Abstract
Background and Purpose
Patients with major depressive disorder receiving racemic ketamine, (R,S)-ketamine, experience transient increases in Clinician-Administered Dissociative States Scale scores and a coincident drop in plasma d-serine levels. The results suggest that (R,S)-ketamine produces an immediate, concentration-dependent pharmacological effect on d-serine plasma concentrations. One potential source of this effect is (R,S)-ketamine-induced inhibition of the transporter ASCT2, which regulates intracellular d-serine concentrations. In this study, we tested this hypothesis by examining the effect of (S)- and (R)-ketamine on ASCT2-mediated transport of d-serine in PC-12 and 1321N1 cells and primary neuronal cells in culture.
Experimental Approach
Intracellular and extracellular d-serine levels were determined using capillary electrophoresis–laser-induced fluorescence and liquid chromatography–mass spectrometry respectively. Expression of ASCT2, Asc-1 and serine racemase was determined utilizing Western blotting.
Key Results
(S)-Ketamine produced a concentration-dependent increase in intracellular d-serine and reduced extracellular d-serine accumulation. In contrast, (R)-ketamine decreased both intracellular and extracellular d-serine levels. The ASCT2 inhibitor, benzyl-d-serine (BDS), and ASCT2 gene knockdown mimicked the action of (S)-ketamine on d-serine in PC-12 cells, while the Asc-1 agonist d-isoleucine reduced intracellular d-serine and increased extracellular d-serine accumulation. This response to d-isoleucine was not affected by BDS or (S)-ketamine. Primary cultures of rat neuronal cells expressed ASCT2 and were responsive to (S)-ketamine and BDS. (S)- and (R)-ketamine increased the expression of monomeric serine racemase in all the cells studied, with (S)-ketamine having the greatest effect.
Conclusions and Implications
(S)-Ketamine decreased cellular export of d-serine via selective inhibition of ASCT2, and this could represent a possible source of dissociative effects observed with (R,S)-ketamine.
Tables of Links
| LIGANDS |
|---|
| D-serine |
| Ketamine |
| Nicotine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14
Alexander et al., 2013a,b,).
Introduction
(R,S)-Ketamine is a phencyclidine derivative that produces rapid and short-lived anaesthesia with a wide margin of clinical safety (Domino, 2010; Hirota and Lambert, 2011); however, disturbing emergence reactions and dissociative and hallucinogenic effects have been reported following the use of (R,S)-ketamine (White et al., 1982). Recent studies have shown that (R,S)-ketamine is also an effective antidepressant agent, as illustrated by the work of Zarate et al. (2006), in which a single sub-anaesthetic dose of the drug produced rapid antidepressant responses in patients with treatment-resistant major depressive disorder (MDD). While the use of (R,S)-ketamine in the treatment of MDD and bipolar depression is an effective and increasingly popular therapeutic approach, its administration is also accompanied by dissociative side effects as measured by an increase in the Clinician-Administered Dissociative States Scale (CADSS) (Pomarol-Clotet et al., 2006; Zarate et al., 2012; Luckenbaugh et al., 2014). In the study by Luckenbaugh et al. (2014), the maximum increases in CADSS scores were observed at the end of a 40 min infusion of (R,S)-ketamine (0.5 mg·kg−1), which was followed by a rapid decline to below baseline at the 80 min sampling point. Moreover, a significant negative correlation was found between the magnitude of the increase in CADSS scores at 40 min and the (R,S)-ketamine-associated antidepressant effect at 230 min, determined as % improvement in the 17-item Hamilton Depression Rating Scale (Luckenbaugh et al., 2014).
We have recently re-analysed a subset of the MDD patients from the study of Luckenbaugh et al. (2014), and demonstrated that the % change in the Montgomery–Åsberg Depression Rating Scale (MADRS) scores between baseline and 230 min after initiation of the study segregates the patients into responders and non-responders to (R,S)-ketamine antidepressant therapy, with response defined as a ≥50% decrease in the MADRS score (Moaddel et al., 2015). Baseline plasma concentrations of d-serine, a key NMDA receptor co-agonist, were compared with the antidepressant response to (R,S)-ketamine treatment and were found to be significantly lower in responders than non-responders (Moaddel et al., 2015). In addition, there was a significant relationship between baseline d-serine plasma concentrations and percentage change in MADRS.
The alanine–serine–cysteine transporter 2 (ASCT2) and neutral amino acid transporter Asc-1 are involved in the cellular uptake and release of d-serine (Rosenberg et al., 2013; receptor and transporter nomenclature follows Alexander et al., 2013b). The first objective of the current study was to determine the effect of (R,S)-ketamine on the intracellular and extracellular concentrations of d-serine in PC-12 phaeochromocytoma cells, 1321N1 astrocytoma cells and primary rat neuronal cells, and to examine the role that ASCT2 and Asc-1 have in mediating (R,S)-ketamine responsiveness. The immortalized cell lines were chosen based upon previous data showing that incubation with the (R,S)-ketamine metabolite, (R,S)-dehydronorketamine, decreased the intracellular d-serine concentration in both cell lines (Singh et al., 2013) and primary neuronal cells were studied based upon the report that these cells are a major source of d-serine (Kartvelishvily et al., 2006). Of significance, (R,S)-ketamine is a chiral molecule existing as (S)-ketamine and (R)-ketamine enantiomers, with different pharmacological properties (Kohrs and Durieux, 1998; Domino, 2010; Hirota and Lambert, 2011). Moreover, (R)-ketamine exhibits a more potent and longer lasting antidepressant effect in mice than (S)-ketamine (Zhang et al., 2014). For these reasons, the experiments were designed to investigate the effect of (S)-ketamine and (R)-ketamine on d-serine synthesis and transport in immortalized cell lines and primary rat neuronal cells.
Methods
Cell lines
The PC-12 phaeochromocytoma cell line derived from rat adrenal medulla was obtained from American Type Culture Collection (Manassas, VA, USA). The human-derived 1321N1 astrocytoma cell line was obtained from European Collection of Cell Cultures (Sigma-Aldrich). DMEM with glutamine, RPMI-1640, trypsin solution, PBS, FBS, sodium pyruvate (0.1 M), l-glutamine (0.2 M) and penicillin/streptomycin solution (containing 10 000 u·mL−1 penicillin and 10 000 μg·mL−1 streptomycin) were obtained from Quality Biological (Gaithersburg, MD, USA), heat-inactivated horse serum was purchased from Biosource (Rockville, MD, USA) and HEPES buffer (1 M, pH 7.4) was obtained from Mediatech, Inc. (Manassas, VA, USA). The PC-12 cells were maintained in RPMI-1640 supplemented with 1 mM HEPES, pH 7.4, 10% horse serum, 5% FBS, 1% sodium pyruvate, 5% l-glutamine and 1% penicillin/streptomycin, and the 1321N1 cells were maintained in DMEM with l-glutamine supplemented with 10% FBS and 1% penicillin/streptomycin.
Primary neuronal cultures
Cultures of cortical and hippocampal neurons were prepared from embryonic day 18 rat brains, as described previously (Mattson et al., 1988). Dissociated neurons were plated on 60 × 15 mm tissue culture plates coated with polyethyleneimine and grown in neurobasal medium supplemented with B27 (Invitrogen, Carlsbad, CA, USA). All experimental treatments were performed in the same media on 7-day-old cultures.
Determination of intracellular and extracellular d-serine concentrations
Intracellular d-serine concentrations were measured using a previously described and validated capillary electrophoresis–laser-induced fluorescence (CE-LIF) method using a P/ACE MDQ system equipped with a LIF detector (Beckman Instruments, Fullerton, CA, USA) (Singh et al., 2012). The extracellular concentrations of d-serine were determined using a previously reported assay employing liquid chromatography with mass spectrometric detection (Xie et al., 2014), which was optimized for use in this study. In brief, the chromatographic experiments were carried out on a Shimadzu Prominence HPLC system (Shimadzu, Columbia, MD, USA) coupled to a 5500 QTRAP triple quadruple mass spectrometer equipped with a Turbo V electrospray ionization source (AB Sciex, Concord, ON, Canada). A 100 μL aliquot of the incubation media was combined with 20 μL aliquot of IS (10 μM D-arginine in acetone) and 400 μL acetone, and then centrifuged at 13 000× g for 10 min at 4°C. A 400 μL aliquot of the supernatant was derivatized with 300 μL of a 1 mM solution of (R)-1-Boc-2-piperidinecarbonyl chloride. The solution was evaporated to dryness and the residue was dissolved in 100 μL of methanol/water (10:90, v v−1) before being transferred to the autosampler for analysis. (R)-1-Boc-2-piperidinecarbonyl chloride was prepared by mixing 28 μL of a 72 mM triethylamine solution in acetone with 2 mM (R)-1-Boc-2-piperidineacetic acid in 500 μL of acetone and 500 μL of a 1 mM cyanuric chloride solution in acetone. This mixture was incubated by stirring at 1000 rpm for 3 h at 28°C. The reaction was terminated on ice and the product, (R)-1-Boc-2-piperidinecarbonyl chloride, was stored at −70°C before use. Chromatographic separation was achieved on a Zorbax Eclipse XDB-C18 column (4.6 mm × 150 mm, 5 μm; Agilent Technologies, Santa Clara, CA, USA) protected with an Agilent C18 guard column at room temperature. The mobile phase consisted of water with 0.3% trifluoroacetic acid (elute A) and methanol with 0.3% trifluoroacetic acid (elute B). The gradient eluent was set at a flow rate of 0.4 mL·min−1 and programmed as follows: 0–15 min, 5–9% B; 15–22 min, 15% B; 22–25 min, 5% B. Positive electrospray ionization data were acquired using multiple reaction monitoring (MRM). The TIS instrumental source settings for temperature, curtain gas, ion source gas 1 (nebulizer), ion source gas 2 (turbo ion spray), collision energy and ion spray voltage were 550°C, 20 psi, 45 psi, 80 psi, 15 V and 4500 V respectively. The TIS compound parameter settings for declustering potential, entrance potential and collision cell exit potential were 80, 10 and 10 V respectively. The standards were characterized using the following MRM ion transitions: D-Ser derivatization product (m/z 231.5–106.1) and d-arginine derivatization product (m/z 300.4–175.0).
Western blotting
PC-12 and 1321N1 cell lines and primary rat neuronal cells were lysed in RIPA containing EDTA and EGTA (Boston BioProducts, Ashland, MA, USA) and supplemented with a protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitor cocktail sets I and II (EMD Millipore, Billerica, MA, USA). Protein concentrations were determined in the clarified lysates using the bicinchoninic acid reagent (Thermo Fisher Scientific, Waltham, MA, USA). Proteins (20 μg per well) were separated on 4–12% precast gels (Invitrogen) using SDS-PAGE under reducing conditions and then electrophoretically transferred onto PVDF membranes (Invitrogen). Western blotting experiments were performed according to standard methods, which involved a blocking step in 5% non-fat milk/0.1% Tween-20 in PBS and incubation with the primary antibody of interest, followed by incubation with a secondary antibody conjugated with the enzyme HRP. The detection of immunoreactive bands was performed using the ECL Plus Western Blotting Detection System (GE Healthcare, Piscataway, NJ, USA). The quantification of bands was done by volume densitometry using ImageJ software (National Institutes of Health, Bethesda, MD, USA) and normalization to β-actin. The primary antibodies for the determination of monomeric and dinmeric serine racemase (Cat. No. ab45434) and β-actin (Cat. No. ab6276) were purchased from Abcam, Inc. (Cambridge, MA, USA), whereas the primary antibodies raised against the Na+-dependent alanine–serine–cysteine transporter 1 (ASCT1) (Cat. No. sc-134846), ASCT2 (Cat. No. sc-130963) and Na+-independent alanine–serine–cysteine transporter 1 (Asc1) (Cat. No. sc-292032) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibodies were used at a dilution recommended by the manufacturer.
ASCT2 silencing in PC-12 cells and treatment with (S)-ketamine and BDS
The siRNA oligos targeting ASCT2 (SMARTpool: siGENOME Rat Slc1a5 siRNA) were obtained from GE Dharmacon (Lafayette, CO, USA). PC-12 cells were seeded into 6-well plates (200 000 cells per well) and cultured for 24 h. Adherent cells were subjected to siRNA transfection using lipofectamine-RNAiMAX (Invitrogen) according to the manufacturer’s recommendations. The final concentration of siRNA in each well was 100 nM. After 24 h of transfection, culture medium containing 10% serum was added and the expression of ASCT2 determined by Western blot. In the next series of experiments, ASCT2-silenced PC-12 cells were incubated for an additional 36 h with S-ketamine (0.25 μM) and BDS (50 μM). The intracellular and extracellular d-serine levels were determined using three replicate dishes. This experiment was repeated in three independent cell preparations (n = 3).
Effects of (R)-ketamine, (S)-ketamine and BDS on intracellular and extracellular d-serine levels and expression of serine racemase in PC-12 and 1321N1 cells
Cells were seeded on 100 × 20 mm tissue culture plates and maintained at 37°C under humidified 5% CO2 in air until they reached >70% confluence. The original medium was replaced with a medium containing the test compounds and the plates were incubated for an additional 36 h, unless otherwise indicated. The medium was removed, and the cells were collected for analysis. (R)-ketamine and (S)-ketamine (0–10 μM), BDS (0–1000 μM) or the combination (S)-ketamine (0.25 μM) + BDS (50 μM) were tested. Intracellular and extracellular d-serine levels were determined, as well as the expression of monomeric and dimeric serine racemase (m-SR, d-SR). The intracellular and extracellular d-serine levels were determined in triplicate dishes, while the determination of serine racemase protein expression was carried out on one set of dishes. Both analyses were repeated in three independent cell cultures (n = 3).
Effects of (R)-ketamine, (S)-ketamine and BDS on intracellular and extracellular d-serine levels in primary rat neuronal cells
Cells were seeded on 60 × 15 mm tissue culture plates and maintained at 37°C under humidified 5% CO2 in air for 7 days, during which time neurons grew axons and dendrites, and formed synapses. The original medium was replaced with a medium containing the test compounds and the plates were incubated for an additional 36 h, unless otherwise indicated. The medium was removed, and the cells were collected for analysis. In the first series of experiments, the effects of (R)-ketamine (1.0 μM) and (S)-ketamine (0.5 μM) were determined. In the second series of experiment, (S)-ketamine (0.5 μM) and BDS (50 μM), or the combination (S)-ketamine (0.5 μM) + BDS (50 μM) were tested. The intracellular and extracellular d-serine levels were determined in triplicate dishes.
Effects of d-isoleucine and d-isoleucine plus (S)-ketamine on the intracellular and extracellular D-serine levels and expression of serine racemase in PC-12 cells
Cells were seeded on 100 × 20 mm tissue culture plates and maintained at 37°C under humidified 5% CO2 in air until they reached >70% confluence. The original medium was replaced with the medium containing the test compounds and the plates were incubated for an additional 36 h. The medium was removed, and the cells were collected for analysis. In the first series of experiments, d-isoleucine concentrations ranged from 0 to 2000 μM. In the second series of experiment, incubations were conducted using d-isoleucine (200 μM), (S)-ketamine (0.6 μM) and d-isoleucine (200 μM) plus (S)-ketamine (0–10 μM). Intracellular and extracellular d-serine levels were determined, as well as the expression of monomeric serine racemase (m-SR) and d-SR. The intracellular and extracellular d-serine levels were determined in triplicate dishes, while the determination of serine racemase protein expression was carried out on one set of dishes. Both analyses were repeated in three independent cell cultures (n = 3).
Statistical analysis
Data are presented as average relative change ± SD. All statistical analyses were performed using one-way anova and Dunnett’s test for post hoc multiple comparisons. GraphPad Prism 4 software package running on a personal computer (GraphPad Software, La Jolla, CA, USA) was used to carry out statistical analyses. P-values of 0.05 or less were considered statistically significant. The observed changes in the d-serine levels was used to determine EC50 and/or IC50 sigmoidal dose–response curves using the ‘nonlinear regression (curve fit)’ model contained within the Prism 4 software package.
Materials
(R)-Ketamine and (S)-ketamine were prepared as previously described (Moaddel et al., 2010). d-Serine, benzyl-d-serine (BDS), d-arginine, d-isoleucine, acetonitrile, methanol, trifluoroacetic acid, triethylamine, cyanuric chloride and (R)-1-Boc-2-piperidineacetic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). Deionized water was obtained from a Milli-Q system (Millipore, Billerica, MA, USA). All other chemicals used were of analytical grade.
Results
The effects of the test agents on d-serine concentration in the intracellular compartment and the incubation medium (extracellular) are presented as % change relative to the initial amount of d-serine in the respective milieu. Significant differences between data sets are set at P < 0.05, unless stated otherwise.
Incubation of PC-12 cells with (S)-ketamine (0–10 μM) produced a concentration-dependent increase in intracellular d-serine levels, with a maximum increase of 58.6 ± 7.3% and an EC50 value of 0.82 ± 0.29 μM (Figure 1A). There was a reciprocal significant decrease in extracellular d-serine levels, with a maximum decrease of 40.7 ± 3.1% and a calculated IC50 value of 0.76 ± 0.13 μM (Figure 1B). Unlike the effect observed with (S)-ketamine, incubation of PC-12 cells with (R)-ketamine (0–10 μM) produced a significant concentration-dependent decrease in the intracellular concentration of d-serine with a maximum decrease of 33.73 ± 8.1% and a calculated IC50 value of 0.94 ± 0.16 μM (Figure 1A). There was a concomitant significant decrease in the extracellular d-serine concentration with a maximum effect of 27.8 ± 2.3% and a calculated IC50 value of 0.70 ± 0.10 μM (Figure 1B).
Figure 1.
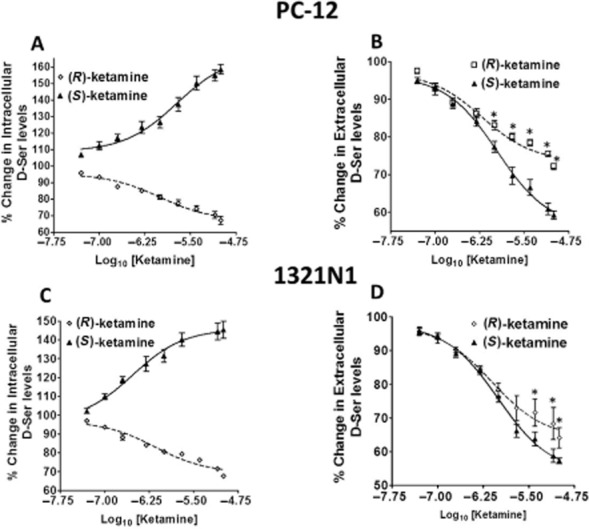
Effects of ketamine enantiomers on the cellular partitioning of d-serine (D-Ser) in PC-12 and 1321N1 cells. PC-12 (A, B) and 1321N1 (C, D) cells were incubated with increasing concentrations of (R)-ketamine (0–10 μM) or (S)-ketamine (0–10 μM) for 36 h followed by the determination of intracellular (panels A and C) and extracellular (panels B and D) d-serine levels. The EC50 and IC50 values were calculated and are presented in the Results section. The EC50 and IC50 values for (S)-ketamine, based upon the intracellular and extracellular D-serine levels in PC12 cells, were 0.82 ± 0.29 and 0.76 ± 0.13 μM respectively. In (S)-ketamine-treated 1321N1 cells, the EC50 and IC50 values were 0.46 ± 0.25 and 0.57 ± 0.32 μM respectively. IC50 values for (R)-ketamine, based upon the extracellular and intracellular D-Ser levels in PC12 cells, were 0.94 ± 0.16 and 0.70 ± 0.10 μM, whereas in 1321N1 cells, the IC50 values were 0.75 ± 0.27 and 0.88 ± 0.25 μM. Data represent the average ± SD of three independent experiments, where *P < 0.05.
Incubation of 1321N1 cells with (S)-ketamine and (R)-ketamine produced similar significant effects on the intracellular and extracellular concentrations of d-serine. (S)-Ketamine produced an increase in the intracellular d-serine concentration of 45.6 ± 8.9%, while (R)-ketamine produced a 32.3 ± 1.0% decrease with calculated EC50 and IC50 values of 0.46 ± 0.25 and 0.75 ± 0.27 μM, respectively (Figure 1C). Incubation with (S)-ketamine and (R)-ketamine resulted in decreased extracellular d-serine concentrations of 42.7 ± 2.1 and 38.3 ± 5.9%, with IC50 values of 0.57 ± 0.32 and 0.88 ± 0.25 μM, respectively (Figure 1D).
The incubation of primary rat neuronal cells obtained from cultures of cortical and hippocampal neurons with (S)-ketamine (0.5 μM) and (R)-ketamine (1.0 μM) produced the same qualitative and significant changes in the intracellular and extracellular concentrations of d-serine as observed in the immortalized cell lines. (S)-Ketamine increased the intracellular d-serine concentration by 18.7 ± 2.5% in the cortex-derived cells and by 19.8 ± 2.6% in the hippocampus-derived cells, while (R)-ketamine decreased the amount of intracellular d-serine by 32.3 ± 1.0% (cortex-derived) and 32.3 ± 1.0% (hippocampus-derived), respectively (Table 2013a). Incubation with (S)-ketamine and (R)-ketamine resulted in the lowering of the extracellular d-serine levels of 18.6 ± 1.9 and 16.4 ± 3.2% in the cortex-derived cells, and 18.7 ± 2.0 and 18.6 ± 1.9% decreases in the hippocampus-derived cells (Table 2013a).
Table 1.
The change in intracellular and extracellular d-serine concentrations in primary neuronal cells derived from rat cortex and hippocampus produced by incubation with R)-ketamine, (S)-ketamine, BDS and BDS plus (S)-ketamine
| Samples | % change in intracellular d-serine levels | % change in extracellular d-serine levels | ||
|---|---|---|---|---|
| Primary neuronal cells derived from cortex | Primary neuronal cells derived from hippocampus | Primary neuronal cells derived from cortex | Primary neuronal cells derived from hippocampus | |
| Control | 100.00 | 100.00 | 100.00 | 100.00 |
| (R)-Ket (1 μM) | −17.7 ± 2.5%* | −19.8 ± 2.6%* | −16.4 ± 3.2%* | −18.6 ± 1.9%* |
| (S)-Ket (0.5 μM) | +22.1 ± 3.5%* | +24.6 ± 2.6%* | −19.5 ± 1.9%* | −18.7 ± 2.0%* |
| BDS (50 μM) | +21.6 ± 1.9%* | +22.2 ± 1.9%* | −18.5 ± 2.8%* | −16.5 ± 1.6%* |
| BDS + (S)-Ket | +47.2 ± 5.1%* | +45.3 ± 3.4%* | −44.1 ± 3.6%* | −42.5 ± 2.4%* |
Results are expressed as % change relative to concentrations measured in control experiments Each value represents the average ± SD (n = 3).
P < 0.05 as compared with the control cells.
The data demonstrate that there is a significant enantioselective difference in the effect of (S)-ketamine and (R)-ketamine on the intracellular d-serine concentration in both PC-12 and 1321N1 cell lines. Significance was reached at (S)-ketamine and (R)-ketamine concentrations of 0.100 μM in both cell lines (Figure 1A,C). The same enantioselective property was observed in the cortex-derived and hippocampus-derived cells (Table 2013a). (S)-Ketamine and (R)-ketamine also produced distinct effects on the extracellular d-serine levels in both PC-12 and 1321N1 cells. However, these effects did not reach significance when concentrations of (S)-ketamine and (R)-ketamine lower than 2.0 μM were used in PC-12 cells, while a concentration of 4.0 μM was required to produce a significant difference between the enantiomers in 1321N1 cells (Figure 1B,D). In the studies with the cortex-derived and hippocampus-derived cells, (S)-ketamine (0.5 μM) and (R)-ketamine (1.0 μM) did not result in a significant enantioselective difference in the ketamine-associated decrease in the amount of extracellular d-serine (Table 2013a).
The expression of ASCT2 and Asc-1 was established in PC-12, 1321N1, cortex-derived and hippocampus-derived cells by Western blotting, using lysate from the C6 glioblastoma cell line as positive control (Sikka et al., 2010) (Figure 2). The data established the presence of these transporters, as well as ASCT1, in each of the cell types studied. However, the specific interaction between ASCT1 and (S)-ketamine and (R)-ketamine was not pursued because of previous reports indicating that Asc-1 and ASCT2 are primarily responsible for d-serine transport in retinal Müller cells, neuronal cells and astrocytes (Dun et al., 2007; Rosenberg et al., 2013; Martineau et al., 2014).
Figure 2.
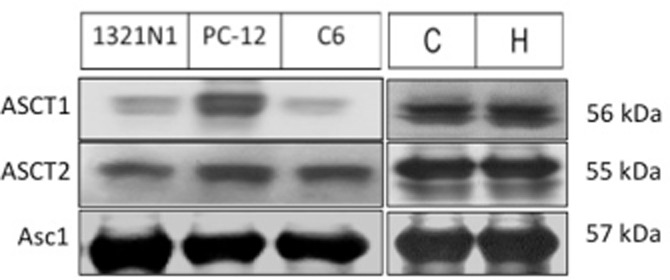
Representative immunoblots depicting the expression of the d-serine transporters ASCT2 and Asc1 in immortalized 1321N1 and PC-12 cells, and primary cultures of rat neuronal cells isolated from the cortex (C) and hippocampus (H). C6 glioma cell lysates were used as a positive control. The molecular mass of each transporter (in kDa) is indicated on the right.
To assess the contribution of ASCT2 to the control of d-serine transport, PC-12, 1321N1, cortex-derived and hippocampus-derived cells were incubated with BDS, a selective ASCT2 competitive inhibitor (Grewer and Grabsch, 2004). Treatment of PC-12 and 1321N1 cells with BDS (0–1000 μM) produced significant concentration-dependent increases in the intracellular d-serine levels, with maximum increases of 35.3 ± 3.8 and 38.2 ± 8.0% and corresponding EC50 values of 53.9 ± 6.5 and 38.1 ± 4.7 μM, respectively (Figure 3A,B). There were corresponding decreases in the extracellular d-serine concentrations, with maximum decreases of 25.2 ± 9.8% (PC-12) and 23.2 ± 9.5% (1321N1) and IC50 values of 64.9 ± 18.3 and 52.5 ± 13.2 μM, respectively (Figure 3A,B).
Figure 3.
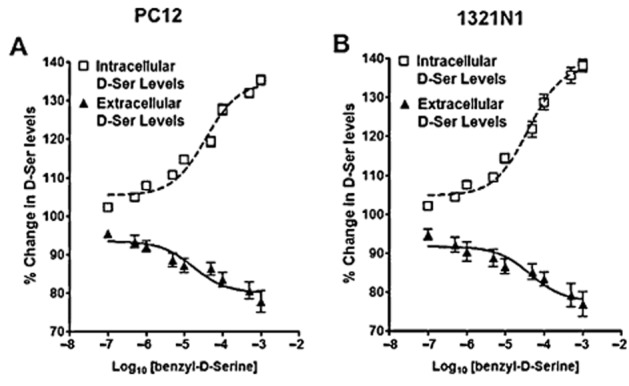
Effect of benzyl-d-serine (BDS) on the cellular partitioning of d-serine in PC-12 and 1321N1 cells. PC-12 (A) and 1321N1 cells (B) were incubated with increasing concentrations of the ASCT2 inhibitor BDS (0–1000 μM) for 36 h, followed by the determination of the intracellular and extracellular d-serine contents. The EC50 and IC50 values were calculated and presented in the Results section. The EC50 and IC50 values for BDS in PC-12 cells, based upon the extracellular and intracellular D-Ser levels, were 64.95 ± 1.83 and 53.92 ± 6.53 μM, whereas in 1321N1 cells, the IC50 values were 38.16 ± 8.01 and 52.50 ± 13.12 μM. Data represent the average ± SD of three independent experiments.
The incubation of PC-12 cells with 0.5 μM (S)-ketamine (the approximate EC50 − IC50 value) produced a significant 26.1 ± 1.9% increase in the intracellular d-serine levels and a 17.9 ± 1.9% decrease in the extracellular d-serine concentration (see Figure 1A,B). The incubation of PC-12 cells with 50 μM BDS (the approximate EC50 − IC50 value) produced a 27.4 ± 5.1% increase in the amount of intracellular d-serine and a 14.8 ± 3.8% decrease in the extracellular d-serine levels (see Figure 3A). Co-incubation with (S)-ketamine (0.5 μM) and BDS (50 μM) resulted in a 47.8 ± 0.9% increase in the intracellular d-serine concentration and a 34.5 ± 3.7% decrease in the extracellular d-serine concentration (data not shown), indicating that the effects of (S)-ketamine and BDS were additive.
The incubation of primary cortical and hippocampal neuronal cells with BDS (50 μM) produced the same qualitative and significant changes in the intracellular and extracellular concentrations of d-serine as observed in the immortalized cell lines. BDS increased the amount of intracellular d-serine by 21.6 ± 1.9% in the cortex-derived cells and 22.2 ± 1.9% in the hippocampus-derived cells, and decreased the extracellular d-serine levels by 18.5 ± 2.8 and 16.5 ± 1.6%, respectively (Table 2013a). Co-incubation with (S)-ketamine (0.5 μM) and BDS (50 μM) resulted in 47.2 ± 5.1% (cortex) and 45.3 ± 3.4% (hippocampus) increases in the intracellular d-serine levels, and corresponding 44.1 ± 3.6 and 42.5 ± 2.4% decreases in the extracellular d-serine concentrations (Table 2013a), indicating that the effects of (S)-ketamine and DBS were also additive in these cells.
The interaction between (S)-ketamine and BDS was further investigated in PC-12 cells by the co-incubation of BDS (50 μM) with (S)-ketamine concentrations ranging from 0.1 to 10 μM. The resulting effect on the intracellular and extracellular d-serine levels was then determined (Figure 4A,B). The presence of BDS in the incubation media shifted the concentration–response curves produced by (S)-ketamine to the left and resulted in approximately threefold reductions in the EC50 and IC50 values to 0.28 ± 0.02 and 0.27 ± 0.01 μM respectively.
Figure 4.
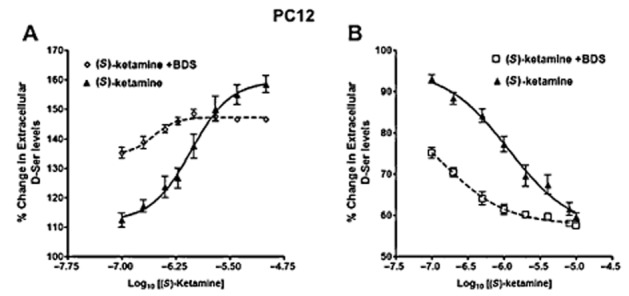
Interaction between (S)-ketamine and benzyl-d-serine (BDS) in PC-12 cells. Cells were incubated with increasing concentrations of (S)-ketamine (0–10 μM) in the presence or absence of BDS (50 μM) for 36 h followed by the determination of intracellular (panel A) and extracellular (panel B) d-serine content. Data represent the average ± SD of three independent experiments.
In the next series of experiments, ASCT2 gene knockdown was accomplished in PC-12 cells using a pool of siRNA. An initial experiment established that the expression of ASCT2 protein was significantly reduced (P < 0.01) after 24 h of siRNA treatment without an effect on β-actin expression (Figure 5A). Reducing the expression of ASCT2 produced a significant 14.1 ± 2.2% increase in the intracellular d-serine concentrations (Figure 5B) and lower extracellular d-serine levels (12.4 ± 1.9%; Figure 5C), while cell treatment with the transfection reagent alone had no effect (data not shown). Incubation with BDS (50 μM) and (S)-ketamine (0.50 μM) produced the same significant increases in the intracellular d-serine content and corresponding decreases in the extracellular concentration of d-serine as those observed in the siRNA-treated cells and without additive effects (Figure 5B,C). Thus, ASCT2 silencing conferred refractoriness to BDS and (S)-ketamine signalling with regard to the cellular accumulation and export of d-serine.
Figure 5.
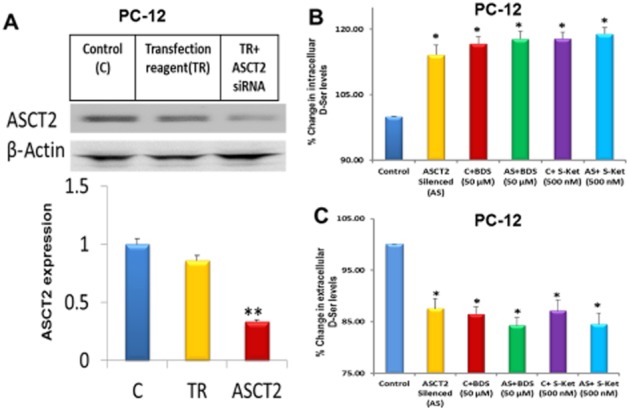
Effect of ASCT2 silencing on the cellular response to benzyl-d-serine (BDS) and (S)-ketamine. (A) Top panel: Representative autoradiogram depicting the expression of ASCT2 and β-actin proteins after siRNA-mediated ASCT2 knockdown in PC-12 cells. Bottom panel: bars represent the relative expression levels of ASCT2 after normalization with β-actin. (B, C) Effect of BDS (50 μM) and (S)-ketamine (250 nM) on the intracellular (panel B) and extracellular (panel C) d-serine levels in both control, untransfected PC-12 cells (C), cells incubated with transfection reagent alone (TR), and ASCT2-silenced PC-12 cells (AS). Data represent the average ± SD of three independent experiments. *P < 0.05; **P < 0.01 as compared with the control cells.
The contribution of the Asc-1 transporter to the observed effects produced by (S)-ketamine and BDS was investigated using d-isoleucine, a selective agonist of Asc-1 antiporter activity (Rosenberg et al., 2013). Incubation of PC-12 cells with increasing concentrations of d-isoleucine (0–2000 μM) produced a concentration-dependent increase in the intracellular d-serine levels with a calculated EC50 value of 197.20 ± 6.84 μM (Figure 6A). There were corresponding decreases in the amount of extracellular d-serine with an IC50 value of 179.6 ± 9.88 μM (Figure 6B). Incubation of PC-12 cells with 200 μM d-isoleucine (the ∼EC50/IC50 concentration) decreased the intracellular d-serine concentration by 19 ± 2%, while incubation with 0.6 μM d-isoleucine (the ∼EC50/IC50 concentration) increased the intracellular d-serine levels by 22 ± 4% (Table 2013b). Co-incubation with d-isoleucine (200 μM) and (S)-ketamine (0.6 μM) produced a 16 ± 2% decrease in intracellular d-serine, which was slightly lower, albeit significant, relative to the decrease produced by d-isoleucine alone. Incubation of PC-12 cells with 200 μM d-isoleucine increased the extracellular d-serine concentration by 21 ± 2%, while incubation with 0.6 μM (S)-ketamine decreased the amount of extracellular d-serine by 20 ± 4% (Table 2013b). Co-incubation with d-isoleucine (200 μM) and (S)-ketamine (0.6 μM) produced a 17 ± 1% increase, which was slightly lower, albeit significant, than the effect produced by d-isoleucine alone.
Figure 6.
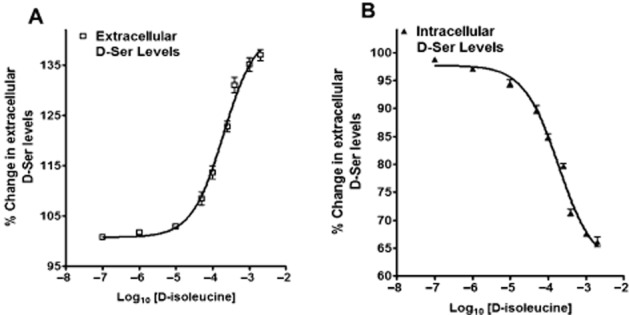
Effect of d-isoleucine on the cellular partitioning of d-serine in PC-12 cells. Cells were incubated with increasing concentrations of d-isoleucine (0–2000 μM) for 36 h followed by the determination of (A) extracellular and (B) intracellular D-serine levels. Data represent the average ± SD of three independent experiments.
Table 2.
The change in intracellular and extracellular d-serine concentrations in PC-12 cells produced by incubation with D-isoleucine and (S)-ketamine
| Samples | % change in intracellular d-serine levels | % change in extracellular d-serine levels |
|---|---|---|
| Control | 100.00 | 100.00 |
| d-IL (200 μM) | −19.2 ± 1.8%* | +21.4 ± 1.7%* |
| S-Ket (0.5 μM) | +22.0 ± 3.5%* | −20.2 ± 3.6%* |
| d-IL + S-Ket | −15.7 ± 1.9%* | +16.6 ± 0.7%* |
The results are expressed as % change relative to concentrations measured in control experiments. Each value represents the average ± SD (n = 3).
P < 0.05 as compared with the control cells.
Western blotting analysis established that (S)-ketamine and (R)-ketamine produced significant increases in the monomeric form of serine racemase (m-SR) protein in PC-12 and 1321N1 cells (Figure 7). In PC-12 cells, the maximum increase (∼2.0-fold) in m-SR expression was observed at 4 and 0.2 μM for (R)-ketamine and (S)-ketamine, respectively (Figure 7A,B). Similarly, the maximum effects on m-SR expression were observed at 2 μM for (R)-ketamine and 0.5 μM for (S)-ketamine in 1321N1 cells (Figure 7C,D). The data indicate that (S)-ketamine and (R)-ketamine produced U-shaped concentration-dependent responses on m-SR expression and that the effect was enantiospecific, with (S)-ketamine being the more potent enantiomer. Treatment with BDS or d-isoleucine and the siRNA-mediated attenuation of ASCT2 expression had no effect on m-SR expression in these two immortalized cell lines (data not shown). Incubation of cortex-derived cells and hippocampus-derived cells with (R)-ketamine (1 μM) increased the expression of m-SR by ∼1.5-fold, while an ∼2.0-fold increase was observed with (S)-ketamine (0.5 μM) (Figure 7E,F). The expression of d-SR was not affected by any of the treatments used in this study (data not shown).
Figure 7.
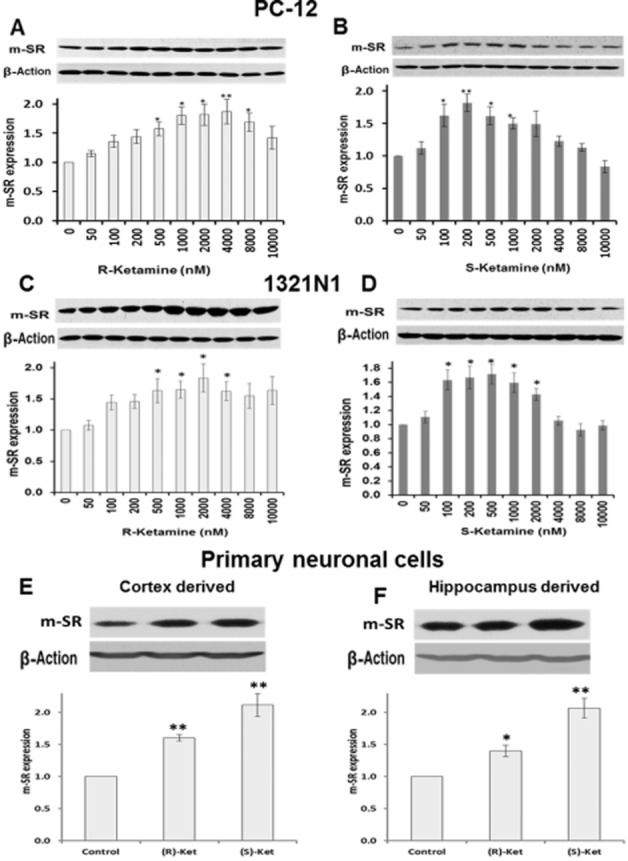
Effect of (R)-ketamine and (S)-ketamine on the expression of monomeric serine racemase protein. (A–D) Thirty-six hours after treatment with the indicated concentrations of (R)-ketamine (panels A and C) or (S)-ketamine (panels B and D), PC-12 (panels A and B) and 1321N1 (panels C and D) cell lysates were prepared and then immunoblotted with anti-serine racemase (m-SR) antibody. Relative levels of m-SR after quantification and normalization with β-actin are shown in the bars. (E, F), Primary cultures of rat neuronal cells isolated from cortex (panel E) and hippocampus (panel F) were treated with vehicle, (R)-ketamine (1 μM) or (S)-ketamine (0.5 μM) for 36 h and then processed for m-SR immunoblot analysis. Data represent the average ± SD of three independent experiments. *P < 0.05; **P < 0.01 as compared with the control cells.
Discussion and conclusions
The anaesthetic and antinociceptive properties of (R,S)-ketamine have been associated with direct non-competitive inhibition of the NMDA receptor through binding at the receptor’s PCP-binding site (Kohrs and Durieux, 1998; Hirota and Lambert, 2011). (S)-ketamine displays an approximately threefold higher affinity for the NMDA receptor relative to (R)-ketamine, and this variance occurs in conjunction with clinical differences between the two enantiomers, including the dissociative effects associated with the sub-anaesthetic dose of the drug (Vollenweider et al., 1997; Persson et al., 2002; Domino, 2010). In particular, studies in healthy volunteers indicate that (S)-ketamine produces more profound dissociative effects, ego-disintegration and hallucinatory phenomena than (R)-ketamine (Vollenweider et al., 1997; Persson et al., 2002).
Although the direct inhibition of NMDA receptor activity and the resulting NMDA receptor hypofunction, the ‘hypoglutamatergic hypothesis’, is the accepted explanation of the psychosis-inducing effect of (R,S)-ketamine in healthy volunteers (Pomarol-Clotet et al., 2006), an indirect attenuation of NMDA receptor function via the down-modulation of d-serine plasma concentrations could also explain the increase in CADSS scores (Moaddel et al., 2015), as illustrated in Figure 8A. This hypothesis is based upon the function of d-serine as a key and potent NMDA receptor co-agonist (Wolosker et al., 2008). Synaptic NMDA receptors have a preferential affinity for d-serine relative to the NMDA receptor co-agonist glycine, and d-serine plays a key role in LTP and NMDA-induced neurotoxicity (Henneberger et al., 2010; 2013; Papouin et al., 2012). In addition, subjects with schizophrenia have significantly lower baseline d-serine plasma concentrations relative to healthy controls and there is a negative association of d-serine plasma levels with severity of the symptoms of the disease (Hashimoto et al., 2003; Calcia et al., 2012).
Figure 8.
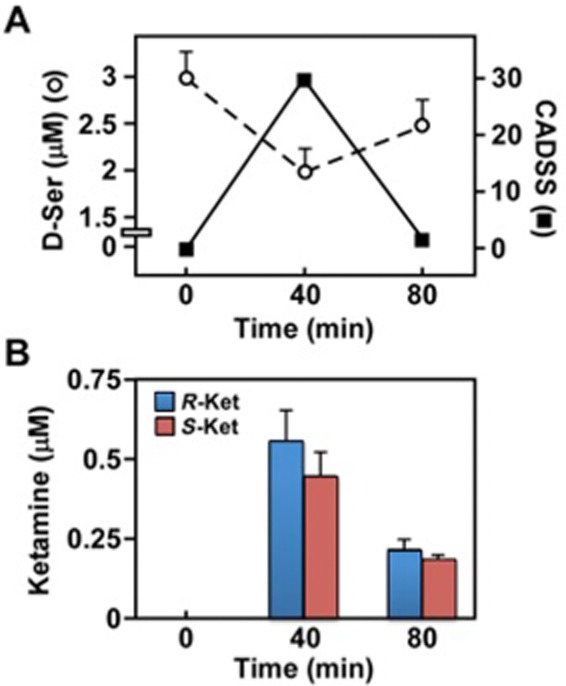
Correlation between CADSS score, plasma d-serine concentration and (R)- and (S)-ketamine levels in MDD patients, based upon the data reported by Moaddel et al. (2015). (A) Left Y-axis: The effect of a 40 min i.v. infusion of 0.5 mg·kg−1 (R,S)-ketamine on the plasma concentration of d-serine in MDD patients was determined from baseline post-infusion levels. Right Y-axis: Changes in the average CADSS scores over time in MDD patients following administration of (R,S)-ketamine. (B) The plasma concentrations of (R)-ketamine and (S)-ketamine following a 40 min i.v. infusion of 0.5 mg·kg−1 (R,S)-ketamine in MDD patients were determined from baseline post-infusion levels.
The effect of d-serine on LTP has been linked to its release from astrocytes (Henneberger et al., 2010; Kang et al., 2013), and d-serine release from primary neuronal cultures obtained from rat cortex and hippocampus has been associated with NMDA receptor activation (Kartvelishvily et al., 2006). d-Serine release is mediated by ASCT2 and Asc-1 (Sikka et al., 2010; Maucler et al., 2013; Rosenberg et al., 2013; Martineau et al., 2014). In particular, the Asc-1-mediated release of neuronal d-serine has been shown to play a key role in LTP in rat and mouse models (Rosenberg et al., 2013), while ASCT2 plays an important role in d-serine release from astrocytes and postsynaptic neurons (Martineau et al., 2014).
In the current study, we have investigated the effect of (S)-ketamine and (R)-ketamine on ASCT2- and Asc-1-mediated cellular export of d-serine in PC-12 and 1321N1 cells and in primary neuronal cultures obtained from rat cortex and hippocampus. The immortalized cell lines and primary neuronal cells expressed Asc-1 and ASCT2 proteins. Incubation of PC-12 and 1321N1 cells with (R)-ketamine produced a concentration-dependent decrease in the intracellular and extracellular d-serine levels. Moreover, incubation of the cortex-derived and hippocampus-derived primary neuronal cells with (R)-ketamine (1.0 μM) also decreased the amount of intracellular and extracellular d-serine. The observed reductions were consistent with previous studies showing that the diminution in the intracellular d-serine concentrations was associated with the inhibition of the de novo synthesis of d-serine by the ketamine metabolites (R,S)-norketamine, (R,S)-dehydronorketamine and (2S,6S)-hydroxynorketamine (Singh et al., 2013; Paul et al., 2014). This effect was attributed to the non-competitive inhibition of the α7 and α3β4 subtypes of the nicotinic acetylcholine (nACh) receptor. These nACh receptor subtypes are expressed in PC-12 and 1321N1 cells (Singh et al., 2013) and Western blot analysis of the cortex-derived and hippocampus-derived neuronal cells used in this study confirmed the presence of the α7 and α3 nACh receptor subunits (data not shown). (R,S)-ketamine has been characterized as a non-competitive inhibitor of the α7 nACh receptor (Coates and Flood, 2001) and α3β4 subtypes (Moaddel et al., 2013), and (R)-ketamine and (S)-ketamine have been identified as non-competitive inhibitors in PC-12 cells (Sasaki et al., 2000) and human neuroblastoma SH-SY5Y cells (Friederich et al., 2000); the latter cell type also expressing α7 and α3β4 nACh receptors (Dajas-Ballador et al., 2002; Dunckley and Lukas, 2006). Thus, the inhibition of d-serine synthesis by (R)-ketamine in the immortalized and primary cells was expected and consistent with previous data.
Since (S)-ketamine is also a non-competitive nACh receptor inhibitor, it was assumed that (S)-ketamine would also produce a concentration-dependent decrease in the intracellular d-serine concentrations. Unexpectedly, (S)-ketamine induced a concentration-dependent increase in the intracellular d-serine levels both in immortalized PC-12 and 1321N1 cells and following incubation of the cortex-derived and hippocampus-derived primary neuronal cells with (S)-ketamine (0.5 μM). The corresponding decrease in the extracellular d-serine levels suggested that the intracellular and extracellular changes in d-serine might be associated with the inhibition of the active export of the compound by Asc-1 and/or ASCT2. This mechanism was investigated using the specific ASCT2 inhibitor BDS (Grewer and Grabsch, 2004) in all of the experimental cells and ASCT2 gene knockdown in PC-12 cells. Both approaches produced the same change in the intracellular/extracellular d-serine distribution in response to (S)-ketamine. The results establish that (S)-ketamine reduces d-serine transport through ASCT2 inhibition, while (R)-ketamine has no effect.
The action of (S)-ketamine on ASCT2 transport was further examined by co-incubation of PC-12 cells with (S)-ketamine and BDS using the approximate EC50 − IC50 values of both compounds. An apparent additive increase in the amount of intracellular d-serine with a corresponding reduction in the extracellular concentrations of d-serine was observed. The incubation of primary cortical and hippocampal neuronal cells with either BDS or (S)-ketamine alone and in combination produced the same qualitative and significant changes in the intracellular and extracellular levels of d-serine as observed in the immortalized cell lines. The data suggest that both compounds are competitive inhibitors of ASCT2 vis-à-vis d-serine transport. The interaction between (S)-ketamine and BDS was further investigated in PC-12 cells by the co-incubation of BDS (50 μM) with (S)-ketamine concentrations ranging from 0.1 to 10 μM. The presence of BDS in the incubation media shifted the concentration–response curves produced by (S)-ketamine to the left and resulted in approximately threefold reductions in the EC50 (increase in the intracellular d-serine concentrations) and IC50 values (decrease in the extracellular d-serine levels). The results of these studies indicate that the inhibition of ASCT2 transport of d-serine by (S)-ketamine is multimodal with both competitive and high affinity non-competitive inhibition of ASCT2.
The data from this study also demonstrate that incubation with (R)-ketamine and (S)-ketamine resulted in a significant increase in the m-SR expression with an inverted U-shaped dose–response curve in all the experimental cell types. (S)-ketamine was ∼10-fold more potent than (R)-ketamine in PC-12 and 1321N1 cells, and similar enantioselectivity was observed in the cortex-derived and hippocampus-derived primary neuronal cells as incubation with (S)-ketamine (0.5 μM) produced a significantly greater increase in the expression of m-SR than (R)-ketamine (1.0 μM). The results are consistent with our previous findings, which showed that the incubation of PC-12 cells with (R,S)-ketamine concentrations increased the m-SR expression via activation of the mammalian target of rapamycin (mTOR) pathway (Paul et al., 2014). The increase in de novo protein synthesis was initiated by non-competitive allosteric inhibition of the α7-nACh receptor (Singh et al., 2013; Paul et al., 2014), a process that was blocked by co-incubation with (S)-nicotine (Paul et al., 2014). The data presented herein suggest that the antagonistic effect of ketamine at nACh receptors is enantioselective, with (S)-ketamine being the more potent inhibitor. Earlier reports have demonstrated that (S)-ketamine is an approximately fourfold more potent inhibitor of nACh receptor activity than (R)-ketamine in human SH-SY5Y neuroblastoma cells (Friederich et al., 2000), while Sasaki et al. (2000) found no significant difference between ketamine enantiomers in PC-12 cells. Both of these studies were conducted as part of the investigations into the anaesthetic effect of ketamine and may have missed enantioselective differences at the lower drug concentrations used in antidepressant therapy.
The modulation in the m-SR expression by both (S)-ketamine and (R)-ketamine indicates that these isomers should produce similar reductions in the intracellular and extracellular d-serine concentrations through the inhibition of nACh receptors. This is difficult to observe even though dramatic and opposite concentration-dependent changes in the intracellular d-serine concentrations were noted in PC-12 and 1321N1 cells. However, the enantioselective effect on the extracellular d-serine levels is more subtle and quantitative. While both (S)-ketamine and (R)-ketamine had a significantly different effect on the extracellular d-serine concentrations, these effects did not reach significance in the PC-12 cells until a 2.0 μM concentration of (S)-ketamine and (R)-ketamine, and, in 1321N1 cells, a concentration of 4.0 μM was required to produce a significant difference between the enantiomers (Figure 1B,D). These results suggest that the effect of (S)-ketamine on the amount of extracellular d-serine is due to both the reduction in intracellular synthesis and the inhibition of active export.
Previous studies have determined that d-serine release from primary neuronal cultures and immortalized cell lines is primarily mediated by Asc-1 (Kartvelishvily et al., 2006; Sikka et al., 2010; Maucler et al., 2013; Rosenberg et al., 2013; Martineau et al., 2014). d-isoleucine is an Asc-1 agonist that increases cellular export of d-serine (Rosenberg et al., 2013). As expected, incubation of PC-12 cells with d-isoleucine led to a significant decrease in intracellular d-serine and a corresponding increase in the extracellular d-serine levels. Co-incubation of PC-12 cells with d-isoleucine (200 μM) and (S)-ketamine (0.6 μM), the approximate IC50/EC50 concentrations of the two agents, produced a slight, but significant, attenuation of the d-isoleucine response; moreover, increasing the concentration of (S)-ketamine to 10 μM did not alter d-isoleucine responsiveness (data not shown). The results suggest that (S)-ketamine does not directly compete with d-isoleucine and that the observed reduction in d-isoleucine actions stemmed from the pharmacological inhibition of ASCT2 by (S)-ketamine. Similar results were observed when BDS was used as co-incubate (data not shown).
The ∼20–25% decrease in d-serine plasma levels observed at the end of the (R,S)-ketamine infusion in MDD patients appears to be clinically relevant and is associated with a corresponding increase in the CADDS scores of these patients (Moaddel et al., 2015) (Figure 8A). Moreover, the rapid fall in the CADSS scores between the 40 and 80 min sampling points was associated with the rapid plasma clearance of (S)-ketamine (an ∼50% drop during that time period) (Moaddel et al., 2015) (Figure 8B), suggesting the contribution of (S)-ketamine in this effect. (R,S)-ketamine is extensively metabolized by microsomal enzymes with a major metabolic pathway involving N-demethylation to norketamine and further transformation to (R,S)-dehydronorketamine and a series of diastereomeric hydroxynorketamines (Kharasch and Labroo, 1992; Portmann et al., 2010; Desta et al., 2012). It is possible that one or more of these metabolites also contribute to the rapid drop in d-serine plasma concentrations. A recent study examined the contribution of (S)-norketamine to (S)-ketamine-induced acute pain relief and neurocognitive impairment in healthy volunteers and concluded that (S)-norketamine made no contribution to the cognitive impairment produced by the administration of (S)-ketamine (Olofsen et al., 2012). We have also demonstrated that (R,S)-dehydronorketamine reduces the intracellular d-serine concentrations (Singh et al., 2013) and preliminary data from recent studies indicate that the individual stereoisomers of norketamine, dehydronorketamine and hydroxynorketamine attenuate the intracellular d-serine concentrations and, like (R)-ketamine, have no effect on d-serine transport by ASCT2 (unpublished data). In addition, previous studies in MDD patients indicate that d-serine plasma levels return to the approximate pre-dose level by 80–120 min post-dosing and then slowly decrease over a 7 day period to an average of 39% decrease in patients who respond to (R,S)-ketamine treatment and 28% in patients who do not respond (Moaddel et al., 2015). Thus, it appears that d-serine plasma concentrations are affected by two independent mechanisms, an immediate and steep decrease associated with (S)-ketamine inhibition of ASCT2-mediated transport and a longer, gradual decrease caused by ketamine and ketamine metabolite inhibition of nACh receptors and the resulting decrease in SR activity.
The data from this study expand our understanding of the clinically relevant mechanisms associated with the use of (R,S)-ketamine in the treatment of depression. The additional insight is related to the dissociative effect of the drug through the selective inhibition of ASCT2 by (S)-ketamine, as illustrated in Figure 9. This property of (S)-ketamine may be associated with the increase in the cerebral metabolic rates of glucose in the frontal cortex and ego-disintegration and hallucinatory phenomena produced by the drug. In contrast, the lack of ASCT2 inhibitory activity by (R)-ketamine may be reflected in the development of a state of relaxation (Vollenweider et al., 1997). A recent report has suggested that (R)-ketamine may be a better antidepressant than (S)-ketamine (Zhang et al., 2014). Our study did not investigate the relative antidepressant efficacy of (S)-ketamine and (R)-ketamine and, therefore, the data provide no insight into the overall clinical response. However, the results indicate that the treatment-associated dissociative effects observed with the administration of (R,S)-ketamine might be reduced by utilization of the (R)-ketamine alone and provide a mechanistic basis for this hypothesis.
Figure 9.
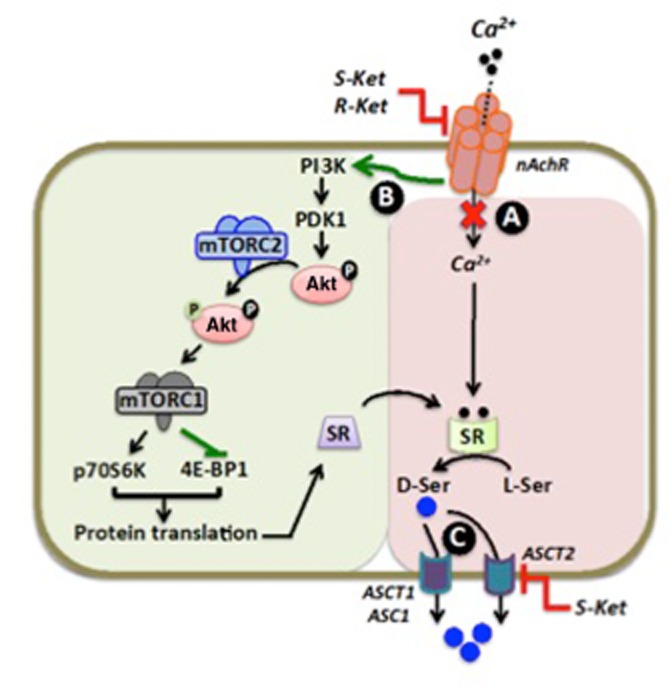
Schematic representation of the regulation of endogenous d-serine level. (A) Inhibition of nACh receptors by (R)-ketamine and (S)-ketamine attenuates the entry of extracellular Ca2+. (B) Activation of the PI3K/Akt/mTOR pathway increases serine racemase (SR) expression; however, SR activity is reduced due to a decrease in intracellular Ca2+. (C) The contribution of the neutral amino acid transporters, ASCT2, ASCT1 and Asc1, to the export of d-serine and the enantioselective inhibition of ASCT2 by (S)-ketamine is also depicted.
Acknowledgments
This work was supported by funding from the Intramural Research Program of the National Institute on Aging/NIH and by NIA Contract No. HHSN271201000008I.
Glossary
- BDS
benzyl-d-serine
- CADSS
Clinician-Administered Dissociative States Scale
- d-IL
d-isoleucine
- Ket
(R,S)-ketamine
- MADRS
Montgomery–Åsberg Depression Rating Scale
- MDD
major depressive disorder
- m-SR
monomeric serine racemase
- R-Ket
(R)-ketamine
- S-Ket
(S)-ketamine
Author contributions
N. S. S. did the experimental design and execution, interpretation of data and manuscript preparation. M. B. contributed conceptually and assisted in manuscript preparation. M. A. K. and R. M. provided technical support. S. C. and M. P. M. provided primary culture of rat neuronal cells. I. W. W contributed conceptually, did the interpretation of data, manuscript preparation and was the project leader.
Conflict of interest
The authors declare the absence of conflict of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: ligand-gated ion channels. Br J Pharmacol. 2013a;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to PHARMACOLOGY 2013/14: transporters. Br J Pharmacol. 2013b;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia MA, Maderia C, Alheira FV, Silva TCS, Tannos FM, Vargas-Lopes C, et al. Plasma levels of d-serine in Brazilian individuals with schizophrenia. Schizophr Res. 2012;142:83–87. doi: 10.1016/j.schres.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Coates KM, Flood P. Ketamine and its preservative benzethonium chloride both inhibit human recombinant α7 and α4β2 neuronal nicotinic acetylcholine receptors in Xenopus oocytes. Br J Pharmacol. 2001;134:871–879. doi: 10.1038/sj.bjp.0704315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Ballador F, Mogg A, Wonnacott S. Intracellular Ca2+ signals evoked by stimulation of nicotinic acetylcholine receptors in SH-SY5Y cells: contribution of voltage-operated Ca+2 channels and Ca+2 stores. J Neurochem. 2002;81:606–614. doi: 10.1046/j.1471-4159.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SLV, et al. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012;42:1076–1087. doi: 10.3109/00498254.2012.685777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113:678–686. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- Dun Y, Mysona B, Itagaki S, Martin-Studdard A, Ganapathy V, Smith SB. Functional and molecular analysis of d-serine transport in retinal Müller cells. Exp Eye Res. 2007;84:191–199. doi: 10.1016/j.exer.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley T, Lukas RJ. Nicotinic modulation of gene expression in SH-SY5Y neuroblastoma cells. Brain Res. 2006;1116:39–49. doi: 10.1016/j.brainres.2006.07.111. [DOI] [PubMed] [Google Scholar]
- Friederich P, Dybek A, Urban BW. Stereospecific interaction of ketamine with nicotinic acetylcholine receptors in human sympathetic ganglion-like SH-SY5Y cells. Anesthesiology. 2000;93:818–824. doi: 10.1097/00000542-200009000-00032. [DOI] [PubMed] [Google Scholar]
- Grewer C, Grabsch E. New inhibitors for the neutral amino acid transporter ASCT2 reveal its Na+-dependent anion leak. J Physiol. 2004;557(Pt 3):747–759. doi: 10.1113/jphysiol.2004.062521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, et al. Decreased serum levels of d-serine in patients with schizophrenia. Evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 2003;60:572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of d-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Bard L, King C, Jennings A, Rusahov DA. NMDA receptor activation: two targets for two co-agonists. Neurochem Res. 2013;38:1156–1162. doi: 10.1007/s11064-013-0987-2. [DOI] [PubMed] [Google Scholar]
- Hirota K, Lambert DG. Ketamine: new uses for an old drug? Br J Anaesth. 2011;107:123–126. doi: 10.1093/bja/aer221. [DOI] [PubMed] [Google Scholar]
- Kang N, Peng H, Yu Y, Stanton PK, Guilarte TR, Kang J. Astrocytes release d-serine by a large vesicle. Neuroscience. 2013;240:243–257. doi: 10.1016/j.neuroscience.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived d-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J Biol Chem. 2006;281:14151–14162. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Labroo R. Metabolism of ketamine stereoisomers by human liver microsomes. Anesthesiology. 1992;77:1201–1207. doi: 10.1097/00000542-199212000-00022. [DOI] [PubMed] [Google Scholar]
- Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, et al. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau M, Parpura V, Mothet JP. Cell-type specific mechanisms of d-serine uptake and release in the brain. Front Synaptic Neurosci. 2014;6:1–12. doi: 10.3389/fnsyn.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Dou P, Kater SB. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci. 1988;8:2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maucler C, Pernot P, Vasylieva N, Pollegioni L, Marinesco S. In vivo d-serine hetero-exchange through alanine-serine-cysteine (ASC) transporters detected by microelectrode biosensors. ACS Chem Neurosci. 2013;4:772–781. doi: 10.1021/cn4000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Venkata SL, Tanga MJ, Bupp JE, Green CE, Iyer L, et al. A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta. 2010;82:1892–1904. doi: 10.1016/j.talanta.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698:228–234. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Luckenbaugh DA, Xie Y, Villaseñor A, Brutsche NE, Machado-Vieira R, et al. d-serine plasma concentration is a potential biomarker of (R,S)-ketamine antidepressant response in subjects with major depressive disorder. Psychopharmacology (Berl) 2015;232:399–409. doi: 10.1007/s00213-014-3669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsen E, Sigtermans M, Noppers I, Niesters M, Mooren R, Bauer M, et al. The dose-dependent effect of S(+)-ketamine on cardiac output in healthy volunteers and complex regional pain syndrome type 1 chronic pain patients. Anesth Analg. 2012;115:536–546. doi: 10.1213/ANE.0b013e31825496f6. [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, et al. (R,S)-Norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin (mTOR) function. Anesthesiology. 2014;121:149–159. doi: 10.1097/ALN.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Hasselstrom J, Maurset A, Oye I, Svensson JO, Almqvist O, et al. Pharmacokinetics and non-analgesic effects of S- and R-ketamines in healthy volunteers with normal and reduced metabolic capacity. Eur J Clin Pharmacol. 2002;57:869–875. doi: 10.1007/s002280100353. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Honey GD, Murray GK, Corlett PR, Absalom AR, Lee M, et al. Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry. 2006;189:173–179. doi: 10.1192/bjp.bp.105.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portmann S, Kwan HT, Theurillat R, Schmitz A, Mevissen M, Thormann W. Enantioselective capillary electrophoresis for the identification and characterization of human cytochrome P450 enzymes, which metabolize ketamine and norketamine in vitro. J Chromatogr A. 2010;1217:7942–7948. doi: 10.1016/j.chroma.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Rosenberg D, Artoul S, Segal AC, Kolodney G, Radzishevsky I, Dikopoltsev E, et al. Neuronal d-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J Neurosci. 2013;33:3533–3544. doi: 10.1523/JNEUROSCI.3836-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Andoh T, Watanabe I, Kamiya Y, Itoh H, Higashi T, et al. Nonstereoselective inhibition of neuronal nicotinic acetylcholine receptors by ketamine. Anesth Analg. 2000;91:741–748. doi: 10.1097/00000539-200009000-00046. [DOI] [PubMed] [Google Scholar]
- Sikka P, Walker R, Cockayne R, Wood MJ, Harrison PJ, Burnet PW. d-serine metabolism in C6 glioma cells: involvement of alanine-serine-cysteine transporter (ASCT2) and serine racemase (SRR) but not D-amino acid oxidase (DAO) J Neurosci Res. 2010;88:1829–1840. doi: 10.1002/jnr.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NS, Paul RK, Sichler M, Moaddel R, Bernier M, Wainer IW. Capillary electrophoresis-laser-induced fluorescence (CE-LIF) assay for measurement of intracellular d-serine and serine racemase activity. Anal Biochem. 2012;421:460–466. doi: 10.1016/j.ab.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NS, Paul RK, Ramamoorthy A, Torjman MC, Moaddel R, Bernier M, et al. Nicotinic acetylcholine receptor antagonists alter the function and expression of serine racemase in PC-12 and 1321N1 cells. Cell Signal. 2013;25:2634–2646. doi: 10.1016/j.cellsig.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur Neuropsychopharmacol. 1997;7:25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- White PF, Way WL, Trevor AJ. Ketamine – its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119–136. doi: 10.1097/00000542-198202000-00007. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Dumin E, Balan L, Foltyn V. D-Amino acids in the brain: d-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- Xie Y, Alexander GM, Schwartzman RJ, Singh N, Torjman M, Goldberg M, et al. Development and validation of a sensitive LC-MS/MS method for the determination of d-serine in human plasma. J Pharm Biomed Anal. 2014;89:1–5. doi: 10.1016/j.jpba.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K. R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]


