Abstract
CD4 regulatory T cells play a critical role in establishment of immune tolerance and prevention of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. The recovery and maintenance of regulatory T cells is dependent on homeostatic factors including the generation of naïve regulatory T cells from hematopoietic precursor cells, the proliferation and expansion of mature regulatory T cells, and the survival of regulatory T cells in vivo. In this study, quantitation of mitochondrial apoptotic priming was used to compare susceptibility of regulatory T cells, conventional CD4 T cells and CD8 T cells to intrinsic pathway apoptosis in 57 patients after allogeneic hematopoietic stem cell transplantation and 25 healthy donors. In healthy donors, regulatory T cells are more susceptible to mitochondrial priming than conventional T cells. Mitochondrial priming is increased after hematopoietic stem cell transplantation in all T-cell subsets and particularly in patients with chronic graft-versus-host disease. Regulatory T cells express high levels of CD95 and are also more susceptible than conventional T cells to apoptosis through the extrinsic pathway. However, CD95 expression and extrinsic pathway apoptosis is not increased after hematopoietic stem cell transplantation. Decreased expression of BCL2 and increased expression of BIM, a mitochondrial cell death activator protein, in regulatory T cells contributes to increased mitochondrial priming in this T-cell subset but additional factors likely contribute to increased mitochondrial priming following hematopoietic stem cell transplantation.
Introduction
After allogeneic hematopoietic stem cell transplantation (HSCT) donor T cells are the primary immune cells responsible for graft-versus-leukemia (GvL) effect and graft-versus-host disease (GvHD). T-cell engraftment and the establishment of normal T-cell homeostasis play an important role in the clinical outcome of patients undergoing transplantation.1–3 Within the T-cell compartment, both murine and clinical studies have shown that CD4 regulatory T cells (Treg) play a critical role in the establishment of immune tolerance and prevention of GvHD after allogeneic HSCT.4–13 The maintenance of adequate numbers of Treg is dependent on the balance of homeostatic factors that include the generation of mature Treg from hematopoietic precursor cells, the proliferation of mature Treg, and the survival of these cells in vivo.14,15 These observations led us to undertake a more detailed analysis of apoptotic pathways in Treg and to examine the role that these pathways play in regulating Treg survival after allogeneic HSCT.
Two major pathways provide complementary mechanisms of apoptosis in T lymphocytes.16 The extrinsic pathway includes surface membrane molecules such as Fas/APO-1/CD95, which directly initiate death receptor-induced apoptosis. The intrinsic pathway involves a complex set of mitochondrial-associated death signaling molecules. Activation of the intrinsic pathway results in mitochondrial outer membrane permeabilization (MOMP) and release of cytochrome C.17–19 This pathway is regulated by interactions between a large number of BCL2 family proteins, which include antiapoptotic, pro-apoptotic and effector proteins.20 Often, cell death initiated by the extrinsic pathway can recruit the intrinsic pathway via caspase 8-mediated cleavage and activation of the pro-apoptotic BCL2 family protein BIM.
Apoptosis via the intrinsic pathway is a threshold event, with some cells starting closer than others to the threshold. Proximity to the threshold of apoptosis, or apoptotic ‘priming’, can be measured using BH3 profiling.21–24 In this assay, mitochondria are exposed to peptides derived from the BH3 (BCL-2 homology 3) domains of pro-death BCL2 family proteins. The level of MOMP induced by each peptide is then measured. In our experiments, MOMP is quantified by the fluorescent dye TMRE, which accumulates in intact mitochondria but is released following MOMP. BH3 profiling thus provides an assessment of mitochondrial susceptibility to MOMP that integrates the functional activity of all of the BCL2 family proteins that regulate the intrinsic apoptosis pathway in individual cells. This method also allows us to simultaneously compare BH3 peptide-induced mitochondrial membrane depolarization (‘priming’) in different T-cell subsets: Treg, conventional CD4 T cells (Tcon), and CD8 T cells. Using this approach, our analysis of apoptotic pathways demonstrated that Treg are more susceptible to apoptosis through both intrinsic and extrinsic pathways than other T-cell subsets. This difference is evident in healthy donors indicating that increased susceptibility to apoptosis is reflective of normal Treg homeostasis. In addition, mitochondrial apoptotic priming of Treg and other T-cell subsets is significantly increased after HSCT and particularly in patients with active chronic GvHD (cGvHD).
Methods
Patients and sample collection
Blood samples were obtained from 57 patients who had undergone allogeneic HSCT at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital (Boston, MA, USA). Written informed consent was obtained from each patient before sample collection in accordance with the Declaration of Helsinki. This protocol has been reviewed and approved by the Institutional Review Board of the Dana-Farber Harvard Cancer Center. The severity of cGvHD was classified according to NIH criteria. We also studied 25 healthy individuals.
BH3 profiling assay
The BH3 profiling assay has been previously described.21,25,26 PBMC were incubated with anti-CD4, anti-CD25, anti-CD127, and anti-CD8 at 4°C for 20 min. After incubation, 2.4 × 106 cells were washed and suspended in T-EB Buffer.26 Individual BH3 peptides were added to 50 μl of cell suspension for 30 min at room temperature. After incubation, 12.5 μl of 22.5 μM TMRE (Invitrogen) was added for 30 min.25 Cells were analyzed on the BD LSRFortessa using FACS Diva software (BD Biosciences). CD4 T-cell subsets were defined by surface antigen expression: Tcon as CD4+CD25neg-lowCD127med-hi and Treg as CD4+CD25med-hiCD127low.14,27 Examples of flow cytometry plots comparing mitochondrial depolarization in Treg, Tcon and CD8 T cells in response to BMF peptide are shown in the Online Supplementary Figure S1. Comparison of mitochondrial priming in Treg in a healthy donor and individual patients with no GvHD or chronic GvHD is shown in Online Supplementary Figure S2.
Other methods
Additional flow cytometry methods are detailed in the Online Supplementary Methods.
Statistical analysis
Descriptive statistics were used for patients’ and transplant-related characteristics. Fisher’s exact test or a χ2 test was used for group comparisons for categorical variables in Table 1. The Wilcoxon rank sum test was used for pair-wise group comparisons for continuous variables. The Wilcoxon signed test was used for the difference in BH3 profiling, expression levels of BCL2 family proteins and proliferation and functional assays between two T-cell subsets within the same blood samples. For the Wilcoxon signed test, percent change was calculated between two T-cell subsets and an absolute change of 10% or higher was regarded as significantly different between two T-cell subsets. Multivariate linear regression analysis was also performed to compare T-cell subsets after adjusting for age, steroid use, donor type, conditioning intensity, grade II-IV aGvHD, cGvHD status, and time from HSCT to sampling date using PROC MIXED in SAS 9.2 (SAS Institute Inc., Cary, NC, USA). For comparisons between cGvHD and no cGvHD within each T-cell subset, multivariable linear regression analysis was also performed adjusting the same factors as above in the model using PROC GLM in SAS 9.2. Distributions of individual variables as well as their residuals were investigated for normality prior to performing regression analysis. An appropriate transformation (e.g. loge, log10 or square root) was made wherever needed. All tests were two-sided at the significance level of 0.05, and multiple comparisons were not adjusted. Heatmaps were generated using dChip software.28
Table 1.
Patients’ characteristics.
Results
Patients’ clinical characteristics
Immunological studies were undertaken in blood samples obtained from 57 adult patients who had undergone allogeneic HSCT between February 2001 and July 2010 and who had survived more than two years (Table 1). Patients were divided into two groups: 27 patients with no cGvHD and 30 patients with cGvHD (15 had mild cGvHD, 8 moderate cGvHD, and 7 severe cGvHD). Blood samples were also obtained from 25 healthy adults (median age 52 years; range 21–71). Median age of patients was 57 years (range 25–73). As expected, the use of immune suppressive medications (prednisone, tacrolimus) was more frequent in the cGvHD group (P<0.001 and P=0.02). Prior history of acute GvHD (grade 2–4) was also more frequent in the cGvHD group (P=0.003). CD4 T-cell counts were relatively low in both groups and CD4 Treg counts were significantly decreased in the cGvHD group (P=0.02).
BH3 profiling of T-cell subsets in healthy donors
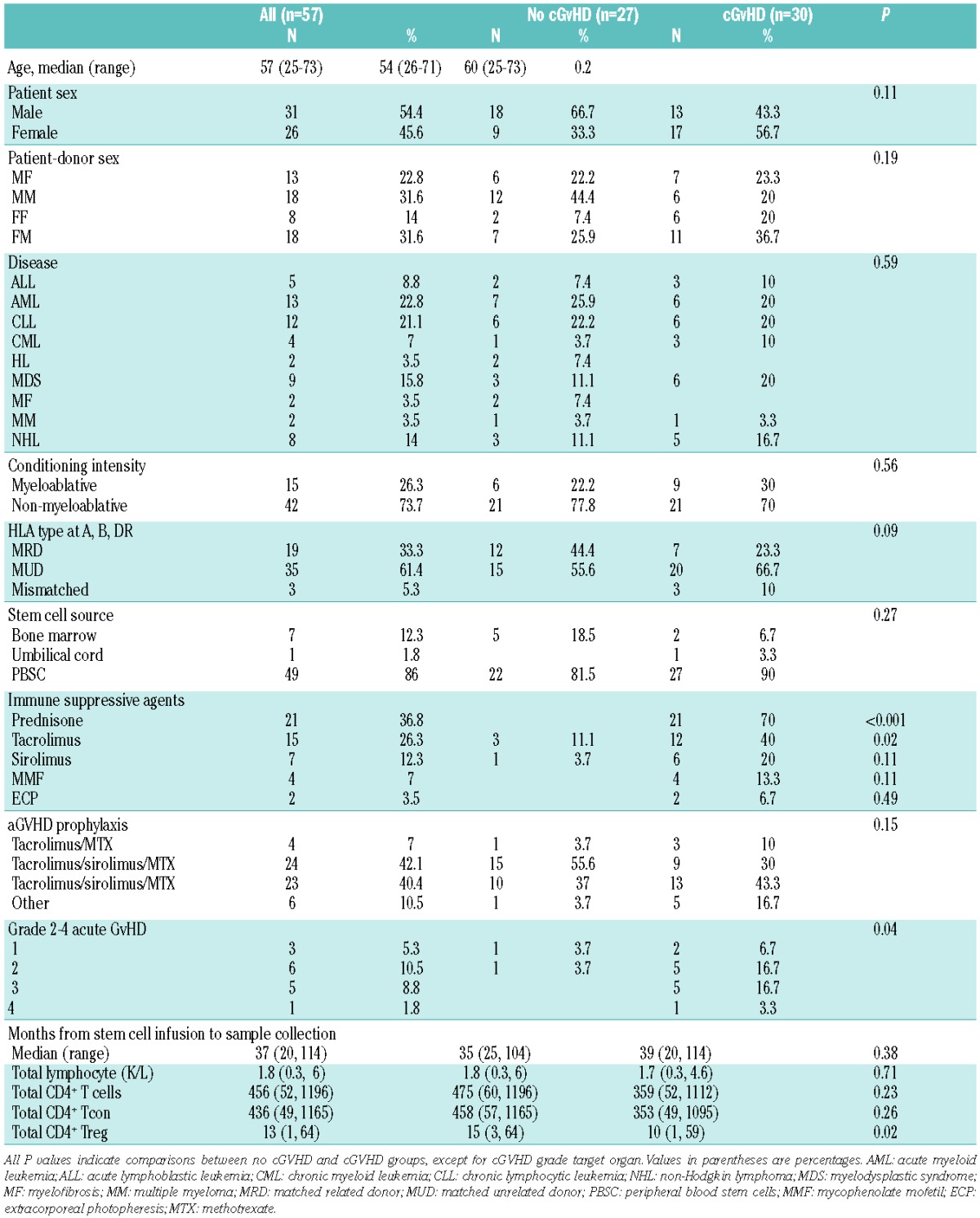
BH3 profiling was used to compare apoptotic priming of Treg, Tcon and CD8 T cells to intrinsic pathway apoptosis in 25 healthy donors (Figure 1A). Treg were more primed than Tcon when challenged with low concentration of BIM (BIM 0.03 μM), BAD, BAD+NOXA, PUMA and BMF peptides (P=0.003, P=0.002, P=0.0005, P<0.0001 and P=0.004, respectively). When compared with CD8 T cells, Treg were more primed when challenged with PUMA peptide (P=0.008). We also directly measured expression of anti-apoptotic (BCL2, BCLXL and MCL1) and pro-apoptotic (BIM) proteins in each T-cell subset by flow cytometry (Figure 1B). When compared to Tcon, Treg had lower levels of BCL2 (P<0.0001) and higher levels of BIM (P<0.0001). When compared to CD8 T cells, Treg had higher levels of BIM (P=0.002). These results are consistent with the results of BH3 profiling showing that Treg are more primed than Tcon and CD8 T cells.
Figure 1.
Apoptosis pathways in T-cell subsets: healthy donors (n=25). (A) BH3 profiling. Mitochondrial membrane depolarization after challenge with BH3 peptides in each T-cell subset (CD8: green; Tcon: blue; Treg: red). The percentage of depolarization was determined after challenge with individual peptides indicated on the x-axis. (B) Expression of anti-apoptotic proteins and BIM in each T-cell subset. Protein expression was measured by flow cytometry. Relative levels of BCL2, BCLXL, MCL1 and BIM were calculated by dividing the median MFI for each protein by the median MFI of isotype control IgG. (C) Expression of Fas (CD95) and cell proliferation (Ki67) in each T-cell subset were measured by flow cytometry. (D) Apoptosis induction after in vitro stimulation with staurosporine (STS) or anti-CD95 monoclonal antibody. Rapid induction of apoptosis in each T-cell subset was assessed by annexin V/7-AAD co-staining. CD95-induced apoptosis data were log-transformed. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
We also measured expression of CD95 as a marker of the death receptor pathway and Ki67 as a marker of proliferation in each T-cell subset (Figure 1C).29 Treg expressed higher levels of CD95 and Ki67 than Tcon and CD8 T cells (P<0.0001). These results suggest that Treg are more susceptible to apoptosis than Tcon and CD8 subsets through the extrinsic pathway and this was confirmed after in vitro stimulation with anti-CD95 antibody (P=0.0005 and P=0.006, respectively) (Figure 1D). Consistent with results of BH3 profiling and differential expression of apoptotic proteins, STS induced apoptosis of Treg was significantly greater than Tcon (P<0.0001) and CD8 T cells (P=0.003).
Characterization of apoptotic pathways of CD4 Treg after allogeneic HSCT
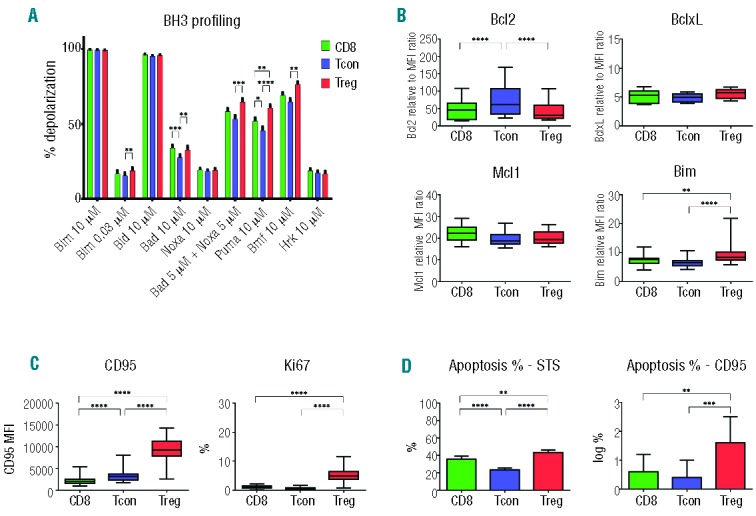
BH3 profiling was used to assess susceptibility of Treg, Tcon and CD8 T cells to intrinsic pathway apoptosis in 57 patients after allogeneic HSCT (Figure 2A). Treg were more primed than Tcon when their mitochondria were challenged with BIM 0.03, BAD, BAD+NOXA, PUMA and BMF peptides (P≤0.0001, except BMF P=0.016). Similarly, CD8 T cells were more primed than Tcon when challenged with BIM 0.03, BAD, BAD+NOXA, PUMA and BMF peptides. There were no significant differences in priming of Treg and CD8 T cells. When compared to Tcon, post-transplant Treg expressed lower levels of BCL2, higher levels of BCLXL and BIM (P<0.0001 for all comparisons) (Figure 2B). When compared to CD8 T cells, post-transplant Treg expressed higher levels of BIM (P<0.0001) but there was no difference in expression of BCLXL, BCL2 and MCL1. Post-transplant Treg also expressed higher levels of CD95 and Ki67 than Tcon and CD8 T cells (Figure 2C). Functional assays for apoptosis after in vitro stimulation with staurosporin (STS) and anti-CD95 antibody (Figure 2D) confirmed that Treg were more susceptible to apoptosis than Tcon through both intrinsic (STS) and extrinsic (CD95) pathways. CD8 T cells were also more susceptible to apoptosis through both pathways than Tcon, but the differences between CD8 T cells and Treg were relatively small. These differences were confirmed in multivariate linear regression analysis on CD95, Ki67, Apoptosis STS, and Apoptosis CD95 in which base-line characteristics, grade II-IV aGvHD, cGvHD, and time from HSCT to sample were adjusted for (Online Supplementary Table S1A). The observation that priming was increased in response to several BH3 peptides, suggests that changes in multiple pro- and anti-apoptotic proteins contribute to increased susceptibility to mitochondrial apoptosis after HSCT.
Figure 2.
Apoptosis pathways in T-cell subsets: post-HSCT patients (n=57). (A) BH3 profiling. Mitochondrial membrane depolarization after challenge with BH3 peptides in each T-cell subset (CD8: green; Tcon: blue; Treg: red). The percentage of depolarization was determined after challenge with individual peptides indicated on the x-axis. (A) Expression of anti-apoptotic proteins and BIM in each T-cell subset. Protein expression was measured by flow cytometry. Relative levels of BCL2, BCLXL, MCL1 and BIM were calculated by dividing the median MFI for each protein by the median MFI of isotype control IgG. (B) Expression of CD95 and Ki67 in each T-cell subset was measured by flow cytometry. (C) Apoptosis induction after in vitro stimulation with STS or anti-CD95 monoclonal antibody. Rapid induction of apoptosis in each T-cell subset was assessed by annexin V/7-AAD co-staining. CD95-induced apoptosis data were log-transformed. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Increased priming associated with cGvHD
Figure 3 compares mitochondrial BH3 profiling for samples obtained from patients with and without cGvHD. Results in healthy donors are also included and each T-cell subset is shown individually. When compared with healthy donors, Treg were more primed in post-transplant samples. This difference was evident when Treg were challenged with BIM 0.03, BAD, NOXA, BAD+NOXA, PUMA, BMF and HRK peptides. However, in most samples, only Treg from patients with active cGvHD showed increased priming and Treg priming from patients without cGvHD (BIM 0.03, BAD+NOXA, PUMA and BMF) was similar to healthy donors. In general, BH3 profiling of Tcon and CD8 T cells showed very similar patterns of priming. Both Tcon and CD8 subsets were more primed after transplant, but in most samples this was only in patients with cGvHD. The only exception to this pattern was challenge with HRK peptide, where there was either no difference in priming (CD8 T cells) or post-transplant samples from patients without GvHD (Tcon and Treg) showed increased priming.
Figure 3.

BH3 priming in T-cell subsets after allogeneic HSCT. Percentage of depolarization was determined in each T-cell subset after challenge with individual BH3 peptides. Within each T-cell subset, results are compared for healthy donors (HD), patients without cGvHD (no cGvHD) and patients with cGvHD. *, **, *** denote significant differences between HD and cGvHD and between HD and no cGvHD: *P<0.05; **P<0.01; ***P<0.001. +, ++ denote significant differences between cGvHD and no cGvHD: +P<0.05; ++P<0.01.
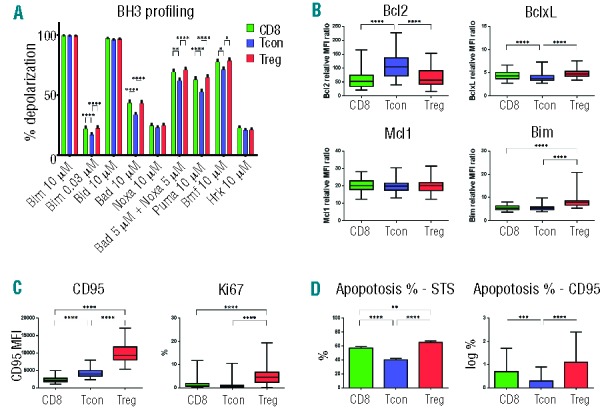
Levels of several key apoptotic proteins in Treg, Tcon and CD8 T cells are shown in Figure 4A. Remarkably, BCL2 expression was increased in each T-cell subset in patients with cGvHD. In contrast, expression of BCL2 in patients without GvHD was similar to the levels observed in each subset in healthy donors. Expression of BCLXL was reduced in all T-cell subsets in post-transplant samples compared to healthy donors. In contrast to BCL2 expression, BCLXL levels were similar in patients with and without cGvHD. Expression of MCL1 was similar in all T-cell subsets from post-transplant patients and healthy donors. Expression of BIM varied widely in Treg but was similar in post-transplant samples and healthy donors. Expression of BIM was reduced in Tcon and CD8 T cells in post-transplant samples compared to healthy donors.
Figure 4.
Expression of apoptotic proteins in T-cell subsets in patients with and without cGvHD and healthy donors. (A) Expression of anti-apoptotic (BCL2, BCLXL, MCL1) and pro-apoptotic proteins (BIM) in each T-cell subset. (B) Expression of cell surface CD95 and Ki67 in each T-cell subset. (C) Apoptosis induction after in vitro stimulation with STS or anti-CD95 monoclonal antibody in each T-cell subset. CD95-induced apoptosis data were log-transformed. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Expression of CD95 and Ki67 in T-cell subsets from patients with cGvHD is shown in Figure 4B. Treg expressed the highest levels of CD95, but expression was similar in patients with and without cGvHD and in healthy donors. CD95 expression in Tcon was increased in post-transplant samples compared with healthy donors. However, CD95 expression was similar in patients with and without cGvHD, and the overall level of increase was relatively small. CD95 expression in CD8 T cells was relatively low and was similar in patients with and without cGvHD and in healthy donors. Proliferation was highest in Treg, but expression of Ki67 was similar in patients with and without cGvHD and in healthy donors. Expression of Ki67 in Tcon and CD8 T cells was similar in patients with and without cGvHD and in healthy donors.
Although expression of BCL2 was increased in each T-cell subset in patients with cGvHD, this difference was not reflected in STS-induced apoptosis. As shown in Figure 4C, STS-induced apoptosis was increased in each T-cell subset in post-transplant samples. This finding is consistent with results of BH3 profiling except that differences in priming with HRK peptide was not reflected in increased apoptosis in samples from patients with cGvHD. CD95-induced apoptosis was highest in Treg; however, extrinsic pathway apoptosis was not significantly different in patients with cGvHD compared to patients without GvHD. For each cell type (CD8, Treg, Tcon), cGvHD versus no cGvHD was compared in multivariable analysis adjusting for age, grade 2–4 aGvHD (with/without), prednisone use (Yes/No), donor type, conditioning intensity, time from HSCT to sample date. The results of this analysis confirm the similarity between patients with and without cGvHD shown in Figure 4B and C (Online Supplementary Table S1B).
Apoptotic pathways of T-cell subsets associated with severity of cGvHD
Increased mitochondrial priming associated with cGvHD was further examined by dividing the cGvHD patients into 3 groups according to severity of clinical manifestations. As shown in Figure 5, increased priming was observed primarily in patients with mild and moderate cGvHD. For Treg, this was evident when cells were challenged with BIM 0.03, BAD+NOXA and BMF. In each of these assays, Treg priming was significantly increased in patients with mild or moderate cGvHD compared with patients with severe cGvHD. Similar patterns of priming were observed in Tcon and CD8 T cells, but differences in these subsets were less marked and only samples from patients with mild cGvHD were significantly more primed than patients with severe cGvHD. Measurement of apoptotic proteins showed that the differences in mitochondrial priming observed in patients with severe cGvHD appeared to reflect significantly increased expression of BCL2 in these samples (Figure 6A). This was evident in Treg, Tcon and CD8 T cells where BCL2 expression was similar in patients with mild or moderate cGvHD but was lower in these samples when compared to patients with severe cGvHD. Expression of BCLXL, MCL1 and BIM did not vary with severity of cGvHD in any T-cell subset. Similarly, expression of CD95 and Ki67 did not vary with severity of cGvHD (Figure 6B). Although BCL2 expression was relatively higher in T cells from patients with severe cGvHD, this was not reflected in lower levels of STS-induced apoptosis (Figure 6C).
Figure 5.
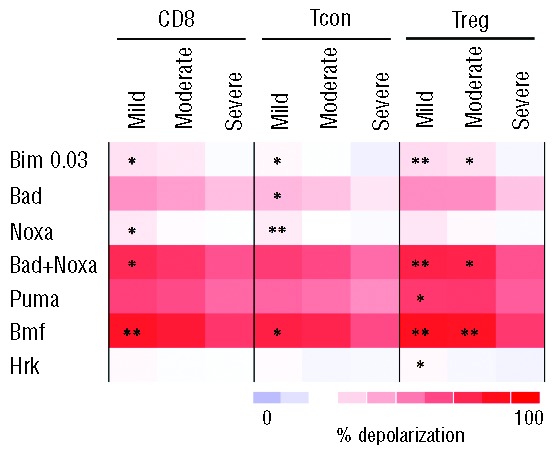
BH3 priming in T-cell subsets in patients with cGvHD. Percentage of depolarization was determined in each T-cell subset after challenge with individual BH3 peptides. Within each T-cell subset, results are compared for patients with mild, moderate or severe cGvHD. *, ** denote significant differences in BH3 priming between mild or moderate cGvHD and severe cGvHD. *P<0.05; **P<0.01. There were no significant differences between mild and moderate cGvHD.
Figure 6.
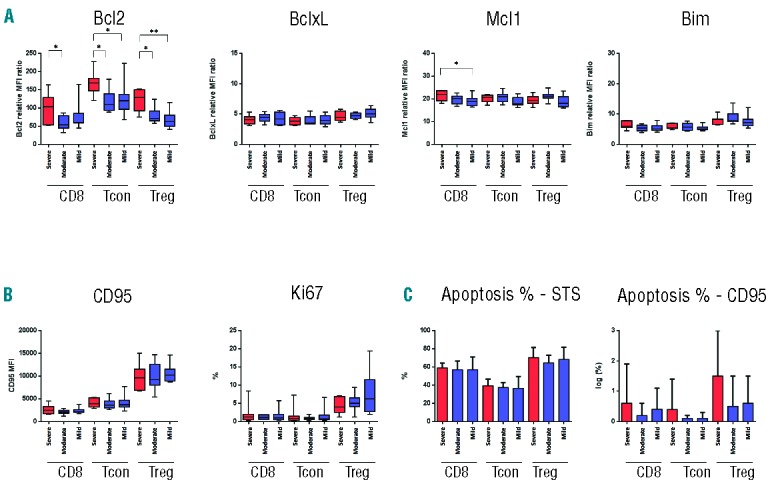
Expression of apoptotic proteins in T-cell subsets in patients with severe, moderate or mild cGvHD. (A) Expression of anti-apoptotic (BCL2, BCLXL, MCL1) and pro-apoptotic proteins (BIM) in each T-cell subset. (B) Expression of cell surface CD95 and Ki67 in each T-cell subset. (C) Apoptosis induction after in vitro stimulation with STS or anti-CD95 monoclonal antibody in each T-cell subset. CD95-induced apoptosis data were log-transformed. *P<0.05; **P<0.01; ***P<0.001.
To examine whether decreased priming in patients with severe cGvHD might reflect more intensive immunosuppressive therapy (especially prednisone), we performed multivariable linear regression analysis (Online Supplementary Table S2). In this analysis, severe cGvHD is significantly associated with Treg expression of BCL2 and priming with BIM 0.03, BAD+NOXA and BMF at the significance level of <0.01, but was independent of prednisone use, aGvHD and time from transplant. To examine the influence of various factors on BH3 profiling, we also created two heatmaps based on unsupervised hierarchical clustering of Treg data: one for all patients (Online Supplementary Figure S3A) and the other for patients with cGvHD only (Online Supplementary Figure S3B). In these heatmaps, steroid use does not appear to be associated with severity of cGvHD or results of BH3 profiling.
Discussion
The establishment of immune tolerance after allogeneic HSCT is a complex process that involves various cell types including CD4 Treg, CD8 Treg, invariant NKT cells (iNKT), dendritic cell subsets and B-regulatory cells as well as soluble mediators and multiple immune pathways.9,12,30–40 CD4 Treg have clearly defined phenotypic and functional characteristics and, as a result, have been studied extensively in various models of GvHD as well as in clinical studies. The relative deficiency of CD4 Treg after allogeneic HSCT appears to contribute to both acute and cGvHD, and this observation has prompted efforts to understand the mechanisms responsible for the inadequate reconstitution of this important T-cell subset after transplant.6,41–45 These studies have revealed that Treg reconstitution after allogeneic HSCT is primarily driven by proliferation and expansion of mature memory Treg. However, Treg proliferation is offset by relatively low levels of thymic Treg production. Increased susceptibility to apoptosis also appears to limit the ability to maintain adequate numbers of Treg.14 Previous studies have shown that Treg have relatively short telomeres and cGvHD is associated with low levels of telomerase activity.27 Treg also express high levels of CD95 (Fas) and are highly susceptible to Fas-mediated death through the extrinsic apoptosis pathway.46
To assess Treg susceptibility to apoptosis through the intrinsic pathway, we used BH3 profiling, a functional assay that measures mitochondrial membrane depolarization after challenge with a panel of BH3 peptides.21,22 This approach provides an integrated functional assessment of individual cell susceptibility to intrinsic pathway apoptosis, termed ‘priming’. In previous studies, priming has been used to characterize tumor cell susceptibility to chemotherapeutic agents, and levels of priming have been correlated with clinical response after treatment.22,24 We were able to examine priming of different T-cell subsets within the same blood samples and compare priming in patients after stem cell transplant with healthy controls. When comparing results in different T-cell subsets and patient populations, the absolute differences in priming were relatively small. To ensure that these differences were functionally significant, we required that the absolute difference in priming be at least 10%. Moreover, statistically significant differences in priming were further correlated with functional assays of apoptosis and quantitative assessment of several key pro- and anti-apoptotic proteins. The results of these comparisons provide several insights into mechanisms that modulate survival of Treg in vivo in normal individuals as well as in patients with cGvHD.
In healthy adults, both Treg and CD8 T cells are more primed than Tcon. It was not possible to quantify expression of all pro-apoptotic, anti-apoptotic and effector proteins in the BCL2 family, but the relatively low level of Tcon priming compared with other T-cell subsets appears to primarily reflect higher levels of BCL2 and lower levels of BIM in Tcon. Treg and CD8 had similar levels of priming and express similar levels of BCL2. Further analysis of BH3 profiling in a cross-sectional cohort of 57 patients who were more than two years after allogeneic HSCT revealed that, similar to healthy donors, both Treg and CD8 T cells are more primed than Tcon. In this setting, the relatively low level of priming in Tcon compared to Treg and CD8 T cells also appears to primarily reflect higher levels of BCL2 and lower levels of BIM in this subset. Notably, direct comparison of priming in patients’ samples and those of healthy donors revealed generally higher levels of priming in all T-cell subsets that was most evident in patients with cGvHD. Except when challenged with NOXA and HRK peptides, priming in patients without GvHD was similar to healthy donors. This increased level of priming associated with cGvHD could not be explained by differences in expression of any of the BCL2 family proteins we measured (BCL2, BCLXL, MCL1 and BIM). In fact, BCL2 levels were increased in all T-cell subsets in patients with cGvHD compared to patients without cGvHD and healthy donors. Since BH3 profiling provides an integrated functional assessment of mitochondrial susceptibility to membrane depolarization, these findings suggest that other cellular changes occur in all T cells in association with cGvHD to enhance susceptibility to intrinsic pathway apoptosis. These changes do not appear to be associated with administration of corticosteroids or other individual immune suppressive agents, but further studies are needed to define the mechanisms responsible for increased priming of all major T-cell subsets in patients with cGvHD.
We further examined whether the severity of cGvHD influenced the level of T-cell priming. This analysis revealed that priming was decreased in all T-cell subsets in patients with severe cGvHD. In this setting, decreased priming appeared to reflect higher levels of BCL2 in severe cGvHD, but there were no differences in STS-induced apoptosis associated with severity of cGvHD. Although STS induces mitochondrial membrane depolarization, this agent also has other direct effects on apoptotic signaling and measurements of STS-induced apoptosis do not only reflect the level of mitochondrial priming in individual cells. Similarly, expression of CD95 was not affected by the severity of cGvHD, which remained higher in Treg than other T-cell subsets.
Taken together, this analysis of apoptotic pathways in T cells after allogeneic HSCT demonstrates that CD4 Treg are significantly more primed than CD4 Tcon and this regulatory subset is highly susceptible to both intrinsic and extrinsic apoptosis pathways. The relative differences in mitochondrial priming between Treg and Tcon likely contribute to the relative deficiency of Treg after transplantation. These differences are also found in healthy donors suggesting that the high level of priming in Treg represents a normal response to high levels of homeostatic proliferation and constitutes a natural mechanism for limiting the overall number of Treg and preventing excessive levels of immune suppression. After allogeneic HSCT, priming is increased in patients with cGvHD, but this effect is observed in all T-cell subsets without a selective effect on Treg. This increased level of priming in cGvHD is reversed in the subset of patients with severe GvHD. These patients are typically lymphopenic and also receive more intensive immune suppressive therapy. In our cohort, total lymphocytes, total CD4, Tcon and Treg counts were all significantly reduced in patients with severe cGvHD compared with moderate or mild cGvHD (P<0.03) (data not shown). The non-selective lowering of the level of priming in this setting may, therefore, reflect the selective persistence of T cells that are less susceptible to apoptosis as well as the effects of immune suppressive therapeutic agents.
Our studies focused on CD4 Treg in patients with well established cGvHD who were at least 20 months post transplant (median 37 months). CD4 Treg also likely play a role in the modulation of acute GvHD and the establishment of immune tolerance at earlier times after transplant.10,11,44,47,48 In the early post-transplant period, CD4 Treg are primarily derived from mature Treg present in the hematopoietic stem cell graft. In this setting, the number of CD4 Treg and the proliferative and functional capacity of Treg in the stem cell product likely play an important role in their ability to modulate acute GvHD.4,5 Previous studies examined in vitro-expanded umbilical cord blood-derived CD4 Treg and found that expression of various pro- and anti-apoptotic proteins was markedly influenced by the signaling pathways used to stimulate and facilitate in vitro expansion.49 Umbilical cord blood Treg are predominately naïve cells compared to adult Treg that are predominately memory cells. These differences affect the ability of these cells to undergo homeostatic expansion after transplantation as memory CD4 Treg have more limited proliferative capacity and are more susceptible to apoptosis.27 Activation of various signaling pathways also has distinct effects on CD4 Treg function in vivo. For example, activation of STAT3 limits the function and survival of CD4 Treg and limits the ability of these cells to control GVHD in vivo.50,51 In contrast, activation of STAT5 in CD4 Treg promotes their survival and ability to suppress GVHD reactions.7,51–54
The observation that CD4 Treg deficiency contributes to the development of GvHD has led to the development of therapeutic approaches to selectively expand these cells or enhance their function after transplant.36,55–57 These approaches have included adoptive therapy with highly purified CD4 Treg expanded in vitro as well as daily administration of low-dose IL-2.58–64 CD4 Treg that have undergone extensive proliferation in vitro may become more susceptible to apoptosis and this may limit the survival of these cells in vivo after adoptive transfer. Our analysis of patients receiving low-dose IL-2 revealed that this treatment had multiple effects on CD4 Treg, which included induction of proliferation, generation of new Treg, and increased expression of BCL2.65 In some experiments, we also compared priming in memory and naive CD4 Treg subsets. Memory Treg had higher levels of priming and lower levels of BCL2 than naive Treg (data not shown). CD4 memory Treg are the predominant regulatory population in patients with cGvHD and the high level of priming in this subset further contributes to the inability to maintain adequate numbers of CD4 Treg after transplant. Naïve Treg exhibit relatively low levels of priming and strategies to increase the generation of new CD4 Treg in the early post-transplant period may, therefore, be one approach to promote immune tolerance and prevent the development of cGvHD. As clinical efforts to enhance CD4 Treg recovery and function after allogeneic HSCT are evaluated in clinical trials, it will be important to consider the inherent susceptibility of CD4 Treg to intrinsic pathway apoptosis and to develop concurrent strategies to enhance the survival of these cells in vivo. Other immune regulatory cells including CD8 Treg, iNKT cells, dendritic cell subsets and B-regulatory cells also likely have distinct homeostatic characteristics and further studies to identify factors that promote the function and survival of these cells in vivo may lead to new approaches to sustain immune tolerance and prevent chronic GvHD.
Acknowledgments
We thank John Daley, Suzan Lazo-Kallanian and Kristen Cowens for assistance with flow cytometry analysis and cell sorting; Doreen Hearsey and Lauren Gaffny for obtaining clinical blood samples.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This study was supported by NIH grants CA142106, CA183559, CA183560, AI056299, the Jock and Bunny Adams Education and Research Endowment and the Ted and Eileen Pasquarello Research Fund.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Bhushan V, Collins RH., Jr Chronic graft-vs-host disease. JAMA. 2003;290(19):2599–603. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–50. [DOI] [PubMed] [Google Scholar]
- 6.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108(5):1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mielke S, Rezvani K, Savani BN, Nunes R, Yong AS, Schindler J, et al. Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood. 2007;110(5): 1689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114(4):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews K, Lim Z, Afzali B, Pearce L, Abdallah A, Kordasti S, et al. Imbalance of effector and regulatory CD4 T cells is associated with graft-versus-host disease after hematopoietic stem cell transplantation using a reduced intensity conditioning regimen and alemtuzumab. Haematologica. 2009;94(7):956–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magenau JM, Qin X, Tawara I, Rogers CE, Kitko C, Schlough M, et al. Frequency of CD4(+)CD25(hi)FOXP3(+) regulatory T cells has diagnostic and prognostic value as a biomarker for acute graft-versus-host-disease. Biol Blood Marrow Transplant. 2010;16(7):907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM. Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. J Exp Med. 2011;208(12):2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma H, Lu C, Ziegler J, Liu A, Sepulveda A, Okada H, et al. Absence of Stat1 in donor CD4(+) T cells promotes the expansion of Tregs and reduces graft-versus-host disease in mice. J Clin Invest. 2011;121(7):2554–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120(5): 1479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beres AJ, Drobyski WR. The role of regulatory T cells in the biology of graft versus host disease. Front Immunol. 2013;4:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. [DOI] [PubMed] [Google Scholar]
- 17.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–19. [DOI] [PubMed] [Google Scholar]
- 19.van Delft MF, Smith DP, Lahoud MH, Huang DC, Adams JM. Apoptosis and noninflammatory phagocytosis can be induced by mitochondrial damage without caspases. Cell Death Differ. 2010;17(5):821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37(3):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan JA, Brunelle JK, Letai A. Heightened mitochondrial priming is the basis for apoptotic hypersensitivity of CD4+ CD8+ thymocytes. Proc Natl Acad Sci U S A. 2010;107(29):12895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334(6059):1129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol. 2012;30(25):3127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151(2):344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–65. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi R, Andreyev A, Murphy AN, Perkins GA, Ellisman MH, Newmeyer DD. Mitochondria frozen with trehalose retain a number of biological functions and preserve outer membrane integrity. Cell Death Differ. 2007;14(3):616–24. [DOI] [PubMed] [Google Scholar]
- 27.Kawano Y, Kim HT, Matsuoka K, Bascug G, McDonough S, Ho VT, et al. Low telom-erase activity in CD4+ regulatory T cells in patients with severe chronic GVHD after hematopoietic stem cell transplantation. Blood. 2011;118(18):5021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Wong W. DNA-Chip Analyzer (dChip). In: Parmigiani G, Garrett E, Irizarry R, Zeger S, eds. The analysis of gene expression data: methods and software. New York: Springer, 2003:120–41. [Google Scholar]
- 29.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710–5. [PubMed] [Google Scholar]
- 30.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467(7313):328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, Cantor H. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Semin Immunol. 2011;23(6):446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varthaman A, Clement M, Khallou-Laschet J, Fornasa G, Gaston AT, Dussiot M, et al. Physiological induction of regulatory Qa-1-restricted CD8+ T cells triggered by endogenous CD4+ T cell responses. PLoS One. 2011;6(6):e21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varthaman A, Khallou-Laschet J, Clement M, Fornasa G, Kim HJ, Gaston AT, et al. Control of T cell reactivation by regulatory Qa-1-restricted CD8+ T cells. J Immunol. 2010;184(12):6585–91. [DOI] [PubMed] [Google Scholar]
- 34.Hu D, Weiner HL, Ritz J. Identification of cytolytic CD161- CD56+ regulatory CD8 T cells in human peripheral blood. PLoS One. 2013;8(3):e59545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaidos A, Patterson S, Szydlo R, Chaudhry MS, Dazzi F, Kanfer E, et al. Graft invariant natural killer T-cell dose predicts risk of acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Blood. 2012;119(21):5030–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneidawind D, Pierini A, Negrin RS. Regulatory T cells and natural killer T cells for modulation of GVHD following allogeneic hematopoietic cell transplantation. Blood. 2013;122(18):3116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin KL, Fulton LM, Berginski M, West ML, Taylor NA, Moran TP, et al. Intravital imaging of donor allogeneic effector and regulatory T cells with host dendritic cells during GvHD. Blood. 2014;213(10)1604–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sega EI, Leveson-Gower DB, Florek M, Schneidawind D, Luong RH, Negrin RS. Role of lymphocyte activation gene-3 (lag-3) in conventional and regulatory T cell func tion in allogeneic transplantation. PLoS One. 2014;9(1):e86551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaunat O, Morelon E, Defrance T. Am“B”valent: anti-CD20 antibodies unravel the dual role of B cells in immunopathogenesis. Blood. 2010;116(4):515–21. [DOI] [PubMed] [Google Scholar]
- 40.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491(7423):264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107(4):1717–23. [DOI] [PubMed] [Google Scholar]
- 42.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9(3):239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010; 11(1):7–13. [DOI] [PubMed] [Google Scholar]
- 44.Ukena SN, Velaga S, Geffers R, Grosse J, Baron U, Buchholz S, et al. Human regulatory T cells in allogeneic stem cell transplantation. Blood. 2011;118(13):e82–92. [DOI] [PubMed] [Google Scholar]
- 45.Baron F, Humblet-Baron S, Ehx G, Servais S, Hannon M, Belle L, et al. Thinking out of the box-new approaches to controlling GVHD. Curr Hematol Malig Rep. 2014;9(1):73–84. [DOI] [PubMed] [Google Scholar]
- 46.Weiss EM, Schmidt A, Vobis D, Garbi N, Lahl K, Mayer CT, et al. Foxp3-mediated suppression of CD95L expression confers resistance to activation-induced cell death in regulatory T cells. J Immunol. 2011;187(4): 1684–91. [DOI] [PubMed] [Google Scholar]
- 47.Ukena SN, Geffers R, Buchholz S, Stadler M, Franzke A. Biomarkers for acute and chronic graft-versus-host disease in regulatory T cells. Transpl Immunol. 2012;27(4):179–83. [DOI] [PubMed] [Google Scholar]
- 48.Fujioka T, Tamaki H, Ikegame K, Yoshihara S, Taniguchi K, Kaida K, et al. Frequency of CD4(+)FOXP3(+) regulatory T-cells at early stages after HLA-mismatched allogeneic hematopoietic SCT predicts the incidence of acute GVHD. Bone Marrow Transplant. 2013;48(6):859–64. [DOI] [PubMed] [Google Scholar]
- 49.Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A, Taylor DK, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112(7): 2847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M, et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37(2):209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujino M, Li XK. Role of STAT3 in regulatory T lymphocyte plasticity during acute graft-vs.-host-disease. JAKSTAT. 2013;2(4): e24529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jenks JA, Seki S, Kanai T, Huang J, Morgan AA, Scalco RC, et al. Differentiating the roles of STAT5B and STAT5A in human CD4+ T cells. Clin Immunol. 2013;148(2):227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidl C, Hansmann L, Lassmann T, Balwierz PJ, Kawaji H, Itoh M, et al. The enhancer and promoter landscape of human regulatory and conventional T-cell subpopulations. Blood. 2014;24(4):68–78 [DOI] [PubMed] [Google Scholar]
- 54.Carbone F, De Rosa V, Carrieri PB, Montella S, Bruzzese D, Porcellini A, et al. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med. 2014;20(1):69–74. [DOI] [PubMed] [Google Scholar]
- 55.Veerapathran A, Pidala J, Beato F, Yu XZ, Anasetti C. Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood. 2011;118(20): 5671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3(83):83ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Lu L, Jiang S. Regulatory T cells: customizing for the clinic. Sci Transl Med. 2011;3(83):83ps19. [DOI] [PubMed] [Google Scholar]
- 58.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–8. [DOI] [PubMed] [Google Scholar]
- 60.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, 3rd, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011; 365(22):2055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veerapathran A, Pidala J, Beato F, Betts B, Kim J, Turner JG, et al. Human regulatory T cells against minor histocompatibility antigens: ex vivo expansion for prevention of graft-versus-host disease. Blood. 2013;122(13):2251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hannon M, Lechanteur C, Lucas S, Somja J, Seidel L, Belle L, et al. Infusion of clinical-grade enriched regulatory T cells delays experimental xenogeneic graft-versus-host disease. Transfusion. 2014;54(2):353–63. [DOI] [PubMed] [Google Scholar]
- 63.Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu MF, Liu H, et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20(8):2215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parmar S, Liu X, Tung SS, Robinson SN, Rodriguez G, Cooper LJ, et al. Third-party umbilical cord blood-derived regulatory T cells prevent xenogenic graft-versus-host disease. Cytotherapy. 2014;16(1):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5(179):179ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]


