Abstract
Objective
Inadequate medication adherence is a widespread problem that contributes to increase chronic disease complications and health care expenditures. Packaging interventions using pill boxes and blister packs have been widely recommended to address the medication adherence issue. This meta-analysis review determined the overall effect of packaging interventions on medication adherence and health outcomes. In addition, we tested whether effects vary depending on intervention, sample, and design characteristics.
Research design and methods
Extensive literature search strategies included examination of 13 computerized databases and 19 research registries, hand searches of 57 journal, and author and ancestry searches. Eligible studies included either pill-boxes or blister packaging interventions to increase medication adherence. Primary study characteristics and outcomes were reliably coded. Random-effects analyses were used to calculate overall effect sizes and conduct moderator analyses.
Results
Data were synthesized across 22,858 subjects from 52 reports. The overall mean weighted standardized difference effect size for two-group comparisons was 0.593 (favoring treatment over control), which is consistent with the mean of 71% adherence for treatment subjects compared to 63% among control subjects. We found using moderator analyses that interventions were most effective when they used blister packs and were delivered in pharmacies, while interventions were less effective when studies included older subjects and those with cognitive impairment. Methodological moderator analyses revealed significantly larger effect sizes in studies reporting continuous data outcomes instead of dichotomous results and in studies using pharmacy refill medication adherence measures as compared to studies with self-report measures.
Conclusions
Overall, meta-analysis findings support the use of packaging interventions to effectively increase medication adherence. Limitations of the study include the exclusion of packaging interventions other than pill boxes and blister packs, evidence of publication bias, and primary study sparse reporting of health outcomes and potentially interesting moderating variables such as the number of prescribed medications.
Keywords: medication adherence, meta-analysis, intervention, medication compliance
Introduction
Inadequate medication adherence (MA) is a pervasive global hidden epidemic with devastating health and economic consequences1, 2. The cost of nonadherence has been estimated at over €25 billion in the European Union and $100 billion yearly in the United States3–5. Overall, MA is suboptimal, estimated at around 50%1, 6–8. Between 20% and 25% of prescriptions are never filled, and another 20% of prescriptions are filled, but are not consumed due to patient-initiated drug holidays9. Rates of MA have not improved over the decades10, 11. Considering these findings, it is not surprising that the World Health Organization (WHO) calls poor adherence a “worldwide problem of striking magnitude”1.
The consistent evidence of widespread inadequate MA, as well as the importance of the issue, has led to considerable research testing diverse interventions to remedy the problem. Packaging interventions have long been recommended12–17, and several trials have tested various packaging types with inconclusive results. A few small reviews of six to twelve primary studies have attempted to summarize the effectiveness of packaging interventions12–16, 18. Very limited meta-analyses have been reported across two, three, and six primary studies15, 16, 18. These reviews have been hampered by narrow searches and very small numbers of primary studies. Moderator analysis, which examines the associations between study characteristics and MA behavior outcomes, is a strength of meta-analytic work. Previous reviews have retrieved too few studies to conduct moderator analyses to determine sample, design, and intervention characteristics linked to better MA outcomes.
Primary studies testing packaging interventions have not been adequately synthesized, which seriously impedes research progress and effective practice. This project aimed to provide the most comprehensive integration of scientific knowledge about packaging interventions to increase MA. This meta-analysis addressed the following research questions: 1) What are the overall effects of packaging interventions on MA? 2) Do the effects of packaging interventions on MA outcomes vary depending on intervention characteristics? 3) Do the effects of packaging interventions on MA outcomes vary depending on study design or sample characteristics? 4) What are the overall effects of packaging interventions on health outcomes?
Methods
We used standard meta-analysis review methods to identify and secure potential studies, assess eligibility, code data from primary study reports, meta-analyze results across studies, and interpret findings19.
Search Strategies
Multiple search strategies were employed to ensure a comprehensive search, move beyond previous narrow reviews, and limit the bias associated with limited searches20, 21. An experienced health sciences reference librarian performed searches in PubMED, MEDLINE, PsychINFO, EBSCO, CINAHL, PQDT, Cochrane Central Trials Register, Cochrane Database of Systematic Reviews, ERIC, IndMed, International Pharmaceutical Abstracts, EBM Reviews - Database of Abstracts of Reviews of Effects, and Communication and Mass Media. Broad search terms were used. For example, the primary MeSH terms upon which searches were constructed were Patient Compliance and Medication Adherence. Patient Compliance was used to locate studies published prior to 2009 because the term 'medication adherence' was not in MeSH usage until that year. Medication adherence (MeSH term) was used to locate studies published after 2008. Other MeSH terms used in constructing search strategies were: pharmaceutical preparations, dosage forms, drugs, generic, or prescription drugs. Keywords used in searches were: medication(s), regimen(s), prescription(s), prescribed, drug(s), pill(s), tablet(s), agent(s), compliant, compliance, adherent, adherence, noncompliant, noncompliance, nonadherent, nonadherence, improve, promote, enhance, encourage, foster, advocate, influence, incentive, ensure, remind, optimize, increase, impact, prevent, address, decrease. Other potential MA search terms, such as persistence, were not used because they are not MeSH terms and medication adherence and patient compliance are broader terms. Nineteen research registers were searched (e.g., Research Portfolio Online Reporting Tool). Hand searches were conducted in 57 journals where multiple eligible studies in the parent project were published. Author searches were conducted for authors of more than one eligible primary study in the parent project. Ancestry searches were conducted on all eligible studies and review papers. We retrieved abstracts from forty-eight conferences that contained, or led to, includable reports. Final searching was completed in 2013.
Inclusion Criteria
We included reports of packaging interventions to increase MA among adult subjects. MA refers to the extent to which patient medication-taking behavior is consistent with health care provider recommendations1, 6.
Packaging interventions provide a physical assembly of medications into an object that indicates the day and/or time medications should be administered16. Examples of packaging interventions include professionally prepared single-use sealed containers of medications, which are called blister packs, unit-packaging, unit-of-use systems, unit-of-dose packaging, and monitored dosage systems in the literature14–16. Blister packs provide correct medications in containers because they are filled by professionals. Pill boxes, reusable multi-compartment containers with designated spaces for medications to be consumed at a particular time, are another common type of packaging16. Unlike blister packs, pill boxes do not require professional action: they may be filled by patients, informal caregivers, or health care providers. While this may reduce costs, pill boxes may contain incorrect medications because they may be filled by patients or informal caregivers. Both blister packs and pill boxes may be recommended for aging adults with multiple chronic diseases. Possible cognitive limitations in this population could increase the incidence of incorrect medications in pill boxes. Other types of medication container changes such as replacing child-resistant caps, placing medications in envelopes instead of bottles, changing labels on medication containers, or instituting individual electronic medication containers caps which display the last medication administration time, were excluded from this review because they were functionally dissimilar to pill boxes and blister packs.
Studies of incarcerated or institutionalized persons were excluded because of institutional control over medication administration. Subjects with psychiatric (e.g., schizophrenia, major clinical depression) or substance abuse problems (e.g., nicotine, alcohol) were excluded because patients often deliberately decide to omit or cease medications. Contraceptive and sexual dysfunction medications were excluded because they are voluntary medications were patient decisions about consuming medications are expected. Although packaging interventions might be beneficial for these patients, the reasons for poor MA may differ significantly from the typical reasons for inadequate MA among persons with acute and chronic physical diseases. Nutraceuticals were excluded because they are food-focused instead of medication-focused.
Since only studies with adequate data to calculate an effect size (ES) were included, strategies to ensure adequate data were used. For reports without adequate data, author searches were completed to locate other reports about the same sample which might include the necessary information such as a measure of variability. Corresponding authors were contacted to secure ES data when such data were not provided in reports nor found in companion papers. Procedures that meta-analysts use for missing ESs are to exclude the study from the analysis, set the ES to 0 for studies reporting lack of statistically significant effect, estimate possible ESs from studies with sample size and direction of effect information, or estimate the ES magnitude derived from other studies with nonsignificant or significant findings. Using 0 may result in underestimating the ESs and distorting estimates of heterogeneity, if the treatment is effective but the primary study exhibited low statistical power. Imputing values from other studies requires assumptions that may not be justified. We excluded from the meta-analysis studies without sufficient ES information.
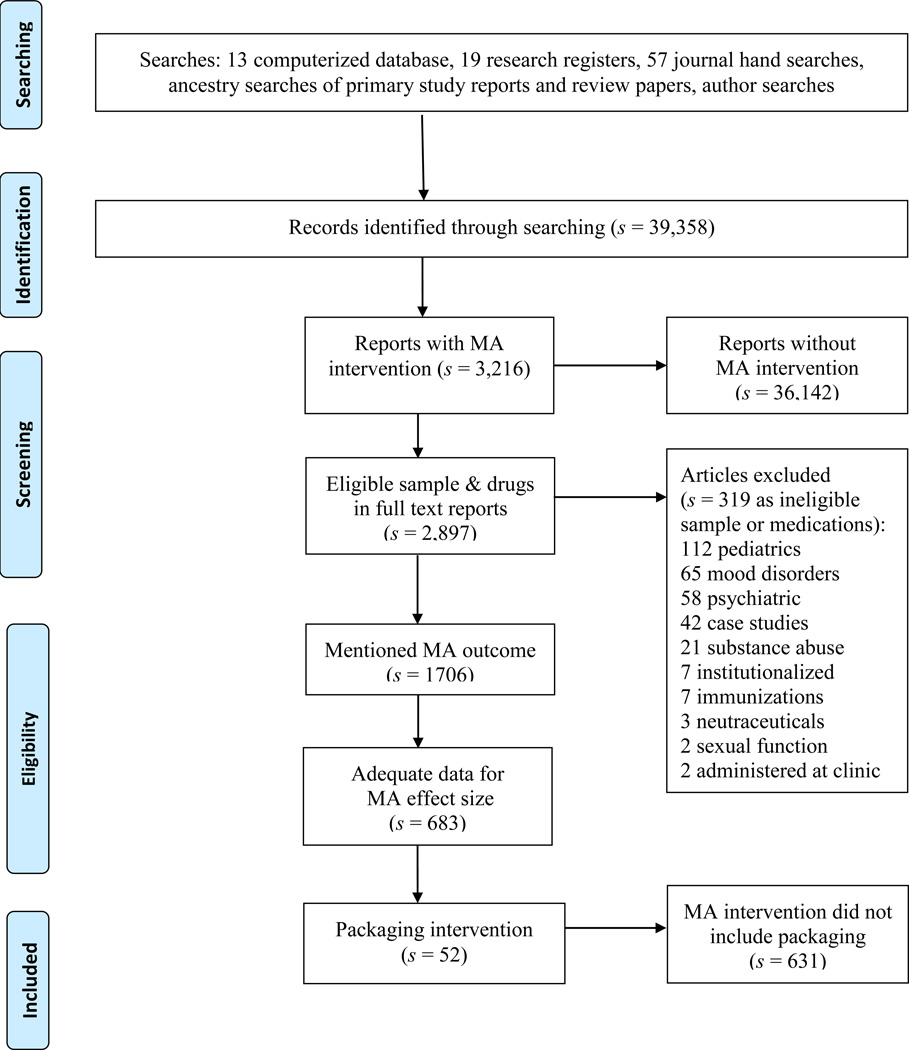
Both unpublished and published studies were included to reduce potential publication bias22, 23. Small-sample and pre-experimental studies were included19. Non-English studies were included if research specialists or investigators were fluent in that language. Studies distributed from 1960 until 2013 were eligible for inclusion. The flow of potential primary studies through the project is displayed in Figure 1.
Figure 1.
Flow diagram
Data Coding and Evaluation
A coding frame was developed from elements in previous related meta-analyses by this research team, suggestions from MA and meta-analysis experts, and a preview of 50 studies with diverse MA interventions. The coding frame includes source, participant, methodology, and intervention characteristics as well as MA outcome data. Extensive pilot testing was used to fine-tune the coding frame. The year of distribution, dissemination medium (e.g. journal article, dissertation), and presence of funding were recorded as source information. Participant characteristics included gender, age, ethnicity, chronic diseases, cognitive impairment, number of prescribed medications, and whether the subjects were selected because of poor MA.
Intervention characteristics coded included whether the intervention was a pill box or blister pack. For pill boxes, we coded whether the device was given to subjects or if subjects were told to obtain a pill box on their own. We also coded other packaging intervention details including cycle (i.e., duration in days that the current packaging lasts before subjects must obtain additional packages or refill the device) and the number of compartments. We recorded other intervention characteristics, such as information about MA intervention components in addition to the packaging, location of intervention delivery, and the professional background of the interventionist.
We coded a wide variety of aspects of how researchers conducted their studies. Of primary interest were MA data necessary for calculating effect sizes: baseline and outcome means, measures of variability, success rates, and sample sizes. If studies reported multiple MA outcome data, we preferentially selected the data from the most distal time point with the largest number of subjects using the most valid MA measure (e.g., coded pharmacy refill data when self-report data were also available). We noted the type of MA measure as an additional indicator of methodological quality in MA research. In addition, methodological features we coded included sample size, attrition rates, random vs. nonrandom assignment of participants to groups, allocation concealment, data collector masking, intention-to-treat analyses, and days between receiving the intervention and MA outcome measurement. Each attribute was analyzed as a potential moderator variable. This sensitivity analysis was used to determine if findings were robust to variations in methodological quality.
All data were independently coded by two extensively trained coders. Every variable was compared between coders to achieve 100% agreement24, 25. A doctorally-prepared coder further verified effect size data. To obtain sample independence, author lists on every study were cross checked with author lists of all other studies to identify and resolve any potentially overlapping samples. Senior authors were contacted when necessary to clarify the uniqueness of samples in their research. When multiple reports about the same sample were located, we kept these ancillary reports and used them to enhance the detail of coding.
Statistical Analysis
Analyses were conducted using Comprehensive Meta Analysis software. The main analyses in this project compared treatment and control groups after interventions. Supplementary analyses examined treatment group pre- versus post-intervention scores. A similar single-group analysis was conducted for control subjects. Unless otherwise stated, all analyses and results in the report address the treatment versus control post-intervention comparisons.
Data calculations were handled by meta-analytic standardized mean difference (d) ES26. For treatment versus control comparisons, a standardized mean difference is the difference between treatment group versus control group post-intervention means divided by the pooled standard deviation. For single group ES, the d represents the outcome scores minus the baseline scores divided by the baseline standard deviation. A positive d reflects more favorable outcomes for treatment groups or following interventions. The ESs were weighted by the inverse of variance to give larger sample studies more influence and adjust for bias27. To acknowledge that ESs vary both from subject-level sampling error and other sources of study-level error such as participant or method variations, random-effect models were used to calculate ESs26. ES confidence intervals were constructed. Homogeneity was assessed using a conventional heterogeneity statistic (Q) and computing the I2 index of heterogeneity beyond within-study sampling error26. Since clinical and statistical heterogeneity is common in behavior change research28, the expected heterogeneity was managed in four ways. Random-effects models were used for analyses because they take into account heterogeneity beyond that explained by moderator analyses. Potential heterogeneity was explored with moderator analyses. Heterogeneity was quantified, along with the location parameter. Finally, the interpretation of findings considered the context of discovered heterogeneity.
Potential outliers were detected by examining the externally standardized residuals of ESs. Potential publication bias was explored using funnel plots of ES against sampling variance26. Larger samples typically yield less sampling error in observed ESs. Observed ESs should be symmetrical around the overall average ES regardless of sample size in the absence of publication bias. Begg’s and Egger’s tests were used to assess publication bias.
We conducted exploratory moderator analyses to examine the association between study characteristics and ESs26. Continuous moderator analyses consisted of testing effects through an unstandardized regression slope, which is a meta-analytic analogue of regression. Dichotomous moderators were examined by testing effects of between-group heterogeneity statistics (Qbetween), which is a meta-analytic analogue of ANOVA.
Results
We identified 52 eligible primary study reports with a total of 22,858 subjects29–80. Eight additional articles reported on the same studies and were used as companion papers for additional coding information81–88. One Spanish language study was included58. One study was included by using ESs data obtained directly from the author because the published article lacked sufficient ES data47. These reports yielded ES data for 51 comparisons for treatment vs. control at outcome, 19 treatment pre- vs. post-intervention, and 7 control baseline vs. outcome comparisons.
Primary Study Characteristics
Most comparisons were disseminated as journal articles (k=50); two dissertation comparisons were included (s=number of reports, k=number of comparisons). The numbers of studies that have examined packaging interventions have increased in recent years. Nine reports were disseminated before 1990, and 31 were disseminated in 2000 or after. Table 1 shows descriptive statistics across the all primary studies. Most studies (k=32) received funding. The median of mean sample size was 104.5 subjects. Attrition was modest and similar between treatment (median=3.45%) and control (2.74%) groups. The mean length of follow-up was 12 weeks, with a range from 1 to 52 weeks. The median value for mean age was 54.4 years. Among the studies that reported gender distribution (s=33), almost half the subjects were women. Ethnicity was very poorly reported; only four comparisons provided this information. Among the seven studies that reported the mean number of medications prescribed to subjects, the median of mean value was 5.94 medications. Length of follow-up was poorly reported, it ranged from one week to one year.
Table 1.
Characteristics of Primary Studies Included in Medication Adherence Meta-analyses
| Characteristic | s | Min | Q1 | Mdn | Q3 | Max |
|---|---|---|---|---|---|---|
| Mean age (years) | 31 | 26.8 | 43.5 | 54.4 | 69.85 | 85 |
| Total post-test sample size per study | 48 | 16 | 53 | 104.50 | 183.75 | 12969 |
| Percentage attrition treatment group | 32 | 0 | 0 | 3.45 | 20.10 | 81.36 |
| Percentage attrition control group | 32 | 0 | 0 | 2.74 | 24.31 | 68 |
| Percentage female | 36 | 0 | 27.28 | 43.5 | 64 | 92 |
| Percentage ethnic minority | 4 | 4.6 | 24.4 | 59.55 | 89.2 | 92.5 |
| Mean number of prescribed medications | 7 | 2.04 | 4.45 | 5.94 | 6.00 | 8.09 |
Note. Includes all studies that contributed to primary analyses at least one effect size for any type of comparison.
s=number of reports providing data on characteristic; Q1=first quartile, Q3=third quartile.
Tables 2 and 3 contain information about individual treatment vs. control comparisons which were included in the meta-analysis. Among the two-group comparisons, 28 were conducted in North America, 9 in Europe, 5 in Asia, 4 in Africa, and 2 in Australia. No studies conducted in South America were retrieved. Eleven studies included samples with diverse chronic diseases. Twenty studies focused on infectious diseases, including eight studies with HIV subjects. Six of the nine studies focused on cardiovascular populations recruited samples with hypertension.
Table 2.
Characteristics and Quality Indicators of Pill Box Intervention Primary Studies.
| Study & location | Sample | Random assignment |
Allocation concealed |
Bundled intervention |
Behavior target |
Masking | Attrition | Intention to treat |
Adherence measure |
|---|---|---|---|---|---|---|---|---|---|
| Bosworth et al. (2008) North America | N = 636 hypertension | yes | NR | yes | MA + | NR | 0% | yes | self-report |
| Burrelle et al. (1987) North America | N = 16 hypertension | yes | NR | yes | MA | NR | 0% | no | pill counts |
| Calvert et al. (2012) North America | N = 143 cardiac diseases | yes | yes | yes | MA | yes | 27% | no | pharmacy refills |
| Goujard et al. (2003) Europe | N = 367 HIV | no | NR | yes | MA | NR | 35% | no | self-report |
| Henry et al. (1999) Australia | N = 119 infections | yes | NR | yes | MA | NR | 2% | yes | combined measures |
| Holzemer et al. (2006) North America | N = 240 HIV | yes | NR | yes | MA + | no | 25% | no | combined measures |
| Kalichman et al. (2011) North America | N = 40 HIV | yes | yes | yes | MA | yes | 3% | yes | self-report |
| Kennedy (1990) North America | N = 65 chronic diseases | yes | NR | yes | MA | yes | 34% | no | pill counts |
| Kripalani et al. (2012) North America | N = 862 chronic diseases | yes | no | yes | MA | yes | 25% | yes | self-report |
| Laramee et al. (2003) North America | N = 287 heart failure | yes | NR | yes | MA + | NR | 20% | no | self-report |
| Lee et al. (1999) North America | N = 125 infections | yes | yes | yes | MA | NR | 0% | yes | pill counts |
| Levensky (2006) North America | N = 54 HIV | yes | NR | yes | MA + | NR | 2% | yes | pill counts |
| MacDonald et al. (1977) Europe | N = 74 chronic diseases | no | NR | yes | MA | NR | 0% | no | combined measures |
| MacIntosh et al. (2007) North America | N = 25 cancer | yes | NR | no | MA | NR | 4% | no | pill counts |
| McPherson-Baker et al. (2000) North America | N = 42 HIV | no | NR | yes | MA | NR | 0% | no | pharmacy refills |
| Moshkovska et al. (2011) Europe | N = 84 gastrointestinal | yes | yes | yes | MA | NR | 0% | yes | drug level |
| Nazareth et al. (2001) Europe | N = 362 chronic diseases | yes | NR | yes | MA | yes | NR | no | self-report |
| Park et al. (1992) North America | N = 31 chronic diseases | yes | NR | no | MA | NR | 0% | no | combined measures |
| Park et al. (1992) North America | N = 31 chronic diseases | yes | NR | no | MA | NR | 0% | no | combined measures |
| Peterson et al. (1984) Australia | N = 27 epilepsy | yes | NR | yes | MA | NR | NR | no | drug level |
| Saafren et al. (2001) North America | N = 56 HIV | no | NR | yes | MA | NR | 5% | no | self-report |
| Suarez-Varela et al. (2009) Europe | N = 182 chronic diseases | yes | NR | no | MA | NR | 0% | no | self-report |
| Sweeney et al. (1989) Europe | N = 103 chronic diseases | no | no | yes | MA | no | 34% | no | pill counts |
| Taylor et al. (2003) North America | N = 81 chronic diseases | yes | NR | yes | MA | NR | 15% | no | self-report |
| Traiger et al. (1997) North America | N = 41 organ transplant | no | NR | yes | MA | NR | 12% | no | self-report |
| Tsuyuki et al (2004) North America | N = 276 heart failure | yes | NR | yes | MA + | NR | 0% | yes | pharmacy refills |
| Wang et al. (2010) Asia | N = 116 HIV | yes | NR | yes | MA + | NR | 16% | no | self-report |
| Zillich et al. (2005) North America | N = 125 hypertension | no | NR | yes | MA + | NR | 6% | no | self-report |
NR: not reported
Some reports contained multiple comparisons of different treatment groups compared to control groups.
Bundled interventions include packaging plus other medication adherence enhancing interventions.
Behavioral target: MA studies focused exclusively on MA. MA + studies targeted MA and other health behaviors such as diet, exercise, etc.
Masking refers to masking of data collectors regarding group assignment.
Combined MA measure indicates primary studies that reported MA outcomes from combined measures. Conn et al. did not combine measures.
Table 3.
Characteristics and Quality Indicators of Blister Pack Intervention Primary Studies included in Meta-Analysis.
| Study & location | Sample | Random assignment |
Allocation concealed |
Bundled intervention |
Behavior target |
Masking | Attrition | Intention to treat |
Adherence measure |
|---|---|---|---|---|---|---|---|---|---|
| Awofeso et al. (1995) Africa | N = 294 infections | no | NR | yes | MA | NR | 0% | no | drug level |
| Becker et al. (1986) North America | N = 171 hypertension | yes | NR | no | MA | NR | 8% | no | pill counts |
| Crome et al. (1982) Europe | N = 78 chronic diseases | yes | NR | no | MA | NR | NR | no | pill counts |
| Eshelman et al (1976) North America | N = 100 hypertension | yes | NR | no | MA | NR | 35% | no | drug level |
| Hirsch et al. (2011) North America | N = 2234 HIV | no | NR | yes | MA | NR | 0% | no | pharmacy refills |
| Linkewich et al. (1974) North America | N = 46 infections | yes | NR | yes | MA | NR | NR | no | pill counts |
| Linkewich et al. (1974) North America | N = 51 infections | yes | NR | yes | MA | NR | NR | no | pill counts |
| Qingjun et al. (1998) Asia | N = 342 malaria | yes | NR | no | MA | NR | 0% | no | self-report |
| Qingjun et al. (1998) Asia | N = 59 malaria | yes | NR | no | MA | NR | 0% | no | tracers |
| Qingjun et al. (1998) Asia | N = 65 malaria | yes | NR | no | MA | NR | 0% | no | tracers |
| Revankar et al. (1993) Asia | N = 189 infections | no | NR | no | MA | NR | 0% | no | drug level |
| Skaer et al. (1993) North America | N = 163 hypertension | yes | NR | yes | MA | NR | 0% | no | pharmacy refills |
| Skaer et al. (1993) North America | N = 131 type 2 diabetes | yes | NR | yes | MA | NR | 0% | no | pharmacy refills |
| Skaer et al. (1993) North America | N = 64 hypertension | yes | NR | yes | MA | NR | 0% | no | pharmacy refills |
| Spriet et al. (1980) Europe | N = 842 neurological | yes | yes | no | MA | NR | 0% | no | pill counts |
| Spriet et al. (1980) Europe | N = 833 neurological | yes | yes | yes | MA | NR | 0% | no | pill counts |
| Wright et al. (1999) Africa | N = 143 STD | no | no | no | MA + | no | NR | no | pill counts |
| Wright et al. (1999) Africa | N = 162 STD | no | no | no | MA + | no | NR | no | pill counts |
| Wright et al. (1999) Africa | N = 142 STD | no | no | no | MA + | no | NR | no | pill counts |
| Zillich et al. (2012) North America | N = 12969 chronic diseases | no | NR | yes | MA | NR | 0% | yes | pharmacy refills |
NR: not reported
Some reports contained multiple comparisons of different treatment groups compared to control groups.
Bundled interventions include packaging plus other medication adherence enhancing interventions.
Behavioral target: MA studies focused exclusively on MA. MA + studies targeted MA and other health behaviors such as diet, exercise, etc.
Masking refers to masking of data collectors regarding group assignment.
Most interventions targeted MA behavior exclusively, ten interventions focused on multiple health behaviors. Packaging interventions were combined with other MA intervention components in 33 comparisons.
Risk of bias was poorly reported in many primary studies. For example, 36 comparisons did not report whether allocation was concealed. Data collector masking is a common risk of bias measure which could be difficult to implement in this research, 38 studies did not report masking data collectors. Most studies randomly assigned subjects to treatment and control conditions, 14 did not.
Overall Effects of Packaging Interventions on Medication Adherence Outcomes
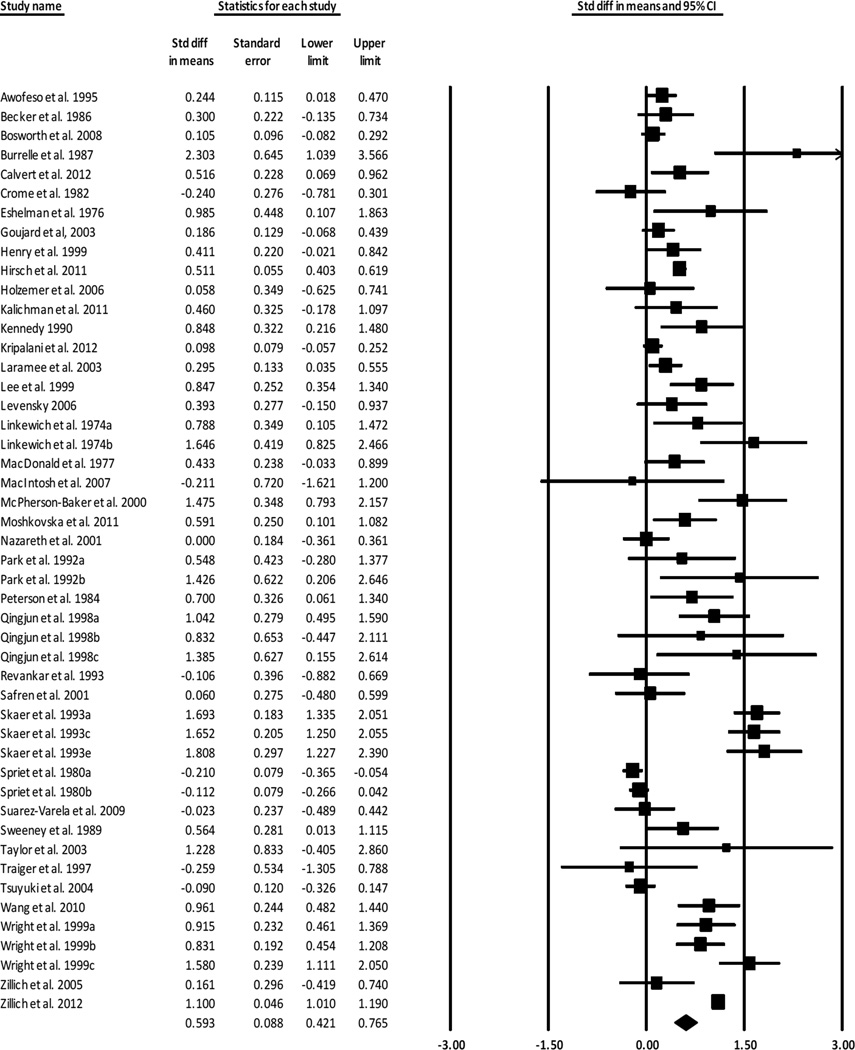
Overall MA ESs are presented in Table 4. We calculated ESs for 48 treatment-vs.-control-group outcome comparisons of 21,944 subjects. The overall standardized mean difference ES was 0.593. For two-group comparisons, three ESs were excluded as outliers (the ES with outliers included was 0.757). The positive ES documents that treatment subjects had significantly better MA outcomes than were reported for control subjects. The 0.593 ES is consistent with the finding of 71% adherence rate among treatment subjects compared to 63% adherence rate among control subjects. The forest plot in Figure 2 includes ES for individual studies which compared treatment and control groups.
Table 4.
Random-Effects Medication Adherence and Health Outcome Estimates and Tests
| k | Effect size |
p (ES) |
95% Confidence interval |
Standard error |
I2 | Q | p (Q) | |
|---|---|---|---|---|---|---|---|---|
| Medication Adherence Outcomes | ||||||||
| Treatment vs. control all studiesa | 48 | 0.593 | <.001 | 0.421, 0.765 | .088 | 91.940 | 583.090 | <.001 |
| Treatment vs. control continuous data studies | 18 | 1.160 | <.001 | 0.699, 1.621 | .235 | 96.186 | 445.729 | <.001 |
| Treatment vs. control dichotomous data studies | 33 | 0.535 | <.001 | 0.327, 0.742 | .106 | 92.052 | 402.636 | <.001 |
| Treatment subjects pre- vs. post-comparisons | 19 | 0.540 | <.001 | 0.374, 0.705 | .084 | 79.338 | 87.117 | <.001 |
| Control subjects pre- vs. post-comparisons | 7 | 0.002 | .995 | −0.181, 0.184 | .093 | 49.981 | 11.995 | .062 |
| Health Outcomesb | ||||||||
| Quality of life | 5 | 0.226 | .168 | −0.112, 0.645 | .193 | 80.502 | 20.515 | <.001 |
| Diastolic blood pressure | 5 | 0.318 | .159 | −0.125, 0.762 | .226 | 86.951 | 30.653 | <.001 |
| Systolic blood pressure | 4 | 0.416 | .092 | −0.068, 0.900 | .247 | 86.335 | 21.954 | <.001 |
| Knowledge | 3 | 0.456 | .082 | −0.058, 0.970 | .262 | 72.843 | 7.364 | .025 |
| Mood | 2 | 0.591 | .011 | 0.135, 1.047 | .233 | 46.093 | 1.855 | .173 |
| HIV viral load | 2 | 0.102 | .476 | −0.178, 0.381 | .143 | 15.921 | 1.189 | .275 |
k denotes number of comparisons, Q is a conventional homogeneity statistic, I2 is the percentage of total variation among studies’ observed ES due to heterogeneity.
Three comparisons were excluded as outliers. The overall effect size with inclusion of outliers was .757 (SE = .099, CI: .563, .952).
Health outcomes were calculated for treatment vs. control comparisons.
Figure 2.
Forest plot for treatment vs. control comparisons
Subgroup analyses were conducted for primary studies that reported continuous outcome data and those that reported dichotomous outcome data16. The overall ES for continuous data was 1.160. The overall ES for dichotomous data studies was significantly smaller at 0.535.
We calculated ESs for 19 treatment group pre-post comparisons of 1,757 subjects and for 7 control pre-post comparisons with 844 subjects. No outliers were found for treatment or control group pre-post comparisons. For treatment baseline vs. outcome comparisons, the overall ES was 0.540. In contrast to treatment subjects, control group subjects did not have improved MA outcomes from participating in studies, the overall ES was 0.002, which was not significantly different from zero.
Treatment vs. control and treatment pre- vs. post-intervention comparisons were significantly heterogeneous (based on Q statistics) with I2 from 79 to 92. The funnel plots of ES vs. sampling variance suggested possible evidence of publication bias among treatment vs. control group comparisons which was confirmed with Begg’s test (p = .021) but not by the Egger’s test (p = .324). The funnel plot for treatment group pre-post comparisons displayed evidence of publication bias which was confirmed by the Begg’s test (p = .010) but not by the Egger’s test (p = .235). No publication bias was evident for the control group pre-post comparisons as confirmed by both the Begg’s (p = .368) and Egger’s (p = .529) tests. (Funnel plots are available from the corresponding author.)
Moderator Analyses
Tables 5 and 6 display dichotomous and continuous moderator analyses. Many additional potential moderators could not be analyzed because they occurred too infrequently or were poorly reported (e.g., ethnicity). Moderator analyses are exploratory and should be interpreted with caution given the small number of studies in some analyses.
Table 5.
Dichotomous Moderator Results for Medication Adherence: Treatment vs. Control at Outcome
| Moderator | k | Effect size | Standard error |
Qbetween |
p (Qbetween) |
|---|---|---|---|---|---|
| Report Moderators | |||||
| Publication status | 0.000 | .996 | |||
| Published articles | 46 | 0.592 | 0.090 | ||
| Dissertations | 2 | 0.591 | 0.225 | ||
| Location of primary research | 2.493 | .114 | |||
| Europe, Asia, Africa, Australia | 20 | 0.444 | 0.113 | ||
| North America | 28 | 0.699 | 0.115 | ||
| Presence of funding for research | 2.024 | .155 | |||
| Funded | 32 | 0.655 | 0.101 | ||
| Unfunded | 16 | 0.420 | 0.131 | ||
| Source of funding for research | 1.672 | .196 | |||
| Funding from for-profit source | 8 | 0.948 | 0.257 | ||
| Funding from not-for-profit source | 22 | 0.583 | 0.117 | ||
| Research Methods Moderators | |||||
| Allocation to treatment groups | 0.003 | .958 | |||
| Randomization of individual subjects | 34 | 0.595 | 0.102 | ||
| Subjects not individually randomized | 14 | 0.586 | 0.132 | ||
| Allocation concealment | 3.807 | .051 | |||
| Allocation concealed | 6 | 0.276 | 0.161 | ||
| Did not report allocation concealed | 42 | 0.636 | 0.090 | ||
| Data collector masking | 4.088 | .043 | |||
| Data collectors masked to group assignment | 5 | 0.289 | 0.136 | ||
| Did not report data collectors masked to group assignment | 43 | 0.625 | 0.096 | ||
| Intention-to-treat approach | 0.804 | .370 | |||
| Reported intention-to-treat approach | 9 | 0.429 | 0.210 | ||
| Did not report intention-to-treat approach | 39 | 0.636 | 0.097 | ||
| Outcome data | |||||
| Continuous outcome data in primary report | 18 | 1.160 | 0.235 | ||
| Dichotomous outcome data in primary report | 33 | 0.535 | 0.106 | ||
| Medication adherence measure: pharmacy refill | 7.522 | .006 | |||
| Pharmacy refill data | 8 | 1.044 | 0.201 | ||
| Study did not use pharmacy refill data | 40 | 0.455 | 0.075 | ||
| Medication adherence measure: pill count | 0.069 | .792 | |||
| Pill count data | 15 | 0.628 | 0.167 | ||
| Study did not use pill counts to measure medication adherence | 33 | 0.577 | 0.100 | ||
| Medication adherence measure: drug metabolite | 1.167 | .280 | |||
| Drug metabolite data | 5 | 0.418 | 0.149 | ||
| Study did not use drug metabolite data | 43 | 0.609 | 0.095 | ||
| Medication adherence measure: self-report | 11.692 | .001 | |||
| Self-report data | 12 | 0.247 | 0.080 | ||
| Study did not use self-report to measure medication adherence | 36 | 0.715 | 0.111 | ||
| Sample Characteristic Moderators | |||||
| Sample socio-economic status | 0.388 | .533 | |||
| Reported low socio-economic status | 6 | 0.737 | 0.245 | ||
| Did not report low socio-economic status | 42 | 0.574 | 0.093 | ||
| Sample with cognitive impairment | 15.682 | <.001 | |||
| Reported subjects had cognitive impairment | 5 | 0.074 | 0.115 | ||
| Did not report cognitively impaired subjects | 43 | 0.649 | 0.088 | ||
| Sample selected for poor medication adherence | 0.719 | 0.396 | |||
| Reported sample selected for poor medication adherence | 6 | 0.835 | 0.301 | ||
| Did not report targeting subjects with poor medication adherence | 42 | 0.568 | 0.093 | ||
| Intervention Feature Moderators | |||||
| Pill boxes vs. blister packs | 6.255 | .012 | |||
| Pill boxes | 28 | 0.384 | 0.072 | ||
| Blister packs | 20 | 0.802 | 0.151 | ||
| Packaging recommended to patients vs. given to patients | 0.300 | .584 | |||
| Recommended to patients | 6 | 0.379 | 0.153 | ||
| Given to patients | 17 | 0.483 | 0.112 | ||
| Intervention exclusively packaging vs. other interventions | 0.019 | .890 | |||
| Medication intervention exclusively packaging | 15 | 0.573 | 0.192 | ||
| Intervention included packaging and other strategies | 33 | 0.602 | 0.101 | ||
| Interventionist: physician | 5.992 | .014 | |||
| Physician interventionist | 9 | 0.269 | 0.121 | ||
| Study did not report physician interventionist | 39 | 0.641 | 0.093 | ||
| Interventionist: pharmacist | 3.126 | .077 | |||
| Pharmacist interventionist | 18 | 0.782 | 0.146 | ||
| Study did not report pharmacist interventionist | 30 | 0.475 | 0.095 | ||
| Interventionist: nurse | 4.734 | .030 | |||
| Nurse interventionist | 10 | 0.295 | 0.135 | ||
| Study did not report nurse interventionist | 38 | 0.661 | 0.101 | ||
| Intervention location: inpatient | 13.930 | <.001 | |||
| Intervention delivered while subjects was an inpatient | 10 | 0.194 | 0.089 | ||
| Study did not report inpatient location | 38 | 0.704 | 0.104 | ||
| Intervention location: ambulatory care clinic | 6.838 | .009 | |||
| Intervention delivered in ambulatory care clinic | 19 | 0.334 | 0.095 | ||
| Study did not report clinic as location for intervention | 29 | 0.710 | 0.108 | ||
| Intervention location: pharmacy | 4.326 | 0.038 | |||
| Intervention delivered in pharmacy | 11 | 0.945 | 0.103 | ||
| Study did not report intervention delivered in pharmacy | 37 | 0.485 | 0.196 | ||
k denotes number of comparisons, effect size is standardized mean difference, Q is a conventional homogeneity statistic.
Table 6.
Continuous Moderator Results for Medication Adherence: Treatment vs. Control at Outcome
| Moderator | k | Slope | Standard Error |
Tau2 | Qmodel | p (slope) |
|---|---|---|---|---|---|---|
| Report Moderator | ||||||
| Year of publication | 48 | 0.018 | 0.002 | .253 | 100.976 | <.001 |
| Method Moderators | ||||||
| Sample size | 48 | <.001 | 0.000 | .209 | 217.352 | <.001 |
| Attrition proportion | 32 | −0.795 | 0.202 | .237 | 15.466 | <.001 |
| Days between intervention completion and outcome measurement | 24 | 0.004 | 0.000 | .276 | 99.876 | <.001 |
| Sample Attribute Moderators | ||||||
| Age | 31 | −0.022 | 0.002 | .207 | 90.021 | <.001 |
| Percent women | 36 | 0.006 | 0.001 | .263 | 21.015 | <.001 |
| Intervention Feature Moderator | ||||||
| Cycle (days when subjects must take action to refill/receive packaging) | 28 | −.0.006 | 0.003 | .518 | 5.985 | .014 |
k denotes number of comparisons, Q is a conventional homogeneity statistic, Tau2 is the between-study variance.
Intervention Moderators
Studies that used blister packs reported significantly larger ESs (0.802) than studies that used pill boxes (0.384). There was no difference in ESs between studies that gave pill boxes to subjects and studies where interventionists merely recommended that subjects acquire a pill box. Medication refill cycle was recorded as the number of days before participants would be required to refill pill boxes or obtain new blister packs. Studies with longer cycles reported slightly lower MA ES than studies with shorter cycles (β̂1 = −0.006).
Packaging was the sole intervention in 15 studies while other researchers (k = 33) combined packaging with other MA interventions. The ESs did not differ between trials with exclusively packaging interventions and studies with packaging as one component of multiple MA interventions. None of the studies combined packaging with telemedicine interventions.
ESs were significantly smaller for studies with physician intervention delivery (0.269) as compared to interventions not delivered by physicians (0.641). The same pattern was present for nurse delivered interventions; studies with nurse interventionists had significantly smaller ESs (0.295) than studies with interventions not delivered by nurses (0.661). While the trend for interventions to be more effective when delivered by pharmacists (0.782) as compared to interventions without pharmacists (0.475) did not achieve statistical significance, interventions delivered in pharmacies reported significantly larger ESs (0.945) than interventions administered elsewhere (0.485). Interventions were less effective when delivered while patients were hospitalized (0.194) than when not delivered in an inpatient setting (0.704). ESs were also smaller for interventions delivered in ambulatory care settings (0.334) than for interventions delivered elsewhere such as subjects’ homes or pharmacies (0.710).
Report and Sample Moderators
The ESs did not differ between published and unpublished studies. Studies completed more recently reported slightly larger ESs than studies distributed earlier (β̂1 =0.018). The ESs did not differ between studies conducted in North America and studies conducted in Asia, Australia, Africa or Europe. Neither the presence of funding for the research nor the source of funding (for-profit vs. not-for-profit) was a significant moderator.
Studies with younger subjects reported larger ESs than studies with older samples (β̂1 =−0.022). The reported socio-economic status of participants was unrelated to ESs. Studies with more female subjects reported slightly larger ESs than studies with fewer female participants (β̂1 = 0.006). Interventions were much less effective in samples with cognitive impairment (0.074) as compared to samples without reported cognitive impairment (0.649). The ES difference between samples recruited because of medication nonadherence (0.835) and studies that did not target nonadherent subjects (0.568) was not statistically significant. The number of chronic illnesses and prescribed medications were too infrequently reported for moderator analyses.
Potential Sources of Bias: Design and Methods Moderators
Studies with larger sample sizes reported slightly larger ESs than studies with smaller samples. Allocation of subjects to treatment groups, individually randomized vs. some other allocation, was not related to ESs. The difference between ESs of studies with allocation concealment (0.276) and studies without concealment (0.636) did not achieve statistical significance. Studies with masked data collectors reported significantly smaller ESs (0.289) than studies that did not report masking (0.625). There was no difference in ESs between studies that reported intention-to-treat analyses and those that did not report such analyses.
Studies with lower attrition rates reported significantly higher MA ESs (β̂1 = −0.795). Studies with longer follow-up, days between completion of the intervention and MA outcome measurement, reported slightly higher MA ES (β̂1 = 0.004).
Primary studies reported either continuous data (e.g., means and measures of variability) or dichotomous data such as success rates. Studies that reported continuous data outcomes had significantly larger ESs (1.160) than studies that reported dichotomous outcomes (0.535). The largest ESs were reported among studies that measured MA with pharmacy refills (1.044) as compared to studies with pill counts (0.628), drug metabolites (0.418), and self-report (0.247). No studies used electronic monitoring to assess MA.
Overall Effects of Packaging Interventions on Health Outcomes
Health outcomes findings should be considered exploratory and interpreted with caution given the small number of comparisons for each health outcome (see Table 4). ESs ranged from 0.102 to 0.591: quality of life (ES=0.226), diastolic blood pressure (ES=0.318), systolic blood pressure (ES=0.416), knowledge (ES=0.456), mood (ES=0.591), and HIV viral load (ES=0.102). ESs were significantly heterogeneous for quality of life and both systolic and diastolic blood pressure.
Discussion
The completed meta-analyses of 48 comparisons between treatment groups receiving packaging interventions and control groups without packaging interventions provided valuable new information not available in the previous meta-analyses of two to six primary studies15, 16,18. The moderate effect sizes that we found document that packaging interventions significantly improve MA.
There are several reasons packaging interventions may be effective at producing good MA. Packaging interventions provide a mechanism for patients to self-monitor medication consumption. Difficulty remembering whether a certain dose had been consumed may be an important aspect of forgetting medications: the most often patient-reported reason for nonadherence14, 16. Packaging interventions also allow third parties, such as informal and home-visiting formal caregivers, to monitor dose removal from the device12.
Packaging interventions may be especially effective for medications that should be consumed at different times of day16, because patients do not need to make decisions about which medications to consume at different times. The number of prescribed medications has been positively linked to lack of MA16, and packaging interventions may be useful for this particular issue, because patients do not need to open multiple containers for each administration. Unfortunately, primary studies rarely reported the number of prescribed medications, so no moderator analyses could be conducted on this possibly relevant variable. Future research should examine possible interactions between the number of medications and effectiveness of packaging interventions.
Most MA interventions, such as pharmacist counseling, are time limited16. Pill boxes are a more persistent intervention than programs that are designed to last a discrete period of time17. The moderator analyses of this study documented improved MA over time using packaging interventions. This contrasts with MA behavior following most MA intervention with a reveal a pattern of diminished MA over time. Since persisting MA is important to achieve positive health outcomes, this is an important benefit of packaging interventions. Future research should continue follow-up months or years after interventions to determine long-term benefits from packaging interventions.
Another benefit of pill boxes is that they do not require much health care provider labor, unless they are filled by providers during home or clinic visits. In contrast, blister packs require pharmacist effort17. The low cost of pill box interventions make them especially attractive for widespread use.
Packaging interventions have limitations. Packaging interventions can be useful for non-intentional nonadherence, but not for intentional nonadherence12, 16. Some packaging may not be child resistant17. A further limitation is that pill boxes and blister packs do not provide feedback to tell patients the time when previous doses were consumed. Packaging interventions may be less useful when patients make voluntary decisions about consuming medications, such as for some psychiatric and substance abuse medications.
The exploratory moderator analyses showed that blister pack interventions were significantly more effective than pill boxes. Because blister packs are prepared by pharmacists, they are more likely to contain the appropriate medications than pill boxes, which are often filled by patients or caregivers. We noted that the observed pattern of interventions being the most effective when delivered in pharmacies (as compared to in-patient or ambulatory care settings) by pharmacists (as compared to physicians and nurses) was not entirely due to pharmacists preparing blister packs; 12 of the comparisons with pharmacist interventionists did not involve blister packs and 8 of the pharmacist-delivered interventions were not located in pharmacies.
Although blister packs are more expensive than pill boxes, because they require pharmacist activity and special technology, the gains in MA may make such expenditure reasonable in light of reducing health care costs arising from disease complications. Unfortunately, none of the packaging primary studies provide data about cost-effectiveness. This is an important limitation in existing primary research. It is crucial that future research examine the cost-benefit of using these interventions. Without such cost-benefit information, policy changes will be difficult to secure.
The blister pack interventions included in this meta-analysis involved medications dispensed by pharmacists in blister packs, rather than medications sold in blister packs. Regulations vary by country regarding the approvals needed for pharmaceutical manufacturers to utilize blister packs, as opposed to other forms of medication packaging. In the U.S., manufacturers must have packaging methods approved by the Food and Drug Administration as part of new drug applications, or as an equivalent change to approved packaging methods89, 90. The European Union has guidelines for plastic packaging; blister packs are regulated separately by each country91. In the U.S., repackaged blister packs are used almost exclusively in long-term care settings, while in other countries such practices are more common.
We found two surprising results analyzing pill box interventions. Pill box interventions in which pill boxes were just suggested to the patient were as effective as interventions that actually provided them to patients. Other studies found that patients are receptive to using pill boxes as descriptive research has documented that 35% to 77% of surveyed adults use pill boxes47, 92,93. Also, MA interventions that exclusively used packaging interventions were as effective as interventions that combined packaging with other MA interventions. The effectiveness and very low cost of recommending pill boxes to patients are sufficient rationale for health care providers to incorporate this minute step into their treatment programs.
We did find circumstances when packaging interventions were not effective. Packaging interventions did not help MA in in primary research studies among patients with documented cognitive impairments as much as in studies that reported samples without cognitive limitations. Perhaps packaging interventions do not provide stimulus to take medications for cognitively impaired adults. Cognitive impairment could also affect accuracy in filling pill boxes. Older subjects also benefited less from packaging interventions than younger subjects. One possible explanation for this finding could be the increased number of medications among older adults and the additional burden that a heavy medication load imposes on MA. Unfortunately, too few studies reported the numbers of medications to explore this possibility through moderator analyses. It is also possible that opening blister packs may be an obstacle among older subjects with greater dexterity problems.
Common methodological weaknesses in primary research on packaging interventions include the infrequent application of steps such as random allocation to groups, concealed allocation, masked data collectors, and intention-to-treat analyses. Poor reporting, such as baseline MA values, prevented analyses controlling for baseline values or determining if baseline MA differed between pill boxes and blister packs. The moderator analyses revealed some lower ESs among studies with stronger methodological features. MA outcome measurement using self-report is a significant methodological weakness associated with significantly lower ES outcomes, leading us to think that intervention effectiveness may be masked by imprecise measurement of MA. Overall, the largest ESs among these primary studies was for research using pharmacy refill data to assess MA. Because this study focused on packaging interventions, electronic medication cap monitoring device data were not available for measuring MA94. In the future, new packaging technology, such as devices that accept blister packs, use an audible cue for dose administration, record administration, and display when previous pills were administered, will provide alternative MA interventions and measures95.
MA is not a unitary construct. Aspects of MA, such as initiation, implementation, and persistence, may be influenced by different MA adherence interventions. Lack of conceptual clarity may have contributed to the scant primary research which has evaluated different aspects of MA. The primary studies in this project examined implementation as the proportion of prescribed drugs which were consumed. As future primary research examines different dimensions of MA, meta-analyses may find variations in effectiveness for initiation, implementation, and persistence.
MA outcomes reported as a dichotomous variable (i.e., success rates of treatment and control groups) is another significant weakness in the MA primary research. In studies that reported dichotomous outcomes, continuous data about MA behavior were recorded and researchers categorized individual subjects as adherent or non-adherent. Significant information about the size of the effect is lost when these continuous data are transformed to dichotomous data. Furthermore, a criterion value for acceptable levels of MA has not been established for most medications, so establishing a cut-off point for success is somewhat arbitrary. Moderator analyses confirmed a larger ES for studies that reported continuous data as compared to those that reported dichotomous data. Future primary research should include continuous data MA outcomes.
This meta-analysis encountered a few factors that could have limited the robustness of the results. We were unable to assess potentially interesting variables that were poorly reported, such as the numbers of medications and chronic illnesses. Another limitation of the project was the dearth of primary studies with health outcomes. Although all of the present health outcomes had overall positive ESs, the scant amount of primary study data limits confidence in these findings. Additional reporting of intermediate and clinical health outcomes in MA research would be very valuable14. Also, although extensive searching was completed, it is possible the investigators missed some potentially eligible studies. This study used a specific operational definition of packaging interventions consistent with extant research. Other aspects of interventions related to packaging, such as labeling, were not examined.
This meta-analysis is the most comprehensive quantitative synthesis of packaging interventions to improve MA to date. Interventions were moderately effective across most populations. Blister packs were more effective than pill boxes, although pill boxes remain an attractive intervention due to low cost. Future research should include pharmacy refill or other objective measures of MA over self-report data. Furthermore, studies should report outcomes as continuous data instead of converting continuous data to dichotomous outcomes. Finally, we recommend that more MA studies report health and health care cost outcomes to fully evaluate the importance of MA interventions.
Acknowledgement
None
Funding: The project described was supported by Award Number R01NR011990 (Conn-PI) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors have no financial support or personal connection which could be perceived to bias their work.
Footnotes
Previous presentations: None
Declaration of financial/other relationships: The authors report no conflicts of interest.
Contributor Information
Vicki S. Conn, University of Missouri
Todd M. Ruppar, University of Missouri
Keith C. Chan, University of Missouri
Jacqueline Dunbar-Jacob, University of Pittsburgh.
Ginette A. Pepper, University of Utah
Sabina De Geest, University of Basel.
References
- 1.World Health Organization. Adherence to long-term therapies: evidence for action. Vol. 2003 Geneva, Switzerland: 2003. [Google Scholar]
- 2.Chaudhry HJ, McDermott B. Recognizing and improving patient nonadherence to statin therapy. Curr Atheroscler Rep. 2008 Feb;10:19–24. doi: 10.1007/s11883-008-0004-4. [DOI] [PubMed] [Google Scholar]
- 3.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002 Sep;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Starner T. The price of noncompliance: Hospitalization rates and other medical costs go down when employees take their medication regularly. Human Resources Executive Online. 2006 [Google Scholar]
- 5.European Policymakers Debate. Summary of policymakers' debate - Just what the doctor ordered: an EU response to medication non-adherence. Brussels, Belgium: 2010. [Google Scholar]
- 6.Bosworth HB. Medication treatment adherence. Patient treatment adherence: Concepts, intervention, and measurement. 2006:147–94. [Google Scholar]
- 7.McDonald HP, Garg AX, Haynes R. Interventions to enhance patient adherence to medication prescriptions: Scientific review. JAMA. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 8.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Christensen AJ. Patient adherence ot medical treatment regimens. New Haven: Yale University Press; 2004. [Google Scholar]
- 10.Frishman WH. Importance of medication adherence in cardiovascular disease and the value of once-daily treatment regimens. Cardiol Rev. 2007 Sep-Oct;15:257–263. doi: 10.1097/CRD.0b013e3180cabbe7. [DOI] [PubMed] [Google Scholar]
- 11.Urquhart J. Pharmionics: research on what patients do with prescription drugs. Pharmacoepidemiol Drug Saf. 2004 Sep;13:587–590. doi: 10.1002/pds.1004. [DOI] [PubMed] [Google Scholar]
- 12.Rivers PH. Compliance aids--do they work? Drugs Aging. 1992 Mar-Apr;2:103–111. doi: 10.2165/00002512-199202020-00004. [DOI] [PubMed] [Google Scholar]
- 13.Connor J, Rafter N, Rodgers A. Do fixed-dose combination pills or unit-of-use packaging improve adherence? A systematic review. Bull World Health Organ. 2004 Dec;82:935–939. [PMC free article] [PubMed] [Google Scholar]
- 14.Zedler BK, Kakad P, Colilla S, Murrelle L, Shah NR. Does packaging with a calendar feature improve adherence to self-administered medication for long-term use? A systematic review. Clin Ther. 2011 Jan;33:62–73. doi: 10.1016/j.clinthera.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Orton LC, Barnish G. Unit-dose packaged drugs for treating malaria. Cochrane Database Syst Rev. 2009;1:1. doi: 10.1002/14651858.CD004614.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahtani KR, Heneghan CJ, Glasziou PP, Perera R. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD005025.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Rudd P. Medication packaging: simple solutions to nonadherence problems? Clin Pharmacol Ther. 1979 Mar;25:257–265. doi: 10.1002/cpt1979253257. [DOI] [PubMed] [Google Scholar]
- 18.Morrison A, Wertheimer AI, Berger ML. Interventions to improve antihypertensive drug adherence: a quantitative review of trials. Formulary. 2000;35:234–236. [Google Scholar]
- 19.Cooper H, Hedges LV, Valentine JC, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. New York: Russell Sage Foundation; 2009. [Google Scholar]
- 20.White H. Scientific communication and literature retrieval. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. New York: Russell Sage Foundation; 2009. pp. 51–71. [Google Scholar]
- 21.Rothstein HR, Hopewell S. Grey literature. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. New York: Russell Sage Foundation; 2009. pp. 103–125. [Google Scholar]
- 22.Conn VS, Valentine JC, Cooper HM, Rantz MJ. Grey literature in meta-analyses. Nurs Res. 2003 Jul-Aug;52:256–261. doi: 10.1097/00006199-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Burdett S, Stewart LA, Tierney JF. Publication bias and meta-analyses: a practical example. Int J Technol Assess Health Care. 2003;19:129–134. doi: 10.1017/s0266462303000126. [DOI] [PubMed] [Google Scholar]
- 24.Wilson D. Systematic coding. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. New York: Russell Sage Foundation; 2009. pp. 159–176. [Google Scholar]
- 25.Orwin R, Vevea J. Evaluating coding decisions. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. New York: Russell Sage Foundation; 2009. pp. 177–203. [Google Scholar]
- 26.Borenstein M, Hedges L, Higgins JPT, Rothstein H. Introduction to Meta-Analysis. West Sussex, England: John Wiley & Sons, Ltd.; 2009. [Google Scholar]
- 27.Hedges L, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 28.Conn VS, Hafdahl AR, Mehr DR, LeMaster JW, Brown SA, Nielsen PJ. Metabolic effects of interventions to increase exercise in adults with type 2 diabetes. Diabetologia. 2007 May;50:913–921. doi: 10.1007/s00125-007-0625-0. [DOI] [PubMed] [Google Scholar]
- 29.Awofeso N, Lammers H, Verschuuren M. Effect of blister calendar packs in enhancing compliance with MDT: The Kaduna State (Nigeria) experience. Int J Lepr Other Mycobact Dis. 1995;63:453–454. [PubMed] [Google Scholar]
- 30.Becker LA, Glanz K, Sobel E, Mossey J, Zinn SL, Knott KA. A randomized trial of special packaging of antihypertensive medications. J Fam Pract. 1986;22:357–361. [PubMed] [Google Scholar]
- 31.Bosworth HB, Olsen MK, Neary A, Orr M, Grubber J, Svetkey L, et al. Take Control of Your Blood Pressure (TCYB) study: A multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Educ Couns. 2008;70:338–347. doi: 10.1016/j.pec.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burrelle TN. Evaluation of an interdisciplinary compliance service for elderly hypertensives. J Geriatr Drug Ther. 1986;1:23–51. [Google Scholar]
- 33.Calvert SB, Kramer JM, Anstrom KJ, Kaltenbach LA, Stafford JA, Allen LaPointe NM. Patient-focused intervention to improve long-term adherence to evidence-based medications: A randomized trial. Am Heart J. 2012;163:657–665. doi: 10.1016/j.ahj.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Crome P, Curl B, Boswell M, Corless D, Lewis RR. Assessment of a new calendar pack: The 'C-Pak'. Age Ageing. 1982;11:275–279. doi: 10.1093/ageing/11.4.275. [DOI] [PubMed] [Google Scholar]
- 35.Desborough JA, Sach T, Bhattacharya D, Holland RC, Wright DJ. A cost-consequences analysis of an adherence focused pharmacist-led medication review service. Int J Pharm Pract. 2012;20:41–49. doi: 10.1111/j.2042-7174.2011.00161.x. [DOI] [PubMed] [Google Scholar]
- 36.Eshelman FN, Fitzloff J. Effect of packaging on patient compliance with an antihypertensive medication. Curr Ther Res Clin Exp. 1976;20:215–219. [PubMed] [Google Scholar]
- 37.Goujard C, Bernard N, Sohier N, Peyramond D, Lancon F, Chwalow J, et al. Impact of a patient education program on adherence to HIV medication: A randomized clinical trial. J Acquir Immune Defic Syndr. 2003;34:191–194. doi: 10.1097/00126334-200310010-00009. [DOI] [PubMed] [Google Scholar]
- 38.Griffiths R, Johnson M, Piper M, Langdon R. A nursing intervention for the quality use of medicines by elderly community clients. Int J Nurs Pract. 2004;10:166–176. doi: 10.1111/j.1440-172X.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 39.Henry A, Batey RG. Enhancing compliance not a prerequisite for effective eradication of Helicobacter pylori: The HelP Study. Am J Gastroenterol. 1999;94:811–815. doi: 10.1111/j.1572-0241.1999.00856.x. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch JD, Gonzales M, Rosenquist A, Miller TA, Gilmer TP, Best BM. Antiretroviral therapy adherence, medication use, and health care costs during 3 years of a community pharmacy medication therapy management program for Medi-Cal beneficiaries with HIV/AIDS. J Managed Care Pharm. 2011;17:213–223. doi: 10.18553/jmcp.2011.17.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holzemer WL, Bakken S, Portillo CJ, Grimes R, Welch J, Wantland D, et al. Testing a nurse-tailored HIV medication adherence intervention. Nurs Res. 2006;55:189–197. doi: 10.1097/00006199-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Ingersoll KS, Farrell-Carnahan L, Cohen-Filipic J, Heckman CJ, Ceperich SD, Hettema J, et al. A pilot randomized clinical trial of two medication adherence and drug use interventions for HIV+ crack cocaine users. Drug Alcohol Depend. 2011;116:177–187. doi: 10.1016/j.drugalcdep.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Insel KC, Cole L. Individualizing memory strategies to improve medication adherence. Appl Nurs Res. 2005;18:199–204. doi: 10.1016/j.apnr.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Kalichman SC, Cherry J, Cain D. Nurse-delivered antiretroviral treatment adherence intervention for people with low literacy skills and living with HIV/AIDS. J Assoc Nurses AIDS Care. 2005;16:3–15. doi: 10.1016/j.jana.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Kalichman SC, Kalichman MO, Cherry C, Swetzes C, Amaral CM, White D, et al. Brief behavioral self-regulation counseling for HIV treatment adherence delivered by cell phone: An initial test of concept trial. AIDS Patient Care STDS. 2011;25:303–310. doi: 10.1089/apc.2010.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kennedy LM. Effectiveness of a self-care medication education protocol on the home medication behaviors of recently hospitalized elderly. Austin: University of Texas; 1990. [Google Scholar]
- 47.Kripalani S, Roumie CL, Dalal AK, Cawthon C, Businger A, Eden SK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: A randomized trial. Ann Intern Med. 2012;157:1–10. doi: 10.7326/0003-4819-157-1-201207030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: A randomized controlled trial. Arch Intern Med. 2003;163:809–817. doi: 10.1001/archinte.163.7.809. [DOI] [PubMed] [Google Scholar]
- 49.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: A randomized controlled trial. JAMA. 2006;296:2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 50.Lee M, Kemp JA, Canning A, Egan C, Tatoronis G, Farraye FA. A randomized controlled trial of an enhanced patient compliance program for Helicobacter pylori therapy. Arch Intern Med. 1999;159:2312. doi: 10.1001/archinte.159.19.2312. [DOI] [PubMed] [Google Scholar]
- 51.Lefante JJ, Jr, Harmon GN, Roy W, Fontenot S, Brown K, Webber L. The effect of medication reviews in a rural community pharmacy assistance program: The Cenla Medication Access Program. J Pharm Pract. 2005;18:486–492. [Google Scholar]
- 52.Leung LB, Busch AM, Nottage SL, Arellano N, Glieberman E, Busch NJ, et al. Approach to antihypertensive adherence: A feasibility study on the use of student health coaches for uninsured hypertensive adults. Behav Med. 2012;38:19–27. doi: 10.1080/08964289.2011.651174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levensky ER. Further development and evaluation of an individualized intervention for increasing adherence to HIV medications. Reno: University of Nevada; 2006. [Google Scholar]
- 54.Linkewich JA, Catalano RB, Flack HL. The effect of packaging and instruction on outpatient compliance with medication regimens. Drug Intell Clin Pharm. 1974;8:10–15. [Google Scholar]
- 55.MacDonald E, MacDonald JB, Phoenix M. Improving drug compliance after hospital discharge. Br Med J. 1977;2:618–621. doi: 10.1136/bmj.2.6087.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacIntosh P, Pond G, Pond B, Leung V, Siu L. A comparison of patient adherence and preference of packaging method for oral anticancer agents using conventional pill bottles versus daily pill boxes. Eur J Cancer Care (Engl) 2007;16:380–386. doi: 10.1111/j.1365-2354.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 57.McPherson-Baker S, Malow RM, Penedo F, Jones DL, Schneiderman N, Klimas NG. Enhancing adherence to combination antiretroviral therapy in non-adherent HIV-positive men. AIDS Care. 2000;12:399–404. doi: 10.1080/09540120050123792. [DOI] [PubMed] [Google Scholar]
- 58.Morales Suarez-Varela M GEMECOR. Study on the use of a smart pillbox to improve treatment compliance. Atencion Primaria/Sociedad Espanola de Medicina de Familia y Comunitaria. 2009;41:185–191. doi: 10.1016/j.aprim.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moshkovska T, Stone MA, Smith RM, Bankart J, Baker R, Mayberry JF. Impact of a tailored patient preference intervention in adherence to 5-aminosalicylic acid medication in ulcerative colitis: Results from an exploratory randomized controlled trial. Inflamm Bowel Dis. 2011;17:1874–1881. doi: 10.1002/ibd.21570. [DOI] [PubMed] [Google Scholar]
- 60.Nazareth I, Burton A, Shulman S, Smith P, Haines A, Timberal H. A pharmacy discharge plan for hospitalized elderly patients--a randomized controlled trial. Age Ageing. 2001;30:33–40. doi: 10.1093/ageing/30.1.33. [DOI] [PubMed] [Google Scholar]
- 61.Nochowitz B, Shapiro NL, Nutescu EA, Cavallari LH. Effect of a warfarin adherence aid on anticoagulation control in an inner-city anticoagulation clinic population. Ann Pharmacother. 2009;43:1165–1172. doi: 10.1345/aph.1L707. [DOI] [PubMed] [Google Scholar]
- 62.Park DC, Morrell RW, Frieske D, Kincaid D. Medication adherence behaviors in older adults: Effects of external cognitive supports. Psychol Aging. 1992;7:252–256. doi: 10.1037//0882-7974.7.2.252. [DOI] [PubMed] [Google Scholar]
- 63.Peterson GM, McLean S, Millingen KS. A randomised trial of strategies to improve patient compliance with anticonvulsant therapy. Epilepsia. 1984;25:412–417. doi: 10.1111/j.1528-1157.1984.tb03436.x. [DOI] [PubMed] [Google Scholar]
- 64.Qingjun L, Jihui D, Laiyi T, Xiangjun Z, Jun L, Hay A, et al. The effect of drug packaging on patients' compliance with treatment for Plasmodium vivax malaria in China. Bull World Health Organ. 1998;76:21–27. [PMC free article] [PubMed] [Google Scholar]
- 65.Revankar CR, Gupta N, Sorensen BH, Naik SS. Further observations on MDT blister-calendar packs in vertical leprosy eradication programmes--a multicentre study (phase II) Lepr Rev. 1993;64:250–254. doi: 10.5935/0305-7518.19930027. [DOI] [PubMed] [Google Scholar]
- 66.Robbins B, Rausch KJ, Garcia RI, Prestwood KM. Multicultural medication adherence: A comparative study. J Gerontol Nurs. 2004;30:25–32. doi: 10.3928/0098-9134-20040701-07. [DOI] [PubMed] [Google Scholar]
- 67.Safren SA, O'Cleirigh C, Reilly LC, Tan JY, Raminani SR, Otto MW, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28:1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Safren SA, Otto MW, Worth JL, Salomon E, Johnson W, Mayer K, et al. Two strategies to increase adherence to HIV antiretroviral medication: Life-steps and medication monitoring. Behav Res Ther. 2001;39:1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 69.Skaer TL, Sclar DA, Markowski DJ, Won JK. Effect of value-added utilities on prescription refill compliance and health care expenditures for hypertension. J Hum Hypertens. 1993;7:515–518. [PubMed] [Google Scholar]
- 70.Skaer TL, Sclar DA, Markowski DJ, Won JK. Effect of value-added utilities on prescription refill compliance and Medicaid health care expenditures--a study of patients with non-insulin-dependent diabetes mellitus. J Clin Pharm Ther. 1993;18:295–299. doi: 10.1111/j.1365-2710.1993.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 71.Skaer TL, Sclar DA, Markowski DJ, Won JKH. Effect of value-added utilities in promoting prescription refill compliance among patients with hypertension. Current Therapeutic Research - Clinical and Experimental. 1993;53:251–255. [Google Scholar]
- 72.Spriet A, Beiler D, Dechorgnat J, Simon P. Adherence of elderly patients to treatment with pentoxifylline. Clin Pharmacol Ther. 1980;27:1–8. doi: 10.1038/clpt.1980.1. [DOI] [PubMed] [Google Scholar]
- 73.Sweeney SJ, Dixon JS, Sutcliffe I. Impact of the clinical pharmacist on compliance in a geriatric population. Pharm J. 1989;242:R4–R6. [Google Scholar]
- 74.Taylor CT, Byrd DC, Krueger K. Improving primary care in rural Alabama with a pharmacy initiative. Am J Health Syst Pharm. 2003;60:1123–1129. doi: 10.1093/ajhp/60.11.1123. [DOI] [PubMed] [Google Scholar]
- 75.Traiger GL, Bui LL. A self-medication administration program for transplant recipients. Transplantation. 1997;17:71–79. [PubMed] [Google Scholar]
- 76.Tsuyuki RT, Fradette M, Johnson JA, Bungard TJ, Eurich DT, Ashton T, et al. A multicenter disease management program for hospitalized patients with heart failure. J Card Fail. 2004;10:473–480. doi: 10.1016/j.cardfail.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 77.Wang H, Zhou J, Huang L, Li X, Fennie KP, Williams AB. Effects of nurse-delivered home visits combined with telephone calls on medication adherence and quality of life in HIV-infected heroin users in Hunan of China. J Clin Nurs. 2010;19:380–388. doi: 10.1111/j.1365-2702.2009.03048.x. [DOI] [PubMed] [Google Scholar]
- 78.Wright JM, Htun Y, Leong MG, Forman P, Ballard RC. Evaluation of the use of calendar blister packaging on patient compliance with STD syndromic treatment regimens. Sex Transm Dis. 1999;26:556–563. doi: 10.1097/00007435-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 79.Zillich AJ, Jaynes HAW, Snyder ME, Harrison J, Hudmon KS, de Moor C, et al. Evaluation of specialized medication packaging combined with medication therapy management: adherence, outcomes, and costs among Medicaid patients. Med Care. 2012;50:485–493. doi: 10.1097/MLR.0b013e3182549d48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zillich AJ, Sutherland JM, Kumbera PA, Carter BL. Hypertension outcomes through blood pressure monitoring and evaluation by pharmacists (HOME study) J Gen Intern Med. 2005;20:1091–1096. doi: 10.1111/j.1525-1497.2005.0226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bakken S, Holzemer WL, Portillo CJ, Grimes R, Welch J, Wantland D. Utility of a standardized nursing terminology to evaluate dosage and tailoring of an HIV/AIDS adherence intervention. J Nurs Scholarsh. 2005;37:251–257. doi: 10.1111/j.1547-5069.2005.00043.x. [DOI] [PubMed] [Google Scholar]
- 82.Bosworth HB, Olsen MK, Dudley T, Orr M, Neary A, Harrelson M, et al. The Take Control of Your Blood pressure (TCYB) study: study design and methodology. Contemp Clin Trials. 2007;28:33–47. doi: 10.1016/j.cct.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 83.Dunnell K, Cartwright A. Medicine Takers, Prescribers and Hoarders. Boston: Routledge and Kegan Paul; 1972. pp. 148–165. [Google Scholar]
- 84.Hirsch JD, Rosenquist A, Best BM, Miller TA, Gilmer TP. Evaluation of the first year of a pilot program in community pharmacy: HIV/AIDS medication therapy management for Medi-Cal beneficiaries. J Managed Care Pharm. 2009;15:32–41. doi: 10.18553/jmcp.2009.15.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holzemer WL, Henry SB, Portillo CJ, Miramontes H. The Client Adherence Profiling-Intervention Tailoring (CAP-IT) intervention for enhancing adherence to HIV/AIDS medications: a pilot study. J Assoc Nurses AIDS Care. 2000;11:36–44. doi: 10.1016/s1055-3290(06)60420-2. [DOI] [PubMed] [Google Scholar]
- 86.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 87.Safren SA, Otto MW, Worth JL. Life-steps: Applying cognitive behavioral therapy to HIV medication adherence. Cogn Behav Pract. 1999;6:332–341. [Google Scholar]
- 88.Schnipper JL, Roumie CL, Cawthon C, Businger A, Dalal AK, Mugalla I, et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) study. Circulation. 2010;3:212–219. doi: 10.1161/CIRCOUTCOMES.109.921833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Food and Drug Administration. Container Closure Systems for Packaging Human Drugs and Biologics. 1999 [Google Scholar]
- 90.Food and Drug Administration. Changes to an approved NDA or ANDA. 2004 [Google Scholar]
- 91.European Medicines Agency. Guideline on plastic immediate packaging materials. London, UK: 2005. [Google Scholar]
- 92.Littenberg B, MacLean CD, Hurowitz L. The use of adherence aids by adults with diabetes: a cross-sectional survey. BMC Family Practice. 2006;7:1. doi: 10.1186/1471-2296-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morello C, Chynoweth M, Kim H, Singh RF, Hirsch JD. Strategies to improve medication adherence reported by diabetes patients and caregivers: results of taking control of your diabetes study. Ann Pharmacother. 2011;45:145–153. doi: 10.1345/aph.1P322. [DOI] [PubMed] [Google Scholar]
- 94.Demonceau J, Ruppar T, Kristanto P, Hughes DA, Fargher E, Kardas P, et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs. 2013 May;73:545–562. doi: 10.1007/s40265-013-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santschi V, Wuerzner G, Schneider M-P, Bugnon O, Burnier M. Clinical evaluation of IDAS II, a new electronic device enabling drug adherence monitoring. Eur J Clin Pharmacol. 2007 Dec;63:1179–1184. doi: 10.1007/s00228-007-0364-7. [DOI] [PubMed] [Google Scholar]