Abstract
Background and Purpose
γ-Oryzanol, derived from unrefined rice, attenuated the preference for dietary fat in mice, by decreasing hypothalamic endoplasmic reticulum stress. However, no peripheral mechanisms, whereby γ-oryzanol could ameliorate glucose dyshomeostasis were explored. Dopamine D2 receptor signalling locally attenuates insulin secretion in pancreatic islets, presumably via decreased levels of intracellular cAMP. We therefore hypothesized that γ-oryzanol would improve high-fat diet (HFD)-induced dysfunction of islets through the suppression of local D2 receptor signalling.
Experimental Approach
Glucose metabolism and regulation of molecules involved in D2 receptor signalling in pancreatic islets were investigated in male C57BL/6J mice, fed HFD and treated with γ-oryzanol. In isolated murine islets and the beta cell line, MIN6, the effects of γ-oryzanol on glucose-stimulated insulin secretion (GSIS) was analysed using siRNA for D2 receptors and a variety of compounds which alter D2 receptor signalling.
Key Results
In islets, γ-oryzanol enhanced GSIS via the activation of the cAMP/PKA pathway. Expression of molecules involved in D2 receptor signalling was increased in islets from HFD-fed mice, which were reciprocally decreased by γ-oryzanol. Experiments with siRNA for D2 receptors and D2 receptor ligands in vitro suggest that γ-oryzanol suppressed D2 receptor signalling and augmented GSIS.
Conclusions and Implications
γ-Oryzanol exhibited unique anti-diabetic properties. The unexpected effects of γ-oryzanol on D2 receptor signalling in islets may provide a novel; natural food-based, approach to anti-diabetic therapy.
Tables of Links
| TARGETS |
|---|
| GPCRsa |
| Dopamine D2 receptor |
| GPR119 |
| GPR120 |
| Transportersb |
| DAT, dopamine transporter |
| VMAT2, vesicular monoamine transporter 2 |
| Enzymesc |
| PKA |
| TH, tyrosine hydroxylase |
| LIGANDS | |
|---|---|
| cAMP | Haloperidol |
| CCK-8, cholecystokinin-octapeptide | Insulin |
| L-DOPA | Oleoylethanolamide |
| GLP-1, glucagon-like peptide 1 | Palmitic acid |
| Glucagon | Quinpirole |
| GW 9508 | Somatostatin |
| H-89 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14
Alexander et al., 2013a,b,c,,).
Introduction
Dopamine is a major catecholamine neurotransmitter that controls a wide range of biological processes important in neurological, cardiovascular and metabolic homeostasis. Previous reports have demonstrated that in patients with Parkinson’s disease, glucose metabolism was markedly impaired by treatment with L-DOPA, a dopamine precursor, in a dose-dependent manner (Sirtori et al., 1972; Marsden and Parkes, 1977). Importantly, molecules involved in dopamine receptor signalling are expressed in both murine and human pancreatic islets (Rubi et al., 2005; Simpson et al., 2012). Notably, a recent study on isolated pancreatic islets from humans demonstrated that pancreatic islet-derived dopamine did attenuate insulin secretion in an autocrine or paracrine fashion via its receptors (Simpson et al., 2012). In particular, studies in dopamine D2 receptor knockout mice suggest a critical role of dopaminergic suppression in function and replication of pancreatic beta cells during development in mice (Garcia-Tornadu et al., 2010).
It is well recognized that two distinct signalling pathways contribute to the control of insulin secretion from pancreatic beta cells, namely the ATP-sensitive K+ channel-dependent pathway (triggering pathway) and the cAMP/PKA pathway (amplifying pathway) (Henquin, 2000; Kahn et al., 2006). Two major incretin hormones, glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide, are crucial regulators for glucose-stimulated insulin secretion (GSIS) through an increase in intracellular cAMP level, thereby activating the cAMP/PKA pathway. On the other hand, dopamine is known to substantially decrease intracellular cAMP level mainly via D2 receptors in striatum and pituitary gland in the brain in rats, pigs and humans (Missale et al., 1998; Vallone et al., 2000).
Based on the notion that chronic feeding with a high fat diet (HFD) causes dysfunction of pancreatic islets and results in whole body glucose dysmetabolism (Giacca et al., 2011), we hypothesized that dopamine receptor signalling would be activated locally in pancreatic islets from HFD-fed mice, thereby causing dyshomeostasis of islet functions, at least partly, through a decrease in intracellular cAMP level. On the other hand, it has been shown that expression of genes involved in D2 receptor signalling in the brain reward system (e.g. striatum, ventral tegmental area) was considerably decreased in HFD-induced obese rodents, resulting in profound addiction to fatty foods (Li et al., 2009; Johnson and Kenny, 2010). This finding suggested that decreased local synthesis of dopamine in the brain could be relevant to this deviation in feeding behaviour.
γ-Oryzanol, derived from unrefined rice, is a unique bioactive substance, consisiting of a mixture of ferulic acid esters with phytosterols or triterpene alcohols (Lerma-Garcia et al., 2009; Kozuka et al., 2013). An earlier study in humans demonstrated that replacement of white rice by brown rice reduced the incidence of type 2 diabetes mellitus (Sun et al., 2010). Based on this report and our interventional trial assessing the metabolically beneficial impact of brown rice on pre-diabetic obese humans (Sun et al., 2010; Shimabukuro et al., 2014), we recently reported in mouse experiments that γ-oryzanol acted directly on the hypothalamus and attenuated preference for dietary fat by decreasing hypothalamic endoplasmic reticulum (ER) stress, thereby ameliorating HFD-induced obesity (Kozuka et al., 2012). We also demonstrated that long-term administration of γ-oryzanol considerably ameliorated HFD-induced glucose dyshomeostasis, independently of body weight and food intake (Kozuka et al., 2012). Moreover, although γ-oryzanol (3.2 mg·g−1 body weight) given orally to mice was distributed predominantly to the brain (83.8 mg per 100 g tissue); it also accumulated particularly in the pancreas (3.5 mg per 100 g tissue) 1 h after supplementation (Kozuka et al., 2015). However, the full mechanism whereby γ-oryzanol ameliorates glucose dysmetabolism throughout the body remained to be elucidated.
In rats, γ-oryzanol increased the dopamine content of the medial basal hypothalamus (Ieiri et al., 1982). This effect was suppressed by an inhibitor of L-tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis (Ieiri et al., 1982), suggesting a potential interaction of γ-oryzanol between dopamine metabolism and signalling via dopamine receptors. Based on all these findings, we tested if γ-oryzanol would improve dysfunction of pancreatic islets through the inhibition of D2 receptor signalling in murine experimental models.
Methods
Animals
All animal care and experimental procedures were approved by the Animal Experiment Ethics Committee of the University of the Ryukyus (Nos. 5352, 5718 and 5943). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 204 animals were used in the experiments described here.
Eight-week-old male C57BL/6J mice obtained from Charles River Laboratories Japan, Inc. (Kanagawa, Japan) were housed at 24°C under a 12 h/12 h light/dark cycle. The mice were allowed free access to food and water.
Administration of γ-oryzanol
γ-Oryzanol (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was dissolved in 0.5% methyl cellulose solution. γ-Oryzanol (20, 80 or 320 μg·g−1 body weight) was delivered into the stomach by a gavage needle every day during feeding with a HFD (Western Diet; Research Diets Inc., New Brunswick, NJ, USA) for 13 weeks. HFD and HFD containing 0.4% γ-oryzanol were manufactured as pellets by Research Diets (Research Diets Inc.). Daily intake of γ-oryzanol by mice, as estimated by mean food intake, was approximately 320 μg·g−1 body weight. The doses of γ-oryzanol were determined as described (Kozuka et al., 2012).
Metabolic parameters
Whole blood was taken from the tail vein and blood glucose was measured using an automatic glucometer (Medisafe Mini; Terumo, Tokyo, Japan). Occasional blood samples were taken from the retro-orbital venous plexuses or tail vein. Plasma insulin, glucagon and active GLP-1 levels were measured using elisa kits (Shibayagi Co. Ltd., Gunma, Japan; Wako Pure Chemical Industries, Ltd.; and Morinaga Institute of Biological Science, Inc., Tokyo, Japan). For glucose tolerance tests (GTTs), mice were intraperitoneally injected with 2.0 g·kg−1 glucose after an 18 h fast. Blood glucose levels were measured at the indicated times.
Sub-diaphragmatic vagotomy
Sub-diaphragmatic vagotomy, or sham surgery, was performed as described earlier (Miyamoto et al., 2012) and mice were used for experiments 2 weeks after the surgery. To test the success of the vagotomy, we assessed the satiety induced by CCK-8 (Bachem, Bubendorf, Switzerland), which is mediated by the abdominal vagus nerves (Smith et al., 1985; 1985). Sham-treated and vagotomized mice were injected i.p. with PBS or 8 μg·kg−1 CCK-8 after an 18 h fast.
Immunohistochemical (IHC) analyses
The pancreas was carefully dissected and fixed in 4% paraformaldehyde, embedded in paraffin and sectioned. The paraffin-embedded sections were stained with haematoxylin and eosin or immunostained for insulin (A0654; Dako Japan, Tokyo, Japan), glucagon (A0565; Dako Japan), somatostatin (AB5495; Merck Millipore, Billerica, MA), dopamine transporter (DAT) (AB1591P; Merck Millipore) and TH (AB152; Merck Millipore). The mean size and ratio of glucagon-positive α-cells, DAT-positive and TH-positive cell areas to the total islet area were calculated based on >100 islets per group using Photoshop (Adobe, San Jose, CA, USA).
Isolation of pancreatic islets and assessment of insulin/glucagon secretion
Pancreatic islets were isolated from mice by collagenase digestion (Liberase TL; Roche Diagnostics GmbH, Mannheim, Germany) and purified on a Histopaque gradient (Histopaque 1077; Sigma-Aldrich, St Louis, MO, USA) as described by Zmuda et al., (2011). Insulin secretion from isolated islets and from a murine pancreatic beta cell line, MIN6 cells, (Miyazaki et al., 1990), was measured as described earlier (Wei et al., 2005). Briefly, the islets were incubated with or without γ-oryzanol (0.2, 2 or 20 μg·mL−1), forskolin (10 mM), Rp-8-Br-cAMPS (10 μM), H-89 (10 μM), haloperidol (1, 10 μM; Wako Pure Chemical Industries, Ltd.), a D2 receptor antagonist, 10 μM L-DOPA, a dopamine precursor, or 5 μM quinpirole, a potent D2 receptor agonist (Sigma-Aldrich), for 1 h, and stimulated with glucose for an additional 1 h with or without γ-oryzanol, haloperidol, L-DOPA or quinpirole. The doses of each compound were decided as described (Simpson et al., 2012). MIN6 cells and an α-cell line (α-TC cells) were seeded at a density of 2.0 × 105 cells·mL−1 on 24-well plates. After 48 h of culture, MIN6 cells were incubated with Krebs–Ringer bicarbonate buffer (KRB; composition; 119 mM NaCl, 4.74 mM KCl, 2.54 mM CaCl2, 1.19 mM MgCl2, 1.19 mM KH2PO4, 25 mM NaHCO3, 0.5 % BSA, 25 mM HEPES, pH 7.4.) containing 2.5 mM glucose for 2 h, subsequently incubated in KRB with or without γ-oryzanol (0.2, 2 or 10 μg·mL−1) for 1 h. The cells were also incubated with a series of insulin secretagogues with or without γ-oryzanol for 2 h. α-TC cells were incubated with KRB containing 16.7 mM glucose for 1 h, subsequently incubated with or without palmitic acid (0.25 or 0.5 mM; Sigma-Aldrich), γ-oryzanol (2 or 10 μg·mL−1) or haloperidol (10 μM) for 2 h. Insulin or glucagon secretion was normalized by cellular protein content. Levels of cAMP and PKA activity were determined by the cyclic AMP EIA Kit (Cayman Chemical, Ann Arbor, MI, USA) and PKA kinase activity kit (Enzo Life Sciences, Farmingdale, NY, USA) respectively. To measure insulin content of islets, 10 islets were placed in 1 mL of acid-ethanol (90 mM HCl in 70% ethanol). Insulin was extracted overnight at −20°C after sonication, as previously described (Ariyama et al., 2008). The acid-ethanol extract was neutralized with 1 M Tris (pH 7.5) and insulin levels were measured using an elisa kit.
Measurement of cytosolic Ca2+ concentration ([Ca2+]i) in isolated islets
[Ca2+]i in isolated islets was measured by fura-2 microfluorometry as described (Nakata et al., 2010). Briefly, islets on coverslips were incubated with 1 μM fura-2/acetoxymethylester (Dojin Chemical Co., Kumamoto, Japan) for 1 h at 37°C in KRB containing 2.8 mM glucose with or without γ-oryzanol or haloperidol. Islets were subsequently mounted in a chamber and superfused at a rate of 1 mL·min−1 at 37°C in KRB with or without γ-oryzanol or haloperidol. Fluorescence following excitation at 340 nm (F340) and that at 380 nm (F380) was measured, and [Ca2+]i was expressed by the ratio (F340/F380).
RNA interference
The small interfering RNA (siRNA) for D2 receptors (the Drd2 gene) and a control scrambled siRNA were designed and purchased from Sigma-Aldrich. Pancreatic islets and MIN6 cells were transfected with each siRNA using Lipofectamine RNAi/MAX (Life technologies, Tokyo, Japan) according to the manufacturer’s protocol. Insulin secretion from MIN6 cells was normalized against cellular DNA content.
Agonist activity assay
Recruitment of β-arrestin to GPCRs, induced by γ-oryzanol was tested by the PathHunter β-Arrestin Assay obtained from DiscoveRx (Fremont, CA, USA). Luminescence was analysed with Envision (PerkinElmer, Waltham, MA, USA) and % activity was expressed as the relative luminescence units of 10 μM γ-oryzanol in comparison with that of each positive ligand. Antagonist activity (% inhibition) was measured against approximately EC80 concentrations of agonists. Duplicate data were obtained. The Z-factor, a parameter of quality control in high throughput screening assays (Zhang et al., 1999), was determined by the following equation: Z-factor = 1 − 3(SDsample + SDcontrol)/|meansample − meancontrol|. SDsample and SDcontrol refer to standard deviation of sample and positive control regions respectively.
Western blotting
Western blotting was performed as described (Tanaka et al., 2007) with antibodies against D2 receptors (AB5084P; Merck Millipore), DAT, TH and β-actin (ab6276; Abcam, Cambridge, MA, USA).
Quantitative real-time PCR
Gene expression was examined as described (Kozuka et al., 2012). Total RNA was extracted using Trizol reagent (Life technologies) and cDNA was synthesized using an iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Quantitative real-time PCR was performed using a StepOnePlusTM Real-Time PCR System and Fast SYBR Green Master Mix (Life Technologies). The mRNA levels were normalized against Rn18s (18S rRNA). The primer sets used for the quantitative real-time PCR analyses are summarized in Table 2013a.
Table 1.
The primer sets used for quantitative real-time PCR analysis
| Gene | GenBank Accession No. | Primer (5′–3′) |
|---|---|---|
| Drd2 (D2R) | NM_010077 | f CCA TTG TCT GGG TCC TGT CC |
| r GTG GGT ACA GTT GCC CTT GA | ||
| Drd3 (D3R) | NM_007877 | f GCA GTG GTC ATG CCA GTT CAC TAT CAG |
| r CCT GTT GTG TTG AAA CCA AAG AGG AGA GG | ||
| Slc6a3 (DAT) | NM_010020 | f GCA CTA CTT CTT CTC CTC CT |
| r CCT GAA GTC TTT ACT CCC TTC C | ||
| Th (TH) | NM_009377 | f CCC TAC CAA GAT CAA ACC TAC C |
| r GAG CGC ATG CAG TAG TAA GA | ||
| Slc18a2 (VMAT2) | NM_172523 | f GTC TGT CTA TGG GAG TGT GTA T |
| r GGG TAC GGC TGG ACA TTA TT | ||
| Rn18s (18S rRNA) | NR_003278 | f TTC TGG CCA ACG GTC TAG ACA AC |
| r CCA GTG GTC TTG GTG TGC TGA |
Forward and reverse primers are designated by f and r respectively. D2R, dopamine D2 receptor; D3R, dopamine D3 receptor.
Data analysis
Data are expressed as the mean ± SEM from n independent experiments. One-way anova and repeated-measures anova followed by multiple comparison tests (Bonferroni/Dunn method) were used where applicable. Student’s t-test was used to analyse the differences between two groups. Differences were considered significant at P < 0.05.
Results
γ-Oryzanol acts directly on pancreatic islets and enhances GSIS in vivo
As a first step in exploring the effects of γ-oryzanol on GSIS in chow-fed mice, the effects of a single oral dose of γ-oryzanol (320 μg·g−1 body weight) on blood glucose and insulin levels were examined during i.p. GTTs (ipGTTs). γ-Oryzanol augmented GSIS and significantly enhanced glucose tolerance even in normal mice (Figure 1A,B). γ-Oryzanol showed a trend towards a decrease in the plasma GLP-1 level, but the change was not statistically significant (P = 0.11) (Figure 1C). To see if γ-oryzanol would enhance GSIS independently of GLP-1 receptors, we evaluated, using PathHunter β-arrestin assays, the agonist activities of γ-oryzanol on GLP-1 receptors and on two other GPCRs, GPR119 and GPR120, both of which potently stimulate GLP-1 secretion from intestine (Hirasawa et al., 2005; Chu et al., 2007; Lauffer et al., 2009). γ-Oryzanol did not show agonist activities on these GPCRs [0% of exendin-4, a potent GLP-1 receptor agonist, Z-factor (a parameter of quality control in high throughput screening assays) (Zhang et al., 1999) was 0.81; 9% of oleoylethanolamide, a potent GPR119 agonist, Z-factor was 0.41; −2% of GW 9508, a potent GPR120 agonist, Z-factor was 0.75 respectively].
Figure 1.
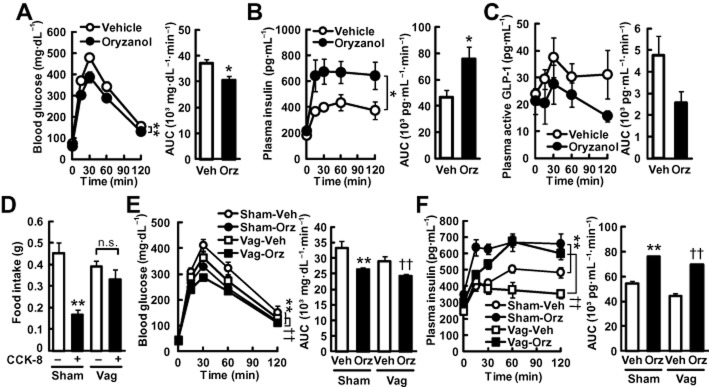
γ-Oryzanol enhances GSIS in mice. (A–C, E, F) Mice on a chow diet were treated with a single oral dose of γ-oryzanol (320 μg·g−1). The concentrations and AUCs of blood glucose (A, E), plasma insulin (B, F) and plasma active GLP-1 (C) during ipGTTs (n = 8) are shown. Chow-fed mice (A–C) and vagotomized mice (Vag) (E, F) were analysed. (D) Satiety effects of CCK-8 were tested in sham-treated mice (Sham) and vagotomized mice (Vag). Sub-diaphragmatic vagotomy abolished the satiety effect of CCK-8. *P < 0.05, **P < 0.01 versus unoperated or sham-operated mice treated with vehicle (Vehicle or Sham-Veh). ††P < 0.01 versus vehicle-treated vagotomized mice (Vag-Veh). Data are expressed as means ± SEM.
To exclude the possibility that γ-oryzanol augments GSIS via a central mechanism, we carried out sub-diaphragmatic vagotomy in mice. Cholecystokinin-octapeptide (CCK-8) reduced the food intake in 1 h by 63% in sham-operated mice, while sub-diaphragmatic vagotomy abolished the satiety effect of CCK-8 (Figure 1D), indicating that the vagotomy was successful. In both sham-operated and vagotomized mice, a single oral dose of γ-oryzanol significantly lowered the blood glucose levels and the AUC of glucose during ipGTTs (Figure 1E). Noticeably, in both sham-operated and vagotomized mice, γ-oryzanol markedly increased plasma insulin levels and the AUC of insulin during ipGTTs (Figure 1F). These results suggest that γ-oryzanol acted directly on the pancreatic islets to enhance GSIS.
γ-Oryzanol enhances GSIS through activation of the cAMP/PKA pathway via the suppression of D2 receptor signalling
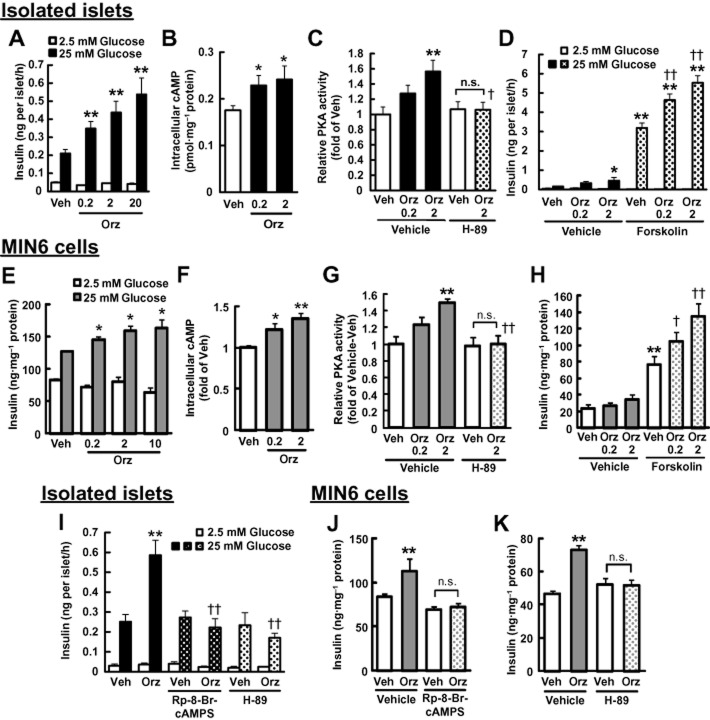
In both isolated murine islets and MIN6 cells, γ-oryzanol markedly enhanced GSIS in a dose-dependent fashion (Figure 2A,E). Furthermore, in both cellular systems, γ-oryzanol significantly increased intracellular cAMP levels and PKA activity (Figure 2B,C,F,G). Similarly, augmentation of PKA activity by γ-oryzanol was abolished by H-89, a PKA inhibitor (Figure 2C,G). To explore the underlying mechanism, isolated murine islets and MIN6 cells were exposed to (i) forskolin, which increases intracellular cAMP level; (ii) Rp-8-Br-cAMPS, a cAMP antagonist; or (iii) H-89 respectively. In both cellular systems, γ-oryzanol augmented forskolin-enhanced insulin secretion (Figure 2D,H), while both Rp-8-Br-cAMPS and H-89 abolished such stimulatory effects of γ-oryzanol on GSIS (Figure 2I–K). These findings suggest that γ-oryzanol reinforces GSIS via the cAMP/PKA amplifying pathway in pancreatic islets.
Figure 2.
γ-Oryzanol enhances GSIS through activation of the cAMP/PKA pathway in murine isolated islets and MIN6 cells. Murine isolated islets (A–D, I) and MIN6 cells (E–H, J, K) were treated with the indicated concentrations of γ-oryzanol (Orz; 0.2, 2, 10 or 20 μg·mL−1). (A, E) Insulin secretion was assessed following 25 mM glucose treated in murine-isolated islets (n = 10) (A) and MIN6 cells (n = 8) (E). (B, C, F, G) γ-Oryzanol (Orz; 0.2 or 2 μg·mL−1) increased intracellular cAMP levels (B, F) and PKA activity (C, G) following 25 mM glucose in islets (n = 12) (B, C) and MIN6 cells (n = 8) (F, G). (D, H) Effects of γ-oryzanol (Orz; 0.2 or 2 μg·mL−1) on insulin secretion enhanced by 10 μM forskolin in islets (n = 10) (D) and MIN6 cells following 2.5 mM glucose (n = 8) (H). (I–K) GSIS by 25 mM glucose was suppressed by 10 μM Rp-8-Br-cAMPS or 10 μM H-89 in islets (n = 10) (I) and MIN6 cells (n = 8) (J, K) treated with γ-oryzanol (Orz; 2 μg·mL−1). Islets used in each experiment were isolated from eight mice, and they were pooled and divided into indicated number of groups. *P < 0.05, **P < 0.01 versus vehicle (Veh)-treated islets. ††P < 0.01 versus cells treated with vehicle (Veh) and γ-oryzanol (2 μg·mL−1). n.s., not significant. Data are expressed as means ± SEM.
On the other hand, haloperidol, a D2 receptor antagonist, significantly enhanced GSIS (Figure 3A) through the elevation of intracellular cAMP (Figure 3B) but γ-oryzanol showed no additive effect with haloperidol (Figure 3C,D), supporting the notion that γ-oryzanol increased intracellular cAMP levels and enhanced GSIS through suppression of D2 receptor signalling. Furthermore, both L-DOPA, a dopamine precursor, and quinpirole, a potent D2 receptor agonist, abolished γ-oryzanol-induced enhancement of GSIS (Figure 3E–G). Of note, the inhibition by L-DOPA and quinpirole was concentration-dependent (Figure 3F,G). To further confirm the involvement of D2 receptor signalling in enhancing GSIS by γ-oryzanol, Drd2 was silenced in vitro by incubating the tissues or cells with specific siRNA for 2 days. In both pancreatic islets and MIN6 cells treated with Drd2 siRNA, the expression of Drd2 was attenuated by 71.4 ± 0.1% and 69.5 ± 0.1% compared with scrambled siRNA-treated cells respectively (Figure 3H,K). There were no significant changes in the expression of Drd3 (dopamine D3 receptor) in both systems (Figure 3H,K). Either γ-oryzanol or haloperidol enhanced GSIS accompanied by the elevation of intracellular cAMP level in cells treated with the scrambled siRNA. In contrast, in Drd2 siRNA-treated cells, γ-oryzanol and haloperidol did not increase GSIS and intracellular cAMP level (Figure 3I,J,L). These results suggest that γ-oryzanol augments GSIS via the suppression of D2 receptor signalling in pancreatic beta cells. Of note, data from the PathHunter β-arrestin assays suggested that there was no significant agonist or antagonist activities of γ-oryzanol for any of the dopamine receptors (Table 2013b).
Figure 3.
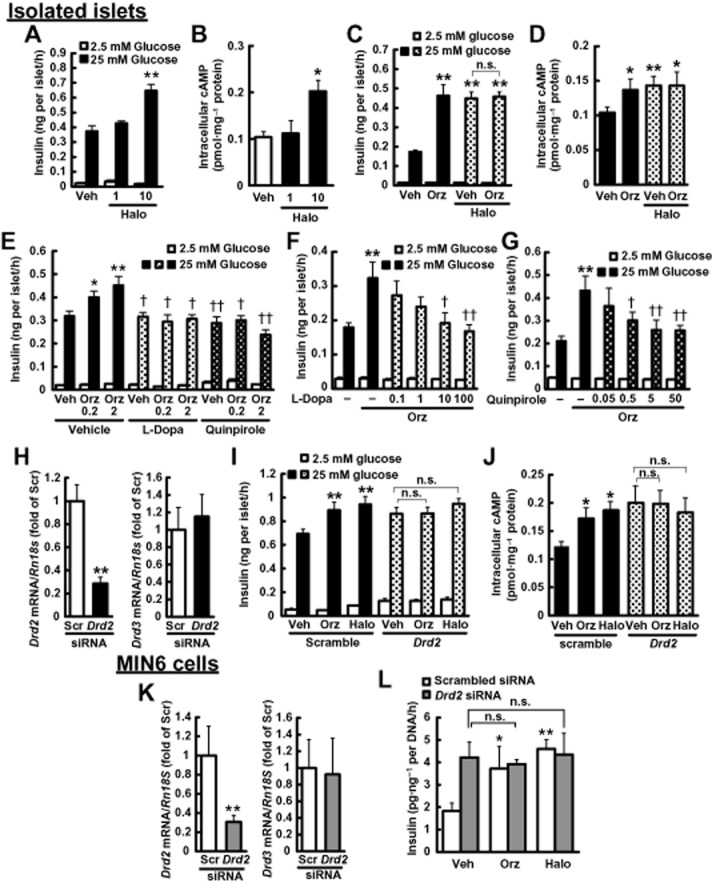
γ-Oryzanol enhances GSIS through the suppression of D2 receptor signalling in murine isolated islets and MIN6 cells. (A, B) Haloperidol (1, 10 μM) increased insulin secretion (A) and intracellular cAMP levels (B) in isolated islets following 25 mM glucose (n = 12). (C, D) γ-Oryzanol (Orz; 2 μg·mL−1) and haloperidol (10 μM) had no additive effect on insulin secretion (n = 12) (C) and intracellular cAMP levels (n = 24) (D) in isolated islets following 25 mM glucose. Islets used in each experiment were isolated from six mice, and they were pooled and divided into indicated number of groups. (E) Insulin secretion enhanced by the indicated concentrations of γ-oryzanol (Orz; 0.2 or 2 μg·mL−1) was suppressed by 10 μM L-DOPA or 5 μM quinpirole and in isolated islets (n = 10; islets isolated from 12 mice were pooled and divided into indicated number of groups). (F, G) Insulin secretion in isolated islets treated with γ-oryzanol (Orz; 2 μg·mL−1) was suppressed by the indicated concentrations of L-DOPA (0.1, 1, 10 or 100 μM) (F) or quinpirole (0.05, 0.5, 5 or 50 μM) (G) (n = 10–14; islets isolated from eight mice were pooled and divided into indicated number of groups). *P < 0.05, **P < 0.01 versus islets treated with vehicle (Veh). †P < 0.05, ††P < 0.01 versus islets treated with vehicle (Veh) and γ-oryzanol. (H–L) Isolated pancreatic islets (H–J) and MIN6 cells (K, L) were treated with Drd2 siRNA. (H, K) Level of mRNA expression for Drd2 and Drd3. The levels were normalized against those of Rn18s. **P < 0.01 versus scrambled siRNA-transfected islets or cells (Scr). (I, L) Insulin secretion in siRNA-treated islets (I) and MIN6 cells (L) was not enhanced by γ-oryzanol (Orz; 2 μg·mL−1) or haloperidol (10 μM) (n = 15–20). (J) γ-Oryzanol (Orz; 2 μg·mL−1) and haloperidol (Halo; 10 μM) had no effect on intracellular cAMP levels in siRNA-treated islets (n = 10). Islets isolated from eight mice were pooled and divided into indicated number of groups. **P < 0.01 versus scrambled siRNA-transfected islets treated with vehicle (Veh). n.s., not significant. Amount of insulin secretion from MIN6 cells was normalized against the cellular protein content. Data are expressed as means ± SEM.
Table 2.
Agonist or antagonist activities of γ-oryzanol for dopamine receptors (DRD1–DRD5)
| Agonist | Antagonist | |||
|---|---|---|---|---|
| % Activity | Z-factor | % Inhibition | Z-factor | |
| DRD1 | 0 | 0.73 | 10 | 0.84 |
| DRD2L | 1 | 0.79 | −5 | 0.81 |
| DRD2S | 2 | 0.81 | 5 | 0.91 |
| DRD3 | 13 | 0.48 | −13 | 0.79 |
| DRD4 | 1 | 0.86 | −2 | 0.77 |
| DRD5 | −2 | 0.75 | 9 | 0.87 |
Percentage of activity in γ-oryzanol for each dopamine receptor was calculated relative to the basal or maximal agonist values of dopamine. Percentage of inhibition by γ-oryzanol for each dopamine receptor was calculated relative to the basal or EC80 values for dopamine (antagonist activity). GPCR targets: DRD1, dopamine D1 receptor; DRD2L, long form of the dopamine D2 receptor; DRD2S, short form of the dopamine D2 receptor; DRD3, dopamine D3 receptor; DRD4, dopamine D4 receptor; DRD5, dopamine D5 receptor.
γ-Oryzanol increases insulin biosynthesis and [Ca2+]i in islets
Elevation of intracellular cAMP enhances the biosynthesis of insulin (Fehmann and Habener, 1992) and insulin secretion induced by increased [Ca2+]i in the presence of insulinotropic glucose concentrations (Yada et al., 1993). We therefore assessed the effect of γ-oryzanol and haloperidol on the biosynthesis of insulin and its secretion in response to increased [Ca2+]i in murine-isolated islets. Both γ-oryzanol and haloperidol significantly increased intracellular insulin contents and the [Ca2+]i response (Figure 4). Of note, both γ-oryzanol and haloperidol enhanced the first phase of [Ca2+]i responses to high glucose (Figure 4B,C). These results also reinforce the notion that γ-oryzanol increases intracellular cAMP levels and subsequently enhances GSIS through suppression of D2 receptor signalling.
Figure 4.
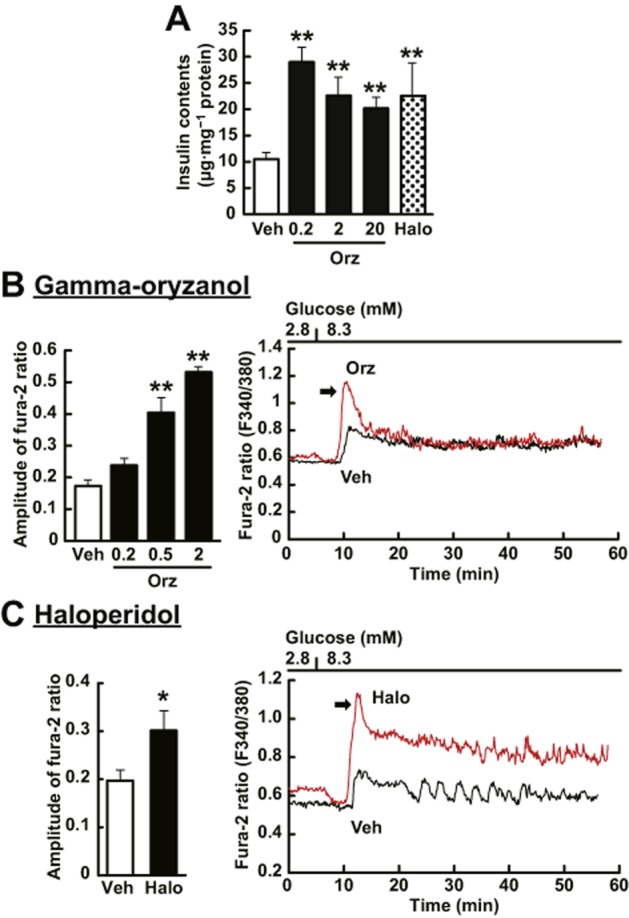
γ-Oryzanol increases intracellular insulin contents and [Ca2+]i in murine isolated islets. (A) γ-Oryzanol (Orz; 0.2, 2 or 20 μg·mL−1) and haloperidol (Halo; 10 μM) increased intracellular insulin contents (n = 14). (B, C) The representative [Ca2+]i responses to 8.3 mM glucose in islets incubated with γ-oryzanol or haloperidol. Both 2 μg·mL−1 γ-oryzanol (B) and 10 μM haloperidol (C) potentiated the first-phase [Ca2+]i response to 8.3 mM glucose in murine single islet. The peak amplitude of [Ca2+]i responses was significantly enhanced by γ-oryzanol (Orz; 0.2, 0.5 or 2 μg·mL−1) (B) (Veh, n = 8, Orz 0.2, n = 12, Orz 0.5, n = 5, Orz 2, n = 3; islets isolated from three mice were pooled and divided into indicated number of groups) and haloperidol (C) (Veh, n = 12, Halo, n = 10; islets isolated from two mice were pooled and divided into indicated number of groups). *P < 0.05, **P < 0.01 versus vehicle (Veh)-treated islets. Data are expressed as means ± SEM.
γ-Oryzanol suppresses D2 receptor signalling in pancreatic islets from HFD-fed mice
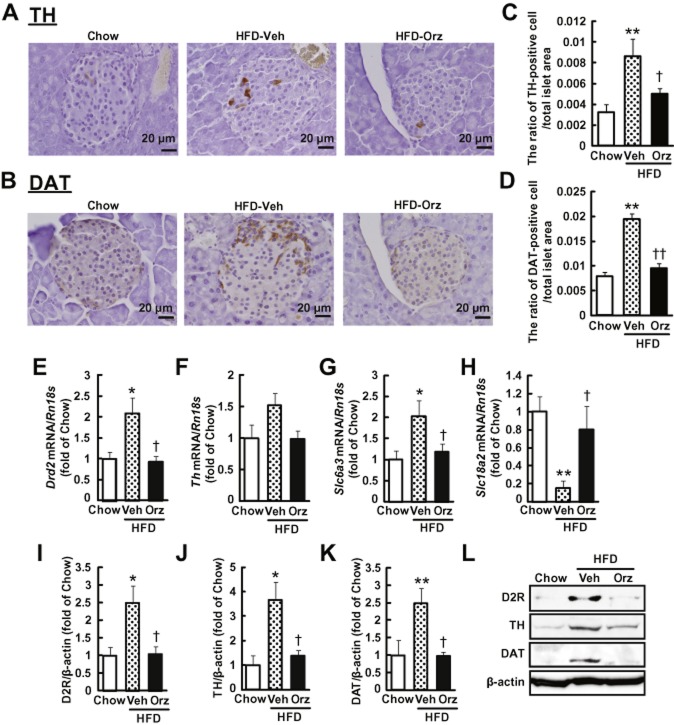
Following treatment of γ-oryzanol (320 μg·g−1 per body weight per day) for 13 weeks, glucose level in mice on a HFD was 1280 ± 50 mg·L−1, which was significantly decreased compared with those in mice on the HFD alone (1570 ± 80 mg·L−1, P < 0.01). Body weight in mice fed HFD with γ-oryzanol was 31.7 ± 0.8 g, which was comparable to that in mice fed HFD alone (30.4 ± 1.2 g). Areas of islet cells stained with antibody to TH, the rate-limiting enzyme of dopamine synthesis (Figure 5A), and antibody to DAT, which mediates dopamine uptake (Figure 5B), were increased in pancreatic islets from HFD-fed mice, whereas the stained areas were markedly decreased after treatment with γ-oryzanol. Consequently, the ratio of TH-positive or DAT-positive cell areas to the total islet area was significantly increased in HFD-fed mice, and was substantially decreased by the treatment with γ-oryzanol (Figure 5C,D). IHC analyses suggested that TH was localized in beta cells, while DAT was not confined to α-cells, beta cells or δ-cells (Figure 6).
Figure 5.
γ-Oryzanol suppresses the expression of molecules involved in D2 receptor signalling in murine pancreatic islets from mice fed HFD. (A, B) IHC analyses of pancreatic islets from HFD-fed mice treated with γ-oryzanol (Orz). Paraffin-embedded sections were stained with anti-TH (A) or anti-DAT (B) antibodies. Scale bar, 20 μm; magnification, ×400. (C, D) The ratios of TH-positive (C) and DAT-positive (D) cell area to the total islet area were attenuated by the treatment with γ-oryzanol in HFD-fed mice (chow, n = 6, HFD-Veh, n = 8, HFD-Orz, n = 8). **P < 0.01 versus chow-fed mice. †P < 0.05, ††P < 0.01 versus vehicle (Veh)-treated HFD-fed mice. (E–H) Expression levels of Drd2 (E), Th (F), Slc6a3 (DAT) (G) and Slc18a2 (VMAT2) (H) mRNAs in pancreatic islets from HFD-fed mice were decreased by γ-oryzanol (Orz; 320 μg·g−1 per body weight per day) (n = 6). The mRNA levels were determined by real-time PCR. The levels were normalized by those of Rn18s (18S rRNA). (I–L) Protein levels of D2 receptors (I), TH (J) and DAT (K) in pancreatic islets from HFD-fed mice were decreased by γ-oryzanol (n = 6). Protein levels were determined by Western blotting. The values were normalized against those of β-actin protein. *P < 0.05, **P < 0.01 versus chow-fed mice. †P < 0.05 versus vehicle (Veh)-treated HFD-fed mice. Data are expressed as means ± SEM.
Figure 6.
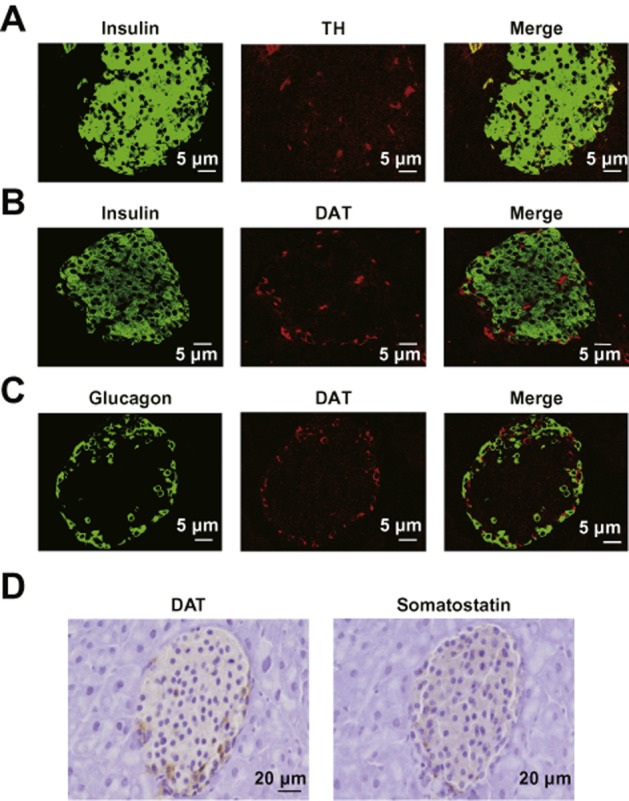
TH was localized in beta cells, whereas DAT was not confined to α-cells, beta cells or δ-cells. IHC analyses of pancreatic islets from HFD-fed mice. Paraffin-embedded sections were co-stained with anti-TH (red) and anti-insulin (green) (A), anti-DAT (red) and anti-insulin (green) (B), or anti-DAT (red) and anti-glucagon (green) (C) antibodies. Scale bar, 5 μm; magnification, ×600. (D) Serial paraffin-embedded sections were stained with anti-DAT and anti-somatostatin antibodies. Scale bar, 20 μm; magnification, ×400.
We assessed protein and mRNA expression levels of genes involved in D2 receptor signalling including D2 receptors (Drd2), TH (Th), DAT (Slc6a3) and the vesicular monoamine transporter type 2 (VMAT2; Slc18a2), which transports dopamine into vesicles. In pancreatic islets from HFD-fed mice, the mRNA levels of Drd2, Th and Slc6a3 were considerably elevated, while that of Slc18a2, also known as a functional marker of insulin production (Harris et al., 2008), was markedly decreased (Figure 5E–H). Importantly, administration of γ-oryzanol depressed the mRNA levels of these genes (Figure 5E–H). In parallel with mRNA levels, protein levels of D2 receptors, TH and DAT were concomitantly decreased by γ-oryzanol (Figure 5I–L).
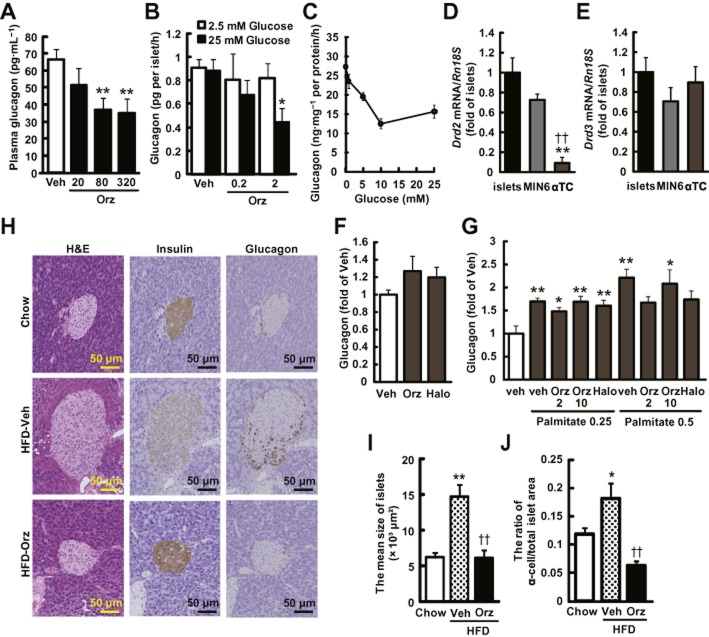
γ-Oryzanol decreases glucagon secretion from murine islets
γ-Oryzanol significantly decreased glucagon levels in plasma of HFD-fed mice (Figure 7A) and in media of isolated islet cultures (Figure 7B). To test the possibility that γ-oryzanol directly acted on α-cells, a murine α-cell line, α-TC cells, was treated with γ-oryzanol. As shown in Figure 7C, glucagon secretion from α-TC cells was reduced, concentration-dependently, by glucose. It should be noted that mRNA level of Drd2 in α-TC cells was extremely low, compared with those in isolated islets and MIN6 cells, while that of Drd3 was about the same in the three types of cells (Figure 7D,E). In α-TC cells, γ-oryzanol and haloperidol did not affect glucagon secretion in both basal and palmitate-stimulated conditions (Figure 7F,G). IHC analyses of pancreatic islets from mice on a HFD demonstrated that γ-oryzanol augmented the intensity of insulin staining, while attenuating the average size of pancreatic islets, as well as the ratio of α-cells to the total islet area (Figure 7H–J). These results raised the possibility that γ-oryzanol reduced the increased secretion of glucagon via mechanisms independent of α-cells.
Figure 7.
γ-Oryzanol ameliorates increased secretion of glucagon in HFD-fed mice and from murine isolated islets. (A) Plasma glucagon levels in HFD-fed mice treated with the indicated doses of γ-oryzanol (Orz) after a 4 h fast (20, 80 or 320 μg·g−1 per body weight per day; n = 6). (B) Glucagon secretion in isolated pancreatic islets was decreased by γ-oryzanol (Orz; 0.2 or 2 μg·mL−1) following the exposure to 25 mM glucose (n = 10; islets isolated from three mice were pooled and divided into indicated number of groups). (C) Glucagon secretion was stimulated by indicated concentrations of glucose (0, 1, 5, 10 and 25 mM). Amount of glucagon secretion was normalized against the cellular protein content. (D, E) Expression level of Drd2 and Drd3 in isolated islets, MIN6 and α-TC cells. Levels of mRNA expression for Drd2 (D) and Drd3 (E) in three types of cells (n = 12). The mRNA levels were determined by real-time PCR. The levels were normalized against those of Rn18s. **P < 0.01 versus islets, ††P < 0.01 versus MIN6 cells. (F, G) In α-TC cells treated with γ-oryzanol (Orz; 2 or 10 μg·mL−1) or haloperidol (10 μM), glucagon secretion was assessed following 5 mM glucose (F) or palmitate (0.25 and 0.5 mM following 16.7 mM glucose (G). *P < 0.05, **P < 0.01 versus HFD-fed mice, islets, or α-TC cells treated with vehicle (Veh). (H) IHC analyses of isolated pancreatic islets from HFD-fed mice treated with γ-oryzanol (Orz; 320 μg·g−1·day−1). Serial paraffin-embedded sections were stained with haematoxylin and eosin (H&E) (upper panel) or anti-insulin (middle panel), anti-glucagon (lower panel) antibodies. Scale bar, 50 μm; magnification, ×200. (I, J) The mean sizes of islets (I) and ratios of glucagon-positive α-cell areas to the total islet area (J) were calculated (n = 6–8). *P < 0.05, **P < 0.01 versus chow-fed mice. ††P < 0.01 versus HFD-fed mice treated with vehicle (Veh). Data are expressed as means ± SEM.
Discussion and conclusions
The major findings in the present study are summarized by the scheme shown in Figure 8. Here, we have demonstrated that, in mice, γ-oryzanol acted directly on pancreatic islets and enhanced GSIS in vivo and in vitro (Figures 1 and 2). Such a reinforcement of GSIS by γ-oryzanol was mediated by the local activation of the cAMP/PKA amplifying pathway (Figures 2 and 4). Along with chemical agonists for a variety of fatty acid receptors, cAMP/PKA amplifying pathways in pancreatic beta cells are promising drug targets for the treatment of type 2 diabetes (Drucker, 2006; Rayasam et al., 2007; Ohishi and Yoshida, 2012). In this context, γ-oryzanol may be potentially useful as an alternative or a partner of combination therapies with incretin-related drugs.
Figure 8.
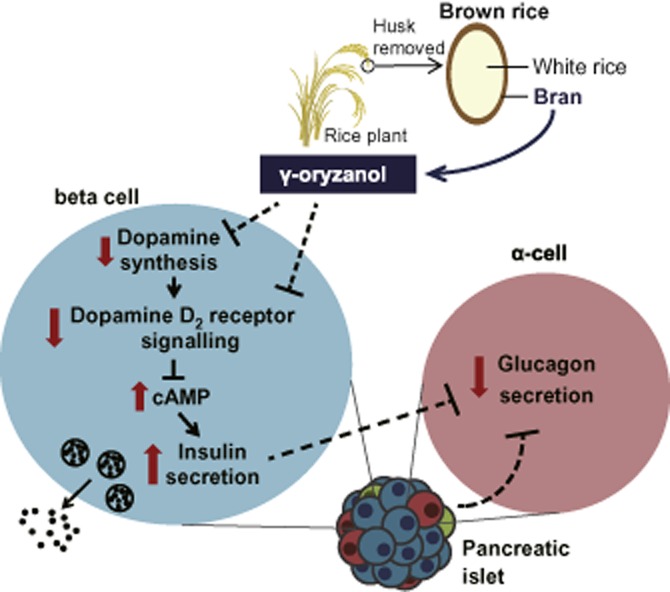
Scheme illustrating the effects of γ-oryzanol on pancreatic islets in mice. In pancreatic islets and beta cells, γ-oryzanol suppresses D2 receptor signalling, at least partly via the inhibition of local dopamine synthesis, leading to an increase in the intracellular cAMP level. Consequently, GSIS is augmented via the cAMP/PKA pathway (amplifying pathway). On the other hand, γ-oryzanol ameliorates exaggerated secretion of glucagon from pancreatic α-cells, not via the direct action on α-cells, but presumably via some intra-islet paracrine factors.
To our knowledge, the present study is the first to demonstrate that protein and mRNA expression of molecules involved in D2 receptor signalling was considerably elevated in pancreatic islets from mice fed on a HFD. Moreover, supplementation with γ-oryzanol corrected the dysregulation of these molecules in vivo (Figure 5). As increased signal transduction by D2 receptors in pancreatic beta cells suppresses the secretion of insulin (Rubi et al., 2005; Simpson et al., 2012), such an effect of γ-oryzanol may be beneficial for individuals with glucose intolerance and type 2 diabetes. To date, how transcription of Drd2 is regulated is largely undefined. It is possible that consensus element of NF-κB in the promoter region of Drd2 (Bontempi et al., 2007) is related to the HFD-induced dysregulation of D2 receptors in isolated islets. Apart from the direct action of γ-oryzanol on pancreatic islets, it is also possible that improvement of hyperglycaemia per se may influence the expression of molecules involved in D2 receptor signalling. In this context, further studies are necessary to elucidate fully the molecular mechanisms involved.
Intriguingly, in HFD-induced obese rodents, expression of genes involved in D2 receptor signalling in the brain reward system (e.g. striatum, ventral tegmental area) was clearly decreased, resulting in a profound addiction to fatty foods (Li et al., 2009; Johnson and Kenny, 2010). Furthermore, recent studies in rodents demonstrated that HFD-induced decrement in D2 receptor expression in the brain reward system was closely associated with the hyper-methylation in the promoter region of the Drd2 gene (Vucetic et al., 2012). Studies are ongoing in our laboratory to investigate whether there is HFD-induced epigenetic dysregulation of the D2 receptor signalling in pancreatic islets or beta cells.
In isolated islets and MIN6 cells, experiments with RNA interference for Drd2 and with exogenous D2 receptor ligands demonstrated that γ-oryzanol augmented GSIS via the suppression of D2 receptor signalling (Figure 3). Enhancement of GSIS by γ-oryzanol was suppressed by L-DOPA (Figure 3E,F), while γ-oryzanol has neither agonist nor antagonist activities at D2 receptors (Table 2013b). These findings suggest that γ-oryzanol has inhibitory effects on local dopamine synthesis.
In the pathophysiology of diabetes mellitus, exaggerated secretion of glucagon from pancreatic α-cells contributes to the vicious cycle of glucose dyshomeostasis (Holst, 2007). We demonstrated that γ-oryzanol substantially ameliorated the exaggerated secretion of glucagon in both HFD-fed mice and murine-isolated islets (Figure 7). As D2 receptors are confined to beta cells in pancreatic islets in mice (Rubi et al., 2005), our data raise the possibility that γ-oryzanol would not directly affect glucagon secretion from α-cells. To support this notion, we demonstrated in an α-cell line, α-TC cells, that γ-oryzanol and haloperidol did not affect glucagon secretion in either basal or palmitate-stimulated conditions (Figure 7F,G). The secretion of glucagon is known to be regulated by the central and peripheral nervous system as well as intra-islet paracrine factors including insulin, GABA and somatostatin (Ishihara et al., 2003; Kawamori et al., 2009; Walker et al., 2011). For instance, postprandial glucagon release is strongly suppressed by GLP-1 and the effect of GLP-1 is mediated, at least partly, by somatostatin (Holst, 2007; Seino et al., 2010). In this context, our results raise the possibility that γ-oryzanol may reduce increased secretion of glucagon via α-cell-independent, intra-islet paracrine factors.
Regarding the effects of γ-oryzanol on food intake in mice, we previously reported that γ-oryzanol did not affect the total amount of food intake (chow: 16.8 ± 0.5 g per week, HFD: 16.4 ± 0.4 g per week, HFD + γ-oryzanol: 16.2 ± 0.5 g per week). However, γ-oryzanol does reduce the preference for fatty foods in mice (Kozuka et al., 2012). Based on these findings, in the current experimental settings, the insulinotropic effects of γ-oryzanol on pancreatic islets should be largely attributed to its direct mechanism. Moreover, as demonstrated in Figure 1, oral administration of γ-oryzanol to mice fed chow diet did not increase plasma GLP-1 level. The results of β-arrestin assays also support the notion that γ-oryzanol did not act as a ligand for GLP-1 receptor. Notably, secretion of GLP-1 is controlled strongly by a vagal nerve-mediated central mechanism (Drucker, 2006). However, even in vagotomized mice, γ-oryzanol markedly increased the plasma insulin levels during ipGTTs (Figure 1). These data suggest that γ-oryzanol acts directly on pancreatic islets and enhances GSIS independently of GLP-1 receptor signalling. Furthermore, we recently demonstrated that γ-oryzanol protects beta cells against ER stress-induced apoptosis in HFD-fed mice (Kozuka et al., 2015). Taken together, γ-oryzanol exhibited metabolically beneficial effects on glucose homeostasis in a GLP-1 independent, unique insulinotropic manner.
The present study unveiled the mechanism, at least in part, whereby γ-oryzanol protects pancreatic islets against HFD-induced dysfunction and augments GSIS via the attenuation of local D2 receptor signalling in mice. This series of unexpected actions of γ-oryzanol may lead to a novel, natural food-based preventive treatment for type 2 diabetes.
Acknowledgments
We thank M. Takaki (University of the Ryukyus, Japan) and F. Y. Wei (Kumamoto University, Japan) for technical help. We are grateful to M. Hirata, I. Asato and C. Noguchi (University of the Ryukyus, Japan) for assistance. The α-TC cells were kindly provided by T. Kitamura (Gunma University, Japan). This work was supported in part by Grants-in-Aid from Japan Society for the Promotion of Science (JSPS; KAKENHI Grant Nos. 24591338 and 25·9668), Council for Science, Technology and Innovation (CSTI), Cross-ministerial Strategic Innovation Promotion Program (SIP), ‘Technologies for creating next-generation agriculture, forestry and fisheries’, Takeda Science Foundation (Specified Research Grant), Society for Woman’s Health Science Research (13-A1-001), the Nestlé Nutrition Council Japan, Narishige Neuroscience Research Foundation, Lotte Foundation, Leave a Nest Grants, Metabolic Syndrome Foundation and Specified Project ‘Establishing a research hub toward the development of an intellectual cluster in Okinawa Prefecture’ (2011–2014). C. K. is a Research Fellow of JSPS.
Glossary
- [Ca2+]i
cytosolic Ca2+ concentration
- CCK-8
cholecystokinin-octapeptide
- DAT
dopamine transporter
- GLP-1
glucagon-like peptide 1
- GSIS
glucose-stimulated insulin secretion
- GTT
glucose tolerance test
- HFD
high-fat diet
- IHC
immunohistochemical
- siRNA
small interfering RNA
- TH
L-tyrosine hydroxylase
- VMAT2
vesicular monoamine transporter 2
Author contributions
C. K., C. S.-O. and M. N. performed the research. C. K. and H. M. designed the research study. S. S., R. U., M. H., Y. O., H. T., C. S.-O., C. T., M. M., M. T., S. I., M. N., T. Y., J. M., S. O. and M. S. provided invaluable advice on research design and data interpretation. J. M. and S. O. contributed essential reagents or tools. C. K. analysed the data. C. K. and H. M. wrote the paper.
Conflict of interest
We declare that we have no conflict of interest.
References
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The concise guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013b;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The concise guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyama Y, Tanaka Y, Shimizu H, Shimomura K, Okada S, Saito T, et al. The role of CHOP messenger RNA expression in the link between oxidative stress and apoptosis. Metabolism. 2008;57:1625–1635. doi: 10.1016/j.metabol.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Bontempi S, Fiorentini C, Busi C, Guerra N, Spano P, Missale C. Identification and characterization of two nuclear factor-kappaB sites in the regulatory region of the dopamine D2 receptor. Endocrinology. 2007;148:2563–2570. doi: 10.1210/en.2006-1618. [DOI] [PubMed] [Google Scholar]
- Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, et al. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007;148:2601–2609. doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Fehmann HC, Habener JF. Insulinotropic hormone glucagon-like peptide-I(7–37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130:159–166. doi: 10.1210/endo.130.1.1309325. [DOI] [PubMed] [Google Scholar]
- Garcia-Tornadu I, Ornstein AM, Chamson-Reig A, Wheeler MB, Hill DJ, Arany E, et al. Disruption of the dopamine d2 receptor impairs insulin secretion and causes glucose intolerance. Endocrinology. 2010;151:1441–1450. doi: 10.1210/en.2009-0996. [DOI] [PubMed] [Google Scholar]
- Giacca A, Xiao C, Oprescu AI, Carpentier AC, Lewis GF. Lipid-induced pancreatic beta-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab. 2011;300:E255–E262. doi: 10.1152/ajpendo.00416.2010. [DOI] [PubMed] [Google Scholar]
- Harris PE, Ferrara C, Barba P, Polito T, Freeby M, Maffei A. VMAT2 gene expression and function as it applies to imaging beta-cell mass. J Mol Med (Berl) 2008;86:5–16. doi: 10.1007/s00109-007-0242-x. [DOI] [PubMed] [Google Scholar]
- Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Ieiri T, Kase N, Hashigami Y, Kobori H, Nakamura T, Shimoda S. [Effects of gamma-oryzanol on the hypothalamo-pituitary axis in the rat] Nihon Naibunpi Gakkai Zasshi. 1982;58:1350–1356. doi: 10.1507/endocrine1927.58.10_1350. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9:350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka C, Yabiku K, Sunagawa S, Ueda R, Taira SI, Ohshiro H, et al. Brown rice and its component, gamma-oryzanol, attenuate the preference for high-fat diet by decreasing hypothalamic endoplasmic reticulum stress in mice. Diabetes. 2012;61:3084–3093. doi: 10.2337/db11-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka C, Yabiku K, Takayama C, Matsushita M, Shimabukuro M, Masuzaki H. Natural food science based novel approach toward prevention and treatment of obesity and type 2 diabetes: recent studies on brown rice and γ-oryzanol. Obes Res Clin Pract. 2013;7:e165–e172. doi: 10.1016/j.orcp.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Kozuka C, Sunagawa S, Ueda R, Higa M, Tanaka H, Shimizu-Okabe C, et al. Gamma-oryzanol protects pancreatic beta-cells against endoplasmic reticulum stress in male mice. Endocrinology. 2015;156:1242–1250. doi: 10.1210/en.2014-1748. [DOI] [PubMed] [Google Scholar]
- Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58:1058–1066. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma-Garcia MJ, Herrero-Martinez JM, Simo-Alfonso EF, Mendonca CRB, Ramis-Ramos G. Composition, industrial processing and applications of rice bran gamma-oryzanol. Food Chem. 2009;115:389–404. [Google Scholar]
- Li Y, South T, Han M, Chen J, Wang R, Huang XF. High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain Res. 2009;1268:181–189. doi: 10.1016/j.brainres.2009.02.075. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Parkes JD. Success and problems of long-term levodopa therapy in Parkinson’s disease. Lancet. 1977;1:345–349. doi: 10.1016/s0140-6736(77)91146-1. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Miyamoto L, Ebihara K, Kusakabe T, Aotani D, Yamamoto-Kataoka S, Sakai T, et al. Leptin activates hepatic 5′ AMP-Activated Protein Kinase through sympathetic nervous system and alpha1 adrenergic receptor: a potential mechanism for improvement of fatty liver in lipodystrophy by leptin. J Biol Chem. 2012;287:40441–40447. doi: 10.1074/jbc.M112.384545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, et al. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- Nakata M, Shintani N, Hashimoto H, Baba A, Yada T. Intra-islet PACAP protects pancreatic beta-cells against glucotoxicity and lipotoxicity. J Mol Neurosci. 2010;42:404–410. doi: 10.1007/s12031-010-9383-4. [DOI] [PubMed] [Google Scholar]
- Ohishi T, Yoshida S. The therapeutic potential of GPR119 agonists for type 2 diabetes. Expert Opin Investig Drugs. 2012;21:321–328. doi: 10.1517/13543784.2012.657797. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayasam GV, Tulasi VK, Davis JA, Bansal VS. Fatty acid receptors as new therapeutic targets for diabetes. Expert Opin Ther Targets. 2007;11:661–671. doi: 10.1517/14728222.11.5.661. [DOI] [PubMed] [Google Scholar]
- Rubi B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, et al. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. 2005;280:36824–36832. doi: 10.1074/jbc.M505560200. [DOI] [PubMed] [Google Scholar]
- Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Investig. 2010;1:8–23. doi: 10.1111/j.2040-1124.2010.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro M, Higa M, Kinjo R, Yamakawa K, Tanaka H, Kozuka C, et al. Effects of the brown rice diet on visceral obesity and endothelial function: the BRAVO study. Br J Nutr. 2014;111:310–320. doi: 10.1017/S0007114513002432. [DOI] [PubMed] [Google Scholar]
- Simpson N, Maffei A, Freeby M, Burroughs S, Freyberg Z, Javitch J, et al. Dopamine-mediated autocrine inhibitory circuit regulating human insulin secretion in vitro. Mol Endocrinol. 2012;26:1757–1772. doi: 10.1210/me.2012-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirtori CR, Bolme P, Azarnoff DL. Metabolic responses to acute and chronic L-dopa administration in patients with parkinsonism. N Engl J Med. 1972;287:729–733. doi: 10.1056/NEJM197210122871501. [DOI] [PubMed] [Google Scholar]
- Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981;213:1036–1037. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249:R638–641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik VS, Willett WC, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med. 2010;170:961–969. doi: 10.1001/archinternmed.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Masuzaki H, Yasue S, Ebihara K, Shiuchi T, Ishii T, et al. Central melanocortin signaling restores skeletal muscle AMP-activated protein kinase phosphorylation in mice fed a high-fat diet. Cell Metab. 2007;5:395–402. doi: 10.1016/j.cmet.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Carlin JL, Totoki K, Reyes TM. Epigenetic dysregulation of the dopamine system in diet-induced obesity. J Neurochem. 2012;120:891–898. doi: 10.1111/j.1471-4159.2012.07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JN, Ramracheya R, Zhang Q, Johnson PR, Braun M, Rorsman P. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes Metab. 2011;13(Suppl. 1):95–105. doi: 10.1111/j.1463-1326.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- Wei FY, Nagashima K, Ohshima T, Saheki Y, Lu YF, Matsushita M, et al. Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat Med. 2005;11:1104–1108. doi: 10.1038/nm1299. [DOI] [PubMed] [Google Scholar]
- Yada T, Itoh K, Nakata M. Glucagon-like peptide-1-(7–36)amide and a rise in cyclic adenosine 3′,5′-monophosphate increase cytosolic free Ca2+ in rat pancreatic beta-cells by enhancing Ca2+ channel activity. Endocrinology. 1993;133:1685–1692. doi: 10.1210/endo.133.4.8404610. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chung TDY, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Zmuda EJ, Powell CA, Hai T. A method for murine islet isolation and subcapsular kidney transplantation. J Vis Exp. 2011;50:e2096. doi: 10.3791/2096. [DOI] [PMC free article] [PubMed] [Google Scholar]