Abstract
The prognostic significance of early response to treatment has not been reported in relapsed pediatric acute myeloid leukemia. In order to identify an early and easily applicable prognostic factor allowing subsequent treatment modifications, we assessed leukemic blast counts in the bone marrow by morphology on days 15 and 28 after first reinduction in 338 patients of the international Relapsed-AML2001/01 trial. Both day 15 and day 28 status was classified as good (≤20% leukemic blasts) in 77% of patients. The correlation between day 15 and 28 blast percentages was significant, but not strong (Spearman correlation coefficient = 0.49, P<0.001). Survival probability decreased in a stepwise fashion along with rising blast counts at day 28. Patients with bone marrow blast counts at this time-point of ≤5%, 6–10%, 11–20% and >20% had 4-year probabilities of survival of 52%±3% versus 36%±10% versus 21%±9% versus 14%±4%, respectively, P<0.0001; this trend was not seen for day 15 results. Multivariate analysis showed that early treatment response at day 28 had the strongest prognostic significance, superseding even time to relapse (< or ≥12 months). In conclusion, an early response to treatment, measured on day 28, is a strong and independent prognostic factor potentially useful for treatment stratification in pediatric relapsed acute myeloid leukemia. This study was registered with ISRCTN code: 94206677.
Introduction
Survival of children after a relapse of acute myeloid leukemia (AML) is generally poor1,2 but has improved for several groups over the last 20 years along with better treatment approaches.3–7 The most recently published study reported a probability of survival after relapse of only 38% at 4 years.8 In general, patients who relapse are currently treated with very intensive reinduction chemotherapy followed by hematopoietic stem cell transplantation from either a related or unrelated donor.3,6
Until now the time to relapse or the duration of first complete remission has been considered the most important prognostic factor for outcome after relapse in pediatric AML.6,9,10 The 3- to 5-year survival rates are reported to be in the range of only 10 to 28% in patients who have an early relapse (relapse occurring within 12 – 18 months from diagnosis) compared to 40–48% in those whose relapse occurs later.7,8,10 Other factors described to be prognostic in the relapse setting are French-American-British subtypes, cytogenetics and intensity of first-line treatment, particularly with or without allogeneic hematopoietic stem cell transplantation.5–7
A good early treatment response in newly diagnosed AML, whether assessed morphologically, molecularly or by immunophenotyping, is accepted as a favorable prognostic indicator, but the significance of early response in relapsed AML in children has not previously been studied. Our aim was to compare the prognostic significance of early treatment response at day 15 and day 28 in order to identify those patients who have only minimal chances of cure with current (standard) relapse therapy. This was studied within the international trial, Relapsed AML 2001/01.8
Methods
Between November 2001 and April 2009, 568 patients under 21 years old of age were enrolled into the prospective international study, Relapsed AML 2001/01: these patients came from 13 study groups and 515 had a first relapse while 53 had primary refractory AML.8 Patients with acute promyelocytic leukemia and myeloid leukemia in Down syndrome were not included. This analysis was based on 546 patients, because 22 patients with isolated extramedullary relapse were excluded.
Early treatment response was evaluated microscopically by bone marrow (BM) blast percentage on day 15 and day 28 of first reinduction (for details see the Online Supplement). The core group for this analysis consisted of 338 patients with data from BM examinations on both day 15 and day 28. Day 15 BM findings did not influence subsequent therapy, while patients with more than 20% blasts on day 28 were off-protocol and eligible for more experimental or even palliative therapy.
The human investigations were performed after approval by all local ethical committees and by the departments of Health and Human Services and in accordance with the Declaration of Helsinki (ISRCTN code: 94206677).
Treatment
Reinduction therapy with fludarabine, cytarabine, and granulocyte colony-stimulating factor (FLAG) was randomized against FLAG plus liposomal daunorubicin (L-DNR/FLAG) in first reinduction. L-DNR (DaunoXome®, DNX) was chosen because of its potentially low cardiotoxicity.8 Second reinduction for all patients was scheduled with FLAG. Patients with ≤20% blasts on day 28 were given consolidation with cytarabine and etoposide or thioguanine and cytarabine to bridge the time until hematopoietic stem cell transplantation. Patients with >20% blasts on day 28 were “off study” and could receive new therapeutic options.
Definitions
Second complete remission was defined as ≤5% leukemic blasts in the BM with regeneration of the peripheral blood counts (platelets >50×109/L, neutrophils >1.0×109/L) and no leukemic blasts. Primary refractory disease was defined as the failure to achieve complete remission in newly diagnosed AML. Relapse was considered to have occurred in the presence of recurrence of ≥10% unequivocal leukemic cells in BM, and/or leukemic infiltrations at any site after the first complete remission. Early relapse was defined as a relapse occurring within 1 year, whereas a late relapse was defined as a relapse occurring 1 year or more after the initial diagnosis. Poor early treatment response was defined by a BM blast count of >20% after induction (on day 15 or day 28). The morphological favorable subgroups were French-American-British subtype M1/2 with Auer rods and M4eo,11 while favorable cytogenetics were t(8;21) or inv(16).
Statistics
The primary endpoint of the study was the day 28 BM status. Secondary endpoints were day 15 BM status, second complete remission and long-term survival. Overall survival was defined as the time to death or last follow-up. The Kaplan-Meier method was used to estimate survival rates; differences were compared with the two-sided log-rank test. Differences in proportions were assessed by the χ2 test. The Cox proportional hazards model was used for univariate and multivariate analyses. P values ≤0.05 were considered statistically significant, and if >0.05 but ≤0.10 of borderline statistical significance. Results are presented as estimated probability of 4-year overall survival with standard error (± SE). All living patients were censored at time of their last follow-up, but no later than January 1, 2011.
Results
Patients’ characteristics
Of the 496 patients for whom day 28 data were available, 338 patients also had data for day 15. Comparing this core-group of 338 patients (data available from both day 15 and day 28) with all the other patients (n=208), the latter cohort contained fewer patients with favorable cytogenetics (P=0.01) or favorable morphology (P=0.01), and fewer patients with a late relapse (P<0.001) or younger age (P<0.001). White blood cell counts were not different between these cohorts (Online Supplementary Table S1).
The survival rates were inferior in the 208 patients without data from both time points than in the core group (4-year probability of overall survival 30%±3% versus 41% ±3%, Plogrank=0.0003). This difference can be partially explained by the fact that patients who died early could not be evaluated on day 15 and day 28.
Table 1 shows the characteristics of the core cohort (n=338) with BM data on day 15 and day 28. Subgroups of patients with favorable morphology and favorable cytogenetics more often had ≤20% blasts on day 15 (P=0.001 and P=0.0001, respectively) and on day 28 (P=0.0001 and P≤0.00001, respectively). Only 2/71 (3%) patients with favorable cytogenetics had ≥20% blasts on day 28 compared to 69/240 (29%) patients with other cytogenetic findings.
Table 1.
Characteristics of the cohorts of patients with ≤ or > 20% BM blasts on day 15 and day 28.
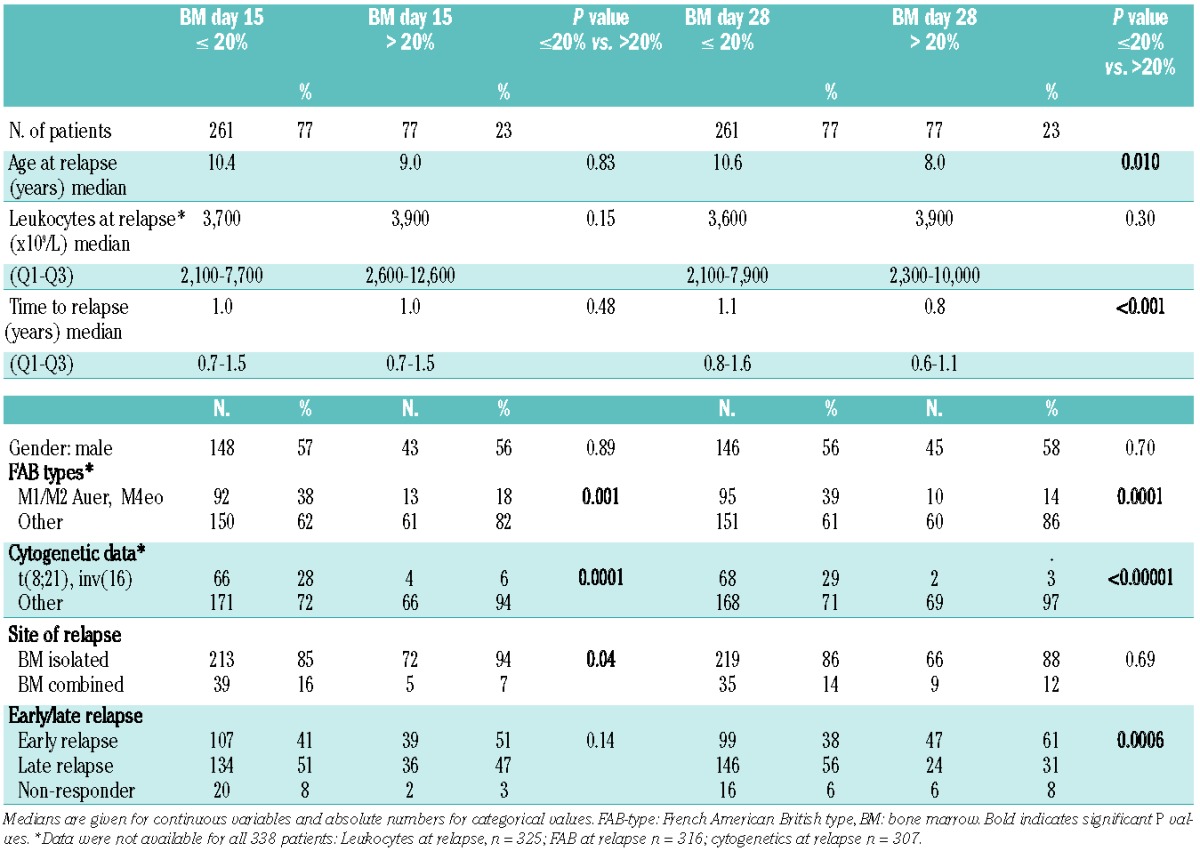
Whereas the treatment response measured on day 15 did not clearly differ between patients according to time to relapse [early relapse ≥20% blasts, n=39/146 (27%) versus late relapse n= 36/170 (21%) P=0.28], significantly more patients with an early relapse (n=47/146, 32%) had a poor treatment response on day 28 compared to those with late relapse (n=24/170, 14%, P=0.00017).
Correlation between the bone marrow blast counts on days 15 and 28
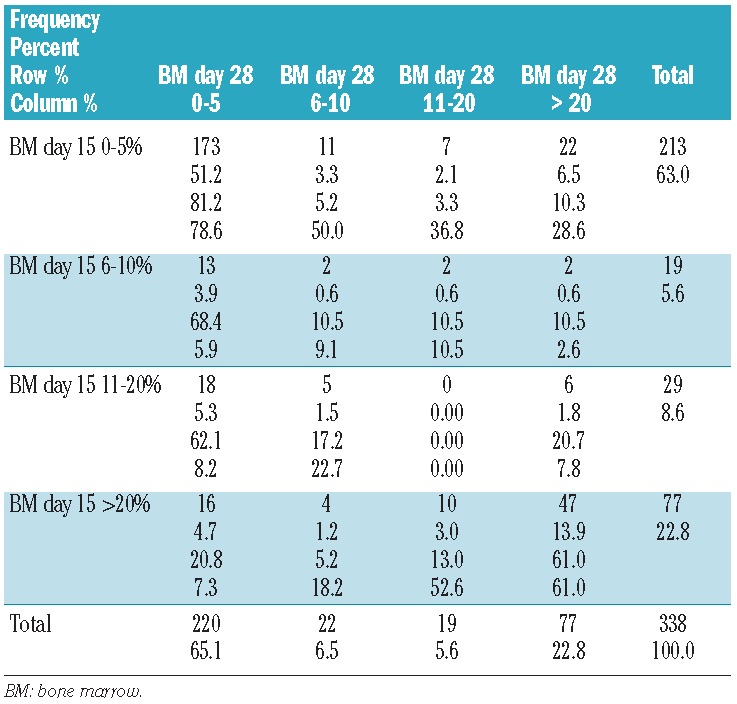
Table 2 shows the correlation between the BM blast counts on day 15 and day 28 in patients with data at both time points. Remarkably few patients were allocated to the subgroups with 6–10% and 11–20% blasts at both time points. The blast percentages on day 15 and day 28 correlated significantly, but not very strongly (Spearman correlation coefficient=0.49, P<0.001). The majority of patients with ≤20% blasts on day 15 (n=261) also had ≤20% blasts on day 28 (231/261 = 89%, Table 2). However, 30 out of 77 (39%) patients with >20% blasts on day 15 had ≤20% blasts on day 28. These 30 patients had a better outcome than the remaining 47 patients (see below). Only 22 of the 77 patients (29%) with >20% blasts on day 28 had <5% blasts on day 15.
Table 2.
Correlation between the BM blast counts on day 15 and day 28.
Outcome in patients according to response on day 15 and/or day 28
Seventy-seven percent of the 338 patients in the core group showed a favorable response to reinduction (≤20% blasts) both on day 15 and day 28. Overall, the 4-year survival in these core-group patients was 41±3%. There were clear differences in survival rates between good and poor responders at both time points (day 15: 47±3% versus 21± 5%, day 28: 48±3% versus 14±4%), showing a trend towards an even worse outcome in poor responders at day 28. Interestingly, while the survival rates were similar in the subgroups with BM blast counts of 0–5%, 6–10% and 11–20% on day 15 (Figure 1), there was a significant, stepwise trend to worsening survival with the categories of increasing blast counts at day 28 (Figure 2) indicating that the day 28 count is a more fine-tuned and reliable predictor of survival (Table 3). When combining the BM data from day 15 and day 28 (Figure 3), the combination “day 15 >20% blasts” but “day 28 <20% blasts” indicated an intermediate survival probability after relapse, while patients with ≤20% blasts on both day 15 and day 28 had a higher survival rate, and patients with >20% blasts on day 28 faced a very poor outcome regardless of the day 15 BM result.
Figure 1.
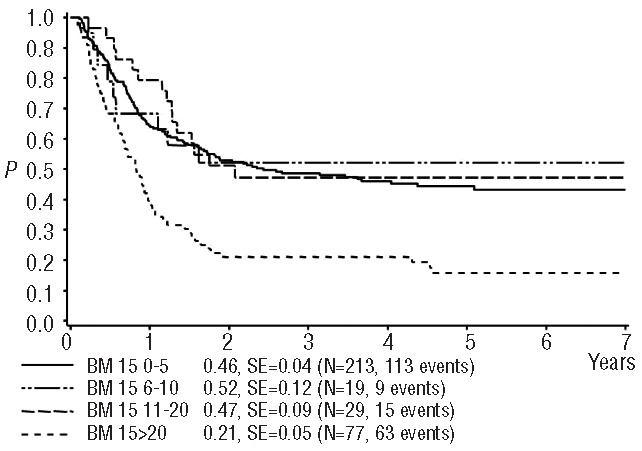
Probability of 4-year survival after relapse according to blast count on day 15. SE = standard error, 0–5% vs. >20%, P(logrank) <0.0001; 6–10% vs. >20% P=0.011; 11–20% vs. >20% P=0.0011; all other comparisons P>0.68.
Figure 2.
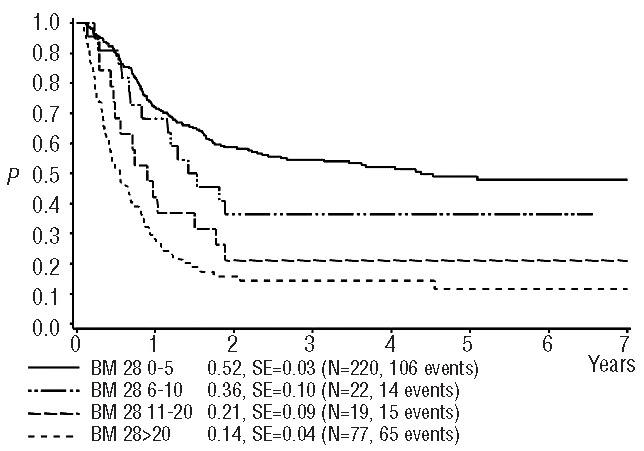
Probability of 4-year survival after relapse according to blast count on day 28. SE = standard error. 0–5% vs. 11–20%, P(logrank)=0.0010; 0–5% vs. >20%, P<0.0001; 6–10% vs. >20%, P=0.0031; all other comparisons P>0.15.
Table 3.
Summary of achievement of second complete remission and 4-year survival results according to day 15 and day 28 BM blasts counts.
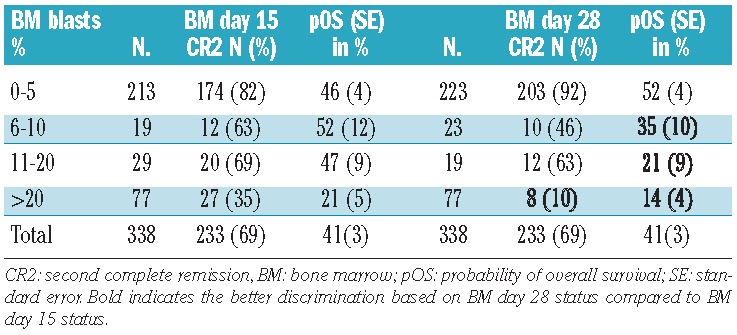
Figure 3.
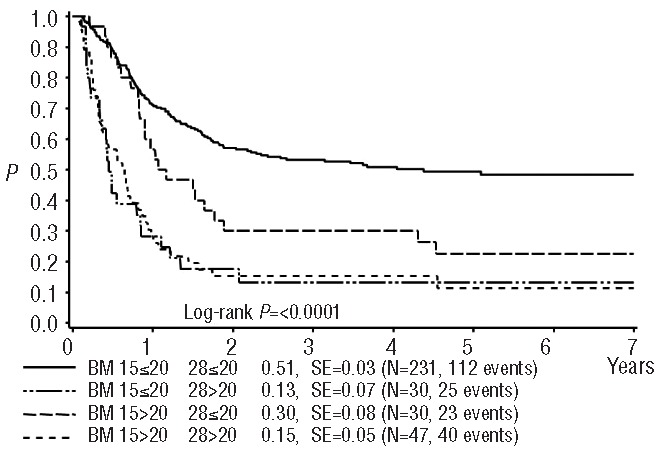
Probability of 4-year survival after relapse combining blast count data on day 15 and day 28. SE = standard error. BM day 15 ≤20% and day 28 ≤20% vs. BM day 15 ≤20% and day 28 >20%, P(logrank)<0.0001; BM day 15 ≤20% and day 28 ≤20% vs. BM day 15 >20% and day 28 ≤20% P=0.0070; BM day 15 ≤20% and day 28 ≤20% vs. BM day 15 >20% and day 28 >20%, P<0.0001; BM day 15 ≤20% and day 28 >20% vs. BM day 15 >20% and day 28 ≤20%, P=0.0089; BM day 15 ≤20% and day 28 >20% vs. BM day 15 >20% and day 28 >20%, P=0.76; BM day 15 >20% and day 28 ≤20% vs. BM day 15 >20% and day 28 >20%, P=0.013.
Results according to achievement of second remission
One-hundred and five patients of the core group did not achieve a second complete remission (4-year survival 13±3%) whereas 233 patients did (4-year survival 53± 3%, P(logrank) ≤0.0001). Patients with a poor early treatment response on day 15 had a second complete remission rate of 35%, while the rate was extremely low (10%) in those with a poor response based on day 28 BM data. Second complete remission rates mirrored survival rates in that they fell proportionately with an increasing blast count on day 28 rather than with the day 15 blast count (Table 3).
Early treatment response in relation to the time to relapse
Early treatment response appeared to have stronger prognostic significance than time to relapse, since patients with either an early or a late relapse and a day 28 BM blast count of ≤20% had only a slightly different, relatively favorable survival rate (4-year overall survival 41±5% and 55±4%, respectively, P=0.05). Likewise, the survival of patients with day 28 BM blast counts >20% was poor in both patients with early and late relapses with 4-year overall survival probabilities of 8±4% and 25±9%, respectively (P=0.02) (Figure 4). However, it should be mentioned that a larger proportion of those with a late relapse had a good response compared to those with an early relapse (86% versus 68%, P<0.001).
Figure 4.
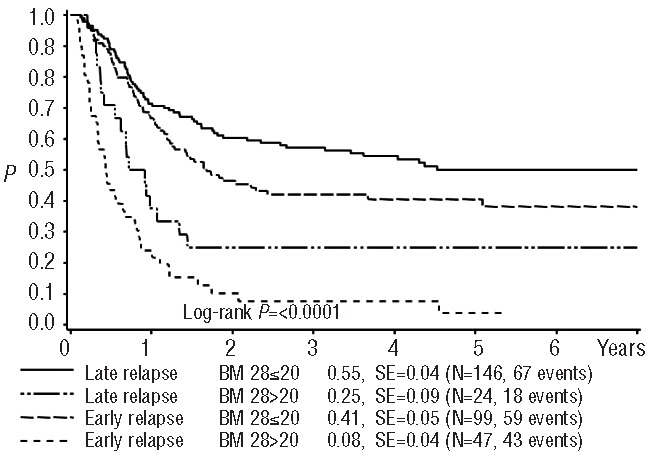
Probability of 4-year survival after relapse according to time of relapse and blast count on day 28. SE = standard error. Late relapse and BM day 28 ≤20% vs. late relapse and BM day 28>20%, P(logrank)=0.0004; late relapse and BM day 28 ≤20% vs. early relapse and BM day 28 ≤20%, P=0.05; late relapse and BM day 28 ≤20% vs. early relapse and BM day 28 >20%, P<0.0001; late relapse and BM day 28 >20% vs. early relapse and BM day 28 ≤20%, P=0.028; late relapse and BM 28 >20% vs. early relapse and BM day 28 >20%, P=0.022; early relapse and BM 28 ≤20% vs. early relapse and BM day 28 >20%, P<0.0001.
Multivariate analysis
In a model combining time to relapse (early versus late) and BM status on day 15 and day 28 (poor versus good), early treatment response, as determined on day 28, was the strongest independent prognostic factor. In a Cox regression analysis of survival adding cytogenetics, favorable cytogenetics was a strong prognostic factor (risk ratio 0.4, 95% confidence interval 0.2–0.6; P<0.001), BM day 15 blast count lost statistical significance (risk ratio 1.2, P=0.43), and a BM day 28 blast count of >20 % (risk ratio 2.5, 95% confidence interval 1.6–4.0; P<0.001) and early relapse (risk ratio 1.6, 95% confidence interval 1.2–2.3; P=0.005) each independently predicted poor outcome after relapse.
The prognostic value of blast count was independent of the treatment effect. There was no significant interaction between treatment effect (FLAG only versus L-DNR/FLAG) and BM blast count on day 15 (P=0.3) or day 28 (P=0.4).
Comparison of day 28 data based on morphology and immunophenotyping
In a limited subset of 20 patients including several whose samples gave an uncertain morphological result at day 28 we piloted a parallel assessment also by immunophenotyping (for technical aspects, including the antibody panel, see Langebrake et al.12).
In eight patients blasts had been suspected on the basis of morphological studies; six of these cases were confirmed by immunophenotyping and two were found not to be evaluable by immunophenotyping because of poor material. Of 12 patients with no blasts by morphology, seven were also negative by immunophenotyping, four patients had 1–2% blasts and one patient had 20% blasts by immunophenotyping.
Discussion
Prognostic factors at diagnosis and relapse are most useful if they influence treatment decisions. While no subgroup has a sufficiently favorable outcome to justify treatment reduction, intensive AML reinduction therapy currently leaves little room for treatment intensification.9 However, this may change upon the introduction of novel drugs and treatment modalities. Moreover, the identification of a subgroup with minimal chances of cure may result in palliative or more experimental therapy.
Response to induction chemotherapy is a well-known prognostic factor in de novo AML.11,13 However, the prognostic value of early treatment response in the setting of pediatric relapsed AML has not previously been reported. According to our results, the day 28 BM status is a strong and independent prognostic factor, allowing an even better discrimination into two risk groups than the time to relapse, which until now has generally been considered to be the most important risk factor.6,7,10 In this study, patients with an early relapse but a good early treatment response on day 28, which was observed in 68% of them, had a long-term outcome which was only slightly inferior (P=0.05) to that of patients with a late relapse and a good early treatment response (Figure 4).
A blast count >20% at day 15 and day 28 is much commoner after relapse (occurring in 23% patients) than in de novo AML (as compared with e.g. day 15 data from the AML-BFM 2004 study: occurring in 37 of 499 patients, 7%; P<0.001), indicating increased resistance to chemotherapy at relapse as compared to de novo AML.
Relapse per se is the strongest risk factor for survival and there is no definable group with relapsed AML which has an outcome sufficiently favorable to consider treatment reduction. Indeed, patients with late relapses with a good early treatment response only had a probability of overall survival at 4 years of 55%. Our main aim was, therefore, to identify a group of patients for which a poor survival can be predicted early and reliably. This group of patients with an estimated survival rate below 10–20% could be a target for experimental therapy, e.g. treatment in phase I or II studies for new agents or new hematopoietic stem cell transplantation approaches, or even palliation only.
Day 28 blast count was already the primary endpoint comparing FLAG or L-DNR/FLAG in the Relapsed AML 2001/01 study.8 The percentage of patients with >20% blasts on day 28 was 10% lower in patients treated with L-DNR/FLAG than in those treated with FLAG only; in corollary, the rate of second complete remissions was higher with L-DNR/FLAG, which thus improved the early response rate significantly. This did not translate into better survival rates in the L-DNR/FLAG group, which may be related to cross-over effects with more treatment intensification, especially in poor responders. However, this study now shows that the prognostic value of the day 28 blast count is not influenced by the treatment effect of L-DNR.
The percentage of patients with BM blast counts of ≤20% on days 15 and 28 was similar at 77%. Likewise, the gaps between the 4-year survival rates of patients with ≤20% and >20% blasts on day 15 and day 28 were almost similar. However, in contrast to the BM day 15 data, which did not discriminate between different survival rates in subgroups by blast count (0–5%, 6–10%, 11–20%), there was a clear and significant trend to worse survival with increasing BM blast counts on day 28. Thirty-nine percent of patients with BM containing >20% blasts on day 15 had ≤20% blasts in the BM on day 28, and this conversion from poor to good early treatment response was associated with a better outcome (Figure 3). This may be explained by the fact that the usually aplastic day 15 BM may still contain residual but non-proliferating leukemic blasts which are bound to subsequent disappearance upon delayed drug-action or to subsequent superimposition by normal regeneration. Conversely, on day 28 normal hematopoiesis is recovering and truly resistant leukemic blasts could regrow together with normal progenitors. In this situation and especially in patients with ≤20% blasts on day 15, but with >20% blasts on day 28 (n=30, 14% of patients) concurrent regeneration of both leukemic blasts and normal progenitors may hamper correct assessment by conventional morphological methods. Obviously, additional techniques, such as immunophenotyping and/or genetic investigations could then be of relevant discriminative value.14–16
Achievement of remission after relapse (second remission) is essential for long-term survival. However, the time point of an eventual second complete remission (according to the classical definition of both BM and peripheral blood parameters)17 may vary considerably because post-induction treatment of AML is often continued before all regeneration parameters needed to fulfill the criteria for complete remission are reached.18 Therefore, from a clinical point of view assessment of classical complete remission will not allow timely treatment modifications.
By multivariate analysis, favorable cytogenetics (present in 23% of relapsed patients) was a strong favorable prognostic factor: only two of 71 patients with favorable cytogenetics had >20% blasts on day 28, and 64 patients had a blast count ≤5%. For the majority of patients, early treatment response on day 28 was the strongest prognostic factor, which, at least partly, also superseded the time to relapse.
In brief, an early response, as assessed microscopically, is an overriding prognostic factor in pediatric relapsed AML – even in an internationally collaborative trial involving many sites. It is robust enough to discriminate between subgroups with significantly different probabilities of second complete remission and of overall survival. This is of great relevance for the design of upcoming trials because it allows for early treatment modifications in selected cohorts of patients. Moreover, microscopic evaluation is usually available in centers dedicated to care of children with leukemia even in case of limited economic or technical resources.
Nevertheless, we are aware that morphological assessment has limitations. First of all, investigator experience is essential and even then, it remains a subjective method. A well-known problem is the discrimination between normal blasts occurring with hyper-regeneration of progenitors and leukemic blasts after aplasia. Also, low numbers of leukemic blasts can be overlooked. In such situations, other diagnostic methods, such as immunophenotyping, can render additional information or proof of cellular benignity.14–16 In a small and selected group of patients in our study, there was a good concordance between blast counts evaluated by morphology and immunophenotyping, but immunophenotyping was superior in evidencing leukemic cells when the counts were <5% as well as even in one case with 20% blasts which were misinterpreted by morphology as being normal. False-positivity of morphological findings was not an issue in this cohort. Notably, immunophenotyping results were experimental and not used for clinical decisions.
In summary, the microscopic day 28 BM blast count is a strong and independent prognostic factor in pediatric relapsed AML. It has better discriminative value than the time to relapse or the day 15 BM status. Treatment evaluation on day 28 of therapy still allows treatment modifications, which might eventually improve the poor outcome that patients with >20% leukemic blasts at that time-point currently face. Additional techniques, such as multicolor immunophenotyping, are likely to improve the discrimination between normal and leukemic blasts at early time points, and may add benefit to risk-group adapted therapy in pediatric relapsed AML.
Acknowledgments
As an investigator-initiated study, this study was not funded nor were drugs provided free-of-charge.
DSMC: we thank the members of the Data Safety and Monitoring Committee for their willingness to perform this task. We also thank the patients, families, clinicians, nurses and data managers in all hospitals and offices for their participation in this study.
We thank Olga Aleinikova, MD from the Belarus Research Center of Pediatric Oncology and Hematology Minsk and Daniel Freigeiro, MD from the Grupo Argentino de Tratamiento de la Leucemia Aguda (GATLA) for their cooperation and the data they provided.
With respect to data management we would like to thank the following persons, other than those who already are co-authors: BFM-AML-G and international data center, Jans-Enno Müller; AIEOP: Roberto Rondelli; BFM-A: Nora Mühlegger; CPH: Alena Vrzalova; DCOG: Karin van der Pal and Ardine Reedijk; FRALLE: Brigitte Ravis; GATLA: Francisco J. Lastiri; HKPHOSG: Joyce Le; Israel: David Steinberg; UK-CCLG: Rachael Clack; NOPHO: Göran Gustafsson; St. Jude: Annette Stone.
This study was done in the setting of the International BFM Study Group (chaired by Martin Schrappe), and was initiated by the AML Relapse Working Group (chair, Gertjan Kaspers) and the AML committee (current chair, Dirk Reinhardt).
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Goemans BF, Tamminga RY, Corbijn CM, Hahlen K, Kaspers GJ. Outcome for children with relapsed acute myeloid leukemia in the Netherlands following initial treatment between 1980 and 1998: survival after chemotherapy only? Haematologica 2008;93(9):1418–20. [DOI] [PubMed] [Google Scholar]
- 2.Gorman MF, Ji L, Ko RH, Barnette P, Bostrom B, Hutchinson R, et al. Outcome for children treated for relapsed or refractory acute myelogenous leukemia (rAML): a Therapeutic Advances in Childhood Leukemia (TACL) Consortium study. Pediatr Blood Cancer. 2010;55(3):421–9. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamsson J, Clausen N, Gustafsson G, Hovi L, Jonmundsson G, Zeller B, et al. Improved outcome after relapse in children with acute myeloid leukaemia. Br J Haematol. 2007;136(2):229–36. [DOI] [PubMed] [Google Scholar]
- 4.Aladjidi N, Auvrignon A, Leblanc T, Perel Y, Benard A, Bordigoni P, et al. Outcome in children with relapsed acute myeloid leukemia after initial treatment with the French Leucemie Aique Myeloide Enfant (LAME) 89/91 protocol of the French Society of Pediatric Hematology and Immunology. J Clin Oncol. 2003;21(23): 4377–85. [DOI] [PubMed] [Google Scholar]
- 5.Rubnitz JE, Razzouk BI, Lensing S, Pounds S, Pui CH, Ribeiro RC. Prognostic factors and outcome of recurrence in childhood acute myeloid leukemia. Cancer. 2007; 109(1):157–63. [DOI] [PubMed] [Google Scholar]
- 6.Sander A, Zimmermann M, Dworzak M, Fleischhack G, von Neuhoff C, Reinhardt D, et al. Consequent and intensified relapse therapy improved survival in pediatric AML: results of relapse treatment in 379 patients of three consecutive AML-BFM trials. Leukemia. 2010; 24(8):1422–8. [DOI] [PubMed] [Google Scholar]
- 7.Webb DK, Wheatley K, Harrison G, Stevens RF, Hann IM. Outcome for children with relapsed acute myeloid leukaemia following initial therapy in the Medical Research Council (MRC) AML 10 trial. MRC Childhood Leukaemia Working Party. Leukemia. 1999;13(1):25–31. [DOI] [PubMed] [Google Scholar]
- 8.Kaspers GJ, Zimmermann M, Reinhardt D, Gibson BE, Tamminga RY, Aleinikova O, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 2013;31(5):599–607. [DOI] [PubMed] [Google Scholar]
- 9.Kaspers GJ. Pediatric acute myeloid leukemia. Expert Rev Anticancer Ther. 2012; 12(3):405–13. [DOI] [PubMed] [Google Scholar]
- 10.Stahnke K, Boos J, Bender-Gotze C, Ritter J, Zimmermann M, Creutzig U. Duration of first remission predicts remission rates and long-term survival in children with relapsed acute myelogenous leukemia. Leukemia. 1998;12(10):1534–38. [DOI] [PubMed] [Google Scholar]
- 11.Creutzig U, Zimmermann M, Ritter J, Henze G, Graf N, Loffler H, et al. Definition of a standard-risk group in children with AML. Br J Haematol. 1999; 104(3):630–9. [DOI] [PubMed] [Google Scholar]
- 12.Langebrake C, Creutzig U, Dworzak M, Hrusak O, Mejstrikova E, Griesinger F, et al. Residual disease monitoring in childhood acute myeloid leukemia by multiparameter flow cytometry: the MRD-AML-BFM Study Group. J Clin Oncol. 2006;24(22): 3686–92. [DOI] [PubMed] [Google Scholar]
- 13.Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003;101(1):64–70. [DOI] [PubMed] [Google Scholar]
- 14.Inaba H, Coustan-Smith E, Cao X, Pounds SB, Shurtleff SA, Wang KY, et al. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol. 2012;30(29):3625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loken MR, Alonzo TA, Pardo L, Gerbing RB, Raimondi SC, Hirsch BA, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children’s Oncology Group. Blood. 2012;120(8):1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6): 543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. [DOI] [PubMed] [Google Scholar]
- 18.Creutzig U, van den Heuvel-Eibrink MM, Gibson B, Dworzak MN, Adachi S, de Bont E, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120(16): 3187–205. [DOI] [PubMed] [Google Scholar]


