Abstract
Background and Purpose
Omecamtiv mecarbil (OM) is a novel cardiac myosin activator drug for inotropic support in systolic heart failure. Here we have assessed the concentration-dependent mechanical effects of OM in permeabilized cardiomyocyte-sized preparations and single skeletal muscle fibres of Wistar-Kyoto rats under isometric conditions.
Experimental Approaches
Ca2+-dependent active force production (Factive), its Ca2+ sensitivity (pCa50), the kinetic characteristics of Ca2+-regulated activation and relaxation, and Ca2+-independent passive force (Fpassive) were monitored in Triton X-100-skinned preparations with and without OM (3nM-10 μM).
Key Results
In permeabilized cardiomyocytes, OM increased the Ca2+ sensitivity of force production (ΔpCa50: 0.11 or 0.34 at 0.1 or 1 μM respectively). The concentration–response relationship of the Ca2+ sensitization was bell-shaped, with maximal effects at 0.3–1 μM OM (EC50: 0.08 ± 0.01 μM). The kinetics of force development and relaxation slowed progressively with increasing OM concentration. Moreover, OM increased Fpassive in the cardiomyocytes with an apparent EC50 value of 0.26 ± 0.11 μM. OM-evoked effects in the diaphragm muscle fibres with intrinsically slow kinetics were largely similar to those in cardiomyocytes, while they were less apparent in muscle fibres with fast kinetics.
Conclusions and Implications
OM acted as a Ca2+-sensitizing agent with a downstream mechanism of action in both cardiomyocytes and diaphragm muscle fibres. The mechanism of action of OM is connected to slowed activation–relaxation kinetics and at higher OM concentrations increased Fpassive production.
Introduction
While heart failure (HF) specialists continue to wait for better opportunity for the characterization of patient populations or clinical conditions so as to optimize the outcome of therapy with conventional positive inotropes (McMurray et al., 2012), the search for new cardiotonic agents remains ongoing (Nagy et al., 2014). In this aspect, pharmacological targeting of the myocardial contractile protein machinery is an appealing concept for the treatment of the weakened cardiac contractions during acute and decompensated HF (Endoh, 2008). Direct activation of the cardiac myosin heads has recently been introduced as a novel approach for the treatment of systolic HF. Hence, the cardiac myosin activator omecamtiv mecarbil (OM) has been proved to be effective in animal models in vitro and in vivo (Malik et al., 2011). Indeed, emerging clinical experience lends support to this therapy (Cleland et al., 2011).
Considering the interaction between Ca2+ and the cardiac troponin C (cTnC) as a reference point within the contractile activation process, inotropic interventions can be classified as those with (i) upstream mechanisms (increasing the amplitude of the intracellular Ca2+ transient); (ii) central mechanisms (promoting the interaction between Ca2+ and cTnC); and (iii) downstream mechanisms (directly modulating the actin–myosin interactions; Endoh, 2001). The latter two actions are conventionally lumped together as Ca2+-sensitizing positive inotropy (Vannier et al., 1997; Szilagyi et al., 2004). By accelerating the inorganic phosphate (Pi) release from the cardiac myosin heads, myosin activation implicates a molecular mechanism, similar to those with a downstream inotropic action. Therefore, it is not surprising that OM increases the Ca2+ sensitivity of the myosin ATPase activity (Malik et al., 2011), but an OM-dependent Ca2+-sensitizing effect on isometric force production has not been documented to date.
Downstream Ca2+ sensitizer agents have been found to increase the Ca2+-independent passive force (Fpassive) of the cardiomyocytes, and thereby to impair the diastolic function of the heart. Accordingly, prolonged relaxation has been observed in the presence of EMD-57033, CGP-48506 and ORG-30029 (Miller and Steele, 1990; Brixius et al., 2000; Papp et al., 2004; Choi et al., 2010). The relative proximity of the OM docking site on the myosin heavy chain (MHC) to the binding site of EMD-57033 implies potential similarities between their mechanisms of action (Radke et al., 2014) but a potential effect of OM on the Fpassive remained unclear.
The effect of OM is considered to be selective for the myocardium, though its target molecule, the cardiac myosin β-heavy chain (β-MHC), is also expressed in certain skeletal muscle fibres (Brenner et al., 2012). OM may therefore influence the contractility of the diaphragm and other slow-twitch muscle fibres which also co-express β-MHC, but these potential effects have not been addressed experimentally so far.
The aims of our present investigations were therefore to identify potential OM-dependent Ca2+-sensitizing effects in cardiomyocytes and in diaphragm muscle fibres, and to determine how OM influences the mechanics and kinetics of their activation–relaxation cycles. These results will hopefully shed new light on the mechanism of action of OM and its tissue selectivity, and hence provide information concerning its clinical applicability.
Methods
Muscle specimens and the animal welfare and ethical statement
All animal care and experimental procedures involved in this work conformed strictly to Directive 2010/63/EU of the European Parliament and were approved by the Ethical Committee of the University of Debrecen (Ethical Statement number: 1/2013/DE MÁB). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 11 animals were used in the experiments described here.
Investigations were carried out on male 8–15-week-old Wistar-Kyoto rats, weighing 250–500 g (Toxi-Coop Toxicological Research Centre, Dunakeszi, Hungary). The animals were fed a standard chow and drank tap water ad libitum. The rats were housed in groups of two to three per polycarbonate cages in a room controlled thermostatically at 20 ± 2°C with a target relative humidity of 55 ± 15%. The room was artificially illuminated with fluorescent lights from 6:00 a.m. to 8:00 p.m. All procedures were as humane as possible to minimize the distress of the animals. Rats were anaesthetized with an intraperitoneal injection of sodium pentobarbital (Release, Garbsen, Germany; 150 mg·kg−1). The heart and the diaphragm were quickly excised and the left ventricle (LV) was dissected in cold isolating solution (MgCl2: 1 mM, KCl: 100 mM, EGTA: 2 mM, ATP: 4 mM, imidazole: 10 mM; pH 7.0). Diaphragm and LV tissue samples were stored at −80°C.
Mechanical measurements on permeabilized myocyte-sized preparations
Deep-frozen LV and diaphragm tissue samples were mechanically disrupted in isolating solution using a tissue homogenizer at 4°C, and thereafter subjected to chemical permeabilization with 0.5% Triton X-100 detergent for 5 min, as described previously (Papp et al., 2004). The Triton X-100 eliminated all membrane structures, allowing characterization of the mechanical properties of single myocytes under standardized conditions with the avoidance of potential disturbing factors present in vivo.
Isolated and permeabilized single cardiomyocytes or skeletal muscle fibres were kept in relaxing solution (BES: 10 mM; KCl: 37.11 mM; MgCl2: 6.41 mM; EGTA: 7 mM; ATP: 6.94 mM; creatine phosphate: 15 mM; pH 7.2) and were attached with silicone adhesive (DAP 100% all-purpose silicone sealant; Baltimore, MD, USA) to two stainless insect needles, connected to a very sensitive force transducer (SensoNor, Horten, Norway) and an electromagnetic motor (Aurora Scientific Inc., Aurora, Canada). After adjustment of the sarcomere length to 2.3 μm, the contractile machinery was activated by transferring the preparation from the relaxing to the activating solution (the same components apart from containing Ca2+-EGTA instead of EGTA). Ca2+ concentrations ([Ca2+]) were indicated as −log of calcium ion concentration (pCa) values, calculated by −lg[Ca2+]. The compositions of the activating (pCa 4.75) and relaxing solution (pCa 9.0) were calculated by using a previously reported approximation (Fabiato and Fabiato, 1979). All solutions contained protease inhibitors: phenylmethylsulfonyl fluoride: 0.5 mM, leupeptin: 40 μM and E-64: 10 μM. All chemicals were from Sigma-Aldrich, St. Louis, MO, USA.
The Ca2+-induced contractions of the preparations were recorded with a custom-built LABVIEW Data Acquisition platform and analysed with LabVIEW analysing software (Myo; National Instruments, Austin, TX, USA). When the Ca2+-activated force production reached its maximal, a quick release-restretch manoeuvre (30 ms) was applied in the activating solution. As a result of this intervention, force first dropped to zero and then started to redevelop allowing the determination of the total force (Ftotal). The Ca2+-independent Fpassive was approximated by shortening of the preparations to 80% of the original lengths in relaxing solution for 8 s. The active isometric force (Factive) was calculated as the difference between Ftotal and Fpassive. Force values were normalized for the cross-sectional area determined from the width and height of the myocytes, approximating their dimensions with elliptic geometry. To test the stability of the preparations, reference activations (pCa 4.75) were performed at the end of each experiment. Preparations were discarded when maximal force level (Fmax) declined to less than 80% of that recorded at the beginning of each experiment.
Analysis of the MHC isoform composition
The MHC isoform composition of the rat diaphragm and LV was analysed, as described elsewhere, using SDS-PAGE (Talmadge and Roy, 1993). Deep-frozen muscle samples were homogenized and then boiled in sample buffer for 2 min. The adjusted final protein concentration was 0.25 mg·mL−1. The stacking gel contained 30% glycerol, 4% acrylamide-bis, 70 mM Tris, 4 mM EDTA and 0.4% SDS. The separating gel consisted of 30% glycerol, 6% acrylamide-bis, 0.2 mM Tris, 4 mM EDTA and 0.4% SDS. The polymerization of the gels was induced by 0.05% tetramethylethylenediamine and 0.1% ammonium persulfate. Separate upper and lower buffers were used. The upper running buffer was composed of 0.1 mM Tris, 150 mM glycine and 0.1% SDS and the lower running gel of 50 mM Tris, 75 mM glycine and 0.05% SDS. The running conditions were 70 V (constant voltage) for 24 h at 4°C. The gels were stained with Coomassie Blue.
Data analysis
Isometric force values (F) at a given [Ca2+] (pCa 5.4–9.0), normalized to Fmax were fitted to a modified Hill equation in Origin 6.0 (Microcal Software, Northampton, MA, USA) or GraphPad Prism 5.02 (GraphPad Software, Inc., La Jolla, CA, USA):
The pCa value for the half-maximal contraction indicated by Ca50 or pCa50 defines the Ca2+ sensitivity of force generation of the contractile machinery, while the steepness of the Ca2+ sensitivity curve describing the myofilamental co-operativity was expressed as a coefficient (nHill).
The force redevelopment following the release-restretch manoeuvre was fitted to a single exponential to evaluate the rate constant of force redevelopment (ktr) indicating the intrinsic kinetics of the actin–myosin cross-bridges. The half-time of activation (t1/2 of activation) was determined by estimating the duration for half-maximal contraction after the preparations were placed in activating solution. The transfer of the preparations into the relaxing solution was associated with a short artefact on the force records. Relaxation time (trelax) was defined as the difference in time between this artefact and the moment when force returned to baseline level. Statistical significance, accepted at P < 0.05, was calculated by anova (repeated measures) followed by Dunnett’s two-tailed test or by paired Student t-test, as appropriate. Statistical analyses were carried out by GraphPad Prism. Values are given as means ± SEM.
Materials
OM was purchased from AdooQ BioScience (Irvine, CA, USA). Stock solutions with a final OM concentration of 10 mM were prepared in DMSO as solvent and stored at 4°C. Appropriate volumes of the concentrated stock solutions were dissolved in activating and relaxing solutions to obtain test solutions containing OM in the concentration range between 3 nM and 10 μM. The final concentration of DMSO never exceeded 0.1%.
Results
The Ca2+-sensitizing effects of OM in isolated cardiomyocytes
The Ca2+-sensitizing effect of OM was first investigated in LV myocyte-sized preparations. As illustrated by typical force recordings, the amplitudes of Factive production increased with OM concentrations upon repeated activations in test solutions at a submaximal [Ca2+] (pCa 6.1). The maximal increase in isometric force was reached in the presence of 1 μM OM. Contractile force was not enhanced further by higher OM concentrations, and it rather tended to decline towards the amplitude in OM-free conditions. This behaviour could not be attributed to preparation run-down as verified by the bracketing of test activations under OM-free conditions at saturating [Ca2+] (resulting in comparable force amplitudes at the beginning and at the end of our protocols; Figure 1A). The OM-evoked increase in Factive at pCa 6.1 was significant in the OM concentration range between 0.1 μM and 3 μM. The concentration–response relationship of OM was bell-shaped, with the highest levels of Ca2+ sensitization between 0.3 μM and 1 μM OM (Factive in OM-free medium: 2.74 ± 0.40 kN·m−2 vs. 8.66 ± 1.32 kN·m−2 and 8.71 ± 1.29 kN·m−2 at 0.3 and 1 μM OM, respectively; P < 0.001 vs. control; Figure 1B), while no Ca2+-sensitizing effect was apparently exerted at 10 μM OM. The concentration–response relationship was fitted to a sigmoid function in the OM concentration range between 3 nM and 1 μM resulting in an EC50 of 0.08 ± 0.01 μM.
Figure 1.
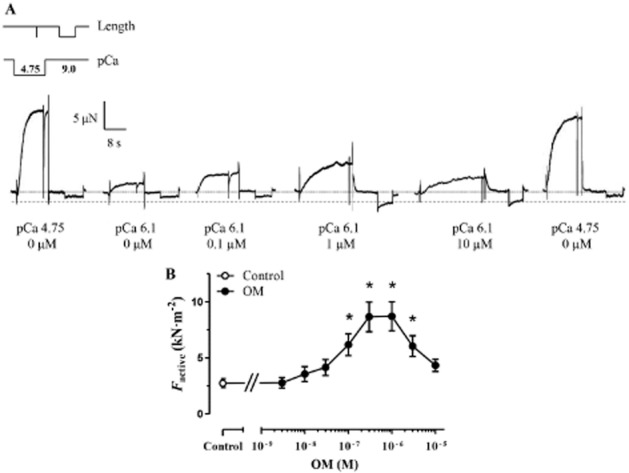
The Ca2+-sensitizing effect of OM in permeabilized cardiomyocytes of the rat. (A) Force recordings illustrate Ca2+-induced contractions with increasing amplitudes at a submaximal [Ca2+] (pCa 6.1), due to the Ca2+-sensitizing effect of increasing OM concentrations in a permeabilized cardiomyocyte under isometric conditions. During repeated activation–relaxation cycles, myocyte-sized preparations were first equilibrated in relaxing solution with an OM concentration between 3 nM and 10 μM for 5 min and subsequently activated in the presence of the same OM concentration at submaximal [Ca2+]. Control maximal activations (pCa 4.75) in the absence of OM were performed to determine Fmax and preparation run-down. Length and [Ca2+] changes are schematically given above the first activation–relaxation cycle. Cardiomyocyte transfers between relaxing (pCa 9.0) and activating solutions (pCa 4.75 or pCa 6.1) were accompanied by brief vertical force artefacts. pCa values and OM concentrations are indicated below the individual force records. The horizontal dotted line depicts the force baseline in relaxing solution and the dashed line the increased Fpassive level seen in the presence of 1 μM or 10 μM OM. (B) OM exhibited a bell-shaped concentration–response relationship on the isometric force production (n = 8 cardiomyocytes from four different animals). Increasing concentrations of OM was applied in the same myocyte-sized preparations. *P < 0.05, significantly different from control; repeated measures ANOVA with Dunnett’s two-tailed test.
Thereafter, the effects of OM on the pCa–force relationship were tested in detail by exposing myocyte-sized preparations to activating solutions with maximal (pCa 4.75) or submaximal [Ca2+] (i.e. between pCa 9.0 and pCa 5.4) in the presence of a low (0.1 μM) or an intermediate (1 μM) concentration of OM. 0.1 μM OM did not affect the force production relative to the OM-free conditions either at the maximal (i.e. at pCa 4.75) or at the lowest applied [Ca2+] (pCa 9), whereas it promoted force production at intermediate levels of activation (between pCa 6.6 and pCa 5.6) and hence shifted the pCa–force relationship to the left. In the presence of 1 μM OM, the force augmentation was pronounced at low–intermediate [Ca2+] levels (i.e. between pCa 9.0 and pCa 6.0), albeit decreases in Fmax together with force reductions at high–intermediate Ca2+ concentrations were also observed (i.e. between pCa 5.6 and pCa 5.4). Means of force amplitudes are expressed in absolute terms (kN·m−2; Figure 2A) and normalized terms (Figure 2B). The leftward shift on the normalized pCa–force relationship indicated increased Ca2+ sensitivity of force production (pCa50 in the OM-free medium: 5.86 ± 0.02 vs. 5.97 ± 0.02 and 6.20 ± 0.03 at 0.1 and 1 μM OM, respectively; P < 0.001 in both groups vs. the control; Figure 2C). 0.1 μM and 1 μM OM decreased nHill of the normalized pCa–force relationships (nHill in OM-free medium: 2.64 ± 0.09 vs. 1.66 ± 0.06 and 0.97 ± 0.07 at 0.1 and 1 μM OM, respectively; P < 0.001 in both groups vs. the control; Figure 2D).
Figure 2.
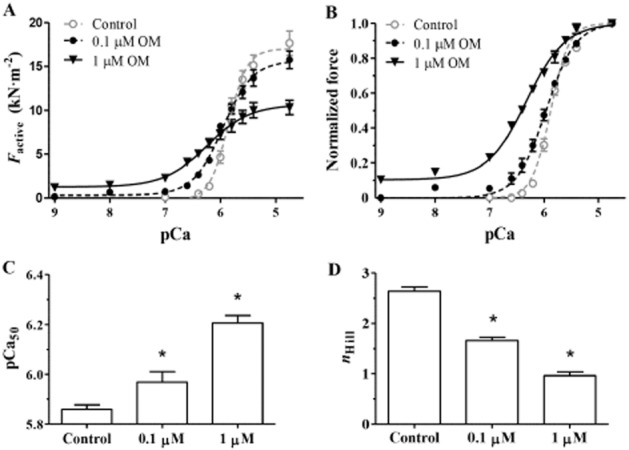
The Ca2+-sensitizing effect of OM in permeabilized cardiomyocytes was quantified by pCa–force relationships ([Ca2+] between pCa 9 and pCa 4.75) in the absence and the presence of 0.1 μM or 1 μM OM. (A, B) Means of force amplitudes are expressed in absolute (kN·m−2) and normalized terms. (C) OM-evoked Ca2+ sensitization and the resultant leftward shifts in the pCa–force relations are illustrated by the means of their midpoints (pCa50) in the absence and presence of 0.1 or 1 μM OM. (D) 0.1 or 1 μM OM decreased the co-operativity of Ca2+-regulated force production (nHill) in permeabilized cardiomyocytes (n = 8 cardiomyocytes from three different animals for each group). Each curve was generated with increasing concentrations of OM in the same cells. *P < 0.05, significantly different from control; repeated measures ANOVA with Dunnett’s two-tailed test.
Effects of OM on the mechanics and kinetics of activation–relaxation cycles in isolated cardiomyocytes
To obtain a more detailed picture of how OM affected the kinetics of Ca2+-dependent activations and relaxations, isometric force measurements were carried out at saturating (pCa 4.75) and submaximal (pCa 6.0) [Ca2+] using a wide range of OM concentrations (between 3 nM and 10 μM) Factive, Fpassive, ktr, t1/2 of activation and trelax were determined (Figure 3A). OM decreased Factive in a monotonous fashion at pCa 4.75, significantly at 1 μM OM concentration or higher (Fmax in OM-free medium: 14.75 ± 0.70 kN·m−2 and at 1 μM OM concentration: 9.42 ± 0.97 kN·m−2, P < 0.001 vs. the control); however, the concentration–response relationship of OM was bell-shaped at pCa 6.0 due to Ca2+ sensitization (Supporting Information Fig. S1a) similarly to that shown in Figure 1B. OM increased Fpassive at 0.3 μM and higher concentration at pCa 4.75 and pCa 6.0 (EC50: 0.30 ± 0.10 μM and 0.26 ± 0.11 μM at pCa 4.75 and pCa 6.0, respectively; Figure 3B). At pCa 4.75, OM decreased ktr at 10 nM and higher concentration (ktr in OM-free medium: 5.70 ± 0.33 s−1 and 4.44 ± 0.37 s−1 at 10 nM OM; P < 0.01 vs. the control; EC50: 0.05 ± 0.01 μM). Interestingly, OM-dependent changes of ktr were biphasic at pCa 6.0: ktr increased in the presence of 0.1 μM OM from the drug-free control of 1.31 ± 0.08 s−1 to 1.57 ± 0.08 s−1 (P < 0.05 vs. the control), albeit ktr decreased in the presence of 0.3 μM and higher OM concentrations (to 1.00 ± 0.11 s−1 at 0.3 μM OM; P < 0.01 vs. the control; Figure 3C). Similar kinetic features were observed on the prolongation of t1/2 of activation. 1 μM and higher concentrations of OM increased significantly the t1/2 of activation at pCa 4.75 (t1/2 in OM-free conditions: 0.77 ± 0.12 s and 4.02 ± 0.56 s at 1 μM OM, P < 0.001 vs. the control) and at pCa 6.0 (t1/2 in OM-free conditions: 2.55 ± 0.19 s and 4.23 ± 0.53 s at 1 μM OM, P < 0.05 vs. the control). Nevertheless, t1/2 of activation tended to decrease at pCa 6.0 in response to intermediate concentrations of OM (30 nM–0.3 μM), without reaching statistical significance (Figure 3D). The relaxation was slowed following Ca2+-removal from the preparations at 1 μM and higher OM concentrations at pCa 4.75 (trelax in the OM-free medium: 1.19 ± 0.10 s and 3.75 ± 0.31 s at 1 μM OM; P < 0.001 vs. control) and at pCa 6.0 (trelax in the OM-free medium: 0.61 ± 0.06 s and 4.36 ± 0.77 s at 1 μM OM; P < 0.001 vs. control; Figure 3E). The t1/2 of activation and trelax could not be determined at higher than 3 μM OM concentrations because of low isometric force production.
Figure 3.
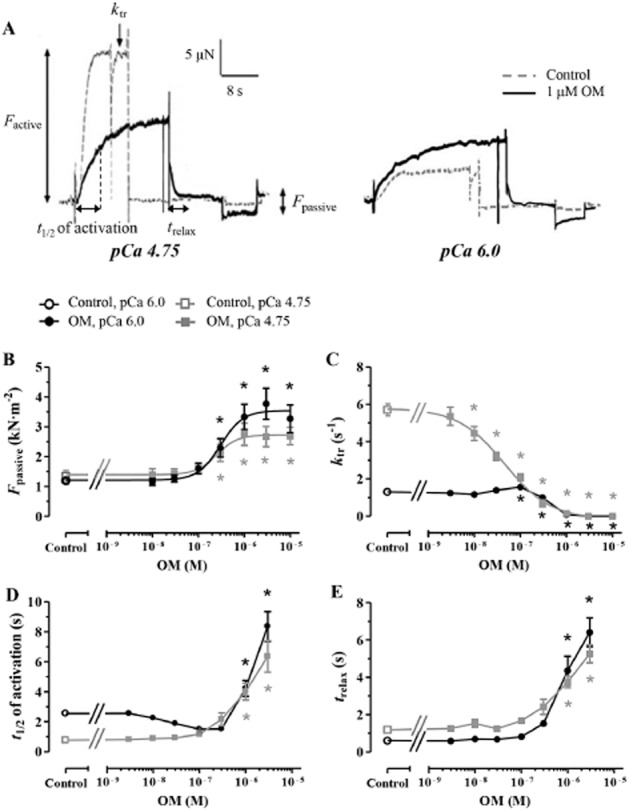
The concentration dependence of OM-induced mechanical and kinetic changes in permeabilized cardiomyocytes at saturating (pCa 4.75) and submaximal (pCa 6.0) [Ca2+]. (A) Representative force records at pCa 4.75 (left panel) or pCa 6.0 (right panel) illustrated the effects of 1 μM OM on Factive, Fpassive, ktr, t1/2 of activation and trelax, together with controls in the absence of OM. (B) OM increased Fpassive similarly at pCa 4.75 and 6.0. (C) The ktr decreased at pCa 4.75 with OM concentration; however, it showed a biphasic OM concentration dependence at pCa 6.0. (D, E) t1/2 of activation and trelax were prolonged after OM treatment at saturating and submaximal [Ca2+]. n = 8 muscle fibres at pCa 4.75 and 7–11 muscle fibres at pCa 6.0, from 3–4 different animals respectively. Increasing concentrations of OM was applied in the same myocyte-sized preparations. *P < 0.05, significantly different from control; repeated measures ANOVA with Dunnett’s two-tailed test.
The Ca2+-sensitizing effects of OM in isolated skeletal muscle fibres with slow intrinsic kinetics
To assess any Ca2+-sensitizing effects of OM in skeletal muscles, measurements similar to those carried out in cardiomyocytes were repeated on isolated muscle fibres derived from the rat diaphragm. The force records depicted in Figure 4A demonstrated that the contractile force of the rat diaphragm was enhanced in response to increasing OM concentrations at a submaximal level of activation (pCa 6.0), as observed in cardiomyocytes. A complicating factor in diaphragm muscle preparations is the composition of muscle fibres with different MHC isoforms. A mixture of abundant proteins with distinct molecular weights close to those of MHC was detected during the SDS-PAGE of the rat diaphragm, reflecting skeletal muscle fibres of types I(β), IIx, IIb and IIa (Talmadge and Roy, 1993). MHC α + β isoforms of the LV samples co-migrated with MHC β isoforms in skeletal muscle fibres (van der Velden et al., 1999) (Figure 4B). Although we could not extend the mechanical measurements on isolated muscle fibres to biochemical MHC isoform subtyping, the rate constant of force redevelopment at pCa 4.75 (ktr,max) parameters allowed the differentiation between muscle fibres with slow (ktr,max < 2 s−1) and fast intrinsic kinetics (ktr,max > 2 s−1), most probably reflecting the isoform compositions of type I vs. type II fibres (Figure 4C). When diaphragm myofibres with distinct kinetic characteristics were analysed separately (on the basis of their ktr,max parameters as indicated above), OM-dependent Ca2+ sensitization was revealed with an apparent EC50 of 0.16 ± 0.03 μM in slow fibres (ktr,max: 1.61 ± 0.22 s−1; Figure 4D).
Figure 4.
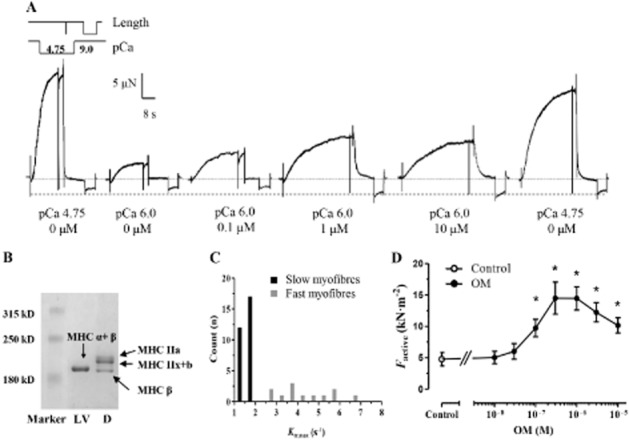
The Ca2+-sensitizing effect of OM in permeabilized rat diaphragm skeletal muscle fibres. (A) Force recordings illustrated Ca2+-induced contractions with increasing amplitudes at a submaximal [Ca2+] (pCa 6.0), due to the Ca2+-sensitizing effect of OM in an individual permeabilized skeletal muscle fibre. The experimental arrangement is similar to that of carried out in isolated cardiomyocytes. (B) MHC isoform compositions of the muscle fibres in the rat diaphragm (D) and the rat cardiomyocytes (LV). (C) Actin–myosin cross-bridge cycle kinetics were assessed through their ktr,max parameters in diaphragm muscle fibres under OM-free conditions; the values obtained were scattered within a range between slow (ktr,max < 2 s−1) and relatively fast kinetics (ktr,max > 2 s−1). (D) OM exhibited a bell-shaped concentration–isometric force relationship in muscle fibres with slow intrinsic kinetics (n = 14 muscle fibres from six different animals). Increasing concentrations of OM was applied in the same muscle fibres. *P < 0.05, significantly different from control; repeated measures ANOVA with Dunnett’s two-tailed test.
The Ca2+-sensitizing effect of OM was demonstrated by comparing pCa–force relationships in diaphragm muscle preparations with low ktr,max (1.50 ± 0.10 s−1) in the absence and presence of OM. Isometric force values were expressed in absolute (Figure 5A) and normalized terms (Figure 5B). 1 μM OM treatment resulted in a pronounced leftward shift of the pCa–normalized force relationship as OM increased the Ca2+ sensitivity of force production in the isolated rat diaphragm muscle fibres (pCa50 in OM-free controls: 5.61 ± 0.02 and at 1 μM OM: 5.85 ± 0.03; P < 0.001; Figure 5C). This OM concentration exerted less effect on the force production at either the highest or the lowest [Ca2+] than in isolated cardiomyocytes. Furthermore, 1 μM OM decreased nHill of the normalized pCa–force relationships (nHill in OM-free medium: 2.68 ± 0.18 and 1.58 ± 0.09 at 1 μM OM; P < 0.01; Figure 5D).
Figure 5.
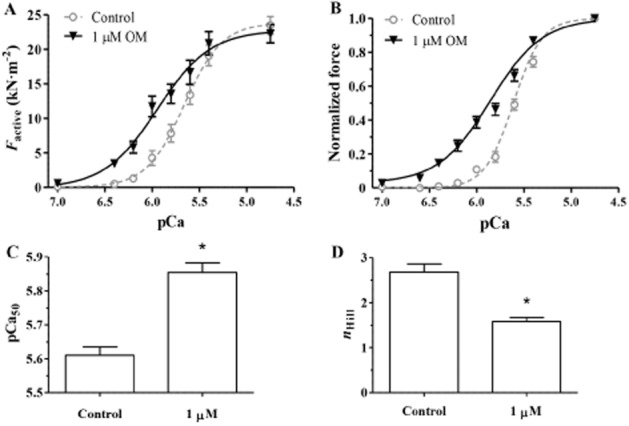
The Ca2+-sensitizing effect of OM in permeabilized skeletal rat diaphragm muscle fibres with slow kinetics was quantified through pCa–force relationships ([Ca2+] between pCa 7.0 and pCa 4.75) in the absence and presence of 1 μM OM. (A, B) Means of force amplitudes are expressed in absolute terms (kN·m−2) and normalized terms, fitted to a modified Hill equation. (C) OM-evoked Ca2+ sensitization and the resultant leftward shift in the isometric pCa–force relations are illustrated by the means of their midpoints (pCa50) in the absence and presence of 1 μM OM. (D) OM decreased the co-operativity of Ca2+-regulated force production (nHill) in the permeabilized skeletal muscle fibres (n = 9 muscle fibres from four different animals). Test activations in the absence and in the presence of OM were carried out in the same muscle fibres. *P < 0.05, significantly different from control; paired Student’s t-test.
Mechanical and kinetic effects of OM in isolated muscle fibres of rat diaphragm with slow intrinsic kinetics
The effects of OM on the kinetics of Ca2+-dependent activations and relaxations were also investigated in isolated diaphragm muscle fibres with slow intrinsic kinetics (ktr,max: 1.57 ± 0.11 s−1) at saturating (pCa 4.75) and submaximal (pCa 6.0) [Ca2+] similarly to that in cardiomyocytes. Although Fmax of the preparations decreased at 3 μM OM and higher at pCa 4.75 (Factive in OM-free medium: 21.37 ± 3.16 kN·m−2 and 13.25 ± 1.78 kN·m−2 at 3 μM OM; P < 0.001 vs. the control; Supporting Information Fig. S1b), OM exhibited a bell-shaped concentration–response relationship due to Ca2+ sensitization at pCa 6.0, similar to that observed in cardiomyocytes. OM increased Fpassive at pCa 4.75 and 6.0 (EC50: 0.79 ± 0.16 μM and 0.69 ± 0.11 μM at pCa 4.75 and pCa 6.0, respectively; Figure 6A). OM decreased ktr both at pCa 4.75 and 6.0, although with a higher potency at pCa 4.75 (EC50: 0.13 ± 0.09 μM) than at pCa 6.0 (EC50: 0.29 ± 0.04 μM; Figure 6B). The kinetic features of the OM effects were also reflected by increases in t1/2 of activation and trelax parameters in diaphragm myofibres with low ktr,max. OM significantly increased the t1/2 of activation at 1 μM and higher concentration both at pCa 4.75 (t1/2 of activation in OM-free medium: 5.99 ± 0.98 s and 13.98 ± 1.46 s at 1 μM OM, P < 0.001 vs. the control) and at pCa 6.0 (t1/2 of activation in OM-free medium: 6.96 ± 0.86 s and 10.50 ± 0.93 s at 1 μM OM, P < 0.001 vs. the control; Figure 6C). 0.3 μM and higher OM slowed the relaxation at pCa 4.75 (trelax in OM-free medium: 3.15 ± 0.68 s and 5.49 ± 0.81 at 0.3 μM OM s; P < 0.05 vs. the control), whereas at pCa 6.0, similar effects were observed at 1 μM OM and higher concentration (trelax in OM-free medium: 0.86 ± 0.09 s and 7.26 ± 0.83 s at 1 μM OM; P < 0.01 vs. the control; Figure 6D).
Figure 6.
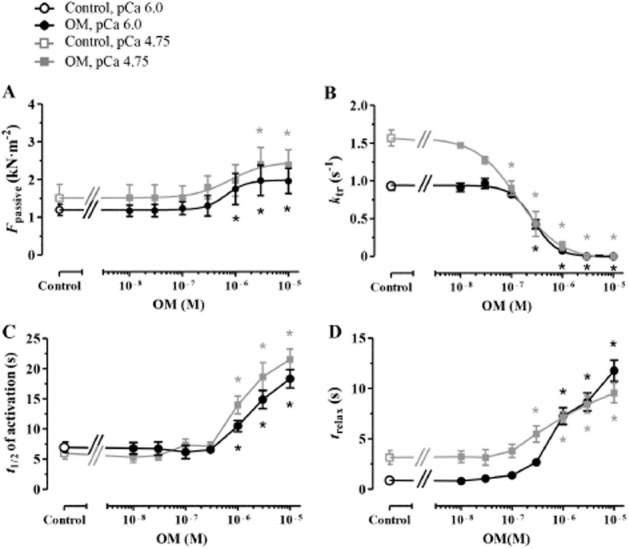
The concentration dependency of OM-induced mechanical and kinetic changes on slow skeletal muscle fibres of the rat diaphragm were illustrated in the function of the applied [Ca2+] (pCa 4.75 and pCa 6.0). (A) OM increased Fpassive of the preparations. (B) The OM-evoked decrease of ktr was more pronounced at pCa 4.75 than at pCa 6.0. (C, D) t1/2 of activation and trelax were prolonged in response to increasing OM concentration in slow fibres, n = 6 muscle fibres at pCa 4.75 and 11–14 muscle fibres at pCa 6.0, from 3–6 different animals respectively). Increasing concentrations of OM was applied in the same muscle fibre preparations. *P < 0.05, significantly different from control; repeated measures ANOVA with Dunnett’s two-tailed test.
Mechanical and kinetic effect of OM in isolated muscle fibres of rat diaphragm with fast intrinsic kinetics
OM-dependent mechanical and kinetic effects were also assessed in isolated skeletal muscle fibres with fast intrinsic kinetics (ktr,max: 4.60 ± 0.31 s−1). Measurements were carried out at saturating (pCa 4.75) and submaximal (pCa 5.8 at this case) [Ca2+]. Fmax was not affected; however, OM exhibited a significant Ca2+-sensitizing effect at 1 μM concentration and higher at pCa 5.8 (Factive in OM-free medium: 4.59 ± 0.99 kN·m−2 and 6.97 ± 1.30 kN·m−2 at 1 μM OM; P < 0.05 vs. the control; Supporting Information Fig. S1c). OM treatment did not increase Fpassive of fast myofibres either at pCa 4.75 or at pCa 5.8 (Figure 7A). OM decreased the ktr at 1 μM concentration and higher at pCa 4.75 (EC50: 1.23 ± 0.28 μM), but had no effect on ktr at pCa 5.8 (Figure 7B). The highest OM concentration was associated with faster Ca2+ contractions at pCa 5.8 (t1/2 of activation in OM-free medium: 8.33 ± 0.70 s and 4.92 ± 0.96 s at 1 μM OM, P < 0.01 vs. the control; Figure 7C), whereas the relaxation of the preparations was not affected following Ca2+ removal either at pCa 4.75 or at pCa 5.8 (Figure 7D).
Figure 7.
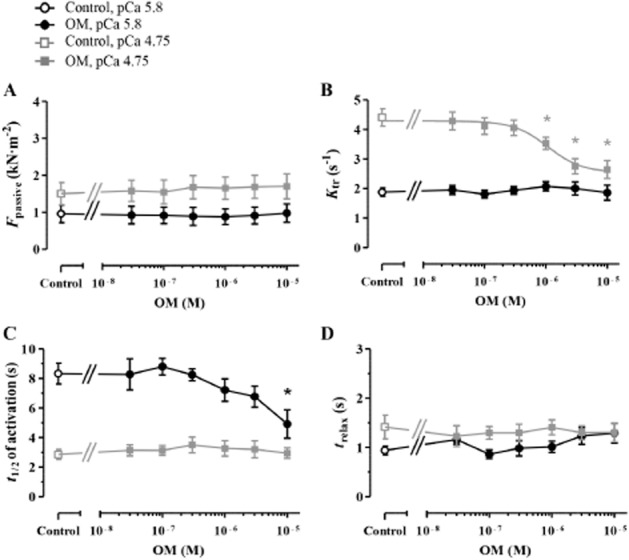
The mechanical and kinetic effects of OM were also tested in permeabilized skeletal muscle fibres of the rat diaphragm with fast intrinsic kinetics at saturating (pCa 4.75) and submaximal (pCa 5.8) [Ca2+]. (A) Fpassive of the preparations was not affected by OM treatment. (B) OM decreased ktr at pCa 4.75; however, this effect was missing at pCa 5.8. (C) t1/2 of activation was unaltered at pCa 4.75 and decreased at pCa 5.8 in response to the highest applied OM concentration. (D) OM did not influence the relaxation of fast skeletal muscle fibres. n = 6 − 6 isolated myofibres for both [Ca2+] from three different animals. Increasing concentrations of OM was applied in the same muscle fibres. *P < 0.05, significantly different from control; repeated measures ANOVA with Dunnett’s two-tailed test.
Discussion
As far as we are aware, this is the first quantitation of the Ca2+-sensitizing effect of the novel myosin activator OM in isolated and skinned cardiomyocytes. OM altered the kinetics of both activations and relaxations in cardiomyocytes during Ca2+-induced contractions. The experimental data additionally revealed that OM could also improve the contractile function of the diaphragm by exerting a Ca2+-sensitizing effect on force production in skeletal muscle fibres.
Myosin activators are novel sarcomere-targeted agents for the treatment of HF without the deleterious side effects of the traditional inotropes (Teerlink, 2009). OM activates the S1 domain of the cardiac myosin heads by binding to the base of the lever arm where the relay helix and the converter domain converge. This pharmacological intervention increases the ATPase activity of the myosin heads (Malik et al., 2011; Wang et al., 2014). Our present data indicate a similar relationship for the OM-evoked changes in the Ca2+-dependence of the contractile force production to that already described for the OM-evoked changes in the Ca2+ sensitivity of myosin ATPase activity (Malik et al., 2011). It is now increasingly accepted, that OM facilitates Pi release while leaving ADP dissociation unchanged thereby increasing the available number of cross-bridges during cardiac contractions without affecting the intracellular Ca2+ homeostasis (Liu et al., 2015). This suggests that myosin activation can be regarded as a downstream Ca2+-sensitizing mechanism. In our present study, OM increased the Ca2+ sensitivity of force production at OM concentrations of 0.1 μM and higher, while OM-dependent changes in ATPase rate and myocardial contractility were also reported at overlapping OM concentrations in preclinical model experiments and clinical trials (Cleland et al., 2011; Malik et al., 2011; Teerlink et al., 2011; Wang et al., 2014). These results imply that OM-dependent Ca2+ sensitization and myosin ATPase activation illustrate different, although closely related facets of the OM-induced sarcomeric changes.
Recruitment of cross-bridges by OM may delay the inactivation of the thin filaments leading to increased number of strongly-attached cross-bridges and prolonged activation of the contractile machinery (Gordon et al., 2000; Poggesi et al., 2005). Our kinetic data on increased t1/2 of activation and decreased ktr corroborate the decreased in vitro motilities of the myosin filaments (Wang et al., 2014; Liu et al., 2015) and decreased contraction and relaxation velocity of the cardiomyocytes (Malik et al., 2011; Shintani et al., 2014), potentially manifesting in increased systolic ejection time. Additionally, sustained thin filament activation and a reduction in the ATP-initiated dissociation rate of acto-myosin can delay the kinetics of the force decay during diastole as indicated by the changes of trelax in this study (Poggesi et al., 2005; Liu et al., 2015). Although the kinetics of force generation in vitro were slower, the maximal rate of LV pressure development (i.e. dP/dtmax) was not affected by OM (Shen et al., 2010). Perhaps the Ca2+-sensitizing effect of OM may partially counterbalance the slowed kinetics of contraction as suggested by the differences in OM concentration dependencies between maximal and submaximal activations (Figure 3C and 3D). Taken together, OM-evoked Ca2+ sensitization may result in stronger, slower and prolonged cardiac contractions consistent with the echocardiographic findings (Cleland et al., 2011; Teerlink et al., 2011).
Ca2+ sensitizers affecting the cross-bridge cycling of the myofilaments may alter force production at both high and low [Ca2+] (Kass and Solaro, 2006). Accordingly, OM exerted robust Ca2+-sensitizing effects over a wide range of [Ca2+], but both the extent and the direction of the evoked changes (force enhancement vs. force mitigation) depended on the applied OM concentration. At 1 μM OM, contractile force development was observed at very low [Ca2+] (pCa 7.0–9.0), as suggested by the upward shift in the pCa–force relationships. Similar findings have been reported after in vitro administration of EMD-57033 or NEM-S1 (Palmer et al., 1995; Fitzsimons et al., 2001; Papp et al., 2004). EMD-57033, the (+)-enantiomer of EMD-53998 and a known downstream Ca2+ sensitizer, also acts as an allosteric activator of the myosin motor by binding at the base of the lever arm located close to the proposed binding site of OM (Radke et al., 2014). NEM-S1, the N-ethylmaleimide derivative of myosin S-1 facilitates cross-bridge binding to the thin filaments, potentiating thereby the acto-myosin ATPase activity (Swartz and Moss, 2001). Isometric force production by downstream Ca2+-sensitizers in the absence of Ca2+ implies that the thin filaments are not completely switched off without Ca2+ (Moss et al., 2004). Furthermore, the Ca2+-sensitizing effect of OM was absent at pCa 4.75, suggesting that the available number of strongly bound cross-bridges are maximal under this condition (Moss et al., 2004).
An increased Ca2+ sensitivity of force production has been observed in advanced stages of HF (van der Velden et al., 2003). Moreover, Ca2+ sensitization at diastolic [Ca2+] may be complicated by an already elevated cytosolic [Ca2+] during diastole in HF (Webster et al., 1993). We have now demonstrated that OM can exert a robust Ca2+-sensitizing effect at diastolic [Ca2+] and can additionally increase the Fpassive of the cardiomyocytes. Additionally, OM substantially prolonged the relaxation of the myocyte-sized preparations following the dissociation of Ca2+ from the contractile apparatus. All of these components may complicate ventricular relaxation during OM treatment especially at high doses. Accordingly, the worsened time constant of isovolumic relaxation (τ) and rate of the LV pressure decrease (dP/dtmin) reflected impaired diastolic performance after i.v. OM administration in rats with volume overload HF (Wilson et al., 2014).
Despite the in vitro mechanical and kinetic effects, OM treatment did not impair the diastolic functions in healthy volunteers or in patients with systolic HF at plasma concentrations similar to those applied in this study (approximately 0.25–2.5 μM; Cleland et al., 2011; Teerlink et al., 2011). It must therefore be acknowledged that extrapolation from in vitro data to in vivo conditions can be complicated by the distinct interactions of OM with the actin–myosin system in permeabilized preparations and in the structurally intact myocardium (Solaro et al., 1993; Palmer et al., 1995). Through the use of an isolated single-cell experimental arrangement, the contractile machinery was directly exposed to OM, in contrast with the structurally intact fibres, and hence resulting in a potentially greater extent of myosin activation at the applied concentration range than that achieved in vivo.
HF is also associated with the decreased contractility of the diaphragm attributable to its decreased Ca2+ sensitivity (Empinado et al., 2014). Further, mechanical ventilation is accompanied by the impaired contractile performance of the respiratory muscles (Jaber et al., 2011). Previous studies have revealed that levosimendan and EMD-57033 improved the contractility of the failing diaphragm through their Ca2+-sensitizing mechanisms (Ochala et al., 2010; Doorduin et al., 2012). In the present study, OM exerted its Ca2+-sensitizing effect in the skeletal muscle fibres of the rat diaphragm because the slow-skeletal muscle fibres co-express the same MHC isoform (MHC-β) as found in the heart (Bers and Harris, 2011; Schiaffino and Reggiani, 2011). Our present data additionally demonstrated that OM exerted its effect on the Ca2+ sensitivity of force production and on the kinetic characteristics of the slow diaphragm preparations at a higher concentration range than in isolated cardiomyocytes. Moreover, our data probably suggest that OM may influence the contractility of the muscle fibres with fast skeletal MHCs, although its Ca2+-sensitizing potency was even lower in isolated muscle fibres with high ktr,max than in those developing low ktr,max, with less effects on Fpassive production and the kinetics of activation–relaxation cycles (Table 2011). As reported on the ATOMIC-HF trial, the treatment of HF patients with OM may decrease the incidence of dyspnoea (Meijs et al., 2012; Valentova and Von Haehling, 2014). Our present study suggests that this might be due in part to the enhanced contractility of the diaphragm resulting particularly from Ca2+ sensitization.
Table 1.
OM concentration-dependent mechanical and kinetic effects in permeabilized cardiomyocytes and diaphragm muscle fibres
| Cardiomyocytes | Diaphragm muscle fibres with low ktr,max | Diaphragm muscle fibres with high ktr,max | |
|---|---|---|---|
| EC50 for Ca2+ sensitization of force production | 0.08 ± 0.01 | 0.16 ± 0.03 | # |
| EC50 for Fpassive increase | 0.26 ± 0.11 | 0.69 ± 0.11 | No effect |
| EC50 for ktr decrease | # | 0.29 ± 0.04 | No effect |
EC50 values (in μM), determined at submaximal [Ca2+] (pCa 6.0 or pCa 5.8) for each muscle preparations are shown as means ± SEM.
EC50 could not be determined.
One of the limitations of our study relates to the choice of isolated single-cell preparations, in consequence of which the myosin isoform composition of the investigated skeletal muscle preparations had to be addressed through their respective OM-free ktr,max parameters and not through protein biochemistry. Nevertheless, it has previously been shown that ktr,max in single-fibre preparations may be appropriate for the differentiation of slow and fast twitching myofibres (Bodnar et al., 2014).
Overall, the findings of the present study have revealed that the mechanism of action of OM-evoked myosin activation is similar to that of Ca2+-sensitizing positive inotropes with targets downstream from cTnC in the heart. Moreover, myosin activation may improve the contractility of the diaphragm via a Ca2+-sensitizing mechanism, most effectively in muscle fibres with slow intrinsic kinetics.
Acknowledgments
This work was supported by the Social Renewal Operational Programme (TÁMOP-4.2.2.A-11/1/KONV-2012-0045), by a grant from the Hungarian Scientific Research Fund (OTKA: K 109083), and by the European Union Project FP7-HEALTH-2010: ‘MEDIA-Metabolic Road to Diastolic Heart Failure’ MEDIA-261409. We wish to thank Duncan van Groen (VU University Medical Centre Amsterdam, the Netherlands) for his kind help in transforming data extracted from LabVIEW recording and analysing software.
Glossary
- cTnC
cardiac troponin C
- Factive
active force
- Fmax
maximal force level
- Fpassive
passive force
- Ftotal
total force level
- HF
heart failure
- ktr
rate constant of force redevelopment at submaximal [Ca2+]
- ktr,max
rate constant of force redevelopment at pCa 4.75
- LV
left ventricle
- MHC
myosin heavy chain
- nHill
Hill coefficient
- OM
omecamtiv mecarbil
- pCa
−log of calcium ion concentration
- pCa50
−log of calcium ion concentration at half-maximal isometric force production
- Pi
inorganic phosphate
- trelax
relaxation time
Author contributions
L. N., Á. K., B. B., E.T. P. and G.Á. F. performed the research, A. T., I. É. and Z. P. designed the research study, L. N., Á. K., B. B., G.Á. F., E.T. P. and Z. P. carried out the data and statistical analysis, L. N., A. T., I. É. and Z. P. wrote the paper.
Conflict of interest
The authors have nothing to disclose.
Supporting Information
Figure S1 OM-evoked changes in Factive at saturating [Ca2+] and at a submaximal level of activation in cardiomyocytes, in skeletal myofibres with slow intrinsic kinetics and in skeletal myofibres with high intrinsic kinetics. (A,B) In cardiomyocytes and slow skeletal muscle fibres, Factive decreased linearly at pCa 4.75 with OM concentration, whereas at pCa, 6.0 the OM concentration–Factive relationships were bell-shaped. (C) OM did not affect Factive at pCa 4.75 while exhibiting a weak Ca2+-sensitizing effect at the submaximal [Ca2+] in fast skeletal muscle preparations. n = 6 − 6 isolated myofibres from three different animals respectively. Increasing concentrations of OM were applied in the same preparations. *P < 0.05, significantly different from control; repeated measures ANOVA with Dunnett’s two-tailed test.
References
- Bers DM, Harris SP. Translational medicine: to the rescue of the failing heart. Nature. 2011;473:36–39. doi: 10.1038/473036a. [DOI] [PubMed] [Google Scholar]
- Bodnar D, Geyer N, Ruzsnavszky O, Olah T, Hegyi B, Sztretye M, et al. Hypermuscular mice with mutation in the myostatin gene display altered calcium signalling. J Physiol. 2014;592:1353–1365. doi: 10.1113/jphysiol.2013.261958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B, Hahn N, Hanke E, Matinmehr F, Scholz T, Steffen W, et al. Mechanical and kinetic properties of beta-cardiac/slow skeletal muscle myosin. J Muscle Res Cell Motil. 2012;33:403–417. doi: 10.1007/s10974-012-9315-8. [DOI] [PubMed] [Google Scholar]
- Brixius K, Mehlhorn U, Bloch W, Schwinger RH. Different effect of the Ca(2+) sensitizers EMD 57033 and CGP 48506 on cross-bridge cycling in human myocardium. J Pharmacol Exp Ther. 2000;295:1284–1290. [PubMed] [Google Scholar]
- Choi YH, Cowan DB, Wahlers TC, Hetzer R, Del Nido PJ, Stamm C. Calcium sensitisation impairs diastolic relaxation in post-ischaemic myocardium: implications for the use of Ca(2+) sensitising inotropes after cardiac surgery. Eur J Cardiothorac Surg. 2010;37:376–383. doi: 10.1016/j.ejcts.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland JG, Teerlink JR, Senior R, Nifontov EM, McMurray JJ, Lang CC, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–683. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- Doorduin J, Sinderby CA, Beck J, Stegeman DF, Van Hees HW, Van Der Hoeven JG, et al. The calcium sensitizer levosimendan improves human diaphragm function. Am J Respir Crit Care Med. 2012;185:90–95. doi: 10.1164/rccm.201107-1268OC. [DOI] [PubMed] [Google Scholar]
- Empinado HM, Deevska GM, Nikolova-Karakashian M, Yoo JK, Christou DD, Ferreira LF. Diaphragm dysfunction in heart failure is accompanied by increases in neutral sphingomyelinase activity and ceramide content. Eur J Heart Fail. 2014;16:519–525. doi: 10.1002/ejhf.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M. Mechanism of action of Ca2+ sensitizers – update 2001. Cardiovasc Drugs Ther. 2001;15:397–403. doi: 10.1023/a:1013385305567. [DOI] [PubMed] [Google Scholar]
- Endoh M. Cardiac Ca2+ signaling and Ca2+ sensitizers. Circ J. 2008;72:1915–1925. doi: 10.1253/circj.cj-08-0838. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- Fitzsimons DP, Patel JR, Moss RL. Cross-bridge interaction kinetics in rat myocardium are accelerated by strong binding of myosin to the thin filament. J Physiol. 2001;530:263–272. doi: 10.1111/j.1469-7793.2001.0263l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–371. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- Kass DA, Solaro RJ. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation. 2006;113:305–315. doi: 10.1161/CIRCULATIONAHA.105.542407. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, White HD, Belknap B, Winkelmann DA, Forgacs E. Omecamtiv mecarbil modulates the kinetic and motile properties of porcine beta-cardiac myosin. Biochemistry. 2015;54:1963–1975. doi: 10.1021/bi5015166. [DOI] [PubMed] [Google Scholar]
- Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- Meijs MF, Asselbergs FW, Doevendans PA. Omecamtiv mecarbil: a promising new drug in systolic heart failure. Eur J Heart Fail. 2012;14:232–233. doi: 10.1093/eurjhf/hfr178. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Steele DS. The ‘calcium sensitising’ effects of ORG30029 in saponin- or Triton-skinned rat cardiac muscle. Br J Pharmacol. 1990;100:843–849. doi: 10.1111/j.1476-5381.1990.tb14102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RL, Razumova M, Fitzsimons DP. Myosin crossbridge activation of cardiac thin filaments: implications for myocardial function in health and disease. Circ Res. 2004;94:1290–1300. doi: 10.1161/01.RES.0000127125.61647.4F. [DOI] [PubMed] [Google Scholar]
- Nagy L, Pollesello P, Papp Z. Inotropes and inodilators for acute heart failure: sarcomere active drugs in focus. J Cardiovasc Pharmacol. 2014;64:199–208. doi: 10.1097/FJC.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochala J, Radell PJ, Eriksson LI, Larsson L. EMD 57033 partially reverses ventilator-induced diaphragm muscle fibre calcium desensitisation. Pflugers Arch. 2010;459:475–483. doi: 10.1007/s00424-009-0744-1. [DOI] [PubMed] [Google Scholar]
- Palmer S, Di Bello S, Herzig JW. The effects of EMD 57033 on rigor tension in porcine skinned cardiac trabecula. Eur J Pharmacol. 1995;294:83–90. doi: 10.1016/0014-2999(95)00509-9. [DOI] [PubMed] [Google Scholar]
- Papp Z, Van Der Velden J, Borbely A, Edes I, Stienen GJ. Effects of Ca2+-sensitizers in permeabilized cardiac myocytes from donor and end-stage failing human hearts. J Muscle Res Cell Motil. 2004;25:219–224. doi: 10.1023/b:jure.0000038365.74532.75. [DOI] [PubMed] [Google Scholar]
- Poggesi C, Tesi C, Stehle R. Sarcomeric determinants of striated muscle relaxation kinetics. Pflugers Arch. 2005;449:505–517. doi: 10.1007/s00424-004-1363-5. [DOI] [PubMed] [Google Scholar]
- Radke MB, Taft MH, Stapel B, Hilfiker-Kleiner D, Preller M, Manstein DJ. Small molecule-mediated refolding and activation of myosin motor function. Elife. 2014;3:e01603. doi: 10.7554/eLife.01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Shen YT, Malik FI, Zhao X, Depre C, Dhar SK, Abarzua P, et al. Improvement of cardiac function by a cardiac Myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail. 2010;3:522–527. doi: 10.1161/CIRCHEARTFAILURE.109.930321. [DOI] [PubMed] [Google Scholar]
- Shintani SA, Oyama K, Kobirumaki-Shimozawa F, Ohki T, Ishiwata S, Fukuda N. Sarcomere length nanometry in rat neonatal cardiomyocytes expressed with alpha-actinin-AcGFP in Z discs. J Gen Physiol. 2014;143:513–524. doi: 10.1085/jgp.201311118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro RJ, Gambassi G, Warshaw DM, Keller MR, Spurgeon HA, Beier N, et al. Stereoselective actions of thiadiazinones on canine cardiac myocytes and myofilaments. Circ Res. 1993;73:981–990. doi: 10.1161/01.res.73.6.981. [DOI] [PubMed] [Google Scholar]
- Swartz DR, Moss RL. Strong binding of myosin increases shortening velocity of rabbit skinned skeletal muscle fibres at low levels of Ca(2+ J Physiol. 2001;533:357–365. doi: 10.1111/j.1469-7793.2001.0357a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi S, Pollesello P, Levijoki J, Kaheinen P, Haikala H, Edes I, et al. The effects of levosimendan and OR-1896 on isolated hearts, myocyte-sized preparations and phosphodiesterase enzymes of the guinea pig. Eur J Pharmacol. 2004;486:67–74. doi: 10.1016/j.ejphar.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol (1985) 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- Teerlink JR. A novel approach to improve cardiac performance: cardiac myosin activators. Heart Fail Rev. 2009;14:289–298. doi: 10.1007/s10741-009-9135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, et al. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378:667–675. doi: 10.1016/S0140-6736(11)61219-1. [DOI] [PubMed] [Google Scholar]
- Valentova M, Von Haehling S. An overview of recent developments in the treatment of heart failure: update from the ESC Congress 2013. Expert Opin Investig Drugs. 2014;23:573–578. doi: 10.1517/13543784.2014.881799. [DOI] [PubMed] [Google Scholar]
- van Der Velden J, Klein LJ, Van Der Bijl M, Huybregts MA, Stooker W, Witkop J, et al. Isometric tension development and its calcium sensitivity in skinned myocyte-sized preparations from different regions of the human heart. Cardiovasc Res. 1999;42:706–719. doi: 10.1016/s0008-6363(98)00337-x. [DOI] [PubMed] [Google Scholar]
- van Der Velden J, Papp Z, Zaremba R, Boontje NM, De Jong JW, Owen VJ, et al. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003;57:37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- Vannier C, Lakomkine V, Vassort G. Tension response of the cardiotonic agent (+)-EMD-57033 at the single cell level. Am J Physiol. 1997;272:C1586–C1593. doi: 10.1152/ajpcell.1997.272.5.C1586. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ajtai K, Burghardt TP. Analytical comparison of natural and pharmaceutical ventricular myosin activators. Biochemistry. 2014;53:5298–5306. doi: 10.1021/bi500730t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KA, Bodi I, McNamara JP, Tracy M, Discher DJ, Bishopric NH. Negative lusitropy and abnormal calcium handling in hypoxic cardiac myocytes exposed to the calcium-sensitizer EMD 53998. J Mol Cell Cardiol. 1993;25:747–751. doi: 10.1006/jmcc.1993.1087. [DOI] [PubMed] [Google Scholar]
- Wilson K, Guggilam A, West TA, Zhang X, Trask AJ, Cismowski MJ, et al. Effects of a myofilament calcium sensitizer on left ventricular systolic and diastolic function in rats with volume overload heart failure. Am J Physiol Heart Circ Physiol. 2014;307:H1605–H1617. doi: 10.1152/ajpheart.00423.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 OM-evoked changes in Factive at saturating [Ca2+] and at a submaximal level of activation in cardiomyocytes, in skeletal myofibres with slow intrinsic kinetics and in skeletal myofibres with high intrinsic kinetics. (A,B) In cardiomyocytes and slow skeletal muscle fibres, Factive decreased linearly at pCa 4.75 with OM concentration, whereas at pCa, 6.0 the OM concentration–Factive relationships were bell-shaped. (C) OM did not affect Factive at pCa 4.75 while exhibiting a weak Ca2+-sensitizing effect at the submaximal [Ca2+] in fast skeletal muscle preparations. n = 6 − 6 isolated myofibres from three different animals respectively. Increasing concentrations of OM were applied in the same preparations. *P < 0.05, significantly different from control; repeated measures ANOVA with Dunnett’s two-tailed test.


