Abstract
Clinical significance of the JAK2V617F mutation in patients with a myeloproliferative neoplasm has been the target of intensive research in recent years. However, there is considerably uncertainty about prognosis in JAK2V617F positive individuals without overt signs of myeloproliferative disease. In this study, we tested the hypothesis that increased JAK2V617F somatic mutation burden is associated with myeloproliferative neoplasm progression rate in the general population. Among 49,488 individuals from the Copenhagen General Population Study, 63 (0.1%) tested positive for the JAK2V617F mutation in the time period 2003–2008. Of these, 48 were available for re-examination in 2012. Level of JAK2V617F mutation burden was associated with myeloproliferative neoplasm progression rate, consistent with a biological continuum of increasing JAK2V617F mutation burden across increasing severity of myeloproliferative neoplasm from no disease (n=8 at re-examination) through essential thrombocythemia (n=20) and polycythemia vera (n=13) to primary myelofibrosis (n=7). Among those diagnosed with a myeloproliferative neoplasm only at re-examination in 2012, in the preceding years JAK2V617F mutation burden increased by 0.55% per year, erythrocyte volume fraction increased by 1.19% per year, and erythrocyte mean corpuscular volume increased by 1.25% per year, while there was no change in platelet count or erythropoietin levels. Furthermore, we established a JAK2V617F mutation burden cut-off point of 2% indicative of disease versus no disease; however, individuals with a mutation burden below 2% may suffer from a latent form of myeloproliferative disease revealed by a slightly larger spleen and/or slightly higher lactic acid dehydrogenase concentration compared to controls. Of all 63 JAK2V617F positive individuals, 48 were eventually diagnosed with a myeloproliferative neoplasm.
Introduction
The JAK2V617F somatic mutation has a central role in the pathogenesis of Philadelphia negative (Ph-) myeloproliferative neoplasms, i.e. essential thrombocythemia, polycythemia vera, and primary myelofibrosis.1 This mutation is also found in patients with different types of venous thromboses but without an overt chronic myeloproliferative neoplasm,2 and in otherwise healthy individuals.3,4
The JAK2V617F mutation has a prevalence of 0.1–0.2% in the general population,5,6 but its clinical implications are still unknown for those individuals harboring the mutation without overt signs of a myeloproliferative neoplasm. These individuals, who often have less than 10% mutation burden,6 may suffer from a latent form of myeloproliferative neoplasm; however, a mutation burden cut-off point indicative of disease versus no disease has not been established for JAK2V617F mutation positive individuals. For these individuals, it is also unknown whether the JAK2V617F mutation burden will change over time, and if such alterations in mutation burden are reflected in an altered hematologic phenotype. Among patients with a chronic myeloproliferative neoplasm, a biological continuum of phenotypic presentation has been described, partly influenced by increasing JAK2V617F mutation burden.7–9 However, a similar correlation between JAK2V617F mutation burden and blood counts or other laboratory tests has not yet been demonstrated among those individuals from the general population who are without overt signs of a myeloproliferative neoplasm.
In this study, we tested the hypothesis that JAK2V617F somatic mutation burden is associated with the development and evolution of myeloproliferative neoplasm in the general population. Among 49,488 individuals from the Copenhagen General Population Study, 63 tested positive for the JAK2V617F mutation in the time period 2003–2008. Of these, 48 were available for re-examination in 2012, which gave us the opportunity to examine increase in allelic burden, and changes in clinical phenotype, hematologic parameters, splenic volume, and morbidity.
Methods
Study population
Among 49,488 individuals from the Copenhagen General Population Study,10–12 we found 63 individuals harboring the JAK2V617F mutation. Of these, 52 were still alive and were re-invited for testing in 2012; 48 of them were re-examined (Figure 1). Selection of control groups is described in the Online Supplementary Appendix.
Figure 1.
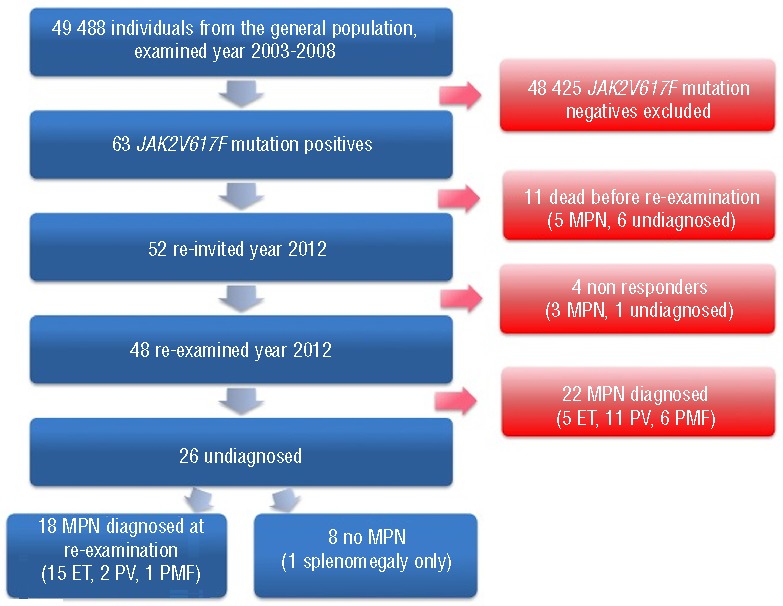
Flow chart of study population. MPN: myeloproliferative neoplasm. ET: essential thrombocythemia; PV: polycythemia vera; PMF: primary myelofibrosis.
The study was approved by a Danish ethical committee (H-KF 01-144/01) and by Herlev Hospital, Copenhagen University Hospital. Written informed consent was obtained from all study participants.
Covariates
At the general population examination in 2003–2008, the 63 JAK2V617F mutation positive individuals and all those mutation negative filled in a self-administered questionnaire concerning present and past life-style and health status. This was completed together with an investigator during the visit, prior to physical examination and blood sampling; participants were unaware of their mutation status at the time of examination.
At their re-examination visit, the 48 JAK2V617F mutation positive individuals filled in an additional self-administered questionnaire concerning known hematologic diagnoses, symptoms, and manifestations. This was completed together with two of the Authors (CN and HSB) during the visit, prior to physical examination and blood sampling. Diagnostic criteria for myeloproliferative neoplasms were in accordance with the World Health Organization criteria for myeloproliferative neoplasms.1
Somatic mutation detection assays
A highly sensitive real-time quantitative PCR assay, using DNA isolated from whole blood, including all leukocytes, was used. This assay, based on a previously published assay,8 briefly described in the Online Supplementary Appendix, was used to re-quantify the mutation burden in the 63 individuals from the general population examination in 2003–2008 as well as the 48 individuals re-examined in 2012; this assay had been previously validated against the Baxter assay,5 where all participants with a mutant allele burden over 2% were also positive on the Baxter assay.
Genotyping
The rs10974944 germline genotype was chosen as a marker of the JAK2 haplotype designated 46/1, associated with risk of developing the JAK2V617F mutation.13–15 Genotyping was performed as reported in our previously published paper.6
Hematologic phenotype
Hematologic parameters were measured with a flow cytometer-based hematology analyzer, ADVIA™120 (Siemens, Healthcare Diagnostics, Deerfield, IL, USA), in the laboratory of Herlev Hospital, Copenhagen University Hospital.
Erythropoietin
Serum samples were analyzed using an Immulite autoanalyzer (Siemens, Healthcare Diagnostics, Deerfield, IL, USA) in the laboratory of Herlev Hospital, Copenhagen University Hospital.
Lactic acid dehydrogenase
Lactic acid dehydrogenase was determined in plasma by using a colorimetric assay performed on a Konelab 60i autoanalyzer (Helsinki, Finland).
Spleen imaging
At re-examination in 2012, all 48 JAK2V617F mutation positive individuals were offered a computed tomography (CT) scan of the spleen; 31 of them accepted. Splenomegaly was defined as splenic volumes higher than the 97.5 percentile in the control group, corresponding to splenic volumes over 353 cubic centimetres.
Registries
Living status until 2012 was obtained from the national Danish Civil Registration System.16 This information is 100% complete for the participants of the Copenhagen General Population Study.
Statistical analyses
The statistical software package STATA release 12.1 was used for all analyses. We used linear regression, Wilcoxon rank-sum test, and Cuzick’s trend test. Two-sided P<0.05 was considered significant.
Results
Myeloproliferative neoplasm progression rate
Of 49,488 individuals from the Copenhagen General Population Study, 63 were found positive for the JAK2V617F somatic mutation at the examination from 2003 through 2008 (Figure 1). This corresponds to a prevalence of 0.1% in this sample of the general population with a median age of 63 years at the time of blood sampling (Table 1). Of these, 30 individuals had already been diagnosed with a myeloproliferative neoplasm (5 essential thrombocythemia, 17 polycythemia vera, 7 primary myelofibrosis, and 1 acute myeloid leukemia), while the remaining 33 individuals (6 dead, 1 non-responder, and 26 re-examined) were undiagnosed (Table 2 and Figure 2A). Hematologic parameters of these 33 JAK2V617F mutation positive individuals not diagnosed with a myeloproliferative neoplasm at the general population examination in 2003–2008 are shown in Online Supplementary Table S1.
Table 1.
Characteristics of JAK2V617F somatic mutation positives and negatives from the Danish general population.
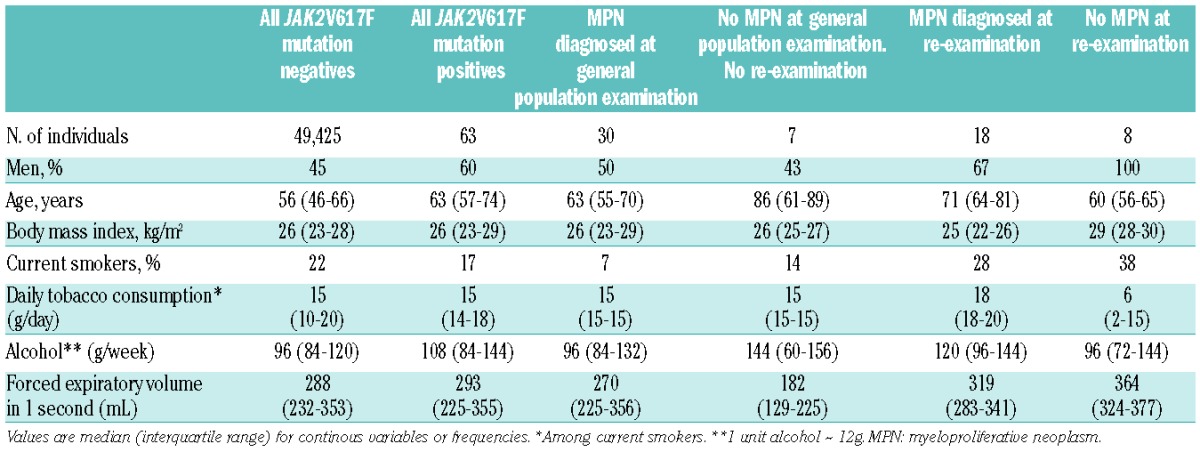
Table 2.
Clinical diagnoses of 63 JAK2V617F mutation positives from the Danish general population.
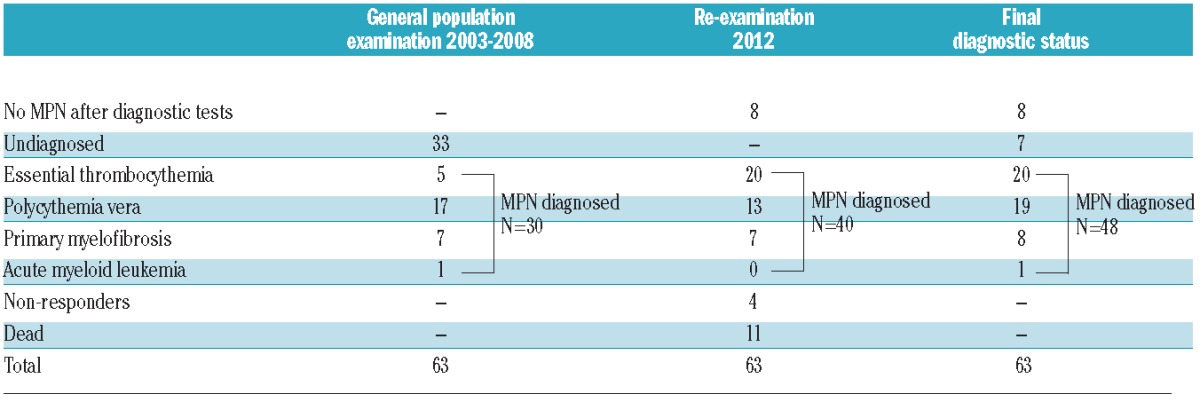
Figure 2.
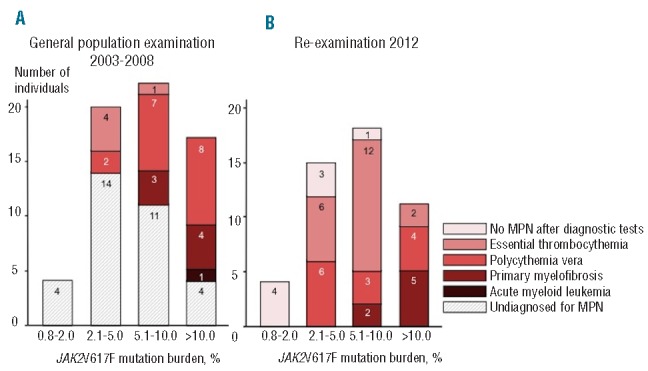
JAK2V617F mutation positive individuals at the general population examination and at re-examination across JAK2V617F mutation burden. The term “No MPN after diagnostic tests” designates individuals without hematologic parameters indicative of a myeloproliferative neoplasm at the time of re-examination. The term “Undiagnosed for MPN” designates individuals without a diagnosis of a myeloproliferative neoplasm at the time of the general population examination. MPN: myeloproliferative neoplasm.
In 2012, the 52 individuals still alive were invited for a re-examination, and 48 individuals attended; their median follow-up time was 5.4 years. Of these 48 re-examined individuals, 22 had already been diagnosed with a myeloproliferative neoplasm before 2012 (5 essential thrombocythemia, 11 polycythemia vera, 6 primary myelofibrosis) while 26 were still undiagnosed (Figure 1). At re-examination in 2012, 18 of these 26 were diagnosed with a myeloproliferative neoplasm based on hematologic parameters (15 essential thrombocythemia, 2 polycythemia vera, and 1 primary myelofibrosis) (Figure 1). These individuals were subsequently offered further medical examination, including a bone marrow examination, and this was performed on 14 individuals, confirming their diagnosis. The remaining 8 JAK2V617F mutation positive individuals (“No MPN after diagnostic tests” in Figure 2B) did not have hematologic parameters indicative of a myeloproliferative disorder; however, one had splenomegaly.
The majority of the JAK2V617F mutation positive individuals had mutation burden levels below 10% at the general population examination in 2003–2008 as well as at reexamination in 2012 (Figure 2). Of the 8 JAK2V617F mutation positive individuals with no hematologic parameters indicative of a myeloproliferative neoplasm at re-examination, 7 had mutation burden levels below 5% whereas the only case with splenomegaly as the only indicator of myeloproliferative disorder had a mutation burden of 5.7%. No individuals with a myeloproliferative neoplasm were found in the group with allele burden below 2%, neither at the general population examination in 2003–2008, nor at re-examination in 2012. At mutation levels of 2.1% and higher, individuals were diagnosed with essential thrombocythemia and polycythemia vera, while individuals with primary myelofibrosis had mutation burden levels of 6.2% or higher at both examinations. One single individual, only attending the general population examination, was diagnosed with acute myeloid leukemia one year after the examination and had a mutation burden of 11.2% at examination. Medical records of this patient did not provide information on the preceding myeloproliferative neoplasm.
Taken together, these results suggest that the JAK2V617F mutation burden level was associated with myeloproliferative neoplasm development and progression rate, consistent with a biological continuum of increasing JAK2V617F mutation burden across increasing severity of myeloproliferative neoplasm from no disease (n=8 at re-examination) through essential thrombocythemia (n=20) and polycythemia vera (n=13) to primary myelofibrosis (n=7).
JAK2V617F somatic mutation progression rate
Figure 3 shows the progression rate of the JAK2V617F mutation burden among the 26 individuals undiagnosed with a myeloproliferative neoplasm until re-examination in 2012. In the 18 individuals diagnosed with a myeloproliferative neoplasm at re-examination, the JAK2V617F mutation burden increased by 0.55% per year (P=0.01) during their follow-up time from the general population examination in 2003–2008 through to the re-examination in 2012; if the person with a 4-fold increase in mutation burden was excluded, the increase was 0.31% per year (P=0.09). In the 8 individuals without a hematologically proven myeloproliferative neoplasm, the JAK2V617F mutation burden was unchanged (P=0.98) during their follow-up period.
Figure 3.
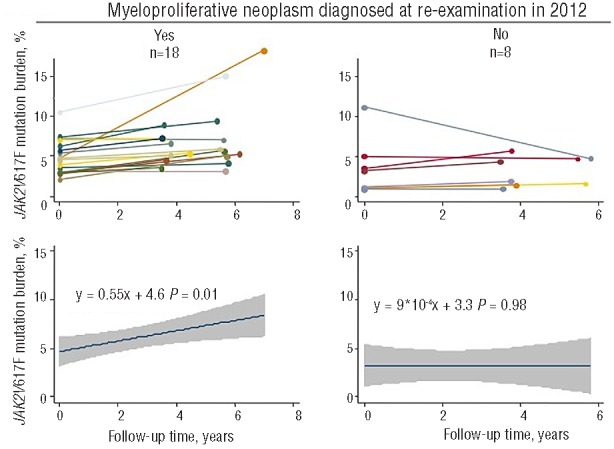
Progression rate of JAK2V617F mutation burden in individuals undiagnosed with a myeloproliferative neoplasm until re-examination. Of the JAK2V617F mutation positive individuals without diagnosis of a myeloproliferative neoplasm at the time of the general population examination in 2003–2008, 26 could be re-examined in 2012. The left side panels show the 18 individuals diagnosed with a myeloproliferative neoplasm at re-examination. The right side panels show the 8 individuals without hematologic parameters indicative of a myeloproliferative neoplasm at re-examination. In the upper panels the JAK2V617F mutation burden measurements at the general population examination and at re-examination are shown for each individual separately, while lower panels show corresponding linear regression analyses with 95% confidence intervals and P values for the combined group.
Hematologic and erythropoietin progression rate
Hematologic phenotypic changes among the 26 individuals without diagnosis of a myeloproliferative neoplasm until re-examination in 2012 are shown in Figure 4. In the 18 individuals diagnosed with a myeloproliferative neoplasm at re-examination, erythrocyte volume fraction increased by 1.19% per year (P=0.001) during their follow-up period, but this parameter was unchanged in the 8 individuals without a hematologically proven myeloproliferative neoplasm. Erythrocyte mean corpuscular volume increased by 1.25 fL per year in both diagnosed (P=0.001) and undiagnosed (P=0.02) individuals. Platelet counts and erythropoietin levels did not change during follow up in diagnosed or undiagnosed individuals.
Figure 4.
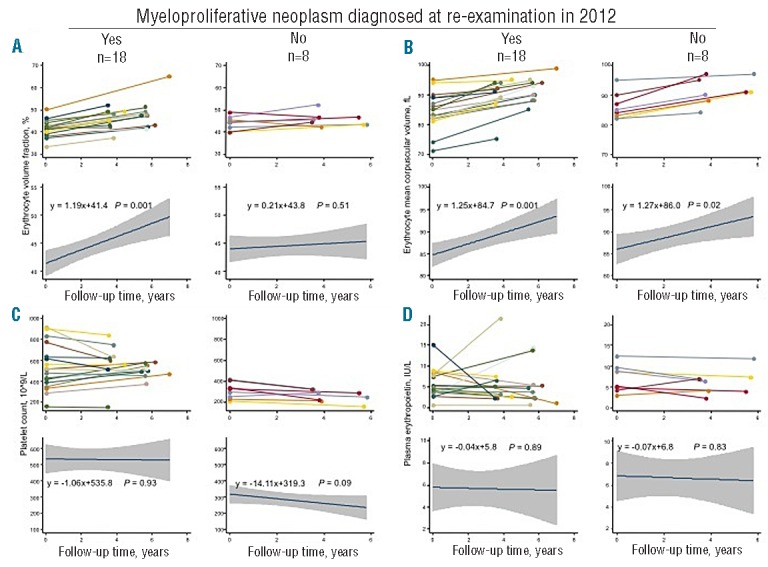
Progression rate of clinically relevant hematologic parameters in individuals undiagnosed with a myeloproliferative neoplasm at reexamination. (A) Erythrocyte volume fraction. (B) Erythrocyte mean corpuscular volume. (C) Platelet count. (D) Erythropoietin plasma concentration. For each panel, the left side shows the 18 individuals diagnosed with a myeloproliferative neoplasm at re-examination while the right side shows the 8 individuals without hematologic parameters indicative of a myeloproliferative neoplasm at re-examination. In the upper part of the panels A–D, measurements of hematologic parameters at the general population examination and at re-examination are shown for each individual separately, while the lower part of these panels shows corresponding regression analyses with 95% confidence intervals and P values for the combined group.
JAK2V617F mutation burden, splenic volume, and lactic acid dehydrogenase
JAK2V617F mutation burden increased across the severity of myeloproliferative neoplasm diagnoses (Figure 5A). Individuals with no hematologically proven myeloproliferative neoplasm had a median JAK2V617F mutation burden of 3.1% (2.5th–97.5th percentiles: 0.9–5.7%), individuals with essential thrombocythemia had a median JAK2V617F mutation burden of 5.5% (3.1–62%), individuals with polycythemia vera had a median JAK2V617F mutation burden of 5.9% (2.1–27%), while individuals with primary myelofibrosis had a median JAK2V617F mutation burden of 13% (6.9–24%); the trend test across the 4 groups showed P=3*10−4.
Figure 5.
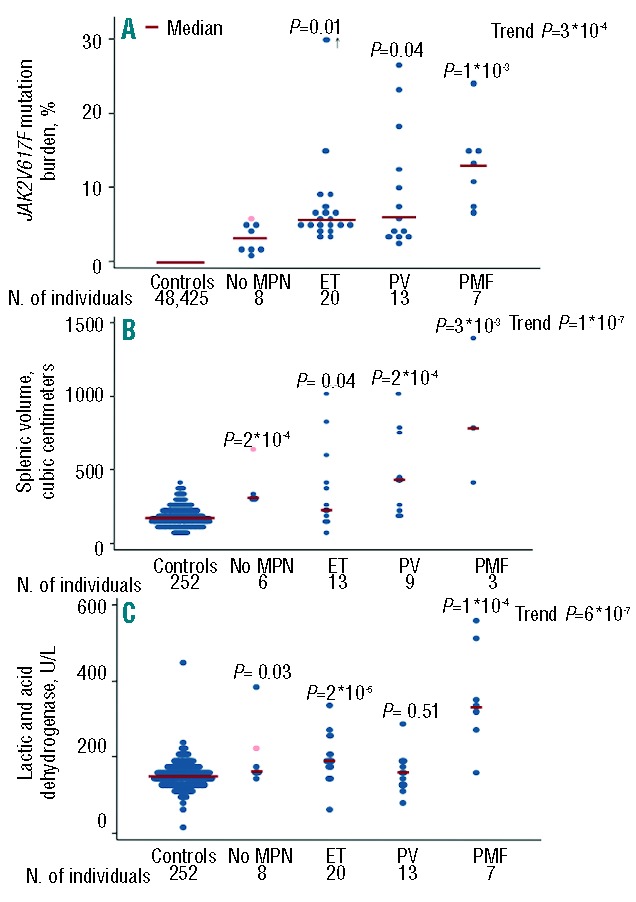
JAK2V617F mutation burden, splenic volume, and lactic acid dehydrogenase concentrations in JAK2V617F positive individuals and in controls. The term “No MPN” designates individuals without hematologic parameters indicative of a myeloproliferative neoplasm at the time of re-examination. One of these 6 individuals had splenomegaly (pink dot). ET: essential thrombocythemia; PV: polycythemia vera; PMF: primary myelofibrosis. The numbers are slightly lower for splenic volumes because not all JAK2V617F mutation positive individuals were examined with a CT scan. Among individuals diagnosed with essential thrombocythemia, one individual had a JAK2V617F mutation burden of 62%, which in the figure is plotted at 30% (+ arrow). P values are from Wilcoxon rank-sum test of the comparison with the control group and from Cuzick’s trend test across the 5 groups.
Splenic volumes were measured in 31 JAK2V617F positive individuals at re-examination in 2012 and were higher compared to controls (Figure 5B). Six of the 8 individuals without a hematologically proven myeloproliferative neoplasm had a splenic volume measurement; all 6 presented with splenic volumes higher than the median control (P=2*10−4). One individual with no sign of a myeloproliferative neoplasm had a splenic volume of 636 cubic centimeters (pink dot in Figure 5B). Even when omitting this single individual with splenomegaly, the “No MPN” individuals had larger splenic volumes than the controls (P=8*10−4). Individuals diagnosed with a myeloproliferative neoplasm all had larger splenic volumes compared to controls: for essential thrombocythemia P=0.04, for polycythemia vera P=2*10−4, and for primary myelofibrosis P=3*10−3; the trend test across the 5 groups showed P=1*10−7.
Lactic acid dehydrogenase concentrations exceeded control levels among individuals diagnosed with essential thrombocythemia (P=2*10−5), primary myelofibrosis (P=1*10−4), and among individuals with no hematologic parameters indicative of a myeloproliferative neoplasm (P=0.03) (Figure 5C). Although individuals with polycythemia vera did not have higher lactic acid dehydrogenase concentration compared to controls, the overall trend test across the 5 groups showed P=6*10−7.
Influence of rs10974944 on myeloproliferative neoplasm status
Of all 63 JAK2V617F positive individuals, 48 were eventually diagnosed with a myeloproliferative neoplasm, 8 had no myeloproliferative neoplasm after diagnostic tests, and 7 were undiagnosed for a myeloproliferative neoplasm as they did not attend the re-examination in 2012 (1 was a non-responder and 6 died with no medical record of a myeloproliferative neoplasm diagnosis) (Table 2 and Figure 1). There was no difference in distribution of rs10974944 genotype among those with and without a diagnosis of a myeloproliferative neoplasm (X2: P=0.45) (Figure 6).
Figure 6.
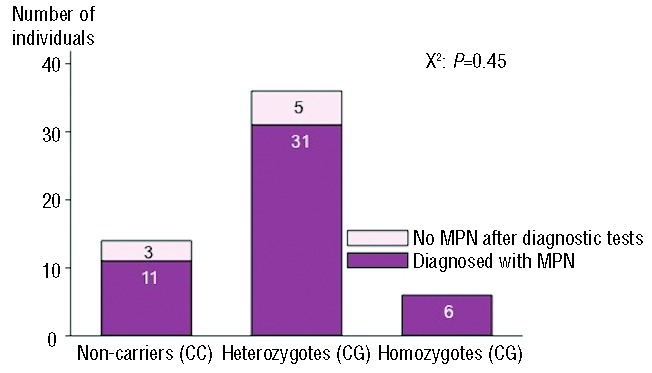
Rs10974944 genotype by myeloproliferative neoplasm status in JAK2V617F mutation positive individuals from the Danish general population. The 48 individuals diagnosed with myelproliferative neoplasm include 30 individuals (5 dead, 3 non-responders, and 22 with a known myeloproliferative neoplasm) diagnosed before the reexamination in 2012 and 18 individuals diagnosed at the re-examination. MPN: myeloproliferative neoplasm.
Discussion
In this study of 63 and 48 JAK2V617F somatic mutation positive individuals found among 49,488 individuals from the Danish general population, we observed that JAK2V617F mutation burden was associated with myeloproliferative neoplasm development and progression rate. Furthermore, we propose a cut-off point of 2% for disease versus no disease for JAK2V617F mutation positive individuals; however, even individuals with JAK2V617F mutation burden below 2% should receive medical attention as our results suggest that, in time, many of them will develop a myeloproliferative neoplasm.
Our findings are consistent with the hypothesis of a biological continuum from JAK2V617F mutation positive individuals from no disease through essential thrombocythemia and polycythemia vera to primary myelofibrosis, like that described in recent studies,7–9 as we did find an increase in JAK2V617F mutation burden across the severity of myeloproliferative neoplasm diagnoses. Furthermore, a novel observation was that no individuals with a myeloproliferative neoplasm were found in cases with an allele burden below 2%, which may represent a cut-off point of disease versus no disease for JAK2V617F mutation positive individuals. Also, as there was a median time span of 5.4 years between the first measurement of JAK2V617F mutation burden at the general population examination in 2003–2008 and at re-examination in 2012, we were able to describe the increase in the rate of allelic burden in JAK2V617F mutation positive individuals from the general population with no diagnosis of myeloproliferative neoplasms. This is also a novel observation. Among individuals diagnosed with a myeloproliferative neoplasm at re-examination, the JAK2V617F mutation burden increased by 0.55% per year between the general population examination and the re-examination. This is unlikely to be explained by error of the mutation detection technique as our assay had coefficient of variation of 1.4% at 3% mutation burden and 2.5% at 30% mutation burden; nevertheless, if we excluded the person with the 4-fold increase in mutation burden over time, the mutation burden increase was lower at 0.31% per year. In comparison, Theocharides et al.17 found a 9% increase in mutation burden among 16 JAK2V617F positive patients without cytoreductive therapy, during a follow up of 36±13 months. Our study is, however, not directly comparable to the study performed by Theocharides et al. as they also included JAK2 exon 12 allele burden and used DNA from purified granulocytes. Nevertheless, our and their findings together support the present understanding of the natural course of myeloproliferative neoplasms as being diseases that develop over several years.5,17,18 The fact that JAK2V617F mutation burden was unchanged in individuals without a hematologically proven myeloproliferative neoplasm also indicates a long subclinical, and consequently a long undiagnosed phase before myeloproliferative neoplasms become clinically overt. Since individuals without hematologic parameters indicative of a myeloproliferative neoplasm at the time of re-examination had higher splenic volumes and plasma lactic acid dehydrogenase concentrations compared to their respective control groups, it seems likely that all individuals testing positive for the JAK2V617F mutation will ultimately develop a myeloproliferative neoplasm.
In this study, we also showed changes during follow up of clinically relevant hematologic parameters in JAK2V617F positive individuals diagnosed with a myeloproliferative neoplasm at re-examination versus individuals without a hematologically proven myeloproliferative neoplasm. As expected, erythrocyte volume fraction increased during follow up in individuals with a myeloproliferative neoplasm. This is probably the natural course of the disease and indicates that without treatment the disease will progress. Erythrocyte mean corpuscular volume also increased during follow up in both those with and those without a diagnosis of a myeloproliferative neoplasm at re-examination. This, however, is rather surprising, as individuals in both groups were untreated. Platelet counts did not change during follow up in either group, which, however, could be explained by the fact that individuals in both groups already had high platelet counts at the general population examination. Therefore, the measurements might represent stability of the high platelet count during the observation period. Similarly, the low erythropoietin concentrations did not decrease further during follow up, which might be due to an already suppressed erythropoietin level at the general population examination that might not decrease any further despite disease progression.
Finally, we analyzed presence of the rs10974944 polymorphism as an expression of the 46/1 haplotype among JAK2V617F mutation positive individuals with and without a diagnosis of myeloproliferative neoplasm showing that there was no difference in distribution of genotype between those with and those without a myeloproliferative neoplasm.
Strengths of our study include the fact that we identified JAK2V617F mutation positive individuals in the general population, thus avoiding ascertainment bias. Also, because we re-examined 48 mutation positive individuals 5.4 years after the initial examination, we could study the natural history of myeloproliferative neoplasm progression rate. Finally, we had 100% complete follow up concerning hospital diagnoses as the Danish registries do not lose track of any persons living in Denmark.
The limitations of our study should also be considered. First, it might seem that statistical power provided by the 63 and 48, respectively, mutation positives among 49,488 individuals from the general population is limited; however, despite these relatively low numbers, our results were mostly highly significant suggesting sufficient statistical power. Second, individuals with the highest JAK2V617F mutation burden may not have attended the Copenhagen General Population Study, as they may already have died or may have been too sick to attend. Such a scenario would, however, bias our results towards the null hypothesis, and thus cannot explain our findings. Third, shortfalls of the screening assay used in this study should also be considered. In our initial study, we found 68 mutation positive individuals6 of which 5 were subsequently found likely to be false positives upon an independent analysis performed two years later using the same assay and performed by the same person (CN). At first hand, this scenario suggests that the assay is not sufficiently robust; however, the 5 individuals originally found positive and later negative only had a mean mutation burden of 0.9% at the original examination. Also, the R2 between the first and the second measurement was 0.84%. Nevertheless, the suitability of a 2% mutant allele burden cut-off point for likely disease manifestation may depend on the individual assay used, as ours like other assays, is not standardized internationally. Importantly, we chose to screen DNA samples from whole blood rather than from purified granulocytes. This means that the mutant allele burden is lower than if lymphoid and mononuclear cells (which are generally not part of the malignant clone) were excluded from measurement. This may be a potential problem in the analysis of samples with an initial low mutant allele burden as fluctuations in the clone size over time may influence the apparent change in allelic burden (positively or negatively). Finally, as our study population was of Caucasian origin, our results may not necessarily apply to other ethnic groups. On the other hand, we are not aware of data suggesting that our results should not be applicable to all races, particularly as the JAK2V617F somatic mutation has been found in people from different races.4,19,20
In conclusion, we demonstrate that increased JAK2V617F mutation burden is associated with myeloproliferative neoplasm progression rate in the general population. Furthermore, we established a JAK2V617F mutation burden cut-off point of 2% indicative of disease versus no disease. However, our results also suggest that individuals with a mutation burden below 2% and no clinical phenotype suggestive of a myeloproliferative neoplasm, may still suffer from a latent form of myeloproliferative neoplasm that may only be traced through a slightly larger spleen and/or slightly higher lactic acid dehydrogenase concentrations compared to controls.
Acknowledgments
The authors would like to thank the staff and participants of the Copenhagen General Population Study for their important contribution and their willingness to participate. We also thank Prof. Dr. W. Fiedler, Universitätsklinikum, Hamburg-Eppendorf, Germany, for providing us with the UKE1 cell line.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was supported by the University of Copenhagen; the Foundation of Anders Hasselbalch against Leukemia; Herlev Hospital, Copenhagen University Hospital; Copenhagen County Foundation; the Chief Physician Johan Boserup and Lise Boserup Foundation; the Danish Cancer Research Foundation; and the Danish Cancer Society.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22. [DOI] [PubMed] [Google Scholar]
- 2.de Stefano V, Fiorini A, Rossi E, Za T, Farina G, Chiusolo P, et al. Incidence of the JAK2 V617F mutation among patients with splanchnic or cerebral venous thrombosis and without overt chronic myeloproliferative disorders. J Thromb Haemost. 2007; 5(4):708–14. [DOI] [PubMed] [Google Scholar]
- 3.Sidon P, El Housni H, Dessars B, Heimann P. The JAK2V617F mutation is detectable at very low level in peripheral blood of healthy donors. Leukemia. 2006; 20(9):1622. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Zhang Q, Luo J, Xing S, Li Q, Krantz SB, et al. JAK2(V617F): Prevalence in a large Chinese hospital population. Blood. 2007; 109(1):339–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen C, Birgens HS, Nordestgaard BG, Kjaer L, Bojesen SE. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica. 2011;96(3):450–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen C, Birgens HS, Nordestgaard BG, Bojesen SE. Diagnostic value of JAK2 V617F somatic mutation for myeloproliferative cancer in 49 488 individuals from the general population. Br J Haematol. 2013; 160(1):70–9. [DOI] [PubMed] [Google Scholar]
- 7.Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366(9501):1945–53. [DOI] [PubMed] [Google Scholar]
- 8.Larsen TS, Pallisgaard N, Moller MB, Hasselbalch HC. The JAK2 V617F allele burden in essential thrombocythemia, polycythemia vera and primary myelofibrosis–impact on disease phenotype. Eur J Haematol. 2007;79(6):508–15. [DOI] [PubMed] [Google Scholar]
- 9.Vannucchi AM, Pieri L, Guglielmelli P. JAK2 Allele Burden in the Myeloproliferative Neoplasms: Effects on Phenotype, Prognosis and Change with Treatment. Ther Adv Hematol. 2011;2(1):21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008; 299(21):2524–32. [DOI] [PubMed] [Google Scholar]
- 11.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. [DOI] [PubMed] [Google Scholar]
- 12.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359(18):1897–908. [DOI] [PubMed] [Google Scholar]
- 13.Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009; 41(4):446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009;41(4):455–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olcaydu D, Harutyunyan A, Jager R, Berg T, Gisslinger B, Pabinger I, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41(4):450–4. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–9. [PubMed] [Google Scholar]
- 17.Theocharides A, Passweg JR, Medinger M, Looser R, Li S, Hao-Shen H, et al. The allele burden of JAK2 mutations remains stable over several years in patients with myeloproliferative disorders. Haematologica. 2008;93(12):1890–3. [DOI] [PubMed] [Google Scholar]
- 18.Larsen TS, Pallisgaard N, Moller MB, Hasselbalch HC. The JAK2 V617F allele burden in essential thrombocythemia, polycythemia vera and primary myelofibrosis–impact on disease phenotype. Eur J Haematol. 2007;79(6):508–15. [DOI] [PubMed] [Google Scholar]
- 19.Anderson LA, Duncombe AS, Hughes M, Mills ME, Wilson JC, McMullin MF. Environmental, lifestyle, and familial/ethnic factors associated with myeloproliferative neoplasms. Am J Hematol. 2012;87(2):175–82. [DOI] [PubMed] [Google Scholar]
- 20.Pagliarini-e-Silva S, Santos BC, Pereira EM, Ferreira ME, Baraldi EC, Sell AM, et al. Evaluation of the association between the JAK2 46/1 haplotype and chronic myeloproliferative neoplasms in a Brazilian population. Clinics (Sao Paulo). 2013;68(1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


