Abstract
Background and Purpose
This study was designed to evaluate the effect of betulinic acid (BA), extracted from Avicennia marina, on the replication of hepatitis C virus (HCV) and to investigate the mechanism of this BA-mediated anti-HCV activity.
Experimental Approach
HCV replicon and infectious systems were used to evaluate the anti-HCV activity of BA. Exogenous COX-2 or knock-down of COX-2 expression was used to investigate the role of COX-2 in the anti-HCV activity of BA. The effects of BA on the phosphorylation of NF-κB and on kinases in the MAPK signalling pathway were determined. The anti-HCV activity of BA in combination with other HCV inhibitors was also determined to assess its use as an anti-HCV supplement.
Key Results
BA inhibited HCV replication in both Ava5 replicon cells and in a cell culture-derived infectious HCV particle system. Treatment with a combination of BA and IFN-α, the protease inhibitor telaprevir or the NS5B polymerase inhibitor sofosbuvir resulted in the synergistic suppression of HCV RNA replication. Exogenous overexpression of COX-2 gradually attenuated the inhibitory effect of BA on HCV replication, suggesting that BA reduces HCV replication by suppressing the expression of COX-2. In particular, BA down-regulated HCV-induced COX-2 expression by reducing the phosphorylation of NF-κB and ERK1/2 of the MAPK signalling pathway.
Conclusions and Implications
BA inhibits HCV replication by suppressing the NF-κB- and ERK1/2-mediated COX-2 pathway and may serve as a promising compound for drug development or as a potential supplement for use in the treatment of HCV-infected patients.
Tables of Links
| TARGETS | |
|---|---|
| COX-2 | IKKβ |
| ERK1 | JNK |
| ERK2 | p38 kinase |
| IKKα |
| LIGANDS | |
|---|---|
| Betulinic acid (BA) | PGE2 |
| Dexamethasone | Sofosbuvir |
| IFN-α2 | Telaprevir |
| Insulin |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Hepatitis C is a chronic infectious disease, resulting in hepatic fibrosis, liver cirrhosis and hepatocellular carcinoma (HCC; Pawlotsky and McHutchison, 2004). Globally, an estimated 200 million people are infected with hepatitis C virus (HCV; Pellicano et al., 2004; Shepard et al., 2005). Although the most widely used standard-of-care (SOC) treatment against HCV is a combination of pegylated IFN-α (PEG-IFN-α) and ribavirin (RBV; Ascione et al., 2010), only 40–50% of patients infected with HCV genotype 1 presents a sustained virological response. Additionally, SOC therapy is also associated with severe side effects, including depression, fatigue, flu-like symptoms and haemolytic anaemia (Schaefer and Mauss, 2008; Thomas et al., 2011). Currently, two US Food and Drug Administration (FDA)-approved HCV protease inhibitors, telaprevir and boceprevir, in combination treatment with PEG-IFN-α and RBV can cause severe rashes or anaemia, resulting in the termination of this treatment (Schlutter, 2011). Drug resistance is a potential drawback for another FDA-approved nucleotide analogue, HCV NS5B polymerase inhibitor sofosbuvir, which is used in clinical therapy (Lam et al., 2012). Clearly, the development of more effective and safer agents is required for HCV therapy.
HCV is an RNA virus containing a positive-sense and single-strand genome of approximately 9.6 kilobases (kb). It is an enveloped virus belonging to the Hepacivirus genus of the Flaviviridae family (Lindenbach and Rice, 2005). Upon HCV entry into the target cells, the RNA genome is translated to a single polyprotein associated with the host endoplasmic reticulum (Mukhopadhyay et al., 2005). Then, the polyprotein is cleaved by viral and host proteases into four structural proteins (C, E1, E2 and p7) and six non-structural proteins (NS2, NS3, NS4A, NS4B, NS5A and NS5B; Penin et al., 2004). HCV infection induces the overexpression of COX-2 through the NF-κB signalling pathway, resulting in pathogenic inflammation and the proliferation of hepatoma cells (Nunez et al., 2004; Joo et al., 2005). COX-2 is an inducible COX isozyme involved in the conversion of arachidonic acid to various prostaglandins (PG), which induce cellular proliferation, cancer invasiveness and angiogenesis. Therefore, abnormal COX-2 expression has been suggested to be highly associated with carcinogenesis (Gee et al., 2008). Several studies have demonstrated that a high level of COX-2 is present in HCV-infected patients (Waris and Siddiqui, 2005a; Morinaga et al., 2007). Our previous reports have demonstrated that inhibiting this HCV-elevated COX-2 expression can efficiently suppress both HCV replication and HCV-induced inflammation (Chen et al., 2013; Lin et al., 2013b). Therefore, the interruption of an aberrant COX-2 signalling pathway may serve as a useful strategy for simultaneously eliminating HCV replication and HCV-related diseases.
Avicennia marina (Fork.) Vierh., a cosmopolitan species resident in the tropical and subtropical regions, belongs to the Verbenaceae family and is primarily found in China, Japan, the Philippines, Malaysia, India and Australia (Feng et al., 2006). In Egypt, the barks, leaves and fruits of Avicennia marina species have been used to treat skin disease (Jain et al., 2014). There are a variety of chemical components that have been isolated from A. marina species, including sterols, flavonoids, triterpines and fatty acids (Sharaf et al., 2000; Feng et al., 2006). In the present study, we isolated several betulinic acid (BA) derivatives from the aerial roots of A. marina. BA was identified as a pentacyclic triterpene and was originally discovered in 1995 in the stem bark of the plant Zizyphus mauritiana. Previous studies have indicated that BA and its derivative exert several biological effects, which include antioxidant, anti-inflammatory, anticancer and anti-HIV effects, and they can also prevent ethanol-induced fatty liver. Hence, we evaluated the inhibitory effect of BA on HCV replication. We showed that BA significantly suppressed HCV replication in replicon cells and in a HCV infectious system by attenuating the COX-2 signalling pathway. We further demonstrated that NF-κB and the MAPKs-ERK signalling pathway, the major regulatory factors of COX-2, are involved in the anti-HCV activity of BA. Finally, a combination treatment of BA with various HCV inhibitors exhibited synergistic suppressive effects on the replication of HCV.
Methods
Cell culture and reagents
Ava5 cells are the human hepatoma cells (Huh-7) harbouring HCV subgenomic replicon RNA (Blight et al., 2000) and were cultured in DMEM containing 10% heat-inactivated FBS, 1% antibiotic-antimycotic, 1% non-essential amino acids and 1 mg·mL−1 G418. Huh-7 cells and Huh7.5 were maintained in DMEM with 10% heat-inactivated FBS, 1% antibiotic-antimycotic, and 1% non-essential amino acids and were incubated at 37°C with a 5% CO2 supplement. Cryopreserved primary human hepatocytes (PHHs) were purchased from Invitrogen (Carlsbad, CA, USA; cat. no. Lot # Hu8116). The PHHs were maintained in Williams E medium supplemented with 5% FBS, dexamethasone (1 μM), penicillin/streptomycin (1%), human recombinant insulin (4 μg·mL−1), GlutaMax (2 mM) and HEPES, pH 7.4 (15 mM). IFN-α2a (Roferon©-A) was purchased from Roche Ltd (Basel, Switzerland). BA was purchased from Sigma-Aldrich (St. Louis, MO, USA). Telaprevir was purchased from Legend Stat International Co., Ltd (Omdurman, Sudan). Sofosbuvir was purchased from Shanghai Haoyuan Chemexpress Co., Ltd (Shanghai, China). Telaprevir or sofosbuvir was stored as 10 mM in 100% DMSO. All treatments were maintained consistently in 0.1% DMSO in each experiment.
Preparation of BA
BA was isolated from the aerial root of A. marina. BA was purified by chromatography over silica gel (60–120 mesh) and by HPLC (an L-2130 pump equipped with an L-2420 UV-vis detector, Hitachi, Tokyo, Japan) using a Supelco Discovery® HS C18 column (250 × 4.8 mm i.d., Supelco, Bellefonte, PA, USA). BA obtained by this method was greater than 99% purity using HPLC analysis (Supporting Information Fig. S1). The presence of BA was confirmed by 1D NMR spectra. BA: colourless solid; melting point, 295–298°C; [α]25D −77.9° (c 0.37, CHCl3), UV (MeOH) λmax (log ε) 216 (2.82) nm; IR (KBr) νmax: 3401, 1686 cm−1. The 1H NMR spectrum (400 MHz in CDCl3) showed the presence of five tertiary methyl groups at δH 0.76, 0.83, 0.94, 0.97 and 0.98 as singlets and follows vinyl methyl at δH 1.69, a secondary carbinol at δH 3.19 (dd, J = 11.2, 4.9 Hz), a characteristic methane at δH 3.0 (m), and an exomethylene group at δH 4.74 (1H, br s) and 4.61 (1H, br s). The 13C NMR spectrum (100 MHz in CDCl3) showed the presence of 30 resonances at δC 180.2 (C28), 150.4 (C20), 109.7 (C29), 79.0 (C3), 56.3 (C17), 55.3 (C5), 50.5 (C9), 49.2 (C18), 46.9 (C19), 42.4 (C14), 40.7 (C8), 38.9 (C4), 38.7 (C1), 38.4 (C13), 37.2 (C10), 37.0 (C22), 34.3 (C7), 32.1 (C16), 30.5 (C15), 29.7 (C21), 28.0 (C23), 27.4 (C2), 25.5 (C12), 20.8 (C11), 19.4 (C30), 18.3 (C6), 16.1 (C25), 16.0 (C26), 15.3 (C24) and 14.7 (C27). (+)ESI-MS data showed a signal with m/z 479 [M + Na]+. These NMR (CDCl3) and MS data are in agreement with the spectral data of BA (C30H48O3) reported in the literature (Anjaneyulu et al., 2003; Khaliq et al., 2007). The chemical structure of BA is given in Figure 1A. A stock solution of BA was prepared in DMSO and further diluted with DMEM culture medium.
Figure 1.
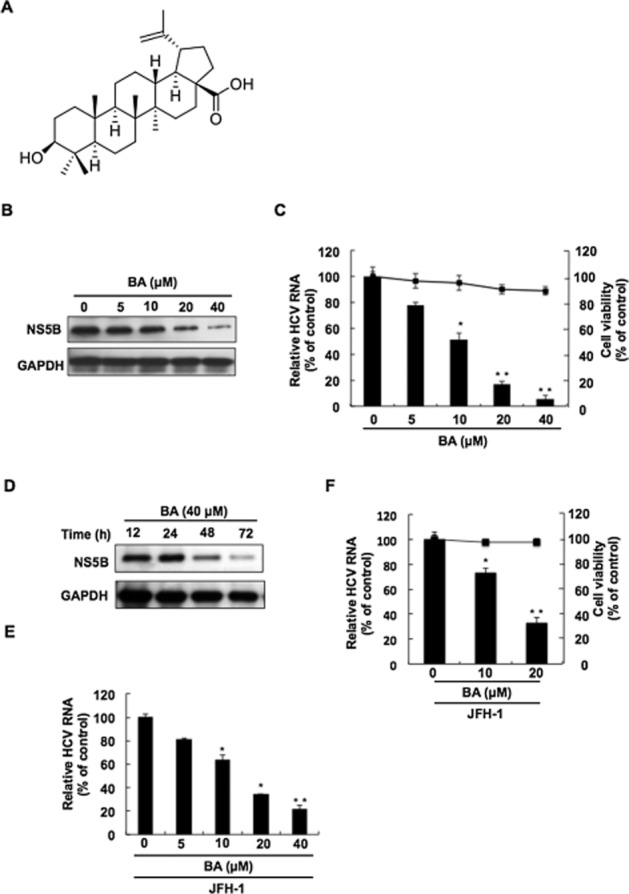
The inhibitory effect of BA on HCV replication. (A) The chemical structure of BA [3β-hydroxy-20(29)-lupaene-28-oic acid] C30H48O3 (MW 456.7). The inhibitory effect of BA on HCV (B) protein synthesis and (C) RNA replication without significant cell cytotoxicity. (D) Time-dependent reduction of HCV protein synthesis in BA-treated HCV replicon cells. Ava5 cells were treated with BA at the indicated concentration (0, 5, 10, 20 and 40 μM) for 3 days or with 40 μM of BA for the indicated times. Total cell lysates were subjected to Western blotting with anti-NS5B or anti-GAPDH antibody. Relative HCV RNA levels were analysed by RT-qPCR following the normalization of cellular gapdh mRNA levels. Cell viability was evaluated by the MTS assay in BA-treated Ava5 cells at the indicated concentrations after 3 days. (E) Concentration-dependent inhibition of HCV JFH-1 replication in BA-treated Huh7.5 cells. Huh7.5 cells were infected with HCV JFH-1 virus for 6 h, and Huh7.5-infected cells were treated with BA for another 3 days. The relative HCV RNA level was determined by RT-qPCR analysis with specific primer against HCV NS5B or gapdh gene. (F) Concentration -dependent inhibition of HCV JFH-1 replication in BA-treated PHHs. PHHs were infected with HCV JFH-1 for 8 h, and then were incubated with BA 0, 10 and 20 μM for 3 days. Relative HCV RNA levels were analysed by RT-qPCR, and cell viability was evaluated by the MTS assay in BA-treated PHHs. The efficiency of inhibition was assessed as a percentage of the control, BA-untreated cells. Data are presented as the mean ± SD of three independent experiments performed in triplicate (n = 9). Statistical significance of differences between BA-treated and BA-untreated cells was determined using Student’s t-test. *P < 0.05; **P < 0.01.
Immunoblotting analysis
Ava5 cells were plated on a 24-well plate at a density of 5 × 104 cells per well and a 6-well plate at a density of 4 × 105 cells per well. After 12 h incubation, cells were treated with the indicated concentration of reagents. Cell lysates were collected with RIPA lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 2% SDS and 1% NP-40) at apropriate times, and a Western blotting assay was performed as previously described (Lee et al., 2011b). The protein-transferred membranes were probed with specific antibodies against HCV NS5B (1:5000; Abcam, Cambridge, MA, USA), GAPDH, lamin B, Myc (1:10 000; GeneTex, Irvine, CA, USA), anti-phosphorylated IκBα, IKKα/β, p38, JNK, ERK and anti-total IκBα, IKKα, IKKβ, p65, p38, JNK, ERK antibodies (1:1000; Cell Signaling Technology, Inc. Danvers, MA, USA), or COX-2 (1:1000; Cayman, ML, USA). The blotting signal was developed using an ECL detection kit (PerkinElmer, CT, USA) and was counted by the software Quantity One (Bio-Rad, CA, USA).
Quantitative real-time RT-PCR (qRT-PCR) assay
Total cellular RNA were extracted using a Total RNA Miniprep Purification Kit (GMbiolab, Kaohsiung, Taiwan) according to the manufacturer’s instructions and transcribed to cDNA by M-MLV reverse transcriptase (Promega, Madison, WI, USA) with HCV 3′UTR (5′-acttgatctgcagagaggcc-3′) or oligo dT primer. The level of cDNA was determined through qRT-PCR with specific primers as previously described (Lee et al., 2011b). The CT value of each sample was determined by the ABI Step One Real-Time PCR-System (ABI, Warrington, UK). The PCR primers were as follows: GAPDH, 5′-gtcttcaccaccatggagaa-3′ (forward), and 5′-atggcatggactgtggtcat-3′ (reverse); NS5B, 5′-ggaaaccaagctgcccatca-3′ (forward) and 5′-cctccacggatagaagttta-3′ (reverse). Each sample was normalized by the endogenous cellular gapdh gene.
Cytotoxicity assay
Ava5 cells were seeded, 5 × 103 cells per well, in 96-well plates and treated with BA at the indicated concentration for 3 days. Cell viability was determined by colorimetric 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl) -2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega) according to the manufacturer’s instructions. The plate was re-incubated at 37°C for 2∼4 h, and the absorbance of the plate was determined at 490 nm using a 550 BioRad plate-reader (Bio-Rad, Hertfordshire, UK).
Transfection and luciferase activity assay
Ava5 cells (5 × 104) were transfected with 1 μg of the reporter plasmid pCOX-2-Luc or pNF-κB-Luc (BD Biosciences Clontech, Palo Alto, CA, USA) by using T-pro reagent (Ji-Feng Biotechnology CO., Ltd., Taipei, Taiwan) following the instruction of the manufacturer. After 6 h of transfection, the transfection reagents were changed with fresh medium containing BA at different concentrations. After 3 days of incubation, cell extracts were subjected to luciferase activity assay by using Bright-Glo Luciferase Assay System (Promega) in accordance with manufacturer’s instruction. To determine exogenous gene expression, Ava5 cells were transfected with either vehicle vector pcDNA4/myc-His-A (Life Technologies, Carlsbad, CA, USA) or COX-2 expression vector pCMV-COX-2-Myc with several concentrations (0.5, 1.0 and 1.5 μg) for 6 h. The transfection reagents were replaced with fresh medium containing BA at 40 μM. In the knock-down of COX-2 gene expression, Ava5 cells were transfected with either a control vector β-galactosidase (LacZ) short hairpin RNA (shRNA) or COX-2 shRNA expression vector (National RNAi Core Facility, Academia Sinica, Taipei, Taiwan) for 6 h. After 3 days, protein and RNA levels were analysed by Western blotting with a specific antibody or qRT-PCR with NS5B and GAPDH primers as previous described respectively.
HCV particles preparation and viral infection assay
Infectious HCV genomic type 2a JFH-1 virus particles were produced by transfecting full-length and linearized JFH-1 RNA into Huh7.5 cells (Kato et al., 2006). The supernatant with JFH-1 virus was filtered (0.45 μm) and stored at −70°C until use. The JFH-1 virus infectivity titre was determined by immunostaining with an anti-core antibody as previous described (Kato et al., 2006). Huh7.5 cells were infected with 50 μL of HCV JFH-1 particles at a multiplicity of infection (M.O.I.) of 0.1 for 6 h, and then were treated with BA at various concentrations for 3 additional days. PHHs were seeded onto 24-well collagen-coated plates at a density of 3 × 105 per well for 16–18 h. PHHs were infected with 50 μL of HCV JFH-1 particles at a M.O.I. of 0.01 for 8 h, and then the virus-containing medium were changed to fresh medium with BA at various concentrations for 3 additional days. Total protein and cellular RNA were subjected to Western blot analysis and qRT-PCR, respectively, as described above.
Analysis of the drug synergism
Ava5 cells were seeded at a density of 5 × 104 cells on a 24-well plate and treated with serially diluted BA at 2.5, 5, 10 and 20 μM in combination with diluted IFN-α (7.5, 15, 30 and 60 U·mL−1), the HCV protease inhibitor telaprevir (0.075, 0.15, 0.3 and 0.6 μM) or the RNA-dependent RNA polymerase nucleoside inhibitor sofosbuvir (10, 20, 40 and 80 nM). Each of the combination treatments was performed by adding BA horizontally with various HCV inhibitors vertically in a checkerboard cross in the 24-well plate. Total cellular RNA was extracted after 3 days of treatment with the compounds, and the RNA levels were quantified through qRT-PCR with specific primers as described above. The combination index (CI) values of each combination achieving 50%, 75% or 95% reduction in HCV RNA level were calculated using the CalcuSyn2™ computer programme (Biosoft, Cambridge, UK), which was based on the Chou and Talalay analysis method (Chou and Talalay, 1981; 1984,). Principally, a CI value of 1, <1 and >1 indicate additive, synergistic and antagonistic effects respectively.
Preparation of cytoplasmic and nuclear fractions
The cytoplasmic and nuclear fractions were prepared through NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Science Inc., Waltham, MA, USA) according to the manufacturer’s protocol. Briefly, Ava5 cells (4 × 105) were plated on the 6-well plate and then treated with BA at various concentrations for 3 days. Total cells were incubated with hypotonic buffer (10 mM HEPES, 1 mM MgCl2, 1 mM EDTA, 10 mM KCl, 0.5 mM DTT, 0.5% Nonidet P-40, 4 mg·mL−1 leupeptin, 20 mg·mL−1 aprotinin and 0.2 mM PMSF) for 15 min on ice, and the cytoplasmic fractions were prepared by centrifugation at 7000× g for 15 min. The pellets containing crude nuclei were suspended in the hypertonic buffer (20 mM HEPES, 1.5 mM MgCl2, 0.2 mM EDTA, 0.6 M KCl, 0.5 mM DTT, 25% glycerol) at 4°C for 30 min. The nuclear fraction was collected after centrifugation at 20 000× g for 15 min and stored at −80°C until use.
Statistical analysis
All data are presented as the mean ± SD. Statistical significance was determined using Student’s t-test for differences between two data groups (BA-treated and -untreated cells). The n value indicates the number of experiments used. P < 0.05 was considered to be significant.
Results
BA inhibits HCV replication in the HCV replicon, HCV JFH-1-infected Huh7.5 cells and primary human hepatocytes
To investigate the anti-HCV activity of BA, Ava5 cells (Blight et al., 2000) were incubated with BA at the indicated concentrations. After 3 days, Western blotting was performed to analyse the HCV protein expression. Simultaneously, the cytotoxicity of BA was measured by MTS assay. The results showed that BA reduced HCV protein levels in a concentration-dependent manner compared with the BA-untreated cells (Figure 1B). Consistently, the results of RT-qPCR showed that BA reduced HCV RNA levels without significant cytotoxicity in a concentration-dependent fashion (Figure 1C). To compare the anti-HCV effect between extracted BA from A. marina and commercially available BA with ≥98% purity, we treated Ava5 cells with commercially available BA under the same experimental conditions. Similar results were observed after treatment with commercially available BA (Supporting Information Fig. S2), which confirmed the anti-HCV activity of BA. In addition, BA induced a time-dependent reduction in HCV protein levels at a concentration of 40 μM (Figure 1D). We then performed the JFH-1 infectious assay to confirm the inhibitory effect of BA on HCV replication using qRT-PCR analysis under the same experimental conditions described above (Figure 1E), and obtained an EC50 value of 11.2 ± 0.3 μM. To further investigate the bioactivity of BA on PHHs, HCV JFH-1-infected PHHs were treated with BA at the same experimental conditions described above. Simultaneously, the cytotoxic effect of BA on PHHs was measured by MTS assay. As shown in Figure 1F, BA reduced HCV RNA levels with an EC50 value of 13.2 ± 0.5 and no significant cytotoxic effects were observed in BA-treated PHHs, which is consistent with the results obtained using human hepatoma cells.
BA down-regulates COX-2 expression in HCV replicon, HCV JFH-1-infected Huh7.5 cells and primary human hepatocytes
COX-2 is an inflammatory mediator and is aberrantly induced upon HCV infection. In turn, COX-2-derived PGE2 promotes HCV RNA replication (Nunez et al., 2004; Waris and Siddiqui, 2005a). Due to the anti-inflammatory activity of BA, we first investigated whether BA could inhibit HCV-induced COX-2 expression in HCV replicon cells. As shown in Figure 2A, the results of Western blotting analysis demonstrated that BA markedly suppressed COX-2 protein levels in a concentration-dependent manner. A COX-2 promoter activity assay using the COX-2 promoter-linked luciferase gene was then performed in parental Huh-7 and Ava5 cells in the presence of various concentrations of BA. The results of the luciferase activity assay showed that BA decreased the elevated COX-2 promoter activity in the Ava5 cells compared with the Huh-7 cells in a concentration-dependent fashion (Figure 2B), indicating that BA inhibited COX-2 expression at the transcriptional level. Next, we performed the HCV infectious assay to confirm the inhibitory effect of BA on HCV-induced COX-2 protein expression and promoter activity (Figure 2C and D). To further confirm the inhibitory effect of BA on HCV-induced COX-2 expression in normal hepatocytes, we used HCV JFH-1 to infect the PHHs and then incubated them with BA at the indicated concentrations. The results of Western blotting and RT-qPCR showed that BA significantly reduced HCV-induced COX-2 protein and RNA levels in a concentration-dependent fashion (Figure 2E and F), which are consistent with the results observed above using a human hepatoma cell line.
Figure 2.
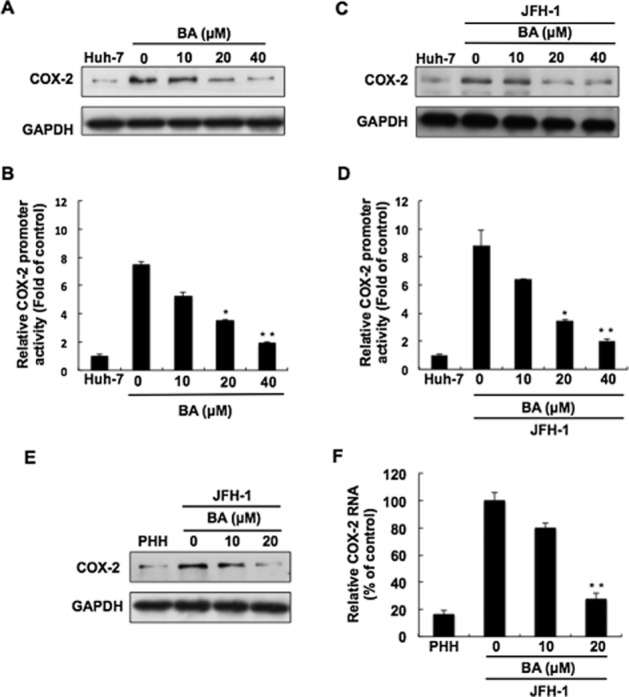
Inhibitory effect of BA on HCV-induced COX-2 expression. (A) Concentration-dependent reduction of HCV-induced COX-2 expression by BA. Ava5 cells were treated with BA at the indicated concentrations for 3 days. Cell lysates were subjected to Western blotting with antibodies against COX-2 or GAPDH. (B) Concentration-dependent reduction of HCV-induced COX-2 gene promoter activity by BA in Ava5 cells. Ava5 cells were transfected with pCOX-2-Luc reporter plasmid encoding firefly luciferase under the control of the COX-2 promoter. After treatment with BA at the indicated concentrations for 3 days, cell lysates were subjected to a luciferase activity assay. (C) Concentration-dependent reduction of HCV JFH-1-induced COX-2 expression by BA. The JFH-1-infected cells were treated with BA at the indicated concentrations for 3 days. Cell lysates were subjected to Western blotting with antibody against COX-2 or GAPDH. (D) Concentration-dependent reduction of HCV-induced COX-2 gene promoter activity by BA in HCV JFH-1-infected Huh-7 cells. The pCOX-2-Luc-transfected Huh-7 cells were infected with JFH-1 virus for 6 h. JFH-infected Huh-7 cells were treated with BA at the indicated concentrations for 3 days, and cell lysates were subjected to a luciferase activity assay. (E and F) Concentration-dependent reduction of HCV-induced COX-2 expression by BA in human primary hepatocytes (PHHs). HCV JFH-1-infected PHHs were incubated with BA at the indicated concentrations for 3 days. Cell lysates and RNA were subjected to Western blotting or RT-qPCR. Data are presented as the mean ± SD of three independent experiments performed in triplicate (n = 9). Statistical significance of differences between BA-treated and BA-untreated cells was determined using Student’s t-test. *P < 0.05; **P < 0.01.
BA inhibits HCV replication by suppressing COX-2 expression
Numerous reports have demonstrated that pharmacological inhibition of HCV-induced COX-2 expression can efficiently interfere with HCV replication (Trujillo-Murillo et al., 2008; Lee et al., 2011a; Chen et al., 2013; Lin et al., 2013b). To further clarify whether the inhibitory effect of BA on COX-2 expression contributes to its anti-HCV action, we examined the effect of BA on HCV replication by adding exogenous COX-2. Briefly, the Ava5 cells were transfected with various concentrations of the COX-2 expression vector, pCMV-COX-2-Myc, followed by incubation with 40 μM of BA for 3 days, in which the transfection of vehicle vector pcDNA4/myc-His-A served as a control. As shown in Figure 3A, increasing exogenous COX-2-Myc expression (middle panel, lanes 3–5) gradually restored HCV NS5B protein levels (upper panel, lanes 3–5) compared with untreated Ava5 cells incubated with BA (lane 1), and the vehicle plasmid transfected Ava5 cells in the presence of BA (lane 2). Consistently, the examination of the HCV RNA levels revealed that exogenous COX-2 expression can efficiently restore the HCV RNA level in the presence of BA (Figure 3B). To clarify the inhibitory effect of COX-2 expression on HCV replication, we transfected different concentrations of COX-2 or LacZ control shRNA in Ava5 cells. As shown in Figure 3C, COX-2 expression was gradually knocked-down by COX-2 shRNA (upper panel, lanes 3 and 4), and HCV protein synthesis was simultaneously reduced in a concentration-dependent manner (middle panel, lanes 3 and 4), compared to un-treated parental Huh-7 cells (lane 1), whereas LacZ shRNA, a control shRNA, had no effect on either COX-2 or HCV protein levels (lane 2), revealing that HCV-elevated COX-2 is required for HCV replication and supporting the hypothesis that the anti-HCV activity of BA is due to the down-regulation of COX-2 expression.
Figure 3.
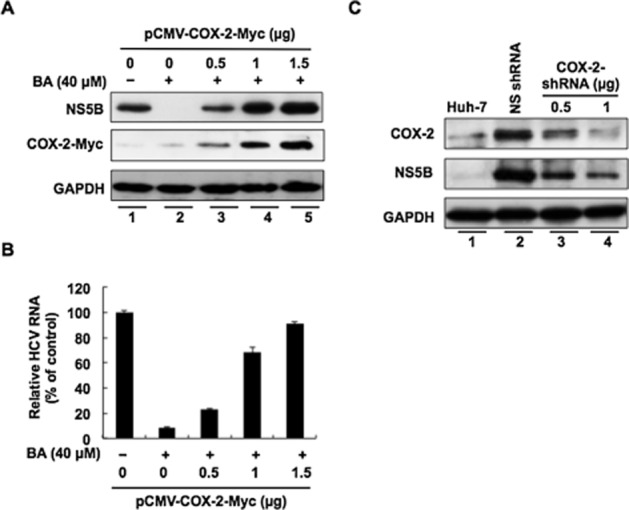
Restoration of HCV replication by exogenous COX-2 expression in BA-treated Ava5 cells. Ava5 cells were transfected with the indicated amount of pCMV-COX-2-Myc encoding cox-2 for 6 h, and then COX-2-transfected cells were treated with BA 40 μM for 3 days, in which vehicle pcDNA4/myc-His-A-transfected Ava5 cells in the presence of BA served as a mock control. (A) Concentration-dependent restoration of the HCV protein level. Cell lysates were subjected to Western blotting with an antibody against NS5B, Myc or GAPDH. (B) Concentration-dependent restoration of HCV RNA level. Total cellular RNAs were subjected to qRT-PCR analysis with specific primers against HCV NS5B or gapdh gene. (C) Concentration-dependent reduction of HCV protein synthesis induced by knock-down of the COX-2 gene. Ava5 cells were transfected with either 2 μg of LacZ shRNA vector (control group) or different amounts (0.5 and 1 μg) of COX-2 shRNA. After 3 days, cell lysates were subjected to Western blotting with anti-COX-2, anti-NS5B and anti-GAPDH antibodies. Data are presented as the mean ± SD of three independent experiments performed in triplicate (n = 9).
BA-induced down-regulation of COX-2 expression correlates with reduced NF-κB signalling and the activation of MAPK-ERK1/2
The activation of NF-κB is a critical transcription factor for HCV-induced COX-2 expression (Gong et al., 2001). To investigate whether an effect of BA on the NF-κB signalling pathway induces the down-regulation of COX-2 expression, we first determined the phosphorylation level of IKKα/β, IκBα, and the NF-κB subunit p65 from 0 to 6 h in Ava5 cells treated with BA at 40 μM. Western blot results revealed that BA significantly decreased the amount of phospho-IKKα/β, IκBα and NF-κB subunit p65 in a time-dependent manner (Figure 4A). As expected, BA also reduced the levels of nuclear NF-κB subunit p65 protein after 3 days of treatment in a concentration-dependent manner (Figure 4B). We next employed a NF-κB p65 DNA-binding activity assay using a pNF-κB-Luc reporter plasmid to confirm the inhibitory effect of BA on the NF-κB signalling pathway. In brief, pNF-κB-Luc-transfected Ava5 cells were incubated with BA at the indicated concentrations for 3 days. As expected, BA diminished the luciferase activity in a concentration-dependent manner (Figure 4C). In addition to the NF-κB signalling pathway, the MAPKs signalling pathway is also involved in the regulation of COX-2 levels (Hou et al., 2007). We then examined the effect of BA on a variety of regulators including ERK1/2, p38 kinase (p38) and cJNK. We treated Ava5 cells with BA at 40 μM and then performed Western blotting to monitor the phosphorylation levels of these regulators from 0 to 6 h. We observed that BA reduced the phosphorylation levels of ERK protein in a time-dependent fashion but did not alter the phosphorylation levels of p38 and JNK, compared with the time point of 0 h (Figure 5).
Figure 4.
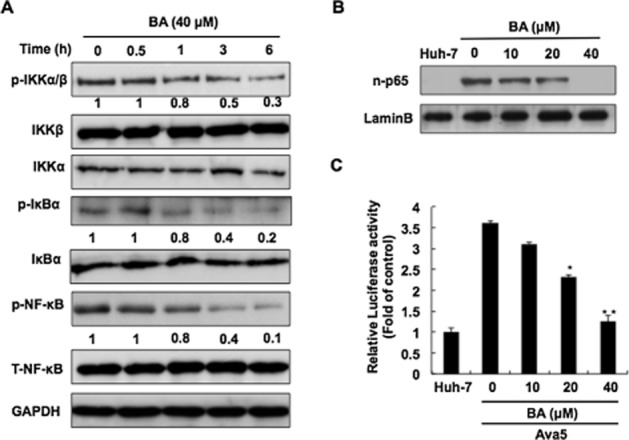
Inhibitory effect of BA on HCV-induced NF-κB signalling pathway. (A) Time-dependent reduction in the phosphorylation of NF-κB signalling transduction factors by BA. Ava5 cells were treated with BA at 40 μM for the indicated times (0, 0.5, 1, 3 and 6 h), and total lysates were subjected to Western blotting with the relevant antibodies. The relative blot intensities were quantified by densitometric scanning. (B) Dose-dependent suppression by BA of the nuclear translocation of p-p65. Ava5 cells were treated with the indicated concentrations of BA for 3 days. The nuclear extracts were subjected to Western blotting with antibody against p-p65 or Lamin B. (C) Dose-dependent reduction by BA of p65 promoter transcriptional activity. Huh-7 and Ava5 cells were transfected with pNF-κB-Luc for 6 h, and then treated with BA at the indicated concentrations for 3 days. Total cell lysates were subjected to a luciferase activity assay. Data are presented as the mean ± SD of three independent experiments performed in triplicate (n = 9). Statistical significance of differences between BA-treated and BA-untreated cells was determined using Student’s t-test. *P < 0.05; **P < 0.01.
Figure 5.
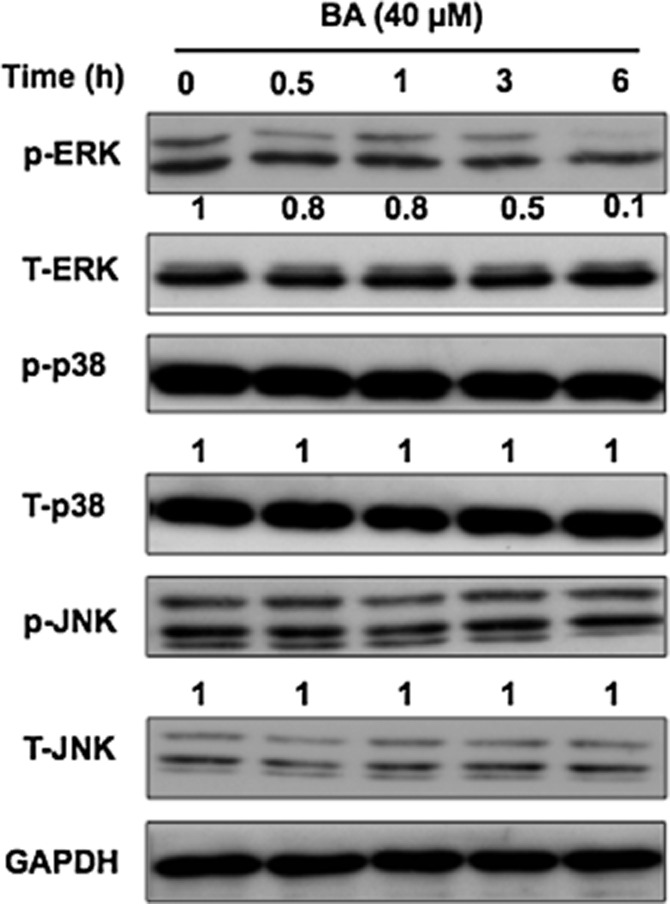
Inhibitory effect of BA on the phosphorylation of ERK 1/2. Ava5 cells were treated with 40 μM BA for the indicated times (0, 0.5, 1, 3 and 6 h), and total lysates were subjected to Western blotting with specific antibodies against p-ERK, T-ERK, p-p38, T-p38, p-JNK, T-JNK and GAPDH (loading control). The relative blot intensities were quantified by densitometric scanning. Data are presented as the mean ± SD of three independent experiments performed in triplicate (n = 9).
BA synergistically inhibits HCV replication when used in combination with various HCV inhibitors
To examine the anti-HCV activity of BA in combination treatments with various HCV inhibitors, we treated Ava5 cells with BA in combination with either IFN-α, the NS3/4A protease inhibitor telaprevir, or the NS5B polymerase inhibitor sofosbuvir, the US FDA-approved direct-acting antivirals (DAAs), at various concentrations with three different molar ratios as described in Methods. After 3 days, total RNA was extracted and quantified by qRT-PCR analysis. As shown in Table 2013, CI values of the combination treatments were less than 1 (ranging from 0.3 to 0.84) for ED50, ED75 or ED90, revealing a synergistic effect of BA against HCV RNA replication. No apparent cytotoxicity was observed in any of these assays (data not shown).
Table 1.
The synergistic effect of BA when combined with various HCV inhibitors on the suppression of HCV RNA replication
| Combination compound | Mean CI ± SD at | Influence | ||
|---|---|---|---|---|
| EC50 | EC75 | EC90 | ||
| IFN-α | 0.84 ± 0.061 | 0.68 ± 0.052 | 0.55 ± 0.022 | Synergistic |
| Telaprevir | 0.81 ± 0.072 | 0.56 ± 0.035 | 0.38 ± 0.048 | Synergistic |
| Sofosbuvir | 0.58 ± 0.022 | 0.43 ± 0.072 | 0.30 ± 0.032 | Synergistic |
Ava5 cells were treated with a combination of BA and IFN-α, telaprevir or sofosbuvir at various concentrations for 3 days. Total RNA was extracted and the levels of HCV RNA were quantified by qRT-PCR analysis. The combination index (CI) values for the effective dose for 50% (ED50), 75% (ED75), or 90% (ED90) inhibition were calculated using the CalcuSyn computer programme. Data are presented as the mean of two independent experiments performed in triplicate (n = 6). The definition of a CI value of 1 indicates additivity, CI values of <1 indicates synergism, and a CI value of >1 indicates an antagonistic effect.
Discussion
HCV infection activates host cellular effectors and soluble factors to stimulate inflammatory responses, and COX-2-regulated PGE2 is one of these factors that has recently been suggested as a major effector of severe tissue injury and fibrogenesis in HCV-related liver diseases (Waris and Siddiqui, 2005a). Upon HCV infection, the elevation of COX-2-derived PEG2 contributes to the prolonged acceleration of chronic inflammation and resistance to therapy, either mono-treatment or combination treatment, of HCV-associated cirrhosis patients (Morinaga et al., 2002). Furthermore, the overexpression of COX-2 in the cirrhotic livers of HCV-infected patients has been strongly associated with the recurrence of HCC in the liver (El-Bassiouny et al., 2007). Currently, the suppression of COX-2 has been implicated as a potential therapeutic target for selective compounds or crude plant extracts against HCV infection (Kern et al., 2002). In the present study, we revealed that BA strongly inhibited HCV-elevated COX-2 expression at both the transcriptional and translation level (Figure 2) and further confirmed that the suppression of COX-2 by BA contributed to its anti-HCV activity (Figure 3). The maximum inhibition (more than 80%) was observed at a concentration of 40 μM. Previous reports have also demonstrated that BA exhibits anti-tumorigenesis by reducing the expression of NF-κB-regulated genes, such as COX-2 and MMP-9, with maximum inhibition (more than 80%) at 30 μM (Takada and Aggarwal, 2003). These findings support the use of BA as a potential supplemental agent against HCV replication and HCV-related diseases, as it appears to target the dysregulated COX-2 expression during viral infection.
HCV infection stimulates COX-2 overexpression by oxidative stress and HCV NS3 protein elevates COX-2 expression through the activation of the host transcription factor NF-κB (Waris et al., 2005b; Lu et al., 2008). Furthermore, HCV-evoked NF-κB activation has been shown to increase the time for viral replication by blocking apoptosis and prolonging the survival of the host cell (Roulston et al., 1999). Here, we observed that BA treatment, at 40 μM, resulted in almost complete inhibition of HCV-induced NF-κB activation in HCV replicon cells (Figure 4), which play an important role in the inhibitory effect of BA on HCV-induced COX-2 expression and HCV replication. A previous study by Viji et al. also demonstrated that BA, at 2 μg·mL−1 (4.37 μM), almost completely inhibited LPS-induced pro-inflammatory PGE2 production by modulating the ERK and IκB phosphorylation in human peripheral blood mononuclear cells (Viji et al., 2011). Furthermore, Szuster-Ciesielska et al., reported that BA 1 μM could effectively attenuate ethanol-induced TNF-α production by inhibiting NF-κB signalling in liver stellate cells (Szuster-Ciesielska et al., 2011). Therefore, BA has been shown to have an inhibitory effect on the NF-κB signalling pathway upregulated by different stimuli in different cell lines. Additionally, as the NF-κB-mediated pathway can be regulated by other upstream kinases, such as PI3K/Akt, PKC and glycogen synthase kinase, the effect of BA on these kinases involved in HCV replication will be further investigated. In addition to the NF-κB signalling pathway, a number of MAPKs can affect the expression of COX-2. In the present study, we demonstrated that BA 40 μM also effectively suppressed the phosphorylation of ERK1/2 of the MAPK signalling pathway (Figure 5). At a similar concentration, BA has also been shown to inhibit IBMX-induced melanogenesis in B16F10 cells and high-fat-diet-induced fatty liver in primary rat hepatocytes by down-regulating the phosphorylation of ERK1/2 and up-regulating AMPK phosphorylation, respectively (Jin et al., 2014; Kim et al., 2014), revealing that BA can be widely used in the treatment of other diseases in addition to the HCV-related ones described above. The MAPK-ERK pathway and its downstream cell cycle regulating factors have been reported to play a crucial role in HCV protein synthesis in HCV 1b replicon cells and the production of infectious HCV 2a progeny (Menzel et al., 2012; Pei et al., 2012). Therefore, in addition to the down-regulation of COX-2, the targeting of MAPK by BA maybe an alternative signalling pathway that contributes to its effects on HCV replication; this will be further elucidated in the future studies.
Resistance to clinical antiviral therapy has become a major problem in the cure or management of chronic viral infection (Lin et al., 2013a). Cocktail therapy is a promising strategy to achieve a sustained viral response, such as a combination of inhibitors with IFN-α and/or the ribavirin, which have a synergistic effect on chronic genotype 1 HCV infection (Saxena and Terrault, 2012). Although many DAAs against HCV infection have been successfully developed in recent years, the variability of the viral genome due to the high viral replication rates has resulted in DAA-relative resistant variants (Halfon and Sarrazin, 2012; Sarrazin et al., 2012). Currently, targeting distinct HCV viral targets or host factors that are critical for HCV infection have been suggested as a viable strategy for preventing viral resistance and increasing the range of drug susceptibility in HCV genotypes (Wohlfarth and Efferth, 2009). In the present study, we identified a synergistic inhibitory effect of BA in combination with either IFN-α or other FDA-approval DAAs on HCV replication (Table 2013), which serves as a strong basis for the further examination of the inhibitory effect of BA on HCV replication in a suitable animal model.
Acknowledgments
We are grateful to Dr Charles Rice (Rockefeller University) and Apath, LLC, NY, USA for kindly providing the human hepatoma cell line (Huh-7 and Huh-7.5) and hepatitis C virus replicon cell line Ava5. We also thank T. Wakita (National Institute of Infectious Diseases, Japan) for HCVcc plasmid. This work was supported by a grant from the Ministry Science and Technology of Taiwan (NSC101-2311-B-037-002-MY3 and MOST103-2622-B-037-007-CC3) and a grant from the National Sun Yat-Sen University-KUM Joint Research Project (NSYSU-KMU 103-I005 and NSYSU-KMU 104-I011).
Glossary
- BA
betulinic acid
- CI
combination index
- DAA
direct-acting antivirals
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- PEG-IFN-α
pegylated IFN-α
- RBV
ribavirin
Author contributions
J-C L, C-C L and S-H W performed the experimental design. C-K L, C-K T and K-H C performed the research as described in the Methods. K-H C and C-C L contributed essential reagents. C-K L and C-K T analysed the data. J-C L and C-K L wrote the paper.
Conflict of interest
None.
Supporting Information
Figure S1 The LC-MS/MS data of the extracted betulinic acid by the Bruker amaZon SL system with Dionex UltiMate 3000 UHPLC system.
Figure S2 The inhibition effect of commercially available betulinic acid (BA) on HCV replication. The inhibitory effect of BA on HCV (A) protein synthesis and (B) RNA replication without significant cell cytotoxicity. Ava5 cells were treated with commercially available BA (Sigma-Aldrich) at the indicated concentration (0, 5, 10, 20 and 40 μM) for 3 days. Total cell lysates were subjected to western blotting with anti-NS5B or anti-GAPDH antibody. Relative HCV RNA levels were determined by qRT-PCR following the normalization of cellular gapdh mRNA levels. Cell variability was evaluated by the MTS assay. Data are presented as the mean ± SD of three independent experiments performed in triplicate (n = 9). Statistical significance were determined using Student’s t-test for difference between BA-treated and BA-untreated cells. *P < 0.05; **P < 0.01.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjaneyulu ASR, Murthy YLN, Lakshman Rao V, Sreedhar K. Chemical examination of the mangrove plant Avicennia officinalis. Indian J Chem. 2003;42B:3117–3119. [Google Scholar]
- Ascione A, De Luca M, Tartaglione MT, Lampasi F, Di Costanzo GG, Lanza AG, et al. Peginterferon alfa-2a plus ribavirin is more effective than peginterferon alfa-2b plus ribavirin for treating chronic hepatitis C virus infection. Gastroenterology. 2010;138:116–122. doi: 10.1053/j.gastro.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- Chen KJ, Tseng CK, Chang FR, Yang JI, Yeh CC, Chen WC, et al. Aqueous extract of the edible Gracilaria tenuistipitata inhibits hepatitis C viral replication via cyclooxygenase-2 suppression and reduces virus-induced inflammation. PLoS ONE. 2013;8:e57704. doi: 10.1371/journal.pone.0057704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem. 1981;115:207–216. doi: 10.1111/j.1432-1033.1981.tb06218.x. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- El-Bassiouny AE, Zoheiry MM, Nosseir MM, El-Ahwany EG, Ibrahim RA, El-Bassiouni NE. Expression of cyclooxygenase-2 and transforming growth factor-beta1 in HCV-induced chronic liver disease and hepatocellular carcinoma. Medgenmed. 2007;9:45–47. [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Li XM, Duan XJ, Wang BG. Iridoid glucosides and flavones from the aerial parts of Avicennia marina. Chem Biodivers. 2006;3:799–806. doi: 10.1002/cbdv.200690082. [DOI] [PubMed] [Google Scholar]
- Gee J, Lee IL, Grossman HB, Sabichi AL. Forced COX-2 expression induces PGE(2) and invasion in immortalized urothelial cells. Urol Oncol. 2008;26:641–645. doi: 10.1016/j.urolonc.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci U S A. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon P, Sarrazin C. Future treatment of chronic hepatitis C with direct acting antivirals: is resistance important? Liver Int. 2012;32(Suppl. 1):79–87. doi: 10.1111/j.1478-3231.2011.02716.x. [DOI] [PubMed] [Google Scholar]
- Hou DX, Masuzaki S, Hashimoto F, Uto T, Tanigawa S, Fujii M, et al. Green tea proanthocyanidins inhibit cyclooxygenase-2 expression in LPS-activated mouse macrophages: molecular mechanisms and structure-activity relationship. Arch Biochem Biophys. 2007;460:67–74. doi: 10.1016/j.abb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Jain R, Monthakantirat O, Tengamnuay P, De-Eknamkul W. Avicequinone C isolated from Avicennia marina exhibits 5alpha-reductase-type 1 inhibitory activity using an androgenic alopecia relevant cell-based assay system. Molecules. 2014;19:6809–6821. doi: 10.3390/molecules19056809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin KS, Oh YN, Hyun SK, Kwon HJ, Kim BW. Betulinic acid isolated from Vitis amurensis root inhibits 3-isobutyl-1-methylxanthine induced melanogenesis via the regulation of MEK/ERK and PI3K/Akt pathways in B16F10 cells. Food Chem Toxicol. 2014;68:38–43. doi: 10.1016/j.fct.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Joo M, Hahn YS, Kwon M, Sadikot RT, Blackwell TS, Christman JW. Hepatitis C virus core protein suppresses NF-kappaB activation and cyclooxygenase-2 expression by direct interaction with IkappaB kinase beta. J Virol. 2005;79:7648–7657. doi: 10.1128/JVI.79.12.7648-7657.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Date T, Murayama A, Morikawa K, Akazawa D, Wakita T. Cell culture and infection system for hepatitis C virus. Nat Protoc. 2006;1:2334–2339. doi: 10.1038/nprot.2006.395. [DOI] [PubMed] [Google Scholar]
- Kern MA, Schubert D, Sahi D, Schoneweiss MM, Moll I, Haugg AM, et al. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36:885–894. doi: 10.1053/jhep.2002.36125. [DOI] [PubMed] [Google Scholar]
- Khaliq S, Volk FJ, Frahm AW. Phytochemical investigation of Perovskia abrotanoides. Planta Med. 2007;73:77–83. doi: 10.1055/s-2006-951766. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Quan HY, Jeong KJ, Kim do Y, Kim G, Jo HK, et al. Beneficial effect of betulinic acid on hyperglycemia via suppression of hepatic glucose production. J Agric Food Chem. 2014;62:434–442. doi: 10.1021/jf4030739. [DOI] [PubMed] [Google Scholar]
- Lam AM, Espiritu C, Bansal S, Micolochick Steuer HM, Niu C, Zennou V, et al. Genotype and subtype profiling of PSI-7977 as a nucleotide inhibitor of hepatitis C virus. Antimicrob Agents Chemother. 2012;56:3359–3368. doi: 10.1128/AAC.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Tseng CK, Wu SF, Chang FR, Chiu CC, Wu YC. San-Huang-Xie-Xin-Tang extract suppresses hepatitis C virus replication and virus-induced cyclooxygenase-2 expression. J Viral Hepat. 2011a;18:e315–e324. doi: 10.1111/j.1365-2893.2010.01424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Chen WC, Wu SF, Tseng CK, Chiou CY, Chang FR, et al. Anti-hepatitis C virus activity of Acacia confusa extract via suppressing cyclooxygenase-2. Antiviral Res. 2011b;89:35–42. doi: 10.1016/j.antiviral.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Lin HM, Wang JC, Hu HS, Wu PS, Wang WH, Wu SY, et al. Resistance studies of a dithiazol analogue, DBPR110, as a potential hepatitis C virus NS5A inhibitor in replicon systems. Antimicrob Agents Chemother. 2013a;57:723–733. doi: 10.1128/AAC.01403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Wu YH, Tseng CK, Lin CK, Chen WC, Hsu YC, et al. Green tea phenolic epicatechins inhibit hepatitis C virus replication via cycloxygenase-2 and attenuate virus-induced inflammation. PLoS ONE. 2013b;8:e54466. doi: 10.1371/journal.pone.0054466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- Lu L, Wei L, Peng G, Mu Y, Wu K, Kang L, et al. NS3 protein of hepatitis C virus regulates cyclooxygenase-2 expression through multiple signaling pathways. Virology. 2008;371:61–70. doi: 10.1016/j.virol.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel N, Fischl W, Hueging K, Bankwitz D, Frentzen A, Haid S, et al. MAP-kinase regulated cytosolic phospholipase A2 activity is essential for production of infectious hepatitis C virus particles. PLoS Pathog. 2012;8:e1002829. doi: 10.1371/journal.ppat.1002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga S, Yamamoto Y, Noguchi Y, Imada T, Rino Y, Akaike M, et al. Cyclooxygenase-2 mRNA is up-regulated in cirrhotic or chronic hepatitis liver adjacent to hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17:1110–1116. doi: 10.1046/j.1440-1746.2002.02836.x. [DOI] [PubMed] [Google Scholar]
- Morinaga S, Tarao K, Yamamoto Y, Nakamura Y, Rino Y, Miyakawa K, et al. Overexpressed cyclo-oxygenase-2 in the background liver is associated with the clinical course of hepatitis C virus-related cirrhosis patients after curative surgery for hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1249–1255. doi: 10.1111/j.1440-1746.2006.04367.x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- Nunez O, Fernandez-Martinez A, Majano PL, Apolinario A, Gomez-Gonzalo M, Benedicto I, et al. Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: role of viral core and NS5A proteins. Gut. 2004;53:1665–1672. doi: 10.1136/gut.2003.038364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlotsky JM, McHutchison JG. Hepatitis C. Development of new drugs and clinical trials: promises and pitfalls. Summary of an AASLD hepatitis single topic conference, Chicago, IL, February 27-March 1, 2003. Hepatology. 2004;39:554–567. doi: 10.1002/hep.20065. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei R, Zhang X, Xu S, Meng Z, Roggendorf M, Lu M, et al. Regulation of hepatitis C virus replication and gene expression by the MAPK-ERK pathway. Virol Sin. 2012;27:278–285. doi: 10.1007/s12250-012-3257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano R, Mladenova I, Dimitrova SM, Bruno CM, Sciacca C, Rizzetto M. The epidemiology of hepatitis C virus infection. An update for clinicians. Minerva Gastroenterol Dietol. 2004;50:1–7. [PubMed] [Google Scholar]
- Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology. 2004;39:5–19. doi: 10.1002/hep.20032. [DOI] [PubMed] [Google Scholar]
- Roulston A, Marcellus RC, Branton PE. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- Sarrazin C, Hezode C, Zeuzem S, Pawlotsky JM. Antiviral strategies in hepatitis C virus infection. J Hepatol. 2012;56(Suppl. 1):S88–S100. doi: 10.1016/S0168-8278(12)60010-5. [DOI] [PubMed] [Google Scholar]
- Saxena V, Terrault N. Hepatitis C virus treatment and liver transplantation in the era of new antiviral therapies. Curr Opin Organ Transplant. 2012;17:216–224. doi: 10.1097/MOT.0b013e3283534d64. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Mauss S. Hepatitis C treatment in patients with drug addiction: clinical management of interferon-alpha-associated psychiatric side effects. Curr Drug Abuse Rev. 2008;1:177–187. doi: 10.2174/1874473710801020177. [DOI] [PubMed] [Google Scholar]
- Schlutter J. Therapeutics: new drugs hit the target. Nature. 2011;474:S5–S7. doi: 10.1038/474S5a. [DOI] [PubMed] [Google Scholar]
- Sharaf M, El-Ansari MA, Saleh NA. New flavonoids from Avicennia marina. Fitoterapia. 2000;71:274–277. doi: 10.1016/s0367-326x(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Szuster-Ciesielska A, Plewka K, Daniluk J, Kandefer-Szerszen M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-alpha, TGF-beta) production and by influencing intracellular signaling. Toxicology. 2011;280:152–163. doi: 10.1016/j.tox.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Takada Y, Aggarwal BB. Betulinic acid suppresses carcinogen-induced NF-kappa B activation through inhibition of I kappa B alpha kinase and p65 phosphorylation: abrogation of cyclooxygenase-2 and matrix metalloprotease-9. J Immunol. 2003;171:3278–3286. doi: 10.4049/jimmunol.171.6.3278. [DOI] [PubMed] [Google Scholar]
- Thomas E, Feld JJ, Li Q, Hu Z, Fried MW, Liang TJ. Ribavirin potentiates interferon action by augmenting interferon-stimulated gene induction in hepatitis C virus cell culture models. Hepatology. 2011;53:32–41. doi: 10.1002/hep.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo-Murillo K, Rincon-Sanchez AR, Martinez-Rodriguez H, Bosques-Padilla F, Ramos-Jimenez J, Barrera-Saldana HA, et al. Acetylsalicylic acid inhibits hepatitis C virus RNA and protein expression through cyclooxygenase 2 signaling pathways. Hepatology. 2008;47:1462–1472. doi: 10.1002/hep.22215. [DOI] [PubMed] [Google Scholar]
- Viji V, Helen A, Luxmi VR. Betulinic acid inhibits endotoxin-stimulated phosphorylation cascade and pro-inflammatory prostaglandin E(2) production in human peripheral blood mononuclear cells. Br J Pharmacol. 2011;162:1291–1303. doi: 10.1111/j.1476-5381.2010.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris G, Siddiqui A. Hepatitis C virus stimulates the expression of cyclooxygenase-2 via oxidative stress: role of prostaglandin E2 in RNA replication. J Virol. 2005a;79:9725–9734. doi: 10.1128/JVI.79.15.9725-9734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Waris G, Turkson J, Hassanein T, Siddiqui A. Hepatitis C virus (HCV) constitutively activates STAT-3 via oxidative stress: role of STAT-3 in HCV replication. J Virol. 2005b;79:1569–1580. doi: 10.1128/JVI.79.3.1569-1580.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wohlfarth C, Efferth T. Natural products as promising drug candidates for the treatment of hepatitis B and C. Acta Pharmacol Sin. 2009;30:25–30. doi: 10.1038/aps.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The LC-MS/MS data of the extracted betulinic acid by the Bruker amaZon SL system with Dionex UltiMate 3000 UHPLC system.
Figure S2 The inhibition effect of commercially available betulinic acid (BA) on HCV replication. The inhibitory effect of BA on HCV (A) protein synthesis and (B) RNA replication without significant cell cytotoxicity. Ava5 cells were treated with commercially available BA (Sigma-Aldrich) at the indicated concentration (0, 5, 10, 20 and 40 μM) for 3 days. Total cell lysates were subjected to western blotting with anti-NS5B or anti-GAPDH antibody. Relative HCV RNA levels were determined by qRT-PCR following the normalization of cellular gapdh mRNA levels. Cell variability was evaluated by the MTS assay. Data are presented as the mean ± SD of three independent experiments performed in triplicate (n = 9). Statistical significance were determined using Student’s t-test for difference between BA-treated and BA-untreated cells. *P < 0.05; **P < 0.01.


