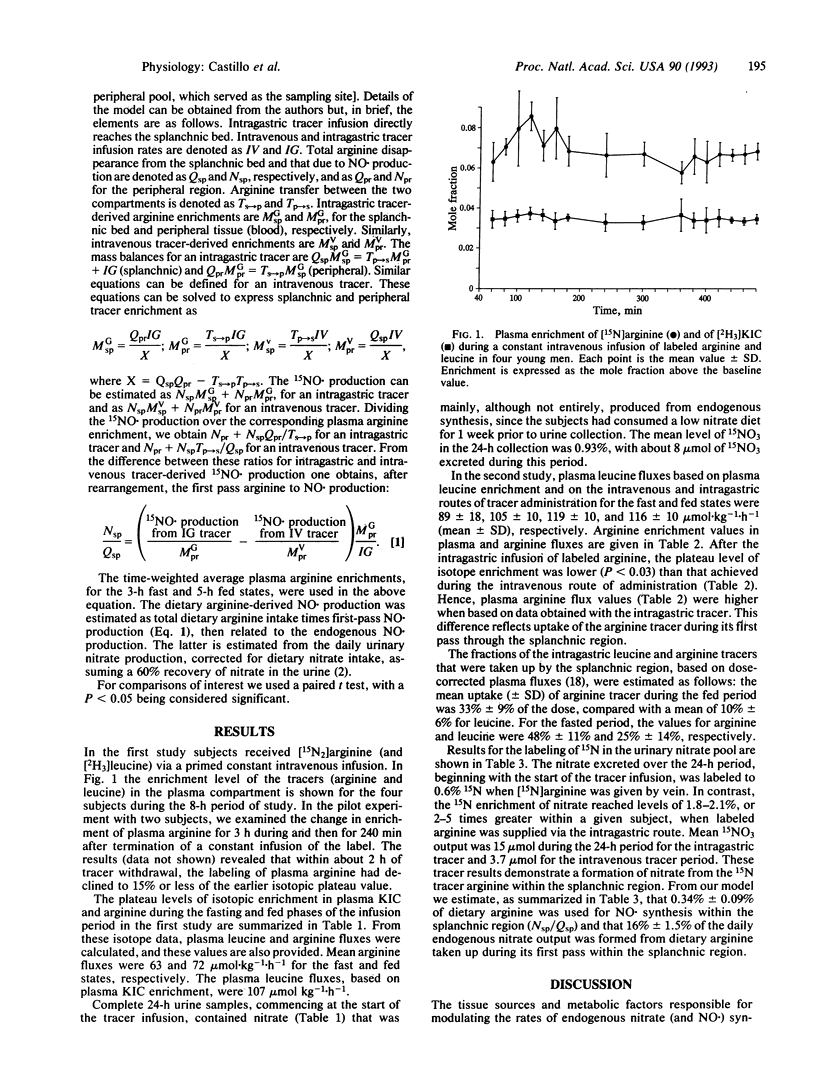
Abstract
Urinary nitrate (NO3) is the stable end product of nitric oxide, which is formed, in turn, from a guanidino nitrogen of arginine. We have conducted two experiments, each in four healthy adult men receiving a low nitrate diet for 7-10 days, to investigate the in vivo conversion of arginine to nitrate. In the first study [guanidino-15N2, 5,5-2H2]arginine was given on day 7 via a primed continuous intravenous infusion for 8 h. In the second study, the labeled arginine was given for 8 h by the intragastric route on day 7 and by the intravenous route on day 10. Measurement of 15NO3 output in urine collected for 24 h beginning at the time of the arginine tracer infusion revealed a more extensive transfer of 15N when the arginine tracer was given intragastricly. From the comparative labeling of 15NO3 after administration of the tracer arginine via the intragastric and intravenous routes, we estimate that 16% +/- 2% of the daily production of nitrate arises from the metabolism of dietary arginine that is taken up during its "first pass" in the splanchnic region. Hence, nitric oxide production occurs, to a measurable extent, in this area in healthy subjects, raising the question as to how various pathophysiological states might alter the relations between exogenous and endogenous sources of arginine as precursors of NO. and the relative contributions made by various organs to whole body (NO.) NO3 formation. These results also raise important questions about the use of nitric oxide synthase inhibitors in animal and human studies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. F. Determination of amino acid profiles in biological samples by gas chromatography. J Chromatogr. 1974 Aug 14;95(2):189–212. doi: 10.1016/s0021-9673(00)84078-9. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Montesano R. Relevance of nitrosamines to human cancer. Carcinogenesis. 1984 Nov;5(11):1381–1393. doi: 10.1093/carcin/5.11.1381. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., Ferrari F. K., Simmons R. L. Evidence that activation of Kupffer cells results in production of L-arginine metabolites that release cell-associated iron and inhibit hepatocyte protein synthesis. Surgery. 1989 Aug;106(2):364–372. [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Chaves Das Neves H. J., Vasconcelos A. M. Capillary gas chromatography of amino acids, including asparagine and glutamine: sensitive gas chromatographic-mass spectrometric and selected ion monitoring gas chromatographic-mass spectrometric detection of the N,O(S)-tert.-butyldimethylsilyl derivatives. J Chromatogr. 1987 Apr 17;392:249–258. doi: 10.1016/s0021-9673(01)94270-0. [DOI] [PubMed] [Google Scholar]
- Cheung C. W., Cohen N. S., Raijman L. Channeling of urea cycle intermediates in situ in permeabilized hepatocytes. J Biol Chem. 1989 Mar 5;264(7):4038–4044. [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. Channeling of extramitochondrial ornithine to matrix ornithine transcarbamylase. J Biol Chem. 1987 Jan 5;262(1):203–208. [PubMed] [Google Scholar]
- Evans T., Carpenter A., Cohen J. Purification of a distinctive form of endotoxin-induced nitric oxide synthase from rat liver. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5361–5365. doi: 10.1073/pnas.89.12.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Haüssinger D. Nitrogen metabolism in liver: structural and functional organization and physiological relevance. Biochem J. 1990 Apr 15;267(2):281–290. doi: 10.1042/bj2670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992 Mar;89(3):867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerr R. A., Matthews D. E., Bier D. M., Young V. R. Leucine kinetics from [2H3]- and [13C]leucine infused simultaneously by gut and vein. Am J Physiol. 1991 Jan;260(1 Pt 1):E111–E117. doi: 10.1152/ajpendo.1991.260.1.E111. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989 Jul;69(3):708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Langenbeck U., Luthe H., Schaper G. Keto acids in tissues and biological fluids: O-t-butyldimethylsilyl quinoxalinols as derivatives for sensitive gas chromatographic/mass spectrometric determination. Biomed Mass Spectrom. 1985 Sep;12(9):507–509. doi: 10.1002/bms.1200120912. [DOI] [PubMed] [Google Scholar]
- Leaf C. D., Wishnok J. S., Tannenbaum S. R. L-arginine is a precursor for nitrate biosynthesis in humans. Biochem Biophys Res Commun. 1989 Sep 15;163(2):1032–1037. doi: 10.1016/0006-291x(89)92325-5. [DOI] [PubMed] [Google Scholar]
- Leone A. M., Palmer R. M., Knowles R. G., Francis P. L., Ashton D. S., Moncada S. Constitutive and inducible nitric oxide synthases incorporate molecular oxygen into both nitric oxide and citrulline. J Biol Chem. 1991 Dec 15;266(35):23790–23795. [PubMed] [Google Scholar]
- Lowenstein C. J., Glatt C. S., Bredt D. S., Snyder S. H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons C. R., Orloff G. J., Cunningham J. M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992 Mar 25;267(9):6370–6374. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991 Aug;21(4):361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nissim I., Yudkoff M., Terwilliger T., Segal S. Rapid determination of [guanidino-15N]arginine in plasma with gas chromatography--mass spectrometry: application to human metabolic studies. Anal Biochem. 1983 May;131(1):75–82. doi: 10.1016/0003-2697(83)90137-9. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rocchiccioli F., Leroux J. P., Cartier P. Quantitation of 2-ketoacids in biological fluids by gas chromatography chemical ionization mass spectrometry of O-trimethylsilyl-quinoxalinol derivatives. Biomed Mass Spectrom. 1981 Apr;8(4):160–164. doi: 10.1002/bms.1200080406. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Biological roles of nitric oxide. Sci Am. 1992 May;266(5):68-71, 74-7. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- Srere P. A., Ovadi J. Enzyme-enzyme interactions and their metabolic role. FEBS Lett. 1990 Aug 1;268(2):360–364. doi: 10.1016/0014-5793(90)81286-w. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F., Griffith O. W., Feldman P. L., Wiseman J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J Biol Chem. 1991 Apr 5;266(10):6259–6263. [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. A., Schultz D. S., Deen W. M., Young V. R., Tannenbaum S. R. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: effect of diet supplementation with ascorbic acid. Cancer Res. 1983 Apr;43(4):1921–1925. [PubMed] [Google Scholar]
- Wagner D. A., Young V. R., Tannenbaum S. R. Mammalian nitrate biosynthesis: incorporation of 15NH3 into nitrate is enhanced by endotoxin treatment. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4518–4521. doi: 10.1073/pnas.80.14.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K. A., Marletta M. A. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992 Jul 28;31(29):6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- White M. F., Christensen H. N. Simultaneous regulation of amino acid influx and efflux by system A in the hepatoma cell HTC. Ouabain simulates the starvation-induced derepression of system A amino acid transport. J Biol Chem. 1983 Jul 10;258(13):8028–8038. [PubMed] [Google Scholar]
- White M. F. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1985 Dec 9;822(3-4):355–374. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]



