Abstract
The vast majority of chronic myeloid leukemia patients express a BCR-ABL1 fusion gene mRNA encoding a 210 kDa tyrosine kinase which promotes leukemic transformation. A possible differential impact of the corresponding BCR-ABL1 transcript variants e13a2 (“b2a2”) and e14a2 (“b3a2”) on disease phenotype and outcome is still a subject of debate. A total of 1105 newly diagnosed imatinib-treated patients were analyzed according to transcript type at diagnosis (e13a2, n=451; e14a2, n=496; e13a2+e14a2, n=158). No differences regarding age, sex, or Euro risk score were observed. A significant difference was found between e13a2 and e14a2 when comparing white blood cells (88 vs. 65 × 109/L, respectively; P<0.001) and platelets (296 vs. 430 × 109/L, respectively; P<0.001) at diagnosis, indicating a distinct disease phenotype. No significant difference was observed regarding other hematologic features, including spleen size and hematologic adverse events, during imatinib-based therapies. Cumulative molecular response was inferior in e13a2 patients (P=0.002 for major molecular response; P<0.001 for MR4). No difference was observed with regard to cytogenetic response and overall survival. In conclusion, e13a2 and e14a2 chronic myeloid leukemia seem to represent distinct biological entities. However, clinical outcome under imatinib treatment was comparable and no risk prediction can be made according to e13a2 versus e14a2 BCR-ABL1 transcript type at diagnosis. (clinicaltrials.gov identifier:00055874)
Introduction
The reciprocal translocation between chromosomes 9 and 22 resulting in a shortened chromosome 22 (Philadelphia chromosome) is the genetic hallmark of chronic myeloid leukemia (CML). Consequently, the vast majority of patients express a BCR-ABL1 mRNA fusion gene encoding a 210 kDa tyrosine kinase (p210) which is constitutively activated and hence the mainspring of leukemic transformation.1 With rare exceptions, the p210 BCR-ABL1 is encoded by e13a2 and e14a2 BCR-ABL1 transcripts, or in some cases by both of them.
One genomic breakpoint can result in variable transcript types that differ in the number of involved BCR and (very rarely) ABL exons due to alternative splicing.2 The p210 BCR-ABL1 is associated with breakpoints located within the “major” breakpoint cluster region (M-bcr) that comprises exons 12–16, historically referred to as b1–b5. In these cases, BCR exon 13 (e13) or BCR exon 14 (e14) is fused to ABL exon 2 (a2). Thereby the e14a2 fusion (3′ M-bcr breakpoint) as well as the e13a2 fusion (5′ M-bcr breakpoint) can result in an e13a2 mRNA since e14 is subjected to alternative splicing and occasionally both transcripts occur together in multiplex PCR as a product of the same clone.3 Both mRNA variants differ in the presence or absence of the 75 base pairs of e14: the e13a2, (lacking exon 14, also referred to as “b2a2”) and the e14a2 BCR-ABL1 transcript (“b3a2”).
Little is known about the functional relevance of the additional 25 amino acid residues of the e14a2 BCR-ABL1 oncoprotein. It has been hypothesized that spatial rearrangements of the adjacent domains might be a consequence and altered cellular interactions might result in particular due to the sequential arrangement of six hydrophobic residues coded by exon e14.4 Interestingly, a novel lysine residue is introduced at the e14a2 junction site due to codon disruption, which is not the case in the e13a2 fusion because the resulting codon is identical with the non-rearranged a1a2 sequence. It has been assumed that this might contribute to the immunogenicity of the e14a2 junction peptide. The HLA-associated expression of this junction peptide as a 9-mer on the surface of CML cells has been shown to provoke a cytotoxic T-lymphocyte response.5
The clinical relevance of the additional 25 residues and the specific fusion peptide of the e14a2 BCR-ABL1 oncoprotein were extensively studied in the pre-imatinib era. However, the influence of the genomic breakpoint location and the predominantly spliced mRNA variant on disease phenotype and outcome remained controversial.6 Regarding hematologic characteristics at diagnosis, the observation of higher platelet counts in the e14a2 group must now be considered in the light of reports to the contrary over the past two decades.4,7,8
There has still been no evaluation of distinct disease characteristics and the prognostic implications of different p210 BCR-ABL1 transcript types in a larger data set of CML patients on imatinib treatment. In this study, we analyze the long-term molecular, cytogenetic, and clinical outcome of 1105 chronic-phase CML patients receiving an imatinib-based treatment within the randomized German CML Study IV.9
Methods
The randomized German CML Study IV compares four imatinib-based treatment modalities in newly diagnosed chronic-phase CML patients. Treatment arms comprise monotherapy with imatinib 400 mg/d or 800 mg/d and combinations of imatinib 400 mg/d with interferon alpha (IFN-α) and low-dose cytarabine.9 IFN-α was administered at a dose of 1.5–3.0 × 106 IU three times per week according to tolerability. Cytarabine was given in an intermittent dose of 10 mg absolute up to 20 mg/m2 subcutaneously according to tolerability. The study was approved by the ethics committees of the participating centers. Written informed consent was obtained from all patients according to the Declaration of Helsinki. The study is registered at clinicaltrials.gov identifier:00055874 (http://clinicaltrials.gov).
Definition of treatment response
Major cytogenetic response (MCyR), complete cytogenetic response (CCyR), major molecular remission (MMR), accelerated phase (AP), and blastic phase (BP) were defined according to European LeukemiaNet (ELN) criteria.10,11 Deep molecular remission was defined as a 4-log reduction in BCR-ABL1 transcript levels (MR4), i.e. a decline of BCR-ABL1 equal or below 0.01% compared to the standardized baseline according to the International Scale (IS).12 Disease progression was defined by the occurrence of AP, BP, or death from any cause.
Multiplex-PCR, quantitative RT-PCR
The type of BCR-ABL1 transcript was determined via multiplex RT-PCR from cDNA synthesized from total leukocyte RNA at diagnosis.13 Expression of BCR-ABL1 and total ABL1 was determined by quantitative RT-PCR from cDNA and standardized according to the IS as previously described.14,15
Statistical analysis
Comparisons of base-line blood variables were made by the Mann-Whitney-Wilcoxon test. Probabilities of overall survival (OS) and progression-free survival (PFS) were calculated by the Kaplan-Meier method and compared by log rank statistics. For response parameters, cumulative incidences were calculated in consideration of competing risks defined by AP, BC, and death.16 Comparisons between cumulative incidences were performed by the Gray test. P=0.05 was considered significant. All calculations were performed with SAS software v. 9.1.3 (SAS Institute, Cary, NC, USA).
Results
Patients and transcripts
A total of 1311 patients were recruited until April 30, 2009. Ten patients had to be excluded from the study: no CML (n=4), not in first chronic phase (n=4), withdrawal of consent (n=2). Patients with primary IFN-α treatment were switched to imatinib in case of failure and not subjected to the transcript analysis (n=131). In 51 patients the BCR-ABL1 transcript type was unknown. Atypical transcripts were detected in 14 patients: e1a2 (n=3), e13a3 (n=1), e14a3 (n=4), e19a2 (n=6). The remaining 1105 patients with typical BCR-ABL1 transcripts were evaluable for response and survival analysis and presented at diagnosis with e13a2 (n=451, 41%), e14a2 (n=496, 45%) or both transcript types (n=158, 14%) (Online Supplementary Table S1). According to protocol, patients could be pre-treated with hydroxyurea or imatinib before first clinical examination and differential blood count. Therefore, 67 patients (hydroxyurea, n=48; imatinib, n=19) were excluded from the analysis of base-line characteristics (CONSORT diagram) (Online Supplementary Figure S1).
A total of 105 patients were switched to second- or third-line tyrosine kinase inhibitor (TKI) treatment at the investigators’ discretion, usually due to intolerance or a lack of response. The distribution of patients receiving nilotinib (n=34), dasatinib (n=73) or bosutinib (n=3) according to BCR-ABL transcript type is shown in the Online Supplementary Table S2.
Disease characteristics at diagnosis
No correlation of transcript type with age or sex was observed (Table 1). The analysis of initial blood counts for both transcript groups revealed a significant difference in total leukocytes (WBC) and platelets. At diagnosis, e14a2 patients presented with a median WBC of 65 × 109/L compared to 88 × 109/L in the e13a2 group (P<0.001) and 78 × 109/L in the e13a2+e14a2 group (P=0.030) (Figure 1A) indicating a lower tumor load. With regard to platelets, there was a significant difference that is inverse to the difference in WBC: 296 × 109/L in the e13a2 group compared to 430 × 109/L in the e14a2 group and 420 × 109/L in the e13a2+e14a2 group (P<0.001). (Figure 1B). No differences were observed in hemoglobin concentration, differential counts of peripheral blood, or spleen size (Table 1). The different platelet counts did not transform into a significant difference between patients’ risk status according to Euro scores. According to the absence of a systematic difference in Euro score, age, and sex, the transcript groups were compared without stratification for those covariates.
Table 1.
Patients’ characteristics at diagnosis (median, range).
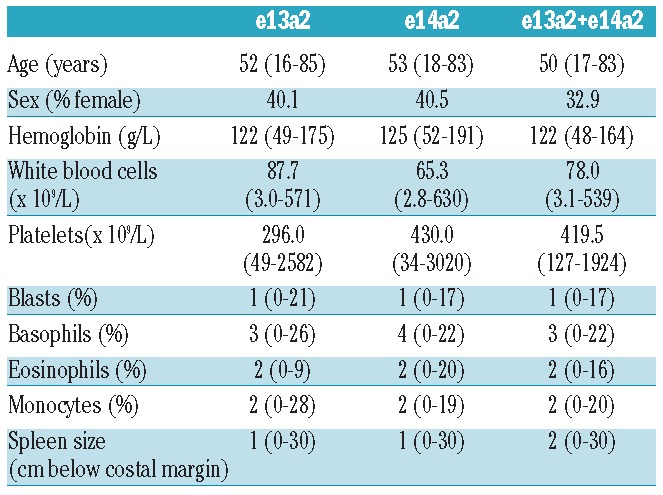
Figure 1.
(A) Box plots of leukocyte counts (WBC) at diagnosis. E14a2 is associated with lower WBC (65 × 109/L, median) as compared to e13a2 (88 × 109/L, P<0.001) and e13a2+e14a2 (78 × 109/L; P=0.030). (B) Box plots of platelet counts at diagnosis. E13a2 is associated with lower platelet count (296 × 109/L, median) as compared to e14a2 (430 × 109/L; P<0.001) and e13a2+e14a2 (420 × 109/L; P<0.001).
Molecular response in total patient sample
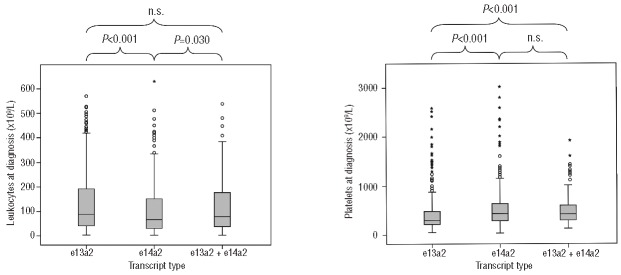
Patients expressing one single transcript showed a difference in the cumulative incidence (CI) of MMR (P=0.002), whereas the e13a2+e14a2 transcript group did not differ to either side (not significant) (Figure 2). There was a substantial difference in median time to MMR when e13a2 was compared to e14a2 (18.4 vs. 14.2 months), while CI of MMR after five years of treatment was 81% versus 85%.
Figure 2.
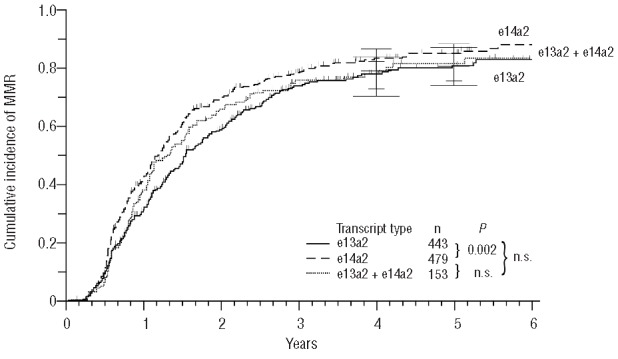
Cumulative incidence of major molecular remissions (MMR).
Regarding the CI of MR4, the difference between e13a2 and e14a2 was also significant (P<0.001) (Figure 3) and even more pronounced: median time to MR4 55.2 versus 32.4 months, while CI of MR4 after five years of treatment was 58% versus 76%. In terms of MR4, e13a2+e14a2 differed from e14a2 (P=0.004) (Figure 3) but not from e13a2.
Figure 3.
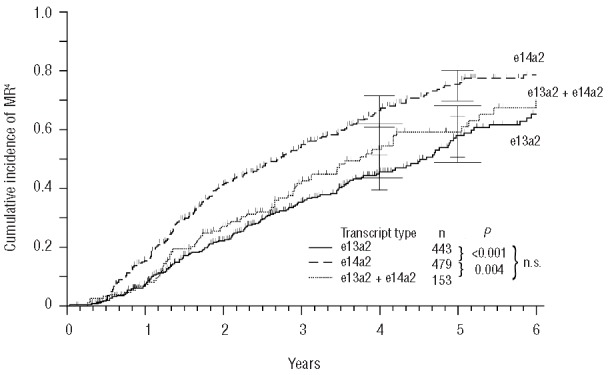
Cumulative incidence of 4-log molecular remissions (MR4).
These findings suggest a better molecular response rate in the e14a2 group as compared to e13a2, indicated by a difference of approximately four months of median time to MMR and 23 months to MR4.
Molecular response in single treatment arms
If single treatment arms were analyzed with regard to MMR, a significant difference in favor of e14a2 was only seen for the imatinib 400 mg + IFN-α arm (n=331; P=0.004) as compared to e13a2 (data not shown). Concerning MR4, the imatinib 400 mg + IFN-α group (n=331; P<0.001), the imatinib 400 mg + cytarabine group (n=150; P=0.004) and the imatinib 800 mg group (n=324; P=0.028) all showed significant differences if e14a2 and e13a2 were compared.
Cytogenetic response
In contrast to molecular findings, no significant difference emerged when cumulative incidence of MCyR (data not shown) and CCyR (Figure 4) were compared. The three transcript groups varied between 6.8 and 7.8 months in median time to MCyR, and between 10.1 and 11.4 months in median time to CCyR, therefore only slight differences were observed.
Figure 4.
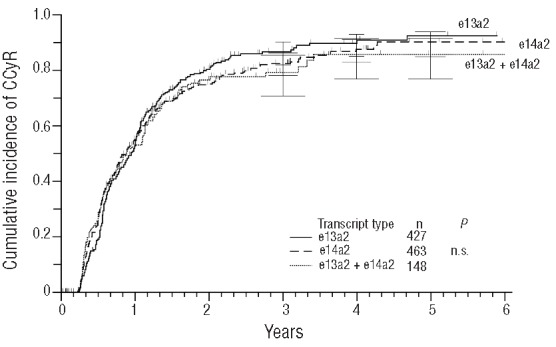
Cumulative incidence of complete cytogenetic remissions (CCyR).
Clinical outcome
No difference between the three transcript groups was observed with regard to PFS and OS (Figures 5 and 6).
Figure 5.
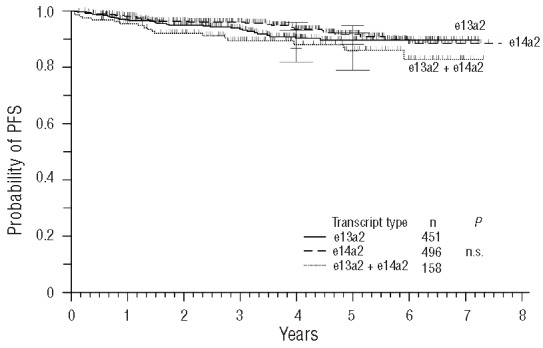
Probability of progression-free survival (PFS).
Figure 6.
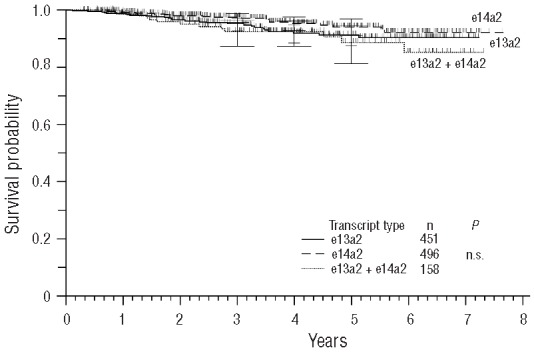
Probability of overall survival (OS).
Adverse events
Despite the disparities in blood counts at diagnosis, no significant differences in the incidence of adverse hematologic events (grade 3/4) under imatinib treatment were observed.
BCR-ABL kinase domain mutations
In case of suspected resistance, a total of 690 mutation analyses were performed in the study center, 108 of which were positive. Twenty-seven different mutations could be detected in 63 patients. The most common mutations were T315I (n=13 patients), M244V (n=9), Y253H (n=7) and E255K (n=7). No difference in the incidence of mutations was observed in a comparison between BCR-ABL transcript groups (Online Supplementary Table S3).
Discussion
The question as to whether or not the type of the individually expressed BCR-ABL1 fusion transcript determines the phenotype of CML and might influence course and prognosis had already been investigated extensively in the pre-imatinib era. While distinct CML features could be associated with the three major BCR-ABL1 types, p190, p210, and p230,6 the biological significance of the two p210 subtypes occurring in the vast majority of CML cases remained controversial.17 Several studies could not find any impact on clinical course or survival.18,19 Here we report on the hematologic phenotype and outcome of 1105 imatinib-treated patients expressing one or both of the different p210 BCR-ABL1 transcript types.
Our data reveal a distinct phenotype of e13a2 and e14a2 CML, the latter of which is characterized by lower WBC count (88 vs. 65 × 109/L) and higher platelet count (296 vs. 430 × 109/L), whereas there was no significant difference in any of the other hematologic features, including spleen size. A first report of a link between the e14a2 transcript type and high platelet counts at diagnosis was provided in 199120 and questioned shortly after.21 Both papers reported on small series of patients (n=57,20 n=4521). In a later analysis of 119 patients, a higher platelet count was found only in patients with WBC less than 100 × 109/L.22 In a cohort of 88 patients, a significantly higher platelet count in e14a2 patients was observed (306 vs. 616 × 109/L).4 A more recent analysis of 202 cases could not find any hematologic differences at all.8 Our findings, derived from a large cohort of patients, strongly support the former reports of distinct blood counts for both transcript types, which might reflect a specific disease biology.
Assuming a distinct CML phenotype according to the individual BCR-ABL1 transcript leads to the question as to whether these phenotypes might be associated with specific patterns of TKI response. Regarding molecular response, we found a clear advantage for the e14a2 group of patients: median time to MMR was 14 versus 18 months, median time to MR4 was 32 versus 55 months (Figures 2 and 3). This is in agreement with results of the Italian GIMEMA working party that investigated 559 patients on imatinib treatment and found a significantly shorter time to MMR in the e14a2 group.23 Vega-Ruiz et al. analyzed 480 patients and found significantly lower BCR-ABL1 levels in the e14a2 group at three, six, and nine months.24
In contrast, both studies found no difference in the achievement of CCyR for e14a2 and e13a2 patients, which is somewhat unexpected in the face of superior molecular response in the e14a2 group. Likewise, our data show no difference in the cumulative incidence of MCyR and CCyR (Figure 4), which is in contrast to the molecular findings. Distinct BCR-ABL1 transcript levels in the presence of identical proportions of leukemic bone marrow cells given by cytogenetic response would imply a specific transcription frequency of the particular BCR-ABL1 transcript type. To our knowledge, there are no data on this question. Besides this explanation, a possible bias in PCR amplification efficiency should also be examined. We perform quantitative RT-PCR on the LightCycler™ 1.5 and 2.0 instruments (Roche Diagnostics, Mannheim, Germany) using hybridization probes which allows the use of relatively long PCR amplicons of 671 and 596 base pairs for e14a2 and e13a2 transcripts. A similar PCR assay is performed in many European laboratories. The effect of the 75 base pair difference might be more significant in PCR assays using shorter amplicons, e.g. using the TaqMan™ approach.25 An evaluation of disparate efficiencies affecting the comparability of molecular assessments of patients with different transcript types is warranted.
Lucas et al. investigated 78 patients and showed a faster achievement of CCyR in the e14a2 group, which was explained by a higher kinase activity of e13a2 BCR-ABL1 reflected by pCRKL.26 Taking into account that in the study of Lucas et al. CCyR was also given as CCyR equivalence, i.e. BCR-ABL1 transcript levels less than 1%, this result might also be affected by disparate PCR efficiencies.
In contrast to the above findings, better response rates for e13a2 have also been reported. A small series of Brazilian patients (n=22) showed better molecular response,27 while a cohort of Indian patients (n=87) showed superior cytogenetic response.28 Another analysis of Indian patients (n=202) showed no difference in molecular and cytogenetic response rates.8
With regard to clinical end points such as failure-free, transformation-free, progression-free, and overall survival, none of the larger cohorts, including our own, showed a significantly different outcome on imatinib treatment according to the BCR-ABL1 transcript type (Figure 5 and 6).23,24,26 Despite conflicting data regarding molecular and cytogenetic response in several studies, this might suggest a general agreement that the type of BCR-ABL1 transcript does not serve as a prognostic marker at diagnosis.
It has been reported that patients develop circulating T cells that react with an HLA BCR-ABL1 junction peptide complex on the surface of e14a2 BCR-ABL1 positive cells.5 A differential immunogenicity of e14a2 BCR-ABL1 cells could be postulated that might lead to a distinct response to treatment with IFN-α, which is known to stimulate T-cell response. In the subgroup of CML Study IV treated with a combination of IFN-α and imatinib (n=331), no difference in survival was observed according to transcript type. However, a significant difference regarding MMR and MR4 was still observed despite the lower number of cases. No other treatment arm showed the same effect for MMR. This might support the notion of a distinct immune response to e14a2 BCR-ABL1 cells. On the genomic level, the group of patients expressing both transcripts at diagnosis is identical to the e14a2 group and does not harbor two distinct clones.2 However, in this group, exon e14 is spliced to a significant extent resulting in two mRNA transcripts. The group expressing both transcripts seems to be similar to e13a2 with regard to base-line leukocyte count (Figure 1A) and similar to e14a2 with regard to base-line platelet count (Figure 1B). In terms of molecular response, the group expressing both transcripts might resemble e13a2, as suggested by CI of MR4 (Figure 3). This might argue against an altered immune response conferred by e14a2.
We conclude that the type of BCR-ABL1 transcript is reflected by different leukocyte and platelet counts at diagnosis, which might represent a distinct phenotype and disease biology. In our data, superior molecular response was observed in e14a2 patients. This is not paralleled by cytogenetic response where no difference was observed with regard to CCyR and MCyR. The differences in molecular response do not translate into differences in survival. Therefore, the type of BCR-ABL1 transcript should be taken into account when BCR-ABL1 levels are interpreted to assess imatinib response. However, the type of transcript allows no risk stratification at diagnosis.
Acknowledgments
We thank Michelle Giehl, Katrin Ackermann, Alla Elkovskaia, Elena Felde, Maike Haas, Melanie Hartmann, Carolin Hölting, Cathrin Huber, Vanessa Leins, Melanie Müller, Iris Palme, and Irina Tarnapolscaia for reporting of laboratory results and technical assistance. The contributions of Gabriele Bartsch, Sabine Dean, Michaela Hausmann, Ute Kossak, Elke Matzat, Barbara Müller, Regina Pleil-Lösch, Nicole Schomber, Annette Schreiber, Inge Stalljann, Nicole Steidl, Cornelia Willersinn, Uwe Böhm, the Steering Group (Rüdiger Hehlmann, Gerhard Ehninger, Joerg Hasford, Andreas Hochhaus, Dieter Hossfeld, Hans-Jochem Kolb, Stefan Krause, Christoph Nerl, Hans Pralle, Dominik Heim, Gabriela M. Baerlocher, Hermann Heimpel) and all CML trial participants are acknowledged.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Goldman JM, Melo JV. Chronic myeloid leukemia–advances in biology and new approaches to treatment. N Engl J Med. 2003;349(15):1451–64. [DOI] [PubMed] [Google Scholar]
- 2.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96(10):3343–56. [PubMed] [Google Scholar]
- 3.Shtivelman E, Lifshitz B, Gale RP, Roe BA, Canaani E. Alternative splicing of RNAs transcribed from the human abl gene and from the bcr-abl fused gene. Cell. 1986;47(2):277–84. [DOI] [PubMed] [Google Scholar]
- 4.Perego RA, Costantini M, Cornacchini G, Gargantini L, Bianchi C, Pungolino E, et al. The possible influences of B2A2 and B3A2 BCR/ABL protein structure on thrombopoiesis in chronic myeloid leukaemia. Eur J Cancer. 2000;36(11):1395–401. [DOI] [PubMed] [Google Scholar]
- 5.Clark RE, Dodi IA, Hill SC, Lill JR, Aubert G, Macintyre AR, et al. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood. 2001;98(10):2887–93. [DOI] [PubMed] [Google Scholar]
- 6.Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood. 1996;88(7):2375–84. [PubMed] [Google Scholar]
- 7.Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10(2):203–22. [DOI] [PubMed] [Google Scholar]
- 8.Polampalli S, Choughule A, Negi N, Shinde S, Baisane C, Amre P, et al. Analysis and comparison of clinicohematological parameters and molecular and cytogenetic response of two Bcr/Abl fusion transcripts. Genet Mol Res. 2008;7(4):1138–49. [DOI] [PubMed] [Google Scholar]
- 9.Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Muller MC, Pletsch N, et al. Tolerability-Adapted Imatinib 800 mg/d Versus 400 mg/d Versus 400 mg/d Plus Interferon-{alpha} in Newly Diagnosed Chronic Myeloid Leukemia. J Clin Oncol. 2011;29(12):1634–42. [DOI] [PubMed] [Google Scholar]
- 10.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108(6):1809–20. [DOI] [PubMed] [Google Scholar]
- 12.Cross NC, White HE, Muller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26(10):2172–5. [DOI] [PubMed] [Google Scholar]
- 13.Cross NC, Feng L, Chase A, Bungey J, Hughes TP, Goldman JM. Competitive polymerase chain reaction to estimate the number of BCR-ABL transcripts in chronic myeloid leukemia patients after bone marrow transplantation. Blood. 1993;82(6):1929–36. [PubMed] [Google Scholar]
- 14.Cross NC. Standardisation of molecular monitoring for chronic myeloid leukaemia. Best Pract Res Clin Haematol. 2009;22(3):355–65. [DOI] [PubMed] [Google Scholar]
- 15.Muller MC, Cross NC, Erben P, Schenk T, Hanfstein B, Ernst T, et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia. 2009;23(11):1957–63. [DOI] [PubMed] [Google Scholar]
- 16.Pfirrmann M, Hochhaus A, Lauseker M, Saussele S, Hehlmann R, Hasford J. Recommendations to meet statistical challenges arising from endpoints beyond overall survival in clinical trials on chronic myeloid leukemia. Leukemia. 2011;25(09):1433–8. [DOI] [PubMed] [Google Scholar]
- 17.Mills KI, Benn P, Birnie GD. Does the breakpoint within the major breakpoint cluster region (M-bcr) influence the duration of the chronic phase in chronic myeloid leukemia¿ An analytical comparison of current literature. Blood. 1991;78(5):1155–61. [PubMed] [Google Scholar]
- 18.Tefferi A, Bren GD, Wagner KV, Schaid DJ, Ash RC, Thibodeau SN. The location of the Philadelphia chromosomal breakpoint site and prognosis in chronic granulocytic leukemia. Leukemia. 1990;4(12):839–42. [PubMed] [Google Scholar]
- 19.Jaubert J, Martiat P, Dowding C, Ifrah N, Goldman JM. The position of the M-BCR breakpoint does not predict the duration of chronic phase or survival in chronic myeloid leukemia. Br J Haematol. 1990;74(1):30–5. [DOI] [PubMed] [Google Scholar]
- 20.Inokuchi K, Inoue T, Tojo A, Futaki M, Miyake K, Yamada T, et al. A possible correlation between the type of bcr-abl hybrid messenger RNA and platelet count in Philadelphia-positive chronic myelogenous leukemia. Blood. 1991;78(12):3125–7. [PubMed] [Google Scholar]
- 21.Opalka B, Wandl UB, Stutenkemper R, Kloke O, Seeber S, Niederle N. No correlation between the type of bcr-abl hybrid messenger RNA and platelet counts in chronic myelogenous leukemia. Blood. 1992;80(7):1854–5. [PubMed] [Google Scholar]
- 22.Shepherd P, Suffolk R, Halsey J, Allan N. Analysis of molecular breakpoint and m-RNA transcripts in a prospective randomized trial of interferon in chronic myeloid leukaemia: no correlation with clinical features, cytogenetic response, duration of chronic phase, or survival. Br J Haematol. 1995;89(3):546–54. [DOI] [PubMed] [Google Scholar]
- 23.Castagnetti F, Gugliotta G, Palandri F, Breccia M, Specchia G, Abruzzese E, et al. BCR-ABL Fusion Transcript Do Not Significantly Influence the Outcome of Chronic Myeloid Leukemia Patients In Early Chronic Phase Treated with Imatinib Mesylate: a GIMEMA CML WP Analysis. ASH Annual Meeting Abstracts. 2010;116(21):1230. [Google Scholar]
- 24.Vega-Ruiz A, Kantarjian H, Shan J, Wierda W, Burger J, Verstovsek S, et al. Better Molecular Response to Imatinib for Patients (pts) with Chronic Myeloid Leukemia (CML) in Chronic Phase (CP) Carrying the b3a2 Transcript Compared to b2a2. ASH Annual Meeting Abstracts. 2007;110(11):1939. [Google Scholar]
- 25.Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003;17(12):2318–57. [DOI] [PubMed] [Google Scholar]
- 26.Lucas CM, Harris RJ, Giannoudis A, Davies A, Knight K, Watmough SJ, et al. Chronic myeloid leukemia patients with the e13a2 BCR-ABL fusion transcript have inferior responses to imatinib compared to patients with the e14a2 transcript. Haematologica. 2009;94(10):1362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lemos JA, de Oliveira CM, Scerni AC, Bentes AQ, Beltrao AC, Bentes IR, et al. Differential molecular response of the transcripts B2A2 and B3A2 to imatinib mesylate in chronic myeloid leukemia. Genet Mol Res. 2005;4(4):803–11. [PubMed] [Google Scholar]
- 28.Sharma P, Kumar L, Mohanty S, Bose S, Kochupillai V. Does BCR-ABL transcript type predict cytogenetic response to imatinib mesylate in chronic phase, chronic myeloid leukemia patients¿ J Clin Oncol. 2007;7043. [Google Scholar]


