Abstract
Aging is associated with progressive loss of cardiovascular and skeletal muscle function. The impairment in physical capacity with advancing age could be related to an insufficient peripheral O2 delivery to the exercising muscles. Furthermore, the mechanisms underlying an impaired blood flow regulation remain unresolved. Cyclic guanosine monophosphate (cGMP) is one of the main second messengers that mediate smooth muscle vasodilation and alterations in cGMP signaling could, therefore, be one mechanism by which skeletal muscle perfusion is impaired with advancing age. The current study aimed to evaluate the effect of inhibiting the main enzyme involved in cGMP degradation, phosphodiesterase 5 (PDE5), on blood flow and O2 delivery in contracting skeletal muscle of young and older humans. A group of young (23 ± 1 years) and a group of older (72 ± 2 years) male human subjects performed submaximal knee-extensor exercise in a control setting and following intake of the highly selective PDE5 inhibitor sildenafil. Sildenafil increased leg O2 delivery (6–9%) and leg O2 uptake (10–12%) at all three exercise intensities in older but not young subjects. The increase in leg O2 delivery with sildenafil in the older subjects correlated with the increase in leg O2 uptake (r2 = 0.843). These findings suggest an insufficient O2 delivery to the contracting skeletal muscle of aged individuals and that reduced cGMP availability is a novel mechanism underlying impaired skeletal muscle perfusion with advancing age.
Keywords: Blood flow, exercise, sildenafil
Introduction
Life expectancy has steadily increased over the past two centuries, resulting in an increasing number of older individuals (Oeppen and Vaupel 2002). As aging is associated with progressive loss of cardiovascular and skeletal muscle function that often leads to progressive disability (Doherty 2003) and increased risk of cardiovascular disease (Buchner 2009), a better understanding of the mechanisms that contribute to the age-related changes in vascular and skeletal muscle function is needed.
Oxidative metabolism is the dominant source of energy for skeletal muscle and to ensure sufficient availability of O2, blood flow, and O2 delivery to the contracting muscles are closely regulated to match the O2 demand (Andersen and Saltin 1985; Saltin et al. 1998; Roach et al. 1999; Gonzalez-Alonso et al. 2001). Aging has consistently been reported to be associated with reduced blood flow and O2 delivery to the exercising limb (Wahren et al. 1974; Proctor et al. 1998, 2003; Lawrenson et al. 2003; Poole et al. 2003; Kirby et al. 2012; Nyberg et al. 2012) but the mechanisms underlying the altered regulation of exercise hyperemia have not been resolved (Proctor and Parker 2006). Furthermore, to what extent a reduced blood flow and O2 delivery to contracting skeletal muscle of older individuals have metabolic and functional consequences remain unclear (Proctor and Moore 2012). Blood flow to skeletal muscle is determined by perfusion pressure and vascular tone of which the latter is the result of the balance between vasoconstrictor and vasodilator signaling pathways in vascular smooth muscle cells (VSMC). Among these regulatory mechanisms is the cyclic nucleotide cyclic guanosine monophosphate (cGMP), which mediates its effects via activation of protein kinase G (PKG), is considered one of the main second messengers that mediate vasodilation (Morgado et al. 2012). Intracellular cGMP is degraded by cGMP-binding phosphodiesterase 5 (PDE5), and concentrations of cGMP are tightly controlled by this enzyme (Rybalkin et al. 2003). Sildenafil has been shown to specifically inhibit the catalytic site of PDE5 with a very high selectivity for PDE5 and this compound can, therefore, be used to potentiate cGMP signaling via inhibition of cGMP degradation (Ballard et al. 1998; Corbin et al. 2003). This effect of sildenafil on cGMP signaling in the vasculature can be assessed in vivo by infusion of the endothelium-dependent vasodilator ACh and the nitric oxide (NO) donor sodium nitroprusside (SNP) (endothelium-independent vasodilator) as the vasoactive effects of these compounds are mediated via formation of cGMP (Hellsten et al. 2012).
Evidence in the literature indicates that cGMP signaling may be impaired in aging. In older rats, the ability of cGMP to stimulate PKG in VSMC has been shown to be blunted compared to VSMC of young rats (Lin et al. 2001). Furthermore, the bioavailability of NO, which mediates its vasodilator effect via production of cGMP, is reduced in aging (Taddei et al. 2001; Nyberg et al. 2012). To what extent alterations in cGMP signaling are involved in the reduced blood flow and O2 delivery to exercising skeletal muscle in aging humans remain unknown.
We tested the hypothesis that potentiation of cGMP signaling would increase blood flow, O2 delivery and O2 uptake in contracting skeletal muscle of older but not young human subjects during submaximal exercise engaging a small muscle mass. To accomplish this, we examined the effect of the highly selective PDE5 inhibitor sildenafil on central and peripheral hemodynamics during small muscle mass exercise in a group of young and older subjects matched for physical activity level.
Methods
A total of 17 healthy male subjects of which 9 were young (23 ± 1 years) and 8 were older (72 ± 2 years) participated in the study (Table1). The subjects underwent screening by means of a medical examination, 12-lead electrocardiogram and blood sampling from the antecubital vein. Exclusion criteria were history or symptoms of cardiovascular disease, renal dysfunction, insulin resistance, diabetes, or hypercholesterolemia. All subjects were nonsmokers and none of the subjects were taking prescription medicine. The study was approved by the Ethics Committee of Copenhagen (H-3-2012-176) and conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all subjects before enrollment into the study.
Table 1.
Subject characteristics
| Young | Older | |
|---|---|---|
| Age (years) | 23 ± 1 | 72 ± 2* |
| Height (m) | 1.83 ± 0.01 | 1.80 ± 0.02 |
| Body weight (kg) | 75.8 ± 3.3 | 79.3 ± 4.8 |
| Body fat (%) | 13.7 ± 1.4 | 25.6 ± 1.4* |
| Systolic blood pressure (mmHg) | 117 ± 3 | 138 ± 5* |
| Diastolic blood pressure (mmHg) | 60 ± 1 | 62 ± 3 |
| Mean arterial blood pressure (mmHg) | 80 ± 2 | 90 ± 2* |
| Vo2max (l/min) | 3.42 ± 0.24 | 2.42 ± 0.12* |
| Vo2max relative to body weight (l/min/kg) | 45.9 ± 2.7 | 30.6 ± 0.8* |
| Experimental leg mass (kg) | 11.9 ± 0.6 | 11.7 ± 0.4 |
| Experimental lean leg mass (kg) | 10.0 ± 0.5 | 8.9 ± 0.2 |
| Peak workload during knee-extensor exercise (W) | 48 ± 3 | 37 ± 2* |
| HbA1c (mmol/mol) | 32 ± 1 | 37 ± 1* |
| Glucose, average (from HbA1c) (mmol/l) | 5.4 ± 0.1 | 6.1 ± 0.1* |
| Total cholesterol (mmol/l) | 4.3 ± 0.3 | 5.4 ± 0.3* |
| HDL-C (mmol/l) | 1.4 ± 0.1 | 1.7 ± 0.2 |
| LDL-C (mmol/l) | 2.6 ± 0.2 | 3.2 ± 0.2* |
Values are means ± SEM. Significant difference from young
P < 0.05.
Initial testing
Before the experimental day the subjects visited the laboratory to become accustomed to the one-leg knee-extensor model (Andersen and Saltin 1985) and to perform an incremental bicycle ergometer exercise test in which pulmonary maximal oxygen uptake (L min−1, 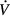 o2max) was determined (Oxycon Pro, Intramedic, Gentofte, Denmark; Table1). An incremental test was also performed in a one-leg knee-extensor ergometer to determine maximal workload (Wmax).
o2max) was determined (Oxycon Pro, Intramedic, Gentofte, Denmark; Table1). An incremental test was also performed in a one-leg knee-extensor ergometer to determine maximal workload (Wmax).
Experimental protocol
Subjects refrained from caffeine, alcohol, and exercise for 24 h before the experimental day. On the day of the experiment the subjects arrived at the laboratory after eating breakfast. After local anesthesia (lidocaine, 20 mg mL−1; Astra Zeneca, Copenhagen, Denmark), catheters (20 Ga.; Arrow Int., Reading, PA) were placed in the femoral artery and vein of the experimental leg (right) and in the femoral artery of the nonexperimental leg (left). Following 30 min of rest, subjects were positioned in a supine position where they received femoral arterial infusion of: (1) ACh (25 and 100 μg min−1 kg leg per mass; Miochol-E, Bausch & Lomb Inc., Bridgewater, NJ) and (2) SNP (4 μg min−1 kg leg per mass; Nitropress, Hospira Inc., Lake Forest, IL). Each dose of ACh and SNP was infused for 2.5 min and measurements (blood flow, blood pressure and arterial and venous blood samples [∼2 mL]) were obtained after 2.0 min. Infusion of SNP was performed 30 min after infusion of the last dose of ACh was terminated. After an additional 60 min of rest, subjects performed knee-extensor exercise at three different intensities: 6 W, 12 W and 40% of the maximal workload obtained in the incremental test (40% Wmax; 19 ± 1 and 15 ± 1 W: young and older). Each workload was sustained for 5 min and measurements were obtained after 3.5 min. Exercise performed at 6 and 12 W were completed consecutively whereas 40% Wmax was performed after 5 min of rest. Following exercise, subjects received an oral dose of the PDE5 inhibitor sildenafil (100 mg; Actavis, Hafnarfjordur, Iceland). One hour after sildenafil intake, infusion of ACh and SNP and knee-extensor exercise were repeated in the same order as during the control condition.
Measurements and calculations
Femoral arterial blood flow (FABF) was measured with ultrasound Doppler (Vivid E9; GE Healthcare, Brondby, Denmark) equipped with a linear probe operating at an imaging frequency of 11 MHz and Doppler frequency of 5.0 MHz and as previously described (Nyberg et al. 2014).
Intra-arterial and intra-venous pressure was monitored with transducers (Pressure Monitoring Set, Edwards Lifesciences, Irvine, CA) positioned at the level of the catheters. Blood gases, hemoglobin, glucose, and lactate were measured using an ABL800 FLEX analyzer (Radiometer, Bronshoj, Denmark). Total cholesterol, low-density lipoprotein (LDL-C), and high-density lipoprotein (HDL-C) were analyzed using an automatic analyzer using enzymatic kits (Cobas 8000, Roche, Hvidovre, Denmark), HbA1c using HPLC and noradrenaline using ELISA (Research ELISA, LDN, Nordhorn, Germany). Leg mass was calculated from whole-body dual-energy x-ray absorptiometry scanning (Prodigy; GE Healthcare), leg vascular conductance (LVC) as FABF/(mean femoral arterial pressure [FAP] − mean femoral venous pressure [FVP]), leg O2 delivery as arterial O2 content × FABF, leg O2 uptake as arteriovenous O2 difference × FABF, leg lactate release as arteriovenous lactate difference × FABF, change in leg lactate release as leg lactate release with sildenafil – leg lactate release in the control setting, change in leg O2 delivery as leg O2 delivery with sildenafil – leg O2 delivery in the control setting, change in leg O2 uptake as leg O2 uptake with sildenafil – leg O2 delivery in the control setting and difference in leg O2 delivery – leg O2 uptake was calculated by subtracting leg O2 uptake from leg O2 delivery.
Statistical analysis
Specific hypothesis testing during rest, infusion, and exercise was performed with two-way repeated-measures analyses of variance. After a significant F test, pairwise differences were identified using a Student-Newman–Keuls post hoc test. Differences between young and older subjects at specific time points were assessed with unpaired t-tests and changes within each group with one-sample t-tests. Pearson correlation analysis was used to determine relations of interest. The number of subjects was selected on basis of detecting ∼10% differences in blood flow, O2 delivery, and O2 uptake within each group with intake of sildenafil, as these were the main outcomes of the study. Statistical significance was set at a priori at 0.05 and data are presented as means ± SEM.
Results
Arterial ACh infusion
Sildenafil increased FABF at rest in both young (P < 0.05) and older (P < 0.05) subjects (Fig.1). In the older subjects, FABF was lower (P < 0.05) than that of the young subjects during both infusion doses without and with sildenafil. FAP was lower with sildenafil at rest (P < 0.05) and during infusion of the low (P < 0.05) and high dose (P < 0.05) of ACh. In the older subjects, FAP was higher (P < 0.05) at rest and during infusion of the low dose of ACh, but this difference was abolished with sildenafil. At rest and during each infusion, FAP decreased to a larger extent (P < 0.05) in the older (14 ± 2, 13 ± 2 and 15 ± 2 mmHg; rest, low dose and high dose) compared to the young (7 ± 2, 5 ± 2 and 5 ± 2 mmHg) subjects with sildenafil. At rest, femoral venous plasma noradrenaline increased to a similar extent in the young (1.3 ± 0.2 nmol L−1) and older (1.6 ± 0.7 nmol L−1) subjects with sildenafil. Following intake of sildenafil, LVC increased (P < 0.05) at rest and during the highest dose of ACh in both groups whereas it only increased (P < 0.05) in the older subjects during the low dose of ACh. LVC was lower (P < 0.05) in the older subjects during both infusion doses with and without sildenafil. Blood variables, heart rate and FVP are presented in Table S1A and B.
Figure 1.
Femoral arterial blood flow (A), mean femoral arterial blood pressure (B), and leg vascular conductance (C) in young and older subjects at rest and during femoral arterial ACh infusion without (CON) and with sildenafil. Significant difference from CON within same condition: *P < 0.05; significant difference from young within same condition: #P < 0.05.
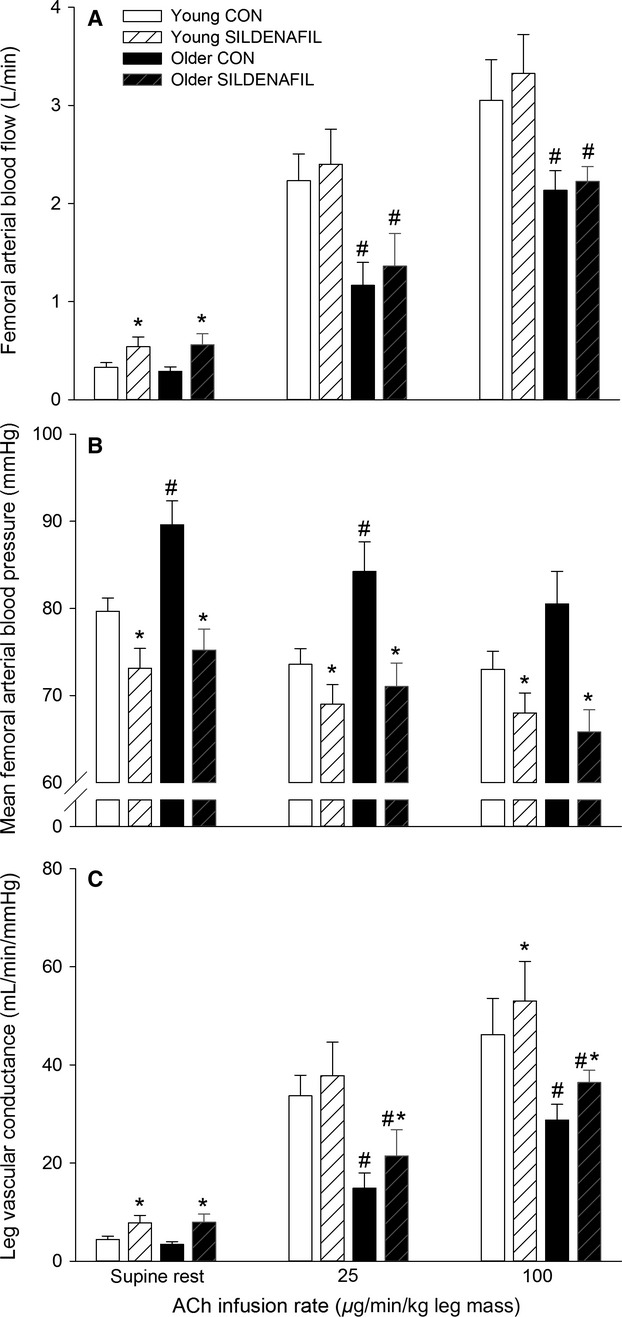
Arterial SNP infusion
Sildenafil increased (P < 0.05) FABF during SNP infusion in the young subjects whereas no significant difference was detected in the older group (Fig.2). FAP was lower (P < 0.05) with sildenafil during infusion of SNP in young and older subjects, resulting in higher (P < 0.05) LVC in both groups during infusion of SNP. The decrease in FAP with SNP was more pronounced (P < 0.05) in the older (14 ± 2 mmHg) compared to the young (4 ± 2 mmHg). A difference in the magnitude of change in FABF (0.17 ± 0.12 and 0.15 ± 0.14 L min−1, young and older subjects) and LVC (4.1 ± 1.9 and 7.3 ± 1.8 mL min−1 mmHg−1) with SNP without and with sildenafil was not detected between the young and older groups. Blood variables, heart rate, and FVP are presented in Table S2A and B.
Figure 2.
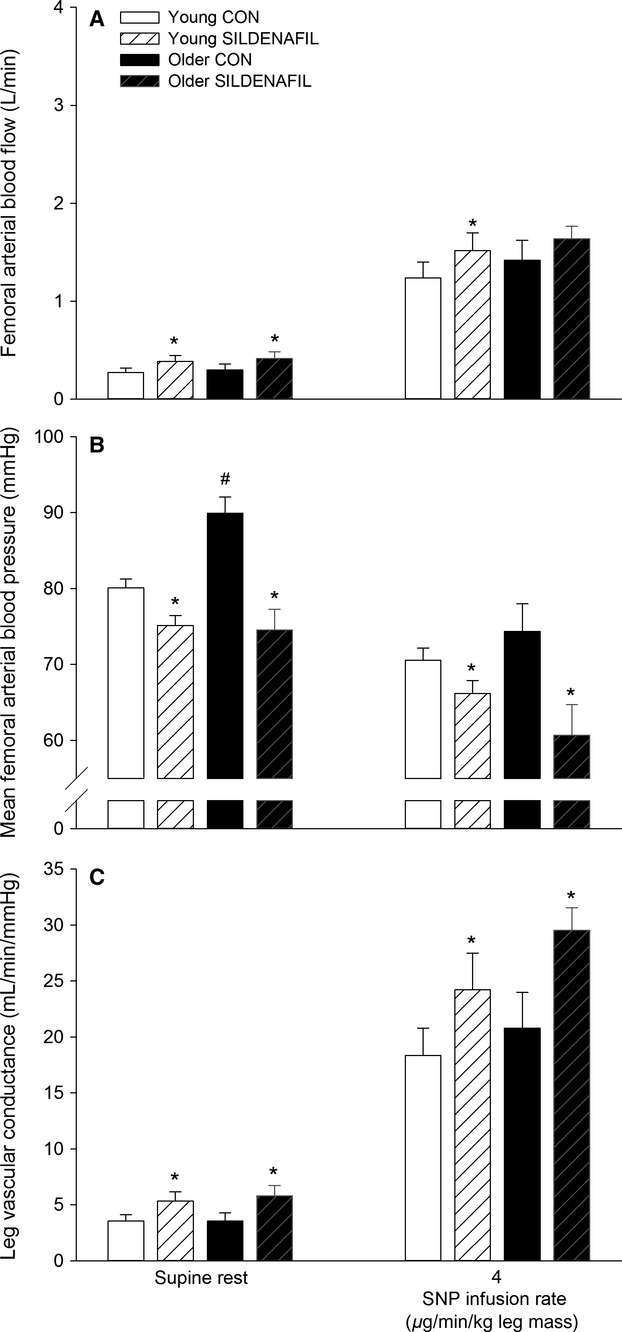
Femoral arterial blood flow (A), mean femoral arterial blood pressure (B), and leg vascular conductance (C) in young and older subjects at rest and during femoral arterial sodium nitroprusside (SNP) infusion without (CON) and with sildenafil. Significant difference from CON within same condition: *P < 0.05; significant difference from young within same condition: #P < 0.05.
Knee-extensor exercise
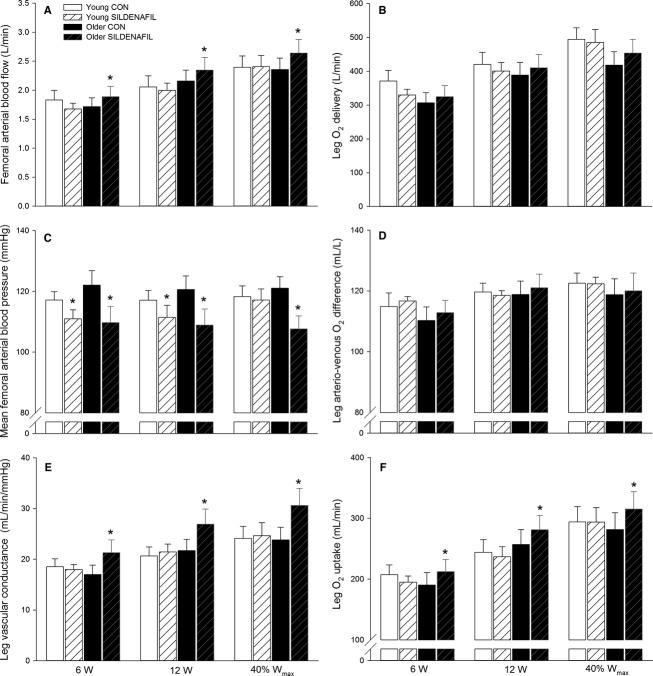
Sildenafil increased (P < 0.05) FABF at all exercise intensities in the older subjects (Fig.3). Leg O2 uptake increased (P < 0.05) at all intensities in the older group with sildenafil. In the young group, FAP was lower (P < 0.05) with sildenafil at 6 and 12 W and lower (P < 0.05) at all exercise intensities in the older group. LVC with sildenafil was unaltered in the young and increased (P < 0.05) at all exercise intensities in the older subjects.
Figure 3.
Femoral arterial blood flow (A), leg O2 delivery (B), mean femoral arterial blood pressure (C), leg arteriovenous O2 difference (D), leg vascular conductance (E), and leg O2 uptake (F) in young and older subjects during knee-extensor exercise performed at 6 W, 12 W, and 40% Wmax (19 ± 1 and 15 ± 1 W: young and older) without (CON) and with sildenafil. Significant difference from CON within same condition: *P < 0.05.
When expressed as change in response to sildenafil intake, leg O2 delivery (6.3 ± 2.4, 6.0 ± 1.7 and 9.3 ± 4.0%; 6, 12 and 40% Wmax), and leg O2 uptake (11.6 ± 3.9, 10.4 ± 2.8 and 12.8 ± 4.8%) increased (P < 0.05) at all exercise intensities in the older group (Fig.4). The change in leg O2 delivery with sildenafil correlated with the change in leg O2 uptake in the older (r2 = 0.843, P < 0.001) but not young (r2 = 0.024) subjects. The difference between leg O2 delivery and O2 uptake was greater (P < 0.05) in the young compared to the older subjects at all exercise intensities in the control condition and a negative association between the change in leg O2 uptake and leg lactate release was detected in the older (r2 = 0.445, P = 0.001) and young (r2 = 0.281, P = 0.004) subjects (Fig.5). The change in leg O2 uptake for a given change in power output during knee-extensor exercise (calculated as the slope of the linear regression of leg O2 uptake on power output for each subject; Fig.6A) was lower (P < 0.05) in older compared to young subjects in the control condition (Fig.6B). No significant difference was detected in the change in power output for a given change in leg O2 uptake with sildenafil in either group. Blood variables, heart rate, and FVP are presented in Table2.
Figure 4.
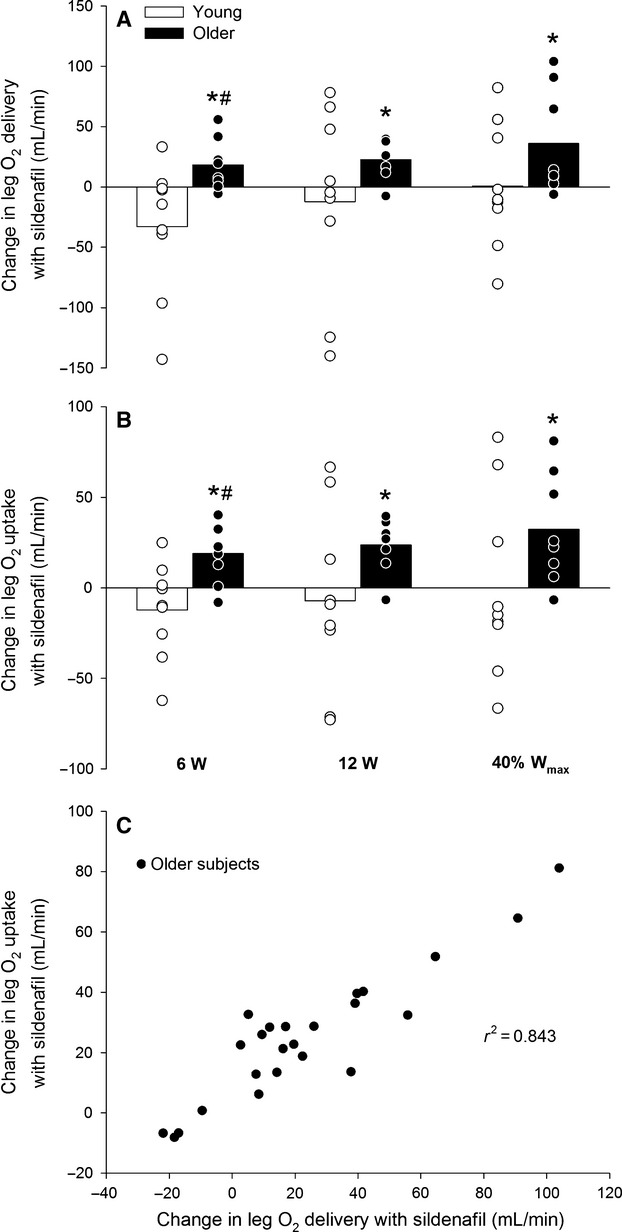
Change in leg O2 delivery with sildenafil (A), change in leg O2 uptake with sildenafil (B) and the association between the change in leg O2 delivery and the change in leg O2 delivery with sildenafil (C) in young and older subjects performing knee-extensor exercise at 6 W, 12 W, and 40% Wmax (19 ± 1 and 15 ± 1 W: young and older). Significant difference: *P < 0.05; significant difference from young within same condition: #P < 0.05.
Figure 5.
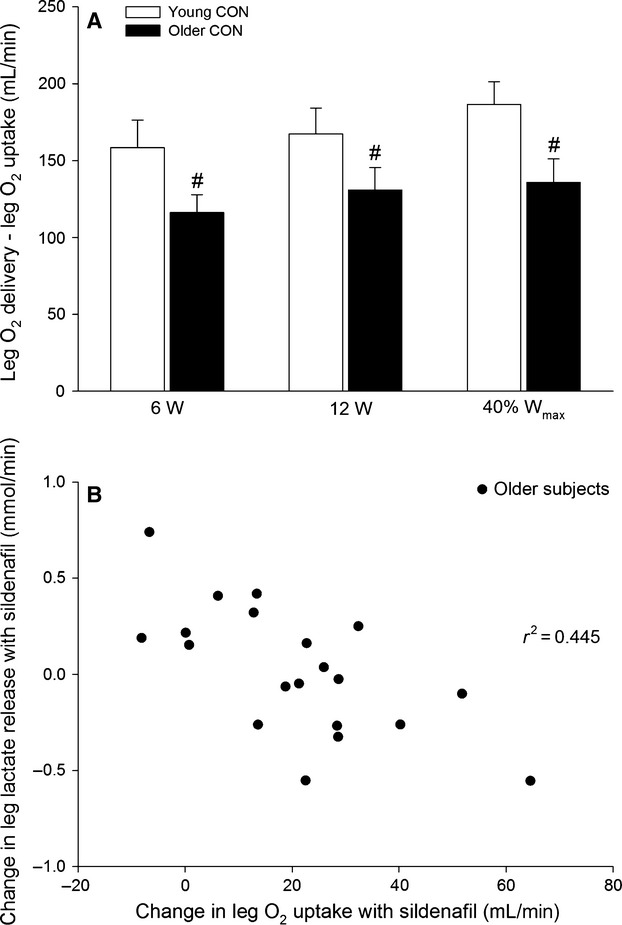
Difference between leg O2 delivery and leg O2 uptake (A) and association between change in leg O2 uptake and lactate release with sildenafil (B) in young and older subjects performing knee-extensor exercise at 6 W, 12 W, and 40% Wmax (19 ± 1 and 15 ± 1 W: young and older). Significant difference from young within same condition: #P < 0.05.
Figure 6.
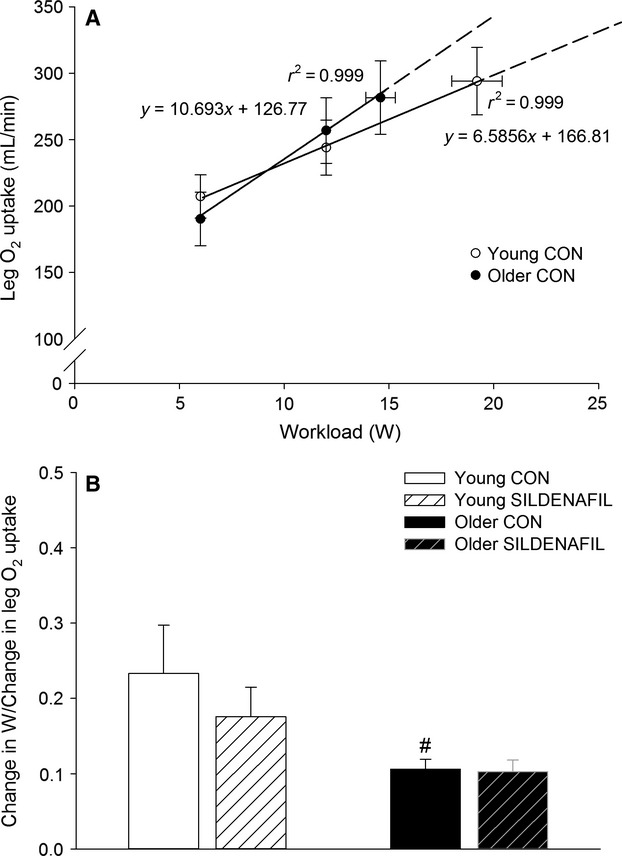
Association between workload and leg O2 uptake (A) and change in power output for a given change in leg O2 uptake (B) in young and older subjects performing knee knee-extensor exercise at 6 W, 12 W, and 40% Wmax (19 ± 1 and 15 ± 1 W: young and older). Significant difference from young within same condition: #P < 0.05.
Table 2.
Blood variables during knee-extensor exercise
| Blood variable | 6 W | 12 W | 40% Wmax | |||
|---|---|---|---|---|---|---|
| CON | SILD | CON | SILD | CON | SILD | |
| Young | ||||||
| PO2 (mmHg) | ||||||
| a | 107 ± 3 | 102 ± 2* | 103 ± 3 | 102 ± 2 | 104 ± 2 | 103 ± 2 |
| v | 26 ± 1 | 25 ± 1 | 25 ± 1 | 25 ± 1 | 25 ± 1 | 25 ± 1 |
| Hemoglobin (g dL−1) | ||||||
| a | 14.6 ± 0.2 | 14.5 ± 0.2 | 14.7 ± 0.2 | 14.7 ± 0.2 | 14.7 ± 0.3 | 14.7 ± 0.2 |
| v | 14.4 ± 0.3 | 14.2 ± 0.3 | 14.4 ± 0.2 | 14.1 ± 0.2 | 14.2 ± 0.3 | 14.2 ± 0.3 |
| O2 saturation (%) | ||||||
| a | 98.2 ± 0.1 | 98.0 ± 0.1 | 98.1 ± 0.1 | 97.9 ± 0.1 | 98.1 ± 0.1 | 98.0 ± 0.1 |
| v | 40.7 ± 2.3 | 39.8 ± 1.2 | 38.9 ± 2.1 | 40.1 ± 1.4 | 38.2 ± 1.8 | 38.5 ± 1.5 |
| O2 content (mL L−1) | ||||||
| a | 195 ± 2 | 194 ± 3 | 196 ± 3 | 195 ± 3 | 196 ± 3 | 196 ± 3 |
| v | 80 ± 6 | 77 ± 3 | 76 ± 5 | 77 ± 3 | 74 ± 5 | 74 ± 3 |
| Lactate (mmol L−1) | ||||||
| a | 1.5 ± 0.3 | 1.2 ± 0.2 | 1.8 ± 0.4 | 1.3 ± 0.3 | 2.1 ± 0.4 | 1.6 ± 0.4 |
| v | 2.2 ± 0.5 | 1.5 ± 0.3* | 2.4 ± 0.6 | 1.5 ± 0.4* | 2.6 ± 0.6 | 2.1 ± 0.6 |
| Lactate release (mmol min−1) | ||||||
| 1.1 ± 0.3 | 0.5 ± 0.3* | 1.2 ± 0.6 | 0.4 ± 0.4* | 1.2 ± 0.7 | 1.1 ± 0.6 | |
| pH | ||||||
| a | 7.394 ± 0.007 | 7.393 ± 0.005 | 7.384 ± 0.006 | 7.396 ± 0.004 | 7.380 ± 0.005 | 7.394 ± 0.007* |
| v | 7.304 ± 0.011 | 7.327 ± 0.005* | 7.296 ± 0.012 | 7.320 ± 0.010* | 7.289 ± 0.011 | 7.306 ± 0.012* |
| Heart rate (beats per minute) | ||||||
| 89 ± 7 | 87 ± 5 | 94 ± 8 | 94 ± 6 | 99 ± 6 | 100 ± 6 | |
| FVP (mmHg) | ||||||
| 18.4 ± 0.9 | 17.4 ± 1.2 | 17.6 ± 1.0 | 17.3 ± 1.0 | 17.5 ± 0.8 | 18.0 ± 1.0 | |
| Older | ||||||
| PO2 (mmHg) | ||||||
| a | 85 ± 4† | 76 ± 3*† | 83 ± 3† | 77 ± 3† | 80 ± 4† | 78 ± 4† |
| v | 25 ± 1 | 23 ± 0** | 24 ± 1 | 22 ± 1 | 23 ± 1 | 22 ± 1 |
| Hemoglobin (g dL−1) | ||||||
| a | 13.6 ± 0.3† | 13.3 ± 0.4*† | 13.7 ± 0.4† | 13.5 ± 0.3† | 13.5 ± 0.3† | 13.3 ± 0.4† |
| v | 13.4 ± 0.3† | 13.1 ± 0.4† | 13.3 ± 0.4† | 13.0 ± 0.3† | 13.6 ± 0.3 | 13.3 ± 0.3 |
| O2 saturation (%) | ||||||
| a | 96.1 ± 0.3† | 94.8 ± 0.4*† | 95.9 ± 0.3† | 94.9 ± 0.5*† | 95.5 ± 0.5† | 95.1 ± 0.6† |
| v | 37.0 ± 1.8 | 33.1 ± 1.5*† | 33.3 ± 1.7† | 30.1 ± 2.2*† | 31.2 ± 2.2† | 28.5 ± 2.0† |
| O2 content (mL L−1) | ||||||
| a | 177 ± 4† | 172 ± 5*† | 178 ± 5† | 174 ± 6*† | 176 ± 4† | 172 ± 5*† |
| v | 67 ± 3† | 59 ± 3*† | 60 ± 3*† | 53 ± 4† | 57 ± 4† | 51 ± 4*† |
| Lactate (mmol L−1) | ||||||
| a | 1.2 ± 0.2 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| v | 1.5 ± 0.2 | 1.2 ± 0.1 | 1.4 ± 0.2 | 1.2 ± 0.1 | 1.6 ± 0.2 | 1.4 ± 0.2 |
| Lactate release (mmol min−1) | ||||||
| 0.6 ± 0.1 | 0.4 ± 0.2 | 0.5 ± 0.2 | 0.3 ± 0.2 | 0.7 ± 0.3 | 0.7 ± 0.3 | |
| pH | ||||||
| a | 7.413 ± 0.006† | 7.421 ± 0.005† | 7.412 ± 0.005† | 7.424 ± 0.006† | 7.410 ± 0.008† | 7.431 ± 0.005*† |
| v | 7.336 ± 0.007† | 7.350 ± 0.004* | 7.337 ± 0.005† | 7.342 ± 0.006 | 7.333 ± 0.006† | 7.339 ± 0.006† |
| Heart rate (beats per minute) | ||||||
| 74 ± 5 | 74 ± 5 | 78 ± 5 | 79 ± 6 | 82 ± 6 | 81 ± 6 | |
| FVP (mmHg) | ||||||
| 19.7 ± 1.6 | 19.0 ± 1.8 | 19.7 ± 1.8 | 19.8 ± 1.9 | 20.2 ± 1.8 | 19.7 ± 1.9 | |
a, femoral arterial; v, femoral venous.
Significant difference from CON within same condition (P < 0.05).
Significant difference from young within same condition (P < 0.05).
There were no associations between the changes in leg O2 delivery with sildenafil and age, systolic blood pressure, FAP, HbA1c, total cholesterol, and LDL cholesterol.
Discussion
The findings from the current study demonstrate that potentiation of cGMP signaling by inhibition of PDE5 activity increases blood flow, O2 delivery, and O2 uptake in the exercising lower limb of older but not young human subjects during submaximal exercise engaging a small muscle mass. This effect of cGMP potentiation suggests an insufficient blood flow and O2 delivery to the contracting skeletal muscle of aged individuals and that a reduced cGMP level is a novel mechanism by which skeletal muscle blood flow and O2 delivery are impaired with advancing age in humans.
Blood flow to contracting skeletal muscle is closely regulated as a function of O2 bound to hemoglobin and arterial O2 content so that O2 delivery matches the O2 demand (Saltin et al. 1998; Roach et al. 1999; Gonzalez-Alonso et al. 2001). Aging is associated with a decline in hemoglobin levels and arterial O2 content (Ershler et al. 2005). Therefore, a lowered (Proctor et al. 1998; Lawrenson et al. 2003; Poole et al. 2003; Kirby et al. 2012; Nyberg et al. 2012) or unaltered (Beere et al. 1999; Proctor et al. 2003) blood flow to contracting skeletal muscle in aged individuals could result in an insufficient O2 delivery to meet the O2 demand of the contracting skeletal muscles. In the current study, hemoglobin levels, arterial O2 content, and the difference between O2 delivery and O2 uptake were lower in the older group, suggesting that O2 delivery could limit oxidative metabolism. We did not detect a difference in O2 uptake during submaximal exercise between the young and the older group in the control setting and although this would indicate that O2 delivery was sufficient to meet the O2 demand, age-related differences in mechanical efficiency with the possibility of anaerobic energy contribution needs to be taken into account. Accordingly, impaired mitochondrial and contractile efficiency has been documented in the human quadriceps muscle of aged subjects (Conley et al. 2013; Layec et al. 2015), suggesting that the metabolic demand for the same absolute workload was higher in the older group in the current study. The change in power output for a given change in leg O2 uptake during knee-extensor exercise was also found to be lower in the older group (Fig.6A and B), which is in support of a lower mechanical efficiency. Hence, the increase in O2 uptake with sildenafil in the older subjects is likely to reflect that oxidative metabolism in the control setting was compromised by insufficient O2 delivery. Notably, the lower difference between O2 delivery and O2 uptake in the older group was unaltered with sildenafil. This finding is explained by the close association between the increase in O2 delivery and O2 uptake and is in agreement with an insufficient O2 delivery in the control setting.
An insufficient O2 delivery to meet the oxidative demand in the older subjects would entail that anaerobic metabolism compensated for the lower aerobic metabolism. With regard to quantification of anaerobic metabolism, although lactate is formed and utilized continuously under fully aerobic conditions (Brooks 2009). Lactate leaving the exercising limb is still descriptive of anaerobic glycolysis in skeletal muscle (Juel and Halestrap 1999). As no difference in leg lactate release in the control condition was detected between the two groups, it may be that the intracellular level of lactate in the muscle of the older subjects was higher as the capacity to release lactate from the active muscle fibers may have been reduced. In line with this suggestion, the two important lactate transporters MCT1 and MCT4 have been shown to be downregulated in aging skeletal muscle (Masuda et al. 2009). Furthermore, in a previous study on older lifelong physically sedentary subjects, lactate release from the exercising leg was found to be similar to that of young adults despite a lower leg O2 uptake in the older group (Nyberg et al. 2012), indicating that lactate release was reduced as anaerobic metabolism would be expected to compensate for the lower oxidative metabolism. Importantly, the change in leg O2 uptake with sildenafil in the current study was negatively correlated with the change in lactate release, indicating that the increase in oxidative metabolism reduced anaerobic metabolism.
An insufficient O2 delivery to meet the metabolic demand of the contracting skeletal muscles in aging was also supported by a close association (r2 = 0.843) between the change in O2 delivery and O2 uptake with sildenafil. Alternatively, the increase in O2 delivery with sildenafil could be a result of an increased metabolic demand due to reduced mitochondrial efficiency, however, sildenafil has been shown not to affect oxidative phosphorylation in isolated mitochondria (Fernandes et al. 2008) and lactate levels and the change in power output for a given change in leg O2 uptake did not change with sildenafil.
In the current study, blood flow, O2 delivery, and O2 uptake was found to increase by ∼6–12% during exercise in the older subjects with sildenafil. Previous studies have shown reductions of ∼10–20% in blood flow and O2 uptake (Proctor et al. 1998; Lawrenson et al. 2003; Poole et al. 2003; Kirby et al. 2012; Nyberg et al. 2012) in aged individuals compared to young, indicating that the increase in these variables in the current study are of physiological relevance. It would, therefore, be of interest in future studies to examine the extent to which PDE5 inhibition can improve the functional capacity of older subjects. In this context, it may be that during exercise involving a large muscle mass, arterial blood pressure following sildenafil intake could compromise perfusion of the contracting muscles, thereby negating the effect observed during small muscle mass exercise.
Despite many efforts to determine the effects of aging on exercise hyperemia, a fundamental question regarding the mechanisms by which blood flow is altered remains unanswered. The cyclic nucleotide cGMP is considered one of the main second messengers that mediate vasodilation (Morgado et al. 2012). Acute potentiation of cGMP signaling via inhibition of PDE5 has been shown to increase blood flow following forearm contractions in hypertensive subjects (Attina et al. 2008) and skeletal muscle oxygenation during exercise in patients with atherosclerotic disease (Roseguini et al. 2014) and an altered cGMP signaling could be one mechanism by which blood flow regulation during exercise is impaired in aging. In the present study, potentiation of cGMP signaling by inhibition of PDE5 increased LVC and blood flow during exercise in the older but not young group. This effect of PDE5 inhibition specifically in the older group could reflect that aged individuals have generally higher activity of PDE5 and/or lower cGMP formation in contracting skeletal muscle. Although it is difficult to differentiate between these mechanisms based on an integrative response to PDE5 inhibition, the finding that sildenafil increased LVC to a similar extent in response to infusion of an NO donor is indicative of a similar activity of PDE5 in the young and older subjects. Hence, this finding on the vascular response to an NO donor is in line with a reduced cGMP formation in contracting skeletal muscle of older individuals.
The vasoactive substance NO plays a key role in the regulation of systemic blood pressure (Rees et al. 1989). Thus, the observation that sildenafil was found to induce a more marked reduction in blood pressure in the older subjects is in agreement with a reduced cGMP availability in the older subjects. This effect of PDE5 inhibition on blood pressure in the older group could be related to a higher initial blood pressure and hence less pronounced baroreceptor buffering in these subjects. However, a difference in the change in venous noradrenaline, which has been shown to correlate closely with acute changes in skeletal muscle sympathetic nervous activity (Grassi et al. 2008), was not found between the two groups following intake of sildenafil. This observation is also in line with a previous study demonstrating that the reduction in blood pressure with sildenafil was more related to age than blood pressure per se (Vardi et al. 2002).
It has been shown that aging is associated with a reduced vascular response to infusion of the endothelium-dependent vasodilator ACh (Taddei et al. 2001; Mortensen et al. 2012) as a consequence of a lower NO bioavailability (Taddei et al. 2001). In the current study, the vasodilator response to ACh was accordingly found to be lower in the older than the young group in the control setting. Although speculative, this could indicate that a diminished endothelial NO bioavailability was one factor contributing to lower cGMP levels during exercise. Notably, NO does not appear to be obligatory for exercise hyperemia during lower limb exercise in young subjects (Radegran and Saltin 1999) and a lower NO bioavailability would, therefore, entail an altered role of the NO system and/or that redundant systems that would normally compensate for a reduced NO formation are also affected by aging (Mortensen et al. 2007; Schrage et al. 2007).
Many biological events associated with advancing age are due to complex and integrated alterations in physiological systems that are influenced by genetic and life-style factors. One important lifestyle factor is the level of physical activity as evidenced by studies on enforced inactivity such as bed rest that initiates an “accelerated aging” process (Saltin et al. 1968; McGuire et al. 2001), and it has been argued that being physically active is the default requirement for maintaining health and physiological function throughout the life span (Lazarus and Harridge 2010). Therefore, we chose to include subjects that were characterized by a moderate level of physical activity in order to limit the potential adverse effects of skeletal muscle disuse in an attempt to elucidate the effects of primary aging. Hence, the finding that PDE5 inhibition increased blood flow and O2 uptake in these recreationally active subjects indicates that groups characterized by pronounced impaired endothelial function such as hypertension (Taddei et al. 1997; Attina et al. 2008), diabetes (Schalkwijk and Stehouwer 2005) and hypercholesterolemia (Creager et al. 1990) may benefit even more from potentiation of cGMP signaling.
Summary
The current study shows that potentiation of cGMP signaling increases blood flow, O2 delivery, and oxidative metabolism in the exercising lower limb of older but not young healthy human subjects during submaximal exercise engaging only a small muscle mass. This finding suggest that a reduced O2 delivery to contracting skeletal muscle of older individuals have metabolic consequences that, at least in part, may explain the impairment in physical capacity associated with aging and that reduced levels of cGMP is one mechanism underlying the insufficient O2 delivery.
Acknowledgments
Rasmus Damsgaard is gratefully acknowledged for his excellent medical assistance.
Conflict of Interest
None declared.
Supporting Information
Table S1. Blood variables at rest and during ACh infusion. (A) Young, (B) older.
Table S2. Blood variables at rest and during SNP infusion. (A) Young, (B) older.
References
- Andersen P. Saltin B. Maximal perfusion of skeletal muscle in man. J. Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attina TM, Malatino LS, Maxwell SR, Padfield PL. Webb DJ. Phosphodiesterase type 5 inhibition reverses impaired forearm exercise-induced vasodilatation in hypertensive patients. J. Hypertens. 2008;26:501–507. doi: 10.1097/HJH.0b013e3282f382ff. [DOI] [PubMed] [Google Scholar]
- Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME. Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J. Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW. Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Cell-cell and intracellular lactate shuttles. J. Physiol. 2009;587:5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner DM. Physical activity and prevention of cardiovascular disease in older adults. Clin. Geriatr. Med. 2009;25:661–675. doi: 10.1016/j.cger.2009.08.002. viii. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Cress ME. Esselman P. Exercise efficiency is reduced by mitochondrial uncoupling in the elderly. Exp. Physiol. 2013;98:768–777. doi: 10.1113/expphysiol.2012.067314. [DOI] [PubMed] [Google Scholar]
- Corbin JD, Blount MA, Weeks JL, Beasley A, Kuhn KP, Ho YS, et al. [3H]sildenafil binding to phosphodiesterase-5 is specific, kinetically heterogeneous, and stimulated by cGMP. Mol. Pharmacol. 2003;63:1364–1372. doi: 10.1124/mol.63.6.1364. [DOI] [PubMed] [Google Scholar]
- Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, Loscalzo J, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J. Clin. Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. J. Appl. Physiol. (1985) 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Sheng S, McKelvey J, Artz AS, Denduluri N, Tecson J, et al. Serum erythropoietin and aging: a longitudinal analysis. J. Am. Geriatr. Soc. 2005;53:1360–1365. doi: 10.1111/j.1532-5415.2005.53416.x. [DOI] [PubMed] [Google Scholar]
- Fernandes MA, Marques RJ, Vicente JA, Santos MS, Monteiro P, Moreno AJ, et al. Sildenafil citrate concentrations not affecting oxidative phosphorylation depress H2O2 generation by rat heart mitochondria. Mol. Cell. Biochem. 2008;309:77–85. doi: 10.1007/s11010-007-9645-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Richardson RS. Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J. Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Bolla G, Quarti-Trevano F, Dell’Oro R, Arenare F, et al. Heart rate as a sympathetic marker during acute adrenergic challenge. J. Hypertens. 2008;26:70–75. doi: 10.1097/HJH.0b013e3282f112e6. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Nyberg M, Jensen LG. Mortensen SP. Vasodilator interactions in skeletal muscle blood flow regulation. J. Physiol. 2012;590:6297–6305. doi: 10.1113/jphysiol.2012.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C. Halestrap AP. Lactate transport in skeletal muscle – role and regulation of the monocarboxylate transporter. J. Physiol. 1999;517(Pt 3):633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Voyles WF. Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ. Res. 2012;111:220–230. doi: 10.1161/CIRCRESAHA.112.269571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P. Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Layec G, Hart CR, Trinity JD, Le FY, Jeong EK. Richardson RS. Skeletal muscle work efficiency with age: the role of non-contractile processes. Clin. Sci. (Lond.) 2015;128:213–223. doi: 10.1042/CS20140274. [DOI] [PubMed] [Google Scholar]
- Lazarus NR. Harridge SD. Exercise, physiological function, and the selection of participants for aging research. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:854–857. doi: 10.1093/gerona/glq016. [DOI] [PubMed] [Google Scholar]
- Lin CS, Liu X, Tu R, Chow S. Lue TF. Age-related decrease of protein kinase G activation in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2001;287:244–248. doi: 10.1006/bbrc.2001.5567. [DOI] [PubMed] [Google Scholar]
- Masuda S, Hayashi T, Egawa T. Taguchi S. Evidence for differential regulation of lactate metabolic properties in aged and unloaded rat skeletal muscle. Exp. Gerontol. 2009;44:280–288. doi: 10.1016/j.exger.2008.12.003. [DOI] [PubMed] [Google Scholar]
- McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, et al. A 30-year follow-up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation. 2001;104:1350–1357. [PubMed] [Google Scholar]
- Morgado M, Cairrao E, Santos-Silva AJ. Verde I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell. Mol. Life Sci. 2012;69:247–266. doi: 10.1007/s00018-011-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B. Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J. Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Winding K. Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J. Physiol. 2012;590:6227–6236. doi: 10.1113/jphysiol.2012.240093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y. Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J. Physiol. 2012;590:5361–5370. doi: 10.1113/jphysiol.2012.239053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Christensen PM, Mortensen SP, Hellsten Y. Bangsbo J. Infusion of ATP increases leg oxygen delivery but not oxygen uptake in the initial phase of intense knee-extensor exercise in humans. Exp. Physiol. 2014;99:1399–1408. doi: 10.1113/expphysiol.2014.081141. [DOI] [PubMed] [Google Scholar]
- Oeppen J. Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C. Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1251–H1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- Proctor DN. Moore DJ. Lifelong physical activity and blood flow to active muscles: sufficient supply to meet the demand. J. Physiol. 2012;590:5927–5928. doi: 10.1113/jphysiol.2012.245183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor DN. Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation. 2006;13:315–327. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, et al. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J. Appl. Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA. Leuenberger UA. Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J. Appl. Physiol. (1985) 2003;94:1859–1869. doi: 10.1152/japplphysiol.00898.2002. [DOI] [PubMed] [Google Scholar]
- Radegran G. Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am. J. Physiol. 1999;276:H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Rees DD, Palmer RM. Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl Acad. Sci. USA. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach RC, Koskolou MD, Calbet JA. Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am. J. Physiol. 1999;276:H438–H445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- Roseguini BT, Hirai DM, Alencar MC, Ramos RP, Silva BM, Wolosker N, et al. Sildenafil improves skeletal muscle oxygenation during exercise in men with intermittent claudication. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R396–R404. doi: 10.1152/ajpregu.00183.2014. [DOI] [PubMed] [Google Scholar]
- Rybalkin SD, Yan C, Bornfeldt KE. Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ. Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K. Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38:VII1–VII78. [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD. Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol. Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Schalkwijk CG. Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin. Sci. (Lond.) 2005;109:143–159. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH. Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J. Physiol. 2007;579:227–236. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, et al. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29:736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Vardi Y, Klein L, Nassar S, Sprecher E. Gruenwald I. Effects of sildenafil citrate (viagra) on blood pressure in normotensive and hypertensive men. Urology. 2002;59:747–752. doi: 10.1016/s0090-4295(02)01510-8. [DOI] [PubMed] [Google Scholar]
- Wahren J, Saltin B, Jorfeldt L. Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand. J. Clin. Lab. Invest. 1974;33:79–86. doi: 10.3109/00365517409114201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Blood variables at rest and during ACh infusion. (A) Young, (B) older.
Table S2. Blood variables at rest and during SNP infusion. (A) Young, (B) older.