Abstract
Background
Interindividual variability and drug interaction studies suggest that blood-brain barrier drug transporters mediate human methadone brain biodistribution. In vitro and animal studies suggest that methadone is a substrate for the efflux transporter P-glycoprotein, and that P-glycoprotein-mediated transport influences brain access and pharmacologic effect. This investigation tested whether methadone is a transporter substrate in humans.
Methods
Healthy volunteers received oral (N=16) or IV (N=12) methadone in different crossover protocols after nothing (control) or the validated P-glycoprotein inhibitor cyclosporine (4.5 mg/kg orally twice daily for 4 days, or 5 mg/kg IV over 2 hr). Plasma and urine methadone and metabolite concentrations were measured by mass spectrometry. Methadone effects were measured by miosis and thermal analgesia (maximally tolerated temperature and verbal analog scale rating of discreet temperatures).
Results
Cyclosporine marginally but significantly decreased methadone plasma concentrations and apparent oral clearance, but had no effect on methadone renal clearance or on hepatic N-demethylation. Cyclosporine had no effect on miosis, or on R-methadone concentration-miosis relationships after either oral or IV methadone. Peak miosis was similar in controls and cyclosporine-treated subjects after oral methadone (1.4 ± 0.4 and 1.3 ± 0.5 mm/mg, respectively) and IV methadone (3.1 ± 1.0 and 3.2 ± 0.8 mm respectively). Methadone increased maximally tolerated temperature, but analgesia testing was confounded by cyclosporine-related pain.
Conclusions
Cyclosporine did not affect methadone pharmacodynamics. This result does not support a role for cyclosporine-inhibitable transporters mediating methadone brain access and biodistribution.
INTRODUCTION
Methadone use for opioid addiction and analgesia is influenced by variable clinical effects and untoward side effects. Numerous investigations have focused on pharmacokinetic variability and drug interactions, yet less is known about pharmacodynamic-based variability and drug interactions, and their causes. For example, well-maintained methadone patients experience withdrawal symptoms,1 and toxicity may occur at seemingly therapeutic methadone plasma concentrations.2 Rifampin, ritonavir, nelfinavir, and efavirenz shifted methadone plasma concentration–effect (miosis) curves leftward and upward, increasing apparent potency and maximum effect.3-6 These latter findings provided insight into previously unexplained lack of opioid withdrawal despite methadone concentrations decreased by certain antiretrovirals. This implicated brain transport-mediated methadone drug interactions, hence suggesting that blood brain barrier (BBB) efflux and/or influx proteins influence methadone clinical effects.
Drug transporters of the adenosine triphosphate-binding cassette (ABC) family, including the efflux transporters P-glycoprotein (P-gp, ABCB1, multi-drug resistance protein 1), breast cancer resistance protein (BCRP, ABCG2), and multidrug resistance proteins (MRP, ABCC), are expressed in human brain capillary endothelial cells.7,8 BBB ABC transporters have been implicated in brain opioid biodistribution,9 and some evidence in vitro suggests methadone is an ABC transporter substrate.10,11 Methadone did not accumulate in ABCB1-transfected pig kidney cells compared to controls, suggesting methadone was a P-gp substrate.12 In human P-gp-overexpressing cells, the P-gp inhibitors verapamil and GF120918 (elacridar) significantly decreased basal-to-apical methadone transport.13 In vivo, and consistent with these data, methadone brain uptake clearance or concentrations were about 3-fold higher in multidrug-resistant (mdr)-deficient mdr1a/b(−/−) mice relative to wild-type mdr1a/b(+/+) mice, and methadone produced greater analgesia.13-15 Cerebral methadone concentrations were substantially greater in mdr1a (−/−) compared with wild-type mice.16 Upregulation of BBB P-gp activity in wild-type mice reduced methadone antinociception.17 In rats, methadone co-administration with the ABC transport inhibitor PSC833 (valspodar) increased methadone brain concentrations and antinociception, and reduced the dose for half-maximal effect (ED50).18 Together, these studies suggest that methadone is a substrate for P-gp, and brain P-gp-mediated transport influences brain access and pharmacologic effect.
In contrast to cellular and animal studies, little information exists on the role of P-gp in determining methadone brain access in humans. Indirect evidence from a pharmacogenetic study of P-gp genetic variants and dose requirements in methadone-maintained patients suggested P-gp substrate potential for methadone.19 In contrast, the P-gp inhibitor quinidine did not alter intravenous methadone-dependent changes in pupil diameter (miosis) or methadone concentration-effect relationships.20 Although quinidine did increase miosis after oral methadone, this was attributed to intestinal P-gp inhibition, increased methadone absorption, and increased plasma concentrations rather than enhanced brain penetration and altered BBB P-gp activity.20 It was recognized that quinidine is a non-potent P-gp inhibitor, and plasma quinidine concentrations possibly insufficient to inhibit brain P-gp and P-gp-mediated methadone transport (if present).20 Therefore, the potential role of BBB P-gp in influencing human methadone brain penetration is unknown.
A recent study in human volunteers, conducted because in vitro and animal studies implicated P-gp in morphine transport, suggested a role for P-gp or other efflux transporters in morphine brain access and pharmacodynamics.21 Specifically, morphine miosis was more pronounced and prolonged in subjects pretreated with cyclosporine, reported to be an effective inhibitor of human BBB P-gp activity.21-23
The present study therefore tested the hypothesis that methadone is a substrate for human BBB drug transporters, such as P-gp, and that transport activity influences methadone plasma concentration-effect relationships (pharmacodynamics). The secondary aim was to evaluate the role of intestinal and renal transporters in the oral absorption and renal excretion of methadone. Cyclosporine was used as a drug transport inhibitor. Methadone concentration-effect relationships were studied using pupil diameter and analgesia as primary and secondary effect measures, in a single-center, open-label, crossover study in healthy volunteers.
MATERIALS AND METHODS
Clinical protocol
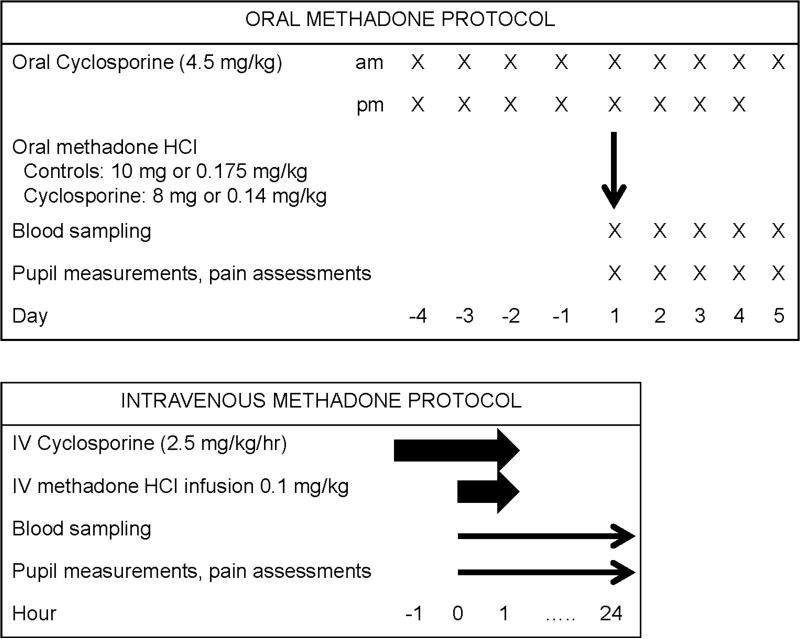
The clinical investigation was comprised of two separate protocols, for oral and intravenous (IV) drug administration, in healthy volunteers (Figure 1). Both were approved by the Institutional Review Board of Washington University in St. Louis. The protocols were 2-period sequential crossovers in healthy volunteers (control session first, for logistical considerations) with each subject as their own control. All subjects provided written informed consent. Healthy males and females, age 18-40 years and body mass index 20-33 kg/m2 were eligible. Exclusion criteria were a history of major medical problems, including a history of liver or kidney disease, use of prescription or non-prescription medications, herbals, or foods known to be substrates of P-gp or to affect its activity, pregnant or nursing females, and a known history of addiction to drugs or alcohol. For both protocols, intravenous catheters were inserted for drug administration and blood sampling and subjects received IV ondansetron (4 mg) for antiemetic prophylaxis. Subjects were monitored with a pulse oximeter and automated blood pressure cuff, and received supplemental oxygen for saturations less than 94%. Subjects were fed a standard breakfast two hr after drug dosing and had free access to food and water thereafter. Methadone doses were chosen to target a small change (2-3 mm) in pupil diameter based on previous studies.
Figure 1.
Protocol scheme
Protocol 1 (oral methadone) consisted of two sessions at least ten days apart, the second of which was preceded by oral cyclosporine 4.5 mg/kg twice per day (maximally used therapeutic dose) (Gengraf, Abbott, Abbott Park IL) for four days before and on the morning of the study day. The first four subjects were given 10 or 8 mg of racemic methadone hydrochloride orally for the control (session 1) or cyclosporine (session 2) sessions, respectively, in anticipation of a potentially increased methadone effect when co-administered with cyclosporine. The 10 mg dose was chosen to target a small change (2-3 mm) in pupil diameter. Methadone was administered 2 hr after the final oral cyclosporine dose. Due to greater than anticipated intersubject variability in weight, the remaining twelve subjects received weight-based dosing (0.175 and 0.14 mg/kg methadone hydrochloride, respectively, in control and cyclosporine sessions) to diminish potential interindividual variability in plasma concentrations.
For protocol 2 (intravenous methadone), also on two occasions at least a week apart, twelve subjects received 0.1 mg/kg methadone as a one hr intravenous infusion for both control (session 1) and cyclosporine (session 2) sessions. In session 2, subjects received an intravenous infusion of 2.5 mg/kg/h cyclosporine (Bedford Laboratories, Bedford, OH) for two hr. This cyclosporine dose produced a 79% increase in intracerebral concentrations of the P-gp substrate verapamil,23 and was used in the previous investigation of morphine pharmacodynamics.21 Methadone was administered starting at the beginning of the second hr of the cyclosporine infusion.
Dark-adapted pupil diameter was measured in triplicate coincident with blood sampling using a handheld infrared pupillometer (Neuroptics, Irvine, CA).24 Pupil diameter change from pre-drug baseline (miosis) was determined at each time. Analgesia was assessed by response to thermal stimulus (Pathway, Medoc Advanced Medical Systems, Ramat Yishai, Israel) using both the maximum tolerated temperature (method of limits) and the verbal analog pain rating of several predetermined temperatures (ramp-and-hold method). Thermode temperature started at 36°C and increased 0.5°C/sec, and subjects pressed a button when the maximum tolerable temperature was reached. The average result of three stimuli was recorded in °C. Subjects then rated pain intensity on a verbal analog scale (VAS, 0-100) in response to discrete stimuli (41.0, 43.0, 44.8, 46.5, 48.2, and 50.0°C in random order). The probe was moved to a different region for each thermal stimulus.
Sixteen subjects (8 males, 8 females, 79 ± 14 kg, body mass index 27 ± 4) completed both arms of protocol 1. The average oral racemic methadone hydrochloride doses were 13 ± 3 and 10 ± 2 mg (control and cyclosporine sessions), corresponding to 5.8 ± 1.2 and 4.7 ± 1.0 mg of each methadone enantiomer base, and the cyclosporine dose was 361 ± 64 mg BID for four days. Twelve subjects (8 males, 4 females, 75 ± 14 kg, body mass index 25 ± 4) completed both arms of protocol 2. The average IV methadone hydrochloride dose was 7.5 ± 1.5 mg for control and cyclosporine sessions, corresponding to 3.4 ± 0.7 mg of each methadone enantiomer base. The cyclosporine dose was 424 ± 84 mg IV over 2 h.
Analytical methods
Venous blood samples were obtained before and periodically for 96 (protocol 1) and 24 hr (protocol 2) after methadone administration, and all urine was collected during these times. Plasma was separated and stored at −20°C for later analysis. Cyclosporine blood concentrations were determined by the clinical laboratory of Barnes-Jewish Hospital as trough (pre-dose) and peak (2 hr after oral cyclosporine) concentrations on the methadone study day (protocol 1), and 1 (mid-infusion), 2 (end-infusion), and 4 hr after starting the IV cyclosporine infusion (protocol 2). Serum creatinine was determined before and after the study.
Methadone and 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) in plasma and urine were quantified by solid phase extraction and stereoselective high pressure liquid chromatography–mass spectrometry using a previous method.25 Interday coefficients of variation in plasma averaged 6% and 6% for EDDP enantiomers (0.2, 2 ng/ml) and 7%, 5%, and 4% for methadone enantiomers (1, 10, and 80 ng/ml).
Data and Statistical Analysis
The intended primary outcome measure was the EC50 (plasma concentration causing 50% attenuation of response to thermal stimulation) and secondarily, EC50 for miosis, determined using a standard sigmoid Emax model, where Emax is the maximum possible effect (e.g. pupil diameter change, miosis) and C is plasma methadone concentration:
Because methadone concentrations high enough to cause maximum miosis (Emax) were not attempted or achieved, Emax was fixed at 7 mm for the modeling, assuming typical minimum and maximum pupil diameters of 2.5 and 9.5 mm.21
Methadone metabolism and clearance were assessed, and standard pharmacokinetic parameters were determined by non-compartmental analysis, as described previously.3-6 Pharmacokinetic data were assessed using paired t-tests and effect data were analyzed by two-way repeated measure analysis of variance (ANOVA) with Student-Neumann-Keuls post-hoc analysis, with 2-tailed hypothesis testing (SigmaPlot, Systat Software Inc., San Jose CA). P < 0.05 was considered statistically significant.
Sample size was based on a secondary outcome (area under the plasma methadone concentration vs time curve, AUC), because intraindividual viariability in methadone analgesia was not known a priori. Based on prior 22 and 33% interday-intrasubject variability in intravenous and oral methadone AUC, respectively,3-6 to detect a 25% change using a paired t test (1-β=0.8, α=0.05) would require 9 and 16 subjects.
RESULTS
Oral methadone
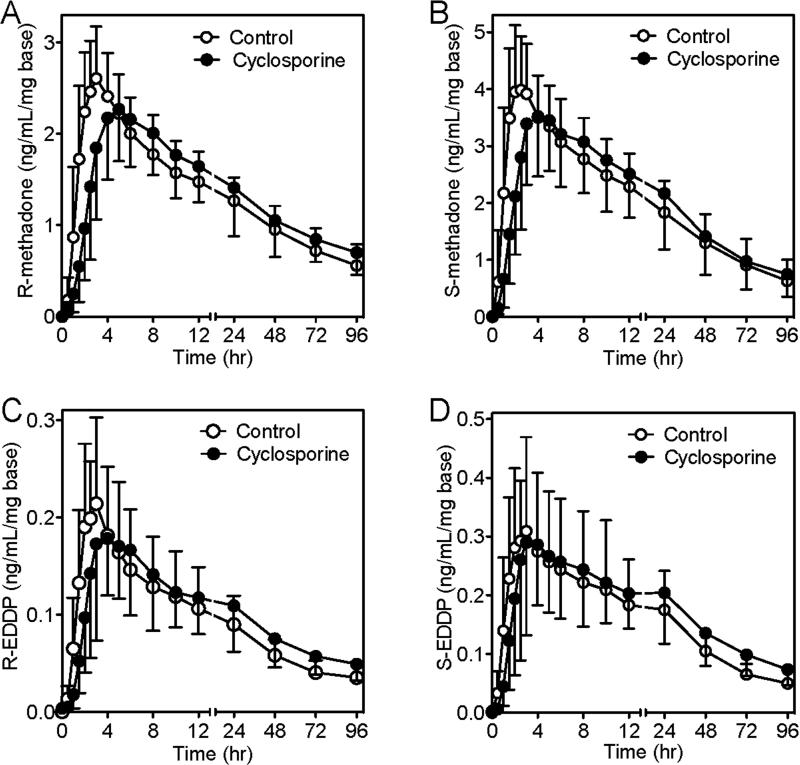
Cyclosporine blood concentrations after 4 days of oral administration were 451 ± 158 ng/ml (trough) and 1163 ± 248 ng/ml (peak). Dose-adjusted plasma methadone and EDDP concentrations versus time are shown in figure 2, and pharmacokinetic parameters in table 1. In cyclosporine-treated subjects, compared with untreated controls, dose-adjusted R- and S-methadone Cmax was slightly (about 10%) albeit significantly lower, Tmax was delayed, and dose-adjusted methadone enantiomer concentrations were lower between 1 and 3 hr after dosing, yet AUC0-24/dose (during and most immediately after cyclosporine dosing) was not different between groups. Together this suggests marginally impaired oral methadone absorption by cyclosporine. In contrast, methadone enantiomers AUC0-96/dose and AUC0-∞/dose were somewhat (about 10%) but significantly greater in the cyclosporine-treated subjects. Cyclosporine marginally (about 10%) but significantly decreased methadone apparent oral clearance, without affecting methadone renal clearance or the fraction eliminated in urine, either in the 24 hr most immediately after cyclosporine dosing or throughout the 96 hr follow-up period. Cyclosporine had no effect on EDDP apparent formation clearance, but did diminish EDDP elimination, evidenced by a greater elimination half-life, and thereby somewhat (11-15%) increased the EDDP/methadone AUC ratio.
Figure 2.
Effects of cyclosporine on dose-normalized methadone and 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) enantiomer plasma concentrations after oral methadone in subjects receiving nothing (controls, 0.175 mg/kg methadone hydrochloride, open circles) or 4 days of cyclosporine (4.5 mg/kg twice per day), where the methadone hydrochloride dose was 0.14 mg/kg (closed circles). (A) R-methadone, (B) S-methadone, (C) R-EDDP, and (D) S-EDDP. Results are the mean ± standard deviation (N=16). Methadone and EDDP dose (enantiomer base)-normalized concentrations were significantly lower in cyclosporine-treated subjects between 1 and 3 hr after dosing (p<0.05).
Table 1.
Oral methadone pharmacokinetic parameters
| Control | Cyclosporine | Control | Cyclosporine | |
|---|---|---|---|---|
| R-Methadone | S-Methadone | |||
| Plasma Cmax/dose(ng/ml/mg) | 2.8 ± 0.5 | 2.5 ± 0.6* | 4.6 ± 0.8 | 4.0 ± 0.9* |
| Plasma Tmax (h) | 2.6 ± 0.8 | 5.1 ± 2.2* | 2.0 ± 0.5 | 4.6 ± 2.3* |
| Plasma AUC0-24/dose (ng/mL•h/mg) | 38 ± 7 | 39 ± 8 | 59 ± 13 | 61 ± 17 |
| Plasma AUC0-96/dose (ng/ml•h/mg) | 100 ± 22 | 112 ± 26* | 142 ± 42 | 156 ± 54* |
| Plasma AUC0-∞/dose (ng/ml•h/mg) | 161 ± 64 | 193 ± 71* | 192 ± 82 | 211 ± 89 |
| AUC0-∞/dose ratio (cyclosporine/control) | 1.10 (1.10, 1.30) | 1.10 (1.03, 1.18) | ||
| Plasma Cl/F (ml/kg/min) | 1.64 ± 0.91 | 1.40 ± 0.88* | 1.41 ± 0.79 | 1.28 ± 0.77* |
| Vz/F (L/kg) | 7.7 ± 2.0 | 7.3 ± 2.6 | 4.7 ± 1.4 | 4.5 ± 1.6 |
| Plasma t½ (h) | 66 ± 28 | 73 ± 32 | 46 ± 18 | 46 ± 15 |
| Urine %dose eliminated (0-24 h) | 1.6 ± 0.7 | 2.6 ± 1.8 | 1.2 ± 0.6 | 2.0 ± 1.5 |
| Urine %dose eliminated (0-96 h) | 4 ± 2 | 5 ± 2 | 3 ± 2 | 3 ± 2 |
| Urine clearance (ml/kg/min) | 0.08 ± 0.05 | 0.08 ± 0.08 | 0.04 ± 0.03 | 0.05 ± 0.05 |
| R-EDDP | S-EDDP | |||
| Plasma Cmax/dose(ng/ml/mg) | 0.24 ± 0.09 | 0.22 ± 0.09 | 0.35 ± 0.17 | 0.36 ± 0.14 |
| Plasma Tmax (h) | 2.5 ± 0.5 | 4.1 ± 1.1* | 2.3 ± 0.7 | 4.0 ± 1.6* |
| Plasma AUC0-96/dose (ng/ml•h/mg) | 6.7 ± 1.6 | 8.2 ± 1.9* | 11.6 ± 3.2 | 14.4 ± 3.5* |
| Plasma t½ (h) | 57 ± 22 | 73 ± 38* | 87 ± 25 | 102 ± 39* |
| Plasma AUC0-96 (EDDP/methadone) | 0.07 ± 0.02 | 0.08 ± 0.02* | 0.09 ± 0.04 | 0.12 ± 0.05* |
| Plasma AUC0-96 (EDDP/methadone) ratio (cyclosporine/control) | 1.11 (1.04, 1.08) | 1.15 (1.04, 1.27) | ||
| Urine %dose eliminated (0-24 h) | 4.2 ± 2.3 | 4.2 ± 1.8 | 7.2 ± 3.8 | 7.4 ± 3.7 |
| Urine %dose eliminated (0-96 h) | 9 ± 4 | 10 ± 4 | 15 ± 6 | 16 ± 8 |
| Urine formation clearance (ml/kg/min) | 0.18 ± 0.15 | 0.15 ± 0.12 | 0.26 ± 0.23 | 0.24 ± 0.20 |
All data are reported as mean ± standard deviation except AUC ratios (cyclosporine/control), which are the geometric mean and 90% confidence interval (n=16). Urine data were not available for five subjects.
p<0.05 vs control
AUC=area under the plasma concentration-time curve; Cmax= peak plasma concentration; Tmax=time to maximum concentration, Cl/F, apparent oral clearance
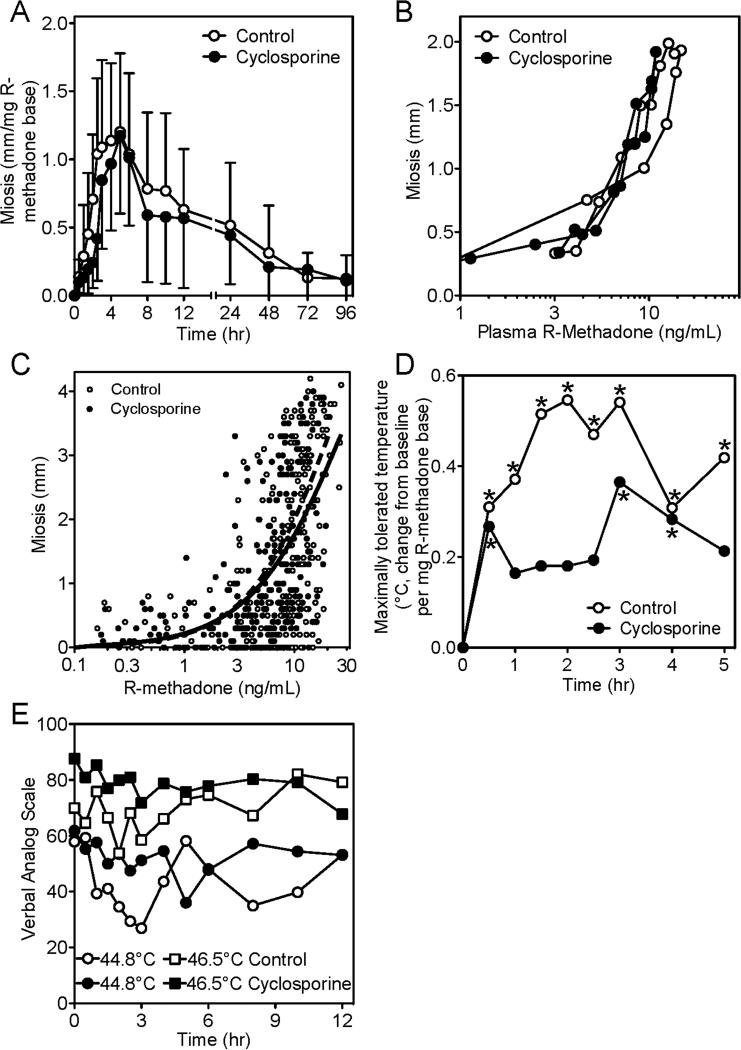
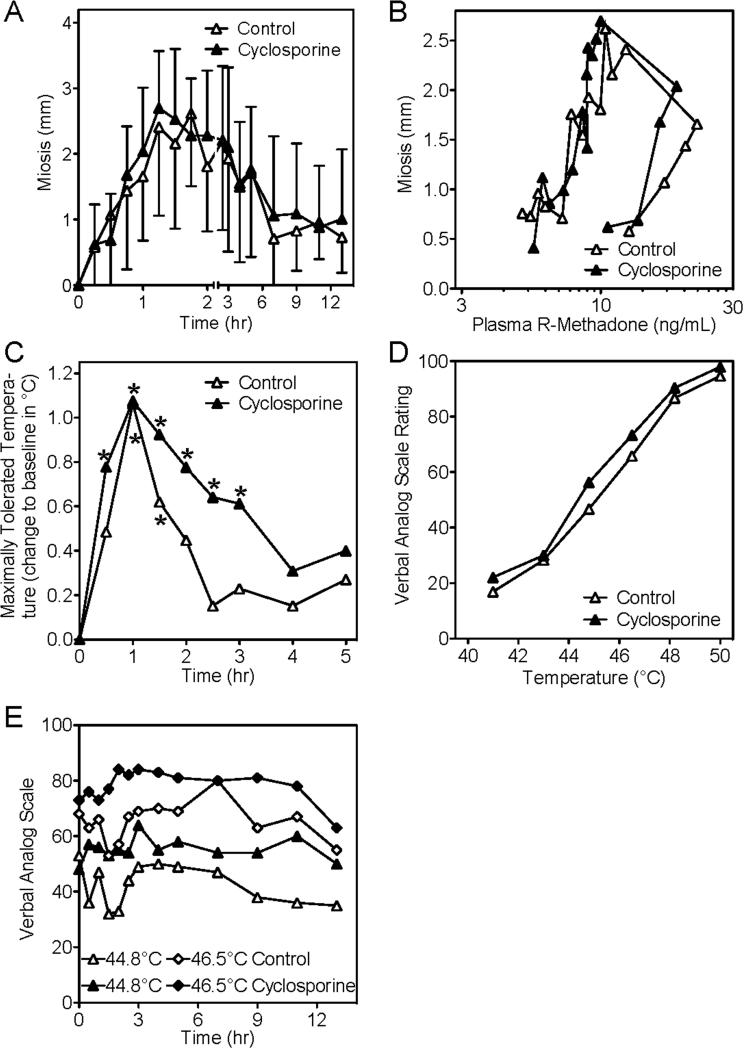
Oral methadone effects are shown in figure 3. Dose-adjusted dark-adapted pupil diameter difference vs pre-drug baseline (miosis) was not different between control and cyclosporine-treated subjects (fig. 3A). Miotic effects coincided with peak plasma methadone concentrations. Peak miosis was not different in controls (1.4 ± 0.4 mm/mg) and cyclosporine-treated subjects (1.3 ± 0.5 mm/mg). R-methadone (the active enantiomer) concentration-effect relationships (hysteresis curves) showed no difference between controls and cyclosporine-treated subjects (fig. 3B). However, at the early times and highest plasma concentrations after methadone dosing (0.5-5 hr), the lack of cyclosporine effects on miosis (fig. 3A) despite slightly lower plasma concentrations (fig 2A), and the apparently minor leftward shift of the mean concentration-effect curve (fig. 3B), prompted closer examination. Individual concentration-effect data for the 0.5-5 hr time period showed no differences between control and cyclosporine-treated subjects (fig. 3C), and modeling of the data using a sigmoid Emax model showed no significant differences between control and cyclosporine-treated subjects in R-methadone EC50 concentrations (29 ± 5 and 23 ± 3 ng/ml, respectively) or gamma (1.0 ± 0.2 and 1.1 ± 0.2). Methadone increased the maximally tolerated temperature in the method of limits paradigm, with the time of maximum analgesia coinciding with peak plasma methadone concentrations (fig. 3D). Maximally tolerated temperature was lower in the cyclosporine-treated subjects. In the paradigm using verbal analog ratings to discrete temperatures, there was a small and brief analgesic effect of methadone (fig. 3E). However, VAS scores were elevated in cyclosporine-treated subjects. Cyclosporine hyperalgesia in both pain paradigms was similar to that reported previously.21
Figure 3.
Influence of cyclosporine on oral methadone effects and pharmacodynamics. Effects were evaluated using dark-adapted pupil diameter and response to thermal stimulus. Open and solid symbols reflect controls and cyclosporine-treated subjects, respectively. (A) R-methadone dose-normalized miosis (time-specific pupil diameter minus pre-drug pupil diameter). Results are the mean ± standard deviation (N=16). (B) Miosis vs plasma R-methadone concentrations. Results are the mean (standard deviation omitted for clarity) (N=16). (C) Miosis vs plasma R-methadone concentrations for 0.5-5 hr after methadone dosing. Data points are individual concentrations. Lines (solid for controls, dashed for cyclosporine) show results predicted by fitting the data using a sigmoid Emax model. (D) R-methadone dose-normalized maximally tolerated temperature difference vs pre-drug baseline using the methods of limits. Results are the mean (standard deviation omitted for clarity, N=16, asterisks denote significant differences vs baseline). (E) Pain ratings (verbal analog scale) in response to thermal stimulus (shown for 44.8 and 46.5°C). Results are the mean (standard deviation omitted for clarity) (N=16).
Intravenous methadone
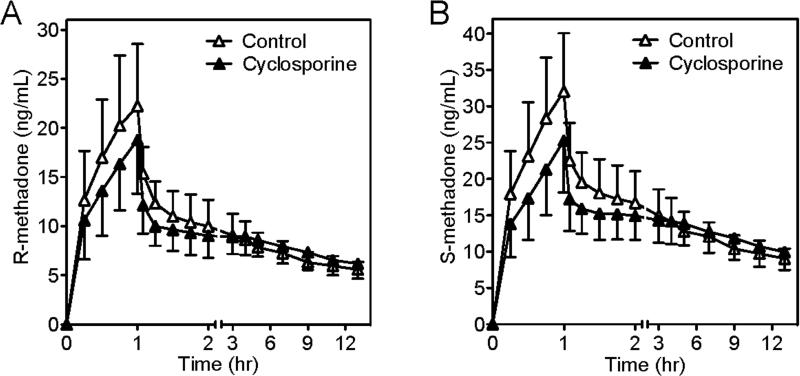
A second protocol using intravenous methadone and intravenous cyclosporine was performed, to achieve higher plasma cyclosporine concentrations than achievable after oral dosing, and to eliminate potential effects of cyclosporine on intestinal methadone absorption. Based on the results of protocol 1 with oral methadone, the same intravenous methadone dose was used in both the control and cyclosporine sessions. Cyclosporine blood concentrations were 321 ± 809 and 3764 ± 1277 ng/ml after one (at the start of the methadone infusion) and two h (at the end of the cyclosporine and methadone infusions) of cyclosporine, respectively, and 750 ± 146 ng/ml two h after the cyclosporine infusion was stopped. R- and S-methadone Cmax were somewhat but significantly lower in the cyclosporine-treated subjects compared to controls, as were methadone enantiomer concentrations between 0.25 and 2 hr after the start of the methadone infusion (fig. 4). Nevertheless, AUC0-24 was not different between groups and cyclosporine had no effect on methadone elimination in urine (table 2). Because the focus of this experiment was on methadone pharmacodynamics, plasma concentrations were not measured after 24 hr, and hence formal pharmacokinetics parameters not determined.
Figure 4.
Effects of cyclosporine on plasma concentrations of (A) R-methadone, (B) S-methadone, after a 1 hr intravenous infusion of methadone hydrochloride (0.1 mg/kg) in subjects receiving nothing (controls, open triangles) or a 2 hr infusion of cyclosporine (2.5 mg/kg/h, closed triangles). Results are shown as the mean ± standard deviation (N=12). Methadone concentrations were significantly lower in cyclosporine-treated subjects between 0.25 and 2 hr after the start of the methadone infusion (p<0.05). Times are relative to the start of the methadone infusion.
Table 2.
Intravenous methadone pharmacokinetic parameters
| Control | Cyclosporine | Control | Cyclosporine | |
|---|---|---|---|---|
| R-Methadone | S-Methadone | |||
| Plasma Cmax (ng/ml) | 22.6 ± 6.4 | 18.8 ± 5.5* | 32.2 ± 7.9 | 25.3 ± 7.2* |
| Plasma AUC0-24 (ng/ml•h) | 174 ± 31 | 180 ± 41 | 276 ± 47 | 282 ± 68 |
| Plasma AUC0-24/dose ratio (cyclosporine/control) | 1.03 (0.97, 1.09) | 1.00 (0.94, 1.08) | ||
| Urine %dose eliminated (0-24 h) | 6.3 ± 2.8 | 7.8 ± 3.2 | 5.1 ± 2.4 | 6.1 ± 2.5 |
All data are reported as mean ± standard deviation except AUC ratios (cyclosporine/control), which are the geometric mean and 90% confidence interval (n=12).
p<0.05 vs control
AUC=area under the plasma concentration-time curve; Cmax= peak plasma concentration
Miosis was not different between control and cyclosporine-treated subjects (fig. 5A). Peak miosis was 3.1 ± 1.0 and 3.2 ± 0.8 mm in controls and cyclosporine-treated subjects. Hysteresis curves showing the relationship between miosis and plasma R-methadone concentrations (fig. 5B) were similar in controls and cyclosporine-treated subjects. A small degree of thermal analgesia compared to baseline was observed at the intravenous methadone doses used (data given from 11 subjects, due to technical problems). The maximum tolerated thermal stimulus increased in both groups, peaked at the end of the methadone infusion, and abated after 3-4 hr (fig. 5C). However, there were no statistically significant differences between control and cyclosporine-treated subjects. In the ramp and hold paradigm of discreet thermal stimuli, there was a clear relationship between temperature and verbal analog scale (VAS) pain rating (fig. 5D), but VAS scores at peak methadone concentrations (end of the methadone infusion) were not different between controls and cyclosporine-treated subjects. Time-specific verbal pain ratings to discrete thermal stimuli were higher in the cyclosporine-treated subjects (fig. 5D). Thus, methadone had minimal analgesic effects in this experiment, and analgesia was not affected by cyclosporine pretreatment. Cyclosporine itself did decrease thermal pain tolerance.
Figure 5.
Influence of cyclosporine on intravenous methadone effects and pharmacodynamics. Effects were evaluated using dark-adapted pupil diameter and response to thermal stimulus. Time is relative to the start of the methadone infusion. The 2 hr cyclosporine infusion (−1 to 1 hr) was started 1 hr before methadone (0 to 1 hr). Open and solid symbols reflect controls and cyclosporine-treated subjects, respectively. (A) Miosis (time-specific pupil diameter minus pre-drug pupil diameter) versus time after start of infusion. Results are the mean ± standard deviation (N=12). (B) Miosis versus plasma R-methadone concentrations. Results are the mean (SD omitted for clarity, N=12). (C) Maximally tolerated temperature difference compared to pre-drug baseline using the methods of limits over the first 5 hr after the start of the methadone infusion. Results are the averages of differences (standard deviation omitted for clarity, N=11, asterisks denote significant differences vs baseline). (D) Stimulus-response relationship (verbal analog scale pain ratings) in response to discreet thermal stimuli, at the end of the 1 hr methadone infusion, and the influence of cyclosporine. Results are the mean (standard deviation omitted for clarity, N=12). (E) Pain ratings (verbal analog scale) in response to thermal stimulus (shown for 44.8 and 46.5 °C). Results are the mean (standard deviation omitted for clarity, N=12).
Adverse Events
During the intravenous cyclosporine infusion, some subjects reported uncomfortable feelings of warmth, which were not considered intolerable, stopped after the infusion was ended, and required no treatment. These side effects resolved after methadone administration. Serum creatinine concentrations were monitored as a safety assessment of renal function after cyclosporine. Creatinine concentrations were 0.9 ± 0.2 and 1.0 ± 0.2 mg/dl, respectively, before and after the cyclosporine session in protocol 1, and 0.9 ± 0.1 and 1.0 ± 0.1 mg/dl, respectively, before and after the cyclosporine session in protocol 2. One subject had a creatinine of 1.5 mg/dl post-cyclosporine, which had normalized when rechecked. Cyclosporine was therefore considered to have had no significant effect on renal function.
DISCUSSION
This investigation tested the hypothesis that methadone is subject to drug transport processes in humans. The primary focus was the blood-brain barrier, and the hypothesis that transport activity, specifically the efflux transporter P-gp, influences methadone pharmacodynamics. Cyclosporine was used as a previously validated inhibitory P-gp probe.22 Cyclosporine was previously found to enhance clinical effects (miosis) and pharmacodynamics of morphine, showing it to be a weak transporter substrate.21,22 In the present investigation, neither oral nor IV cyclosporine had a significant influence on oral or IV methadone plasma concentration–effect relationships, measured primarily as miosis. Since the investigation was initiated with the idea that cyclosporine was a selective inhibitor of P-gp, one conclusion would be to reject the hypothesis that methadone brain access and pharmacodynamics are mediated by P-gp. Nevertheless, it has become apparent that cyclosporine also inhibits other efflux transporters (vide infra). Therefore the primary conclusion is to reject the hypothesis that methadone brain access and pharmacodynamics are mediated by cyclosporine-inhibitable transport processes.
Support for this conclusion is greater from the IV than the oral methadone protocol, because blood cyclosporine concentrations were higher. The EC50 for cyclosporine inhibition of P-gp was previously reported in rats as 7 μM.26 In the present investigation, oral cyclosporine for 4 days achieved peak blood concentrations of 1.0 ± 0.2 μM. IV infusion (5 mg/kg over 2 hr) achieved blood cyclosporine concentrations of 2.7 ± 0.7 and 3.1 ± 1.1 μM after 1 and 2 hr, respectively, comparable to those previously shown to inhibit human brain P-gp activity.22,23,26 Specifically, intracerebral concentrations of the P-gp substrate verapamil, quantified using positron emission tomography imaging, were increased 79% by 2.8 μM cyclosporine (5 mg/kg over 2 hr).23
Accumulated evidence demonstrates however that cyclosporine is a non-selective inhibitor of several transport proteins, including the efflux transporters MRP2 and BCRP,27-30 and several uptake transporters. However, the cyclosporine IC50 for BCRP (26 μM)29 is far greater than systemic concentrations, and, at relevant concentrations, cyclosporine had no effect on OAT1 or OAT3 or MRP4, and only moderate inhibitory activity towards MRP2 in vitro, and is only considered to have significant inhibitory effects on intestinal (but not hepatic) MRP2 activity.31,32 Thus, we refer more broadly to cyclosporine-inhibitable transport rather than to specific inhibition of P-gp.
The human BBB constitutes a formidable defensive bulwark designed to restrict xenobiotic penetration. The most abundantly expressed human BBB ABC efflux transporters are P-gp, BCRP, and MRP4.33-35 These BBB efflux transporters collaborate to exclude drugs and prevent brain accumulation. Many drugs are substrates for more than one efflux transporter, and several murine studies have shown that for these poly-transporter substrates, selective chemical inhibition or genetic knock-out of only one transporter has minimal effect on brain accumulation, but simultaneous inhibition or genetic knock-out of all members of the relevant transporter suite markedly increases brain biodistribution.36-38 Thus, because BBB efflux transporters work in concert, unless cyclosporine were to inhibit all BBB transporters responsible for methadone efflux (assuming methadone is actually an efflux transporter substrate in vivo), inhibiting less than the full efflux transporter suite might not alter methadone pharmacodynamics. Therefore, the present results do not exclude the possibility that methadone is a substrate for one or more (non-cyclosporine inhibitable) efflux transporters at the human BBB. In addition, because cyclosporine can also inhibit uptake transporters such as OATPs, simultaneous inhibition of BBB uptake and efflux by cyclosporine might be offsetting. These considerations compel the need to identify more precisely which BBB transporters are responsible for methadone influx and/or efflux.
Results of this investigation can be compared with previous studies of P-gp-mediated methadone transport, both in vitro and animals. Early in vitro data said to support the P-gp substrate potential of methadone (i.e. inhibition of rhodamine-123 transport in Caco-2 cells), found an IC50 of 7.5 μM, which was far above clinical methadone concentrations, and which caused only a 25% reduction of rhodamine transport.39 Similarly, methadone transport in P-gp overexpressing cells was modest (transport efflux ratio of ~1.9),10,12 or absent.39 A transport ratio >2 is generally accepted as denoting a substrate, and, for comparison, the transport ratio of the well-known P-gp substrate loperamide generally exceeds 10. Although verapamil and GF120918 inhibited methadone transport in Caco-2 cells,13 they may also inhibit other transporters. Thus the present results are consistent with in vitro studies suggesting that methadone is a poor P-gp substrate. In contrast, animal studies in vivo, both with P-gp genetic knock-outs and cyclosporine inhibition, provided strong evidence for methadone brain efflux transport,.13-15 Thus, cyclosporine effects on methadone brain transport in humans are less than that predicted using cell systems, and much less than that by genetic knock-out or cyclosporine experiments in mice. It is now well-recognized that there are species differences in BBB P-gp expression and function, and that rodent models are over-predictive of human P-gp transport.40
Results of this investigation can also be compared with previous studies of BBB methadone transport in humans. They are consistent with the inability of the P-gp inhibitor quinidine to alter methadone miosis and concentration-effect relationships.20 They are not consistent with a report that methadone dose requirements were influenced by P-gp genetic polymorphisms.19
Cyclosporine influence on methadone miosis and pharmacodynamics was less than that observed previously for other opioids. The same cyclosporine regimen (5 mg/kg over 2 hr) increased and prolonged morphine miosis, increased the area under the miosis–time curve, plasma effect-site transfer rate constant, and calculated effect-site morphine concentrations, although the magnitude of the effects was small.21 Cyclosporine more markedly (110%) increased brain uptake of the known P-gp substrate loperamide (assessed by positron emission tomography) in volunteers,41,42 which, when corrected for loperamide metabolism, was even greater (457%).42 Thus, cyclosporine-inhibitable BBB transporters play a greater role in brain access, pharmacodynamics, and clinical effects of morphine, and certainly loperamide, than methadone.
A second conclusion of this investigation was that cyclosporine minimally altered the pharmacokinetics of oral and intravenous methadone. For both oral and IV protocols, plasma methadone enantiomer concentrations were slightly lower in the cyclosporine-treated subjects in the period immediately after methadone dosing. The mechanism for this effect on apparent methadone distribution is not evident, but appears unrelated to methadone elimination. The more mechanistically and clinically relevant observation is that cyclosporine had no significant effect on either methadone hepatic metabolism (N-demethylation to EDDP) or renal clearance. While methadone was originally identified as a substrate in vitro for cytochrome P4503A4 (CYP3A4),43 and assumed therefore to be cleared in vivo by CYP3A4, it is now clear that methadone is also a CYP2B6 substrate in vitro,3,44-47 and cleared predominantly if not exclusively by CYP2B6 in humans in vivo.3,5,25,48-51 Cyclosporine inhibits hepatic and intestinal CYP3A activity and the clearance of CYP3A substrates.32,52 Based on the in vitro Ki for cyclosporine inhibition of CYP3A (1.4 μM),53 and clinical effects of 200 mg/day cyclosporine (24-31% inhibition of CYP3A activity at a trough concentration of 119 ng/ml (0.1 μM)),52 and blood cyclosporine concentrations in the present investigation (>3 μM peak), substantial inhibition of CYP3A activity in the present investigation (approximately 720 mg/d oral cyclosporine) would be expected. The lack of CYP3A inhibition of methadone metabolism to EDDP by cyclosporine is inconsistent with a role for CYP3A in clinical methadone metabolism and clearance, but is consistent with previous studies in which other strong CYP3A inhibitors also had no influence on (or sometimes even increased) methadone N-demethylation and clearance, 3,6,48,51,54 and CYP3A induction also had no effect.55 This further reinforces the predominant role of CYP2B6 in methadone metabolism and clearance.3,5,25,48-51
Another investigational aim was to evaluate whether methadone is subject to intestinal and/or renal drug transport processes. Cyclosporine delayed methadone absorption and slightly reduced Cmax, but this is more consistent with inhibition of an uptake than an efflux transporter. Renal methadone clearance, which can account for up to 25% of total systemic methadone clearance, was not mediated by cyclosporine-inhibitable renal transporters. EDDP elimination did appear slightly reduced by cyclosporine. This may be consistent with observations that EDDP is a substrate for BCRP, OATP1A2, OCT1, and OCT3 (E. Kharasch, unpublished results) and that cyclosporine can affect these transporters.56
The last conclusion of this investigation was that miosis was a much more sensitive measure than thermal analgesia of methadone clinical effects and pharmacodynamics. Plasma R-methadone Cmax in controls averaged 23 and 16 ng/ml after IV and oral administration, respectively. Miotic response was greater and more sustained (average 2.5 and 2 mm, respectively) than thermal analgesia, using either the method of limits (maximally tolerated temperature in an ascending temperature paradigm), or the ramp-and-hold method (VAS scores to specific temperatures). Miosis was detectable at plasma R-methadone concentrations averaging 5 ng/ml. In comparison, the median minimal effective (postoperative) analgesia threshold for (racemic) methadone was 31 ng/ml.57
Both IV and oral cyclosporine caused cutaneous sensitization to heat, similar to that reported previously for IV cyclosporine.21 This sensitization differs from the well-described pain syndrome (bilateral bone pain in the lower extremities) caused by cyclosporine.58 Cyclosporine sensitization confounded the use of analgesia as a metric of cyclosporine influence on methadone effects and pharmacodynamics, and reinforces the value of pupillometry for evaluating these outcomes.
One limitation of this investigation is that cyclosporine is only a partial BBB P-gp inhibitor in humans. For example, the third-generation P-gp inhibitor tariquidar (6 mg/kg) in humans increased brain concentrations of the P-gp substrates [11C]N-desmethyl-loperamide and (R)-[11C]verapamil 4- and 2.5-fold, respectively,40,59 whereas cyclosporine (2.5 mg/kr/hr for 2 hr) increased (RS)-[11C]verapamil by only 88%.22 Nevertheless, third generation P-gp inhibitors were not available when the present investigation was performed.
In summary, this investigation showed that cyclosporine, used as an inhibitory in vivo probe for blood-brain barrier P-glycoprotein and other transporters, had no influence on methadone miosis, or on R-methadone plasma concentration-miosis relationships, for either IV or oral methadone. This suggests little or no role for cyclosporine-inhibitable transporters in methadone brain access and pharmacodynamics.
MS #201401024 Final Box Summary.
What we already know about this topic
Pretreatment with the blood-brain barrier efflux transport inhibitor cyclosporine resulted in more pronounced and more prolonged morphine-induced miosis in healthy volunteers.
In vitro and animal studies have suggested methadone may also be a substrate for blood brain barrier efflux transport.
What this article tells us that is new
Pretreatment of healthy volunteers with either oral or intravenous cyclosporine had no effect on the methadone concentration versus miosis relationship, suggesting there is no role for P-glycoprotein or other cyclosporine-inhibitable transporters in methadone brain access or pharmacodynamics in humans.
ACKNOWLEDGEMENTS
We thank Daniel Brennan, MD, Professor of Medicine (Nephrology), Washington University in St. Louis, St. Louis MO, for valuable guidance regarding cyclosporine dosing, Mitch Scott, PhD, Professor of Pathology & Immunology, Washington University in St. Louis, St. Louis MO, for cyclosporine analysis and interpretation of analytical methods, and Kathryn Vehe, RPh, Investigational Pharmacist, Barnes-Jewish Hospital, St. Louis MO, for drug preparation. The valuable technical assistance of Kristi Stubbert BS, Senior Research Technician, Nichole Meier BS, and Patty Suntrup RT, BA, CCRP, both Clinical Research Coordinators, Department of Anesthesiology, Washington University in St. Louis, St. Louis MO is appreciated.
Funded by a Clinical Scholar Research Award from the International Anesthesia Research Society (San Francisco, CA, USA) (to Konrad Meissner), National Institutes of Health (Bethesda MD, USA) Grants K24-DA00417, R01-DA14211, and R01-DA025931 (to Evan D. Kharasch), and National Institutes of Health Grant UL1-TR000448 to the Washington University in St Louis Institute of Clinical and Translational Sciences
Footnotes
This investigation was conducted before the requirement for clinical trials registration.
No author has any conflict of interest.
REFERENCES
- 1.Dyer KR, Foster DJ, White JM, Somogyi AA, Menelaou A, Bochner F. Steady-state pharmacokinetics and pharmacodynamics in methadone maintenance patients: Comparison of those who do and do not experience withdrawal and concentration-effect relationships. Clin Pharmacol Ther. 1999;65:685–94. doi: 10.1016/S0009-9236(99)90090-5. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Socoteanu C, Reinier K, Waltz J, Jui J, Gunson K. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121:66–71. doi: 10.1016/j.amjmed.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition, and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–69. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Kharasch ED, Bedynek PS, Walker A, Whittington D, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: II. Ritonavir effects on CYP3A and P-glycoprotein activities. Clin Pharmacol Ther. 2008;84:506–12. doi: 10.1038/clpt.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kharasch ED, Walker A, Whittington D, Hoffer C, Bedynek PS. Methadone metabolism and clearance are induced by nelfinavir despite inhibition of cytochrome P4503A (CYP3A) activity. Drug Alcohol Depend. 2009;101:158–68. doi: 10.1016/j.drugalcdep.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kharasch ED, Whittington D, Ensign D, Hoffer C, Bedynek PS, Campbell S, Stubbert K, Crafford A, London A, Kim T. Mechanism of efavirenz influence on methadone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2012;91:673–84. doi: 10.1038/clpt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–8. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem. 2006;54:1159–67. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wandel C, Kim R, Wood M, Wood A. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology. 2002;96:913–20. doi: 10.1097/00000542-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Tournier N, Chevillard L, Megarbane B, Pirnay S, Scherrmann JM, Decleves X. Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2). Int J Neuropsychopharmacol. 2010;13:905–15. doi: 10.1017/S1461145709990848. [DOI] [PubMed] [Google Scholar]
- 11.Tournier N, Decleves X, Saubamea B, Scherrmann JM, Cisternino S. Opioid transport by ATP-binding cassette transporters at the blood-brain barrier: Implications for neuropsychopharmacology. Curr Pharm Des. 2011;17:2829–42. doi: 10.2174/138161211797440203. [DOI] [PubMed] [Google Scholar]
- 12.Crettol S, Digon P, Golay KP, Brawand M, Eap CB. In vitro P-glycoprotein-mediated transport of (R)-, (S)-, (R,S)-methadone, LAAM and their main metabolites. Pharmacology. 2007;80:304–11. doi: 10.1159/000107104. [DOI] [PubMed] [Google Scholar]
- 13.Hassan HE, Myers AL, Coop A, Eddington ND. Differential involvement of P-glycoprotein (ABCB1) in permeability, tissue distribution, and antinociceptive activity of methadone, buprenorphine, and diprenorphine: In vitro and in vivo evaluation. J Pharm Sci. 2009;98:4928–40. doi: 10.1002/jps.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson SJ, Koszdin K, Bernards CM. Opiate-induced analgesia is increased and prolonged in mice lacking P-glycoprotein. Anesthesiology. 2000;92:1392–9. doi: 10.1097/00000542-200005000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Dagenais C, Graff CL, Pollack GM. Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem Pharmacol. 2004;67:269–76. doi: 10.1016/j.bcp.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Wang JS, Ruan Y, Taylor RM, Donovan JL, Markowitz JS, DeVane CL. Brain penetration of methadone (R)- and (S)-enantiomers is greatly increased by P-glycoprotein deficiency in the blood-brain barrier of Abcb1a gene knockout mice. Psychopharmacology (Berl) 2004;173:132–8. doi: 10.1007/s00213-003-1718-1. [DOI] [PubMed] [Google Scholar]
- 17.Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol. 2006;70:1212–9. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez M, Ortega I, Soengas I, Suarez E, Lukas JC, Calvo R. Effect of P-glycoprotein inhibition on methadone analgesia and brain distribution in the rat. J Pharm Pharmacol. 2004;56:367–74. doi: 10.1211/0022357022782. [DOI] [PubMed] [Google Scholar]
- 19.Levran O, O'Hara K, Peles E, Li D, Barral S, Ray B, Borg L, Ott J, Adelson M, Kreek MJ. ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum Mol Genet. 2008;17:2219–27. doi: 10.1093/hmg/ddn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharasch ED, Hoffer C, Whittington D. The effect of quinidine, used as a probe for the involvement of P-glycoprotein, on the intestinal absorption and pharmacodynamics of methadone. Br J Clin Pharmacol. 2004;57:600–10. doi: 10.1111/j.1365-2125.2003.02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meissner K, Avram MJ, Yermolenka V, Francis AM, Blood J, Kharasch ED. Cyclosporineinhibitable blood-brain barrier drug transport influences clinical morphine pharmacodynamics. Anesthesiology. 2013;119:941–53. doi: 10.1097/ALN.0b013e3182a05bd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasongko L, Link JM, Muzi M, Mankoff DA, Yang X, Collier AC, Shoner SC, Unadkat JD. Imaging P-glycoprotein transport activity at the human blood-brain barrier with positron emission tomography. Clin Pharmacol Ther. 2005;77:503–14. doi: 10.1016/j.clpt.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Hsiao P, Bui T, Ho RJ, Unadkat JD. In vitro-to-in vivo prediction of P-glycoprotein-based drug interactions at the human and rodent blood-brain barrier. Drug Metab Dispos. 2008;36:481–4. doi: 10.1124/dmd.107.018176. [DOI] [PubMed] [Google Scholar]
- 24.Kharasch ED, Walker A, Hoffer C, Sheffels P. Intravenous and oral alfentanil as in vivo probes for hepatic and first-pass cytochrome P450 3A activity: Noninvasive assessment by use of pupillary miosis. Clin Pharmacol Ther. 2004;76:452–66. doi: 10.1016/j.clpt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Kharasch ED, Stubbert K. Role of cytochrome P4502B6 in methadone metabolism and clearance. J Clin Pharmacol. 2013;53:305–13. doi: 10.1002/jcph.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsiao P, Sasongko L, Link JM, Mankoff DA, Muzi M, Collier AC, Unadkat JD. Verapamil P-glycoprotein transport across the rat blood-brain barrier: Cyclosporine, a concentration inhibition analysis, and comparison with human data. J Pharmacol Exp Ther. 2006;317:704–10. doi: 10.1124/jpet.105.097931. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M, Saitoh H, Tadano K, Takahashi Y, Hirano T. Cyclosporin A, but not tacrolimus, inhibits the biliary excretion of mycophenolic acid glucuronide possibly mediated by multidrug resistance-associated protein 2 in rats. J Pharmacol Exp Ther. 2004;309:1029–35. doi: 10.1124/jpet.103.063073. [DOI] [PubMed] [Google Scholar]
- 28.Qadir M, O'Loughlin KL, Fricke SM, Williamson NA, Greco WR, Minderman H, Baer MR. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–6. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 29.Xia CQ, Liu N, Miwa GT, Gan LS. Interactions of cyclosporin a with breast cancer resistance protein. Drug Metab Dispos. 2007;35:576–82. doi: 10.1124/dmd.106.011866. [DOI] [PubMed] [Google Scholar]
- 30.Westley IS, Brogan LR, Morris RG, Evans AM, Sallustio BC. Role of Mrp2 in the hepatic disposition of mycophenolic acid and its glucuronide metabolites: Effect of cyclosporine. Drug Metab Dispos. 2006;34:261–6. doi: 10.1124/dmd.105.006122. [DOI] [PubMed] [Google Scholar]
- 31.El-Sheikh AA, Greupink R, Wortelboer HM, van den Heuvel JJ, Schreurs M, Koenderink JB, Masereeuw R, Russel FG. Interaction of immunosuppressive drugs with human organic anion transporter (OAT) 1 and OAT3, and multidrug resistance-associated protein (MRP) 2 and MRP4. Transl Res. 2013;162:398–409. doi: 10.1016/j.trsl.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Gertz M, Cartwright CM, Hobbs MJ, Kenworthy KE, Rowland M, Houston JB, Galetin A. Cyclosporine inhibition of hepatic and intestinal CYP3A4, uptake and efflux transporters: Application of PBPK modeling in the assessment of drug-drug interaction potential. Pharm Res. 2013;30:761–80. doi: 10.1007/s11095-012-0918-y. [DOI] [PubMed] [Google Scholar]
- 33.Shawahna R, Uchida Y, Decleves X, Ohtsuki S, Yousif S, Dauchy S, Jacob A, Chassoux F, Daumas-Duport C, Couraud PO, Terasaki T, Scherrmann JM. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm. 2011;8:1332–41. doi: 10.1021/mp200129p. [DOI] [PubMed] [Google Scholar]
- 34.Decleves X, Jacob A, Yousif S, Shawahna R, Potin S, Scherrmann JM. Interplay of drug metabolizing CYP450 enzymes and ABC transporters in the blood-brain barrier. Curr Drug Metab. 2011;12:732–41. doi: 10.2174/138920011798357024. [DOI] [PubMed] [Google Scholar]
- 35.Sane R, Agarwal S, Mittapalli RK, Elmquist WF. Saturable active efflux by p-glycoprotein and breast cancer resistance protein at the blood-brain barrier leads to nonlinear distribution of elacridar to the central nervous system. J Pharmacol Exp Ther. 2013;345:111–24. doi: 10.1124/jpet.112.199786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang SC, Lagas JS, Lankheet NA, Poller B, Hillebrand MJ, Rosing H, Beijnen JH, Schinkel AH. Brain accumulation of sunitinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int J Cancer. 2012;130:223–33. doi: 10.1002/ijc.26000. [DOI] [PubMed] [Google Scholar]
- 37.Lin F, Marchetti S, Pluim D, Iusuf D, Mazzanti R, Schellens JH, Beijnen JH, van Tellingen O. Abcc4 together with abcb1 and abcg2 form a robust cooperative drug efflux system that restricts the brain entry of camptothecin analogues. Clin Cancer Res. 2013;19:2084–95. doi: 10.1158/1078-0432.CCR-12-3105. [DOI] [PubMed] [Google Scholar]
- 38.Oberoi RK, Mittapalli RK, Elmquist WF. Pharmacokinetic assessment of efflux transport in sunitinib distribution to the brain. J Pharmacol Exp Ther. 2013;347:755–64. doi: 10.1124/jpet.113.208959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stormer E, Perloff MD, von Moltke LL, Greenblatt DJ. Methadone inhibits rhodamine123 transport in Caco-2 cells. Drug Metab Dispos. 2001;29:954–6. [PubMed] [Google Scholar]
- 40.Bauer M, Zeitlinger M, Karch R, Matzneller P, Stanek J, Jager W, Bohmdorfer M, Wadsak W, Mitterhauser M, Bankstahl JP, Loscher W, Koepp M, Kuntner C, Muller M, Langer O. Pgp-mediated interaction between (R)-[11C]verapamil and tariquidar at the human blood-brain barrier: A comparison with rat data. Clin Pharmacol Ther. 2012;91:227–33. doi: 10.1038/clpt.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passchier J, Comley R, Salinas C, Rabiner E, Gunn R, Cunningham V, Wilson A, Houle S, Gee A, Laruelle M. Blood brain barrier permeability of [11C]loperamide in humans under normal and impaired P-glycoprotein function. J Nucl Med. 2008;49:211P. [Google Scholar]
- 42.Hsiao P, Unadkat JD. P-glycoprotein-based loperamide-cyclosporine drug interaction at the rat blood-brain barrier: Prediction from in vitro studies and extrapolation to humans. Mol Pharm. 2012;9:629–33. doi: 10.1021/mp200563a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iribarne C, Berthou F, Baird S, Dreano Y, Picart D, Bail JP, Beaune P, Menez JF. Involvement of cytochrome P450 3A4 enzyme in the N-demethylation of methadone in human liver microsomes. Chem Res Toxicol. 1996;9:365–73. doi: 10.1021/tx950116m. [DOI] [PubMed] [Google Scholar]
- 44.Gerber JG, Rhodes RJ, Gal J. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality. 2004;16:36–44. doi: 10.1002/chir.10303. [DOI] [PubMed] [Google Scholar]
- 45.Totah RA, Allen KE, Sheffels P, Whittington D, Kharasch ED. Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J Pharmacol Exp Ther. 2007;321:389–99. doi: 10.1124/jpet.106.117580. [DOI] [PubMed] [Google Scholar]
- 46.Totah RA, Sheffels P, Roberts T, Whittington D, Thummel K, Kharasch ED. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2008;108:363–74. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- 47.Gadel S, Crafford A, Regina K, Kharasch ED. Methadone N-demethylation by the common CYP2B6 allelic variant CYP2B6.6. Drug Metab Dispos. 2013;41:709–13. doi: 10.1124/dmd.112.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84:497–505. doi: 10.1038/clpt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kharasch ED, Hoffer C, Whittington D, Walker A, Bedynek PS. Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport: Insights from methadone interactions with ritonavir/indinavir. Anesthesiology. 2009;110:660–72. doi: 10.1097/ALN.0b013e3181986a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kharasch ED, Bedynek PS, Hoffer C, Walker A, Whittington D. Lack of indinavir effects on methadone disposition despite inhibition of hepatic and intestinal cytochrome P4503A (CYP3A). Anesthesiology. 2012;116:432–47. doi: 10.1097/ALN.0b013e3182423478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kharasch ED, Stubbert K. Cytochrome P4503A does not mediate the interaction between methadone and ritonavir-lopinavir. Drug Metab Dispos. 2013;41:2166–74. doi: 10.1124/dmd.113.053991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DR. In vivo CYP3A activity is significantly lower in cyclosporine-treated as compared with tacrolimus-treated renal allograft recipients. Clin Pharmacol Ther. 2011;90:414–22. doi: 10.1038/clpt.2011.130. [DOI] [PubMed] [Google Scholar]
- 53.Niwa T, Yamamoto S, Saito M, Shiraga T, Takagi A. Effect of cyclosporine and tacrolimus on cytochrome P450 activities in human liver microsomes. Yakugaku Zasshi. 2007;127:209–16. doi: 10.1248/yakushi.127.209. [DOI] [PubMed] [Google Scholar]
- 54.van Heeswijk R, Vandevoorde A, Verboven P, Boogaerts G, De Paepe E, van Solingen-Ristea R, Garg V, Beumont M. Pharmacokinetic interaction between methadone and the investigational HCV protease inhibitor telaprevir. J Hepatology. 2011;54:S491–492. [Google Scholar]
- 55.Vourvahis M, Wang R, Gruener DM, Bruce RD, Haider S, Tawadrous M. Effect of lersivirine co-administration on pharmacokinetics of methadone in healthy volunteers. Drug Alcohol Depend. 2012;126:183–8. doi: 10.1016/j.drugalcdep.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Picard N, Levoir L, Lamoureux F, Yee SW, Giacomini KM, Marquet P. Interaction of sirolimus and everolimus with hepatic and intestinal organic anion-transporting polypeptide transporters. Xenobiotica. 2011;41:752–7. doi: 10.3109/00498254.2011.573882. [DOI] [PubMed] [Google Scholar]
- 57.Gourlay GK, Wilson PR, Glynn CJ. Pharmacodynamics and pharmacokinetics of methadone during the perioperative period. Anesthesiology. 1982;57:458–67. doi: 10.1097/00000542-198212000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Prommer E. Calcineurin-inhibitor pain syndrome. Clin J Pain. 2012;28:556–9. doi: 10.1097/AJP.0b013e31823a67f1. [DOI] [PubMed] [Google Scholar]
- 59.Kreisl WC, Liow JS, Kimura N, Seneca N, Zoghbi SS, Morse CL, Herscovitch P, Pike VW, Innis RB. P-glycoprotein function at the blood-brain barrier in humans can be quantified with the substrate radiotracer 11C-N-desmethyl-loperamide. J Nucl Med. 2010;51:559–66. doi: 10.2967/jnumed.109.070151. [DOI] [PMC free article] [PubMed] [Google Scholar]