Abstract
Objective
We conducted a systematic review and meta-analysis of the efficacy of aripiprazole once monthly (AOM) for schizophrenia.
Methods
Randomized controlled trials (RCTs) on AOM, published until June 25, 2015, were retrieved from PubMed, Cochrane, and PsycINFO databases. Relative risk (RR), standardized mean difference (SMD), 95% confidence intervals (95% CIs), and numbers needed to treat/harm (NNT/NNH) were calculated.
Results
We identified four relevant RCTs (total n=1,860), two placebo-controlled trials, one noninferiority trial comparing AOM to oral aripiprazole (OA), and one including therapeutic doses of AOM and OA, as well as an AOM dose below therapeutic threshold (control arm). AOM was superior to placebo for decreasing Positive and Negative Syndrome Scale (PANSS) total scores (SMD =−0.65, 95% CI =−0.90 to −0.41, n=1,126). However, PANSS total scores did not differ significantly between pooled AOM and OA groups. The pooled AOM group showed significantly lower incidence of all-cause discontinuation (RR =0.54, 95% CI =0.41–0.71, n=1,139, NNH =4) and inefficacy (RR =0.28, 95% CI =0.21–0.38, n=1,139, NNH =5) than placebo, but was not superior to placebo regarding discontinuation due to adverse events (AEs) or death. The AOM group exhibited a lower incidence of all-cause discontinuation than OA (RR =0.78, 95% CI =0.64–0.95, n=986, NNH =14), but there were no intergroup differences in discontinuation due to inefficacy, AEs, or death. There were no significant differences in extrapyramidal symptoms scale scores between AOM and placebo or between AOM and OA. AOM resulted in higher weight gain than placebo (SMD =0.41, 95% CI =0.18–0.64, n=734) but lower than OA (SMD =−0.16, 95% CI =−0.29 to −0.02, n=847).
Conclusion
AOM has antipsychotic efficacy and low risk of discontinuation due to AEs.
Keywords: schizophrenia, aripiprazole once monthly, efficacy, safety, systematic review, meta-analysis
Introduction
It is crucial that schizophrenia patients adhere to antipsychotic regimens as noncompliance results in higher relapse [risk ratio (RR) =0.40] and readmission (RR =0.38) compared to placebo.1 While inadequate compliance contributes to symptom exacerbation and relapse,2,3 it is difficult for clinicians to monitor drug adherence to oral antipsychotics (OAPs), especially in outpatients.4 Thus, factors contributing to noncompliance must be considered when initiating or changing pharmacotherapy. In this regard, long-acting injectables (LAIs) are advantageous as drug adherence can be monitored simply by checking outpatient visitation adherence and confirmation of LAI inoculation.5,6 On the other hand, LAIs have several disadvantages compared to OAPs, such as injection site pain7 and difficulty in stopping drug delivery quickly in case of severe extrapyramidal symptom (EPS)8 or neuroleptic malignant syndrome.9 Several reports have compared LAIs to OAPs, but the results are controversial because of various biases in trial design.10–12 A mirror image study of risperidone as LAI found strong superiority over OAP regarding relapse prevention as measured by hospitalization rate.11 Aripiprazole once monthly (AOM) is a newer LAI regimen, and four recent randomized controlled trials (RCTs) have evaluated AOM efficacy and safety.13–16 Oral aripiprazole (OA) is widely used because it has similar efficacy and safety to other OAPs but lowers the risk of EPS and other adverse events (AEs) like weight gain, prolactin elevation, QTc prolongation, and sedation.17 Thus, AOM may be a valuable treatment for the management of schizophrenia by combining the safety profile of oral aripiprazole with the assured drug delivery of a LAI formulation.
A meta-analysis can increase the statistical power for group comparisons and overcome the limitations of small sample sizes. Moreover, using random effects models and standardized mean differences (SMDs) analysis, outcomes with different metrics can be combined (Cochrane Collaboration, http://handbook.cochrane.org/).18 We thus performed a systematic review and meta-analysis of four RCTs of AOM13–16 to assess its efficacy and tolerability (as indicated by discontinuation rate, EPS, and individual AEs).
Methods
Inclusion criteria and search strategy, data extraction, and outcomes
This meta-analysis was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.19 We performed a systematic literature review according to the PICO strategy (patients: schizophrenia, intervention: AOM, comparator: placebo, OA, and/or AOM dosing below the therapeutic threshold [50 mg, AOM-50 mg], outcome: efficacy and safety). We included only double-blind RCTs comparing AOM to placebo, OA, and/or AOM-50 mg for schizophrenia. Relevant studies were identified through searches of PubMed, Cochrane Library, and PsycINFO databases. There were no language restrictions, and we accepted data published up to June 25, 2015, using the keyword combinations schizophrenia aripiprazole depot, schizophrenia aripiprazole once monthly, schizophrenia aripiprazole long-acting injection, and schizophrenia aripiprazole long-acting injectable. Additional eligible studies were also sought by examining the reference lists of primary articles and relevant reviews. Two authors (KO and TK) checked the inclusion and exclusion criteria for each of the identified studies and resolved discrepancies in coding by discussion. The same authors independently extracted, checked, and entered data into Review Manager (RevMan) version 5.3 for Windows (Review Manager version 5.3, Cochrane Collaboration, http://tech.cochrane.org/revman). When data required for the meta-analysis were missing, we checked registries of clinical trials such as https://clinicaltrials.gov/ and University hospital Medical Information Network (UMIN, www.umin.ac.jp/) or contacted the first/corresponding authors for additional information. We also assessed the methodological quality of the trials using the Cochrane risk-of-bias criteria.
Data synthesis
The primary efficacy measures were change in Positive and Negative Syndrome Scale (PANSS)20 total scores from baseline to endpoint for all studies and changes in PANSS positive and negative subscales scores for two studies.13,14 Secondary outcomes were as follows: Clinical Global Impression-Severity (CGI-S) scores and Clinical Global Impression-Improvement (CGI-I);21 relapse rate; proportion of responders and remitters; Abnormal Involuntary Movement Scale (AIMS) scores;22 Simpson–Angus Scale (SAS) scores;23 Barnes Akathisia Rating Scale (BARS) scores;24 and discontinuation due to all causes, inefficacy, AEs, and death. We considered discontinuation due to impending relapse with and without AEs as discontinuation due to inefficacy.14,15 We also considered discontinuation due to the cause meeting the criteria of exacerbation of psychotic symptoms, relapse, or exacerbation of the disease as discontinuation due to inefficacy.16 In addition, we pooled the data for individual AEs.
Statistical analysis
We based our analyses on intention-to-treat (ITT) or modified ITT data (ie, at least one dose or at least one follow-up assessment); no observed case data were included. To combine studies, we used the random effects model described by DerSimonian and Laird,25 a conservative model used to address the possibility that the underlying effects differ across studies and populations (ie, are heterogeneous). For continuous data, we used SMD and 95% confidence interval (CI). For dichotomous data, RR was estimated along with 95% CI. When the random effects model showed significant differences between groups, the numbers needed to treat (NNT) or numbers needed to harm (NNH) were calculated from the risk difference (RD) using the formula NNT or NNH =1/RD. Study heterogeneity was measured using the chi-squared and I2 statistics, with values of P<0.05 and I2≥50%, respectively, indicating heterogeneity.26 We did not examine publication bias regarding primary outcome using funnel plots because of the small number of studies included in the meta-analysis.
Results
Study characteristics
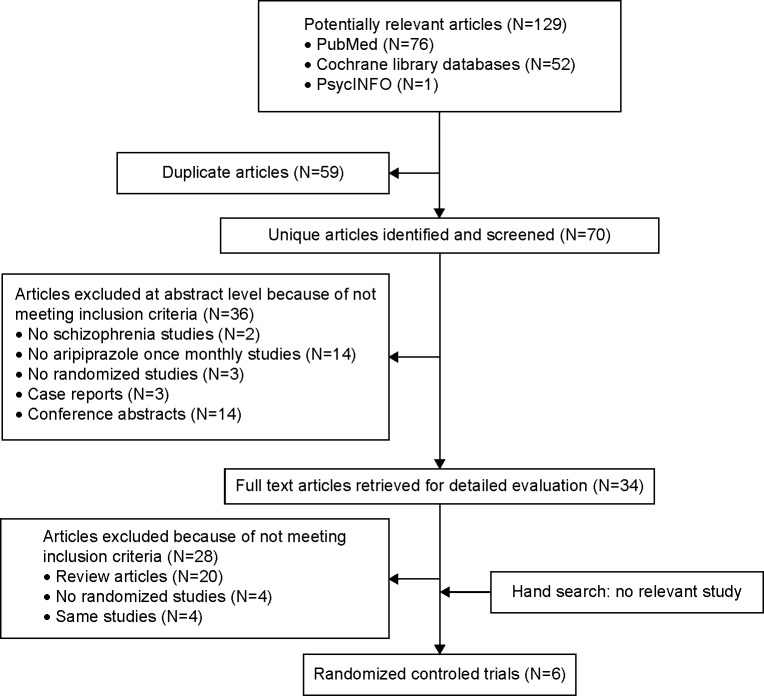
Searches of PubMed, Cochrane Library, and PsycINFO databases yielded 129 hits. We excluded 59 duplicate studies; 36 studies based on title or abstract review; and 30 studies after full text reading, including two RCTs, because of study design. Therefore, four eligible studies were included (Figure 1). Across the four RCTs (mean duration: 38.5 [range, 12–52] weeks), 1,860 adult patients with schizophrenia were randomized to either AOM (n=930), OA (n=493), or control (placebo, n=306 or AOM-50 mg, n=131) groups. The study of Fleischhacker et al15 consisted of three arms comparing AOM, OA, and AOM-50 mg. Sample sizes ranged from 340 to 662 participants. All studies were published in English, and all were sponsored by Otsuka Pharmaceutical Co., Ltd. All four were of high methodological quality based on Cochrane Risk of Bias Criteria (they were double-blind RCTs and contained the required study design detail). Two were placebo-controlled studies,13,14 one OA controlled,16 and the other compared AOM, OA, and AOM-50 mg (defined as placebo as this dose is subthreshold).15 The characteristics of the studies are summarized in Table 1.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram.
Table 1.
Study, patient, and treatment characteristics of the included double-blinded, randomized placebo-controlled trials of patients with schizophrenia
| Study | Total n | Patients | Diagnosis/analyzed population | Duration | Age (mean ± SD) | Male n(%) | Race (%) | Drug/n | Intervention (mg) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Kane et al13 (USA, Croatia, Latvia) supported by Otsuka Pharmaceutical Development and Commercialization, Inc. | 340 | Age: 18–65, schizophrenia (DSM-IV-TR, MINI) acute psychotic episode (PANSS total ≥80, conceptual disorganization, hallucinatory behavior, suspiciousness/persecution and unusual thought content >4) | DSM-IV-TR/m-ITT (LOCF) | 12 weeks | 42.1 (11.0) 42.7(10.9) |
130 (77.4) 139 (80.8) |
White: 31.0, Black or African-American: 65.5, Asian: 1.2, others: 2.4 White: 32.0, Black or African-American: 65.7, Asian: 0, others: 2.3 |
AOM + OA (*1)/168 PLA-IM + OP (*1)/172 |
AOM: 300 or 400/m, OA: 10–20/day PLA-IM, OP | PANSS total: AOM > PLA, CGI-S: AOM > PLA, CGI-I: AOM > PLA PANSS positive: AOM > PLA, PANSS negative: AOM > PLA, PANSS responder rate: AOM > PLA, PSP: AOM > PLA |
| Kane et al14 (USA, Mexico, Argentina, Bulgaria, Romania, Serbia, Slovakia, Russia, India, Taiwan, Malaysia, Philippines), supported by Otsuka Pharmaceutical Commercialization, Inc. | 403 | Inclusion: age: 18–60, schizophrenia (DSM-IV-TR) ≥3 yrs, history of symptom exacerbation or relapse when not receiving AP treatment Allocation: outpatient, PANSS total ≤80, conceptual disorganization, suspiciousness, hallucinatory behavior, unusual thought content ≤4, CGI-S ≤4, CGI-SS part 1 ≤2, part II ≤5 | DSM-IV-TR/ITT (LOCF) | 52 weeks | 40.1 (11.0) 41.7(10.5) |
162 (60.2) 79 (59.0) |
White: 56.5, Black or African-American: 21.9, Asian: 16.7, others: 4.8 White: 68.7, Black or African-American: 16.4, Asian: 9.7, others: 5.2 |
AOM/269 PLA-IM/134 |
AOM: 300 or 400/m PLA-IM |
Time to impending relapse: AOM > PLA, relapse rate: AOM > PLA, time to study discontinuation due to all-cause: AOM > PLA, PANSS total scores: AOM > PLA, mean change in CGI-S score: AOM > PLA |
| Fleischhacker et al15 (Austria, Belgium, Bulgaria, Chile, Croatia, Estonia, France, Hungary, Italy, South Korea, Poland, South Africa, Thailand, USA), supported by Otsuka Pharmaceutical Commercialization, Inc. | 662 | Age: 18–60, schizophrenia (DSM-IV-TR) ≥3 years, history of symptom exacerbation when not receiving AP treatment, responsive to AP treatment (other than CLO) in the past year | DSM-IV-TR/ITT (LOCF) | 38 weeks | 41.7(10.4) 41.2(10.8) 40.2 (9.6) |
160 (60.4) 168 (63.2) 78 (59.5) |
White: 60.4, Black or African-American: 21.1, Asian: 10.9, others: 7.5 White: 57.5, Black or African-American: 24.1, Asian: 9.8, others: 8.6 White: 56.5, Black or African-American: 25.2, Asian: 10.7, others: 7.6 |
AOM-400 mg + OA (*2)/265 OA + PLA-IM/266 AOM-50 mg + OA (*2)/131 |
AOM: 300 or 400/m OA: 10–30/day AOM: 25 or 50/m |
Relapse rate at wk 26: AOM-400 = OA > AOM-50, time to observed impending relapse up to wk 38: AOM-400 = OA > AOM-50, observed impending relapse rate at wk 38: AOM-400 = OA > AOM-50, proportion of responders: AOM-400 > AOM-50, time to discontinuation: AOM-400 > OA, AOM-400 > AOM-50, PANSS total score: AOM-400 > OA, AOM-400 > AOM-50, CGI-S: AOM-400 > OA, AOM-400 > AOM-50, CGI-I; AOM-400 > OA, AOM-400 > AOM-50 |
| Ishigooka et al16 (Japan, Malaysia, Taiwan, Philippines), supported by Otsuka Pharmaceutical Co., Ltd. | 455 | Age ≥18, schizophrenia (DSM-IV-TR), BMI of 18.5–35.0 | DSM-IV-TR/ITT (LOCF) | 52 weeks | 40.2(12.6) 38.2(10.3) |
136 (59.6) 141 (62.1) |
Asian: 100 | AOM-400 mg + OA (*3)/228 OA (*4) + PLA-IM/227 | AOM: 300 or 400/m OA: 6–24/day |
Nonexacerbation of psychotic symptoms/nonrelapse rate: AOM-400 = OA, PANSS total scores, positive, negative, and general subscale-scores, and CGI-S: AOM-400 = OA |
Notes: (*1) Both groups received OA and OP for 14 days beginning on the day of the first injection. (*2) Both AOM groups received OA (10–20 mg/day) for 2 weeks from the date of randomization and then PLA tablets thereafter. (*3) AOM-400 group received 6 mg or 12 mg of OA for 2 weeks after the start of the double blind phase. (*4) The dose could be reduced once by 6 mg/day from week 4 and the dose could be increased back to the original dose.
Abbreviations: AOM, aripiprazole once monthly; AP(s), antipsychotic(s); BMI, body mass index; CGI - (I, S, SS), clinical global impression - (improvement, severity, severity of suicidality); CLO, clozapine; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision; LOCF, last observation carried forward; (m) ITT, (modified) intention-to-treat; m(s), month(s); MINI, Mini-International Neuropsychiatric Interview; n, number of patients; OA, oral aripiprazole; OP, oral placebo; PANSS, Positive and Negative Syndrome Scale; PLA, placebo, PLA-(IM), placebo-(intramuscular injection); PSP, Personal and Social Performance Scale; SD, standard deviation.
Results of the individual studies
Study by Kane et al (2014)13
Kane et al conducted a 12-week double-blind RCT of AOM (initial dose 400 mg, n=168) versus placebo (n=172) for the treatment of schizophrenia. The patients in the AOM-400 mg group were permitted to a dose decrease of 300 mg for tolerability. Least squares mean change in PANSS total scores and CGI-S scores from baseline to study conclusion were primary endpoints for efficacy, and both were significantly greater in the AOM group versus placebo. Additionally, PANSS positive and negative subscale scores and Personal and Social Performance scale scores were significantly improved by AOM compared to placebo. Responder rate (≥30% reduction in PANSS total score) was also significantly higher in the AOM group. Treatment-emergent AEs (TEAEs) were reported in 79.6% of AOM and 70.9% of placebo group patients. The most common AEs (>10%) were weight increase, headache, and akathisia in the AOM group and headache in the placebo group. Serious AEs were reported in 4.8% of AOM- and 3.5% of placebo-treated patients, but no deaths occurred in either group. Study discontinuation rate due to AEs was 4.2% in the AOM and 7.6% in the placebo group. EPS scores, including AIMS, SAS, and BARS scores, did not differ significantly between AOM and placebo groups. Weight increase was significantly larger in the AOM group at endpoint. Fasting glucose level was higher in the AOM group, but other fasting metabolic parameters were comparable between the two groups. Mean prolactin level decreased significantly in the AOM group compared to placebo. Vital signs and changes in the electrocardiogram were comparable between the two groups.
Study by Kane et al (2012)14
In this double-blind RCT, patients were randomized to AOM-400 mg (n=269) or placebo (n=134) for 52 consecutive weeks. Patients in the AOM-400 mg group were permitted to a dose decrease of 300 mg for tolerability. Time to impending relapse was significantly delayed and relapse rate significantly lower in the AOM group (AOM =10.0%, placebo =39.6%). Overall time to all-cause discontinuation was significantly delayed in the AOM group compared to placebo (24.9% vs 54.5%). Mean changes in both total PANSS and CGI-S scores from baseline to final analysis were significantly improved by AOM (PANSS, 1.4 vs 11.6; CGI-S, 0.7 vs 0.1). TEAEs were reported 63.2% of the AOM and 61.9% of the placebo group. Common AEs (>5%) for both groups were akathisia, insomnia, anxiety, headache, and weight increase. Serious AEs were reported in 4.1% of the AOM group and 6.7% of the placebo group. Two deaths were reported during the study, one due to coronary artery insufficiency during the intramuscular-depot stabilization phase and the other from pancreatic carcinoma in the AOM group. Four suicide-related AEs were reported in AOM group, three patients experienced suicidal ideation and the other attempted suicide. Discontinuation rate due to TEAEs during double-blind treatment was 7.1% in the AOM and 13.4% in the placebo group. Mean changes in AIMS movement scores, SAS total scores, and BARS global scores did not differ significantly between the two groups. Similarly, mean weight change and metabolic abnormalities did not differ significantly between the two groups. The incidence of potentially clinically relevant prolactin elevation was lower in the AOM group compared to placebo (AOM, 1.9%; placebo, 7.1%). Incidences of potentially clinically relevant changes in vital signs, orthostatic hypotension, and electrocardiogram parameters were similar between the two groups.
Study by Fleischhacker et al (2014)15
In Fleischhacker et al, patients were randomized 2:2:1 to AOM-400 mg, OA (10–30 mg/day), and AOM-50 mg (considered control in our analysis) groups for 38 consecutive weeks. Patients in AOM-400 mg and AOM-50 mg groups were permitted to a dose decrease of 300 mg and 25 mg, respectively, for tolerability. There were 662 patients randomized to double-blind treatment with AOM-400 mg (n=265), OA (n=266), or AOM-50 mg (n=131). Kaplan–Meier-estimated impending relapse rates at week 26 were similar between AOM-400 mg and OA groups, but significantly lower in the AOM-400 mg group compared to the AOM-50 mg group. Time to impending relapse was similar between AOM-400 mg and OA groups, with both demonstrating statistically significant delays compared to the AOM-50 mg group at week 38. The proportion of responders was significantly higher in the AOM-400 mg group compared to the AOM-50 mg group but similar to the OA group. The proportion of remitters did not show any statistically significant differences among treatment groups. Kaplan–Meier-estimated time to discontinuation favored AOM-400 mg over OA and AOM-50 mg. PANSS total scores, CGI-S scores, and CGI-I scores were significantly improved by AOM-400 mg compared to OA and AOM-50 mg. TEAEs were reported in 82.6% of the AOM-400 mg, 80.1% of the OA, and 80.9% of the AOM-50 mg group. Common AEs (>10%) were akathisia and insomnia with AOM-400 mg, insomnia, headache, and weight increase with OA and insomnia and back pain with AOM-50 mg. Serious AEs were reported in 5.7% of the AOM-400 mg, 5.6% of the OA, and 8.4% of the AOM-50 mg group. Two deaths were reported during the study, one from cardiac arrest in the OA group and the other a suicide in the AOM-50 mg group. Discontinuation due to TEAEs was reported in 7.9% of the AOM-400 mg, 7.1% of the OA, and 18.3% of the AOM-50 mg group. Mean changes in AIMS and SAS did not differ significantly in the AOM-400 mg group compared to the OA or AOM-50 mg group, but the BARS scores were significantly better by AOM-400 mg compared to OA groups. The mean change in body weight at week 38 was significantly higher in the AOM-400 mg group compared to the AOM-50 mg group but did not differ from the OA group. There were no clinically significant differences in metabolic parameters, prolactin, and electrocardiogram parameters among the three groups.
Study by Ishigooka et al (2014)16
The patients were randomized to AOM-400 mg (n=228) or OA (n=227) for 52 consecutive weeks. Patients in the AOM-400 mg group were permitted to a dose decrease of 300 mg for tolerability. For the efficacy outcome, there were no significant differences between the two groups regarding the exacerbation of psychotic symptoms and nonrelapse rates. Time to exacerbation of psychotic symptoms/relapse was similar between the two groups. The mean changes in PANSS total scores, positive, negative, and general subscale scores, and CGI-S and CGI-I scores from baseline were comparable between the two groups at final analysis. Discontinuation due to all causes was comparable between the two groups. TEAEs were reported in 77.2% of the AOM and 79.3% of the OA group. Common AEs (>10%) were pain, erythema, induration of the injection site, and nasopharyngitis with AOM, and injection site pain and nasopharyngitis with OA. Serious AEs were reported in 5.7% of the AOM and 8.8% of the OA group. Two deaths were reported during the study, one from cardiac sudden death in the AOM group and the other from a head injury in the OA group. Two patients in both groups reported suicidal ideation during the trial. Discontinuation rate due to AEs was 7.5% in the AOM and 11.5% in the OA group. Mean changes in Drug-Induced Extrapyramidal Symptoms Scale total scores, AIMS total scores, and BARS global scores were similar between the two groups. The mean weight change from baseline to final analysis was 0.87 kg with AOM and 1.44 kg with OA. Mean changes in glucose, cholesterol levels, and serum prolactin levels did not differ significantly between the two groups.
Results
Efficacy
AOM vs placebo
AOM was superior to placebo for the reduction of PANSS total score (SMD =−0.65, 95% CI =−0.90 to -0.41, P<0.00001, I2=75%, three comparisons, n=1,126), PANSS positive subscale score (SMD =−0.85, 95% CI =−1.01 to −0.69, P<0.00001, I2=0%, two comparisons, n=729), and negative subscale score (SMD =−0.44, 95% CI =−0.59 to −0.28, P<0.00001, I2=0%, two comparisons, n=729). AOM was superior to placebo for the reduction of CGI-S score (SMD =−0.58, 95% CI =0.80 to −0.36, P<0.00001, I2=67%, three comparisons, n=1,118) and CGI-I score (SMD =−0.69, 95% CI =−0.82 to −0.55, P<0.00001, I2=8%, three comparisons, n=1,125). AOM had strong superiority compared to placebo for decreasing observed relapse rate (RR =0.30, 95% CI =0.21–0.44, P<0.00001, I2=26%, two comparisons, n=799, NNT =5) and for enhancing responder rate (RR =0.33, 95% CI =0.23–0.48, P<0.00001, I2=37%, two comparisons, n=794, NNT =4). However, the proportion of remitters was comparable between the two groups (RR =1.00, 95% CI =0.60–1.64, P=0.99, I2=78%, two comparisons, n=405).
AOM vs OA
With respect to psychiatric symptoms, AOM was comparable to OA for the reduction of PANSS total score (SMD =−0.08, 95% CI =−0.31 to 0.14, P=0.46, I2=69%, two comparisons, n=984), CGI-S score (SMD =−0.09, 95% CI =−0.40 to 0.22, P=0.56, I2=83%, two comparisons, n=977), and CGI-I score (SMD =−0.17, 95% CI =−0.49 to 0.16, P=0.31, I2=85%, two comparisons, n=986). With respect to patients’ outcomes, AOM was comparable to OA regarding observed relapse rate (RR =1.03, 95% CI =0.66–1.60, P=0.90, I2=0%, two comparisons, n=986) and proportion of remitters (RR =1.08, 95% CI =0.92–1.28, P=0.34, I2=0%, two comparisons, n=775).
Safety and tolerability
AOM vs placebo
AOM was superior to placebo regarding all-cause discontinuation (RR =0.54, 95% CI =0.41–0.71, P<0.00001, I2=70%, three comparisons, n=1,139, NNH =4) and discontinuation due to inefficacy (RR =0.28, 95% CI =0.21–0.38, P<0.00001, I2=0%, three comparisons, n=1,139, NNH =5). AOM and placebo did not differ in the rate of discontinuation due to AEs (RR =0.64, 95% CI =0.36–1.11, P=0.12, I2=0%, three comparisons, n=1,139) and discontinuation due to death (RR =0.50, 95% CI =0.05–4.77, P=0.55, I2=0%, three comparisons, n=1,139). Regarding EPS, there were no significant differences between the two groups in SAS (SMD =−0.27, 95% CI =−0.80 to 0.25, P=0.30, I2=88%, two comparisons, n=592), AIMS (SMD =0.00, 95% CI =−0.17 to 0.17, P=1.00, I2=0%, two comparisons, n=592), or BARS (SMD =0.22, 95% CI =−0.24 to 0.68, P=0.34, I2=85%, two comparisons, n=592). The pooled AOM group showed no difference in incidence of weight gain compared to placebo (RR =1.58, 95% CI =0.92–2.73, P=0.10, I2=46%, two comparisons, n=1,138), but mean change in body weight at last visit was higher in the AOM group (SMD =0.41, 95% CI =0.18–0.64, P=0.0005, I2=57%, two comparisons, n=734). There were no significant differences in individual AEs, including akathisia, anxiety, headache, injection site pain, insomnia, nasopharyngitis, and parkinsonism, between AOM and placebo groups.
AOM vs OA
AOM was superior to OA regarding all-cause discontinuation (RR =0.78, 95% CI =0.64–0.95, P=0.01, I2=0%, two comparisons, n=986, NNH =14). AOM and OA did not differ in discontinuation due to AEs (RR =0.75, 95% CI =0.45–1.24, P=0.27, I2=0%, two comparisons, n=986), discontinuation due to inefficacy (RR =0.93, 95% CI =0.61–1.42, P=0.73, I2=0%, two comparisons, n=986), and discontinuation due to death (RR =0.62, 95% CI =0.08–5.05, P=0.66, I2=0%, two comparisons, n=986). Regarding EPS, AOM, and OA did not differ in AIMS score (SMD =−0.06, 95% CI =−0.38 to 0.26, P=0.73, I2=78%, two comparisons, n=680) or BARS score (SMD =0.25, 95% CI =−0.24 to 0.74, P=0.31, I2=90%, two comparisons, n=680). AOM did not increase the incidence of weight gain compared to OA (RR =0.97, 95% CI =0.46–2.06, P=0.94, I2=68%, two comparisons, n=986), but mean change in body weight at last visit was lower in the AOM group (SMD =−0.16, 95% CI =−0.29 to -0.02, P=0.02, I2=0%, two comparisons, n=847). There were no significant differences in AEs, including akathisia, injection site pain, insomnia, nasopharyngitis, and suicide ideation, between AOM and OA groups, while incidence of injection site pain was marginally higher in the AOM group (RR =2.00, 95% CI =0.92–4.36, P=0.08, I2=65%, two comparisons, n=986).
Discussion
To the best of our knowledge, this is the first comprehensive systematic review and meta-analysis of AOM for patients with schizophrenia. With respect to efficacy, AOM was superior to placebo regarding reduction of the PANSS total score, positive and negative subscale scores, and both CGI-S and CGI-I scores. AOM was also superior to placebo for relapse and response rates, with medium to large effect sizes, respectively. In contrast, there were no differences in PANSS, CGI-S, and CGI-I scores or in longitudinal outcomes such as relapse rate and response rate between pooled AOM and OA groups. With respect to safety and tolerability, AOM was superior to placebo regarding all-cause discontinuation and discontinuation due to inefficacy. However, discontinuation due to AEs and death did not differ between the two groups. The incidence of weight increase was comparable between AOM and placebo groups, but the mean change in body weight was significantly higher in the AOM group. Finally, AOM was comparable to placebo regarding EPS scales such as SAS, AIMS, and BARS. AOM was superior to OA regarding all-cause discontinuation. However, discontinuation due to AEs, inefficacy, and death did not differ between the two groups. The incidence of weight increase was comparable between the two groups, but mean change in body weight was significantly lower in the AOM group. Finally, AOM was comparable to OA regarding EPS scales, such as AIMS and BARS.
We compared the results of our meta-analysis with that of a previous meta-analysis (Table 2).8 The previous meta-analysis of studies comparing the second generation antipsychotics risperidone, paliperidone, and olanzapine as LAIs (SGA-LAIs) found that pooled SGA-LAIs were better than placebo for reducing PANSS total scores (SMD =0.34, 95% CI =0.25–0.43) and enhancing responder rate (RR =0.54, 95% CI =0.45–0.66, NNT =6).8 Regarding safety, pooled SGA-LAIs were superior to placebo in all-cause discontinuation (RR =0.69, 95% CI =0.58–0.83, NNH =25).8 However, pooled SGA-LAIs showed greater incidence of EPS (RR =2.04, 95% CI =1.15–3.61, NNH =10) and weight gain (RR =2.75, 95% CI =1.87–4.03, NNH =16) compared to placebo.8 In our study, pooled AOM was superior to placebo for reducing PANSS total score with moderate effect size (SMD =0.65, 95% CI =0.41–0.90) and strongly superior for enhancing responder rate (RR =0.33, 95% CI =0.23–0.48, NNT =4). AOM was superior to placebo regarding all-cause discontinuation with a smaller NNH than in the previous meta-analysis (RR =0.54, 95% CI =0.41–0.71, NNH =4).8 AOM and placebo showed comparable risks of developing parkinsonism (RR =1.63, 95% CI =0.64–4.15) and weight gain (RR =1.58, 95% CI =0.92–2.73). Aripiprazole itself is used extensively throughout the world not only because of its high efficacy but also due to low risk of cardiometabolic complication and EPS.17 In fact, our meta-analysis showed that there was no significant difference in EPS incidence between AOM and OA groups, while the AOM group had a lower mean individual increase in body weight than the OA group. It is often thought that LAIs are more suitable for patients with a history of poor adherence. In addition, it is also thought that AOM is better suited for well-functioning patients because it frees them from the efforts of daily antipsychotic administration.27
Table 2.
Comparing AOM vs placebo with SGA-LAIsa vs placebo
| AOM vs placebo | SGA-LAIsa vs placebo | |
|---|---|---|
| PANSS total scores | SMD =0.65, 95% CI =0.41–0.90 | SMD =0.34, 95% CI =0.25–0.43 |
| Responder rate | RR =0.33, 95% CI =0.23–0.48, NNT =4 | RR =0.54, 95% CI =0.45–0.66, NNT =6 |
| Discontinuation due to all causes | RR =0.54, 95% CI =0.41–0.71, NNH =4 | RR =0.69, 95% CI =0.58–0.83, NNH =25 |
| Death | RR =0.50, 95% CI =0.05–4.77 | RR =0.33, 95% CI =0.06–1.89 |
| All treatment-emergent adverse events | RR =1.05, 95% CI =0.98–1.13 | RR =1.02, 95% CI =0.95–1.08 |
| Insomnia | RR =0.95, 95% CI =0.63–1.44 | RR =0.80, 95% CI =0.64–1.01 |
| Anxiety | RR =0.87, 95% CI =0.51–1.47 | RR =0.67, 95% CI =0.48–0.94, NNH =35 |
| Extrapyramidal symptom | RR =1.63, 95% CI =0.64–4.15 | RR =2.04, 95% CI =1.15–3.61, NNH =10 |
| Anticholinergic use | RR =1.50, 95% CI =1.04–2.18, NNH =17 | RR =1.51, 95% CI =1.13–2.02, NNH =20 |
| Weight gain | RR =1.58, 95% CI =0.92–2.73 | RR =2.75, 95% CI =1.87–4.03, NNH =16 |
| Injection site pain | RR =3.62, 95% CI =0.50–26.31 | RR =1.14, 95% CI =0.96–1.36 |
Note: Reproduced from Fusar-Poli P, Kempton MJ, Rosenheck RA. Efficacy and safety of second-generation long-acting injections in schizophrenia: a meta-analysis of ran-domized-controlled trials. Int Clin Psychopharmacol. 2013;28(2):57–66.8
SGA-LAIs included risperidone-LAI, paliperidone-LAI, and olanzapine-LAI.
Abbreviations: AOM, aripiprazole once monthly; CI, confidence interval; LAI, long acting injectables; NNH (T), number needed to harm (treat); PANSS, Positive and Negative Syndrome Scale; RR, relative risk; SD, standard deviation; SGA, second generation antipsychotic; SMD, standardized mean difference.
The main limitation of this study is the paucity of studies. In particular, future research should investigate the long-term efficacy of AOM and generate more safety data using larger samples. The second limitation is the difference in the clinical status of patients. One study13 included patients with acute schizophrenia and the others included chronic schizophrenia patients. The comparator also differed among studies, a placebo in two,13,14 OA in one (noninferiority study),15 and both OA and subthreshold AOM in the remaining one study.16 The third limitation is the difficulty comparing AOM with other LAIs and with OAPs other than aripiprazole. This study included only AOM and OA as antipsychotics, and therefore, we could not identify benefits and drawbacks of AOM over OAPs other than aripiprazole.
Conclusion
In conclusion, our results suggest that AOM is a well-tolerated treatment and improves the psychopathology of schizophrenia. Future research should investigate the long-term efficacy and generate more safety data for AOM.
Acknowledgments
We thank Otsuka Pharmaceutical Co., Ltd. for providing data.
Footnotes
Disclosure
Dr Oya has received speaker’s honoraria from Eli Lilly Meiji, and Otsuka. Dr Kishi has received speaker’s honoraria from Abbott, Astellas, Daiichi Sankyo, Dainippon Sumitomo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Shionogi, Tanabe-Mitsubishi, Tsumura, Novartis, and Pfizer. Dr Iwata has received speaker’s honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Shionogi, Novartis, and Pfizer, and research grants from GlaxoSmithKline and Otsuka. The authors declare no other conflicts of interest in this work.
References
- 1.Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. doi: 10.1016/S0140-6736(12)60239-6. [DOI] [PubMed] [Google Scholar]
- 2.Valenstein M, Blow FC, Copeland LA, et al. Poor antipsychotic adherence among patients with schizophrenia: medication and patient factors. Schizophr Bull. 2004;30(2):255–264. doi: 10.1093/oxfordjournals.schbul.a007076. [DOI] [PubMed] [Google Scholar]
- 3.Masand PS, Narasimhan M. Improving adherence to antipsychotic pharmacotherapy. Curr Clin Pharmacol. 2006;1(1):47–56. doi: 10.2174/157488406775268255. [DOI] [PubMed] [Google Scholar]
- 4.Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. doi: 10.1002/wps.20060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEvoy JP. Risks versus benefits of different types of long-acting injectable antipsychotics. J Clin Psychiatry. 2006;67(suppl 5):15–18. [PubMed] [Google Scholar]
- 6.Kirk Morton N, Zubek D. Adherence challenges and long-acting injectable antipsychotic treatment in patients with schizophrenia. J Psychosoc Nurs Ment Health Serv. 2013;51(3):13–18. doi: 10.3928/02793695-20130215-01. [DOI] [PubMed] [Google Scholar]
- 7.Lindenmayer JP, Jarboe K, Bossie CA, Zhu Y, Mehnert A, Lasser R. Minimal injection site pain and high patient satisfaction during treatment with long-acting risperidone. Int Clin Psychopharmacol. 2005;20(4):213–221. doi: 10.1097/00004850-200507000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Fusar-Poli P, Kempton MJ, Rosenheck RA. Efficacy and safety of second-generation long-acting injections in schizophrenia: a meta-analysis of randomized-controlled trials. Int Clin Psychopharmacol. 2013;28(2):57–66. doi: 10.1097/YIC.0b013e32835b091f. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita T, Fujii Y, Misawa F. Neuroleptic malignant syndrome associated with risperidone long-acting injection: a case report. J Clin Psychopharmacol. 2013;33(1):127–129. doi: 10.1097/01.jcp.0000426180.89572.51. [DOI] [PubMed] [Google Scholar]
- 10.Haddad PM, Kishimoto T, Correll CU, Kane JM. Ambiguous findings concerning potential advantages of depot antipsychotics: in search of clinical relevance. Curr Opin Psychiatry. 2015;28(3):216–221. doi: 10.1097/YCO.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957–965. doi: 10.4088/JCP.13r08440. [DOI] [PubMed] [Google Scholar]
- 12.Kane JM, Kishimoto T, Correll CU. Assessing the comparative effectiveness of long-acting injectable vs oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J Clin Epidemiol. 2013;66:8Sul. doi: 10.1016/j.jclinepi.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane JM, Peters-Strickland T, Baker RA, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(11):1254–1260. doi: 10.4088/JCP.14m09168. [DOI] [PubMed] [Google Scholar]
- 14.Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617–624. doi: 10.4088/JCP.11m07530. [DOI] [PubMed] [Google Scholar]
- 15.Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205(2):135–144. doi: 10.1192/bjp.bp.113.134213. [DOI] [PubMed] [Google Scholar]
- 16.Ishigooka J, Nakamura J, Fujii Y, et al. Efficacy and safety of aripiprazole once-monthly in Asian patients with schizophrenia: a multicenter, randomized, double-blind, non-inferiority study versus oral aripiprazole. Schizophr Res. 2015;161(2–3):421–428. doi: 10.1016/j.schres.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 18.Green S. Systematic reviews and meta-analysis. Singapore Med J. 2005;46(6):270–273. quiz 274. [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 21.Guy W, Bonato RR. Manual for the ECDEU Assessment Battery. 2nd ed. Chevy Chase, MD: National Institute of Mental Health; 1970. [Google Scholar]
- 22.Simpson GM, Lee JH, Zoubok B, Gardos G. A rating scale for tardive dyskinesia. Psychopharmacology (Berl) 1979;64(2):171–179. doi: 10.1007/BF00496058. [DOI] [PubMed] [Google Scholar]
- 23.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 24.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heres S, Hamann J, Mendel R, et al. Identifying the profile of optimal candidates for antipsychotic depot therapy A cluster analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1987–1993. doi: 10.1016/j.pnpbp.2008.09.025. [DOI] [PubMed] [Google Scholar]