Abstract
Background
Advanced esophageal dysplasia and early cancers have been treated traditionally with esophagectomy. Endoscopic esophageal mucosectomy (EEM) offers less-invasive therapy, but high-degree stricture formation limits its applicability. We hypothesized that placement of a biodegradable stent (BD-stent) immediately after circumferential EEM would prevent stricturing.
Methods
Ten pigs (five unstented controls, five BD-stent) were utilized. Under anesthesia, a flexible endoscope with a band ligator and snare was used to incise the mucosa approximately 20 cm proximal to the lower esophageal sphincter. A 10-cm, circumferential, mucosal segment was dissected and excised by using snare electrocautery. In the stented group, an 18-×120-mm, self-expanding, woven polydioxanone stent (ELLA-CS, Hradec-Kralove) was deployed. Weekly esophagograms evaluated for percent reduction in esophageal diameter, stricture length, and proximal esophageal dilation. Animals were euthanized when the stricture exceeded 80 % and were unable to gain weight (despite high-calorie liquid diet) or at 14 weeks.
Results
The control group rapidly developed esophageal strictures; no animal survived beyond the third week of evaluation. At 2 weeks post-EEM, the BD-stent group had a significant reduction in esophageal diameter (77.7 vs. 26.6 %, p < 0.001) and degree of proximal dilation (175 vs. 131 %, p = 0.04) compared with controls. Survival in the BD-stent group was significantly longer than in the control group (9.2 vs. 2.4 weeks, p = 0.01). However, all BD-stent animals ultimately developed clinically significant strictures (range, 4–14 weeks). Comparison between the maximum reduction in esophageal diameter and stricture length (immediately before euthanasia) demonstrated no differences between the groups.
Conclusions
Circumferential EEM results in severe stricture formation and clinical deterioration within 3 weeks. BD-stent placement significantly delays the time of clinical deterioration from 2.4 to 9.2 weeks, but does not affect the maximum reduction in esophageal diameter or proximal esophageal dilatation. The timing of stricture formation in the BD-stent group correlated with the loss radial force and stent disintegration.
Keywords: Endoscopic esophageal mucosectomy, Enteral stent, Esophageal cancer, Esophageal dysplasia, Endoscopic submucosal dissection
Although the incidence of squamous cell esophageal cancer (SCC) in the United States is decreasing, the rate of adenocarcinoma (AC) of the esophagus and gastroesophageal junction (GEJ) is dramatically increasing [1–3]. The overwhelming majority of esophageal adenocarcinomas arise from segments of Barrett’s esophagus (BE); intestinal metaplasia induced by gastroesophageal reflux. Barrett’s esophagus is seen in approximately 10 % of patients with reflux who undergo endoscopy [4]. Whereas only a small minority of patients with BE will develop esophageal AC (approximately 0.5 % annually), predictive models have suggested that the rate of progression from BE to high-grade dysplasia or invasive cancer may be as high as 40 % in high-risk populations [5–8].
Esophageal cancers confined to the mucosa (both SCC and AC) and high-grade dysplasia associated with BE have been traditionally treated with esophagectomy, which removes all of the dysplastic/neoplastic epithelium as well as the locoregional lymph nodes. Whereas this therapy is definitive in its extent of resection, esophagectomy is plagued with high rates of morbidity and mortality. As a result of these issues, 30–50 % of patients develop at least one serious postoperative complication and 5–12 % of patients will die [9–12]. Even in the era of the minimally invasive esophagectomy, where laparoscopic and thoracoscopic methods have reduced operative blood loss, intensive care unit length of stay, and postoperative pain, changes in morbidity and mortality have not been seen [13].
Endoscopic ablation of dysplastic mucosa represents an alternative to esophagectomy. By utilizing radiofrequency, thermal or photochemical energy, these methods destroy the diseased segment of mucosa, sparing the remaining normal esophagus; all with minimal morbidity and mortality [14, 15]. Although less invasive, these ablative methods are prone to undertreatment and, by design, destroy the specimen, preventing final histological analysis of the degree of dysplasia or the invasiveness of the cancer. Ablative therapies, including argon plasma coagulation and photodynamic therapy, have been associated with migration of BE into the gastric cardia and with the development of “buried Barrett’s” glands beneath the neosquamous lining within which AC can develop [16, 17].
Endoscopic resection of dysplastic/neoplastic mucosa, endoscopic esophageal mucosectomy (EEM), represents an alternative treatment method to both esophagectomy and mucosal ablation. By removing the diseased portion of the esophagus, the mucosa, this procedure avoids the morbidity and mortality of major thoracoabdominal procedure but still provides an intact tissue specimen for histopathologic analysis. Therefore, EEM can provide valuable staging information, documenting submucosal invasion that would necessitate an esophagectomy while curing the disease if no submucosal involvement is identified [18]. Such information is not available with endoscopic ablation. A recent analysis demonstrated both financial and survival benefits for endoscopic therapy over surgical therapy for early esophageal cancers perhaps due to the low risk of lymph node metastases [19].
In the United States, the general practice is to perform endoscopic mucosal resection (EMR) for small areas of high-grade dysplasia with intramucosal cancer and concomitant endoscopic ablation of other dysplastic segments of BE [20, 21]. This carries a risk of missing synchronous lesions as well as the development of metachronous lesions over time [22]. Endoscopic surveillance is thus continued indefinitely [23]. EMR is less suitable for lesions >2 cm or for multiple lesions since piecemeal resection increases the complication rate and makes it difficult or impossible to determine the adequacy of the resection. For such lesions, endoscopic submucosal dissection (ESD) is performed.
Practiced mainly in Japan, ESD has a steep learning curve and a higher complication rate than EMR but entirely preserves tissue architecture for histopathologic analysis [24]. Importantly, stricture formation after esophageal ESD is common, although the exact rate of clinically significant stricture formation is unknown [25, 26]. Strictures are more likely to occur when the resection involves >75 % of the lumen circumference or ≥3 cm of lumen length [26, 27]. A therapy to reduce stricture formation after circumferential EEM may be the only barrier to replacing a radical esophagectomy with a minimal invasive endoscopic procedure.
Refining EEM techniques have been performed in animal models, but survival studies have been limited by aggressive stricture formation [28–30]. Circumferential EEM resulted in strictures within 2 weeks in all animals in one swine study [31]. Therapies have been proposed to prevent such stricture formation. Transplantation of autologous epithelial cell sheets following hemicircumferential ESD resulted in better healing and reduced strictures in dogs survived for 4 weeks [27]. Injection of keratinocytes isolated from the buccal mucosa resulted in prevention of stricture formation at the site of injection following EEM in a swine model [32]. Extracellular matrix also has been reported to reduce strictures in dogs treated with circumferential EEM [33]. Collectively, these animal studies demonstrate potential of highly sophisticated (and often proprietary) wound healing therapy to reduce stricture formation after EEM.
We hypothesized that the immediate placement of a commercially available biodegradable enteral stent (BD-stent) would prevent stricture formation after circumferential EEM, bypassing the need for the development of a highly engineered product and providing a clinical therapy that can be implemented immediately.
Methods
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University, Cleveland, OH. Ten domestic swine (Sus scrofus domesticus) were utilized (five unstented controls and five BD-stent animals). Animals were provided water and standard chow ad libitum during the mandated 1-week quarantine period followed by food restriction for 18 hours before the procedure.
Immediately before the procedure, anesthesia was induced with tiletamine hydrochloride and zolazepam (10 mg/kg, IM, Telazol, Fort Dodge Animal Health, Fort Dodge, IA). The animals were intubated and anesthesia maintained with 1–2 % isoflurane delivered in 100 % O2 by mechanical ventilation with SpO2, respiratory rate, and pulse rate monitored throughout the procedure. Preoperative antibiotics (tulathromycin, 2.5 mg/kg, IM, Draxxin, Pfizer, New York, NY) and analgesics (fentanyl, 25 μg/hr, Transdermal, Alza Corp, Mountain View, CA) were administered. All endoscopic equipment (except sterile-packaged, disposable pieces) underwent high level disinfection with ortho-phthaladyhdye solution (Cidex; Johnson and Johnson, New Brunswick, NJ) before use.
A dual-channel endoscope (GIF 2T160; Olympus America, Center Valley, PA) preloaded with a commercially available band ligating device (Super 7, BSCI; Duette, Wilson Cook Medical, Winston-Salem, NC), was introduced into the esophagus. The band ligator was used to create a mucosal pseudo-polyp 20 cm proximal to the lower esophageal sphincter (Fig. 1). An endoscopic EMR snare with a 10–15 mm opening (SD-210U-10/15, Olympus) was then used to transect the stalk of the pseudo-polyp, allowing access to the submucosal plane. This process was repeated, completing a circumferential incision through the mucosal layer. A column of mucosa (approximately 10 cm) was subsequently dissected free from the underlying musculature and excised using snare electrocautery (Fig. 2). Hemostasis was maintained throughout the procedure. The excised mucosa was fixed in formalin and stained with hematoxylin and eosin (H&E), and Masson’s Trichrome for analysis of the depth of resection. For the control group, no additional therapy was performed.
Fig. 1.
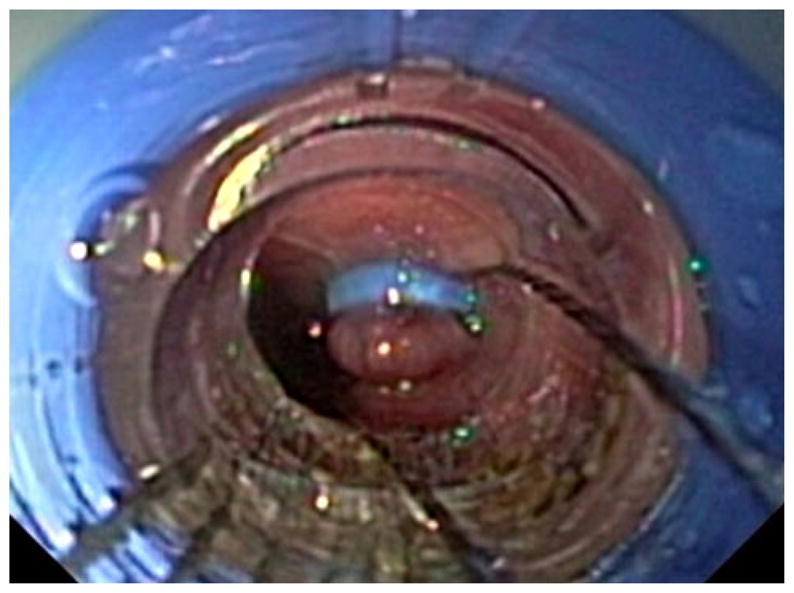
Endoscopic view of the esophageal pseudo-polyp (created with the band ligation device) and the snare being used to begin the mucosal resection
Fig. 2.
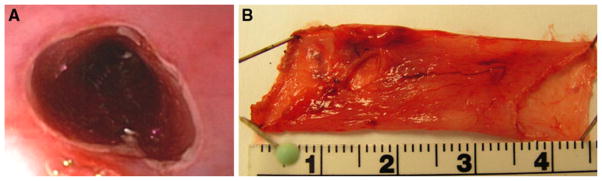
A Endoscopic view of the area of EEM. Normal mucosa ends in a cut edge with underlying intact submucosa and muscular layers appearing more distal in the lumen. B Gross view of the circumferentially resected mucosal segment
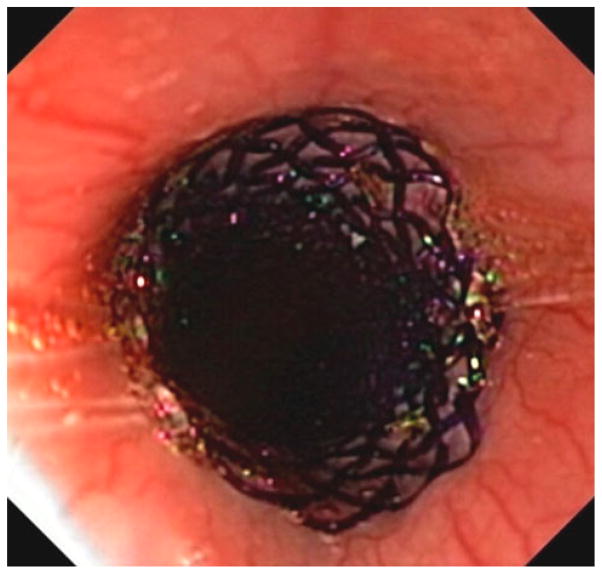
For the stent group, a 0.89-mm (0.035″) guidewire was passed through the working channel of the endoscope into the stomach. The endoscope was then withdrawn over the wire. A commercially available, uncovered, 18- × 120-mm, self-expanding, esophageal stent (SX-ELLA, ELLA-CS, Hradec-Kralove, Czech Republic) was loaded into its sheath delivery system according to the manufacturer’s instructions for use (Fig. 3). Subsequently, the deployment system was passed over-the-wire and into the esophagus. The stent was deployed to cover fully the area of esophageal mucosal defect and position was confirmed endoscopically (Fig. 4). To prevent stent migration, a permanent, monofilament suture was looped through the interstices of the proximal stent and secured percutaneously to the animal’s chin in a position that prevented them from chewing through the suture.
Fig. 3.

SX-ELLA BD stent being loaded into its deployment system
Fig. 4.
Endoscopic view of the deployed BD-stent covering the area of prior EEM
After EEM (in controls) or EEM with BD-stent placement, contrast and noncontrast fluoroscopic images were recorded. The positions of the radiopaque stent markers were first noted on anterior-posterior images. Barium sulfate contrast suspension (E-Z-Paque, E-Z-EM Canada, Inc., Lake Success, NY) was then injected per os to visualize the lumen of the esophagus. A radiopaque ruler was placed under the animal to provide a standard measurement scale. Baseline measurements of the esophageal diameter (at the area of the EEM and proximal to the EEM) and EEM length were recorded. Following imaging, the animals were recovered and returned to their housing where an ad lib diet was resumed.
Weekly, animals were sedated (Telazol, 4 mg/kg) to permit weight assessment and for fluoroscopic and endoscopic surveillance of stricture progression. Contrast fluoroscopic images were obtained, digitized, and esophageal measurements recorded. The reduction in esophageal diameter, the stricture length, and degree of proximal esophageal dilation were all calculated as a percentage of the baseline measurements obtained after the EEM procedure. The average diameter at three locations along the lesion—proximal, middle, and distal—were compared to native tissue diameter proximal to the lesion at week 0 for a determination of the reduction in esophageal diameter. The length of the strictured area also was measured and compared to the original mucosal defect to assess for longitudinal shortening of the esophagus. The change in diameter proximal to the lesion was compared to that of week 0 as an indirect indicator of stricture formation, demonstrating esophageal dilation proximal the stricture.
A high-calorie, liquid diet was provided to animals who displayed symptoms of esophageal stricture (regurgitation or poor weight gain). Animals were euthanized when the esophageal stricture exceeded 80 %, if they were unable to gain weight (despite diet modification) or at 14 weeks.
Animals were first anesthetized by an intramuscular injection of Telazol and then euthanized by an overdose of pentobarbital sodium (>100 mg/kg IV). This method is consistent with the recommendations of the 2000 Panel on Euthanasia of the American Veterinary Medical Association [34]. Samples, including native tissue and stricture zone, were collected for histological analysis. Tissues were fixed in formalin, embedded, sectioned, and stained with H&E and Masson’s Trichrome. Specimens were then analyzed by a veterinary pathologist blinded to the study design and specimen origin. A 5-point Likert scale was used to assess the degree of inflammation, healing, fibrosis, and tissue remodeling.
Power and statistical analysis
Control animals developed a mean reduction in esophageal diameter of approximately 78 %, with a standard deviation of 12 %, 2 weeks following resection. Therefore, a sample size of five per group was adequate to detect a 25 % reduction in stricture size with a significance level of 0.05 and power of 0.8 using t test (SigmaStat, version 3.11, Sysstat Software, Inc.). A 25 % reduction in stricture size compared to control (78 %) would result in a stricture of approximately 50 %, a level at which the therapy would have clinical relevance. All comparisons between groups were made using t test (SigmaStat). A p value <0.05 was considered significant.
Results
All control animals (n = 5) and BD-stent animals (n = 5) successfully underwent EEM without bleeding, perforation, or anesthetic complication. The average starting weight between the groups was similar (31.6 vs. 32.9 kg, p = 0.31). The resected mucosal specimens ranged between 9 and 10 cm, and there was no difference in the resected lengths between groups (p = 0.44). In the BD-stent animals, the ELLA-CS stents were placed successfully without difficulty or stent malposition. There were no stent-related complications (migrations, obstructions, erosions) for the duration of the study.
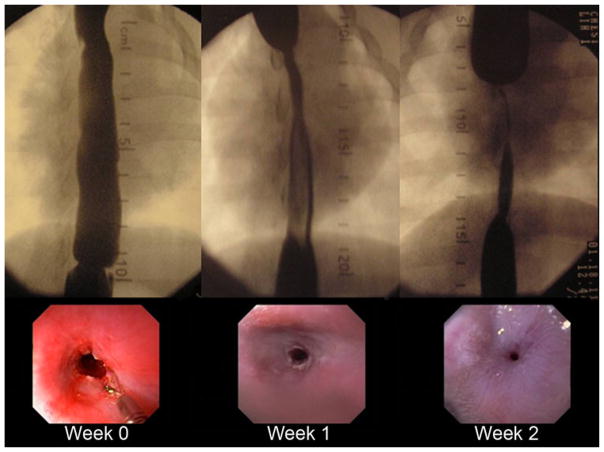
The control group rapidly developed esophageal strictures (Fig. 5). At 1 week post-EEM, esophageal diameter in the resection area was reduced to 62.2 ± 12.9 % of the baseline value; this increased to 77.7 ± 12.1 % by the second week evaluation. Based on defined study endpoints, no control animal survived beyond the third week of evaluation. Table 1 shows the reduction in esophageal diameter, the stricture length, and degree of proximal esophageal dilation in the control group as percentage change from the baseline values obtained immediately post-EEM. As the esophageal diameter reduced, the length of the previous EEM site shortened and the proximal esophagus dilated.
Fig. 5.
Fluoroscopic and endoscopic view of stricture progression in one of the control animals
Table 1.
Control group reduction in esophageal diameter, stricture length, and degree of proximal dilation expressed as a percent change from baseline
| Week | 1 | 2 | 3 |
|---|---|---|---|
| No. of animals | 5 | 5 | 2 |
| % Reduction in diameter (mean ± SD) | 62.2 ± 12.9 | 77.7 ± 12.1 | 90.1 ± 9.6 |
| % Stricture length (mean ± SD) | 77.6 ± 12.4 | 62.7 ± 12.3 | 65.7 ± 1.4 |
| % Proximal dilation (mean ± SD) | 128.2 ± 6.2 | 174.8 ± 27.3 | 179.8 ± 6.8 |
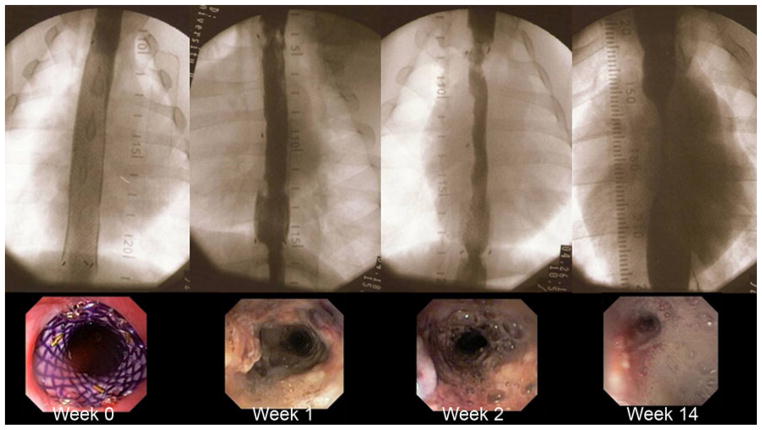
The BD-stent group demonstrated a different pattern of stricture formation (Fig. 6). There was little reduction in the esophageal diameter or proximal esophageal dilation for the first 6 weeks in the majority of animals (Table 2). At 2 weeks post-EEM, the last week that all five control animals were alive, the BD-stent group had a significantly less reduction in esophageal diameter (77.7 % vs. 26.6 %, p < 0.001) and degree of proximal dilation (175 % vs. 131 %, p = 0.04) compared with controls. However, there was no difference in the stricture length between the groups at week 2 (p = 0.52). Figures 7 and 8 compare the percent reduction in esophageal diameter and the percent proximal esophageal dilation between the control and the BD-stent groups, respectively.
Fig. 6.
Fluoroscopic and endoscopic view of stricture progression in one of the BD-stent animals
Table 2.
BD-stent group reduction in esophageal diameter, stricture length, and degree of proximal dilation expressed as a percent change from baseline
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of animals | 5 | 5 | 5 | 5 | 4 | 4 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 |
| % Reduction in diameter (mean ± SD) | 12.5 ± 12.5 | 26.6 ± 16.4 | 26.2 ± 16.3 | 42.7 ± 37.1 | 42.5 ± 13.9 | 45.8 ± 10.3 | 59 ± 14.4 | 50.1 ± 24.5 | 33 ± 11.3 | 32.9 ± 15.1 | 37.2 ± 5.4 | 36.8 ± 20.6 | 35.7 ± 11.2 | 81.2 ± 4.8 |
| % Stricture length (mean ± SD) | 72.6 ± 20.4 | 70.1 ± 21.4 | 69.5 ± 19.4 | 62.2 ± 24.9 | 70.8 ± 20.6 | 61.0 ± 11.8 | 56.9 ± 13.6 | 54.1 ± 8 | 51.9 ± 8.3 | 56 ± 19.8 | 47.6 ± 6.5 | 49.9 ± 9.7 | 50.7 ± 17.9 | 34.5 ± 6.3 |
| % Proximal dilation (mean ± SD) | 112.7 ± 10.1 | 131.6 ± 29.3 | 137.1 ± 27.1 | 143.7 ± 23.8 | 149.6 ± 53.3 | 159.8 ± 52.0 | 144 ± 24.0 | 146.6 ± 27.0 | 134.6 ± 48.9 | 151.3 ± 25.4 | 142.6 ± 48.5 | 115.4 ± 21.8 | 148.8 ± 25.4 | 171.2 ± 29.9 |
Fig. 7.
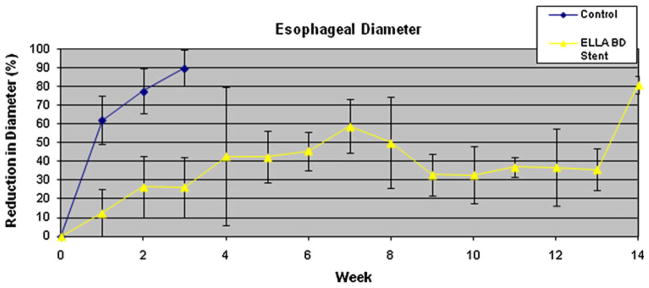
Percent reduction in esophageal diameter over time for the control and BD-stent groups
Fig. 8.
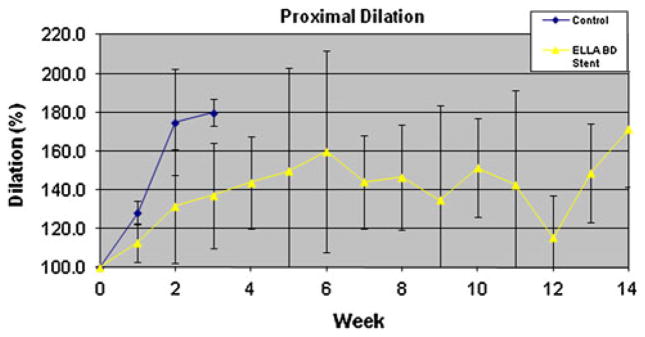
Percent of proximal esophageal dilation over time for the control and BD-stent groups
Survival in the BD-stent group was significantly longer than in the control group (9.2 weeks vs. 2.4, p = 0.01). However, all BD-stent animals ultimately developed clinically significant strictures (range, 4–14 weeks) requiring euthanasia. Comparison between the maximum reduction in diameter and stricture length (immediately before euthanasia) demonstrated no differences between the groups (p = 0.324 and p = 0.788, respectively). However, there was significantly greater shortening of the esophagus in the BD-stent group compared with the control group (43.4 ± 13.6 % vs. 59.6 ± 6.8 %, p = 0.045) immediately before euthanasia (Fig. 9).
Fig. 9.
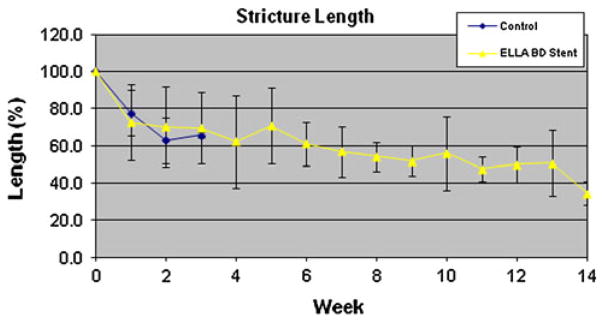
Stricture length (as percentage of original EEM resection length) over time for the control and BD-stent groups
Histological evaluation of the mucosal specimens, demonstrated uniformly comprised normal-appearing, nonkeratinized, squamous mucosa and superficial submucosa. In all animals, no muscularis propria was identified in the mucosal specimen. Necropsy specimens taken proximal to the EEM site included normal-appearing, full-thickness esophagus with no pathological alterations. Specimens from the site of the stricture were more heterogeneous, demonstrating ulceration, granulation (neovascularization with acute and chronic inflammation), repair, and early (cellular) fibrosis (Fig. 10). Evidence of reepithelialization was present in some cases. Evidence of reepithelialization was present in some cases. The fibroinflammatory process involved almost the entirety of the submucosa but spared the muscularis propria in all cases. No statistical differences were noted between control animals and stented animals in terms of histological findings (polymorphonuclear leukocytes, lymphocytes, eosinophils, neovascularization, or amount and cellularity of the fibrosis).
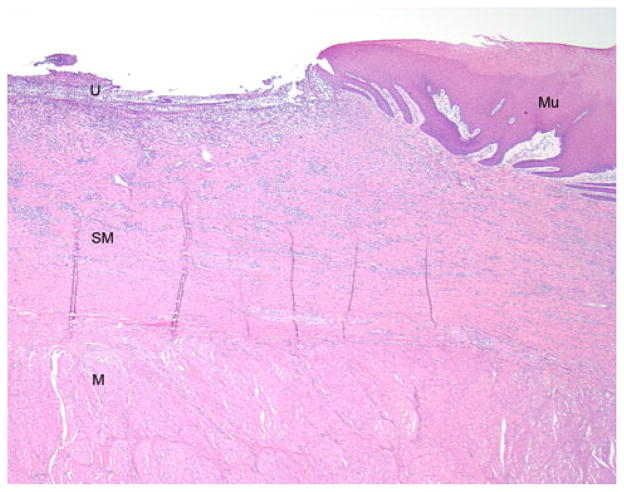
Fig. 10.
Photomicrograph (H&E, original magnification = 20×) from the edge of the stricture zone showing an ulcer (U) where the EEM occurred. The underlying fibroinflammatory reaction involves almost the entirety of the submucosa (SM), and the uninvolved muscularis propria (M) is visible beneath. At the edge of the ulcer, there is reepithelialization over the scar by reactive squamous mucosa (Mu)
Discussion
This study was designed to evaluate the ability of a commercially available, biodegradable enteral stent to reduce the aggressive stricture formation that occurs in a porcine model of circumferential endoscopic esophageal mucosal resection. In control animals, such EEM quickly results in a high degree of stricture formation. These strictures are clinically significant; they result in clinical deterioration (regurgitation and failure to gain weight) and proximal esophageal dilation within 3 weeks. Histology confirmed that the EEM procedure removed an intact mucosal specimen of uniform submucosal depth without damage to the muscularis propria.
Stricture formation after >75 % circumferential ESD occurs at a rate of almost 90 % [26]. Clinically, these strictures can result in dysphagia, weight loss, aspiration pneumonia, and a reduced quality of life. Unfortunately, there are no guidelines to suggest which patients may benefit from stricture prophylaxis regimens, such as preventative balloon dilations or steroid injection [25, 26]. Moreover, dilation regimens are time- and resource-consuming; some require more than 30 sessions before achieving lasting success [25]. We hypothesized that immediate placement of a commercially available biodegradable enteral stent (BD-stent) would prevent stricture formation following circumferential EEM, preventing the need for postprocedure dilatory therapy.
The BD-stent did significantly delay the time of clinical deterioration from 2.4 to 9.2 weeks but ultimately did not alter the maximum degree of luminal narrowing or proximal esophageal dilation. The SX-ELLA is a fully uncovered stent made of woven polydioxanone, a semicrystalline biodegradable polymer belonging to the polyester family. It degrades by random hydrolysis of its molecule ester bonds. This degradation is accelerated by low pH, and under luminal environmental conditions it is partially absorbed and partially passes through the gastrointestinal tract. It maintains its full integrity and radial force for 6 weeks after deployment. At week 7, the radial force is approximately two thirds and at week 9 approximately one half of the initial force [35]. By 11–12 weeks, the stent fully disintegrates. This stent is available in the European market but is not currently FDA approved for use in the United States.
Our results reflect this degradation process, with slow progression in the degree of luminal narrowing for the first 5–6 weeks followed by stricture formation and proximal esophageal dilation for the next 6 weeks as the stent composition continues to decline. The stents were able to alter the degree of radial stricturing significantly (and as a consequence, the degree of proximal esophageal dilation) for at least 2 weeks after EEM.
Interestingly, there was not a significant difference in the shortening of the EEM lesion between the control (62.7 %) and stented (70.1 %) groups at the 2-week evaluation. This suggests that the BD-stent provided adequate radial force to prevent narrowing but did nothing to restrict longitudinal contracture of the stricture segment. The final value for length obtained for controls immediately before euthanasia (59.6 %) was significantly greater than the final value obtained in the BD-stent group (43.4 %). This again seems to attest to ongoing longitudinal contracture in the face of the radial support of the stent. The stent delayed clinically significant luminal narrowing and extended survival, which permitted additional time for longitudinal contracture to occur.
Histologic analysis of the stricture segments showed that the fibrous scar formation did not involve or disrupt the muscularis propria (Fig. 10), suggesting that therapies inhibiting fibrosis may be able to alleviate the stricture formation. Such therapies may include topical anti-inflammatory or antiproliferative agents. Some degree of reepithelialization was seen in the BD-stent strictured segments. It is possible that a biodegradable stent with a longer in vivo half-life might permit a greater degree of such epithelization to occur. More complete remodeling may reduce the amount of stricture formation after loss of stent integrity.
The limitations of this study included the porcine model, which appears to develop strictures in a more aggressive fashion than humans who undergo circumferential EEM. Moreover, no attempts at endoscopic salvage therapy, such as balloon stricturoplasty, were made in either group as would be standard therapy in humans.
This study confirmed previous findings that circumferential mucosal resection quickly results in stricture formation. With no therapy, such strictures become clinically relevant and lead to overall deterioration of the animal. The placement of a BD-stent significantly delays the time to such deterioration but does not alter the final degree of luminal narrowing or proximal esophageal dilatation. The timing of stricture formation correlates with the known loss of radial force (6–8 weeks) of the stent.
Acknowledgments
The stents used in this study were graciously provided by ELLA-CS, Hradec-Kralove, Czech Republic.
Footnotes
Disclosures: Jeffrey M. Marks receives an honorarium as a consultant for Covidien, Olympus, Boston Scientific, WL Gore, and Ethicon and for serving on the advisory board for Apollo Endosurgery. Jeffrey L. Ponsky serves as a consultant for US Endoscopy. None of the other authors have relationships to disclose.
Contributor Information
Eric M. Pauli, Email: Eric.Pauli@uhhospitals.org, Department of Surgery, University Hospitals Case Medical Center, 11100 Euclid Ave, Mail Stop LKS 5047, Cleveland, OH 44106, USA
Steve J. Schomisch, Department of Surgery, University Hospitals Case Medical Center, 11100 Euclid Ave, Mail Stop LKS 5047, Cleveland, OH 44106, USA
Joseph P. Furlan, Department of Surgery, University Hospitals Case Medical Center, 11100 Euclid Ave, Mail Stop LKS 5047, Cleveland, OH 44106, USA
Andrea S. Marks, Department of Surgery, University Hospitals Case Medical Center, 11100 Euclid Ave, Mail Stop LKS 5047, Cleveland, OH 44106, USA
Amitabh Chak, Division of Gastroenterology, University Hospitals Case Medical Center, Cleveland, OH 44106, USA.
Richard H. Lash, Miraca Life Sciences Research Institute, Irving, TX 75039, USA
Jeffrey L. Ponsky, Department of Surgery, University Hospitals Case Medical Center, 11100 Euclid Ave, Mail Stop LKS 5047, Cleveland, OH 44106, USA
Jeffrey M. Marks, Email: Jeffrey.Marks@uhhospitals.org, Department of Surgery, University Hospitals Case Medical Center, 11100 Euclid Ave, Mail Stop LKS 5047, Cleveland, OH 44106, USA
References
- 1.Bollschweiler E, Wolfgarten E, Gutschow C, Hölscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92(3):549–555. doi: 10.1002/1097-0142(20010801)92:3<549::aid-cncr1354>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11(2):23–56. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC, Petersen N, Key CR. Epidemiological differences between adenocarcinoma of the oesophagus and adenocarcinoma of the gastric cardia in the USA. Gut. 2002;50(3):368–372. doi: 10.1136/gut.50.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–1677. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen NJ, Crosby MA, Bozymski EM, et al. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus? Gastroenterology. 2000;119:333–338. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 6.Hage M, Siersema PD, van Dekken H, et al. Oesophageal cancer incidence and mortality in patients with long-segment Barrett’s oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol. 2004;39:1175–1179. doi: 10.1080/00365520410003524. [DOI] [PubMed] [Google Scholar]
- 7.Sikkema M, de Jonge PJ, Steyerberg EW, et al. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8:235–244. doi: 10.1016/j.cgh.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Sikkema M, Looman CW, Steyerberg EW, et al. Predictors for neoplastic progression in patients with Barrett’s esophagus: a prospective cohort study. Am J Gastroenterol. 2011;106(7):1231–1238. doi: 10.1038/ajg.2011.153. [DOI] [PubMed] [Google Scholar]
- 9.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology. 2009;137(3):815–823. doi: 10.1053/j.gastro.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swisher SG, Deford L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119(6):1126–1132. doi: 10.1067/mtc.2000.105644. [DOI] [PubMed] [Google Scholar]
- 11.van Lanschot JJ, Hulscher JB, Buskens CJ, et al. Hospital volume and hospital mortality for esophagectomy. Cancer. 2001;91(8):1574–1578. doi: 10.1002/1097-0142(20010415)91:8<1574::aid-cncr1168>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Young MM, Deschamps C, Trastek VF, et al. Esophageal reconstruction for benign disease: early morbidity, mortality, and functional results. Ann Thorac Surg. 2000;70(5):1651–1655. doi: 10.1016/s0003-4975(00)01916-0. [DOI] [PubMed] [Google Scholar]
- 13.Santillan AA, Farma JM, Meredith KL, et al. Minimally invasive surgery for esophageal cancer. J Natl Compr Canc Netw. 2008;6(9):879–884. doi: 10.6004/jnccn.2008.0066. [DOI] [PubMed] [Google Scholar]
- 14.Sampliner RE. Endoscopic ablative therapy for Barrett’s esophagus: current status. Gastrointest Endosc. 2004;59:66–69. doi: 10.1016/s0016-5107(03)02380-0. [DOI] [PubMed] [Google Scholar]
- 15.Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65(2):185–195. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Fleischer DE, Sharma VK. Endoscopic ablation of Barrett’s esophagus using the halo system. Dig Dis. 2008;26(4):280–284. doi: 10.1159/000177009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Laethem JL, Peny MO, Salmon I, et al. Intramucosal adenocarcinoma arising under squamous re-epithelialisation of Barrett’s oesophagus. Gut. 2000;46(4):574–577. doi: 10.1136/gut.46.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieth M, Ell C, Gossner L, et al. Histological analysis of endoscopic resection specimens from 326 patients with Barrett’s esophagus and early neoplasia. Endoscopy. 2004;36(9):776–781. doi: 10.1055/s-2004-825802. [DOI] [PubMed] [Google Scholar]
- 19.Pohl H, Sonnenberg A, Strobel S, et al. Endoscopic versus surgical therapy for early cancer in Barrett’s esophagus: a decision analysis. Gastrointest Endosc. 2009;70(4):623–631. doi: 10.1016/j.gie.2008.11.047. [DOI] [PubMed] [Google Scholar]
- 20.Pech O, Ell C. Endoscopic therapy of Barrett’s esophagus. Curr Opin Gastroenterol. 2009;25(5):405–411. doi: 10.1097/MOG.0b013e32832d9b71. [DOI] [PubMed] [Google Scholar]
- 21.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer) Gastro-intest Endosc. 2007;65(1):3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 22.Esaki M, Matsumoto T, Hirakawa K, et al. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy. 2007;39(1):41–45. doi: 10.1055/s-2006-945143. [DOI] [PubMed] [Google Scholar]
- 23.Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–580. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc. 2010;72(5):960–966. doi: 10.1016/j.gie.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Isomoto H, Yamaguchi N, Nakayama T, et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2011;11:46–52. doi: 10.1186/1471-230X-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661–665. doi: 10.1055/s-0029-1214867. [DOI] [PubMed] [Google Scholar]
- 27.Katada C, Muto M, Manabe T, et al. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc. 2003;57(2):165–169. doi: 10.1067/mge.2003.73. [DOI] [PubMed] [Google Scholar]
- 28.Kamler JP, Borsatto R, Binmoeller KF. Circumferential endoscopic mucosal resection in the swine esophagus assisted by a cap attachment. Gastrointest Endosc. 2002;55(7):923–928. doi: 10.1067/mge.2002.124738. [DOI] [PubMed] [Google Scholar]
- 29.Ohki T, Yamato M, Murakami D, et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55(12):1704–1710. doi: 10.1136/gut.2005.088518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajan E, Gostout C, Feitoza A, et al. Widespread endoscopic mucosal resection of the esophagus with strategies for stricture prevention: a preclinical study. Endoscopy. 2005;37(11):1111–1115. doi: 10.1055/s-2005-870531. [DOI] [PubMed] [Google Scholar]
- 31.Willingham FF, Gee DW, Sylla P, et al. En bloc esophageal mucosectomy for concentric circumferential mucosal resection (with video) Gastrointest Endosc. 2009;69(1):147–151. doi: 10.1016/j.gie.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai T, Miyazaki S, Miyata G, et al. Autologous buccal keratinocyte implantation for the prevention of stenosis after EMR of the esophagus. Gastrointest Endosc. 2007;66(1):167–173. doi: 10.1016/j.gie.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 33.Nieponice A, McGrath K, Qureshi I, et al. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2009;69(2):289–296. doi: 10.1016/j.gie.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 34.AVMA Panel on Euthansia . 2000 Report of the AVMA Panel on Euthansia. J Am Vet Assn. 2001;218(5):669–701. doi: 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- 35.Repici A, Vleggaar FP, Hassan C, et al. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc. 2010;72(5):927–934. doi: 10.1016/j.gie.2010.07.031. [DOI] [PubMed] [Google Scholar]