIV-1. These primary and secondary receptors, CD4 and CCR5, respectively, represent potential alternatives for blocking HIV-1 entry and have been targets for the development of antiviral drugs, including small-molecule CCR5 antagonists (30, 31). Because CD4 is the primary HIV-1 receptor on T cells, antibodies to CD4 can potently block viral entry in vitro (32–34) and have been evaluated for antiviral effects in clinical trials (35, 36). However, with regard to in vivo prevention of HIV-1 infection, the relative efficacy of mAbs to CD4 compared to those that target conserved Env sites is unknown. To address this question, we have compared the protective efficacy of mAbs to the cellular receptor CD4 and to conserved Env structures in a nonhuman primate (NHP) mucosal SHIV challenge model.
RESULTS
Characterization of an anti-CD4 mAb that potently neutralizes HIV-1
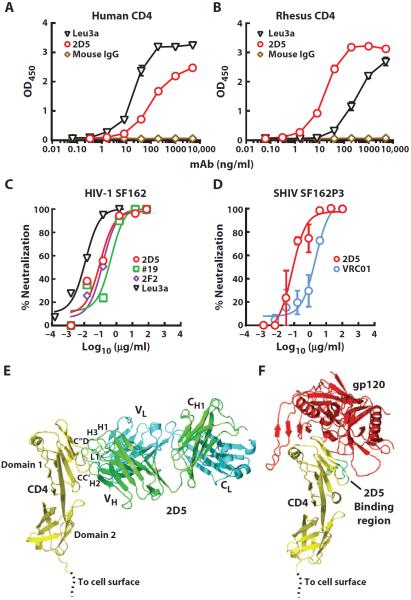
We immunized mice with rhesus CD4 and screened with a human CD4-expressing cell line, thus allowing selection of a mAb clone (2D5) reactive with both human and rhesus CD4 (fig. S1). As expected, 2D5 bound both human and rhesus CD4 (Fig. 1, A and B). This cross-reactive binding was similar to a known anti-CD4 clone, Leu3A (37), though Leu3A preferentially bound human CD4, whereas 2D5 displayed better binding to rhesus CD4. mAb 2D5 also had potent HIV-1 blocking activity using MAGI target cells expressing human CD4 and CCR5. This blocking was similar to another anti-CD4 clone (2F2) isolated from the same hybridoma cultures as 2D5 and the anti-CD4 antibody clone (#19) previously shown to block HIV-1 infection (38). Leu3A displayed somewhat better HIV-1 blocking activity, likely due to its better binding to human CD4 (Fig. 1, A and C). Notably, both R5- and X4-tropic HIV-1 strains were potently blocked by 2D5 (fig. S2). The crystal structure of the 2D5 Fab complexed with the first two extracellular domains of human CD4 was determined to 3.65-Å resolution (tables S1 to S3). The structure revealed a 2D5 interaction with domain 1 of CD4 in a manner that partially overlaps with the CD4bs of HIV gp120 (Fig. 1, E and F, and fig. S3). Thus, 2D5 binding to CD4 would directly block CD4 interaction with gp120. The Leu3A mAb has also been reported to bind to domain 1 of CD4 (39). We next compared the in vitro neutralization potency of 2D5 to mAb VRC01 that targets the CD4bs of gp120, using replication-competent SHIV SF162P3 challenge virus and rhesus peripheral blood mononuclear cell (PBMC) target cells (Fig. 1D). There was potent dose-dependent neutralization by both mAbs, and 2D5 [median inhibitory concentration (IC50), 0.07 μg/ml] was about 30-fold more potent than VRC01 (IC50, 2.15 μg/ml).
Fig. 1. Neutralization of HIV-1 Env by mAbs 2D5 and VRC01.
(A and B) Binding of 2D5 and Leu3A anti-CD4 antibodies to either soluble human (A) or rhesus CD4 (B) as tested by enzyme-linked immunosorbent assay (ELISA). Data are representative of two independent experiments. (C) Neutralization of HIV-1 SF162 by different anti-CD4 mAbs, measured using an Env-pseudotyped lentiviral reporter assay and MAGI-CCR5 target cells that express human CD4 and CCR5. (D) Neutralization of SHIV SF162P3 by 2D5 and VRC01 using a rhesus PBMC infection assay. Means ± SEM from two independent experiments are shown. (E) Ribbon diagram showing CD4 (yellow) complexed to the 2D5 Fab (heavy chain, green; light chain, cyan). Complementarity-determining regions H1, H2, H3, and L1 of 2D5 that contact CD4 are labeled as are 2D5-contacting CD4 loops CC′ and C″D. (F) Ribbon diagram showing CD4 (yellow) complexed to the HIV-1 gp120 core (red) from Protein Data Bank (PDB) entry 1G9M. The 2D5 binding region of CD4 is shown in cyan and green.
Protective efficacy of 2D5 and VRC01 against mucosal SHIV challenge
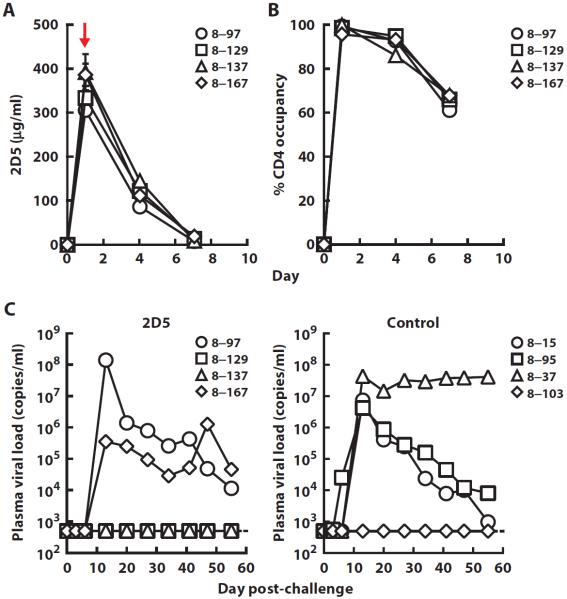
The ability of 2D5 and VRC01 to prevent mucosal SHIV SF162P3 infection was assessed in rhesus macaques. We first assessed mAb 2D5 compared to a control human immunoglobulin G (IgG) using an infusion dose of 40 mg/kg administered intravenously to four animals each, followed by intrarectal inoculation with a single high dose [300 TCID50 (median tissue culture infectious dose)] of SHIV SF162P3 1 day later. The average plasma concentration of plasma 2D5 was 352 μg/ml on the day of challenge (Table 1 and Fig. 2A). At this same time point, we also observed full occupancy of CD4 on the surface of circulating CD4 T cells by 2D5 with no depletion of T lymphocyte populations observed (Fig. 2B and fig. S5). Two of four 2D5-treated animals were protected against infection. One of four control animals remained uninfected. This difference between 2D5 and control IgG was not significant (P = 1, Fisher's exact test; n = 4). These data indicate that, even at this high infusion dose, 2D5 was not highly effective in preventing infection.
Table 1. Pharmacokinetic parameters of the different mAbs and rates of infection after mucosal SHIV challenge of rhesus macaques.
Each mAb was given at the indicated dose intravenously to rhesus macaques, and the level of antibody in the plasma was quantitated by an antibody-specific ELISA using the cognate antigen. Values for concentration of mAb and half-life are the means ± SEM. The plasma half-lives were calculated using the WinNonlin software.
| Antibody (no. of animals) | Antibody dose (mg/kg) | Concentration at day of challenge (μg/ml) | Half-life (days) | Challenge virus (route) | Rate of infection |
|---|---|---|---|---|---|
| mAb2D5 (4) | 40 | 351.6 ± 16.6 | 1.2 ± 0.1 | SHIV SF162P3 (rectal) | 2/4 |
| VRC01 (4) | 20 | 79.2 ± 2.3 | 7.0 ± 0.3 | SHIV SF162P3 (rectal) | 0/4 |
| VRC01 (4) | 20 | 64.6 ± 7.0 | 5.3 ± 1.4 | SHIV SF162P3 (vaginal) | 0/4 |
| VRC01 (6) | 20 | 60.9 ± 2.4 | 6.8 ± 1.6 | SHIV BaLP4 (rectal) | 0/6 |
| 10E8 (6) | 20 | 133 ± 5.5 | 3.2 ± 0.7 | SHIV BaLP4 (rectal) | 0/6 |
| PG9 (6) | 20 | 32.0 ± 3.0 | 2.2 ± 0.4 | SHIV BaLP4 (rectal) | 2/6 |
| VRC01 (6) | 5 | 22.2 ± 1.4 | 8.3 ± 2.3 | SHIV BaLP4 (rectal) | 0/6 |
| 10E8 (6) | 5 | 31.3 ± 1.8 | 5.5 ± 1.4 | SHIV BaLP4 (rectal) | 0/6 |
| PG9 (6) | 5 | 3.7 ± 0.2 | 2.2 ± 0.1 | SHIV BaLP4 (rectal) | 3/6 |
| VRC01 (10) | 0.3 | 1.3 ± 0.1 | 5.1 ± 0.8 | SHIV BaLP4 (rectal) | 6/10 |
| 10E8 (6) | 0.3 | 1.8 ± 0.16 | 6.9 ± 0.7 | SHIV BaLP4 (rectal) | 3/6 |
| PG9 (6) | 0.3 | 0.28 ± 0.03 | 3.2 ± 0.5 | SHIV BaLP4 (rectal) | 6/6 |
Fig. 2. Receptor occupancy, serum mAb levels, and plasma viral loads in rhesus macaques administered 2D5 followed by a single high-dose mucosal challenge with SHIV SF162P3.
(A) The concentration of 2D5 was measured by ELISA in serum taken at different time points from rhesus macaques after administration of a dose (40 mg/kg) of the antibody. The red arrow indicates time of rectal SHIV challenge. (B) The occupancy of cell surface CD4 on peripheral CD4+ T cells by 2D5 was determined using flow cytometry. Day 0 indicates the time point of mAb infusion. (C) Plasma viral loads in rhesus macaques that were administered a single high dose (40 mg/kg) of 2D5 or a control human IgG and rectally challenged 1 day later with a single high dose of SHIV SF162P3 (300 TCID50).
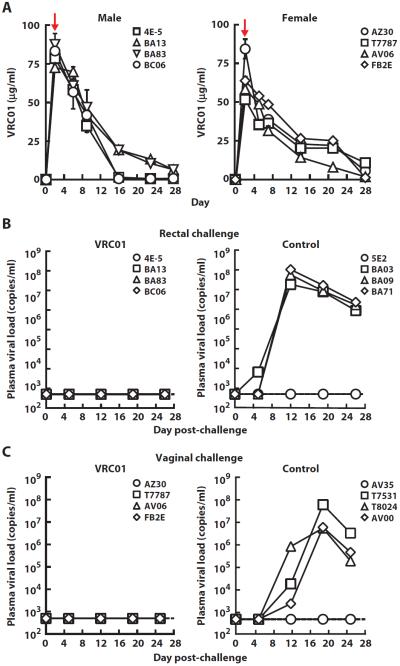
This protection was compared to VRC01, an HIV-1 Env-specific antibody. A twofold lower dose of VRC01 (20 mg/kg) was infused, and rectal challenge was performed 2 days after antibody administration. Control animals received normal human IgG. Despite the lower average plasma concentration (about sixfold) for VRC01 compared to 2D5 on the day of challenge (Table 1 and Fig. 3A), none of the four animals were infected compared to three of four in the control group (Fig. 3B; P = 0.14, Fisher's exact test; n = 4). Because HIV-1 is commonly transmitted from males to females through exposure at the vaginal mucosa, we also tested the ability of VRC01 to protect against this route of challenge. VRC01 or human IgG was administered at a dose of 20 mg/kg to four animals each; 2 days later, the animals were inoculated intravaginally with SHIV SF162P3. Similar to results after intrarectal challenge, none of the four animals were infected compared to three of four in the control group (Fig. 3C; P = 0.14, Fisher's exact test; n = 4). Analysis of the challenge data from VRC01 (eight of eight VRC01 animals protected versus two of eight uninfected controls) demonstrated statistically significant protection (P = 0.01, exact conditional test; n = 8). Thus, the Env-specific mAb VRC01 provided protection against mucosal SHIV SF162P3 challenges and was more effective than mAb 2D5 directed to the CD4 receptor.
Fig. 3. Serum mAb levels and plasma viral loads in rhesus macaques administered VRC01 followed by a single high-dose mucosal challenge with SHIV SF162P3.
(A) The concentration of VRC01 IgG1 was measured by an RSC3 (resurfaced stabilized gp120 core, derivative 3)–based ELISA in blood taken at different time points from male or female rhesus macaques after administration of a dose (20 mg/kg) of the antibody. The red arrows indicate time of mucosal SHIV challenge. (B) Plasma viral loads in rhesus macaques that were administered a single high dose (20 mg/kg) of VRC01 or a control human IgG and rectally challenged 2 days later with a single high dose of SHIV SF162P3 (300 TCID50). (C) Plasma viral loads in rhesus macaques that were administered a single high dose (20 mg/kg) of VRC01 or a control human IgG and vaginally challenged 2 days later with a single high dose of SHIV SF162P3 (300 TCID50).
Protective efficacy of VRC01, PG9, and 10E8 against mucosal SHIV challenge
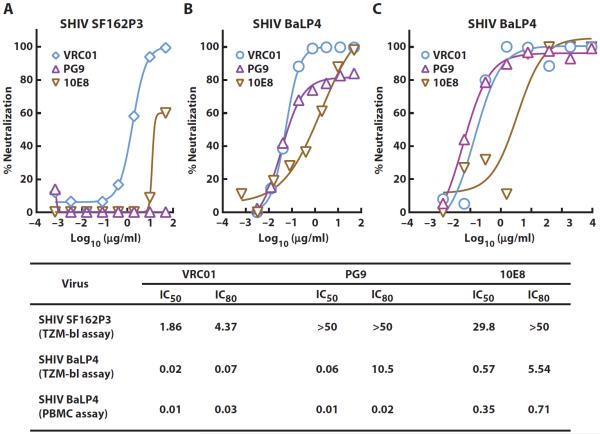
To compare the overall protective efficacy of several well-characterized broadly neutralizing mAbs to HIV-1, we evaluated the relative pharmacokinetics and protection conferred by VRC01, PG9, and 10E8 targeting the CD4bs, V1V2 peptidoglycans, and gp41 membrane proximal Env regions, respectively. Although VRC01 neutralized SHIV SF162P3, 10E8 and PG9 demonstrated weak or no neutralization, respectively, against this SHIV (Fig. 4A). We therefore evaluated an alternative CCR5-tropic strain, SHIV BaLP4. VRC01 and PG9 neutralized this SHIV with similar IC50 concentrations (0.02 and 0.06 μg/ml, respectively), whereas 10E8 had a higher IC50 of 0.57 μg/ml in a single-round entry assay (Fig. 4B). These results were confirmed in a rhesus PBMC infection assay in which both VRC01 and PG9 were more potent than 10E8 in neutralizing SHIV BaLP4 infection (Fig. 4C).
Fig. 4. Neutralization of CCR5-tropic SHIV strains by VRC01, PG9, and 10E8.
(A) Neutralizing activity of VRC01 and PG9 against SHIV SF162P3 in a neutralization assay using a luciferase reporter–based TZM-bl cell line. (B) Neutralizing activity of VRC01, PG9, and 10E8 against SHIV BaLP4 in a neutralization assay using a luciferase reporter–based TZM-bl cell line. (C) Neutralizing activity of VRC01, PG9, and 10E8 against SHIV BaLP4 in a rhesus PBMC infection assay. The table lists the IC50 and IC80 values for the neutralization of SHIVs by the three anti–HIV-1 mAbs in the different assay formats. Average values from two independent experiments are shown.
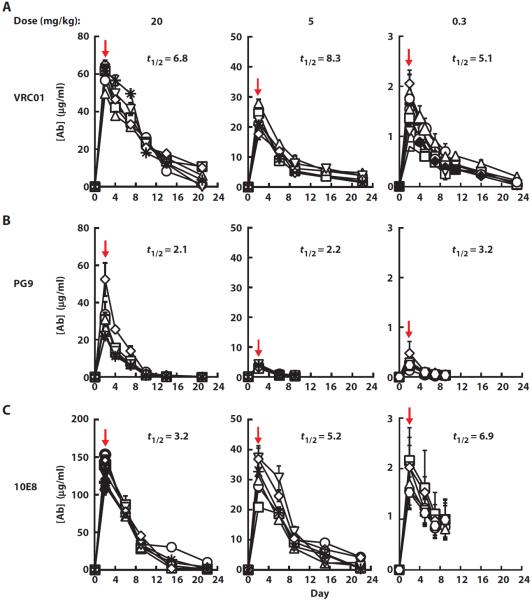
To assess the relative protective efficacies of VRC01, PG9, and 10E8, these mAbs were each administered at doses of 20, 5, and 0.3 mg/kg in a study using at least six animals per treatment arm (Table 1). We first studied the antibody pharmacokinetics. The mean plasma half-lives for VRC01 and 10E8 were comparable (7.0 and 5.2 days, respectively), whereas the half-life of PG9 was only 2.5 days (Table 1 and Fig. 5), possibly because of PG9 reactivity with mammalian carbohydrates. Man5GlcNAc2 glycans are required for PG9 binding to its epitope (22). Notably, GnTI− 293S are 293 cells that lack GnTI activity (40) and consequently produce Man5GlcNAc2 glycans, but not more complex N-glycans. We observed glycan-dependent binding of PG9 to the cell surface of GnTI− 293S cells (fig. S4), raising the possibility that reactivity with host proteins may contribute to its shorter half-life in vivo.
Fig. 5. Plasma levels of mAbs in rhesus macaques administered VRC01, PG9, and 10E8 at three different doses of each antibody.
(A to C) The plasma concentrations of VRC01 (A), PG9 (B), and 10E8 (C) IgG1 were measured by ELISA at different time points after administration of the indicated doses (20, 5, and 0.3 mg/kg) of each antibody in each group. The terminal in vivo half-life (t1/2) is indicated for each antibody dose. The red arrows indicate time of mucosal SHIV challenge. Each treatment group consisted of 6 animals except for the group that received VRC01 (0.3 mg/kg), which had 10 animals.
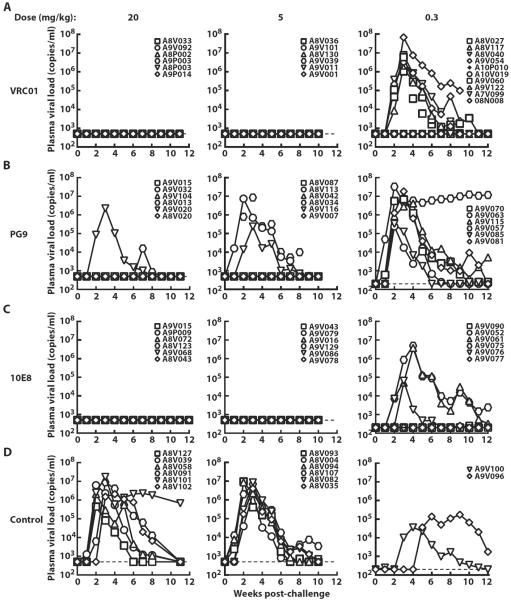
We next evaluated the dosages of antibodies VRC01, PG9, and 10E8 necessary to protect against infection by rectal challenge with SHIV BaLP4. The animals were infused with the respective mAbs (20, 5, or 0.3 mg/kg) and were challenged 2 days after antibody transfer. Fourteen control animals received human IgG followed 2 days later by SHIV BaLP4 challenge, and all became infected (Fig. 6D). At the highest dose of 20 mg/kg, VRC01 and 10E8 protected all animals (n = 6 for each antibody), and PG9 prevented infection in four of six animals (Fig. 6). At 5 mg/kg, VRC01 and 10E8 showed similar complete protection of all six animals, whereas PG9 still conferred partial benefit, protecting three of six animals. At 0.3 mg/kg, both VRC01 and 10E8 showed partial efficacy with 4 of 10 and 3 of 6 animals protected, respectively. All six animals that received PG9 (0.3 mg/kg) became infected. Therefore, although all three mAbs were protective, VRC01 and 10E8 were significantly more effective in protecting against acquisition of SHIV BaLP4 infection, as determined by exact logistic regression analysis that was adjusted for dose groups (P = 0.001 for VRC01 versus PG9, n = 22 for VRC01, n = 18 for PG9; P = 0.004 for 10E8 versus PG9, n = 18).
Fig. 6. Plasma viral loads in rhesus macaques administered VRC01, PG9, and 10E8 at three different doses of each antibody after a single high-dose mucosal challenge with SHIV BaLP4.
(A to D) Plasma viral loads in rhesus macaques that were administered three different doses (20, 5, and 0.3 mg/kg) of VRC01 (A), PG9 (B), 10E8 (C), control IgG (D) and rectally challenged 2 days later with a single high dose of SHIV BaLP4.
DISCUSSION
Passive antibody protection against HIV-1 infection could result from antibodies directed to the viral Env or potentially from antibodies directed to the primary cellular receptor CD4. Here, we show that despite potent in vitro neutralizing activity against the challenge virus and high occupancy of antibody bound to the CD4 receptor of circulating CD4+ T cells, the anti-CD4 mAb 2D5 provided only partial protection against a mucosal SHIV challenge. In contrast, three well-characterized HIV-1 broadly neutralizing mAbs provided robust in vivo protection, suggesting that the potent viral neutralization observed in vitro translated to high level protection in vivo.
The explanation for the relatively poor protection provided by mAb 2D5 compared to the HIV-1 Env-specific mAbs is not fully apparent. In contrast to the human HIV-1–specific mAbs VRC01, PG9, and 10E8, 2D5 is a mouse mAb and had a shorter circulating half-life in macaques. However, SHIV challenges were performed 1 day after 2D5 infusion, when plasma mAb levels were several hundred micrograms per milliliter and about 1000-fold above the in vitro neutralization IC50 value of 2D5 against SHIV SF162P3 (Fig. 2A). Similarly, differences in Fc effector function have been noted previously between species (5, 41), but it is unlikely that these alone would account for the major differences in protection that have been observed when both show strong neutralization potency in vitro. NHP challenge studies have shown that whereas Fc-mediated effector functions may play a small role in antibody-mediated protection (6, 41), viral neutralization is the major effector function associated with in vivo protection against SHIV challenge (6, 41, 42). We also documented that 2D5 achieves full occupancy of the macaque CD4 receptor on T cells in the peripheral circulation at the day of challenge. Despite these results, it is more likely that there was insufficient antibody to block all receptors in mucosal and peripheral lymphoid tissues. Greater receptor blocking might be achieved through multiple infusions of 2D5, but given the large number of CD4+ T cells resident in the gastrointestinal tract as well as in other lymphoid organs, it is not clear whether the levels of anti-CD4 antibody needed to block all such potential receptors can be readily attained. An alternative approach could be to locally administer the anti-CD4 antibody to sites of infection like mucosal sites, which may prevent it from binding to CD4 expressed on cells present in other nonrelevant tissue compartments. Topical applications of entry inhibitors and an anti-HIV-1 antibody have shown protective efficacy against mucosal SHIV challenges, although the protection is transient, requiring challenge within a few hours of application (43–45). These data suggest a narrow window of protection using cell-directed entry inhibitors.
Ibalizumab is an anti-CD4 antibody that has been evaluated in clinical trials and binds to domain 2 of CD4 (46). Although 2D5 binds to domain 1 of CD4, both mAbs show substantial potency in inhibition of HIV-1 infection (32). Notably, the data in this report show that anti-Env mAbs with less potency than the anti-CD4 mAb 2D5 confer greater in vivo protective efficacy. Thus, such anti-CD4 mAbs would seem less attractive candidates for the immune prophylaxis of HIV-1. It remains possible that ibalizumab could have different protective efficacy against SHIV challenge, but no animal model protection studies have yet been published that demonstrate such efficacy. Ibalizumab is derived from a mouse mAb 5A8 that was shown to have a therapeutic effect in chronically SIV-infected macaques (47). Likewise, ibalizumab reduced viremia after infusion into HIV-1–infected subjects (35, 36). Thus, anti-CD4 mAbs may have some benefit in a therapeutic setting, but our data highlight the challenges of targeting host cellular proteins to prevent HIV-1 infection.
There are several potentially related explanations for the lower efficacy of PG9. This mAb did not produce complete in vitro neutralization of SHIV BaLP4, but rather the neutralization curve saturated at about 80% neutralization in a single-round infectivity assay (Fig. 4B). This phenomenon of incomplete in vitro neutralization has been observed for several anti-V1V2 mAbs, including PG9, PG16, and PGT145, and has been observed on a subset of diverse HIV-1 isolates (11, 19). The mechanism of this effect is not well understood but may be related to incomplete or variable glycosylation of the Env glycoprotein on the virions. Anti-V1V2 mAbs such as PG9 bind to both amino acid and glycan sites and are sensitive to the complexity of glycosylation (22, 23, 48). It has been shown that binding of PG9 to HIV-1 Env is dependent on the presence of Man5GlcNAc2 glycan at an N-glycosylation site for antigen recogni tion and that replacement with high mannose–type (Man8GlcNAc2 or Man9GlcNAc2) glycans at this site abrogates PG9 binding. In addition to this requirement for glycan binding, the circulating plasma half-life of PG9 was about twofold shorter than that observed with VRC01 and 10E8. This shorter half-life may reflect properties intrinsic to the variable region or antigen binding site of PG9. PG9 may bind to glycoproteins on the host cell or be taken up by glycosylated scavenger receptors that are present on many cell types, which may contribute to its shorter half-life and lower protective efficacy in vivo. A recent study demonstrated that a V3 glycan mAb, PGT121, provides protection against mucosal SHIV challenge at low doses (49), indicating that this class of antibodies may be different from those that bind to the V1V2 glycopeptide like PG9 in terms of their in vivo efficacy.
It has been shown previously that b12, another mAb to the CD4bs that neutralizes about 40% of circulating HIV-1 strains, can confer complete protection against mucosal SHIV challenge (41, 50). In addition, 2F5 and 4E10, two mAbs to the MPER, have been shown to protect against mucosal challenge, but protection required relatively high doses of antibody infusion (7, 51). In contrast, we show that 10E8, an MPER mAb that neutralizes more than 95% of circulating HIV-1 strains, provided complete protection at an infusion dose of 5 mg/kg and partial protection at 0.3 mg/kg. 10E8 displayed about 10-fold less in vitro neutralization potency against SHIV BaLP4 than did VRC01 (Fig. 4), yet produced similar in vivo protective efficacy (Fig. 6). We observed higher plasma concentrations of 10E8 than VRC01 at the day of challenge, which suggests increased bio-availability of 10E8 compared to VRC01 and may relate to its higher in vivo efficacy.
There are several potential limitations to our study. We tested only one antibody to the CD4 receptor, and it is possible that a different anti-CD4 mAb, possibly one with a different mode of CD4 binding or a lower off-rate, could provide better in vivo protection. We also did not test antibodies to the co-receptor molecule CCR5, and because our SHIV challenge virus enters via both CD4 and CCR5, it is possible that antibodies to CCR5 could have an additional effect on transmission. Last, we tested transmission with one SHIV, and the impact of antibodies to the host cell receptors may vary among viruses and in different mucosal tissues.
In summary, we show that mAb 2D5 binds with high affinity to the CD4 receptor and blocks HIV-1 entry in vitro but lacks robust in vivo protective efficacy despite high plasma levels. In contrast, potent protection against infection was observed for mAbs that target highly conserved epitopes on the HIV-1 Env. Among these mAbs, the ones that target CD4bs and MPER may be preferred to those that target V1V2 sites on the HIV-1 Env. These data suggest that the CD4bs and MPER of the HIV-1 Env represent attractive targets for both active and passive immunization strategies to prevent HIV-1 transmission.
MATERIALS AND METHODS
Animal study design
Healthy male and female Macaca mulatta animals of Indian origin weighing 3 to 4 kg were used in this study. For the studies using anti-CD4 antibody, the antibody was administered intravenously at a dose of 40 mg/kg and challenged with SHIV SF162P3 (300 TCID50, intrarectal) 1 day after passive transfer. For the studies using anti-HIV antibodies, the antibodies were administered intravenously at doses of 20, 5, or 0.3 mg/kg and challenged with either SHIV SF162P3 (300 TCID50, intrarectal and intravaginal) or SHIV BaLP4 (1 ml of stock virus, intrarectal) 2 days after passive transfer. For the intravaginal challenge, the animals were treated with Depo-Provera 30 days before challenge to thin the vaginal epithelium and increase infection (52). Whole blood was collected at different time points to obtain plasma and PBMC samples for measurement of antibody levels and other immune parameters. All animal experiments were reviewed and approved by the Animal Care and Use Committee of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), and all animals were housed and cared for in accordance with local, state, federal, and institute policies in an American Association for Accreditation of Laboratory Animal Care–accredited facility at the NIH.
Challenge viruses
SHIV SF162P3, propagated in phytohemagglutinin-activated rhesus macaque PBMCs, was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH (cat. no. 6526; contributors: J. Harouse, C. Cheng-Mayer, and R. Pal, Aaron Diamond AIDS Research Center). Similarly, the challenge stock of SHIV BaLP4 (53) was generated in concanavalin A–activated human PBMCs, and the TCID50 titer in TZM-bl cells was 12,800/ml.
Neutralization assays
Neutralization of replication-competent SHIV challenge stocks by anti–HIV-1 mAbs was performed in two different assay formats. In one format, neutralization was measured using single-round infection of TZM-bl target cells (HeLa cells engineered to express CD4 and CCR5) with replication-competent SHIV stocks in the presence of the protease inhibitor indinavir, as described previously (54–56). In a second format, neutralization was measured using infection of rhesus PBMCs with replication-competent SHIV stocks, allowing for multiple rounds of replication, as described previously (50). Neutralization of HIV-1 Env-mediated entry into target cells by the anti-CD4 antibody was also measured using a modified Env-pseudotyped reporter virus assay. Briefly, the target MAGI-CCR5 cells were first incubated with serial dilutions of the anti-CD4 antibody for 1 hour, followed by addition of the HIV-1 Env pseudotyped virus and quantitation of luciferase reporter activity in cell lysates 72 hours later. Neutralization of replication-competent SHIV by the anti-CD4 antibody was measured using a modified rhesus PBMC infection assay as described previously (50). Here, instead of incubation of the virus with the antibody, the PBMCs were preincubated with the anti-CD4 antibody for 1 hour before addition of the virus.
Enzyme-linked immunosorbent assays
Quantitative ELISA was used to measure antibody levels in the animal plasma obtained at different time points. For quantitation of mAbs 2D5, VRC01, 10E8, and PG9, soluble CD4, RSC3 (56), MPER peptide, and gp120 (ZM109), respectively, were used to capture the mAbs in the plasma and detected by horseradish peroxidase (HRP)–conjugated anti-mouse or anti-human IgG conjugates (Southern Biotech). Serum half-lives were calculated on the basis of the levels of each mAb measured at different time points after infusion using a noncompartment model by the WinNonlin software (Pharsight).
Binding of anti-CD4 antibodies to soluble CD4 was performed by overnight coating (2 μg/ml) of microtiter plates with either recombinant human CD4 or rhesus CD4 (Immune Technology Corp.), followed by addition of serially diluted mAbs against CD4. Bound mAbs were detected by an HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch).
Receptor occupancy assay
Whole blood, obtained at different time points after administration of 2D5, was stained in replicates of three (50 μl each) with a fluorescently conjugated anti-mouse IgG (Southern Biotech) to detect cell surface–bound antibodies on the lymphocyte population that were gated on the basis of their forward and side scatter (fig. S5). A total of 10,000 events were collected in the lymphocyte gate for each replicate sample. Percent receptor occupancy was calculated by comparing the observed signal to a 100% control, in which a saturating amount of 2D5 (100 μg/ml) was added to the sample that was run in parallel at all time points.
Plasma viral loads
Plasma viral RNA levels were determined using a modified two-step quantitative reverse transcription polymerase chain reaction (PCR) process. Experimental samples were run in parallel with an SIV gag RNA standard on an Applied Biosystems StepOne real-time PCR system. The lower limit of detection using this assay was 250 SIV RNA copies/ml.
Statistical analysis
For SHIV challenge studies, four to six animals per group were evaluated, and sample size was assessed using exact conditional tests. The rate of infection in each mAb group was compared to the corresponding control group using a two-tailed Fisher's exact test and analyzed using the JMP statistical software from SAS Institute Inc. The rates of infection reflect the number of animals infected in each group as noted by at least one weekly time point showing detectable plasma viremia (>250 copies/ml) in a 10-week period after a single high-dose challenge. For direct comparison of the protective efficacy of VRC01, 10E8, and PG9, exact logistic regression analysis was carried out between each pair of antibodies, adjusting for the dose and group sizes at each dose.
Supplementary Material
Acknowledgments
We thank staff members of Southeast Regional Collaborative Access Team (SER-CAT) sector 22 at the Advanced Photon Source, Argonne National Laboratory for assistance in data collection.
Funding: This research was supported by the Intramural Research Program of the Vaccine Research Center, NIAID, NIH. Use of SER-CAT sector 22 at the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under contract W-31-109-Eng-38. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency.
Footnotes
SUPPLEMENTARY MATERIALS www.sciencetranslationalmedicine.org/cgi/content/full/6/243/243ra88/DC1
Author contributions: A.P., Z.-y.Y., S.S.R., J.R.M., and G.J.N. designed the research studies; A.P., Z.-y.Y., L.W., J.C.B., S.-Y.K., S.D.S., K.M., W.-P.K., W.S., X.C., and J.-P.T. performed the research; A.P., Z.-y.Y., J.C.B., N.L.L., J.H., M.C.N., J.A.H., P.D.K., M.C., J.R.M., and G.J.N. analyzed the data; and A.P., J.C.B., J.R.M., and G.J.N. wrote the paper.
Competing interests: J.R.M., Z.-y.Y., G.J.N., P.D.K., and M.C. are listed as inventors on an NIH patent on VRC01 (“Neutralizing antibodies to HIV-1 and their use”; U.S. Patent 8637036 B2). M.C., J.H., P.D.K., G.J.N., and J.R.M. are included on an NIH patent on 10E8 (“Neutralizing gp41 antibodies and their use”; WO 2013070776 A1). The other authors declare no other competing financial interests.
Data and materials availability: Coordinates and structure factors for the 2D5/CD4 complex have been deposited with the PDB under accession code 4Q6I.
REFERENCES AND NOTES
- 1.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 2004;5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 2.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu. Rev. Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 3.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian–human immunodeficiency virus infection. Nat. Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 4.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 5.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLOS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: Good news for an HIV-1 vaccine? Nat. Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 9.Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLOS Pathog. 2010;6:e1001028. doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLOS Pathog. 2011;7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators. Koff WC, Wilson IA, Burton DR, Poignard P. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonsignori M, Alam SM, Liao HX, Verkoczy L, Tomaras GD, Haynes BF, Moody MA. HIV-1 antibodies from infection and vaccination: Insights for guiding vaccine design. Trends Microbiol. 2012;20:532–539. doi: 10.1016/j.tim.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overbaugh J, Morris L. The antibody response against HIV-1. Cold Spring Harb. Perspect. Med. 2012;2:a007039. doi: 10.1101/cshperspect.a007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS. 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: Identification, structures, and B cell ontogenies. Immunity. 2012;37:412–425. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, O'Dell S, Perfetto S, Schmidt SD, Shi W, Wu L, Yang Y, Yang ZY, Yang Z, Zhang Z, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Simek M, Burton DR, Koff WC, Doria-Rose NA, Connors M, NISC Comparative Sequencing Program. Mullikin JC, Nabel GJ, Roederer M, Shapiro L, Kwong PD, Mascola JR. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou T, Zhu J, Wu X, Moquin S, Zhang B, Acharya P, Georgiev IS, Altae-Tran HR, Chuang GY, Joyce MG, Do Kwon Y, Longo NS, Louder MK, Luongo T, McKee K, Schramm CA, Skinner J, Yang Y, Yang Z, Zhang Z, Zheng A, Bonsignori M, Haynes BF, Scheid JF, Nussenzweig MC, Simek M, Burton DR, Koff WC, NISC Comparative Sequencing Program. Mullikin JC, Connors M, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39:245–258. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators. Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pejchal R, Walker LM, Stanfield RL, Phogat SK, Koff WC, Poignard P, Burton DR, Wilson IA. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc. Natl. Acad. Sci. U.S.A. 2010;107:11483–11488. doi: 10.1073/pnas.1004600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonsignori M, Montefiori DC, Wu X, Chen X, Hwang KK, Tsao CY, Kozink DM, Parks RJ, Tomaras GD, Crump JA, Kapiga SH, Sam NE, Kwong PD, Kepler TB, Liao HX, Mascola JR, Haynes BF. Two distinct broadly neutralizing antibody specificities of different clonal lineages in a single HIV-1-infected donor: Implications for vaccine design. J. Virol. 2012;86:4688–4692. doi: 10.1128/JVI.07163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang LX, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pancera M, Shahzad-Ul-Hussan S, Doria-Rose NA, McLellan JS, Bailer RT, Dai K, Loesgen S, Louder MK, Staupe RP, Yang Y, Zhang B, Parks R, Eudailey J, Lloyd KE, Blinn J, Alam SM, Haynes BF, Amin MN, Wang LX, Burton DR, Koff WC, Nabel GJ, Mascola JR, Bewley CA, Kwong PD. Structural basis for diverse N-glycan recognition by HIV-1–neutralizing V1–V2–directed antibody PG16. Nat. Struct. Mol. Biol. 2013;20:804–813. doi: 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stiegler G, Kunert R, Purtscher M, Wolbank S, Voglauer R, Steindl F, Katinger H. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses. 2001;17:1757–1765. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- 25.Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, Chen B, Parks R, Foulger A, Jaeger F, Donathan M, Bilska M, Gray ES, Abdool Karim SS, Kepler TB, Whitesides J, Montefiori D, Moody MA, Liao HX, Haynes BF. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLOS One. 2011;6:e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Z, Qin HR, Chen W, Zhao Q, Shen X, Schutte R, Wang Y, Ofek G, Streaker E, Prabakaran P, Fouda GG, Liao HX, Owens J, Louder M, Yang Y, Klaric KA, Moody MA, Mascola JR, Scott JK, Kwong PD, Montefiori D, Haynes BF, Tomaras GD, Dimitrov DS. Cross-reactive HIV-1-neutralizing human monoclonal antibodies identified from a patient with 2F5-like antibodies. J. Virol. 2011;85:11401–11408. doi: 10.1128/JVI.05312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutje Hulsik D, Liu YY, Strokappe NM, Battella S, El Khattabi M, McCoy LE, Sabin C, Hinz A, Hock M, Macheboeuf P, Bonvin AM, Langedijk JP, Davis D, Forsman Quigley A, Aasa-Chapman MM, Seaman MS, Ramos A, Poignard P, Favier A, Simorre JP, Weiss RA, Verrips CT, Weissenhorn W, Rutten L. A gp41 MPER-specific llama VHH requires a hydrophobic CDR3 for neutralization but not for antigen recognition. PLOS Pathog. 2013;9:e1003202. doi: 10.1371/journal.ppat.1003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ofek G, Zirkle B, Yang Y, Zhu Z, McKee K, Zhang B, Chuang GY, Georgiev IS, O'Dell S, Doria-Rose N, Mascola JR, Dimitrov DS, Kwong PD. Structural basis for HIV-1 neutralization by 2F5-like antibodies m66 and m66.6. J. Virol. 2014;88:2426–2441. doi: 10.1128/JVI.02837-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobritz MA, Ratcliff AN, Arts EJ. HIV-1 entry, inhibitors, and resistance. Viruses. 2010;2:1069–1105. doi: 10.3390/v2051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb. Perspect. Med. 2012;2:a007161. doi: 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkly LC, Olson D, Shapiro R, Winkler G, Rosa JJ, Thomas DW, Williams C, Chisholm P. Inhibition of HIV infection by a novel CD4 domain 2-specific monoclonal antibody. Dissecting the basis for its inhibitory effect on HIV-induced cell fusion. J. Immunol. 1992;149:1779–1787. [PubMed] [Google Scholar]
- 33.Moore JP, Sattentau QJ, Klasse PJ, Burkly LC. A monoclonal antibody to CD4 domain 2 blocks soluble CD4-induced conformational changes in the envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) and HIV-1 infection of CD4+ cells. J. Virol. 1992;66:4784–4793. doi: 10.1128/jvi.66.8.4784-4793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shearer MH, Timanus DK, Benton PA, Lee DR, Kennedy RC. Cross-clade inhibition of human immunodeficiency virus type 1 primary isolates by monoclonal anti-CD4. J. Infect. Dis. 1998;177:1727–1729. doi: 10.1086/517432. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson JM, Kuritzkes DR, Godofsky E, DeJesus E, Larson JA, Weinheimer SP, Lewis ST. Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob. Agents Chemother. 2009;53:450–457. doi: 10.1128/AAC.00942-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fessel WJ, Anderson B, Follansbee SE, Winters MA, Lewis ST, Weinheimer SP, Petropoulos CJ, Shafer RW. The efficacy of an anti-CD4 monoclonal antibody for HIV-1 treatment. Antiviral Res. 2011;92:484–487. doi: 10.1016/j.antiviral.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sattentau QJ, Dalgleish AG, Weiss RA, Beverley PC. Epitopes of the CD4 antigen and HIV infection. Science. 1986;234:1120–1123. doi: 10.1126/science.2430333. [DOI] [PubMed] [Google Scholar]
- 38.Endres MJ, Clapham PR, Marsh M, Ahuja M, Turner JD, McKnight A, Thomas JF, Stoebenau-Haggarty B, Choe S, Vance PJ, Wells TN, Power CA, Sutterwala SS, Doms RW, Landau NR, Hoxie JA. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 39.Truneh A, Buck D, Cassatt DR, Juszczak R, Kassis S, Ryu SE, Healey D, Sweet R, Sattentau Q. A region in domain 1 of CD4 distinct from the primary gp120 binding site is involved in HIV infection and virus-mediated fusion. J. Biol. Chem. 1991;266:5942–5948. [PubMed] [Google Scholar]
- 40.Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: High-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13419–13424. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 42.Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, Dugast AS, Parren PW, Nimmerjahn F, Evans DT, Alter G, Forthal DN, Schmitz JE, Iida S, Poignard P, Watkins DI, Hessell AJ, Burton DR. A non-fucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcγRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J. Virol. 2012;86:6189–6196. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 44.Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, Marx PA, Dufour J, Colonno RJ, Shattock RJ, Springer MS, Moore JP. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus–cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 45.Veazey RS, Ketas TJ, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, Moore JP. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J. Infect. Dis. 2010;202:739–744. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeman MM, Seaman MS, Rits-Volloch S, Hong X, Kao CY, Ho DD, Chen B. Crystal structure of HIV-1 primary receptor CD4 in complex with a potent antiviral antibody. Structure. 2010;18:1632–1641. doi: 10.1016/j.str.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reimann KA, Cate RL, Wu Y, Palmer L, Olson D, Waite BC, Letvin NL, Burkly LC. In vivo administration of CD4-specific monoclonal antibody: Effect on provirus load in rhesus monkeys chronically infected with the simian immunodeficiency virus of macaques. AIDS Res. Hum. Retroviruses. 1995;11:517–525. doi: 10.1089/aid.1995.11.517. [DOI] [PubMed] [Google Scholar]
- 48.Amin MN, McLellan JS, Huang W, Orwenyo J, Burton DR, Koff WC, Kwong PD, Wang LX. Synthetic glycopeptides reveal the glycan specificity of HIV-neutralizing antibodies. Nat. Chem. Biol. 2013;9:521–526. doi: 10.1038/nchembio.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci. U.S.A. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein K, Veazey RS, Warrier R, Hraber P, Doyle-Meyers LA, Buffa V, Liao HX, Haynes BF, Shaw GM, Shattock RJ. Neutralizing IgG at the portal of infection mediates protection against vaginal simian/human immunodeficiency virus challenge. J. Virol. 2013;87:11604–11616. doi: 10.1128/JVI.01361-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 53.Pal R, Taylor B, Foulke JS, Woodward R, Merges M, Praschunus R, Gibson A, Reitz M. Characterization of a simian human immunodeficiency virus encoding the envelope gene from the CCR5-tropic HIV-1 Ba-L. J. Acquir. Immune Defic. Syndr. 2003;33:300–307. doi: 10.1097/00126334-200307010-00003. [DOI] [PubMed] [Google Scholar]
- 54.Wu L, Zhou T, Yang ZY, Svehla K, O'Dell S, Louder MK, Xu L, Mascola JR, Burton DR, Hoxie JA, Doms RW, Kwong PD, Nabel GJ. Enhanced exposure of the CD4-binding site to neutralizing antibodies by structural design of a membrane-anchored human immunodeficiency virus type 1 gp120 domain. J. Virol. 2009;83:5077–5086. doi: 10.1128/JVI.02600-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 58.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adams PD, Mustyakimov M, Afonine PV, Langan P. Generalized X-ray and neutron crystallographic analysis: More accurate and complete structures for biological macromolecules. Acta Crystallogr. D Biol. Crystallogr. 2009;65:567–573. doi: 10.1107/S0907444909011548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.