Abstract
Context
Human immunodeficiency virus (HIV) infection complicates care and contributes to poor outcomes among tuberculosis (TB) patients. The Centers for Disease Control and Prevention recommends that providers test all TB patients for HIV.
Objective
We assessed completeness of HIV status determination among TB patients and identified key gaps in adherence.
Design
We conducted a retrospective review of public health charts to determine the HIV status for all TB patients reported in California during 2008. We then used logistic regression to determine the factors associated with a known (positive or negative) HIV status. A random sample of TB patients was selected for secondary review to characterize the timing of HIV status determination and the providers who had opportunity to test for HIV.
Setting
California TB programs.
Participants
All TB patients reported from California in 2008.
Main Outcome Measures
Proportion of patients with a known HIV status, adjusted odds ratios for having a known HIV status, proportion of patients with a known HIV status before TB diagnosis, and proportion of patients diagnosed with TB by different provider types.
Results
Only 1752 (66%) of 2667 TB patients had a known HIV status. Having a known HIV status was strongly associated with those aged between 15 and 44 years and being managed with any public provider involvement. Of 292 patients in the random sample, 12 patients (4%) had a known HIV status before TB diagnosis. Among the remaining 280 patients, 187 patients (67%) were diagnosed with TB by a private provider.
Conclusions
The HIV status determination of TB patients was selective and not routine as recommended. Private providers can play a key role in testing for HIV at TB diagnosis. California TB programs should ensure that all TB patients have an HIV status by promulgating national recommendations, educating private providers on the benefits of testing TB patients for HIV, and monitoring completeness of HIV status determination.
Keywords: HIV, routine HIV testing, tuberculosis
Since 1989, the Centers for Disease Control and Prevention (CDC) has recommended testing all tuberculosis (TB) patients for human immunodeficiency virus (HIV).1,2 Beginning in 2006, the CDC recommended routine provider-initiated opt-out HIV testing for all patients in health care settings.3 The United States has made progress toward the national standard of routine HIV testing as the percentage of TB patients with a known (positive or negative) HIV status increased from 35% in 1993 to 80% in 2008.4 Data from California have not been included in these national estimates because HIV status of TB patients only recently became reportable to the state. This is a major gap for determining national trends because California reports the greatest number of TB patients of any state.
This also presents a gap in TB management and public health TB control. Human immunodeficiency virus infection is a risk factor for death with TB,5-9 TB recurrence,10 and acquired TB drug resistance with intermittent therapy.11-13 Thus, HIV status determination is needed to identify TB patients who should receive daily directly observed TB therapy and highly active antiretroviral therapy to prevent poor TB outcomes.14-17 Human immunodeficiency virus status determination is also needed for TB programs to link HIV-infected TB patients to HIV care services and to identify HIV-infected contacts, who are at increased risk for progression to disease.18
Ensuring complete HIV status determination among patients is part of TB programs’ broader responsibility to monitor and ensure the quality of all TB-related activities. However, the decision to test patients for HIV ultimately rests with the provider. Although TB programs can directly implement routine HIV testing for patients in public TB clinics, they have less influence over the care given by private providers. Therefore, public health efforts to ensure complete HIV status determination must consider the provider types involved in TB care and target interventions appropriately.
We conducted an evaluation of TB patients reported from California in 2008 to (1) assess completeness of HIV status determination, (2) identify factors associated with having a known HIV status, (3) characterize providers who have opportunity to test for HIV during TB care, and (4) provide recommendations to increase HIV status determination completeness.
Methods
Data sources and evaluation design
There are 58 county and 3 city TB programs in California responsible for receiving TB case reports and maintaining public health records to monitor treatment for all TB patients. If patients are managed directly by TB programs, public health records are compiled from public TB clinic records. If patients are managed by private providers, private providers provide documentation for public health records. Because TB treatment and follow-up can last more than 1 year, patients reported in 2008 had the most complete data at the time of our evaluation.
We abstracted HIV status from public health records for all TB patients reported from California in 2008 and linked these data to individual demographic and clinical data from the state TB registry. From this data set, we assessed completeness of HIV status determination and identified factors associated with having a known HIV status.
We also selected a simple random sample of 300 TB patients from jurisdictions reporting at least 5 TB patients in 2008 for secondary public health record review. This secondary review was done to verify HIV status documentation, to assess timing of HIV status determination, and to characterize the type of provider at specific stages of TB care. This evaluation was reviewed and approved by the California Health and Human Services Agency institutional review board and all relevant county review boards.
Definitions
Negative HIV status was defined as having one of the following before TB treatment completion but no earlier than a year before TB diagnosis: (1) a negative test result or (2) documentation of a negative HIV status from a previous or referring clinician. Positive HIV status was defined as having one of the following before TB treatment completion: (1) a positive test result, (2) a report of highly active antiretroviral therapy medication, (3) documentation of HIV diagnosis from a previous or referring clinician, or (4) self-report of positive HIV status. A known HIV status was defined as having a positive or negative HIV status with all others having an unknown HIV status. The TB diagnosis date was the earliest of the report date, collection date of the first positive sputum culture, or the TB treatment start date.
Individual demographic and clinical data from the state TB registry conformed to the requirements for the national TB case report form.19 To identify those patients managed according to the policies and procedures of the TB programs, patients were categorized as either managed with any public provider involvement or managed solely by private providers. Public providers were defined as those who were part of or directed by TB programs. All other providers, including some county hospital clinicians not associated with TB programs, were classified as private providers.
We characterized the timing of HIV status determination in relation to the TB diagnosis date. Because the time from initial health visit to TB confirmation might be longer than 2 months, the 3 months before TB diagnosis were considered to be the TB diagnostic period. Patients without a known HIV status documented before the TB diagnostic period were considered to have no previously known HIV status. Patients who did not have a previously known HIV status but had subsequent documentation of HIV status were considered to have a newly identified HIV status. For patients with a newly identified HIV status and a documented HIV test, the time to test was calculated as the number of days from the TB diagnosis date to the HIV specimen collection date. Negative values indicated that HIV specimen collection preceded the TB diagnosis date. Patients without a precise HIV specimen collection date were excluded from analysis of the time to test.
Statistical analysis
Analysis was conducted using SAS 9.2 (SAS Institute, Cary, North Carolina). We calculated proportions of patients with a known HIV status and with HIV infection grouped by clinical and demographic characteristics. Because HIV testing is recommended early in TB treatment, we examined patient characteristics apparent on initial TB presentation. These characteristics included age, sex, race, birthplace, site of TB disease, previous TB, and history of incarceration, homelessness, excess alcohol use, or illicit drug use. We also examined patients by type of provider managing TB care. We used logistic regression to identify factors associated with having a known HIV status and to calculate odds ratios, adjusted odds ratios, and confidence intervals. Factors with P < .05 were considered significant. A multivariate logistic model including all patients with a known provider type was chosen using backward-stepwise hierarchical selection. We also tested for interaction between race and nativity.
We characterized the timing and providers relevant to HIV status determination among the patients in our random sample. We calculated the percentage of patients with a previously known HIV status and the percentage with a newly identified HIV status. We determined the frequency of patients by their provider at TB diagnosis, at TB treatment initiation, and for the majority of TB care. We also determined the frequency of patients who ever visited a public TB clinic. Among patients who had a newly identified HIV status and a documented HIV test, we calculated the percentage of patients whose test was ordered by public providers and the time to test. The Wilcoxon-Mann-Whitney test was used to compare the distribution of the time to test stratified by ordering provider type.
Results
HIV status determination among patients
Of the 2697 TB patients reported during 2008 in California, 30 (1%) patients had public health records unavailable, leaving 2667 (99%) patients for review. Of the 2667 patients whose records were reviewed, 132 (5%) patients were HIV positive and 1620 (61%) patients were HIV negative summing to 1752 (66%) patients with a known HIV status. The remaining 915 (34%) patients had an unknown HIV status. Of these, 164 (18%) patients refused an HIV test, 154 (17%) patients were offered a test but did not have locatable results, and 597 (65%) patients had no HIV status or test documentation. Patients managed solely by private providers comprised 819 of 2667 (31%) TB patients. Only 377 of these 819 (46%) patients had a known HIV status compared with 1343 of 1799 (75%) patients managed with any public provider involvement (P < .01, Table 1).
TABLE 1.
Characteristics and HIV status of 2667 TB patients, California, 2008
| Patients |
HIV Status Known |
Positive HIV Status |
||||
|---|---|---|---|---|---|---|
| n | n | % Among All Patients |
n | % Among All Patients |
% Among Patients With a Known Status |
|
| Total patients | 2667 | 1752 | 65.7 | 132 | 4.9 | 7.5 |
| Provider type | ||||||
| Any public provider involvement | 1799 | 1343 | 74.7 | 89 | 4.9 | 6.6 |
| Solely by private providers | 819 | 377 | 46.0 | 40 | 4.9 | 10.6 |
| Unknown | 49 | 32 | 65.3 | 3 | 6.1 | 9.4 |
| Age, ya | ||||||
| ≤14 | 155 | 52 | 33.5 | 0 | 0.0 | 0.0 |
| 15-29 | 490 | 395 | 80.6 | 20 | 4.1 | 5.1 |
| 30-44 | 587 | 452 | 77.0 | 70 | 11.9 | 15.5 |
| 45-59 | 626 | 447 | 71.4 | 36 | 5.8 | 8.1 |
| ≤60 | 809 | 406 | 50.2 | 6 | 0.7 | 1.5 |
| Sexa | ||||||
| Male | 1635 | 1109 | 67.8 | 110 | 6.7 | 9.9 |
| Female | 1032 | 643 | 62.3 | 22 | 2.1 | 3.4 |
| Previous TB | ||||||
| No | 2534 | 1652 | 65.2 | 125 | 4.9 | 7.6 |
| Yes | 133 | 100 | 75.2 | 7 | 5.3 | 7.0 |
| History of homelessness, incarceration, illicit drug use, or alcohol usea |
||||||
| No history of behaviors | 2239 | 1410 | 63.0 | 80 | 3.6 | 5.7 |
| History of 1 behavior | 239 | 182 | 76.2 | 27 | 11.3 | 14.8 |
| History of 2 or more behaviors | 189 | 160 | 84.7 | 25 | 13.2 | 15.6 |
| Site of diseasea | ||||||
| Pulmonary only | 1908 | 1263 | 66.2 | 72 | 3.8 | 5.7 |
| Extrapulmonary | 759 | 489 | 64.4 | 60 | 7.9 | 12.3 |
| Racea | ||||||
| White | 255 | 160 | 62.7 | 17 | 6.7 | 10.6 |
| Black | 209 | 152 | 72.7 | 27 | 12.9 | 17.8 |
| Hispanic | 1046 | 746 | 71.3 | 77 | 7.4 | 10.3 |
| Asian | 1148 | 687 | 59.8 | 10 | 0.9 | 1.5 |
| AI or NA | 8 | 7 | 87.5 | 1 | 12.5 | 14.3 |
| Unknown | 1 | 0 | 0.0 | 0 | 0.0 | |
| Birthplacea | ||||||
| US-born | 654 | 402 | 61.5 | 51 | 7.8 | 12.7 |
| Foreign-born | 2013 | 1350 | 67.1 | 81 | 4.0 | 6.0 |
| Birthplace and race | ||||||
| US-born | ||||||
| White | 193 | 125 | 64.8 | 17 | 8.8 | 13.6 |
| Black | 155 | 111 | 71.6 | 21 | 13.5 | 18.9 |
| Hispanic | 244 | 137 | 56.1 | 12 | 4.9 | 8.8 |
| Asian | 55 | 23 | 41.8 | 0 | 0.0 | 0.0 |
| AI or NA | 7 | 6 | 85.7 | 1 | 14.3 | 16.7 |
| Foreign-born | ||||||
| White | 62 | 35 | 56.5 | 0 | 0.0 | 0.0 |
| Black | 54 | 41 | 75.9 | 6 | 11.1 | 14.6 |
| Hispanic | 802 | 609 | 75.9 | 65 | 8.1 | 10.7 |
| Asian | 1093 | 664 | 60.8 | 10 | 0.9 | 1.5 |
| AI or NA | 1 | 1 | 100.0 | 0 | 0.0 | 0.0 |
| Unknown | 1 | 0 | 0.0 | 0 | 0.0 | |
Abbreviations: AI or NA, American Indian or Native Alaskan; HIV, human immunodeficiency virus; TB, tuberculosis.
Characteristic is associated with HIV-infection prevalence in bivariate analysis.
Human immunodeficiency virus–infection prevalence was 8% among patients with a known HIV status. Human immunodeficiency virus–infection prevalence was associated with age, sex, race, birthplace, site of TB disease, and history of incarceration, homelessness, excess alcohol use, or illicit drug use (Table 1).
Factors associated with having a known HIV status
Factors associated with having a known HIV status were identified through bivariate and multivariate analysis (Table 2). Forty-nine patients were excluded because they had an unknown provider type. Among the 2618 patients remaining, 1720 (66%) patients had a known HIV status. Provider type, age, sex, previous TB, birthplace, race, and history of incarceration, homelessness, excess alcohol use, or illicit drug use were associated with having a known HIV status in bivariate analysis.
TABLE 2.
Bivariate and Multivariate Analysis of Factors Associated With Having a Known HIV Status for 2618 TB Patients, California, 2008a
| No. Patients | OR (95% CI) | P | AOR (95% CI) | P | |
|---|---|---|---|---|---|
| Provider type | |||||
| Any public provider involvement | 1799 | Reference | Reference | ||
| Solely by private providers | 819 | 0.29 (0.24-0.35) | <0.01 | 0.28 (0.23-0.34) | <0.01 |
| Age, y | |||||
| ≤14 | 154 | 0.15 (0.10-0.22) | <0.01 | 0.15 (0.10-0.24) | <0.01 |
| 15-29 | 478 | 1.22 (0.91-1.65) | 0.19 | 1.16 (0.84-1.59) | 0.36 |
| 30-44 | 579 | Reference | Reference | ||
| 45-59 | 610 | 0.75 (0.57-0.97) | 0.03 | 0.67 (0.51-0.89) | 0.01 |
| ≥60 | 797 | 0.30 (0.23-0.38) | <0.01 | 0.31 (0.24-0.40) | <0.01 |
| Sex | |||||
| Male | 1609 | Reference | Reference | ||
| Female | 1009 | 0.78 (0.66-0.91) | <0.01 | 0.78 (0.65-0.95) | 0.01 |
| Previous TB | |||||
| No | 2488 | Reference | Reference | ||
| Yes | 130 | 1.57 (1.05-2.35) | 0.03 | 1.72 (1.11-2.67) | 0.02 |
| History of homelessness, incarceration, illicit drug use, or alcohol use |
|||||
| No history of behaviors | 2201 | Reference | Reference | ||
| History of 1 behavior | 232 | 1.90 (1.38-2.60) | <0.01 | 1.27 (0.89-1.80) | 0.19 |
| History of 2 or more behaviors | 185 | 3.45 (2.27-5.23) | <0.01 | 1.87 (1.18-2.96) | 0.01 |
| Site of disease | |||||
| Pulmonary only | 1877 | Reference | |||
| Extrapulmonary | 741 | 1.19 (0.90-1.57) | 0.23 | ||
| Race | |||||
| White | 255 | Reference | |||
| Black | 209 | 1.58 (1.07-2.35) | 0.02 | ||
| Hispanic | 1024 | 1.47 (1.10-1.96) | 0.01 | ||
| Asian | 1122 | 0.89 (0.67-1.17) | 0.40 | ||
| AI or NA | 8 | 4.15 (0.50-34.28) | 0.19 | ||
| Birthplace | |||||
| US-born | 648 | Reference | |||
| Foreign-born | 1970 | 1.28 (1.07-1.54) | 0.01 | ||
| Birthplace and race | |||||
| US-born | |||||
| White | 193 | Reference | |||
| Black | 155 | 0.92 (0.56-1.53) | 0.75 | ||
| Hispanic | 239 | 0.70 (0.44-1.12) | 0.13 | ||
| Asian | 54 | 0.45 (0.22-0.91) | 0.03 | ||
| AI or NA | 7 | 1.66 (0.18-15.18) | 0.65 | ||
| Foreign-born | |||||
| White | 62 | Reference | |||
| Black | 54 | 2.12 (0.88-5.10) | 0.09 | ||
| Hispanic | 785 | 1.75 (0.98-3.12) | 0.06 | ||
| Asian | 1068 | 1.28 (0.73-2.26) | 0.39 | ||
| AI or NA | 1 | … b | … b |
Abbreviations: AI or NA, American Indian or Native Alaskan; AOR, adjusted odds ratios; HIV, human immunodeficiency virus; OR, odds ratios; TB, tuberculosis.
49 patients did not have a known provider type and were excluded from analysis.
Stratum contains a single patient.
Statistically significant factors in bivariate analysis remained significant in multivariate analysis. Age and provider type were strongly associated with having a known HIV status. A known HIV status was less likely among females but was more likely among patients who had previous TB. History of incarceration, homelessness, excess alcohol use, or illicit drug use was also associated with having a known HIV status, but only if the patient had a history of at least 2 behaviors.
Interaction between race and birthplace was detected. Among foreign-born patients, Asians and Hispanics were more likely to have a known HIV status than whites. However, among US-born patients, Asians and Hispanics were less likely to have a known HIV status.
Timing of HIV status determination
A random sample of 300 patients was selected for secondary record review to ascertain the timing of HIV status determination. Records were unavailable for 3 patients. Of the remaining 297 patients, 5 (2%) patients were dead at diagnosis and had no HIV status documented (the Figure). Of the 292 patients alive at TB diagnosis, 280 (96%) patients did not have a previously known HIV status (an HIV status determined more than 3 months before the TB diagnosis date). Of the 280 patients who did not have a previously known HIV status, 171 (61%) patients had a newly identified HIV status and a documented HIV test, 7 (3%) patients had no documented HIV test but had documentation of a negative HIV status from a referring clinician, and 102 (36%) patients had no documentation of an HIV test or HIV status. Of the 171 patients who had a newly identified HIV status from testing, 164 (96%) patients were HIV negative and 7 (4%) patients were HIV positive. Overall, of the 297 sampled patients, 190 (64%) patients had a known HIV status and 15 (5%) patients were HIV positive. Seven of the 15 (47%) HIV-positive patients were not previously known to be HIV positive, but were subsequently identified through testing.
FIGURE. Timing of HIV Status Determination for Randomly Sampled Patientsa.
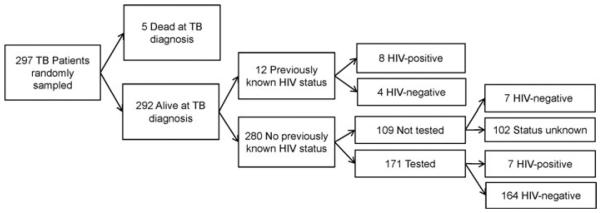
aHuman immunodeficiency virus status known 3 months before TB diagnosis is a previously known HIV status. The 7 HIV-negative patients who did not have a documented HIV test result in the public health chart had documentation of HIV-negative status from a referring clinician after TB diagnosis. HIV indicates human immunodeficiency virus; TB, tuberculosis.
Providers involved with TB treatment and HIV testing
Through secondary review, we characterized the providers at different stages of TB treatment for the 280 patients without a previously known HIV status. Of these patients, 187 (67%) patients were diagnosed with TB exclusively by private providers and 186 (66%) patients had the majority of their TB treatment managed by public providers (Table 3). Among the 171 patients with the recommended HIV testing, 90 (53%) patients had tests ordered by public providers (Table 4). All but 1 of the HIV tests ordered by public providers were done in an outpatient setting. In contrast, 51 of the 70 (73%) HIV tests ordered by private providers were done in an inpatient setting. The median time from TB diagnosis to HIV test was 0 days when HIV tests were ordered by private providers compared with 11 days when HIV tests were ordered by public providers (P < .01).
TABLE 3.
Providers of TB Care for 280 Sampled Patients Alive at Diagnosis With No Previously Known HIV Status, California, 2008
| Among Patients Sampled, n (%) | |
|---|---|
| Provider involved with TB diagnosis | |
| Public | 37 (13.2) |
| Private | 187 (66.8) |
| Both | 53 (18.9) |
| Unknown | 3 (1.1) |
| Provider initiating TB treatment | |
| Public | 103 (36.8) |
| Private | 172 (61.4) |
| Unknown or no treatment | 5 (1.8) |
| Provider for majority of TB treatment | |
| Public | 186 (66.4) |
| Private | 88 (31.4) |
| Unknown or no treatment | 6 (2.1) |
| Ever visited public TB clinic | |
| Yes | 205 (73.2) |
| No | 74 (26.4) |
| Unknown | 1 (0.4) |
Abbreviations: HIV, human immunodeficiency virus; TB, tuberculosis.
TABLE 4.
Providers and Settings of HIV Tests for 171 Sampled Patients Who Were Tested
| Among Patients Tested, n (%) | |
|---|---|
| Provider type | |
| Public | 90 (52.6) |
| Private | 70 (40.9) |
| Unknown | 11 (6.4) |
| Setting | |
| Inpatient | 54 (31.6) |
| Outpatient | 102 (59.6) |
| Unknown | 15 (8.8) |
| Provider type and setting | |
| Public | |
| Inpatient | 1 (0.6) |
| Outpatient | 89 (52.0) |
| Unknown | 0 (0.0) |
| Private | |
| Inpatient | 51 (29.8) |
| Outpatient | 13 (7.6) |
| Unknown | 6 (3.5) |
| Unknown | |
| Inpatient | 2 (1.2) |
| Outpatient | 0 (0.0) |
| Unknown | 9 (5.3) |
Abbreviation: HIV, human immunodeficiency virus.
Discussion
Only 66% of TB patients reported from California in 2008 had a known HIV status documented in public health charts, which was far below the national average of 80%. It is concerning that one-third of all TB patients did not have a known HIV status because patients with undiagnosed HIV might have missed an opportunity for HIV diagnosis and adequate TB treatment. Without adequate treatment, they would be more likely to experience poor TB outcomes including death. Such a large proportion of patients with missing HIV status information also introduces uncertainty regarding the true burden of HIV and TB comorbidity in California and impacts national estimates of HIV and TB comorbidity. We found an HIV-infection prevalence of 8% among those with a known HIV status, but the true prevalence among all TB patients is unknown. To ensure that all HIV-infected TB patients are identified, California must improve HIV status determination efforts.
Human immunodeficiency virus status determination was not uniformly low across all patient groups. Groups that were less likely to have a known HIV status were also those groups with a lower HIV-infection prevalence. Age was most strongly associated with having a known HIV status. Those younger than 15 years and those older than 45 years were less likely to have a known HIV status than those aged between 30 and 44 years. Females, US-born Asians, and patients without previous TB were also less likely to have a known HIV status. Patients without a history of incarceration, homelessness, excess alcohol use, or illicit drug use were less likely to have a known HIV status than those with a history of 2 or more behaviors. This suggests that HIV status determination was driven by provider-perceived risk for HIV infection and was not routine as recommended by the CDC. Other studies of TB programs have reported similar findings.20-22
However, risk-based testing does not effectively identify all HIV infection because clinicians cannot easily identify those at risk.1,3,23 Providers who practice risk-based testing might miss HIV infection among female, foreign-born, or older patients, who are increasingly represented among HIV-infected TB patients.24,25 Although this evaluation detected no or little HIV infection among patients younger than 15 years and patients older than 60 years, infection in these groups does occur. Nationally in 2005, HIV infection was detected in 1% of TB patients younger than 4 years, in 2% of those aged 5 to 14 years, and in 1% of those older than 64 years.4 Human immunodeficiency virus infection is life threatening and should be identified early in any TB patient. Opt-out HIV testing as recommended by the CDC, rather than risk-based testing, is necessary to achieve complete HIV status determination.26 It also increases patient and provider acceptability of HIV testing27,28 and improves linkage to antiretroviral treatment for HIV-infected patients.29 Finally, HIV testing is cost-effective compared with no testing when the HIV-infection prevalence is at least 1.0 per 1000 persons screened, as it was in our population of TB patients.30
Provider type was also an important factor in HIV status determination. Less than half of patients managed solely by private providers had a known HIV status and being managed with any public provider involvement was strongly associated with having a known HIV status. This finding warrants further attention because 31% of all TB patients were managed solely by private providers and HIV-infection prevalence was 11% among such patients who had a known HIV status.
Human immunodeficiency virus testing at TB diagnosis was effective in identifying HIV infection. In our random sample, 96% of patients did not have a known HIV status prior to the TB diagnostic period. Of note, 4% of those tested were HIV positive. Furthermore, almost half of all HIV-positive patients in our sample were not previously known to be HIV positive. Although our random sample examined only 15 of the total HIV-positive patients, similar findings have been reported by other studies.20,31
Private providers were best positioned to determine HIV status at TB diagnosis because they were the provider at TB diagnosis for 67% of patients. Their prominent role in initial TB care also explained why private providers ordered HIV tests earlier than public providers. Also, most HIV tests ordered by private providers were done in inpatient settings suggesting that many patients receive initial TB care in hospitals. Public provider testing is still important to achieve complete HIV status determination because many privately diagnosed patients were transferred to public care. Although it is possible that private providers do not report HIV status to TB programs, TB programs should follow up with private providers or the patient to document HIV status in public health records so it can inform care referral.
Tuberculosis providers, especially private providers in inpatient settings, should be engaged as partners to address incomplete HIV status determination. Patient acceptability of routine HIV tests is higher when providers encourage HIV tests and believe HIV testing benefits the patient.28,32-34 Provider education about their role in HIV status determination and associated patient benefits should accompany implementation of routine opt-out HIV testing.
This was the first statewide evaluation of HIV status determination among TB patients in California and included 99% of all TB patients reported in 2008. Nevertheless, this evaluation has limitations. Our evaluation included TB patients reported in 2008 only. Estimates of HIV-infection prevalence and HIV status determination among specific patient groups may have subsequently changed. Also, it was unclear whether patients lacked a known HIV status because they were not tested or because their test results were not documented. Because our data sources were public health records maintained by TB programs, patients managed solely by private providers were more likely to have incomplete HIV status information. If private providers tested for HIV among TB patients but did not report results to TB programs, it would cause us to underestimate the percentage of patients who have a known HIV status and overestimate the association between provider type and having a known HIV status. Regardless, TB programs use public health records to ensure adequate management for all TB patients. Therefore, our recommendations for TB programs to educate and follow up with private providers still apply irrespective of the reason for not documenting HIV status in the public health record. Education efforts could be improved by surveying private providers and determining whether HIV tests are not performed or test results are simply not reported.
Finally, our evaluation focused on how patient and provider characteristics affect HIV status determination. However other factors such as confidentiality concerns, language difficulties, test cost, and stigma influence whether HIV tests are offered and accepted. Further research is needed to determine whether HIV status determination is low because providers decline to offer HIV tests or because TB patients refuse HIV tests. Studies should also identify patient and provider concerns regarding HIV testing during TB care and evaluate methods to mitigate them. This information would allow TB programs to target specific HIV testing barriers among patients and providers.
Despite these limitations, our evaluation completed its objectives by finding that (1) one-third of California TB patients in 2008 did not have a known HIV status, (2) HIV status determination appeared to be selective based on provider-perceived risk, and (3) private providers usually have the first opportunity to establish HIV status in TB patients, but patients managed solely by private providers were less likely to have a known HIV status. To ensure that all HIV-infected TB patients are detected and receive potentially life-saving treatment, TB programs should document HIV status for every TB patient as recommended. Providers, especially private providers, should also be encouraged to implement routine opt-out HIV testing for TB patients. On the basis of these recommendations, the California Department of Public Health began initiatives targeting TB programs and providers. First, we developed and distributed statewide guidelines to TB programs reflecting the CDC recommendations for routine optout HIV testing. Second, we produced an information sheet explaining the rationale and benefits of routine HIV testing to educate private providers. Finally, we established HIV status determination completeness as a statewide performance measure and began providing real-time feedback of performance to TB programs.
Acknowledgments
The California Department of Public Health received a grant for this work from the Division of Tuberculosis Elimination, a Division of the Centers for Disease Control and Prevention [5U52 PS900515-28].
The authors thank Nicolette Palermo and Alicia Rodriguez as well as the participating local tuberculosis programs for their efforts in data collection.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Mr Darryl G. Kong, Tuberculosis Control Branch, California Department of Public Health, Richmond.
Dr James P. Watt, Division of Communicable Disease Control, California Department of Public Health, Richmond.
Ms Suzanne Marks, Division of Tuberculosis Elimination, Centers for Disease Control and Prevention, Atlanta, Georgia.
Dr Jennifer Flood, Tuberculosis Control Branch, California Department of Public Health, Richmond.
REFERENCES
- 1.Centers for Disease Control and Prevention Tuberculosis and human immunodeficiency virus infection: recommendations of the Advisory Committee for the Elimination of Tuberculosis (ACET) MMWR Morb Mortal Wkly Rep. 1989;38:236–250. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1–81. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Reported HIV status of tuberculosis patients—United States, 1993-2005. MMWR Morb Mortal Wkly Rep. 2007;56:1103–1106. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Mortality among patients with tuberculosis and associations with HIV status—United States, 1993-2008. MMWR Morb Mortal Wkly Rep. 2010;59:1509–1513. [PubMed] [Google Scholar]
- 6.Cayla JA, Caminero JA, Rey R, Lara N, Valles X, Galdos-Tanguis H. Current status of treatment completion and fatality among tuberculosis patients in Spain. Int J Tuberc Lung Dis. 2004;8:458–464. [PubMed] [Google Scholar]
- 7.Horne DJ, Hubbard R, Narita M, Exarchos A, Park DR, Goss CH. Factors associated with mortality in patients with tuberculosis. BMC Infect Dis. 2010;10:258. doi: 10.1186/1471-2334-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nahid P, Jarlsberg LG, Rudoy I, et al. Factors associated with mortality in patients with drug-susceptible pulmonary tuberculosis. BMC Infect Dis. 2011;11:1. doi: 10.1186/1471-2334-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haar CH, Cobelens FG, Kalisvaart NA, van Gerven PJ, van der Have JJ. HIV-related mortality among tuberculosis patients in The Netherlands, 1993-2001. Int J Tuberc Lung Dis. 2007;11:1038–1041. [PubMed] [Google Scholar]
- 10.Korenromp EL, Scano F, Williams BG, Dye C, Nunn P. Effects of human immunodeficiency virus infection on recurrence of tuberculosis after rifampin-based treatment: an analytical review. Clin Infect Dis. 2003;37:101–112. doi: 10.1086/375220. [DOI] [PubMed] [Google Scholar]
- 11.Burman W, Benator D, Vernon A, et al. Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am J Respir Crit Care Med. 2006;173:350–356. doi: 10.1164/rccm.200503-417OC. [DOI] [PubMed] [Google Scholar]
- 12.Vernon A, Burman W, Benator D, Khan A, Bozeman L. Acquired rifamycin monoresistance in patients with HIV-related tuberculosis treated with once-weekly rifapentine and isoniazid. Tuberculosis Trials Consortium. Lancet. 1999;353:1843–1847. doi: 10.1016/s0140-6736(98)11467-8. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Munsiff SS, Driver CR, Sackoff J. Relapse and acquired rifampin resistance in HIV-infected patients with tuberculosis treated with rifampin- or rifabutin-based regimens in New York City, 1997-2000. Clin Infect Dis. 2005;41:83–91. doi: 10.1086/430377. [DOI] [PubMed] [Google Scholar]
- 14.Dheda K, Lampe FC, Johnson MA, Lipman MC. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis. 2004;190:1670–1676. doi: 10.1086/424676. [DOI] [PubMed] [Google Scholar]
- 15.Nahid P, Gonzalez LC, Rudoy I, et al. Treatment outcomes of patients with HIV and tuberculosis. Am J Respir Crit Care Med. 2007;175:1199–1206. doi: 10.1164/rccm.200509-1529OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan FA, Minion J, Pai M, et al. Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clin Infect Dis. 2010;50:1288–1299. doi: 10.1086/651686. [DOI] [PubMed] [Google Scholar]
- 17.King L, Munsiff SS, Ahuja SD. Achieving international targets for tuberculosis treatment success among HIV-positive patients in New York City. Int J Tuberc Lung Dis. 2010;14:1613–1620. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep. 2005;54:1–47. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention . Instruction Manual: Report of Verified Case of Tuberculosis. US Department of Health and Human Services Centers for Disease Control and Prevention; Washington, DC: 2009. http://www.cdc.gov/tb/programs/rvct/InstructionManual.pdf. Accessed 1 June, 2011. [Google Scholar]
- 20.Rodger AJ, Story A, Fox Z, Hayward A. HIV prevalence and testing practices among tuberculosis cases in London: a missed opportunity for HIV diagnosis? Thorax. 2010;65:63–69. doi: 10.1136/thx.2009.122754. [DOI] [PubMed] [Google Scholar]
- 21.Stout JE, Ratard R, Southwick KL, Hamilton CD. Epidemiology of human immunodeficiency virus testing among patients with tuberculosis in North Carolina. South Med J. 2002;95:231–238. [PubMed] [Google Scholar]
- 22.Asch SM, London AS, Barnes PF, Gelberg L. Testing for human immunodeficiency virus infection among tuberculosis patients in Los Angeles. Am J Respir Crit Care Med. 1997;155:378–381. doi: 10.1164/ajrccm.155.1.9001340. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins TC, Gardner EM, Thrun MW, Cohn DL, Burman WJ. Risk-based human immunodeficiency virus (HIV) testing fails to detect the majority of HIV-infected persons in medical care settings. Sex Transm Dis. 2006;33:329–333. doi: 10.1097/01.olq.0000194617.91454.3f. [DOI] [PubMed] [Google Scholar]
- 24.Albalak R, O’Brien RJ, Kammerer JS, et al. Trends in tuberculosis/human immunodeficiency virus comorbidity, United States, 1993-2004. Arch Intern Med. 2007;167:2443–2452. doi: 10.1001/archinte.167.22.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris TG, Li J, Hanna DB, Munsiff SS. Changing sociodemographic and clinical characteristics of tuberculosis among HIV-infected patients, New York City, 1992-2005. Clin Infect Dis. 2010;50:1524–1531. doi: 10.1086/652654. [DOI] [PubMed] [Google Scholar]
- 26.Sturtevant D, Preiksaitis J, Singh A, et al. The feasibility of using an “opt-out” approach to achieve universal HIV testing of tuberculosis patients in Alberta. Can J Public Health. 2009;100:116–120. doi: 10.1007/BF03405519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke RC, Sepkowitz KA, Bernstein KT, et al. Why don’t physicians test for HIV? A review of the US literature. AIDS. 2007;21:1617–1624. doi: 10.1097/QAD.0b013e32823f91ff. [DOI] [PubMed] [Google Scholar]
- 28.Irwin KL, Valdiserri RO, Holmberg SD. The acceptability of voluntary HIV antibody testing in the United States: a decade of lessons learned. AIDS. 1996;10:1707–1717. doi: 10.1097/00002030-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Lawn SD, Fraenzel A, Kranzer K, Caldwell J, Bekker LG, Wood R. Provider-initiated HIV testing increases access of patients with HIV-associated tuberculosis to antiretroviral treatment. S Afr Med J. 2011;101:258–262. doi: 10.7196/samj.4392. [DOI] [PubMed] [Google Scholar]
- 30.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N EnglJ Med. 2005;352:586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 31.Bock NN, McGowan JE, Jr, Blumberg HM. Few opportunities found for tuberculosis prevention among the urban poor. Int J Tuberc Lung Dis. 1998;2:124–129. [PubMed] [Google Scholar]
- 32.Murphy DA, Mitchell R, Vermund SH, Futterman D. Factors associated with HIV testing among HIV-positive and HIV-negative high-risk adolescents: the REACH Study. Reaching for Excellence in Adolescent Care and Health. Pediatrics. 2002;110:e36. doi: 10.1542/peds.110.3.e36. [DOI] [PubMed] [Google Scholar]
- 33.Royce RA, Walter EB, Fernandez MI, Wilson TE, Ickovics JR, Simonds RJ. Barriers to universal prenatal HIV testing in 4 US locations in 1997. Am J Public Health. 2001;91:727–733. doi: 10.2105/ajph.91.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson JE, Koenig LJ, Lampe MA, Wright R, Leiss J, Saul J. Achieving universal HIV screening in prenatal care in the United States: provider persistence pays off. AIDS Patient Care STDS. 2005;19:247–252. doi: 10.1089/apc.2005.19.247. [DOI] [PubMed] [Google Scholar]


