Abstract
Bortezomib is an active agent in AL amyloidosis and responses to this drug in combination with cyclophosphamide and dexamethasone are both rapid and deep. Here we present an international, multicenter series of 60 patients with Mayo Clinic stage III cardiac amyloidosis to assess the impact of this regimen in improving outcomes in this poor-risk group. The median follow-up for the entire cohort is 11.8 months. The overall response rate was 68%. In a landmark analysis, examining patients who survived more than 3 months, the overall response rate was 86%. A cardiac response was seen in 32% of patients. The estimated 1-year survival rate for the whole cohort was 57% and 24 patients (40%) died while on therapy. Although unable to save the poorest risk patients, the combination of bortezomib, cyclophosphamide and dexamethasone can achieve a high number of hematologic and cardiac responses, likely improving overall survival and justifying a prospective trial.
Introduction
AL amyloidosis is a disease of protein misfolding with the monoclonal light chain component of intact immunoglobulin being the precursor protein leading to amyloid deposits within tissues. Progressive deposition leads to organ dysfunction and eventually organ failure.
Cardiac involvement is a source of significant morbidity and mortality in this disease.1 The original Mayo Clinic cardiac staging system developed by Dispenzieri et al. was instrumental in establishing a means of rapidly identifying this high-risk group of patients.2 It is now the standard prognostic model used in the stratification of AL amyloid.
Patients with stage III disease comprise a large proportion of the subjects who undergo the early death often seen in this condition and there is an ongoing unmet need for effective therapies in this population.2–5 Recent, published experience with bortezomib-containing regimens holds promise with high rates of deep clonal responses achieved.6–13 In addition, the responses seem to be rapid, frequently occurring within the first cycle of therapy, and the best response is often achieved within two cycles.11,12 A number of different strategies have since been used in many centers to incorporate bortezomib into different treatment regimens, particularly in patients with advanced cardiac disease. Prospective data on such combinations are still lacking, especially in patients with advanced cardiac involvement. Such patients risk clinical decompensation and sudden death as well as unexpected adverse events related both to the direct toxicity of the treatment and to the amyloid burden during therapy.
In this article we present a multi-institutional study of bortezomib, cyclophosphamide, and dexamethasone (VCD) used as upfront therapy in patients with Mayo Clinic stage III disease. We examine depth of response, and the impact on overall survival and toxicity. In addition, we examine other baseline features in the hope of further characterizing the risk of death in patients with advanced cardiac involvement. A landmark analysis at 3 months was done to evaluate outcomes in patients who lived long enough to benefit from clonal control.
Methods
Additional details regarding the methods of this study can be found in the Online Supplementary Appendix.
Population
This is a retrospective analysis of treatment-naïve patients treated with VCD as upfront therapy. All patients had confirmed symptomatic AL amyloidosis, cardiac involvement and Mayo Clinic stage III disease. The dataset was based on patients’ information collected at four central referral institutions in the United States of America (14 patients), United Kingdom (20 patients), and France (26 patients) between November 2008 and April 2012. Approval for the collection, analysis, and publication of patients’ data was obtained from the ethics committee or institutional review board of each center.
Evaluation
The diagnosis of AL amyloidosis and assessment of organ involvement was performed locally based on consensus criteria published in 200514 and modified in 2012.15 The main outcome assessed was hematologic response, as previously described.15 A complete response was defined as having no evidence of clonal disease in serum or urine, determined by electrophoresis and immunofixation, with normal serum free light chain levels and ratio. The difference between the involved and the uninvolved free light chain serum levels (dFLC) was determined in order to assess responses, with a very good partial response (VGPR) being defined by a post-treatment dFLC level below 40 mg/L and a partial response by a 50% drop in dFLC serum level.15 Mayo stage was determined, when possible, using the original criteria.2 As high-sensitivity troponin (hsT) has been used in France as the standard marker for this parameter, hs-cTnT was used and a cut-off of 0.07 ng/L was chosen instead of the recently proposed cut-off of 0.05 ng/L16 as it seems to be the best threshold in this population using receiver operating characteristics (ROC) curves for prediction of survival and to be sure that the patients selected with this criteria did not have less severe cardiac involvement compared to the original one.
The cohort was analyzed on an intention-to-treat (ITT) basis and patients who died before hematologic response could be assessed were deemed non-responders. Organ responses were assessed based on published criteria.14,15 Overall survival was calculated from diagnosis until death or last follow-up. Given the high proportion of early deaths, a landmark analysis was performed at 3 months to examine outcomes in patients who would have survived long enough to receive at least three cycles of therapy and benefit from clonal control over the long-term. Within the limitations of a retrospective study, tolerability and toxicity data were also collected.
Treatment
The treatment consisted of bortezomib (1.0–1.5 mg/m2) administered either weekly (days 1, 8, 15 and 22 in a 4- or 5-week schedule) or twice weekly (days 1, 4, 8 and 11 in a 3-week schedule) with oral dexamethasone (10 or 20 mg, 4 to 8 doses) and cyclophosphamide [300 mg/m2 (maximum 500 mg) on days 1, 8 and 15]. The regimen was based on those previously used in patients with myeloma.17–19 Doses could be reduced at the discretion of the treating physician. The cycles were given every 21 to 35 days depending on the patients’ tolerance. For patients attaining a complete response it was recommended that treatment be limited to six cycles. For those attaining a partial response or less, an additional two cycles were suggested (for a total of 8 cycles) in an attempt to deepen the response.
Statistics
Survival curves were plotted using the method of Kaplan and Meier and the log-rank test was applied for comparisons among groups. Differences were compared with the test for categorical variables using the Fisher exact test when appropriate. Cox proportional hazards were used for the calculation of hazard ratios (HR) for each variable. Univariate and multivariate analyses were carried out to examine for factors predictive of early death (less than 3 months after the start of treatment).
Results
Sixty patients were analyzed. There were 37 men and 23 women; the median age was 66 years (range, 44–83 years) with 18 patients above 70 years and three patients above 80 years of age. The median number of organs involved was two (range, 1–5). All patients had cardiac amyloidosis, with other organs frequently involved (Table 1). Cardiac biomarkers and echocardiography parameters are described in Table 1. The median value of the different cardiac biomarkers was 7933 pg/mL (range, 661–66000) for N-terminal of pro-B-type natriuretic peptide (NT-proBNP), 766 ng/L (range, 228–5000) for B-type natriuretic peptide (BNP), 0.110 μg/L for troponin T (range, 0.04–0.610), 0.205 μg/L (range, 0.110–0.640) for troponin I and 0.090 μg/L (range 0.070–0.150) for hs-cTnT (Table 1). The median left ventricular septal wall thickness was 15 mm (range, 12–23) and the median dFLC was 313 mg/L (range, 35–6988).
Table 1.
Patients’ characteristics at diagnosis and according to outcome.
All patients began the first cycle of the planned therapy. The median number of cycles delivered was 3.0 (range, 0.5–10.0). Twenty-five percent of patients completed six or more cycles of therapy, 62% received at least three cycles. The median time from diagnosis to treatment initiation was 1 month (range, 0–7). The bortezomib doses were 1.3 mg/m2 in 81% of patients, 1.5 mg/m2 in 15% and 1.0 mg/m2 in 4%. A weekly bortezomib dosing strategy was pursued for 43 patients (72%). Bortezomib was given subcutaneously in 12 patients (23%).
Hematologic responses and relapses
On an intention-to-treat basis, the overall response rate was 68% with ten patients (17%) achieving a complete response and 15 (25%) a VGPR (including 4 patients with normalization of FLC levels but no available serum and/or urine immunofixation data). The median dFLC for patients evaluable at the response assessment time-point dropped significantly from 286 mg/L (range, 35–6988) to 19.6 mg/L (range, 0.6–1037). Thirty-five patients had complete monthly clonal markers. The median time to best response in these 35 patients was 2.1 months (range, 0.7–11).
Forty-two patients were evaluable in the landmark analysis examining those surviving longer than 3 months. In this group, the overall hematologic response rate was 86% with ten patients (24%) achieving a complete response and 14 patients (33%) achieving a VGPR.
There was no significant difference in responses between patients treated with the weekly or bi-weekly schedules. The overall response rate and rate of attaining a VGPR or better in the patients treated with the bi-weekly schedule was 71% and 36% versus 70% and 44% in those treated with the weekly schedule (P=0.90 and P=0.57, respectively).
Nineteen patients did not respond to VCD. Fifteen patients succumbed to an early death and did not, therefore, receive a second line of therapy. The median survival of this cohort was 1.8 months (range, 0.1 to 7 months). Of the remaining patients, one did not respond to second-line treatment with melphalan, thalidomide and dexamethasone, one patient had a complete response to lenalidomide plus dexamethasone, another had a partial response after melphalan, thalidomide and prednisone and a fourth reached a VGPR after bendamustine, thalidomide and dexamethasone.
Among the 41 patients who responded, at a median follow-up of 16 months (range, 2.1–50.0) after starting treatment, we observed seven relapses (17%): two relapses in the complete response group (20%), two among the patients with a VGPR (13%) and three among the 16 patients with a partial response (20%). The median time-to-progression for these seven patients was 12.8 months (range, 7.8–17.9) from the start of treatment.
Five of these patients are still alive. Two patients received VCD again, attaining a partial response. One patient developed plasma cell leukemia 19 months after the diagnosis of AL amyloidosis and received VTD-PACE (bortezomib, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide). She reached a complete response but rapidly relapsed, despite maintenance treatment with VTD, and died 6 months after the relapse. Two patients received BMDEX (bortezomib, melphalan, and dexamethasone), one of whom reached a complete response after nine cycles and remains stable 31 months after relapse. The second patient attained a partial response after two cycles and died of heart failure 17 months after diagnosis (7 months after relapse). Another patient has not yet been retreated and the last one died soon after relapse.
Organ responses
Cardiac responses were documented in 19 patients (32% of the whole cohort and 45% of the patients in the landmark analysis). In the intent-to-treat cohort cardiac response was predictive of survival with an estimated 1-year survival of 89% in patients with a cardiac response versus 41% in those without (P=0.0006). In the landmark analysis the 1-year overall survival was 89% versus 74%, respectively (P=0.16). Eight patients had a renal response and three patients a liver response.
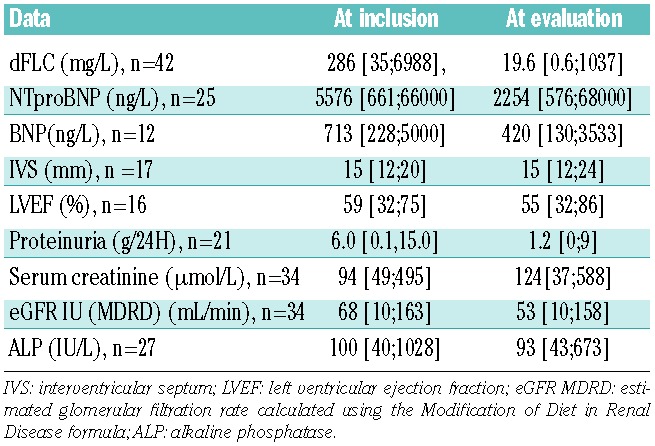
Table 2 gives the median values for dFLC as well as cardiac, renal and liver parameters at inclusion and post-treatment in the landmark analysis.
Table 2.
Characteristics of patients surviving more than 3 months after initiation of VCD at inclusion and evaluation.
Toxicity
Neuropathy was observed in 11 patients (18%) with four requiring withdrawal of treatment. Neuropathy was not seen more frequently in the bi-weekly schedule (21% versus 17%). Two patients stopped treatment because of worsening cardiac failure. Gastrointestinal toxicity occurred in two patients. Twenty-eight patients have died. Twenty-four of the deaths occurred while patients were on treatment. Eighteen patients (30%) died within the first 3 months, of whom eight patients (13%) in the first month of treatment. The causes of death were as follows: plasma cell leukemia (n=1), sepsis (n=4), sudden cardiac arrest (n=9), progressive heart failure (n=10) and progressive extra-cardiac amyloidosis (n=4).
Survival
The median follow-up for the whole cohort was 11.8 months, while that for living patients was 19.5 months. The median overall survival by Kaplan-Meier analysis for the whole cohort was not reached (Figure 1). The estimated 1-year survival is 57%. Survival was significantly influenced by hematologic response (Figure 2). For the landmark cohort, the median follow-up was 16.5 months. The estimated 1-year survival rate is 81% (Figure 3). We found no significant difference in survival between patients receiving the weekly or bi-weekly bortezomib regimen (P=0.88).
Figure 1.
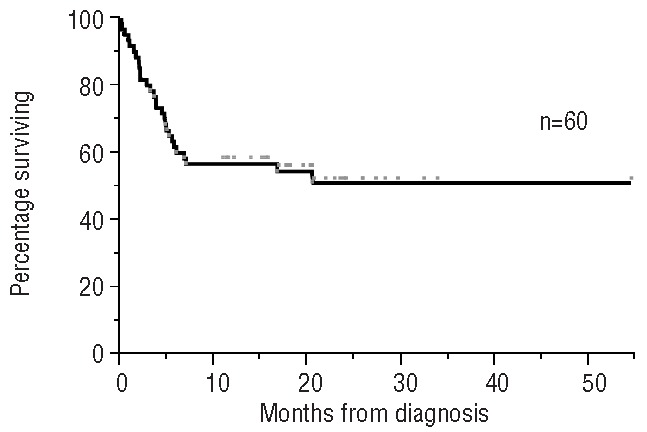
Overall survival for the whole cohort of patients.
Figure 2.
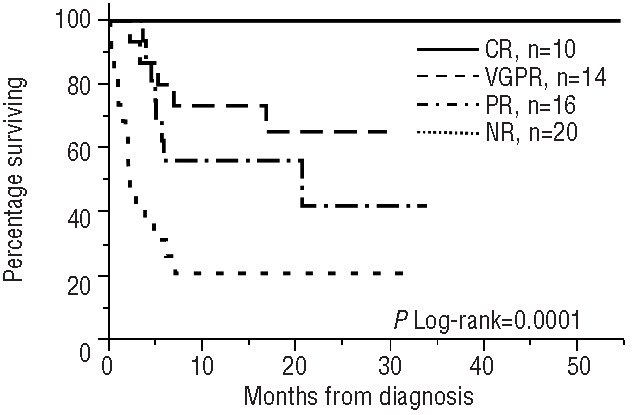
Overall survival according to hematologic responses. CR: complete response; VGPR: very good partial response; PR: partial response; NR: no response.
Figure 3.
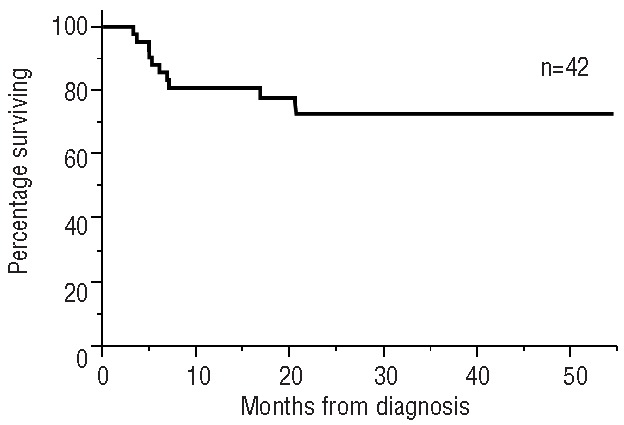
Overall survival for patients in the landmark analysis.
With respect to the risk of early death, age (categorized as below or above the median age), NT-proBNP greater than 9500 ng/L, BNP greater than 1100 ng/mL, estimated glomerular filtration rate below 50 mL/min and dFLC greater than 313 mg/L were found to have a significant impact on outcome (Table 3). The individual variables were then dichotomized and entered into the multivariate model. NT-proBNP remained a significant independent predictor of mortality (Table 3). Survival curves according to NT-proBNP and BNP levels are shown in Figure 4. The median survival in the group with a pre-treatment serum NT-proBNP concentration over 9500 ng/L or BNP over 1100 ng/L was 4.4 months whereas it was not reached in the group with values below these thresholds with an estimated 1-year survival rate of 81% (Figure 4).
Table 3.
Univariate and multivariate analyses.
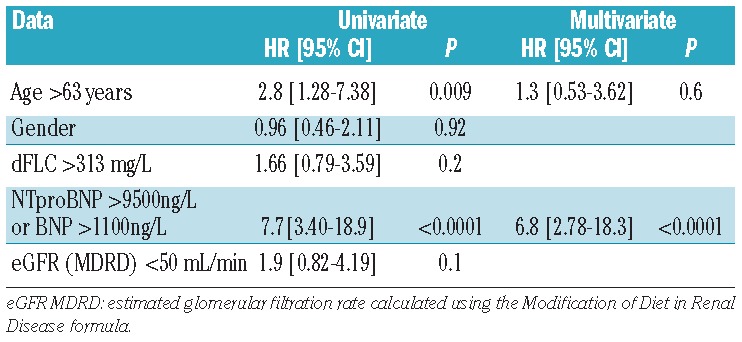
Figure 4.
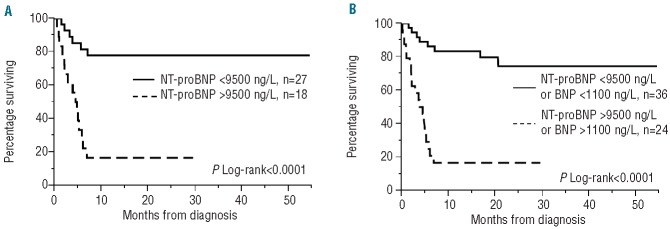
Overall survival stratified by serum NT-proBNP values (A) and serum NT-proBNP or BNP values (B).
Discussion
The introduction of novel agents has changed the therapeutic landscape in the treatment of plasma cell dyscrasias including AL amyloidosis.1 However, early death remains a major barrier in this disease. The most critical prognostic marker with respect to survival from diagnosis is the extent of cardiac involvement. Cardiac biomarkers are important predictors of outcome in amyloidosis, serving as the basis for the now widely accepted Mayo Clinic cardiac staging system.2 When this system was first reported in 2004, a time when the majority of stage III patients were treated with melphalan and prednisone, such patients had a median survival of 3.4 months and 1-year survival rate of 20%. More recent retrospective data indicate that the prognosis of this group of patients remains very poor. A large retrospective European survey reported a 7.1-month median survival5 with less than 30% of patients surviving beyond 3 years. Mayo stage III patients account for the majority of subjects with AL amyloidosis who succumb to an early death, contributing to the poor overall survival observed in previously published retrospective and prospective studies in this disease.20 It has been proposed that a rapid response is integral to overcoming the early deaths seen in this disease and improve outcomes in patients with advanced cardiac involvement. This principle guided the development of VCD as an ideal regimen for use in the upfront setting.
Previous data suggest that bortezomib could lead to prompt and profound responses in both myeloma17–19 and amyloidosis.6–13 In a recent prospective study in relapsed AL amyloidosis using a weekly or twice-weekly schedule the median time to first response was only 1.2 months. A subsequent analysis of the phase I cohort which examined patients with advanced cardiac disease based on echocardiographic criteria and BNP showed no unexpected toxicity.11 However, AL amyloidosis patients with relapsed disease are already deemed “survivors”; that is, those who are well enough to have survived therapy. Hence, one should not extrapolate outcomes with a given regimen in such patients to those treated in the upfront setting. Due to advanced organ failure newly diagnosed patients have an extremely high likelihood of dying before maximal response is achieved, thus failing to benefit from a clonal response.
The addition of bortezomib to the traditional alkylator and steroid backbone further improves response rates. A prospective phase II study of 26 patients with AL amyloidosis showed that a combination of melphalan, dexamethasone and bortezomib produced a hematologic response in 83% of patients, with 45% achieving a complete response.10 Oral cyclophosphamide has the advantage of being easier to dose in patients with renal insufficiency, a common occurrence in patients with stage III disease due to renal amyloidosis or reduced renal perfusion. A recent randomized phase II study testing four different regimens in myeloma showed that such a combination was very promising in terms of response.19 In AL amyloidosis, two small series examining VCD have been reported, with descriptions of unprecedented deep and rapid hematologic responses, especially when the regimen was given in the upfront setting.12,13
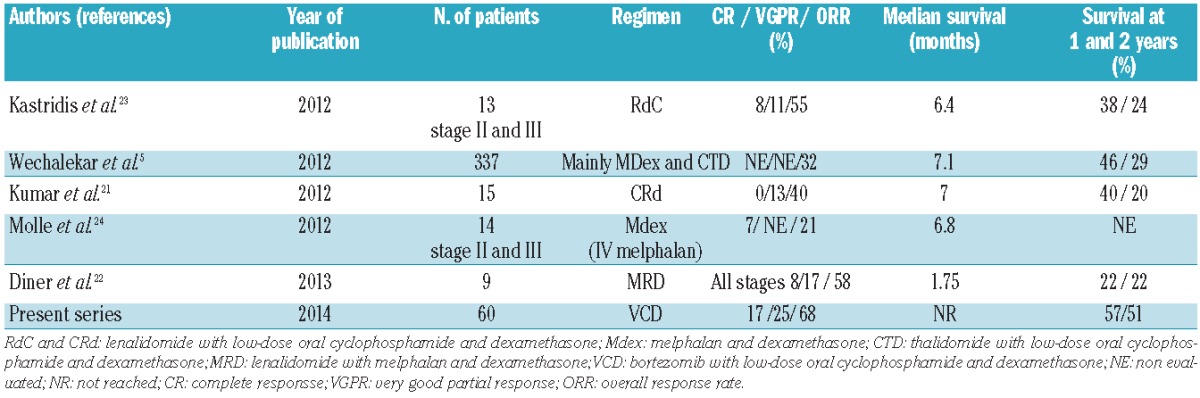
The present study examines the use of VCD in a multicenter setting. It represents the largest cohort of Mayo stage III patients treated with this regimen to date. The 1-year overall survival of 57% compares favorably with previously published data drawn from specific studies of stage III patients treated in the upfront setting, a time when most of the mortality associated with the burden of disease is most likely to manifest (Table 4). This is particularly true if one compares outcomes to those of the recently published retrospective European survey5 or two recently published studies based on similar regimens examining an immunomodulatory drug-based combination with an alkylator and steroid backbone. The 1-year survival rate for stage III patients in these series was only 25%.21,22 This difference in survival may be due to deeper response rates with the bortezomib-containing regimen with faster time to first response. In this series, there appeared to be no difference between patients treated once weekly or bi-weekly with bortezomib. A recent larger analysis from the London group, not limited to Mayo stage III patients, suggested otherwise25 and these data need to be interpreted with caution. It is important to note that no prospective phase III study has compared such regimens in the era of the novel agent.
Table 4.
Results of recent studies in AL amyloidosis Mayo stage III patients.
In the European retrospective survey5 the two main prognostic factors were NT-proBNP >8500 ng/L and supine blood pressure <100 mmHg. In the current cohort, we also found that high values of NT-proBNP or BNP (NT-proBNP > 9500 ng/L or BNP >1100 pg/L) were associated with a dismal outcome (Figure 4A for combined markers and Figure 4B for NT-proBNP alone). Patients with NT-proBNP or BNP below these thresholds had a good outcome with this regimen, with an estimated 81% survival at 1 year. This appears to be better than a previously reported survival of 40% at 2 years from the European survey in this poor-risk group although this conclusion must be considered with caution due to a potential selection bias for patients receiving this regimen.
Despite the improvements in long-term survival these data demonstrate that we still cannot overcome the poor prognosis of the more severely ill stage III patients. We observed a similar survival in such patients using VCD compared to that reported in the multicenter series (4.6 versus 4.4 months).5 In these patients, who are extremely fragile and sensitive to treatment toxicity, few options exist because the cardiac damage may have reached a critically irreversible stage. Despite the great need for therapy to induce deep and durable responses, given the burden of disease such patients have little reserve to tolerate any treatment-related complications that might contribute to toxicity and death. Although achieving a rapid clonal response is important, this must be balanced against the risk of toxicity to preserve a patient’s chance of surviving long enough to benefit from clonal control. The toxicity profile of novel regimens is uncertain in these fragile patients and may easily be missed in retrospective series. It must be noted that 24 patients died while on therapy, and it is very difficult to evaluate the role of this regimen in these early deaths. The survival of our poor-risk patients treated with VCD is comparable to that in the European series suggesting that toxicity is not different from that seen with the two main regimens used in such patients, melphalan and dexamethasone (M-Dex) and cyclophosphamide, thalidomide and dexamethasone (CTD).5 While we await prospective data, this regimen should be used with caution. One must pay meticulous attention to fluid balance before and during treatment, precise adjustment of diuretic dose and close cardiac monitoring.
The very poor prognosis in this group of patients raises the question of whether the addition of more aggressive measures such as cardiac transplantation would be beneficial over the long-term. Presently, few patients will be deemed eligible for such a procedure. That said, with the deep and potentially durable responses achieved with regimens such as VCD such interventions may need to be considered. Outcomes in AL amyloidosis are proving to be more favorable than previously expected, especially in patients who live long enough to benefit maximally from therapy. Even for patients with relapsed disease, post-relapse survival is quite good.26 In a similar vein, the role of assistive cardiac devices, used as a bridge to cardiac transplantation, has recently been studied and shows some promise although further study will be needed before widespread adoption of such a strategy can be considered. These very severely ill patients should be studied as a unique population with interventions incorporating both novel treatment regimens in addition to more intensive supportive care strategies. It is improbable that a complete response alone, even within the first cycle, is enough to alter the natural history of these very high-risk patients.
In conclusion, VCD appears to be an effective regimen in stage III cardiac AL amyloidosis but benefits in the ultra high-risk group of patients remain to be proven. In patients surviving to achieve maximal clonal response, the combination seems to provide deep and rapid responses. Longer follow-up is required to comment on durability of response but the early data are encouraging. In patients with severe cardiac involvement incorporation of novel supportive care strategies will be required to maximize the proportion of patients who will survive long enough to benefit from therapy. Despite the difficulty of performing studies in this high-risk cohort, prospective trials in Mayo stage III patients are warranted to formally establish the utility of VCD in this setting.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Gertz MA. Immunoglobulin light chain amyloidosis: 2011 update on diagnosis, risk stratification, and management. Am J Hematol. 2011;86(2):180–6. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A1, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22(18):3751–7. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich S, Schonland SO, Benner A, Bochtler T, Kristen AV, Beimler J, et al. Treatment with intravenous melphalan and dexamethasone is not able to overcome the poor prognosis of patients with newly diagnosed systemic light chain amyloidosis and severe cardiac involvement. Blood. 2010;116(4):522–8. [DOI] [PubMed] [Google Scholar]
- 4.Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol. 2011;29(14): 1924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121(17):3420–7. [DOI] [PubMed] [Google Scholar]
- 6.Davies FE, Wu P, Jenner M, Srikanth M, Saso R, Morgan GJ. The combination of cyclophosphamide, velcade and dexamethasone induces high response rates with comparable toxicity to velcade alone and velcade plus dexamethasone. Haematologica. 2007;92(8):1149–50. [DOI] [PubMed] [Google Scholar]
- 7.Wechalekar AD, Lachmann HJ, Offer M, Hawkins PN, Gillmore JD. Efficacy of bortezomib in systemic AL amyloidosis with relapsed/refractory clonal disease. Haematologica. 2008;93(2):295–8. [DOI] [PubMed] [Google Scholar]
- 8.Reece DE, Sanchorawala V, Hegenbart U, Merlini G, Palladini G, Fermand JP, et al. Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a phase 1 dose-escalation study. Blood. 2009;114(8):1489–97. [DOI] [PubMed] [Google Scholar]
- 9.Kastritis E, Wechalekar AD, Dimopoulos MA, Merlini G, Hawkins PN, Perfetti V, et al. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J Clin Oncol. 2010;28(6): 1031–7. [DOI] [PubMed] [Google Scholar]
- 10.Zonder J, Sanchorawala V, Snyder R, Matous J, Terebelo H, Janakirama N, et al. Rapid haematologic and organ responses in patients with AL amyloid treated with bortezomib plus melphalan and dexamethasone. Amyloid Journal of Protein Folding Disorders. 2010;17(s1):86–7.20462368 [Google Scholar]
- 11.Reece DE, Hegenbart U, Sanchorawala V, Merlini G, Palladini G, Bladé J, et al. Efficacy and safety of once-weekly and twice- weekly bortezomib in patients with relapsed systemic AL amyloidosis: results of a phase 1/2 study. Blood. 2011;118(4): 865–73. [DOI] [PubMed] [Google Scholar]
- 12.Mikhael JR, Schuster SR, Jimenez-Zepeda VH, Bello N, Spong J, Reeder CB, et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;119(19): 4391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venner CP, Lane T, Foard D, Rannigan L, Gibbs SD, Pinney JH, et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood. 2012;119(19):4387–90. [DOI] [PubMed] [Google Scholar]
- 14.Gertz MA, Comenzo R, Falk RH, Fermand J-P, Hazenberg BP, Hawkins PN, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis. Am J Hematol. 2005;79(4):319–28. [DOI] [PubMed] [Google Scholar]
- 15.Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541–9. [DOI] [PubMed] [Google Scholar]
- 16.Kristen AV, Giannitsis E, Lehrke S, Hegenbart U, Konstandin M, Lindenmaier D, et al. Assessment of disease severity and outcome in patients with systemic light-chain amyloidosis by the high-sensitivity troponin T assay. Blood. 2010;116(7):2455–61. [DOI] [PubMed] [Google Scholar]
- 17.Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23(7):1337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Laumann K, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood. 2010;115(16):3416–7. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375–82. [DOI] [PubMed] [Google Scholar]
- 20.Kumar SK, Gertz MA, Lacy MQ, Dingli D, Hayman SR, Buadi FK, et al. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin Proc. 2011;86(1):12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar SK, Hayman SR, Buadi FK, Roy V, Lacy MQ, Gertz MA, et al. Lenalidomide, cyclophosphamide, and dexamethasone (CRd) for light-chain amyloidosis: long-term results from a phase 2 trial. Blood. 2012;119(21):4860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinner S, Witteles W, Afghahi A, Witteles R, Arai S, Lafayette R, et al. Lenalidomide, melphalan and dexamethasone in a population of patients with immunoglobulin light chain amyloidosis with high rates of advanced cardiac involvement. Haematologica. 2013;98(10):1593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kastritis E, Terpos E, Roussou M, Gavriatopoulou M, Pamboukas C, Boletis I, et al. A phase 1/2 study of lenalidomide with low-dose oral cyclophosphamide and low-dose dexamethasone (RdC) in AL amyloidosis. Blood. 2012;119(23):5384–90. [DOI] [PubMed] [Google Scholar]
- 24.Mollee P, Tiley C, Cunningham I, Moore J, Prince HM, Cannell P, et al. A phase II study of risk-adapted intravenous melphalan in patients with AL amyloidosis. Br J Haematol. 2012;157(6):766–9. [DOI] [PubMed] [Google Scholar]
- 25.Sachchithanantham S, Mahmood S, Sayed R, Patel K, Fontana M, Lane T, et al. Biweekly bortezomib is more efficacious than weekly bortezomib when used as first line in patients with systemic AL amyloidosis. Blood (ASH Annual Meeting Abstracts) 2013;122:Abstract 1981. [Google Scholar]
- 26.Warsame R, Bang S, Kumar S, Lacy M, Buadi F, Dingli D, et al. Outcomes and treatments of relapsed AL amyloidosis following stem cell transplant. Blood (ASH Annual Meeting Abstracts) 2012;120: Abstract 1858. [Google Scholar]


