Abstract
Pneumocystis jirovecii infection causes fulminant interstitial pneumonia (Pneumocystis pneumonia, PCP) in patients with rheumatoid arthritis (RA) who are receiving biological and/or nonbiological antirheumatic drugs. Recently, we encountered a PCP outbreak among RA outpatients at our institution. Hospital-acquired, person-to-person transmission appears to be the most likely mode of this cluster of P. jirovecii infection. Carriage of P. jirovecii seems a time-limited phenomenon in immunocompetent hosts, but in RA patients receiving antirheumatic therapy, clearance of this organism from the lungs is delayed. Carriers among RA patients can serve as sources and reservoirs of P. jirovecii infection for other susceptible patients in outpatient facilities. Development of PCP is a matter of time in such carriers. Considering the poor survival rates of PCP cases, prophylactic antibiotics should be considered for RA patients who are scheduled to receive antirheumatic therapy. Once a new case of PCP occurs, we should take prompt action not only to treat the PCP patient but also to prevent other patients from becoming new carriers of P. jirovecii. Short-term prophylaxis with trimethoprim-sulfamethoxazole is effective in controlling P. jirovecii infection and preventing future outbreaks of PCP among RA patients.
Keywords: Pneumocystis jirovecii pneumonia, rheumatoid arthritis, asymptomatic carrier, nosocomial transmission, short-term prophylaxis
Introduction
Pneumocystis jirovecii is one of the most significant opportunistic fungal pathogens in humans with impaired immune function. An immunocompetent host clears this organism without obvious clinical consequences, while most immunocompromised patients develop interstitial pneumonia (Pneumocystis pneumonia, PCP). With the emergence of human immunodeficiency virus (HIV), there was a dramatic increase in the incidence of PCP. In the 1980s, PCP was the most common opportunistic infection in patients with AIDS: it was an AIDS-defining illness for more than half of the adults and adolescents with AIDS. The routine use of trimethoprim-sulfamethoxazole (TMP-SMX) as PCP chemoprophylaxis and the introduction of highly active antiretroviral therapy led to a substantial decline in the incidence of PCP among HIV-positive individuals.1,2 However, PCP has become a serious public health threat to HIV-negative immunocompromised patients such as those receiving immunosuppressive therapy or anticancer chemotherapy for hematological malignancies and solid tumors, organ and bone marrow transplantation, and autoimmune inflammatory diseases.3–9
The Japanese Ministry of Health, Labor and Welfare Study Group recommended the use of TMP-SMX or pentamidine as a prophylactic against PCP for patients with inflammatory rheumatic diseases over the age of 50 receiving corticosteroids equivalent to 1.2 mg/kg/day of prednisolone or more, those receiving corticosteroids equivalent to 0.8 mg/kg/day of prednisolone or more along with immunosuppressive agents, or those whose peripheral lymphocyte counts are <500/μL during immunosuppressive therapy.10 However, we encountered a PCP outbreak among outpatients with rheumatoid arthritis (RA) who did not satisfy any of these conditions.11,12 All PCP patients in the outbreak had received low-dose methotrexate (MTX) therapy but no biological agents. Their peripheral lymphocyte counts were maintained at levels >500/μL. They had not received high doses of corticosteroids. Which RA patients should receive prophylactic antibiotics? What is the optimal duration of such chemoprophylaxis? Which agent is recommended for prophylaxis for PCP during anti-RA therapy? This article includes an up-to-date review of present literature on P. jirovecii infection and a proposal for preventive strategies against PCP outbreak among RA patients.
Risk Factors of PCP in RA and other Inflammatory Rheumatic Diseases
Biological and nonbiological antirheumatic drugs
Over the past decade, the treatment of RA has changed dramatically through early use of MTX and the advent of biological molecular-targeted agents. With the increased use of such antirheumatic drugs, RA patients have been exposed to an increased risk of PCP.13–18 Recent postmarketing surveillance (PMS) reports by pharmaceutical companies in Japan reported >200 cases of PCP during low-dose MTX therapy as of June 2012.19 Other PMS reports in Japan indicated a high incidence of PCP in RA patients receiving the tumor necrosis factor α (TNFα) inhibitors infliximab (0.4% of 5,000 patients), etanercept (0.2% of 7,091 patients), and adalimumab (0.3% of 3,000 patients).20–22 A review of U.S. Food and Drug Administration data between 1998 and 2003 identified 84 cases of PCP following infliximab therapy, and among those cases, 49 were RA patients.23 Regarding the monoclonal anti-interleukin-6 receptor antibody tocilizumab, a PMS report showed that the incidence of PCP was 0.28/100 patient-years in Japan.24 In addition, there are several reports on PCP cases occurring in RA patients who were treated with newly approved biological drugs, such as rituximab (a monoclonal antibody that binds the CD20 antigen on B lymphocytes)25,26 and abatacept (a biological agent that inhibits the activation and proliferation of T lymphocytes).27 In Table 1, we show the prevalence rates of PCP in RA patients receiving biological antirheumatic therapy. These rates were obtained from the most recent surveillance reports produced by individual pharmaceutical companies in Japan.
Table 1.
Prevalence of Pneumocystis jirovecii pneumonia in RA patients during biological antirheumatic therapy in Japan.
| DRUGS | MECHANISMS OF ACTION | PCP PREVALENCE NUMBER (%) | MORTALITY NUMBER (%) |
|---|---|---|---|
| Infliximab | TNFα inhibitor (anti-TNFα antibody) | 188 (0.3) | 19 (10.1) |
| Etanercept | TNFα inhibitor (soluble TNF receptor) | 81 (0.1) | 15 (18.5) |
| Adalimumab | TNFα inhibitor (anti-TNFα antibody) | 54 (0.3) | 10 (18.5) |
| Tocilizumab | Anti–IL-6 receptor antibody | 14 (0.2) | 2 (14.3) |
| Abatacept | T-cell signaling inhibitor | 9 (0.1) | 2 (22.2) |
Note: Adapted from Mori et al.19
As a result of a literature search of the PubMed and EMBASE, Kourbeti et al showed that no significant differences were found in the incidence of PCP between RA patients receiving biological therapy and those mainly receiving MTX-based regimens without biologics, suggesting that RA patients are susceptible to PCP regardless of the type of antirheumatic therapy. Nevertheless, the absence of a significant association with biological therapy should not be regarded as proof of no difference, because there is concern over the lack of statistical power.28 Using two different population-based hospitalization databases in the United States, Louie et al showed that there was no detectable increase in the frequency of PCP among RA patients in the United States from 1996 to 2007. Data suggested that the introduction and increased use of biological antirheumatic drugs might not have influenced incidence rates in PCP. We should keep in mind, however, that this complication may not be frequent enough to detect any increase in rates of PCP at the population levels.29
Steroids
Systemic corticosteroid therapy has been identified as a risk factor of PCP in patients with RA,30–32 those with Wegener’s granulomatosis (WG),33 those with inflammatory rheumatic diseases including RA,17,34–38 and those with non-RA inflammatory rheumatic diseases.39 Corticosteroids are likely to promote PCP development through depletion of CD4+ T cells.40 Even low or moderate doses of corticosteroids can increase the risk of PCP in these patient populations.17,30–32,37,38
Lymphocyte counts
Several case series and comparison studies between patients with PCP and those without showed that low lymphocyte counts are a risk factor for the development of PCP in patients with systemic lupus erythematosus (SLE) or polymyositis/dermatomyositis (PM/DM),41 those with WG or SLE,42 and those with inflammatory rheumatic diseases including RA.34,35,37,38,43 Means of lymphocyte counts in PCP patients varied among studies, ranging from 88 to 1,053/μL.
The Pneumocystis cell wall contains an abundance of surface antigens that are heavily glycosylated with carbohydrates, including 1,3-β-D-glucan (β-D-glucan), glycoprotein A, and chitins. These components are an important factor not only for cell growth and integrity but also for driving the initiation of lung inflammation during PCP.44,45 Inflammatory responses directed against P. jirovecii are essential for clearance of this organism from the body; however, excessive inflammation can cause severe lung injury and impairment of pulmonary function. It is well known that immune-mediated inflammation directly impairs pulmonary function, which contributes to the pathogenesis of PCP.46 These findings may be related to the fact that PCP often occurs in patients with RA11,12,31,47 and in those with inflammatory rheumatic diseases including RA17,36,48 who have CD4+ T-cell counts >200/μL (mean range, 435–793/μL) and/or lymphocyte counts >500/μL (mean range, 726–1,591/μL).
Through a literature review, Wu et al suggested that the unmasking of PCP following a reversal of immunosuppresion may be more common in both HIV-positive and HIV-negative patients. Withdrawal of corticosteroids led to reconstitution of the immune system, as proven by a consistent rise in CD4+ T-cell counts, thereby triggering the host’s immune-mediated inflammatory response to Pneumocystis organisms and causing damage to the lungs. Under these conditions, PCP may more commonly manifest with an acute and fulminant clinical course compared with the immunosuppressed conditions.49
Preexisting lung diseases
Through an in-depth survey of national databases regarding PCP-associated hospitalization in England, preexisting lung disease has been identified as a new risk factor for this opportunistic disease.50 The association of PCP with preexisting lung disease has also been reported for patients with RA30–32,48,51 or for those with SLE or PM/DM41 in several studies with small patient numbers.
Old age
There are several reports that old age is an independent factor contributing to an increased risk of PCP development in RA patients who are treated with biological agents (mean age range: PCP patients, 66–70 years versus non-PCP patients, 55–60 years).30–32,48,52 Teichtahl et al showed that PCP patients with inflammatory rheumatic diseases including RA were older compared with those without (mean age, 69.6 versus 50.6 years).38
Asymptomatic carriage of P. jirovecii
Asymptomatic carriers of P. jirovecii are at increased risk for developing PCP. Besides HIV-positive individuals, the carriage of this organism has been noted in HIV-negative patients with immunosuppressed conditions, especially in those with a CD4+ T-cell count less than 400/μL.53 Patients with RA or other inflammatory rheumatic diseases often receive immunosuppressive drugs and/or long-term corticosteroid therapy. Mekinian et al reported a high prevalence rate of P. jirovecii colonization in patients with inflammatory rheumatic diseases (16%), and in this patient population, high-dose corticosteroid therapy and low total lymphocyte counts were identified as risk factors for colonization.54 Fritzsche et al also showed that 28.5% of patients with inflammatory rheumatic diseases including RA, especially those over the age of 60, are colonized with this organism, but there were no significant influences of corticosteroid dose or immunosuppressive co-medication.55 We also found that 10.9% of RA patients have asymptomatic carriage of this organism, and the mean age of these carriers is significantly older than that of noncarriers. There were no significant differences in lymphocyte counts or corticosteroid use.18 A high rate of colonization (25.6%) was reported among patients with RA, ankylosing spondylitis, or psoriatic arthritis treated with infliximab. Among those patients, corticosteroid use was one of the significant risk factors, but its effect was not dose-dependent.56
Mortality of PCP in RA Patients
Despite the availability of anti-PCP drugs, the mortality rate of PCP remains high. Most studies indicate better survival rates for HIV-positive PCP patients (86%–92%) than for HIV-negative PCP patients with various underlying conditions (51%–80%).2 The most recent surveillance by pharmaceutical companies in Japan reported high numbers of fatal cases during treatment with biological or nonbiological antirheumatic drugs for RA (MTX, 28 out of 236 patients [11.9%]; tacrolimus, 4 out of 14 [28.6%]; infliximab, 19 out of 188 [10.1%], etanercept, 15 out of 81 [18.5%]; adalimumab, 10 out of 54 [18.5%], tocilizumab, 2 out of 14 [14.3%]; abatacept, 2 out of 9 [22.2%]) (Table 1).19 However, mortality rates have been reported to vary from study to study, ranging from 0% to 67%, in RA patients and those with other rheumatic diseases who developed PCP during immunosuppressive therapy.12,17,23,31,34–36,47,48,51,52,57,58
There is the possibility that an early diagnosis and prompt use of therapeutic antibiotics could decrease the mortality rate of non–HIV-infected patients who are receiving immunosuppressive therapy. Roux et al conducted a prospective, multicenter, cohort study for consecutive patients with or without AIDS who were admitted to 17 hospitals in France from 2007 to 2010, in which PCP was more often fatal in non-AIDS patients than in AIDS patients (mortality rates, 27% versus 4%) and the time interval between admission and treatment initiation was longer in non-AIDS patients (2 days versus 1 day). This delayed implementation of PCP treatment apparently affected the survival of non-AIDS patients.59
Diagnostic Pitfalls for PCP in RA Patients
Clinical features
HIV-negative PCP patients rapidly develop fulminate pneumonia with severe oxygenation impairment, diffuse and progressive alveolar damage, and irreversible respiratory failure, whereas PCP in HIV-positive individuals presents as a subacute disease course.3,4,6,8,9,59–64 Such marked differences between the two types of PCP can be explained by the fact that more excessive inflammatory responses of the lungs are induced in HIV-negative patients.65
In the early stages of PCP in RA patients, respiratory symptoms are nonspecific and nonsevere. Chest radiographs are nearly normal, but high-resolution computed tomographic (HRCT) scans at that time reveal diffuse ground-glass opacities. HRCT seems to play a central role in evaluating immunocompromised patients with suspected pneumonia and almost normal chest radiographs.66 Oxygen saturation in the blood should also be measured, at rest and after motion, by pulse-oximeter, regardless of the presence or absence of respiratory symptoms. Oxygen saturation is often in the normal range at rest, but it drops to low levels after motion.11 Elderly patients do not often complain of dyspnea, even when oxygen saturation is below 90%. In many cases, their chief complaint is a slight general fatigue. We should therefore advise RA patients to visit a doctor immediately if they note any unusual physical conditions. PCP in RA patients is likely to progress to fulminant and irreversible respiratory failure within the first several days.47 RA patients receiving immunosuppressive therapy should be followed up for signs and symptoms of PCP development, with a high index of suspicion.
Detection of P. jirovecii organisms (microbiological examinations)
The timely diagnosis of PCP during biological or nonbiological antirheumatic therapy and the prompt action to treat this complication are critical; however, there are no formal criteria for diagnosis of PCP. Given that P. jirovecii cannot be cultured in the laboratory, the gold standard of diagnosis of PCP is microscopic visualization of P. jirovecii cysts and/or tropic forms in induced sputum and bronchoalveolar lavage (BAL) fluids using various staining techniques. Since the tropic forms predominate over the cystic forms,67 the modified Giemsa staining (the Diff-Quik staining) of the tropic forms may have better sensitivity than the cyst staining. However, the microscopy-based diagnosis must be performed by experienced microscopists. Cysts can be stained with the Grocott-Gomori methenamine-silver and toluidine blue O, which has good specificity, but the sensitivity is weak. Therefore, the cyst staining is not considered satisfactory in HIV-negative patients. The sensitivity of microscopic diagnosis mainly depends on the type and quality of respiratory specimens, the skill and experience of the observers, and the fungal burdens. In HIV-negative individuals, the fungal burden is lower compared with that in AIDS patients.47,61 In addition, it is often difficult to collect optimal specimens from HIV-negative PCP patients because of rapidly worsening pulmonary conditions.6,34,60,62
Molecular diagnosis of polymerase chain reaction (PCR)–based methods, which was developed to overcome the limitations of the microscopic diagnosis, has improved the sensitivity of P. jirovecii detection in HIV-negative patients.68 BAL fluids are optimal specimens, but induced sputum is also acceptable for PCR assays if a patient’s pulmonary condition is severe.69 Because of its high sensitivity, PCR testing has come to be widely used for the diagnosis of PCP in both HIV-positive and HIV-negative patients.70 This technique also makes it possible to identify individuals who carry P. jirovecii but have no clinical or radiological signs of PCP. The asymptomatic carriage of this organism is not an uncommon event in elderly RA patients,18 but conventional or standard PCR testing cannot distinguish the disease from P. jirovecii colonization or subclinical infection, thereby often producing false-positive results (low specificity rates) and low positive-predictive values in this patient population. Positive PCR results should be interpreted in parallel with clinical symptoms and radiological findings. In contrast, quantitative real-time PCR assays have improved the test specificity and positive-predictive value and, therefore, can discriminate between PCP and colonization or subclinical infection in immunocompromised patients according to a threshold of fungal load.71–77 However, the clinically relevant cut-off values remain to be determined and may vary depending on the underlying disease.78–80 In addition, we should be aware that positive- and negative-predictive values are influenced by the disease’s prevalence. Nevertheless, very high negative-predictive values (close to 100%) of real-time PCR assays are especially useful in making a therapeutic decision: a negative result of this analysis may allow treatment providers to eliminate the need for potentially toxic empirical treatment.73,74,79,81,82
Serum β-D-glucan
Although the diagnosis of PCP relies upon microscopic visualization of P. jirovecii organisms and DNA detection in pulmonary specimens, a serological diagnosis is also helpful, especially for patients who cannot undergo invasive sampling of respiratory specimens due to respiratory failure. β-D-glucan is a component of the cell wall of various fungi such as Candida, Aspergillus, and Pneumocystis, the levels of which are increased in the sera of patients with invasive aspergillosis, invasive candidiasis, or PCP.83,84 Although the β-D-glucan assay is not specific to PCP, meta-analysis studies have indicated that its sensitivity for PCP is particularly high, even in HIV-negative patients.85,86 Since a negative predictive value is also high, this assay is helpful in ruling out the possibility of PCP and thereby preventing any unnecessary treatment for this condition.83 Recent studies have shown that the β-D-glucan assay, using a particular threshold, is of practical use for discrimination of PCP from asymptomatic carriage of P. jirovecii.75,76,87 However, we should interpret data carefully in the context of underlying clinical characteristics of an individual patient because there are various factors that may produce false- positive results. In addition, generally accepted cut-off values have not been determined. A correlation of serum β-D-glucan levels to pulmonary fungal load determined by real-time PCR testing remains a matter of controversy.88–90 It also remains to be clarified whether serum β-D-glucan levels may be of prognostic value in HIV-negative patients.17,88,89,91,92 We should keep in mind that β-D-glucan is abundant in the cyst form but is not detectable in the trophic form.45
Elevated levels of serum Krebs von den Lungen-6 (KL-6), lactate dehydrogenase, and S-adenosylmethionine have been reported in PCP patients, but these serological markers have not displayed sufficient performance to suggest that they would be useful for making an early diagnosis of PCP in HIV-negative patients.83,88,93
Differential diagnosis
PCP, MTX pneumonia, and TNFα inhibitor–induced pneumonia are mainly included in the differential diagnosis of acute-onset diffuse interstitial pneumonia occurring in RA patients receiving immunosuppressive therapy. Although there are no established criteria for the diagnosis of PCP, positive results of microbiological examinations with respiratory specimens and increased levels of serum β-D-glucan are helpful in the differential diagnosis. If all of these examinations are negative, MTX pneumonia or TNFα inhibitor–induced pneumonia should be suspected. Serological testing for cytomegalovirus (CMV) antigen pp65 is also useful for differentiating PCP from CMV pneumonia, which shows clinical radiological features similar to PCP. A recent study suggested that the combined use of HRCT findings and serum markers is of great value in the differential diagnosis of PCP and CMV pneumonia in HIV-negative patients.94 Given the rapid progression to respiratory failure and the devastating outcomes of PCP, however, we should consider starting anti-PCP treatment for RA patients who experienced hypoxemia and exhibited abnormal HRCT findings suggestive of interstitial pneumonia. In these cases, we need not wait for the results of microbiological or serum examinations. Instead, the differential diagnosis is what is required to determine whether biological agents and/or MTX can be restarted after recovery from PCP.
Key Points for the Treatment of PCP
TMP-SMX
TMP-SMX is still recommended as the first-line regimen for the treatment of mild-to-severe PCP regardless of the status of HIV infection. The recommended daily use is 15–20 mg/kg of TMP plus 75–100 mg/kg of SMX, and the duration is 21 days for HIV-positive patients and 14 days for HIV-negative patients. Nine to 12 tablets daily (TMP, 720–960 mg; SMX, 360–480 mg) are generally prescribed in Japan. However, these recommendations have not been proven by randomized trials. The difference in duration between HIV-positive and HIV-negative patients is explained by the difference in fungal burdens between both groups, but the degree of immunosuppression and fungal load are different among HIV-negative patients because of the heterogeneity of this patient population. We reported a successful elimination of P. jirovecii from RA patients with PCP through a short-term course of treatment with TMP-SMX (1–2 weeks), as proven by PCR examinations.12 If adjunctive corticosteroid therapy is not introduced, the patient’s condition may temporarily worsen within the first 3–5 days of TMP-SMX therapy because of the inflammatory response triggered by antibiotic- induced lysis and the clearance of P. jirovecii organisms in the lung.95 During this period, changes in anti-PCP therapy should not be considered. Other concomitant infections must be excluded as a cause for respiratory failure.
This therapy contributes to the development of adverse allergic reactions, such as myelosuppression, mild-to-severe skin rash, hepatotoxicity, although such adverse events occur less commonly in HIV-negative patients than in HIV-positive patients.60 Adverse events usually occur during the second week of treatment. In the case of intolerance to TMP-SMX, intravenous pentamidine or oral atovaquon is administered as an alternative. Although several prospective randomized treatment trials have shown that TMP-SMX and pentamidine do not show statistically significant differences in efficacy or frequency of adverse events,96–98 a recent tri-center cohort study revealed that pentamidine was associated with a greater risk of death when used as first- and second-line therapy for HIV-positive PCP patients.99 Atovaquon has less efficacy but better tolerance profiles than TMP-SMX does in the treatment of this patient population,100 but we should nevertheless note that such comparison studies on efficacy and tolerance profiles were performed on HIV-positive patients.
Adjunctive corticosteroid therapy
The usefulness of adjunctive steroid therapy is well established for HIV- positive patients with PCP, especially for those with substantial hypoxemia (arterial oxygen partial pressure <70 mmHg or alveolar–arterial gradient >35 mmHg) on room air,101,102 but the benefit for HIV-negative immunocompromised patients have not been proven by current literature. There are no randomized trials evaluating the efficacy of corticosteroid therapy to reduce morbidity and mortality of PCP in HIV-negative patients. The lower incidence of PCP, compared with that in HIV-positive individuals, makes it difficult to conduct a randomized clinical trial for HIV-negative subjects. Only a very few retrospective studies were previously conducted for HIV-negative patients, which showed controversial results regarding treatment efficacy: benefits or no effects or increased mortality rates.103–106 Such diverse results may be caused by the fact that HIV-negative immunocompromised subjects that were examined constituted a heterogeneous patient population, and most of them had already received corticosteroid therapy at the time of PCP development. Given that immune-mediated inflammatory responses play a critical role in the development of PCP, however, the use of adjunctive corticosteroids during PCP treatment may theoretically benefit HIV-negative patients as well. Further, a multicenter, randomized, controlled trial showed that an addition of corticosteroids decreases the incidence of hypersensitivity reaction to TMP-SMX in HIV-positive patients.107 As mentioned above, it also prevents early and reversible deterioration of respiratory conditions.95 Actually, the use of adjunctive corticosteroids has increased and become more common for HIV-negative patients with PCP.108 Case series studies for RA patients with PCP showed that most cases had received adjunctive corticosteroid therapy.12,31,47,48,51,52,58 In the case of acute interstitial pneumonia occurring in RA patients, there is the possibility of MTX pneumonia or TNFα inhibitor–induced pneumonia, and corticosteroid therapy is the mainstay of the treatment of such drug-induced pneumonia. In our hospital, short-term corticosteroid therapy is currently recommended, immediately after making the diagnosis of interstitial pneumonia, with dosage determined according to the treatment of drug-induced pneumonia.
Our Experience with an Outbreak of PCP Among RA Patients11,12,18
Between March 2005 and October 2009, we performed real-time PCR testing on 132 RA patients who visited our outpatient facility, and we identified nine cases of asymptomatic carriers between November 2006 and October 2008. Among these, three without PCP prophylaxis developed PCP within 1 month, but the other six tested negative for P. jirovecii DNA after 2–4 weeks of prophylaxis with oral TXP-SMX or aerosolized pentamidine. During this period, we also encountered an additional five cases of PCP occurring in RA outpatients who had not yet undergone PCR testing. The members of this cluster had visited our outpatient facility regularly for a routine medical checkup or treatment. We performed contact tracing by extracting the dates of patients’ visits from the medical charts as well as by interviewing patients, and we found that all the members, except two, had potentially infectious contacts at the outpatient facility within at least 4 months before the first positive PCR result. One case was at the same inpatient ward for joint surgery at the time when a PCP patient was hospitalized. The other was a family member of a PCP patient. No geographical clustering by postal code was noted, suggesting that a regional environmental source outside the hospital was less likely. We also checked 42 health staff members who worked in the outpatient facility during this period, but no positive PCR results were obtained. In this outbreak, PCP patients received a very short-term course of treatment with TMP-SMX (1–2 weeks). High doses of corticosteroid were concomitantly administered to PCP patients to reduce inflammatory environments in the lungs. Through the eradication of P. jirovecii from asymptomatic carriers as well as PCP patients, which was confirmed by real-time PCR examinations, the outbreak was resolved and no new PCP outbreak has been observed in our patient group, even though the patients restarted immunosuppressive therapy for RA without secondary prophylaxis.
Person-to-Person Transmission of P. jirovecii
De novo acquisition of P. jirovecii
Primary infection with P. jirovecii occurs early in life, and subclinical infection of these organisms is highly prevalent in healthy infants.109,110 PCP events occurring in adults had therefore been considered to be mostly due to a reactivation of latent childhood infection of P. jirovecii. Currently, however, PCP development is considered to result from new infection rather than from reactivation of latent childhood infection. Several studies showed that a clustering of specific genotypes of P. jirovecii was associated with the places of residence and diagnosis rather than the place of birth, which is consistent with the hypothesis of a new acquisition of P. jirovecii from a common environmental source or person-to-person transmission.111–113 Several studies have also shown that genetically distinct strains were isolated during each recurrent episode of PCP in HIV- positive individuals.114,115
There are three possible infectious sources: the environment, transiently infected carriers, and patients with active PCP. Pneumocystis is a ubiquitous set of organisms that infects a wide variety of mammalian species. Are animals a common environmental source? Several studies indicated that Pneumocystis species were transmissible only to the same host species, and cross-transmission among different mammalian species has not been documented.116,117 Rats and humans harbor genetically distinct types of Pneumocystis species.118 Thus, every mammalian species seems to have its own Pneumocystis species and, among these species, P. jirovecii is the only fungus that is pathogenic in humans. In addition, continuous cultivation of this species outside of the host lung has not as yet been successful; namely, Pneumocystis cannot propagate outside an infected host.119 Given such a nonzoonotic character of Pneumocystis and the inability of continuous cultivation under laboratory conditions, asymptomatic carriers and active PCP patients most likely serve as an infectious source and reservoir in the interhuman transmission cycle.
Asymptomatic carriers of P. jirovecii in non–HIV-infected individuals
Pneumocystis jirovecii colonization is well recognized among HIV-positive individuals who were hospitalized with non-PCP pneumonia (68%) or who died from causes other than PCP (46%).120,121 Carriage of this organism has also been described in non–HIV-infected individuals with immunosuppressive conditions,122,123 those with chronic pulmonary disease,124–135 and even immunocompetent healthy persons.110,136–138 Pneumocystis jirovecii DNA was detected in respiratory samples of 32% of normal infants and in 21.5% of older adults110,137 as well as in the respiratory tract of 20% of healthy adults.136 Pneumocystis jirovecii DNA was also detected in the autopsy lungs of 64.9% of individuals who had died of violent or nonviolent causes.138
Interhuman transmission in the general population
Totet et al reported shared features of P. jirovecii genotypes between immunocompetent infants with a primary infection and immunocompromised adults with PCP, which is consistent with the idea that the infant population may represent an important infectious source and reservoir in the community.139 Rivero et al reported a case of P. jirovecii transmission from colonized grandparents with chronic bronchitis to their infant grandchild via the airborne route. Genotyping of P. jirovecii showed the same genotypes in respiratory samples from the infant and her grandparents. These findings suggested that patients with chronic pulmonary disease who are colonized with P. jirovecii may play an important role as major sources and reservoirs of infection. Considering that patients with chronic pulmonary diseases are sputum producers, they may represent reservoirs with the potential ability to transmit P. jirovecii to susceptible hosts.140
Person-to-person transmission via airborne route in hospital environments
Pneumocystis jirovecii DNA has been identified in air samples obtained from hospital environments, which suggests an environmental risk to susceptible persons. At the same time, this finding suggests the possibility of nosocomial person-to-person transmission via an airborne route.141,142 Choukri et al indicated that fungal burdens in air samples regularly decreased with increased distance from hospitalized patients with PCP. In that study, P. jirovecii DNA was detected in 79.8% of air samples collected 1 meter from patients’ heads, 69.2% at 3 meters, 41.7% at 5 meters, and 33.3% at 8 meters. The authors proposed a possible risk of direct airborne transmission of P. jirovecii from close contact with PCP patients.143 Nevertheless, we cannot exclude the environmental risk completely because air samples taken in the corridor adjacent to their room were still positive in that study. In each PCP patient reported by that study, P. jirovecii genotypes in surrounding air samples closely matched those in respiratory specimens, confirming that P. jirovecii organisms in the air of hospital rooms were exhaled by PCP patients.144 Le Gal et al reported that P. jirovecii was detected in air samples collected 1–5 meters from the heads of patients with diverse underling conditions who were colonized with this organism. Full genotype matches were observed in three out of four pairs or triplets of air and pulmonary specimens, confirming that P. jirovecii was exhaled by colonized patients. Patients harboring this organism can therefore participate in nosocomial transmission of P. jirovecii organism via an airborne route.145
Accumulated molecular evidence indicates that immunocompetent healthcare workers may become colonized with P. jirovecii through occupational close contact with patients who have developed PCP.146,147 Pneumocystis jirovecii can persist, for limited periods of time, in HIV-positive patients who have clinically recovered from PCP.148,149 Thus, transmission of this organism to hospital staff may continue for some time after patients’ recovery from PCP. Healthcare workers may therefore serve as vectors of this infection. Valade et al showed the direct airborne excretion of P. jirovecii by critically ill patients with colonization. Pneumocystis jirovecii DNA was also present in the air exhaled from healthcare workers who had possible contact with colonized patients. In addition, the authors showed a genotype match between the strain found in patients’ BAL fluids and that isolated from healthcare workers, suggesting a possible airborne transmission between patients and healthcare workers.150 In contrast, another group showed that there is no difference in the frequency and level of antibodies to Pneumocystis organism between staff exposed and those unexposed to PCP patients and no detectable Pneumocystis DNA in oropharyngeal washings of any hospital staff, suggesting that immunocompetent staff treating PCP patients are not a potentially infectious source of this organism for immunocompromised patients.151
Using a new genotyping method for PCP patients, Gits-Muselli et al suggested the possibility that nosocomial transmission of P. jirovecii may have occurred between patients with different underlying diseases, including renal transplant recipients, patients with hematological malignancies, and cancer patients. A transmission map showed that contact between these patients occurred in various places of the hospital such as the radiology room, cafeteria, corridors, and hall.152
Prophylaxis of PCP Outbreaks Among RA Patients
Need for measures to control nosocomial outbreaks of PCP among RA patients
Guidelines for PCP prophylaxis are generally based on data that were drawn from experience with HIV-positive patients. There is no consensus on the infection control for inflammatory rheumatic diseases. Isolation of PCP patients during hospitalization is feasible for avoiding close contact with susceptible patients. In contrast, it is difficult to identify and isolate outpatients with subclinical carriage of P. jirovecii in hospital environments. In the case of healthy individuals, this fungus is cleared rapidly from the body without the development of PCP, but RA patients appear to have a persistent infection with active replication of P. jirovecii. Such asymptomatic carriers serve as infectious sources and reservoirs, and, in most cases, they eventually develop PCP. A new infection control measure is required to prevent airborne human-to-human transmission and eventually nosocomial PCP outbreaks among RA patients.
Which RA patients should receive PCP prophylaxis?
Prophylaxis with TMP-SMX is highly effective for prevention of PCP and is associated with a decrease in mortality. Primary PCP prophylaxis is recommended for HIV-infected individuals with CD4+ T-cell counts of <200/μL.153,154 PCP prophylaxis was also warranted for non–HIV-infected adult patients who were transplant recipients, for patients with hematological cancer when the expected risk for PCP is 3.5% or more,155 and also for patients without HIV infection receiving prolonged systemic corticosteroid therapy at a level equivalent to 16–20 mg or more of daily prednisolone.5,156 These recommendations appear to be drawn from the concept that the benefit of PCP prophylaxis relies on the incidence rate of this pneumonia in each patient group. In this context, RA patients may not be subject to PCP chemoprophylaxis because the incidence rate of PCP is lower than that of the high-risk group. Considering the poor survival rates of PCP cases in RA patients, however, prophylaxis of P. jirovecii infection should be discussed for this patient population before starting immunosuppressive therapy. We first need to identify those patients whose risk of developing PCP is great enough to warrant primary prophylaxis in spite of the risk of sometimes life-threatening adverse effects such as myelosuppression, Stevens-Johnson syndrome, and sever hepatotoxicity. Who can obtain the greatest benefit from the prophylactic use of TMP-SMX? Unfortunately, no quantitative markers clearly correlate with the risk of PCP in patients with inflammatory rheumatic diseases in the way that CD4+ T-cell count does in HIV-infected individuals.
Several reports have indicated that PCP prophylaxis with TMP-SMX was effective in patients with inflammatory rheumatic diseases who share the risks mentioned in the first half of this review.37,157,158 Katsuyama et al showed that RA patients with two or three risk factors for PCP including old age (>65 years), preexisting lung disease, and corticosteroid use benefited from primary prophylaxis.32 However, we previously showed that bronchiolar abnormalities and interstitial changes were commonly seen in RA patients (ie, bronchial dilatation and ground-glass attenuation were observed in 41% and 27% of RA patients, respectively).159,160 Such structural modifications in the lungs of RA patients may provide favorable environments for infection with P. jirovecii. In this context, most RA patients should be subject to PCP prophylaxis.
Duration of PCP prophylaxis for RA patients
When can prophylactic antibiotics safely be discontinued? Considering the lifelong use of multiple antirheumatic drugs for RA patients, it is impractical to continue chemoprophylaxis all through anti-RA therapy. As mentioned above, the outbreak of P. jirovecii infection among RA patients was resolved within 1–4 weeks of use of TMP-SMX or pentamidine for PCP patients and asymptomatic carriers, and PCR testing confirmed that 1 week of prophylactic antibiotics is effective in eradicating P. jirovecii from asymptomatic carriers.11,12,18 Saito et al also showed that P. jirovecii disappeared within 7–10 days after commencement of TMP-SMX treatment for patients with PCP and inflammatory rheumatic diseases (SLE, RA, PM/DM, and others), without any recurrence.35 Godeau et al also reported that no relapse of PCP was seen among survivors who had been treated with TMP-SMX for a mean of 17 days, even though they continued to receive immunosuppressive drugs for inflammatory rheumatic diseases (WG, SLE, PM/DM, polyarthritis nodosa, and others) without secondary prophylaxis.34 In contrast, one case series study reported that new PCP developed in three patients with WG or SLE after discontinuation of immunosuppressive therapy (high-dose corticosteroid or cyclophosphamide) and primary PCP prophylaxis (atovaquon or Dapsone). These patients had profound lymphopenia (0–160/μL) at the development of PCP. The data suggested the uncertainty regarding appropriate time of PCP prophylaxis following the tapering or cessation of immunosuppressive therapy.42
Which agent is recommended for prophylaxis for PCP during anti-RA therapy?
Although evidence has accumulated in HIV-positive patients indicating that TMP-SMX is superior to aerosolized pentamidine in prophylactic medication,161,162 there are very few studies comparing TMP-SMX and other anti-PCP agents in terms of prophylactic efficacy for PCP in HIV-negative patients with inflammatory rheumatic diseases. Kimura et al retrospectively compared the prophylactic efficacy of TMP-SMX and aerosolized pentamidine in patients with vasculitis, SLE, PM/DM, adult still disease, or other inflammatory rheumatic diseases, and that study indicated that TMP-SMX was superior to pentamidine inhalation.163 Through a meta-analysis of 12 randomized trial studies for HIV-negative patients who had bone marrow or solid-organ transplant or those who had hematologic cancer, Green et al found that there was no difference between once-daily and three-times weekly administration schedules and suggested the effectiveness of TMP-SMX as a prophylaxis of PCP.155 However, we should be aware that TMP-SMX can sometimes induce allergic pancytopenia in association with MTX treatment of RA.164,165
Beside antimicrobial activity, nonspecific anti-inflammatory and immunomodulatory properties of TMP-SMX have been reported: this medication reduces proliferation of lymphocytes, increases bactericidal activity and chemotaxis of neutrophils, and enhances phagocytosis and intracellular killing of macrophages.166 Improved clinical function and reduced inflammation were reported in RA patients treated with SMX.167 Through a Medline search of the literature from 1966 to 2000, Rozin et al suggested that because of its therapeutic qualities, low cost, and relative nontoxicity, TMP-SMX may warrant a role in the treatment of RA.168 It is clear that the anti-inflammatory and immunomodulatory properties of TMP-SMX require further exploration.
A combination of TMP and SMX shows antimicrobial activity against a broad spectrum of bacterial, fungal, and protozoal pathogens. This medication can, therefore, influence blood culture results, which may make it difficult to isolate other serious infectious pathogens, thereby requiring the need for prolonged courses of broad-spectrum empiric therapy.156 In addition, the increased use of TMP-SMX for both treatment and prevention of PCP has raised concerns about the development of resistant organs. Dihydropteroate synthase (DHPS) is a target enzyme of sulfonamides, and several studies consistently showed a significant association between the use of TMP-SMX for PCP prophylaxis in HIV-positive individuals and the presence of specific mutations in the DHSP gene of P. jirovecii, which suggests that the use of TMP-SMX is responsible for the selection of DHPS mutants. Whether the mutations in the DHPS gene confer clinical resistance to TMP-SMX remains unclear because several published studies offer conflicting results.169
Conclusions
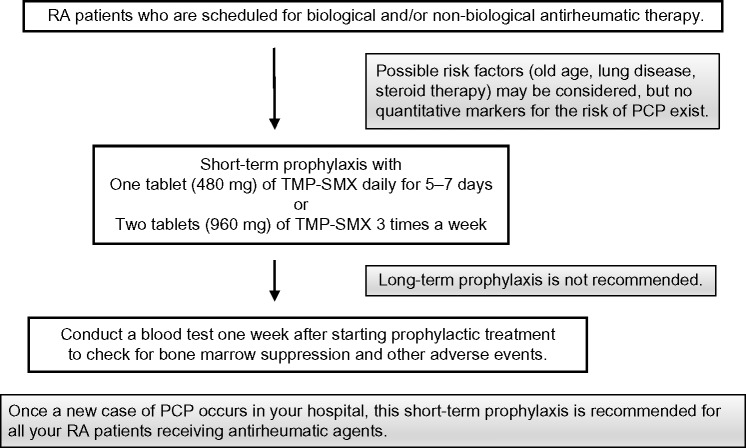
Asymptomatic carriers play a potential role in the circulation of P. jirovecii among RA patients. In our hospital, we usually carry out the following measures to prevent the risk of PCP outbreaks (Fig. 1). First, we prescribe one tablet of TMP-SMX (80 mg of TMX plus 400 mg of SMX) daily for 5–7 days (or two tablets three times a week) for all RA patients who are scheduled to start biological and/or nonbiological antirheumatic therapy. We keep in mind the possibility that a new patient may carry a new infection into our patient cohort. Once a new case of PCP occurs, this short-term prophylaxis is recommended for all RA patients who are receiving biological and/or nonbiological antirheumatic drugs. Regular PCR testing during antirheumatic therapy is now not recommended because of its high cost. Long-term prophylaxis is also not recommended because of the increased risk for developing Pneumocystis resistance and for masking other serious infectious diseases. Considering the possible risk of developing allergic pancytopenia to TMP-SMX, a blood test should be conducted 1 week after starting this course of medication. To avoid close interhuman contact between susceptible RA patients and potential asymptomatic carriers in other clinical sections such as the pulmonary division and HIV clinic, the waiting rooms in our hospital are separated through the use of panels. Since these prophylactic measures were adopted in our rheumatology section, we have not encountered any outbreak of P. jirovecii infection in our hospital.
Figure 1.
Proposed recommendations for the prevention of PCP outbreak among RA outpatients.
Footnotes
ACADEMIC EDITOR: Hussein D Foda, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1634 words, excluding any confidential comments to the academic editor.
FUNDING: This study was supported by research funds from the National Hospital Organization, Japan. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Dr Mori has received research grants from Chugai Pharmaceutical Co., Bristol-Myers Squibb, Eisai Pharmaceutical Co., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., and Astellas Pharma Inc. MS discloses no competing interests.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived the concept and design: SM, MS. Analyzed the data: SM, MS. Wrote the first draft of the manuscript: SM, MS. Contributed to the writing of the manuscript: SM, MS. Agree with manuscript results and conclusions: SM, MS. Jointly developed the structure and arguments for the paper: SM, MS. Made critical revisions and approved final version: SM, MS. Both authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA. 2009;301(24):2578–85. doi: 10.1001/jama.2009.880. [DOI] [PubMed] [Google Scholar]
- 2.Catherinot E, Lanternier F, Bougnoux ME, Lecuit M, Couderc LJ, Lortholary O. Pneumocystis jirovecii pneumonia. Infect Dis Clin North Am. 2010;24(1):107–38. doi: 10.1016/j.idc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17(suppl 2):S416–22. doi: 10.1093/clinids/17.supplement_2.s416. [DOI] [PubMed] [Google Scholar]
- 4.Arend SM, Kroon FP, van’t Wout JW. Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993. An analysis of 78 cases. Arch Intern Med. 1995;155(22):2436–41. [PubMed] [Google Scholar]
- 5.Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71(1):5–13. doi: 10.4065/71.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Nuesch R, Bellini C, Zimmerli W. Pneumocystis carinii pneumonia in human immunodeficiency virus (HIV)-positive and HIV-negative immunocompromised patients. Clin Infect Dis. 1999;29(6):1519–23. doi: 10.1086/313534. [DOI] [PubMed] [Google Scholar]
- 7.Mansharamani NG, Garland R, Delaney D, Koziel H. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest. 2000;118(3):704–11. doi: 10.1378/chest.118.3.704. [DOI] [PubMed] [Google Scholar]
- 8.Roblot F, Godet C, Le Moal G, et al. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur J Clin Microbiol Infect Dis. 2002;21(7):523–31. doi: 10.1007/s10096-002-0758-5. [DOI] [PubMed] [Google Scholar]
- 9.Overgaard UM, Helweg-Larsen J. Pneumocystis jiroveci pneumonia (PCP) in HIV-1-negative patients: a retrospective study 2002–2004. Scand J Infect Dis. 2007;39(6–7):589–95. doi: 10.1080/00365540601150497. [DOI] [PubMed] [Google Scholar]
- 10.Inokuma S, Okada Y, Kanei M, et al. In: Prophylaxis of Pneumocystis jirovecii pneumonia associated with inflammatory rheumatic diseases (in Japanese). Clinical guideline: the Japanese Ministry of Health, Laboratory and Welfare Study Group on complication and treatment of immune diseases. Hashimoto H, editor. Japanese Ministry of Health, Labor and Welfare Study Group; Tokyo: 2004. pp. 24–9. [Google Scholar]
- 11.Mori S, Cho I, Sugimoto M. A cluster of Pneumocystis jirovecii infection among outpatients with rheumatoid arthritis. J Rheumatol. 2010;37(7):1547–8. doi: 10.3899/jrheum.091294. [DOI] [PubMed] [Google Scholar]
- 12.Mori S, Cho I, Sugimoto M. A follow-up study of asymptomatic carriers of Pneumocystis jiroveci during immunosuppressive therapy for rheumatoid arthritis. J Rheumatol. 2009;36(8):1600–5. doi: 10.3899/jrheum.081270. [DOI] [PubMed] [Google Scholar]
- 13.Stenger AA, Houtman PM, Bruyn GA, Eggink HF, Pasma HR. Pneumocystis carinii pneumonia associated with low dose methotrexate treatment for rheumatoid arthritis. Scand J Rheumatol. 1994;23(1):51–3. doi: 10.3109/03009749409102137. [DOI] [PubMed] [Google Scholar]
- 14.LeMense GP Sahn SA. Opportunistic infection during treatment with low dose methotrexate. Am J Respir Crit Care Med. 1994;150(1):258–60. doi: 10.1164/ajrccm.150.1.8025760. [DOI] [PubMed] [Google Scholar]
- 15.Tai TL, O’Rourke KP, McWeeney M, Burke CM, Sheehan K, Barry M. Pneumocystis carinii pneumonia following a second infusion of infliximab. Rheumatology (Oxford) 2002;41(8):951–2. doi: 10.1093/rheumatology/41.8.951. [DOI] [PubMed] [Google Scholar]
- 16.Mori S, Imamura F, Kiyofuji C, et al. Pneumocystis jiroveci pneumonia in a patient with rheumatoid arthritis as a complication of treatment with infliximab, anti-tumor necrosis factor alpha neutralizing antibody. Mod Rheumatol. 2006;16(1):58–62. doi: 10.1007/s10165-005-0454-2. [DOI] [PubMed] [Google Scholar]
- 17.Iikuni N, Kitahama M, Ohta S, Okamoto H, Kamatani N, Nishinarita M. Evaluation of Pneumocystis pneumonia infection risk factors in patients with connective tissue disease. Mod Rheumatol. 2006;16(5):282–8. doi: 10.1007/s10165-006-0502-6. [DOI] [PubMed] [Google Scholar]
- 18.Mori S, Cho I, Ichiyasu H, Sugimoto M. Asymptomatic carriage of Pneumocystis jiroveci in elderly patients with rheumatoid arthritis in Japan: a possible association between colonization and development of Pneumocystis jiroveci pneumonia during low-dose MTX therapy. Mod Rheumatol. 2008;18(3):240–6. doi: 10.1007/s10165-008-0037-0. [DOI] [PubMed] [Google Scholar]
- 19.Mori S, Sugimoto M. Pneumocystis jirovecii infection: an emerging threat to patients with rheumatoid arthritis. Rheumatology (Oxford) 2012;51(12):2120–30. doi: 10.1093/rheumatology/kes244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67(2):189–94. doi: 10.1136/ard.2007.072967. [DOI] [PubMed] [Google Scholar]
- 21.Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol. 2009;36(5):898–906. doi: 10.3899/jrheum.080791. [DOI] [PubMed] [Google Scholar]
- 22.Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol. 2012;22(4):498–508. doi: 10.1007/s10165-011-0541-5. [DOI] [PubMed] [Google Scholar]
- 23.Kaur N, Mahl TC. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig Dis Sci. 2007;52(6):1481–4. doi: 10.1007/s10620-006-9250-x. [DOI] [PubMed] [Google Scholar]
- 24.Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis. 2011;70(12):2148–51. doi: 10.1136/ard.2011.151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin-Garrido I, Carmona EM, Specks U, Limper AH. Pneumocystis pneumonia in patients treated with rituximab. Chest. 2013;144(1):258–65. doi: 10.1378/chest.12-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besada E, Nossent JC. Should Pneumocystis jiroveci prophylaxis be recommended with rituximab treatment in ANCA-associated vasculitis? Clin Rheumatol. 2013;32(11):1677–81. doi: 10.1007/s10067-013-2293-4. [DOI] [PubMed] [Google Scholar]
- 27.Ospina FE, Agualimpia A, Bonilla-Abadía F, Cañas CA, Tobón GJ. Pneumocystis jirovecii pneumonia in a patient with rheumatoid arthritis treated with abatacept. Case Rep Rheumatol. 2014;2014:835050. doi: 10.1155/2014/835050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis. 2014;58(12):1649–57. doi: 10.1093/cid/ciu185. [DOI] [PubMed] [Google Scholar]
- 29.Louie GH, Wang Z, Ward MM. Trends in hospitalizations for Pneumocystis jiroveci pneumonia among patients with rheumatoid arthritis in the US: 1996–2007. Arthritis Rheum. 2010;62(12):3826–7. doi: 10.1002/art.27735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harigai M, Koike R, Miyasaka N. Pneumocystis pneumonia associated with infliximab in Japan. N Engl J Med. 2007;357(18):1874–6. doi: 10.1056/NEJMc070728. [DOI] [PubMed] [Google Scholar]
- 31.Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum. 2009;61(3):305–12. doi: 10.1002/art.24283. [DOI] [PubMed] [Google Scholar]
- 32.Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther. 2014;16(1):R43. doi: 10.1186/ar4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ognibene FP, Shelhamer JH, Hoffman GS, et al. Pneumocystis carinii pneumonia: a major complication of immunosuppressive therapy in patients with Wegener’s granulomatosis. Am J Respir Crit Care Med. 1995;151(3 pt 1):795–9. doi: 10.1164/ajrccm/151.3_Pt_1.795. [DOI] [PubMed] [Google Scholar]
- 34.Godeau B, Coutant-Perronne V, Le Thi Huong D, et al. Pneumocystis carinii pneumonia in the course of connective tissue disease: report of 34 cases. J Rheumatol. 1994;21(2):246–51. [PubMed] [Google Scholar]
- 35.Saito K, Nakayamada S, Nakano K, et al. Detection of Pneumocystis carinii by DNA amplification in patients with connective tissue diseases: re-evaluation of clinical features of P carinii pneumonia in rheumatic diseases. Rheumatology (Oxford) 2004;43(4):479–85. doi: 10.1093/rheumatology/keh071. [DOI] [PubMed] [Google Scholar]
- 36.Sato T, Inokuma S, Maezawa R, et al. Clinical characteristics of Pneumocystis carinii pneumonia in patients with connective tissue diseases. Mod Rheumatol. 2005;15(3):191–7. doi: 10.1007/s10165-005-0395-9. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa J, Harigai M, Nagasaka K, Nakamura T, Miyasaka N. Prediction of and prophylaxis against Pneumocystis pneumonia in patients with connective tissue diseases undergoing medium- or high-dose corticosteroid therapy. Mod Rheumatol. 2005;15(2):91–6. doi: 10.1007/pl00021707. [DOI] [PubMed] [Google Scholar]
- 38.Teichtahl AJ, Morrisroe K, Ciciriello S, Jennens I, Tadros S, Wicks I. Pneumocystis jirovecii pneumonia in connective tissue diseases: comparison with other immunocompromised patients. Semin Arthritis Rheum. 2015;45(1):86–90. doi: 10.1016/j.semarthrit.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Chew LC, Maceda-Galang LM, Tan YK, Chakraborty B, Thumboo J. Pneumocystis jirovecii pneumonia in patients with autoimmune disease on high-dose glucocorticoid. J Clin Rheumatol. 2015;21(2):72–5. doi: 10.1097/RHU.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 40.Walzer PD, LaBine M, Redington TJ, Cushion MT. Lymphocyte changes during chronic administration of and withdrawal from corticosteroids: relation to Pneumocystis carinii pneumonia. J Immunol. 1984;133(5):2502–8. [PubMed] [Google Scholar]
- 41.Kadoya A, Okada J, Iikuni Y, Kondo H. Risk factors for Pneumocystis carinii pneumonia in patients with polymyositis/dermatomyositis or systemic lupus erythematosus. J Rheumatol. 1996;23(7):1186–8. [PubMed] [Google Scholar]
- 42.Suryaprasad A, Stone JH. When is it safe to stop Pneumocystis jiroveci pneumonia prophylaxis? Insights from three cases complicating autoimmune diseases. Arthritis Rheum. 2008;59(7):1034–9. doi: 10.1002/art.23822. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Zheng Y. Pneumocystis jirovecii pneumonia in mycophenolate mofetil-treated patients with connective tissue disease: analysis of 17 cases. Rheumatol Int. 2014;34(12):1765–71. doi: 10.1007/s00296-014-3073-4. [DOI] [PubMed] [Google Scholar]
- 44.Thomas CF Jr Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5(4):298–308. doi: 10.1038/nrmicro1621. [DOI] [PubMed] [Google Scholar]
- 45.Skalski JH, Kottom TJ, Limper AH. Pathobiology of Pneumocystis pneumonia: life cycle, cell wall, and cell signal transduction. FEMS Yeast Res. 2015;15(6):fov046. doi: 10.1093/femsyr/fov046. [DOI] [PubMed] [Google Scholar]
- 46.Wright TW, Gigliotti F, Finkelstein JN, McBride JT, An CL, Harmsen AG. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J Clin Invest. 1999;104(9):1307–17. doi: 10.1172/JCI6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tokuda H, Sakai F, Yamada H, et al. Clinical and radiological features of Pneumocystis pneumonia in patients with rheumatoid arthritis, in comparison with methotrexate pneumonitis and Pneumocystis pneumonia in acquired immunodeficiency syndrome: a multicenter study. Intern Med. 2008;47(10):915–23. doi: 10.2169/internalmedicine.47.0702. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka M, Sakai R, Koike R, et al. Pneumocystis jirovecii pneumonia associated with etanercept treatment in patients with rheumatoid arthritis: a retrospective review of 15 cases and analysis of risk factors. Mod Rheumatol. 2012;22(6):849–58. doi: 10.1007/s10165-012-0615-z. [DOI] [PubMed] [Google Scholar]
- 49.Wu AK, Cheng VC, Tang BS, et al. The unmasking of Pneumocystis jiroveci pneumonia during reversal of immunosuppression: case reports and literature review. BMC Infect Dis. 2004;4(1):57. doi: 10.1186/1471-2334-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maini R, Henderson KL, Sheridan EA, et al. Increasing Pneumocystis pneumonia, England, UK, 2000–2010. Emerg Infect Dis. 2013;19(3):386–92. doi: 10.3201/eid1903.121151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida Y, Takahashi Y, Minemura N, et al. Prognosis of Pneumocystis pneumonia complicated in patients with rheumatoid arthritis (RA) and non-RA rheumatic diseases. Mod Rheumatol. 2012;22(4):509–14. doi: 10.1007/s10165-011-0523-7. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe K, Sakai R, Koike R, et al. Clinical characteristics and risk factors for Pneumocystis jirovecii pneumonia in patients with rheumatoid arthritis receiving adalimumab: a retrospective review and case-control study of 17 patients. Mod Rheumatol. 2013;23(6):1085–93. doi: 10.1007/s10165-012-0796-5. [DOI] [PubMed] [Google Scholar]
- 53.Nevez G, Raccurt C, Vincent P, Jounieaux V, Dei-Cas E. Pulmonary colonization with Pneumocystis carinii in human immunodeficiency virus-negative patients: assessing risk with blood CD4+ T cell counts. Clin Infect Dis. 1999;29(5):1331–2. doi: 10.1086/313478. [DOI] [PubMed] [Google Scholar]
- 54.Mekinian A, Durand-Joly I, Hatron PY, et al. Pneumocystis jirovecii colonization in patients with systemic autoimmune diseases: prevalence, risk factors of colonization and outcome. Rheumatology (Oxford) 2010;50(3):569–77. doi: 10.1093/rheumatology/keq314. [DOI] [PubMed] [Google Scholar]
- 55.Fritzsche C, Riebold D, Munk-Hartig A, Klammt S, Neeck G, Reisinger E. High prevalence of Pneumocystis jirovecii colonization among patients with autoimmune inflammatory diseases and corticosteroid therapy. Scand J Rheumatol. 2012;41(3):208–13. doi: 10.3109/03009742.2011.630328. [DOI] [PubMed] [Google Scholar]
- 56.Wissmann G, Morilla R, Martín-Garrido I, et al. Pneumocystis jirovecii colonization in patients treated with infliximab. Eur J Clin Invest. 2011;41(3):343–8. doi: 10.1111/j.1365-2362.2010.02415.x. [DOI] [PubMed] [Google Scholar]
- 57.Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum. 1999;42(4):780–9. doi: 10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 58.Kameda H, Tokuda H, Sakai F, et al. Clinical and radiological features of acute-onset diffuse interstitial lung diseases in patients with rheumatoid arthritis receiving treatment with biological agents: importance of Pneumocystis pneumonia in Japan revealed by a multicenter study. Intern Med. 2011;50(4):305–13. doi: 10.2169/internalmedicine.50.4508. [DOI] [PubMed] [Google Scholar]
- 59.Roux A, Canet E, Valade S, et al. Pneumocystis jirovecii pneumonia in patients with or without AIDS, France. Emerg Infect Dis. 2014;20(9):1490–7. doi: 10.3201/eid2009.131668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kovacs JA, Hiemenz JW, Macher AM, et al. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med. 1984;100(5):663–71. doi: 10.7326/0003-4819-100-5-663. [DOI] [PubMed] [Google Scholar]
- 61.Limper AH, Offord KP, Smith TF, Martin WJ., II Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis. 1989;140(5):1204–9. doi: 10.1164/ajrccm/140.5.1204. [DOI] [PubMed] [Google Scholar]
- 62.Hardak E, Brook O, Yigla M. Radiological features of Pneumocystis jirovecii pneumonia in immunocompromised patients with and without AIDS. Lung. 2010;188(2):159–63. doi: 10.1007/s00408-009-9214-y. [DOI] [PubMed] [Google Scholar]
- 63.Enomoto T, Azuma A, Kohno A, et al. Differences in the clinical characteristics of Pneumocystis jirovecii pneumonia in immunocompromised patients with and without HIV infection. Respirology. 2010;15(1):126–31. doi: 10.1111/j.1440-1843.2009.01660.x. [DOI] [PubMed] [Google Scholar]
- 64.Tasaka S, Tokuda H, Sakai F, et al. Comparison of clinical and radiological features of Pneumocystis pneumonia between malignancy cases and acquired immunodeficiency syndrome cases: a multicenter study. Intern Med. 2010;49(4):273–81. doi: 10.2169/internalmedicine.49.2871. [DOI] [PubMed] [Google Scholar]
- 65.Hahn PY, Limper AH. The role of inflammation in respiratory impairment during Pneumocystis carinii pneumonia. Semin Respir Infect. 2003;18(1):40–7. doi: 10.1053/srin.2003.50004. [DOI] [PubMed] [Google Scholar]
- 66.Kanne JP, Yandow DR, Meyer CA. Pneumocystis jiroveci pneumonia: high-resolution CT findings in patients with and without HIV infection. AJR Am J Roentgenol. 2012;198(6):W555–61. doi: 10.2214/AJR.11.7329. [DOI] [PubMed] [Google Scholar]
- 67.Aliouat-Denis CM, Martinez A, Aliouat el M, Pottier M, Gantois N, Dei-Cas E. The Pneumocystis life cycle. Mem Inst Oswaldo Cruz. 2009;104(3):419–26. doi: 10.1590/s0074-02762009000300004. [DOI] [PubMed] [Google Scholar]
- 68.Wakefield AE, Pixley FJ, Banerji S, et al. Detection of Pneumocystis carinii with DNA amplification. Lancet. 1990;336(8713):451–3. doi: 10.1016/0140-6736(90)92008-6. [DOI] [PubMed] [Google Scholar]
- 69.Wakefield AE, Guiver L, Miller RF, Hopkin JM. DNA amplification on induced sputum samples for diagnosis of Pneumocystis carinii pneumonia. Lancet. 1991;337(8754):1378–9. doi: 10.1016/0140-6736(91)93062-e. [DOI] [PubMed] [Google Scholar]
- 70.Fan LC, Lu HW, Cheng KB, Li HP, Xu JF. Evaluation of PCR in bronchoalveolar lavage fluid for diagnosis of Pneumocystis jirovecii pneumonia: a bivariate meta-analysis and systematic review. PLoS One. 2013;8(9):e73099. doi: 10.1371/journal.pone.0073099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fillaux J, Malvy S, Alvarez M, et al. Accuracy of a routine real-time PCR assay for the diagnosis of Pneumocystis jirovecii pneumonia. J Microbiol Methods. 2008;75(2):258–61. doi: 10.1016/j.mimet.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 72.Fujisawa T, Suda T, Matsuda H, et al. Real-time PCR is more specific than conventional PCR for induced sputum diagnosis of Pneumocystis pneumonia in immunocompromised patients without HIV infection. Respirology. 2009;14(2):203–9. doi: 10.1111/j.1440-1843.2008.01457.x. [DOI] [PubMed] [Google Scholar]
- 73.Chumpitazi BF, Flori P, Kern JB, et al. Characteristics and clinical relevance of the quantitative touch-down major surface glycoprotein polymerase chain reaction in the diagnosis of Pneumocystis pneumonia. Med Mycol. 2011;49(7):704–13. doi: 10.3109/13693786.2011.566894. [DOI] [PubMed] [Google Scholar]
- 74.Alanio A, Desoubeaux G, Sarfati C, et al. Real-time PCR assay-based strategy for differentiation between active Pneumocystis jirovecii pneumonia and colonization in immunocompromised patients. Clin Microbiol Infect. 2011;17(10):1531–7. doi: 10.1111/j.1469-0691.2010.03400.x. [DOI] [PubMed] [Google Scholar]
- 75.Matsumura Y, Ito Y, Iinuma Y, et al. Quantitative real-time PCR and the (1→3)-beta-D-glucan assay for differentiation between Pneumocystis jirovecii pneumonia and colonization. Clin Microbiol Infect. 2012;18(6):591–7. doi: 10.1111/j.1469-0691.2011.03605.x. [DOI] [PubMed] [Google Scholar]
- 76.Damiani C, Le Gal S, Da Costa C, Virmaux M, Nevez G, Totet A. Combined quantification of pulmonary Pneumocystis jirovecii DNA and serum (1→3)-beta-D-glucan for differential diagnosis of Pneumocystis pneumonia and Pneumocystis colonization. J Clin Microbiol. 2013;51(10):3380–8. doi: 10.1128/JCM.01554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maillet M, Maubon D, Brion JP, et al. Pneumocystis jirovecii (Pj) quantitative PCR to differentiate Pj pneumonia from Pj colonization in immunocompromised patients. Eur J Clin Microbiol Infect Dis. 2014;33(3):331–6. doi: 10.1007/s10096-013-1960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Botterel F, Cabaret O, Foulet F, Cordonnier C, Costa JM, Bretagne S. Clinical significance of quantifying Pneumocystis jirovecii DNA by using real-time PCR in bronchoalveolar lavage fluid from immunocompromised patients. J Clin Microbiol. 2012;50(2):227–31. doi: 10.1128/JCM.06036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mühlethaler K, Bögli-Stuber K, Wasmer S, et al. Quantitative PCR to diagnose Pneumocystis pneumonia in immunocompromised non-HIV patients. Eur Respir J. 2012;39(4):971–8. doi: 10.1183/09031936.00095811. [DOI] [PubMed] [Google Scholar]
- 80.Robert-Gangneux F, Belaz S, Revest M, et al. Diagnosis of Pneumocystis jirovecii pneumonia in immunocompromised patients by real-time PCR: a 4-year prospective study. J Clin Microbiol. 2014;52(9):3370–6. doi: 10.1128/JCM.01480-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hauser PM, Bille J, Lass-Flörl C, et al. Multicenter, prospective clinical evaluation of respiratory samples from subjects at risk for Pneumocystis jirovecii infection by use of a commercial real-time PCR assay. J Clin Microbiol. 2011;49(5):1872–8. doi: 10.1128/JCM.02390-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McTaggart LR, Wengenack NL, Richardson SE. Validation of the Myc Assay Pneumocystis kit for detection of Pneumocystis jirovecii in bronchoalveolar lavage specimens by comparison to a laboratory standard of direct immunofluorescence microscopy, real-time PCR, or conventional PCR. J Clin Microbiol. 2012;50(6):1856–9. doi: 10.1128/JCM.05880-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tasaka S, Hasegawa N, Kobayashi S, et al. Serum indicators for the diagnosis of Pneumocystis pneumonia. Chest. 2007;131(4):1173–80. doi: 10.1378/chest.06-1467. [DOI] [PubMed] [Google Scholar]
- 84.Obayashi T, Negishi K, Suzuki T, Funata N. Reappraisal of the serum (1→3)-beta-D-glucan assay for the diagnosis of invasive fungal infections – a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46(12):1864–70. doi: 10.1086/588295. [DOI] [PubMed] [Google Scholar]
- 85.Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-beta-D-glucan for Pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol. 2012;50(1):7–15. doi: 10.1128/JCM.05267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karageorgopoulos DE, Qu JM, Korbila IP, Zhu YG, Vasileiou VA, Falagas ME. Accuracy of beta-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013;19(1):39–49. doi: 10.1111/j.1469-0691.2011.03760.x. [DOI] [PubMed] [Google Scholar]
- 87.Tasaka S, Kobayashi S, Yagi K, et al. Serum (1 → 3) beta-D-glucan assay for discrimination between Pneumocystis jirovecii pneumonia and colonization. J Infect Chemother. 2014;20(11):678–81. doi: 10.1016/j.jiac.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 88.de Boer MG, Gelinck LB, van Zelst BD, et al. Beta-D-glucan and S-adenosylmethionine serum levels for the diagnosis of Pneumocystis pneumonia in HIV-negative patients: a prospective study. J Infect. 2011;62(1):93–100. doi: 10.1016/j.jinf.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 89.Held J, Koch MS, Reischl U, Danner T, Serr A. Serum (1 → 3)-beta-D-glucan measurement as an early indicator of Pneumocystis jirovecii pneumonia and evaluation of its prognostic value. Clin Microbiol Infect. 2011;17(4):595–602. doi: 10.1111/j.1469-0691.2010.03318.x. [DOI] [PubMed] [Google Scholar]
- 90.Costa JM, Botterel F, Cabaret O, Foulet F, Cordonnier C, Bretagne S. Association between circulating DNA, serum (1→3)-beta-D-glucan, and pulmonary fungal burden in Pneumocystis pneumonia. Clin Infect Dis. 2012;55(2):e5–8. doi: 10.1093/cid/cis412. [DOI] [PubMed] [Google Scholar]
- 91.Koo S, Baden LR, Marty FM. Post-diagnostic kinetics of the (1 → 3)-beta-D-glucan assay in invasive aspergillosis, invasive candidiasis and Pneumocystis jirovecii pneumonia. Clin Microbiol Infect. 2012;18(5):E122–7. doi: 10.1111/j.1469-0691.2012.03777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsumura Y, Ito Y, Yamamoto M, et al. Pneumocystis polymerase chain reaction and blood (1→3)-beta-D-glucan assays to predict survival with suspected Pneumocystis jirovecii pneumonia. J Infect Chemother. 2014;20(2):109–14. doi: 10.1016/j.jiac.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 93.Esteves F, Calé SS, Badura R, et al. Diagnosis of Pneumocystis pneumonia: evaluation of four serologic biomarkers. Clin Microbiol Infect. 2015;21(4):379.e1–379.e10. doi: 10.1016/j.cmi.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 94.Kunihiro Y, Tanaka N, Matsumoto T, Yamamoto N, Matsunaga N. The usefulness of a diagnostic method combining high-resolution CT findings and serum markers for cytomegalovirus pneumonia and Pneumocystis pneumonia in non-AIDS patients. Acta Radiol. 2015;56(7):806–13. doi: 10.1177/0284185114539320. [DOI] [PubMed] [Google Scholar]
- 95.Kaplan JE, Benson C, Holmes KK, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207. quiz CE1-4. [PubMed] [Google Scholar]
- 96.Klein NC, Duncanson FP, Lenox TH, et al. Trimethoprim-sulfamethoxazole versus pentamidine for Pneumocystis carinii pneumonia in AIDS patients: results of a large prospective randomized treatment trial. AIDS. 1992;6(3):301–5. doi: 10.1097/00002030-199203000-00007. [DOI] [PubMed] [Google Scholar]
- 97.Wharton JM, Coleman DL, Wofsy CB, et al. Trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A prospective randomized trial. Ann Intern Med. 1986;105(1):37–44. doi: 10.7326/0003-4819-105-1-37. [DOI] [PubMed] [Google Scholar]
- 98.Sattler FR, Cowan R, Nielsen DM, Ruskin J. Trimethoprim-sulfamethoxazole compared with pentamidine for treatment of Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A prospective, noncrossover study. Ann Intern Med. 1988;109(4):280–7. doi: 10.7326/0003-4819-109-4-280. [DOI] [PubMed] [Google Scholar]
- 99.Helweg-Larsen J, Benfield T, Atzori C, Miller RF. Clinical efficacy of first- and second-line treatments for HIV-associated Pneumocystis jirovecii pneumonia: a tri-centre cohort study. J Antimicrob Chemother. 2009;64(6):1282–90. doi: 10.1093/jac/dkp372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hughes W, Leoung G, Kramer F, et al. Comparison of atovaquone (566C80) with trimethoprim-sulfamethoxazole to treat Pneumocystis carinii pneumonia in patients with AIDS. N Engl J Med. 1993;328(21):1521–7. doi: 10.1056/NEJM199305273282103. [DOI] [PubMed] [Google Scholar]
- 101.Briel M, Boscacci R, Furrer H, Bucher HC. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection: a meta-analysis of randomised controlled trials. BMC Infect Dis. 2005;5:101. doi: 10.1186/1471-2334-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ewald H, Raatz H, Boscacci R, Furrer H, Bucher HC, Briel M. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection. Cochrane Database Syst Rev. 2015;4:CD006150. doi: 10.1002/14651858.CD006150.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pareja JG, Garland R, Koziel H. Use of adjunctive corticosteroids in severe adult non-HIV Pneumocystis carinii pneumonia. Chest. 1998;113(5):1215–24. doi: 10.1378/chest.113.5.1215. [DOI] [PubMed] [Google Scholar]
- 104.Delclaux C, Zahar JR, Amraoui G, et al. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in non-human immunodeficiency virus-infected patients: retrospective study of 31 patients. Clin Infect Dis. 1999;29(3):670–2. doi: 10.1086/598651. [DOI] [PubMed] [Google Scholar]
- 105.Moon SM, Kim T, Sung H, et al. Outcomes of moderate-to-severe Pneumocystis pneumonia treated with adjunctive steroid in non-HIV-infected patients. Antimicrob Agents Chemother. 2011;55(10):4613–8. doi: 10.1128/AAC.00669-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lemiale V, Debrumetz A, Delannoy A, Alberti C, Azoulay E. Adjunctive steroid in HIV-negative patients with severe Pneumocystis pneumonia. Respir Res. 2013;14:87. doi: 10.1186/1465-9921-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walmsley S, Levinton C, Brunton J, et al. A multicenter randomized double-blind placebo-controlled trial of adjunctive corticosteroids in the treatment of Pneumocystis carinii pneumonia complicating the acquired immune deficiency syndrome. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8(4):348–57. [PubMed] [Google Scholar]
- 108.McKinnell JA, Cannella AP, Kunz DF, et al. Pneumocystis pneumonia in hospitalized patients: a detailed examination of symptoms, management, and outcomes in human immunodeficiency virus (HIV)-infected and HIV-uninfected persons. Transpl Infect Dis. 2012;14(5):510–8. doi: 10.1111/j.1399-3062.2012.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pifer LL, Hughes WT, Stagno S, Woods D. Pneumocystis carinii infection: evidence for high prevalence in normal and immunosuppressed children. Pediatrics. 1978;61(1):35–41. [PubMed] [Google Scholar]
- 110.Vargas SL, Hughes WT, Santolaya ME, et al. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis. 2001;32(6):855–61. doi: 10.1086/319340. [DOI] [PubMed] [Google Scholar]
- 111.Beard CB, Carter JL, Keely SP, et al. Genetic variation in Pneumocystis carinii isolates from different geographic regions: implications for transmission. Emerg Infect Dis. 2000;6(3):265–72. doi: 10.3201/eid0603.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Montes-Cano MA, de la Horra C, Martin-Juan J, et al. Pneumocystis jiroveci genotypes in the Spanish population. Clin Infect Dis. 2004;39(1):123–8. doi: 10.1086/421778. [DOI] [PubMed] [Google Scholar]
- 113.Miller RF, Lindley AR, Copas A, Ambrose HE, Davies RJ, Wakefield AE. Genotypic variation in Pneumocystis jirovecii isolates in Britain. Thorax. 2005;60(8):679–82. doi: 10.1136/thx.2004.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Keely SP, Stringer JR, Baughman RP, Linke MJ, Walzer PD, Smulian AG. Genetic variation among Pneumocystis carinii hominis isolates in recurrent pneumocystosis. J Infect Dis. 1995;172(2):595–8. doi: 10.1093/infdis/172.2.595. [DOI] [PubMed] [Google Scholar]
- 115.Latouche S, Poirot JL, Bertrand V, Roux P. Pneumocystis carinii hominis sequencing for reactivation or de novo contamination and for hypothetic transmission from person to person. APMIS Suppl. 1997;77:11–3. doi: 10.1111/j.1600-0463.1997.tb05374.x. [DOI] [PubMed] [Google Scholar]
- 116.Gigliotti F, Harmsen AG, Haidaris CG, Haidaris PJ. Pneumocystis carinii is not universally transmissible between mammalian species. Infect Immun. 1993;61(7):2886–90. doi: 10.1128/iai.61.7.2886-2890.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Durand-Joly I, Aliouat el M, Recourt C, et al. Pneumocystis carinii f. sp. hominis is not infectious for SCID mice. J Clin Microbiol. 2002;40(5):1862–5. doi: 10.1128/JCM.40.5.1862-1865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stringer JR, Stringer SL, Zhang J, Baughman R, Smulian AG, Cushion MT. Molecular genetic distinction of Pneumocystis carinii from rats and humans. J Eukaryot Microbiol. 1993;40(6):733–41. doi: 10.1111/j.1550-7408.1993.tb04468.x. [DOI] [PubMed] [Google Scholar]
- 119.Sloand E, Laughon B, Armstrong M, et al. The challenge of Pneumocystis carinii culture. J Eukaryot Microbiol. 1993;40(2):188–95. doi: 10.1111/j.1550-7408.1993.tb04902.x. [DOI] [PubMed] [Google Scholar]
- 120.Morris A, Kingsley LA, Groner G, Lebedeva IP, Beard CB, Norris KA. Prevalence and clinical predictors of Pneumocystis colonization among HIV-infected men. AIDS. 2004;18(5):793–8. doi: 10.1097/00002030-200403260-00011. [DOI] [PubMed] [Google Scholar]
- 121.Davis JL, Welsh DA, Beard CB, et al. Pneumocystis colonisation is common among hospitalised HIV infected patients with non-Pneumocystis pneumonia. Thorax. 2008;63(4):329–34. doi: 10.1136/thx.2007.088104. [DOI] [PubMed] [Google Scholar]
- 122.Nevez G, Raccurt C, Jounieaux V, Dei-Cas E, Mazars E. Pneumocystosis versus pulmonary Pneumocystis carinii colonization in HIV-negative and HIV-positive patients. AIDS. 1999;13(4):535–6. doi: 10.1097/00002030-199903110-00020. [DOI] [PubMed] [Google Scholar]
- 123.Hauser PM, Blanc DS, Bille J, Nahimana A, Francioli P. Carriage of Pneumocystis carinii by immunosuppressed patients and molecular typing of the organisms. AIDS. 2000;14(4):461–3. doi: 10.1097/00002030-200003100-00022. [DOI] [PubMed] [Google Scholar]
- 124.Helweg-Larsen J, Jensen JS, Dohn B, Benfield TL, Lundgren B. Detection of Pneumocystis DNA in samples from patients suspected of bacterial pneumonia – a case-control study. BMC Infect Dis. 2002;2:28. doi: 10.1186/1471-2334-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maskell NA, Waine DJ, Lindley A, et al. Asymptomatic carriage of Pneumocystis jiroveci in subjects undergoing bronchoscopy: a prospective study. Thorax. 2003;58(7):594–7. doi: 10.1136/thorax.58.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Montes-Cano MA, de la Horra C, Dapena FJ, et al. Dynamic colonisation by different Pneumocystis jirovecii genotypes in cystic fibrosis patients. Clin Microbiol Infect. 2007;13(10):1008–11. doi: 10.1111/j.1469-0691.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- 127.Calderon EJ, Regordan C, Medrano FJ, Ollero M, Varela JM. Pneumocystis carinii infection in patients with chronic bronchial disease. Lancet. 1996;347(9006):977. doi: 10.1016/s0140-6736(96)91468-3. [DOI] [PubMed] [Google Scholar]
- 128.Sing A, Roggenkamp A, Autenrieth IB, Heesemann J. Pneumocystis carinii carriage in immunocompetent patients with primary pulmonary disorders as detected by single or nested PCR. J Clin Microbiol. 1999;37(10):3409–10. doi: 10.1128/jcm.37.10.3409-3410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Probst M, Ries H, Schmidt-Wieland T, Serr A. Detection of Pneumocystis carinii DNA in patients with chronic lung diseases. Eur J Clin Microbiol Infect Dis. 2000;19(8):644–5. doi: 10.1007/s100960000329. [DOI] [PubMed] [Google Scholar]
- 130.Vidal S, de la Horra C, Martín J, et al. Pneumocystis jirovecii colonisation in patients with interstitial lung disease. Clin Microbiol Infect. 2006;12(3):231–5. doi: 10.1111/j.1469-0691.2005.01337.x. [DOI] [PubMed] [Google Scholar]
- 131.Sing A, Geiger AM, Hogardt M, Heesemann J. Pneumocystis carinii carriage among cystic fibrosis patients, as detected by nested PCR. J Clin Microbiol. 2001;39(7):2717–8. doi: 10.1128/JCM.39.7.2717-2718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Armbruster C, Hassl A, Kriwanek S. Pneumocystis carinii colonization in the absence of immunosuppression. Scand J Infect Dis. 1997;29(6):591–3. doi: 10.3109/00365549709035900. [DOI] [PubMed] [Google Scholar]
- 133.Morris A, Sciurba FC, Lebedeva IP, et al. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med. 2004;170(4):408–13. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]
- 134.Calderón E, de la Horra C, Medrano FJ, et al. Pneumocystis jiroveci isolates with dihydropteroate synthase mutations in patients with chronic bronchitis. Eur J Clin Microbiol Infect Dis. 2004;23(7):545–9. doi: 10.1007/s10096-004-1151-3. [DOI] [PubMed] [Google Scholar]
- 135.Respaldiza N, Montes-Cano MA, Dapena FJ, et al. Prevalence of colonisation and genotypic characterisation of Pneumocystis jirovecii among cystic fibrosis patients in Spain. Clin Microbiol Infect. 2005;11(12):1012–5. doi: 10.1111/j.1469-0691.2005.01276.x. [DOI] [PubMed] [Google Scholar]
- 136.Medrano FJ, Montes-Cano M, Conde M, et al. Pneumocystis jirovecii in general population. Emerg Infect Dis. 2005;11(2):245–50. doi: 10.3201/eid1102.040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vargas SL, Pizarro P, López-Vieyra M, Neira-Avilés P, Bustamante R, Ponce CA. Pneumocystis colonization in older adults and diagnostic yield of single versus paired noninvasive respiratory sampling. Clin Infect Dis. 2010;50(3):e19–21. doi: 10.1086/649869. [DOI] [PubMed] [Google Scholar]
- 138.Ponce CA, Gallo M, Bustamante R, Vargas SL. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin Infect Dis. 2010;50(3):347–53. doi: 10.1086/649868. [DOI] [PubMed] [Google Scholar]
- 139.Totet A, Respaldiza N, Pautard JC, Raccurt C, Nevez G. Pneumocystis jiroveci genotypes and primary infection. Clin Infect Dis. 2003;36(10):1340–2. doi: 10.1086/374844. [DOI] [PubMed] [Google Scholar]
- 140.Rivero L, de la Horra C, Montes-Cano MA, et al. Pneumocystis jirovecii transmission from immunocompetent carriers to infant. Emerg Infect Dis. 2008;14(7):1116–8. doi: 10.3201/eid1407.071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bartlett MS, Vermund SH, Jacobs R, et al. Detection of Pneumocystis carinii DNA in air samples: likely environmental risk to susceptible persons. J Clin Microbiol. 1997;35(10):2511–3. doi: 10.1128/jcm.35.10.2511-2513.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Olsson M, Lidman C, Latouche S, et al. Identification of Pneumocystis carinii f. sp. hominis gene sequences in filtered air in hospital environments. J Clin Microbiol. 1998;36(6):1737–40. doi: 10.1128/jcm.36.6.1737-1740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Choukri F, Menotti J, Sarfati C, et al. Quantification and spread of Pneumocystis jirovecii in the surrounding air of patients with Pneumocystis pneumonia. Clin Infect Dis. 2010;51(3):259–65. doi: 10.1086/653933. [DOI] [PubMed] [Google Scholar]
- 144.Damiani C, Choukri F, Le Gal S, et al. Possible nosocomial transmission of Pneumocystis jirovecii. Emerg Infect Dis. 2012;18(5):877–8. doi: 10.3201/eid1805.111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Le Gal S, Pougnet L, Damiani C, et al. Pneumocystis jirovecii in the air surrounding patients with Pneumocystis pulmonary colonization. Diagn Microbiol Infect Dis. 2015;82(2):137–42. doi: 10.1016/j.diagmicrobio.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 146.Vargas SL, Ponce CA, Gigliotti F, et al. Transmission of Pneumocystis carinii DNA from a patient with P. carinii pneumonia to immunocompetent contact health care workers. J Clin Microbiol. 2000;38(4):1536–8. doi: 10.1128/jcm.38.4.1536-1538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miller RF, Ambrose HE, Wakefield AE. Pneumocystis carinii f. sp. hominis DNA in immunocompetent health care workers in contact with patients with P. carinii pneumonia. J Clin Microbiol. 2001;39(11):3877–82. doi: 10.1128/JCM.39.11.3877-3882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.O’Donnell WJ, Pieciak W, Chertow GM, Sanabria J, Lahive KC. Clearance of Pneumocystis carinii cysts in acute P carinii pneumonia: assessment by serial sputum induction. Chest. 1998;114(5):1264–8. doi: 10.1378/chest.114.5.1264. [DOI] [PubMed] [Google Scholar]
- 149.Wakefield AE, Lindley AR, Ambrose HE, Denis CM, Miller RF. Limited asymptomatic carriage of Pneumocystis jiroveci in human immunodeficiency virus-infected patients. J Infect Dis. 2003;187(6):901–8. doi: 10.1086/368165. [DOI] [PubMed] [Google Scholar]
- 150.Valade S, Azoulay E, Damiani C, Derouin F, Totet A, Menotti J. Pneumocystis jirovecii airborne transmission between critically ill patients and health care workers. Intensive Care Med. 2015 doi: 10.1007/s00134-015-3835-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 151.Lundgren B, Elvin K, Rothman LP, Ljungström I, Lidman C, Lundgren JD. Transmission of Pneumocystis carinii from patients to hospital staff. Thorax. 1997;52(5):422–4. doi: 10.1136/thx.52.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gits-Muselli M, Peraldi MN, de Castro N, et al. New short tandem repeat-based molecular typing method for Pneumocystis jirovecii reveals intrahospital transmission between patients from different wards. PLoS One. 2015;10(5):e0125763. doi: 10.1371/journal.pone.0125763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. U.S. Public Health Service (USPHS) and Infectious Diseases Society of America (IDSA) MMWR Recomm Rep. 1999;48(RR-10):1–59. 61–6. [PubMed] [Google Scholar]
- 154.Kovacs JA, Masur H. Prophylaxis against opportunistic infections in patients with human immunodeficiency virus infection. N Engl J Med. 2000;342(19):1416–29. doi: 10.1056/NEJM200005113421907. [DOI] [PubMed] [Google Scholar]
- 155.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82(9):1052–9. doi: 10.4065/82.9.1052. [DOI] [PubMed] [Google Scholar]
- 156.Sepkowitz KA, Brown AE, Armstrong D. Pneumocystis carinii pneumonia without acquired immunodeficiency syndrome. More patients, same risk. Arch Intern Med. 1995;155(11):1125–8. [PubMed] [Google Scholar]
- 157.Okada J, Kadoya A, Rana M, Ishikawa A, Iikuni Y, Kondo H. Efficacy of sulfamethoxazole-trimethoprim administration in the prevention of Pneumocystis carinii pneumonia in patients with connective tissue disease. Kansenshogaku Zasshi. 1999;73(11):1123–9. doi: 10.11150/kansenshogakuzasshi1970.73.1123. [DOI] [PubMed] [Google Scholar]
- 158.Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum. 2011;41(3):497–502. doi: 10.1016/j.semarthrit.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 159.Mori S, Cho I, Koga Y, Sugimoto M. Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. J Rheumatol. 2008;35(8):1513–21. [PubMed] [Google Scholar]
- 160.Mori S, Koga Y, Sugimoto M. Small airway obstruction in patients with rheumatoid arthritis. Mod Rheumatol. 2011;21(2):164–73. doi: 10.1007/s10165-010-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bozzette SA, Finkelstein DM, Spector SA, et al. A randomized trial of three antipneumocystis agents in patients with advanced human immunodeficiency virus infection. NIAID AIDS Clinical Trials Group. N Engl J Med. 1995;332(11):693–9. doi: 10.1056/NEJM199503163321101. [DOI] [PubMed] [Google Scholar]
- 162.Schneider MM, Hoepelman AI, Eeftinck Schattenkerk JK, et al. A controlled trial of aerosolized pentamidine or trimethoprim-sulfamethoxazole as primary prophylaxis against Pneumocystis carinii pneumonia in patients with human immunodeficiency virus infection. The Dutch AIDS Treatment Group. N Engl J Med. 1992;327(26):1836–41. doi: 10.1056/NEJM199212243272603. [DOI] [PubMed] [Google Scholar]
- 163.Kimura M, Tanaka S, Ishikawa A, Endo H, Hirohata S, Kondo H. Comparison of trimethoprim-sulfamethoxazole and aerosolized pentamidine for primary prophylaxis of Pneumocystis jiroveci pneumonia in immunocompromised patients with connective tissue disease. Rheumatol Int. 2008;28(7):673–6. doi: 10.1007/s00296-007-0505-4. [DOI] [PubMed] [Google Scholar]
- 164.Govert JA, Patton S, Fine RL. Pancytopenia from using trimethoprim and methotrexate. Ann Intern Med. 1992;117(10):877–8. doi: 10.7326/0003-4819-117-10-877_2. [DOI] [PubMed] [Google Scholar]
- 165.Chevrel G, Brantus JF, Sainte-Laudy J, Miossec P. Allergic pancytopenia to trimethoprim-sulphamethoxazole for Pneumocystis carinii pneumonia following methotrexate treatment for rheumatoid arthritis. Rheumatology (Oxford) 1999;38(5):475–6. doi: 10.1093/rheumatology/38.5.475. [DOI] [PubMed] [Google Scholar]
- 166.Church JA, Fitzgerald F, Walker AS, Gibb DM, Prendergast AJ. The expanding role of co-trimoxazole in developing countries. Lancet Infect Dis. 2015;15(3):327–39. doi: 10.1016/S1473-3099(14)71011-4. [DOI] [PubMed] [Google Scholar]
- 167.Ash G, Baker R, Rajapakse C, Swinson DR. Study of sulphamethoxazole in rheumatoid arthritis. Br J Rheumatol. 1986;25(3):285–7. doi: 10.1093/rheumatology/25.3.285. [DOI] [PubMed] [Google Scholar]
- 168.Rozin A, Schapira D, Braun-Moscovici Y, Nahir AM. Cotrimoxazole treatment for rheumatoid arthritis. Semin Arthritis Rheum. 2001;31(2):133–41. doi: 10.1053/sarh.2001.27734. [DOI] [PubMed] [Google Scholar]
- 169.Huang L, Crothers K, Atzori C, et al. Dihydropteroate synthase gene mutations in Pneumocystis and sulfa resistance. Emerg Infect Dis. 2004;10(10):1721–8. doi: 10.3201/eid1010.030994. [DOI] [PMC free article] [PubMed] [Google Scholar]