Abstract
Listeria monocytogenes is a psychrotolerant food borne pathogen, responsible for the high fatality disease listeriosis, and expensive food product recalls. Branched-chain fatty acids (BCFAs) of the membrane play a critical role in providing appropriate membrane fluidity and optimum membrane biophysics. The fatty acid composition of a BCFA-deficient mutant is characterized by high amounts of straight-chain fatty acids and even-numbered iso fatty acids, in contrast to the parent strain where odd-numbered anteiso fatty acids predominate. The presence of 2-methylbutyrate (C5) stimulated growth of the mutant at 37°C and restored growth at 10°C along with the content of odd-numbered anteiso fatty acids. The C6 branched-chain carboxylic acids 2-ethylbutyrate and 2-methylpentanoate also stimulated growth to a similar extent as 2-methylbutyrate. However, 3-methylpentanoate was ineffective in rescuing growth. 2-ethylbutyrate and 2-methylpentanoate led to novel major fatty acids in the lipid profile of the membrane that were identified as 12-ethyltetradecanoic acid and 12-methylpentadecanoic acid respectively. Membrane anisotropy studies indicated that growth of strain MOR401 in the presence of these precursors increased its membrane fluidity to levels of the wild type. Cells supplemented with 2-methylpentanoate or 2-ethylbutyrate at 10°C shortened the chain length of novel fatty acids, thus showing homeoviscous adaptation. These experiments use the mutant as a tool to modulate the membrane fatty acid compositions through synthetic precursor supplementation, and show how existing enzymes in L. monocytogenes adapt to exhibit non-native activity yielding unique ‘unnatural’ fatty acid molecules, which nevertheless possess the correct biophysical properties for proper membrane function in the BCFA-deficient mutant.
Keywords: Branched-chain fatty acids, branched-chain carboxylic acids, novel fatty acids, membrane biophysical properties, fatty acid composition, membrane fluidity
1. Introduction
Listeria monocytogenes is a Gram-positive, foodborne, intracellular pathogen that is the causative agent of listeriosis. The organism is also responsible for periodic expensive food product recalls when food is found to be contaminated with L. monocytogenes. Early this year, a total of 35 people were infected from a multistate Listeria outbreak from prepackaged Granny Smith and Gala apples. This was closely followed by a major statewide outbreak from Blue Bell creamery products followed by immediate recalls and reports that the contamination dated back several years (http://www.cdc.gov/listeria/outbreaks). The ability of L. monocytogenes to grow at refrigeration temperatures is an important factor in its role as a foodborne pathogen [1].
The fatty acid composition of the L. monocytogenes cytoplasmic membrane is unusual in that it is composed almost entirely of branched-chain fatty acids (BCFAs) [2, 3, 4]. Typically, the major fatty acids of the organism are anteiso C15:0, anteiso C17:0 and iso C15:0. This fatty acid composition enables L. monocytogenes to adapt to growth at low temperatures, mainly by increasing the content of anteiso C15:0 by a combination of fatty acid chain shortening and branched-chain switching from iso to anteiso [2, 3, 4, 5]. This is a homeoviscous adaptation to maintain appropriate membrane fluidity [6].
BCFAs are biosynthesized from the branched-chain amino acids isoleucine (anteiso fatty acids), leucine (odd-numbered iso fatty acids) and valine (even-numbered iso fatty acids) via branched-chain amino acid transaminase and branched- chain α-keto acid dehydrogenase (Bkd) [7]. Mutants in the bkd gene cluster are cold-sensitive, deficient in BCFAs, and have lower membrane fluidity that the parent strain, and all these defects can be corrected with the short-branched- chain carboxylic acids (BCCAs) that act as precursors for BCFAs via a pathway that bypasses the branched- chain amino acids and Bkd [2, 5, 8, 9].
Studies with the cold-sensitive mutants, cld-1 and cld-2 revealed a switch in the fatty acid composition to one where the major fatty acids are straight-chain fatty acids (SCFAs) and iso-even BCFAs [2, 5, 8, 9], a fatty acid profile incompatible with good growth at low temperature due to high membrane viscosity. In the absence of branched-chain keto acid dehydrogenase activity it is proposed that the SCFAs originate from butyryl CoA, and isobutyryl CoA. Isobutyryl CoA, the precursor of even-numbered iso BCFAs, may be produced via valine dehydrogenase or isomerization of butyryl CoA [2]. However, the biophysical properties of the membrane have broader impacts in listerial physiology and pathogenicity than just cold adaptation. Giotis et al. [10] have shown that the BCFA-deficient cld mutants tolerate acidic and alkaline pH less well than the parent strain, a tolerance that can be restored by medium supplementation with 2-methyl butyrate (2-MB). Sun and O'Riordan [11] showed that BCFA-deficient mutants grew and survived less well in macrophages, exhibited decreased production of the key virulence factor listeriolysin O, and were highly attenuated in a murine model of infection. In an extension of these studies, BCFAs played a critical role in protection against antimicrobial peptides and peptidoglycan hydrolases [12]. In all these cases 2-MB restored the anteiso C15:0 and anteiso C17:0 fatty acid content and the defects in the mutants to a large extent.
In 1971 Kaneda [13] showed that various C6 BCCAs led to the production of novel fatty acids derived from these precursors in Bacillus subtilis. 2-ethylbutyrate (2-EB) and 2-methylpentanoate (2-MP) were the most effective precursors. These two precursors in high (100 mM) concentrations also led to the production of novel “unnatural” fatty acids when added to the cultures of wild type L. monocytogenes [14]. Also, Willecke and Pardee [15] studied a set of chemical analogues of natural BCFA-yielding precursors and found that a B. subtilis bkd mutant used them in the same pattern as noted by Kaneda (1971) [13]. However, neither of these studies examined the effects of the C6 BCCA precursors on growth at low temperatures. It was of interest to see whether these precursors would stimulate the growth of BCFA-deficient L. monocytogenes mutant MOR401 at various temperatures, and see what impact they had on the fatty acid composition of the mutants and their membrane biophysical properties. 2-MP and 2-EB supplementation led to major amounts of novel fatty acids, increased membrane fluidity, and stimulated the growth of the BCFA-deficient mutant at 37°C and 10°C.
2. Materials and methods
2.1 Bacterial strains and growth conditions
L. monocytogenes strains used in this study were parent strain 10403S and cold-sensitive mutant MOR401 (kindly provided by Yvonne Sun and Mary X. D. O'Riordan) harboring a Tn917 transposon insertion in the lpd gene of the bkd gene cluster (lipoamide dehydrogenase, E3) created by transduction of the mutation from strain cld-2 [2, 5] into strain 10403S [11]. The strains were grown in Brain-Heart Infusion (BHI) broth (Difco Laboratories, Detroit, MI). Starter cultures of mutant strain MOR401 were grown in medium supplemented with erythromycin (1 ug ml-1).
For growth and fatty acid composition studies 50 ml of BHI medium, not supplemented with any antibiotic, in a 300 ml Erlenmeyer flask were inoculated with 2% (vol/vol) of overnight starter culture. The cells were grown at 37°C and 10°C with continuous shaking at 200 rpm in the presence of 1 mM concentrations of 2-MB and the C6 BCCAs 2-EB, 2-MP and 3-methyl pentanoate (3-MP) along with a wide range of other short BCCAs with varying chain lengths shown in Table 1. Straight chain precursor, butyrate, was used as a negative control. These BCCAs and butyrate were neutralized with 10 M NaOH to a final pH of 7.0 and added to BHI as filter sterilized solutions. The growth kinetics of the strains were monitored by measuring OD600 using a Beckman DU-65 spectrophotometer. Cultures were appropriately diluted after the OD600 reached 0.5. Growth experiments were carried out on three separate occasions, and results of representative experiments are shown.
Table 1. Various branched-chain carboxylic acids with diverse branching pattern and chain lengths.
| Fatty acid precursors | Chemical structure | Branch | Chain length of hydrocarbon backbone |
|---|---|---|---|
| Trimethyl acetate (C5) |
|
2 (dimethyl) | 3C |
| 2-methylbutyrate (C5) |
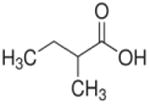
|
2 methyl | 4C |
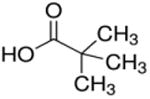
| 2,2-dimethylbutyrate (C6) |
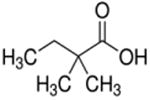
|
2,2-dimethyl | 4C |
| 2-ethylbutyrate (C6) |
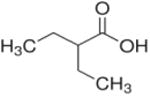
|
2 ethyl | 4C |
| 2-methylpentanoate (C6) |
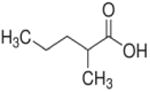
|
2 methyl | 5C |
| 3-methylpentanoate (C6) |
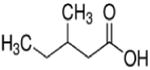
|
3 methyl | 5C |
| 4-methylpentanoate (C6) |
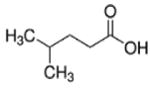
|
4 methyl | 5C |
| 2-methylhexanoate (C7) |
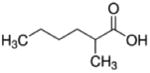
|
2 methyl | 6C |
| 2-ethylhexanoate (C8) |
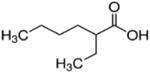
|
2 ethyl | 6C |
| 4-methylhexanoate (C7) |
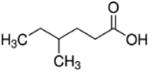
|
4 methyl | 6C |
| 2-methylheptanoate (C8) |
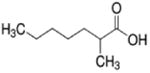
|
2 methyl | 7C |
2.2 Membrane fatty acid analysis
Cells grown in BHI with or without the BCFA precursors were harvested in mid-exponential phase (OD600 0.4-0.6) by centrifugation at 3000 × g at 4°C for 15 minutes, and the pellet was washed 3 times with cold sterile distilled water. The fatty acids in the bacterial cells (30 to 40 mg [wet weight]) were saponified, methylated, and extracted. The resulting methyl ester mixtures were separated using an Agilent 5890 dual-tower gas chromatograph and identified using the MIDI microbial identification system (Sherlock 4.5 microbial identification system) at Microbial ID, Inc. (Newark, DE) [14, 5]. Minor fatty acids (<1% of the total) are not reported in the tables. Novel BCFAs were further characterized by electron ionization mass spectroscopy [16].
2.3 Determination of the membrane fluidity
Membrane fluidity was determined through anisotropy measurements using the fluorophore 1,6-diphenyl-1,3,5-hexatriene (DPH) which specifically fluoresces in the hydrophobic domain of fatty acyl chains in the lipid bilayer of the membrane [17]. Exponential phase (OD600 0.4-0.6) cells grown with or without precursors were pelleted by centrifugation at 3000 × g at 4°C for 15 minutes and washed twice with 0.85% (wt/vol) NaCl solution. The cells were resuspended in 0.85% (wt/vol) NaCl containing 2 μM DPH (Sigma, MO) to an OD600 of 0.3 and incubated at 37°C for 1 hr. The resulting fluorescence polarization values of DPH were measured in a PTI fluorescence spectrophotometer using FelixGX software. Excitation of the fluorescent probe was accomplished with vertically polarized monochromatic light at 360 nm for DPH, with emission intensity quantified at 426 nm, using a detector oriented either parallel to or perpendicular to the direction of the polarized excitation source. Lower fluidity leads to decreased movement of the probe in the membrane. This subsequently results in lesser distortion of the emitted signal and higher anisotropy values recorded by the fluorimeter.
2.4 Light microscopy
The bacterial cells were grown in BHI broth or BHI broth supplemented with BCCA precursors at 37°C and harvested at mid log phase. The pellets were washed with PBS and Gram-stained. The cells were then observed via light microscopy using differential interference contrast with the 100× objective.
3. Results
3.1 The C6 BCCAs stimulate growth of the BCFA-deficient mutant
It is well established that C5 BCCA 2-MB enhances the growth of BCFA-deficient strains of L. monocytogenes [2,5], and this is confirmed in Fig. 1a and 1b. The growth of the parent and MOR401 in unsupplemented BHI medium at 37°C is shown in Fig. 1a. The lag phase of strain MOR401 was significantly longer than the parent strain, the growth rate was slower and the final cell density achieved was considerably lower. As expected, 2-MB markedly stimulated the growth of the mutant at 37°C such that the growth rate and the final cell density achieved were similar to the parent strain. Interestingly the lag phase of strain MOR401 was not reduced significantly by the presence of 2-MB. C6 BCCA 2-MP had an effect on growth very similar to 2-MB, as did 2-EB except for a slightly longer period to exit lag phase with this precursor. The extended lag phase of the mutant may be related to its impairment in cell division that we noted-see Fig. 4. The precursor 3-MP had a negligible stimulating effect on growth compared to 2-MB, 2-MP or 2-EB. Butyrate, a C4 straight-chain carboxylic acid, which can generate SCFAs [14], served as a negative control that had no stimulatory effect on growth.
Fig.1. Influence of the BCCA precursors on the growth of the BCFA-deficient mutant MOR401 (a) at 37°C and (b) at 10°C.
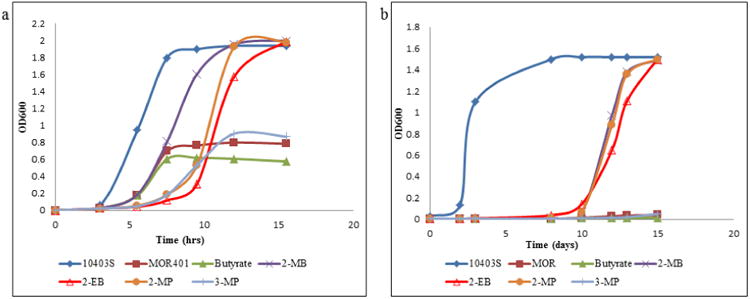
Symbols: (◆) parent strain 10403S; all other symbols are strain MOR401 with no supplementation (■) or supplemented with butyrate (▲) 2-MB (×) 2-EB (Δ) 2-MP (•) 3-MP (+) Representative figures from triplicate experiment sets are shown.
Fig.4. Light microscopic analysis of the BCFA-deficient mutant MOR401 under the influence of different fatty acid precursors.
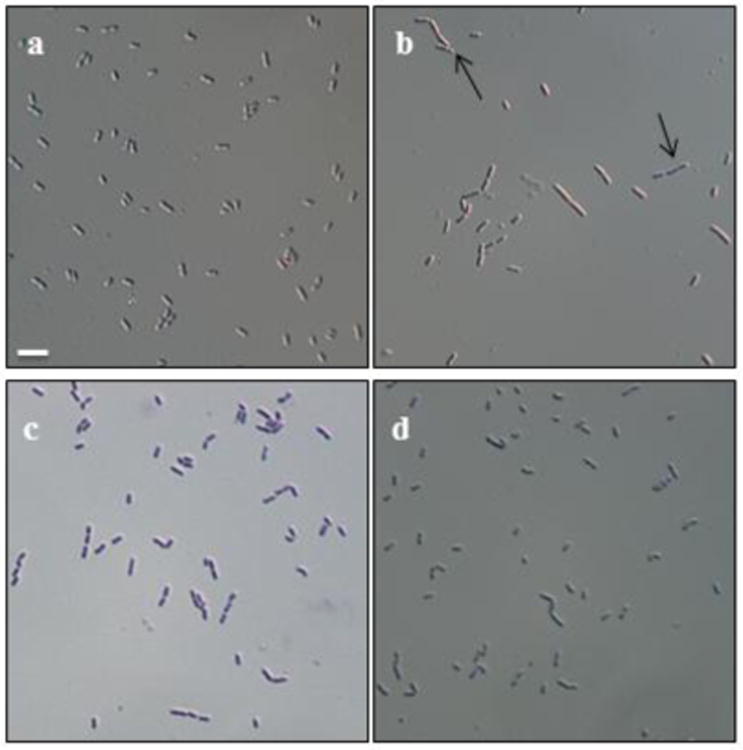
Compared to the wild type Listeria monocyogenes 10403S cells (a), the BCFA-deficient mutant grown in unsupplemented BHI (b) is unusually long and exhibits irregular division sites (pointed out by arrows) Precursors 2-MB (c) and 2-EB (d) restored the cell dimensions and normal cell division. Scale bar= 4 μm
At 10°C (Fig. 1b) the results were more striking. The mutant barely grew at this temperature, and growth was further diminished by inclusion of the C4 straight-chain carboxylic acid butyrate in the medium. Strikingly, 2-MP and 2-EB were equally effective if not more effective than 2-MB in stimulating growth. It is proposed that 2-MP and 2-EB act as precursors for novel unnatural fatty acids that are incorporated into the membrane [13,14,15], yet which appear to have properties that result in ideal membrane fluidity and appropriate membrane biophysical properties to allow the mutant to grow at 10°C.
3.2 Growth in the presence of C6 BCCAs results in novel membrane fatty acids
Given the growth stimulatory effects of the C6 BCCAs it seemed likely that they were altering the membrane fatty acid composition of strain MOR401. Accordingly, the fatty acid compositions of cells grown in the absence and presence of 1 mM concentrations of the BCCAs at 37°C were determined. The gas liquid chromatograph traces are shown in Fig. 1 (Supplemental) and the fatty acid compositions are shown in Table 2. Strain MOR401 has a significantly different fatty acid composition from its parent strain 10403S (Table 2). Ninety eight per cent of the fatty acids were BCFAs in the parent strain, with the major fatty acids being anteiso C15:0 and C17:0 and iso C15:0. In contrast BCFAs only made up 33% of the total in strain MOR401 and the major fatty acids were C16:0, C14:0 and iso C16:0 (Table 2 and Fig 1a supplemental). However, when strain MOR401 was grown in the presence of 2-MB two major peaks appeared in the gas chromatograph trace with retention times of 2.44 and 3.08 minutes (Fig. 1b supplemental) that were identified as anteiso C15:0 (52.3%) and anteiso C17:0 (40.8%). The results are in perfect accord with previous studies on strain cld-2 [2, 5, 12]. Growth in the presence of 2-MP led to two novel major peaks in the chromatograph with retention times of 2.61 and 3.24 min (Fig. 1c supplemental), respectively, constituting 64.5 and 12.3% of the total fatty acids. In fatty acid biosynthesis two carbon atoms are added at a time to a precursor CoA molecule until the fatty acid chain reaches the required length for incorporation into the membrane [18]. In this case the precursor molecule is postulated to be 2-MP-CoA and the two fatty acids are postulated to be 12-methylpentadecanoic acid (C16) and 14-methylheptadecanoic acid (C18). Similarly, the presence of 1 mM 2-EB in the medium resulted in two major peaks of retention times 2.75 and 3.38 min, respectively constituting 50.4 and 31.6% of the total fatty acids (Fig. 1d supplemental). These fatty acids are postulated to be 12-ethyltetradecanoic acid (C16) and 14-ethylhexadecanoic acid (C18) respectively. Very similar results were found when the experiments were performed with strain cld-2 (unpublished observations). Mass spectral analysis (Fig. 2a and b) revealed a spectrum corresponding to an ethyl branch on the 12th carbon of a 14 carbon chain (peak one) and an ethyl branch on the 14th carbon of a 16 carbon chain (peak two) of these two major peaks in the gas chromatogram. Clearly 2-MP and 2-EB led to the production of novel fatty acids that under normal circumstances would not normally be found in a bacterial membrane. However, their physical structures apparently endow the membrane with biophysical properties that result in stimulation of the growth of the BCFA-deficient mutant at both temperatures.
Table 2. The membrane fatty acid profile of L. monocytogenes parent strain 10403S and the BCFA-deficient mutant MOR401 grown in BHI at 37ᵒC with or without BCCA precursors.
% (wt/wt) of total fatty acids
| Strain, growth conditions |
Anteiso odd | Iso odd |
Iso even |
Straight even |
Novel fatty acids | BCFA | SCFA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C15:0 | C17:0 | SUM | |||||||||
| 10403S | 47.77±0. 59 | 34.76±0. 4 | 82.5 | 11.7 | 3.82 | 1.94 | ND | ND | ND | 98.07 | 1.94 |
| MOR401 | 5.4±1.9 | 2±1.8 | 7.4 | 1.6 | 24.1 | 57.2 | ND | ND | ND | 33.2 | 66.0 |
| MOR401+2 MB | 52.3±1.5 | 40±0.7 | 93.1 | ND | 0.74 | 6.2 | ND | ND | ND | 93.8 | 6.2 |
| MOR401+2EB | 0.6±0.01 | 1.8±0.3 | 2.3 | ND | 2.39 | 12.4 | ND | 12-ethyltetradecanoic acid 50.4±2.7 | 14-ethylhexadecanoic acid 31.6±0.6 | 86.8 | 13.3 |
| MOR401+2 MP | 1.4±0.5 | 2.4±0.7 | 3.8 | ND | 10.5 | 5.4 | 10-methyltridecanoic acid 1.04±0.08 | 12-methylpentadecanoic acid 64.5±0.6 | 14-methylheptadecanoic acid 12.3±2.6 | 92.4 | 6.8 |
| MOR401+trimethylacetate | 7.6±1.3 | 10.2±0.1 | 17.8 | ND | 28.1 | 11.2 | ND | 12-dimethyltridecanoic acid 21.1±1.4 | 14-dimethylpentadecanoic acid 20.2±1.4 | 87.2 | 11.2 |
| MOR401+2,2-dimethylbut yrate | 9.5±1.5 | 12.02±0. 01 | 21.5 | ND | 35.3 | 10.8 | ND | 12,12-dimethyltetradecanoic acid 21.5±1.6 | 14,14-dimethylhexadecanoic acid 8±0.6 | 86.3 | 13.7 |
All supplements were used at 1 mM.
The percentages of respective fatty acids are means from three independent experiments with standard deviations
ND- Not detected
The minor fatty acids (<1%) are not reported. This includes minor percentages of odd numbered SCFAs in some cases.
Fig. 2. Mass spectral analysis of membrane fatty acids of the BCFA-deficient mutant strain MOR401 when grown in presence of 2-EB.
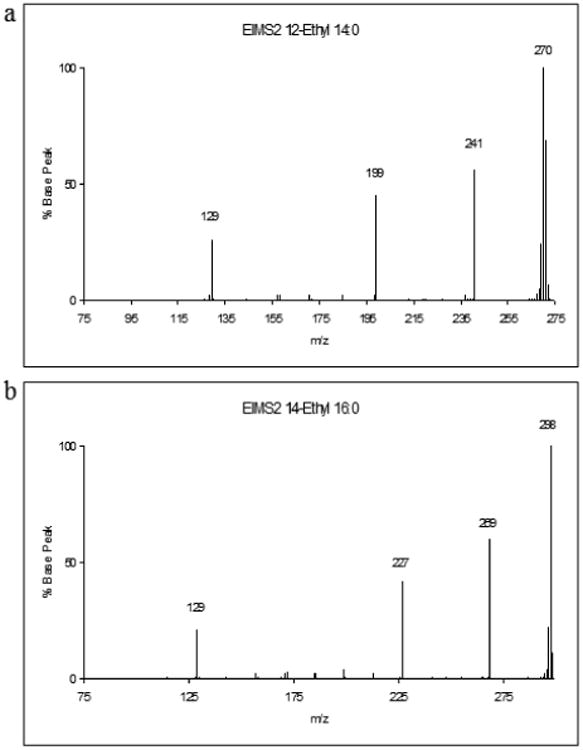
Two novel fatty acid methyl ester peaks with retention times (a) 2.75 min and (b) 3.38 min from the GLC analysis were subjected to mass spectral analysis. The peaks yielded spectra that correspond to (a) an ethyl branch on the 12 carbon of a 14 carbon chain, and (b) on the 14th carbon on a 16 carbon chain.
In order to achieve membrane homeoviscosity at low temperatures L. monocytogenes increases the proportion of fatty acid anteiso C15:0 in its lipids [2, 5, 8], by fatty acid chain shortening and branching switching. It was therefore of interest to observe whether any changes occurred in the proportions of the unnatural even-numbered fatty acids in response to growth at low temperatures. When grown in the presence of 2-MP at 10°C, 10-methyltridecanoic acid increased from 1.04 to 14.4%, 14-methylheptadecanoic acid decreased from 12.3 to 0.5% in strain MOR401 and 12-methylpentadecanoic acid increased to 66% from 64% (Table 3) compared to cells grown at 37°C (Table 2). Similarly, when grown at 10°C in the presence of 2-EB, 12-ethyltetradecanoic acid increased to 65.9% from 50%, and 14-ethylhexadecanoic acid decreased to 14.2% from 31.6% (Table 3) compared to cells grown at 37°C (Table 2). Thus, even with the novel BCFAs the cells can execute successful homeoviscous adaptation at low temperature due to fatty acid shortening.
Table 3. The membrane fatty acid profile of L. monocytogenes parent strain 10403S and the BCFA-deficient mutant MOR401 supplemented with BCCA precursors grown in BHI at 10°C.
% (wt/wt) of total fatty acids
| Strain, growth conditions |
Anteiso odd | Iso odd |
Iso even |
Novel fatty acids | BCFA | SCFA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| C15:0 | C17:0 | SUM | ||||||||
| 10403S | 69.9±1.5 | 7.6±0.8 | 78.6 | 15.2 | 4.9 | ND | ND | ND | 98.8 | 0.6 |
| MOR401+2MB | 77.9±1.4 | 15.8±0.5 | 94.7 | ND | 0.1 | ND | ND | ND | 95.3 | 3.6 |
| MOR401+ 2EB | 1.4±0.21 | 1.5±0.7 | 2.9 | ND | 4.3 | ND | 12-ethyltetradecanoic acid 65.9±5.5 | 14-ethylhexadecanoic acid 14.2±2.6 | 87.7 | 10.8 |
| MOR401+2MP | 1.7±0.18 | 0.6±0.8 | 2.3 | ND | 11.8 | 10-methyltridecanoic acid 14.4±5 | 12-methylpentadecanoic acid 65.9±0.42 | 14-methylheptadecanoic acid 0.5±0.63 | 94.2 | 4.5 |
All supplements were used at 1 mM.
The percentages of respective fatty acids are means from three independent experiments with standard deviations
ND- Not detected
The minor fatty acids (<1%) are not reported.
3.3 Incorporation of unnatural even-numbered BCFAs results in increased membrane fluidity
It was expected that given the incorporation of large amounts of the novel fatty acids and the enhancement of the growth of the mutant the membrane fluidity of the cells grown in the presence of 2-MP and 2-EB would be enhanced compared to growth in unsupplemented BHI medium. When strain MOR401 was grown in unsupplemented BHI medium at 37°C it exhibited a polarization value of 0.24, which was much higher than that observed in the parent strain (0.17) under the same conditions, and indicates a less fluid membrane (Fig. 3). This directly correlates with the deficiency of odd-numbered anteiso fatty acids along with a high proportion of SCFAs and even iso fatty acids in the mutant. When the medium was supplemented with the different BCFA precursors, there was a considerable decrease in the anisotropy values confirming an increase in the fluidity of the membrane (Fig. 3). Inclusion of 2-MB restored the fluidity close to the wild-type, as did 2-EB and 2-MP (Fig. 3).
Fig. 3. Influence of the short chain carboxylic acid precursors on the membrane fluidity of the BCFA-deficient mutant strain MOR401 at 37°C.
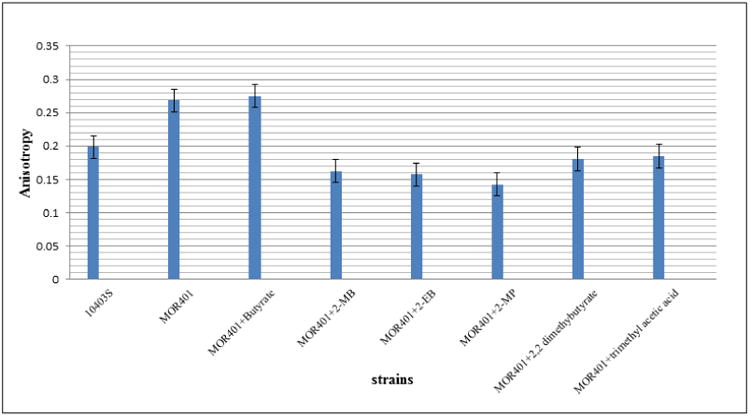
The strains were grown in medium supplemented with the indicated fatty acid precursors and membrane anisotropy was measured by fluorescence polarization.
3.4 BCCA supplementation helps restore normal cell division of the BCFA-deficient mutant
Light microscopic analysis of the bacterial cells showed that when grown in unsupplemented BHI broth, strain MOR401 appeared to be unusually long (almost 3μm) with irregular division sites (identified by arrows in Fig. 4), in contrast to the wild type cells which are short rods. It seems that the BCFA deficiency and decreased membrane fluidity interfere with proper division of the mutant. Supplementation with 2-MB, which reinstates BCFA content and fluidity of the membrane, restored normal division of the cells. 2-EB also showed similar effects on cell division which indicates the novel BCFAs generated from synthetic substrates can also provide the appropriate biophysical properties to the membrane for normal cell division.
3.5 A 2-position branch in the exogenous BCCA precursors effectively rescued the growth of the mutant
A range of BCCAs, with variations in chain lengths and branching patterns, were studied for their ability to stimulate the growth of the strain MOR401 in order to study the fundamental structural requirements for functioning as an efficient fatty acid precursor. The mutant was grown in the presence of each of these precursors at 37°C and the growth kinetics were determined (Fig. 5) Like butyrate none of the corresponding straight-chain substrates -pentanoate (C5), hexanoate (C6) or heptanoate (C7) had any influence on growth of the mutant (data not shown). Among the various BCCA precursors studied of varying chain lengths, the position of branching and nature of branching, a methyl, dimethyl or ethyl branch at the 2 position were found to be effective in stimulating the growth of the mutant at 37°C (Fig. 5). Trimethyl acetate and 2,2-dimethylbutyric acid were efficient in rescuing growth at 37°C, but to a lesser extent than 2-MB. Trimethyl acetate supplementation yielded two primary novel peaks of retention times 2.26 and 2.89 which are postulated to be 12-dimethyltridecanoic acid and 14-dimethylpentadecanoic acid respectively, each constituting about 20% of the total membrane fatty acid composition (Table 2). 2,2-dimethylbutyrate supplementation also had a similar outcome with the membrane having a total of 85% BCFA of which 12,12-dimethyltetradecanoic acid (retention time 2.57 min) and 14,14-dimethylhexadecanoic acid(retention time 3.21 min) were postulated to be the novel fatty acids. However these precursors were unable to support the growth of the mutant at low temperatures. The fluidity of the membrane was moderately increased by the dimethyl branched precursors (Fig. 3).
Fig.5. Influence of various short BCCA precursors on the growth of the BCFA-deficient mutant strain MOR401 at 37°C.
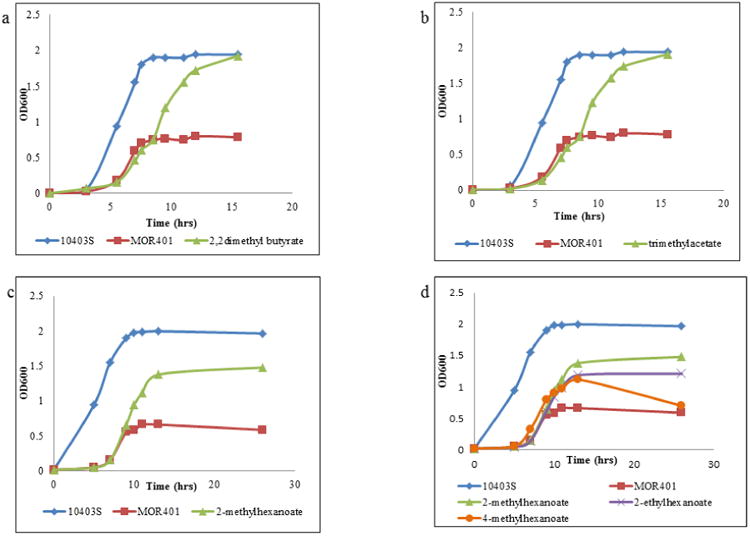
- ◆ parent strain 10403S; ■ MOR401 with no supplementation; ▲ MOR401 with 2,2-dimethylbutyrate
- ◆ parent strain 10403S; ■ MOR401 with no supplementation; ▲ MOR401 with trimethylacetate
- ◆ parent strain 10403S; ■ MOR401 with no supplementation; ▲ MOR401 with 2-methylhexanoate
- ◆ parent strain 10403S; ■ MOR401 with no supplementation; ▲ MOR401with 2-methylhexanoate; x MOR401with 2-ethylhexanoate; • MOR401with 4-methylhexanoate
Representative figures from triplicate experiment sets are shown.
Although we observed that a branch at the second position was crucial for the precursors to rescue the growth of the mutant, the length of the carbon chain plays a role too. Among the 2 carbon-branched BCCAs we tested, IB, 2-MB, 2-MP (chain lengths of 3, 4 and 5 respectively) were highly efficient in stimulating the growth of the BCFA mutant. However, the efficacy decreased drastically when 2-methylhexanoate was used as a substrate, which has a chain length of 6 carbons. This decreased efficiency may be because of inefficient conversion to the corresponding CoA derivative by the bypass pathway enzymes, or inefficient utilization of the CoA precursors by FabH.
4 Discussion
4.1 Fatty acid biosynthesis in the BCFA-deficient mutant
Strain MOR401 contains a Tn917 insertion in the lpd gene of the bkd operon and thus has a nonfunctional branched-chain keto acid dehydrogenase enzyme complex. This leads to a strikingly different fatty acid composition than the parent strain, 10403S, in which even numbered SCFAs make up about 65% of the total fatty acids and iso-even numbered fatty acids about 25% in the mutant. The iso-even numbered fatty acids are biosynthesized from isobutyryl-CoA. Under normal circumstances this fatty acid primer is produced from the branched-chain amino acid valine via branched-chain amino acid transaminase followed by branched-chain keto acid dehydrogenase activities (Fig. 6). This route is not operative in strain MOR401 [5]. Potential alternative routes to isobutyryl-CoA include from valine via valine dehydrogenase, and by isomerization of butyryl-CoA [2].
Fig.6.

Endogenous pathway for membrane BCFA production and the putative pathway for exogenous BCCA utilization in L. monocytogenes
FabH carries out the first condensation reaction in fatty acid biosynthesis [18]. Butyryl-CoA is likely to be the precursor for the SCFAs found in high amounts in MOR401 because L. monocytogenes FabH has very low activity with acetyl-CoA, the only other likely possible precursor of even numbered SCFAs [19]. However, there does not appear to be any information on how butyryl-CoA might be formed endogenously in L. monocytogenes. It is not known whether butyrate is present in BHI medium to act as a source of butyryl-CoA. However, when strain cld-2 (from which MOR401 is derived) is grown in defined medium without butyrate the strain's fatty acid composition is also characterized by a high proportion of even numbered SCFAs [2]. This would also argue against longer chain fatty acids being incorporated into the phospholipids of the organism from the growth medium, such as happens in S. aureus [20]. A possible route to butyryl-CoA is via the condensation of two molecules of acetyl-CoA to form acetoacetyl-CoA, a reaction catalyzed by the enzyme thiolase followed by a series of enzymatic reactions studied extensively in butyrate-producing bacteria such as species of Clostridium [21]. When a genome-wide search for these enzymes or their homologs was carried out throughout the Listeria genus including L. monocytogenes strains 10403S and EGDe, they all seem to be present (data not shown), although not well characterized. Thus it seems feasible that in absence of pathways producing BCFAs in the membrane, butyryl-CoA production and its corresponding elongation leads to SCFAs dominating the membrane profile of the BCFA-deficient mutant.
When MOR401 (or cld-2) is supplied with 2-MB in the medium it causes a dramatic switch in fatty acid composition such that anteiso C15:0 and C17:0 become almost 90% of the total fatty acid composition (Table 2) [2,5]. This clearly results in a membrane with much more ideal biophysical properties for the organism, enabling it to grow efficiently even at low temperatures. Thus there appears to be a bypass to allow the organism to utilize an exogenous supply of short BCCA precursors yielding acyl-CoA primers ready to enter FASII elongation pathway. 2-MBCoA is the preferred L. monocytogenes FabH substrate with the highest activity among various precursors tested [19]. Although the pathway to 2-MBCoA from an exogenous source has not been ascertained yet, the bkd operon contains two genes upstream of the bkd cluster, buk and ptb, encoding butyrate kinase (Buk) and phosphotransbutyrylase (Ptb) respectively [5], which could function to produce 2-MBCoA after 2-MB crosses the L. monocytogenes cytoplasmic membrane as depicted in Fig 6.
Supply of 2-MP and 2-EB in the growth medium also results in very high proportions of fatty acids in the membrane derived from them, not observed under normal circumstances. Clearly, a pathway must exist to produce 2-MPCoA and 2-EBCoA and these C6 primer molecules are then used efficiently by FabH for elongation and incorporation. Buk and Ptb, which we hypothesize form 2-MBCoA from 2-MB, are also likely to catalyze the formation of the CoA derivatives from 2-MP and 2-EB suggesting a relatively wide substrate specificity of the enzymes. It would be interesting to characterize the kinetic parameters of FabH with these substrates, as well as those of the enzymes in the pathway leading to production of 2-MPCoA and 2-EBCoA. Our unpublished observations show that the C6 BCCA substrates can be efficiently utilized by Ptb.
4.2 Membrane fluidity and physiological properties of 2-MP- and 2-EB-grown BCFA-deficient mutant
Both 2-MP and 2-EB stimulated growth of MOR401 at 37°C and restored growth of the strain at 10°C. The main fatty acids generated from these precursors, 12-methylpentadecanoic acid and 12-ethyltetradecanoic acid respectively, although unnatural provide the membrane with biophysical properties that restore its functional efficiency. Strain MOR401 is impaired in cell division producing short chains of unusually long and irregularly dividing cells. Inclusion of 2MB, 2-MP, or 2-EB in the medium corrects this and restores the normal cellular arrangement of the strain. This suggests that low membrane fluidity somehow interferes with the complex process of cell division carried out by the divisome [22], and when the ideal fluidity is restored through sufficient BCFA production in the membrane the cell regains its normal physiology and can divide successfully. This proves that as long as the fatty acid molecule can provide the correct biophysical properties it does not necessarily have to be a natural cellular product. A suitable synthetic precursor can be very well utilized by the bacterial system to restore its ideal membrane parameters. Mercier et al. [23] have demonstrated a crucial role for BCFAs and their associated membrane fluidity in membrane scission in “L-form” B. subtilis cells.
The impact of the natural anteiso fatty acids having a methyl branch at the antepenultimate position such as anteiso C15:0 on the fluidity of the cytoplasmic membranes is attributed to the larger cross-sectional area they occupy than the corresponding SCFAs. This increases the area per lipid, and disrupts close packing of the fatty acyl chains and chain order along with reducing thickness of the lipid bilayer [15, 24]. A recent study on the effects of methyl branched fatty acids on the structural properties of the lipid bilayer using a 1,2-dipalmitoyl-sn glycerol-3-phosphocholine lipid bilayer, showed that the position of the methyl branch on the fatty acyl chain directly influences the membrane fluidity, the fluidizing ability of a mid-chain branch being greater than a terminal one [24]. The branching of 2-MP and 2-EB-derived fatty acids also no doubt occupy a large cross-sectional area similar to the naturally occurring BCFAs, thereby also imparting significant fluidity to the membrane.
4.3 Psychrotolerance of L. monocytogenes
In order to survive in the cold, psychrophiles must have enzymes that perform effectively at low temperatures. Indeed, cold environments reduce enzyme reaction rates and increase membrane viscosity. Cold-adapted organisms cope with these conditions by increasing enzyme turnover or improved catalytic efficiency at low temperatures compared to homologous enzymes in mesophiles. A commonly accepted hypothesis for cold adaptation is that psychrophilic enzymes have an increased flexibility of their structure to compensate for the “freezing effect” at cold temperatures [25]. Such changes are not without cost as this increased flexibility is likely responsible for the lower protein stability generally associated with cold-adapted enzymes, especially at higher temperatures.
L. monocytogenes is not psychrophilic, but rather psychrotolerant, which sets up a paradoxical survival problem for this organism. It must be able to thrive at 37°C and thus cannot afford thermally unstable enzymes, yet it maintains the ability to grow at refrigeration temperatures, which presumably requires increased enzymatic flexibility. Previously, we revealed that L. monocytogenes was uniquely equipped to handle just such a paradox, in that even during exponential growth at 37°C, anteiso-C15:0 accounted for 48% of the total fatty acids [2]. Although other Gram positive bacteria incorporate BCFAs into their membranes, their levels of anteiso-C15:0 are consistently ∼30%, comparable to the cold-sensitive L. monocytogenes mutants, cld-1 and cld-2 and MOR401 [2, 5]. Comparative analysis of the crystal structures of several bacterial FabH enzymes suggests a molecular basis for their substrate specificity [26]. Steric interactions between conserved physically close phenylalanines (distant in primary structure, e.g., between F305 and F208 in L. monocytogenes) cause a narrowing of the FabH active site in Gram-positive bacteria. The perturbing residue (i.e. F208) is not well conserved in Gram-negative bacteria (e.g. V216 in E. coli), which then allows the active site phenylalanine residue to swing away from the active site and open the substrate cavity [26]. Substrate specificity of FabH is the determining factor in the biosynthesis of BCFAs by type II fatty acid synthases [27, 28]. Accordingly, FabH enzymes from organisms that produce BCFAs exhibit broader substrate specificity than FabH homologues from organisms which produce SCFAs [27]. It is this broader substrate utilization that permits the phenomenon of homeoviscous adaptation to low temperatures by fatty acid branch switching and chain length shortening which occurs in wild-type L. monocytogenes and BCFA-deficient mutants in growth medium supplemented with 2MB [2, 5, 8].
An increase in the proportion of the shorter fatty acids derived from 2-MP and 2-EB accompanied by a decrease in the proportion of the longer ones was also observed in adaptation to low temperature. Thus L.monocytogenes can also carry out homeovisocus adaptation with the novel fatty acids derived from these unique precursors.
4.4 Structural requirements for a precursor of fatty acid biosynthesis
A variety of short BCCAs were evaluated for their ability to act as fatty acid primers. Along with the various ones with a methyl branch at position 2 on the acyl chain, trimethyl acetate and 2,2-dimethylbutyric acid also rescued the growth of the BCFA- deficient mutant to significant extents at 37°C but not at 10°C. Precursors having a branch at the third position such as 3-MP or isovalerate failed to support growth of MOR401 or cld-2 [5]. This indicates that a branch at the 2-position is an important structural parameter of the precursors for fulfillment of this function. Both Kaneda [13] and Willecke and Pardee [15] found that branching at the 2-position was important for BCCAs to act as fatty acid primers in B.subtilis. However, they did not include studies of the efficiency of these precursors at low temperatures. Although it is possible that the poor ability of 3-branched BCCAs to act as primers may lie in a low efficiency of forming the CoA derivatives, we feel that it is more likely that the defect lies in the substrate preferences of FabH.
The fatty acid profile of the membrane does show incorporation of novel BCFAs when the mutant is grown in presence of trimethyl acetate or 2,2-dimethylbutyrate. The novel fatty acids generated from trimethyl acetate and 2,2-dimethylbutyrate totaled 41.3% and 29.5% respectively compared to 82%, 76.8% and 93% from 2-EB, 2-MP and 2-MB respectively, all of which had a much larger impact on the fatty acid composition of MOR401 grown in unsupplemented medium. The major impact of trimethyl acetate and 2,2-dimethylbutyrate was to diminish the even-SCFA content, whereas the iso-odd BCFAs actually increased. Assuming these two BCCAs permeate the membrane efficiently we suspect the two groups at the 2-position confer a bulkier structure that renders these compounds to be poor substrates for either the enzymes in the pathway forming their CoA derivatives, or their utilization by FabH (or both). These BCCA precursors stimulate growth at 37°C, and presumably increase the fluidity of the membrane sufficiently, even though we did not detect this in our anisotropy measurements, which may miss subtle, but important fluidity changes. We propose that conversion of trimethyl acetate and 2,2-dimethylbutyrate to CoA derivatives and/or their utilization by FabH are too inefficient at 10°C for stimulation of growth of MOR401.
Our observations raise the question of how the preference for branched-chain precursors can be reconciled with a sterically narrowed FabH substrate access site as identified by Gajiwala [26]. Also how does the structure of the active site account for a selective difference between 2-and 3-branched precursors? The definitive answers await a L. monocytogenes FabH crystal structure with a bound branched-chain precursor. However, one hypothesis is that the impinging phenylalanine may allow stabilizing Van der Waals interactions with an alkyl group on the 2-position of the CoA precursor. The increased contact likely stabilizes the acyl-CoA precursor for efficient catalysis to occur. In the case of a branch in the 3-position, the same stabilizing contact would leave a two-carbon overhang which would likely be a similar poor substrate for L. monocytogenes FabH as acetyl-CoA [19].
4.5 Concluding remarks
Sun et al. [11, 12] reported 2-MB supplementation in the BCFA-deficient mutant plays a key role in expression of virulence properties such as listeriolysin O and survival against CAMPs, and peptidoglycan hydrolases and survival in an in vivo model. Since the novel fatty acids produced from 2-MP and 2-EB also lead to similar membrane biophysical properties in the mutant as when anteiso C15:0 and C17:0 are produced from 2-MB, we can predict that they may have similar effects on the physiology and pathogenicity of the pathogen. In support of this 2MB, 2-MP and 2-EB all corrected the defective cell division of the mutant. In terms of modulating the fatty acid composition of wild-type L. monocytogenes in order to inhibit growth in food, or in vivo, increasing the content of SCFAs from butyrate appears to be the most effective way. The current studies illustrate an interesting picture as to how the putative fatty acid metabolic enzymes in Listeria adapt themselves efficiently to form novel products from synthetic precursors by non-native promiscuity to support the membrane integrity for proper survival. Future studies will investigate the functional properties of the membranes containing large amounts of unnatural fatty acids.
Supplementary Material
Highlights.
The branched-chain fatty acids of a mutant were restored by 2-methylbutyrate
2-ethylbutyrate and 2-methylpentanoate resulted in novel unnatural fatty acids
A branch in the 2-position was required to be an efficient fatty acid precursor
The biophysical properties of the novel fatty acids restored normal physiology
Acknowledgments
This work was supported by grant 1 R15 AI099977-01 from the National Institutes of Health to Brian J. Wilkinson and Craig Gatto and R15-GM61583 to Craig Gatto. The funding sources had no role in study design, collection, analysis and interpretation of data, writing of this manuscript or the decision to submit it for publication. We would like to thank Dr Charitha Galva, at Illinois State University, for her help with the images for our manuscript. We would also like to acknowledge Lily Fernandez-Flores, a MS student in our lab, for her preliminary studies with the BCFA-deficient mutant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chan YC, Wiedmann M. Physiology and genetics of Listeria monocytogenes survival and growth at cold temperatures. Critical Reviews in Food Science and Nutrition. 2009;49:237–253. doi: 10.1080/10408390701856272. [DOI] [PubMed] [Google Scholar]
- 2.Annous BA, Becker LA, Bayles DO, Labeda DP, Wilkinson BJ. Critical role of anteiso-C15:0fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl Environ Microbiol. 1997;63:3887–3894. doi: 10.1128/aem.63.10.3887-3894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mastronicolis SK, Arvanitis N, Karaliota A, Litos C, Stavroulakis G, Moustaka H, Tsakirakis A, Heropoulos G. Cold dependence of fatty acid profile of different lipid structures of Listeria monocytogenes. Food Microbiol. 2005;22:213–219. [Google Scholar]
- 4.Nichols DS, Presser KA, Olley J, Ross T, McMeekin TA. Variation of branched-chain fatty acids marks the normal physiological range for growth in Listeria monocytogenes. Appl Environ Microbiol. 2002;68:2809–2813. doi: 10.1128/AEM.68.6.2809-2813.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu K, Bayles DO, Xiong A, Jayaswal RK, Wilkinson BJ. Precursor and temperature modulation of fatty acid composition and growth of Listeria monocytogenes cold-sensitive mutants with transposon-interrupted branched-chain alpha-keto acid dehydrogenase. Microbiology. 2005;151:615–623. doi: 10.1099/mic.0.27634-0. [DOI] [PubMed] [Google Scholar]
- 6.Sinensky M. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci USA. 1974;71:522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edgcomb MR, Sirimanne S, Wilkinson BJ, Drouin P, Morse RP. Electron paramagnetic resonance studies of the membrane fluidity of the foodborne pathogenic psychrotroph Listeria monocytogenes. Biochim Biophys Acta. 2000;1463:31–42. doi: 10.1016/s0005-2736(99)00179-0. [DOI] [PubMed] [Google Scholar]
- 9.Jones SL, Drouin P, Wilkinson BJ, Morse PD., II Correlation of long-range membrane order with temperature-dependent growth characteristics of parent and a cold-sensitive, branched-chain-fatty-acid-deficient mutant of Listeria monocytogenes. Arch Microbiol. 2002;177:217–222. doi: 10.1007/s00203-001-0380-4. [DOI] [PubMed] [Google Scholar]
- 10.Giotis ES, McDowell DA, Blair IS, Wilkinson BJ. Role of branched-chain fatty acids in pH stress tolerance in Listeria monocytogenes. Appl Environ Microbiol. 2007;73:997–1001. doi: 10.1128/AEM.00865-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Yvonne, O'Riordan Mary XD. Branched-chain fatty acids promote Listeria monocytogenes intracellular infection and virulence. Infect Immun. 2010;78:4667–4673. doi: 10.1128/IAI.00546-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Yvonne, O'Riordan Mary XD. Fatty Acids Regulate Stress Resistance and Virulence Factor Production for Listeria monocytogenes. J Bacteriol. 2012;19:5274–5284. doi: 10.1128/JB.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneda T. Incorporation of branched-chain C6-fatty acid isomers into the related long-chain fatty acids by growing cells of Bacillus subtilis. Biochemistry <v>. 1971;10:340–347. doi: 10.1021/bi00778a022. [DOI] [PubMed] [Google Scholar]
- 14.Julotok M, Singh AK, Gatto C, Wilkinson BJ. Influence of fatty acid precursors, including food preservatives, on the growth and fatty acid composition of Listeria monocytogenes at 37 and 10 degrees C. Appl Environ Microbiol. 2010;76:1423–1432. doi: 10.1128/AEM.01592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willecke K, Pardee AB. Fatty acid-requiring mutant of Bacillus subtilis defective in branched chain alpha-keto acid dehydrogenase. J Biol Chem. 1971;246:5264–5272. [PubMed] [Google Scholar]
- 16.Ran-Ressler Rinat R, Lawrence Peter, Thomas Brenna J. Structural Characterization of Saturated Branched Chain Fatty Acid Methyl Esters by Collisional Dissociation of Molecular Ions Generated by Electron Ionization. Journal of Lipid Research. 2012;53:195–203. doi: 10.1194/jlr.D020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh VK, Hattangady DS, Giotis ES, Singh AK, Chamberlain NR, Stuart MK, Wilkinson BJ. Insertional inactivation of branched-chain alpha-keto acid dehydrogenase in Staphylococcus aureus leads to decreased branched-chain membrane fatty acid content and increased susceptibility to certain stresses. Appl Environ Microbiol. 2008;74:5882–5890. doi: 10.1128/AEM.00882-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang YM, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol. 2008;6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 19.Singh AK, Zhang YM, Zhu K, Subramanian C, Li Z, Jayaswal RK, Gatto C, Rock CO, Wilkinson BJ. FabH selectivity for anteiso branched-chain fatty acid precursors in low-temperature adaptation in Listeria monocytogenes. FEMS Microbiol Lett. 2009;301:188–192. doi: 10.1111/j.1574-6968.2009.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons JB, Yao J, Frank MW, Jackson P, Rock CO. Membrane disruption by antimicrobial fatty acids releases low molecular weight proteins from Staphylococcus aureus. J Bacteriol. 2012;194:5294–5304. doi: 10.1128/JB.00743-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aboulnaga O, Pinkenburg J, Schiffels A, El-Refai W, Buckel T. Selmer Effect of an oxygen-tolerant butyril-coenzyme A dehydrogenase/electron-transferring flavoprotein complex from Clostridium difficile on butyrate production in Escherichia coli. Journal of Bacteriology. 2013;195:3704–3713. doi: 10.1128/JB.00321-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutkenhaus J, Pichoff S, Du S. Bacterial cytokinesis: From Z ring to divisome. Cytoskeleton. 2012;69:778–790. doi: 10.1002/cm.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mercier R, Domínguez-Cuevas P, Errington J. Crucial role for membrane fluidity in proliferation of primitive cells. Cell Reports. 2012;1:417–423. doi: 10.1016/j.celrep.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Poger D, Caron B, Mark A. E.Effect of Methyl-Branched Fatty Acids on the Structure of Lipid Bilayers. J Phys Chem B. 2014;118:13838–13848. doi: 10.1021/jp503910r. [DOI] [PubMed] [Google Scholar]
- 25.Feller G, Gerday C. Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol. 2003;1:200–208. doi: 10.1038/nrmicro773. [DOI] [PubMed] [Google Scholar]
- 26.Gajiwala KS, Margosiak S, Lu J, Cortez J, Su Y, Nie Z, Appelt K. Crystal structures of bacterial FabH suggest a molecular basis fo the substrate specificity of the enzyme. FEBS Letters. 2009;583:2939–2946. doi: 10.1016/j.febslet.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Choi KH, Heath RJ, Rock CO. Beta-ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. J Bacteriol. 2000;182:365–370. doi: 10.1128/jb.182.2.365-370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Florova GK, Reynolds KA. Alteration of the fatty acid profile of Streptomyces coelicolor by replacement of the initiation enzyme 3-ketoacyl acyl carrier protein synthase III (FabH) J Bacteriol. 2005;187:3795–3799. doi: 10.1128/JB.187.11.3795-3799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


